
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Energy Res., 23 June 2020
Sec. Electrochemical Energy Storage
Volume 8 - 2020 | https://doi.org/10.3389/fenrg.2020.00125
This article is part of the Research TopicThree-Dimensional Carbon Architectures for Energy Conversion and StorageView all 10 articles
The past decade has witnessed the boom of porous carbon materials, especially three-dimensional (3D) ones, as supercapacitor electrode materials. Their large surface areas, tunable pore volumes, adjustable degrees of pore interconnectivity, and the potential to possess hierarchical porous networks have enabled them to achieve simultaneously high capacitance and excellent rate capability. This feature is challenging for non-3D counterparts but favorable for supercapacitors. However, behind the prosperity come the problems, and unfortunately, most of the issues are left unacknowledged and unaddressed in the literature available. This perspective aims to identify these problems, discuss the reasons for overlooking, and provide possible solutions to address these issues. The author wishes this article will draw the attention of researchers in electrochemical energy storage to tackle these roadblocks in the future development of 3D porous carbon electrodes in supercapacitors.
Accompanying with the boom of electrochemical energy storage, three-dimensional (3D) porous carbon materials have emerged as rising stars of electrodes in supercapacitors. Supercapacitors, including electrical double-layer capacitors and pseudocapacitors, are electrochemical energy storage devices with power density typically about 100 times higher than those of rechargeable batteries. Their high power density bestows their applications in ultrafast charging and discharging scenarios (Miller and Simon, 2008; Salunkhe et al., 2014). As indispensable components in supercapacitors, electrodes having ultrasmall electrical resistance and ultrahigh surface areas are preferred. Therefore, a diverse array of highly conductive carbon materials with tunable morphologies have become supercapacitor electrode candidates for decades.
One of the representative conventional supercapacitor carbon electrodes is activated carbon. These carbon particles possess abundant micropores and surface areas surpassing 1,000 m2 g−1. They store charges mainly via the formation of electrical double layers and are used in electrical double-layer capacitors (Ji et al., 2014) or capacitive electrodes in supercapacitor-battery hybrids (Liu H. et al., 2020). Activated carbon, however, is far from ideal. Its disadvantage is two-folded. On the one hand, its powdered form demands polymer binders when being processed into electrodes, which introduces unnecessary internal resistance born from the particle-particle contact resistance as well as the low electrical conductivity of the binders (Liu T. et al., 2020). On the other hand, the micropore-dominated porous structure of activated carbon impedes ion diffusion throughout a particle, limiting the pore accessibility and, subsequently, the capacitance and energy density (Zhang et al., 2017). These two drawbacks make activated carbon unsuitable for storing large amounts of charges at elevated charge and discharge rates, the conditions under which supercapacitors are supposed to work.
The shortcomings of conventional powdered porous carbon materials have motivated the development of three-dimensional porous carbons as supercapacitor electrodes within the past decade. 3D porous carbons with high surface areas and easy ion-accessible pores allow capacitance and remarkable rate capability mutually achievable (Liu et al., 2017). Besides, the structural and morphological diversity and tunability derived from the versatility in sources and synthesis processes have generated a tremendous number of 3D carbon supercapacitor electrodes in the literature (Zhu et al., 2016; Zhang et al., 2017, 2019; Yang et al., 2019). The variety in structural parameters, including surface areas, pore interconnectivity, pore volumes, pore size distributions, and surface functionalities, is far more significant than those of non-3D carbon counterparts. These characteristics have also pushed the capacitance of carbon materials to beyond 300 F g−1 (Liu et al., 2017), a level unattainable by conventional porous carbon materials such as activated carbon.
The thrive of 3D porous carbon materials, however, brings about issues that compromise the scientific quality and integrity of relevant works. Unfortunately, most of the problems have been left unacknowledged and unaddressed (Figure 1). Without exhaustively examining the literature, this perspective aims to analyze these critical issues that the author hopes to initiate discussions and cultivate consensuses among the researchers in the field of electrochemical energy storage. Corresponding solutions are given in each section below.
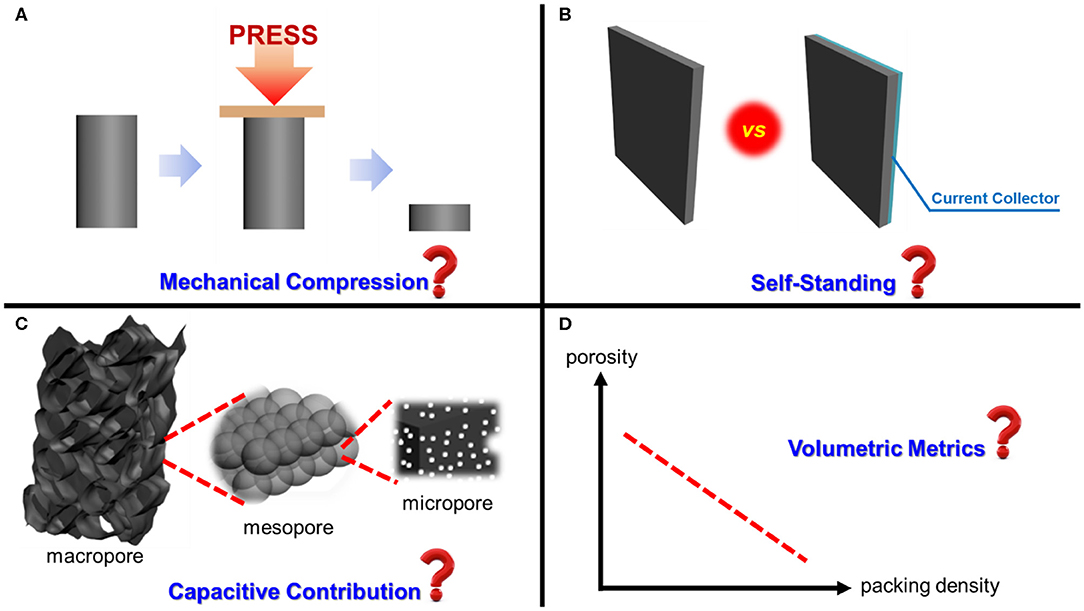
Figure 1. Four typical overlooking issues associated with three-dimensional porous carbon supercapacitor electrodes: (A) Compressing damages 3D structures; (B) Correctly use of “self-standing;” (C) Explaining capacitive contributions of different pores; (D) Acknowledging the trade-off between porosity and packing density in terms of volumetric metrics.
Mechanical pressing or calendering, which improve the contact between carbon materials and their current collectors, are needed for manufacturing supercapacitor electrodes. These processes not only reduce the contact resistance of supercapacitor electrodes (Dsoke et al., 2013), but also increase the volumetric performance metrics such as volumetric capacitance, energy density, and power density, due to volumetric densification. As a result, most researchers mechanically compress their as-prepared 3D carbon materials onto conductive substrates, e.g., nickel foam (Zhang et al., 2017) and aluminum foil (Shah et al., 2020). In some cases, 3D porous carbon materials are ground into powders, blended with binders and carbon black (electrically conductive additive) when making electrodes (Deng et al., 2019; Nawwar et al., 2020). Inevitably, the delicate 3D architectures of the active materials are substantially altered, if not entirely demolished, after compression or grinding. Therefore, claiming that hierarchical porous structures facilitate ion diffusion or render outstanding rate capability based on morphologies before compression or grinding is inappropriate to reflect the actual working conditions of 3D porous carbon materials.
The most straightforward resolution is to avoid compressing or grinding 3D porous carbon materials. For example, Zhu et al. attached compressed Ni foam onto 3D-printed graphene aerogels using silver paste (Zhu et al., 2016), and Zhai et al. soldered copper foil onto Ni-foam-templated graphene foam (Zhai et al., 2015), both of which preserved the 3D morphologies of the active materials. However, silver or solder are prone to oxidation under positive biases, giving rise to unwanted oxidation current and redox peaks. These problems require proper sealing of the contact points (e.g., by epoxy) that complicates the fabrication, adds electrode dead mass, and leads to substantial performance deviations due to the variation in the sealant coverage. Additionally, the contact resistance might be large or small, depending on the adhesion strength between the electrodes and the current collectors. This factor makes the electrochemical performance irreproducible.
A relatively simple solution is to acknowledge the necessity of compression, but provide comprehensive characterizations to support discussions. Probing the morphologies of compressed 3D porous carbon materials is necessary. Based on the author's experience, compression only shrinks macropores. Still, it preserves mesopores and micropores, so the surface area of a 3D porous carbon material will unlikely decrease significantly compared to its uncompressed form. If macropores are partially maintained, the 3D porous network of the carbon material is still retained, though the morphology is different. Grinding, on the contrary, will most likely destroy 3D architectures entirely to broken particles and hence, is not recommended for processing 3D porous carbon materials. Top-view and cross-sectional-view scanning electron microscopy images are critical to illustrate the compressed morphologies of the active materials. Characterizing the compressed carbon materials with gas physisorption is imperative to gauge any subtle changes in the surface areas and pore size distributions. Both techniques provide foundations for discussing the structure-performance relationship of 3D porous carbon electrodes. For readers to gain comprehensive understandings of the reported materials, supplementing typical characterization results of as-prepared 3D porous carbon materials with those of compressed counterparts is encouraged.
Self-standing has emerged as a fashion term to describe the feature of 3D porous carbon electrodes. However, arbitrarily using this word without considering the characteristic of the electrodes can lead to false and misleading information.
The difference between “self-standing material” and “self-standing electrode” must be strictly distinguished. Monolithic 3D porous carbon materials should be self-standing by nature, but whether they constitute self-standing electrodes depend on the fabrication method. If binders are used to bind 3D carbon materials or their grounded granules when making electrodes, such binder-containing electrodes can no longer be called self-standing. Rigorously speaking, electrodes are not self-standing if current collectors are used; however, the author believes that removing current collectors will create unnecessary technical challenges to the evaluation of supercapacitor electrodes, so calling binder-free electrodes self-standing is acceptable.
This issue calls for the transparency of experimental conditions. Authors reporting 3D carbon electrodes in supercapacitors or electrochemical energy storage, if broadly speaking, need to present explicitly how the electrodes are made. These conditions include the use of current collectors, compositions and manufacturers of current collectors, how carbon materials are adhered to current collectors (e.g., use of binders or direct compressing), as well as the mass loadings of active materials. The bottom line is that any researchers should be able to reproduce the experiments following the provided technical details with absolutely no confusion.
One of the unique structural features of 3D porous carbon materials is their hierarchically porous structures, that is, structures with interconnecting pores of different sizes (Figure 2) assembled into hierarchical networks. Porous materials that contain multiscale but spatially isolated pores are not hierarchical, because their pores cannot “communicate” with each other during charge and discharge processes. Due to the diversified pore sizes and shapes, differentiating the capacitive contributions of the pores becomes increasingly critical to rationalize the design of next-generation porous carbon supercapacitor electrodes.
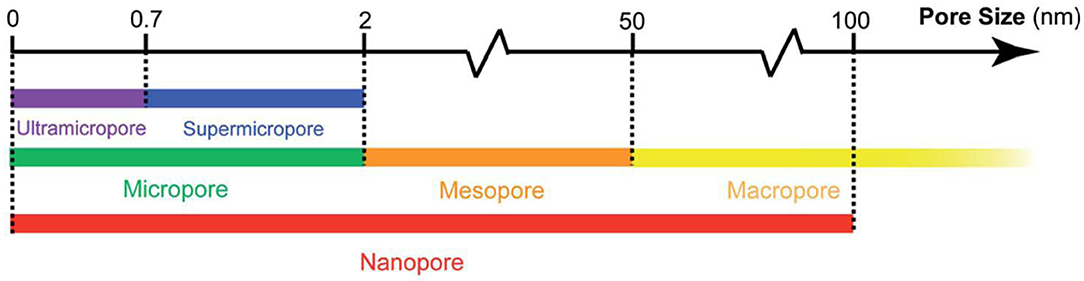
Figure 2. Nomenclatures of pores, according to the International Union of Pure and Applied Chemistry (IUPAC) (Thommes et al., 2015). Figure reproduced with permission from Liu et al. (2017). Copyright 2017, Royal Society of Chemistry.
Despite extensive investigations, the contributions of different pores have yet reached an agreement. The general belief is that macropores and mesopores contribute little to capacitance, but they help electrolyte infiltration and facilitate ion diffusion, both of which improve the ion accessibility to micropores (Yang et al., 2017). Zhang et al. have experimentally verified that sub-micron macropores and mesopores in a chitosan-derived carbon aerogel are indispensable for maintaining its excellent rate capability (Zhang et al., 2017). Micropores are mainly responsible for high surface areas and ion storage, resulting in capacitance. The general belief, however, is challenged by simulation works that show macropores and mesopores are unnecessary, as they facilitate electrolyte infiltration but not ion diffusion (Borchardt et al., 2018). Others have revealed that micropores of pore openings less than 1 nm can accelerate ion diffusion due to the ion packing patterns in ionophobic pores (Mo et al., 2020). Although the author is puzzled about the exact causes of the discrepancy between the simulation results and experimental observations, a possible reason is the different experimental setups. Most simulations choose ionic liquids as study objects because of their simplicity (no solvent molecules and ion solvation) to reduce calculation costs. In contrast, the majority of laboratory supercapacitors adopt aqueous electrolytes because of their safety, ease of fabrication, and intrinsically rapid ion diffusion.
Besides simulations, some experimental observations also contradict the general belief. For example, depending on the source, commercial activated carbon can contain mesopores slightly bigger than 2 nm, according to N2-physisorption (Liu T. et al., 2020), but their capacitance still decreases dramatically at elevated discharging rates. Carbide-derived carbons, though possessing large numbers of mesopores and macropores, still exhibit satisfactory rate capability in aqueous electrolytes (Huang et al., 2016).
These outliers in the literature highlight the complex roles of various pores. Perhaps the pore geometry, surface functionality, and graphitization degrees of 3D porous carbon electrodes all profoundly alter the electrochemical responses. For example, Dyatkin et al. simulated that the oxygen functional groups inside pores attracted planar anions in ionic liquids. This attraction aligned the anions in parallel to the pore surface and provided relatively clear transport channels to enhance rate capability (Dyatkin et al., 2016). The alliance between experimentalists and theorists, as well as alignment between experimental conditions and computational inputs, are undoubtedly indispensable to drive future investigations on the charge-storage mechanisms of 3D porous carbon electrodes. Additionally, the progress of advanced characterization methods, including quasi-elastic neutron scattering (Osti and Mamontov, 2020), quartz crystal microbalance (Levi et al., 2016), in-situ nuclear magnetic resonance spectroscopy (Forse et al., 2017), are vital tools for future investigations. Supporting experimental findings with simulation results in a research article is strongly encouraged if condition permits. This practice may become a new norm for publishing manuscripts in top-tier journals and can reward interdisciplinary researches with crystal-clear mutual communications among all the practitioners involved. The author believes that the role-determination of pores in versatile 3D porous carbon materials will remain as frontiers in electrochemical energy storage.
Perhaps the most common criticism of 3D porous carbon electrodes is their large porosity that declines the packing density of an electrode. Small packing density leads to low volumetric performance metrics, such as volumetric capacitance.
While the author fully agrees that volumetric metrics are crucial for rechargeable batteries whose working space is tight (Choi and Aurbach, 2016), the working environments of supercapacitors are far more diversified than those of batteries. 3D porous carbon materials with large porosity have the advantage of low volumetric density or lightweight. This characteristic makes them especially suitable for portable electronics needing high power density, such as drills and drones (Lin et al., 2018). In such cases, the volume of an energy-storage device is less considerable than its weight. Therefore, the author believes that it is unfair to criticize 3D porous carbon electrodes based on their low packing density. Comprehensive evaluations of a 3D porous carbon electrode and reporting a full set of gravimetric, areal, and volumetric performance metrics are highly recommended.
Another issue calling attention is the report of the gravimetric capacitance of 3D porous carbon materials. The relationship between the total capacitance (CT) and gravimetric capacitance, or specific capacitance (Cm), of an electrode, is:
where m is the mass of active materials. Based on this equation, achieving ultrahigh numbers of gravimetric capacitance becomes diminishingly meaningful if the mass loading of the active material is limited, as the total capacity is insignificant. Therefore, it is always a good practice to report gravimetric performance in the context of mass loading. For commercial viability, the mass loading of an electrode material must be 10 mg cm−2 or above (Chen et al., 2020). Still, this standard should not be rigid to restrict the development of laboratory-scale supercapacitors, which usually involve mass loadings of active materials between 1 and 10 mg cm−2. Nevertheless, explicitly reporting mass loading and accurately characterizing the influence of mass loading on electrochemical performance are always welcome and practically meaningful (Lin et al., 2015). Additionally, as areal capacitance depends on mass loading, reporting both gravimetric capacitance and areal capacitance for single electrodes is beneficial to avoid deceitfully excellent charge-storage performance.
This perspective highlights four main issues behind the prosperity of 3D porous carbon materials as supercapacitor electrodes. They are mostly overlooked in the existing literature, and if continually left unaddressed, are harmful to the healthy development of supercapacitors. Hence, the article presents the current understanding, points out the existing challenges, and discusses the possible resolutions of each issue. The author hopes this perspective will draw the attention of the practitioners in the electrochemical energy storage community to address the issues by intimate collaboration, protocol unification, and frequent idea exchange in the ensuing years.
The author confirms being the sole contributor of this work and has approved it for publication.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We acknowledges Dr. Francesco Coneri for his assistance in arranging the submission of this article.
Borchardt, L., Leistenschneider, D., Haase, J., and Dvoyashkin, M. (2018). Revising the concept of pore hierarchy for ionic transport in carbon materials for supercapacitors. Adv. Energy Mater. 8:1800892. doi: 10.1002/aenm.201800892
Chen, R., Yu, M., Sahu, R. P., Puri, I. K., and Zhitomirsky, I. (2020). The development of pseudocapacitor electrodes and devices with high active mass loading. Adv. Energy Mater. 10:1903848. doi: 10.1002/aenm.201903848
Choi, J. W., and Aurbach, D. (2016). Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 1:16013. doi: 10.1038/natrevmats.2016.13
Deng, X., Zhua, S., Li, J., He, F., Liu, E., He, C., et al. (2019). Bio-inspired three-dimensional carbon network with enhanced mass-transfer ability for supercapacitors. Carbon 143, 728–735. doi: 10.1016/j.carbon.2018.11.055
Dsoke, S., Tian, X., Täubert, C., Schlüter, S., and Wohlfahrt-Mehrens, M. (2013). Strategies to reduce the resistance sources on electrochemical double layer capacitor electrodes. J. Power Sources 238, 422–429. doi: 10.1016/j.jpowsour.2013.04.031
Dyatkin, B., Zhang, Y., Mamontov, E., Kolesnikov, A. I., Cheng, Y., Meyer, H. M., et al. (2016). Influence of surface oxidation onion dynamics and capacitance in porous and nonporous carbon electrodes. J. Phys. Chem. C 120, 8730–8741. doi: 10.1021/acs.jpcc.6b01204
Forse, A. C., Griffin, J. M., Merlet, C., Carretero-Gonzalez, J., Raji, A.-R. O., Trease, N. M., et al. (2017). Direct observation of ion dynamics in supercapacitor electrodes using in situ diffusion NMR spectroscopy. Nat Energy 2:16216. doi: 10.1038/nenergy.2016.216
Huang, P., Lethien, C., Pinaud, S., Brousse, K., Laloo, R., Turq, V., et al. (2016). On-chip and freestanding elastic carbon films for micro-supercapacitors. Science 351, 691–695. doi: 10.1126/science.aad3345
Ji, H., Zhao, X., Qiao, Z., Jung, J., Zhu, Y., Lu, Y., et al. (2014). Capacitance of carbon-based electrical double-layer capacitors. Nat. Commun. 5:3317. doi: 10.1038/ncomms4317
Levi, M. D., Daikhin, L., Aurbach, D., and Presser, V. (2016). Quartz crystal microbalance with dissipation monitoring (EQCM-D) for in-situ studies of electrodes for supercapacitors and batteries: a mini-review. Electrochem. Commun. 67, 16–21. doi: 10.1016/j.elecom.2016.03.006
Lin, T., Chen, I. W., Liu, F., Yang, C., Bi, H., Xu, F., et al. (2015). Nitrogen-doped mesoporous carbon of extraordinary capacitance for electrochemical energy storage. Science 350, 1508–1513. doi: 10.1126/science.aab3798
Lin, Z., Goikolea, E., Balducci, A., Naoi, K., Taberna, P. L., Salanne, M. G., et al. (2018). Materials for supercapacitors: When Li-ion battery power is not enough. Mater. Today 21, 419–436. doi: 10.1016/j.mattod.2018.01.035
Liu, H., Liu, X., Wang, S., Liu, H.-K., and Li, L. (2020). Transition metal based battery-type electrodes in hybrid supercapacitors: a review. Energy Stor. Mater. 28, 122–145. doi: 10.1016/j.ensm.2020.03.003
Liu, T., Serrano, J., Elliott, J., Yang, X., Cathcart, W., Wang, Z., et al. (2020). Exceptional capacitive deionization rate and capacity by block copolymer–based porous carbon fibers. Sci. Adv. 6:eaaz0906. doi: 10.1126/sciadv.aaz0906
Liu, T., Zhang, F., Song, Y., and Li, Y. (2017). Revitalizing carbon supercapacitor electrodes with hierarchical porous structures. J. Mater. Chem. A 5, 17151–17173. doi: 10.1039/c7ta05646j
Miller, J. R., and Simon, P. (2008). Materials science. Electrochemical capacitors for energy management. Science 321, 651–652. doi: 10.1126/science.1158736
Mo, T., Bi, S., Zhang, Y., Presser, V., Wang, X., Gogotsi, Y., et al. (2020). Ion structure transition enhances charging dynamics in subnanometer pores. ACS Nano 14, 2395–2403. doi: 10.1021/acsnano.9b09648
Nawwar, M., Sahu, R. P., Puri, I. K., and Zhitomirsky, I. (2020). Functionally decorated carbon nanotube networks for energy storage in supercapacitors. Front. Energy Res. 8:46. doi: 10.3389/fenrg.2020.00046
Osti, N. C., and Mamontov, E. (2020). Microscopic dynamics in room-temperature ionic liquids confined in materials for supercapacitor applications Sust. Energy Fuels 4, 1554–1575. doi: 10.1039/C9SE00829B
Salunkhe, R. R., Lee, Y. H., Chang, K. H., Li, J. M., Simon, P., Tang, J., et al. (2014). Nanoarchitectured graphene-based supercapacitors for next-generation energy-storage applications. Chemistry 20, 13838–13852. doi: 10.1002/chem.201403649
Shah, S. A., Kulhanek, D., Sun, W., Zhao, X., Yu, S., Parviz, D., et al. (2020). Aramid nanofiber-reinforced three-dimensional graphene hydrogels for supercapacitor electrodes. J. Colloid Interface Sci. 560, 581–588. doi: 10.1016/j.jcis.2019.10.066
Thommes, M., Kaneko, K., Neimark, A. V., Olivier, J. P., Rodriguez-Reinoso, F., Rouquerol, J., et al. (2015). Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 87, 1051–1069. doi: 10.1515/pac-2014-1117
Yang, J., Wu, H., Zhu, M., Ren, W., Lin, Y., Chen, H., et al. (2017). Optimized mesopores enabling enhanced rate performance in novel ultrahigh surface area meso-/microporous carbon for supercapacitors. Nano Energy 33, 453–461. doi: 10.1016/j.nanoen.2017.02.007
Yang, S., Wang, S., Liu, X., and Li, L. (2019). Biomass derived interconnected hierarchical micro-meso-macro- porous carbon with ultrahigh capacitance for supercapacitors. Carbon 147, 540–549. doi: 10.1016/j.carbon.2019.03.023
Zhai, T., Lu, X., Wang, H., Wang, G., Mathis, T., Liu, T., et al. (2015). An electrochemical capacitor with applicable energy density of 7.4 Wh/kg at average power density of 3000 W/kg. Nano Lett. 15, 3189–3194. doi: 10.1021/acs.nanolett.5b00321
Zhang, F., Liu, T., Li, M., Yu, M., Luo, Y., Tong, Y., et al. (2017). Multiscale pore network boosts capacitance of carbon electrodes for ultrafast charging. Nano Lett. 17, 3097–3104. doi: 10.1021/acs.nanolett.7b00533
Zhang, Y., Yang, S., Wang, S., Liu, X., and Li, L. (2019). Microwave/freeze casting assisted fabrication of carbon frameworks derived from embedded upholder in tremella for superior performance supercapacitors. Energy Storage Mater. 18, 447–455. doi: 10.1016/j.ensm.2018.08.006
Keywords: three-dimensional, pore, carbon, supercapacitor, electrodes, overlooking issue
Citation: Liu T (2020) Overlooking Issues and Prospective Resolutions Behind the Prosperity of Three-Dimensional Porous Carbon Supercapacitor Electrodes. Front. Energy Res. 8:125. doi: 10.3389/fenrg.2020.00125
Received: 29 April 2020; Accepted: 25 May 2020;
Published: 23 June 2020.
Edited by:
Jun Yan, Harbin Engineering University, ChinaReviewed by:
Hao Chen, Zhejiang Agriculture and Forestry University, ChinaCopyright © 2020 Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tianyu Liu, dGxpdTIzQHZ0LmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.