
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Energy Res. , 05 July 2018
Sec. Bioenergy and Biofuels
Volume 6 - 2018 | https://doi.org/10.3389/fenrg.2018.00063
 Maria Joseph Angelaalincy1
Maria Joseph Angelaalincy1 Rathinam Navanietha Krishnaraj2
Rathinam Navanietha Krishnaraj2 Ganeshan Shakambari1
Ganeshan Shakambari1 Balasubramaniem Ashokkumar3
Balasubramaniem Ashokkumar3 Shanmugam Kathiresan4
Shanmugam Kathiresan4 Perumal Varalakshmi1*
Perumal Varalakshmi1*Microbial fuel cells (MFCs) are emerging as a promising future technology for a wide range of applications in addition to sustainable electricity generation. Electroactive (EA) biofilms produced by microorganisms are the key players in the bioelectrochemical systems involving microorganism mediated electrocatalytic reactions. Therefore, genetically modifying the organism for increased production of EA biofilms and improving the extra electron transfer (EET) mechanisms may attribute to increase in current density of a MFC and an increased COD removal in wastewater treatment plant coupled MFC systems. Extracellular polysaccharides (EPS) produced by the organisms attribute to both biofilm formation and electron transfer. Although cell surface modification, media optimization and operation parameters validation are established as enhancement strategies for a fuel cell performance, engineering the vital genes involved in electroactive biofilm formation is the future hope. Therefore, in this review we critically address the biofilm formation mechanisms in electro active microorganisms, strategies for improving the biofilm formation leading to improved electrocatalytic rates for applications in bioelectrochemical systems.
The inadequate supply of fossil fuels (Demirbas, 2005; Panwar et al., 2011), ever growing population and the escalating energy demand, in the recent years, has become one of the biggest bottlenecks to human survival and economy. Other than this, the associated problems like global warming and pollution (Davis and Higson, 2007) are major impetus for researchers to explore alternative energy sources which are renewable, sustainable and economical. Though wind power and solar cells have already been harnessed and brought into commercial use, fuel cells, which are equally promising, are still the least explored (Angelaalincy et al., 2016).
Another prime concern other than energy demand, in developing countries, is the increasing levels of wastewater (Liu and Ramnarayanan, 2004; Gude, 2015). Incidentally both these concerns can be alleviated by harnessing the microbes for remediating the wastes while colonizing on electrodes with a biofilm and serving as live or microbial fuel cells (MFC) (Aelterman et al., 2006; Li et al., 2013; Chaturvedi and Verma, 2016). The microbes employed in MFCs convert the chemical energy present in organic compounds to electrical energy through catalysts (Chaudhuri and Lovley, 2003). Most commonly, MFCs may at times employ bacteria on the anode to carry out oxidation of organic matters and bacteria or microalgae on the cathode to undergo reduction. Compared to other bioenergy conversion processes like anaerobic digestion, gasification and fermentation, MFCs have an added advantage of reduced amounts of secondary pollutants production (Chouler et al., 2016) and cost-effective operation, as they operate under ambient environmental conditions (Park and Zeikus, 2003). Irrespective of their role in wastewater treatment and electricity generation, MFC based biosensors are of great interest in the recent years pertaining to their advantages such as high sensitivity, stability and remote site applicability without electricity supply. MFC-based biosensor devices have been to test microorganism load, BOD, presence of corrosive biofilms, cytotoxic elements and microbial activity monitoring (Yang et al., 2015a).
Therefore, identifying the loop-holes that hinder MFC performance and its multi facetted applications, has become essential in setting up an effective MFC system. While discussing the role of microorganisms in MFC, it is essential to understand the mechanism of electron transfer contributing to electricity generation. Microorganisms mostly grow on the electrodes to form a biofilm, which is an extracellular polysaccharide (EPS) enthralled surface harboring the microbial community (Rollefson et al., 2011). The EPS physically immobilizes the bacteria, however paves way for cell to cell contact and communication. This cell to cell communication, involves in the electron transfer and electron- electrode interaction in MFCs (Patil et al., 2012; Sarjit et al., 2015). Moreover, the efficiency in electron transfer is inversely proportional to the distance over which the electrons travel to reach the electron acceptor (Breuer et al., 2013).
Therefore, a clear view on the role of biofilms on electron transfer will provide an insight on development of new approaches for improving the performances of microbial fuel cells apart from increased wastewater treatment efficiency. Irrespective of the several operational parameters that influence a fuel cell performance, the extracellular electron transfer (EET) mechanism (Schröder, 2007) and biofilm production (Zhang et al., 2011) in the employed microorganisms always have a positive influence toward power production. Therefore, approaches for biofilm engineering, which involves genetic and surface modification techniques are considered as new avenues in fuel cell research. This review aims to provide a deep insight on the role of extracellular polysaccharides (EPS) in biofilm formation and the role of biofilm in current generation in microbial fuel cells.
An overview of the basic elements that comprise a MFC is mandatory to understand the major role played by biofilm producing microbes in a fuel cell. Microbial fuel cells are generally made of a cathode, an anode, a PEM (proton exchange membrane) and a resistor, through which the electrons travel to the anode. In most cases, the anode is entrapped within the bacterial consortium (Gouveia et al., 2014). Sometimes, it may contain the organic material to be oxidized or the fuel source (Zhao et al., 2005), however, oxidation occurs at the anode. The cathode is provided with the desired source microbe. After oxidation at the anode protons pass through the PEM to the cathode, where they get reduced into water (He et al., 2014; Figure 1). This is a double—chambered MFC that exists more commonly. A single chambered MFC, on the other hand, contains a single chamber harboring both the anode and the cathode together (Singh et al., 2016) or only the anodic compartment, coupled with an air—cathodic chamber (Tharali et al., 2016). The single chamber MFC lacks a PEM. The various proposed designs for devising a single chamber MFCs have been mentioned in Tharali et al. (2016).
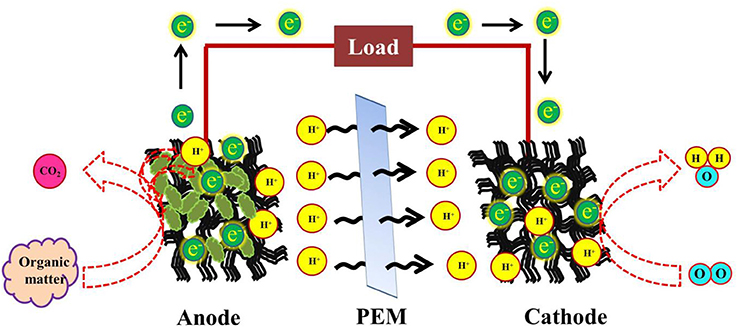
Figure 1. Schematic representation of simultaneous waste reduction and electricity generation in a Microbial fuel cell, employing photosynthetic organisms at the anode.
The types of MFCs that operate with a trielectrode system are commonly employed in electrochemical studies (Angelaalincy et al., 2017). The setup consists of three electrodes: the working electrode which acts as a cathode, mostly made up of glassy carbon or platinum electrode that accommodates the microbial consortium; the counter electrode, that functions as the electricity conductor and the reference electrode which is the standard electrode made up of silver in potassium chloride or silver. The current produced is recorded with the help of a cyclic voltammeter.
MBFC (Microbial biofuel cells) contain two chambers made up of polycarbonate, acrylic glass or glass, plexiglass (Du et al., 2007), or plastic bottles (Parkash et al., 2015), or cans (Obasi et al., 2012), holding the two electrodes. In MBFCs a large surface area and a robust structure are mandatory for supporting the biofilm, withstanding water current. Therefore, an assortment of electrode materials such as carbon paper, carbon cloth, graphite plates, granules, rods and RVCs (Reticulated vitreous carbon) are employed in the construction of a fuel cell. The range of cathodic materials is quiet stringent and is limited to the usage of precious metals like platinum (Pt). At times, Pt is replaced with transient materials such as iron and cobalt mixture catalysts, which are yet to be studied in detail (Cheng et al., 2006; Zhao et al., 2006). However, the platform for ambient microbial growth and biofilm formation is mandatory while constructing a MBFC.
The biofilm that forms under natural circumstances increases in density with the age of the culture, however is not electrochemically active throughout, due to the formation of an inner lining of dead or inactive cells in the biofilm matrix (Sun et al., 2016). Therefore, a strategy that induces live or active microbes to produce more electro active biofilms is considered to be a promising approach for improving the power performance of MFCs. In order to achieve this, the major constituents of a biofilm and their role in biofilm formation needs to be understood.
Microbial biofilms have encountered an evolutionary increase in their complexity with due course of time (Kreft and Wimpenny, 2001). A metaphorical representation of biofilms states them as the “city of microbes,” where the EPS are mentioned as the “house of the biofilm cells.” The water content, charge, porosity, hydrophobicity, density, sorption properties and mechanical stability of the biofilm cells are affected by the EPS thus determining the immediate conditions of life (Flemming et al., 2007). In fact, the organisms are found embedded in these biopolymers. The higher the secretion of EPS by the organism, the greater is the density of the biofilm. A denser biofilm harbors more number of organisms than a lighter one. Therefore, the density of the biofilm increases with respect to the age of the organisms. As mentioned early, biofilm formation on the anode, has a pivotal role in MFCs. However, the electrochemical performance is not determined by the density but by the temporal and spatial locations of live and dead cells within a biofilm (Sun et al., 2015). Studies in electro active bacteria Geobacter sulferreducens have evidenced the rapid drop of charge transfer resistance in the presence of rapidly multiplying live cells. However, with time, as the dead cells start to accumulate in the inner layer of the biofilms, there has been a high diffusion resistance observed in the electrochemical system. In such cases, it is inferred that, not the density of the biofilms, but the active electron transferring live organisms present in the outer layer of the biofilms, contribute to the high current generation of the system (Sun et al., 2016). In composition, apart from EPS, the biofim is also comprised of a major portion of polysaccharides and minor portions of glycoproteins, proteins, glycolipids, and negligible amount of nucleotides and in rare cases, some metals (Angelaalincy et al., 2017) that contribute to the structural and functional outlook of the biofilm. However, EPS comprises the major component of the biofilm matrix. Among the known bacterial EPS, at least three polysaccharides have been found to be active in biofilm formation.
They are the Psl, Pel, and alginate polysaccharides. Psl polysaccharides are EPS produced from Psl genes. Reports since 2004, evidently states the pivotal role of Psl polysaccharides in biofilm formation in Pseudomonas aeroginosa (Colvin et al., 2012; Wei and Ma, 2013). The Psl loci consist of 15 co-transcribed genes, out of which 11 contribute for the synthesis of Psl dependent biofilm (Friedman and Kolter, 2004; Jackson et al., 2004; Matsukawa and Greenberg, 2004). The formation of biofilm comprises of five sequential steps including initial attachment, irreversible attachment, microcolony formation, biofilm maturation and biofilm dispersion. Therefore, overexpression of these genes might attribute to increased biofilm formation by live or active microbes in a MFC.
Zhang et al. (2017) reported the role of these polysaccharides in biofilm initiation, which influenzes the surface motility of subsequent cells (Zhao et al., 2013). It has been observed that overproduction of Psl polysaccharides intensifies cell to cell interaction and intercellular adhesion, promoting the first and crucial step in biofilm formation (Ma et al., 2006; Byrd et al., 2009). The Psl polysaccharide attaches firmly in a helical shape on the bacterial cell wall promoting strong intercellular interactions (Ma et al., 2009) thus serving as a scaffold during biofilm matrix formation. During biofilm maturation, they are found attached on the periphery of the three dimensional structured colonies thus providing structure support and enabling biofilm dispersion at the end (Ma et al., 2009). Thus, EPS have a pivotal role in the formation, structural integrity, adhesion property, stability and life of biofilms and thus could be employed as crucial elements in biofilm engineering processes by increasing the copy number of these EPS producing genes.
Although EPS plays an irresistible role in biofilm formation, the process also depends on the external environmental factors and the gene expression mechanisms that contribute to the biofilms development in individual cells (Toyofuku et al., 2016). The physical and environmental factors to which the cells are subjected influence the biofilms formation along with the surface and extracellular components of the organisms. To be more specific, the lipopolysaccharides (LPS) and exopolysaccharides (EPS) alongs with quorum sensing (QS) signaling molecules prescribe the fate of biofilms formation (Nocelli et al., 2016). Henceforth, it is clear that the factors that externally influence EPS production, quorum sensing signaling molecules production and other stress factors such as heavy metal stress (Chen et al., 2015), salinity (Hong et al., 2016), pH (Christenson, 2011), nutrient starvation (Angelaalincy et al., 2017), nutrient depletion, pathogen invasion, growth substrate, water current in moving water bodies etc. (Angelaalincy et al., 2016) also contribute in influencing biofilm formation. However, the major factors involved in biofilms formation are QS signaling molecules and EPS and genetically engineering these molecules can overcome the other environmental impacts involved in biofilm formation. However, this needs substantial research.
It is well known that the bacterial community communicates with each other through cell to cell interaction thus coordinating their collective behavior. This requires release of autoinducers from the cells resulting in the phenomenon called quorum sensing. Monzon et al. (2016) reported a 95% increase in biofilm mass of Halanaerobium praevalence thereby contributing to a 30% increase in power density upon addition of 100 nM quinolone type signaling molecules. Quinolone are signaling molecules belonging to the LuxR family proteins coded by hmqF genes (Agarwal et al., 2012) in Halanaerobium species. On one hand the entire promotion of biofilms formation in Pseudomonas aeroginosa has been demonstrated (Diggle et al., 2002), where on the other hand, little is known about the effect of autoinducers from other bacteria in stimulating QS of Halanaerobium sp. (Monzon et al., 2016).
It has already been registered that apart from the QS signaling molecules, EPS and LPS are also found to play pivotal role in biofilms formation. Bacterial generation of c-di-GMP by diguanylate cyclases (DGC) at high levels enhance matrix exopolysaccharides such as Pel and Alginate synthesis attributing to biofilms formation. Among the three major polysaccharides, Psl, Pel, and alginate, enhanced production of Psl contributes for initiation and maintenance of biofilms structure (Ma et al., 2009), where Pel, a glucose rich extracellular matrix which is specific to gram negative bacteria, aids in the formation of solid surface associated biofilms for non-pilated organisms (Vasseur et al., 2005). Moreover, a bifunctional enzyme produced from algC gene in P. aeroginosa has been identified to be crucial for the biosynthesis of four polysaccharides viz. LPS, Psl, Pel, and alginate which influenze biofilms formation (Wei and Ma, 2013). Almost all the genes that produce any of the above said polysaccharides are invariably contributing biofilms formation in the organism (Wei and Ma, 2013).
Though the extracellular polysaccharides (EPS) play variety of roles in different cells, its function in electron transfer is largely intriguing. Polysaccharides have long since been known to be associated with cell to cell interaction and surface attachments and variations in composition of the EPS can have pleiotropic effects where the surfacecharge is modified and alters surface attachment, which provide an anchor for holding the peripheral proteins that involve cell-cell recognition events as revealed in studies with Shewanella (Korenevsky and Beveridge, 2007). Studies with Geobacter sulfurreducens have been a matter of intense research in this context where a report showed a role for EPS as attachment sites for peripheral redox proteins that allow multicellular communities to transfer electrons to distant acceptors, where the mutant that lacked the gene encoding exopolysaccharidematrix production failed to develop electrogenic biofilms onelectrodes. It was thus observed that G. sulfurreducenspossessed genes (e.g., xapA or xapK) that encode extracellular anchoring polysaccharides that contain binding sites for c-type cytochromes, are essential for the electron transfer to the electrode (Rollefson et al., 2011). Thus the EPS is evidenced to be a crucial factor not only in formation of biofilm but also in electron transfer in a fuel cell.
As mentioned earlier, a widely proposed use of MFCs, one of the several bioelectrochemical systems (BESs), is to focus on simultaneous wastewater treatment and electricity generation (Pant et al., 2012; He et al., 2016a; Santoro et al., 2017). The bottleneck of BESs is the ability of the electroactive microorganisms to participate in extracellular electron transfer (EET) (Yang et al., 2012). In this process of EET, microorganisms serve as electron transfer systems, using direct or mediated mechanisms (Franks, 2015). Direct EET occurs via electron transfer through outer membrane proteins (Shi et al., 2016) or through electrically active bacterial surfaces (Wrighton et al., 2011) which physically contacts the electrode, most probably the anode or the bacteria in the vicinity. In mediated EET, endogenously (e.g., phenazines) or exogenously soluble (eg. humics) mediator molecules, also called as redox shuttles (Qiao et al., 2008) that shuttles electrons from the cells to the anode through the extracellular aqueous matrix (Lovley, 2011) act as mediators. EET where metabolites or mediators are produced by one species and are consumed by another species in a consortia are called mediated interspecies electron transfer or MIET (Cheng and Call, 2016).
A detailed characterization of the various microbes present in the consortia of exoelectrogenic biofilms provides insight into the processes to convert complex organic matter in wastewater streams into electrical current in bioelectrochemical systems (BESs) (Kiely et al., 2011). The past decade has also provided evidence of yet another method, direct interspecies electron transfer (DIET), that happen between organisms or in association with electrically conductive materials and it has been reviewed that they can be stimulated in engineered systems to improvise on required waste treatment goals and also for energy recovery in microbial electrochemical technologies (Cheng and Call, 2016). Thus the role of biofilm in BESs can hardly be understated and hence it allows the focus on extracellular polysaccharides production by the microorganisms that facilitate biofilm formation.
The mechanism of electricity generation in MFC can be classified is of two types: the direct electron transfer and indirect electron transfer.
In the first type of MFC, the bacteria transfer the electrons from its membrane to the electrode directly without the intervention of an intermediate fermentation product (Angelaalincy et al., 2016). This is called as the direct transfer. These microbial fuel cells impose the selection of highly active microbial consortium which is either mixed or pure, as these microbes are the catalysts functioning in electrons transfer. The transfer is aided through proteins (cytochrome) that are immobilized on the bacterial cell wall. Rhodoferax ferrireducens (Liu et al., 2007) and Geobacter sulfurreducens (Bond and Lovley, 2003) can be stated as examples of this type of bacteria (Roller et al., 2008). Physical contact of the bacterial membrane possessing EPS or membrane organelle with the fuel cell electrode, an anode in most cases, is crucial for direct electron transfer (Read et al., 2010). No diffusional redox species are involved in this electron transfer process. This type of electron transfer between the organism and the electrode is possible only with an electrochemically active (EA) microorganism or bacteria (Chang et al., 2006). Exoelectrogens possesses the ability to transfer electrons directly to an electrode without employing artificial electron shuttles, by three mechanisms (Figure 2): (i)short-range electron transfer with the aid of redox-active proteins like cytochromes found on the outer surface of bacterialcell membrane and (iii) long-range electron transfer through conductive pili also known as nanowires.
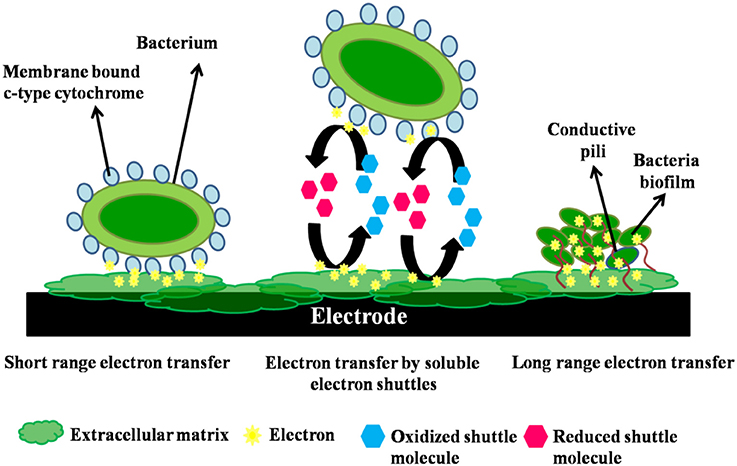
Figure 2. Mechanism of short range, electron shuttle mediated and long range electron transfers in a bacterium.
Living cells, in general are believed to be electrically inactive because of their non-conducting nature (Di Domenico et al., 2015). However, studies revealed that an organism possessing a membrane bound electron transport protein entrapped inside an EA biofilm can be efficiently used for the mechanism (Pinto, 2016). But the transfer of electrons from inside the cell to its outside it governed by transport proteins, whereas the transfer of the electrons to an external, solid terminal electron acceptor, here it is an electrode, is mediated by outermembrane (OM) redox proteins (Wrighton et al., 2011). Some sediment inhabiting metal reducing microorganisms like Geobacter (Seeliger et al., 1998), Rhodoferax (Hochkoeppler et al., 1997) and Shewanella (Myers and Myers, 1997) are found to contain c- type cytochromes, which are multi-heme proteins, found along with EA biofilms in these organisms.
As mentioned, the DET requires physical contact of the EA bacteria and the bacterial cytochrome with the fuel cell anode. However, only the first monolayer existing bacteria in the biofilm will be electrochemically active, at the anode (Babauta et al., 2012). Therefore the performance of the MFC is dependent on the cell density in the first monolayer. As stated previously, the density of the biofilm increases with the age of the culture and with the secretion of more EPS. Therefore, the presence of EA biofilms with dense monolayer have been reported to play a significant role in the MFC performance (Eaktasang et al., 2013).
Other than the cytochromes, the bacteria also possess adherence fimbriae—the pili, made up of proteins and sortase enzyme (Proft and Baker, 2009). Reguera et al. (2006) have reported that some Geobacter and Shewanella strains produce pili that possess electroconductivity. These pili enable organisms to reach a distant anode or solid electron acceptors and utilize the electron transfer potency. The organisms also utilize an electrode, which is not in direct contact with the cell, as its sole electron acceptor, with the aid of their pili. These pili, also called as nanowires, also attach to the membrane bound cytochromes of the cells through which electron transfer to the cell's periphery is adepted (Yang et al., 2012). The nanowires also entangle the development of dense EA biofilms thus enhancing the anode performance.
In the indirect way, a mediator is employed for electron transfer. In indirect transfer, the secondary metabolites (endogenous redox mediators) are especially of great interest, as their synthesis makes the electron transfer independent of the presence of exogenous redox shuttles (Schröder, 2007). This is called as Mediated electron transfer (MET) (Zhou et al., 2013). This can also be attributed by microbially secreted soluble electron shuttles, for example, pyocyanin and flavins (Figure 2). The mediator serves as a reversible terminal electron acceptor, transferring electrons from the bacterial cell either to a solid oxidant (the MFC anode) or into aerobic layers of the biofilm, where it becomes re-oxidized and is again available for subsequent redox processes. One molecule can thus serve for thousands of redox cycles (Santoro et al., 2017).
Consequently, the production of small amounts of these compounds (directly in the anodic biofilm) enables the organism to dispose of electrons at sufficiently high rates. For example, the pigment pyocianine produced by Pseudomonas aeruginosa has been found responsible for its electrochemical activity (Rabaey et al., 2004). Quinone-mediator (2-amino-3-dicarboxyl-1,4 naphthoquinone) produced by Shewanella oneidensis increases the power density of MFC by a factor of 2 when compared with the one without the mediator (Schröder, 2007). The bacteria Pseudomonas alcaliphila is also capable of producing its own redox mediators. Other than the redox mediators, the by-products produced as a result of bacterial metabolism also contribute for indirect electron transfer, through the oxidation of the produced by-products. Oxidation of the fermentative hydrogen produced by bacteria (Chen, 2006) at the anode is an example.
In both the cases, electron transfer is through bacterial contact with the electrode either directly or through soluble shuttles that act as mediator molecules such as ubiquinones, pigments, dyes and metal complexes forming reversible redox couples that are readily soluble and non-toxic to the microbial consortium, biologically non-degradable and are highly stable in both the oxidized and reduced forms (Aghababaie et al., 2015).
Attributing to the above said potencies, a broad range of photosynthetic and anerobic microrganisms have been employed as electron donors and acceptors in MFCs. They include Chlorella vulgaris (Jeon et al., 2012), Phormidium sp. (Bradley et al., 2012), Saccharomyces cerevisiae (Permana et al., 2015), Leptothrix discophora (Rhoads et al., 2005), Scenedesmus armatus (Angelaalincy et al., 2017), Rhodispirullum rubrum (Bensaid et al., 2015), Thiobacillus ferrooxidance (Ter Heijne et al., 2007), Desulfovibrio desulfuricans (Kang et al., 2014), Klebsiellapneumoniae (Deng et al., 2010), Pseudomonas fluroscens (Friman et al., 2013), Geobacter metallireducens (Poddar and Khurana, 2011), and some anaerobic bacteria. Some of these organisms are genetically engineered to provide exponential results in terms of current production and sustainable biomass generation than the wild type strains. Reports on the usage of algae in fuel cells are limited when compared to those on bacteria.
Apart from the microorganisms employed, electro active nature of the biofilm produced and the mode of electron transfer used by the organisms, parameters such as temperature, pH, applied potential, flow conditions etc. influence the performance of a microbial fuel cell during field application (Jadhav and Ghangrekar, 2009). Ringeisen et al. (2007) has explored parameters such as electrodic materials, surface area of the electrode and special aerobic cultures for enhanced fuel cell performances. Inspite of the several reviews on the performance of MFCs under controlled conditions, there are still researches going on to determine the influence of various operational parameters on the fuel cell performance. Jadhav and Ghangrekar (2009) have reported a varying MFC performance with variations in the operationg parameters such as pH, temperature and external resistance. The study has reported that a reduction in temperature range (8–22°C) resulted in an increased current upto 1.4 mA from 0.7 mA and increased coulombic efficiency of 5% from 1.5%. However, the COD removal efficiency decreased to 59% from 90%. On the contrary, certain wastewater derived organisms showed increased bioelectrocatalytic performance and increased COD removal (Chan and Li, 2014). Its presumed that increasing temperature increases the oxygen reduction kinetics, thus decreasing the internal resistance of the cell. This results in increased current density and increased Coulombic efficiency. The COD removal rate has also been observed to increase with increase in temperature, which may be the result of an increased biomass due to increased biochemical reaction rate. Therefore, the substrate utility rate increases, resulting in an efficient COD removal (Scott and Yu, 2015). However, there are also reports that a decreased temperature increases current density, power density and cell voltage (Chan and Li, 2014). Therefore, the optimal operation temperature of a MFC can only be determined based on the anodic consortium employed for current production.
Similiarly, a variation in the anodic pH between 5.5 and 7.5 inferred that, a steady pH maintanence at 6.5 resulted in increased current and coulombic efficiency of 4% where a pH more than 7 and less than 6 resulted in decreased current (Jadhav and Ghangrekar, 2009). In general, MFCs are operated at a neutral pH to attain higher power output, because the anodic microbial consortium performs well in a neutral pH rather than in an increased or decreased pH. However, an increased pH at the anodic chamber shall attribute to increased COD removal, whereas an increased pH at the cathodic chamber results in an increased power output (Scott and Yu, 2015). Further, a carbon source, which is soluble has been reported to significantly change the MFC power output than a particulate carbon source (Borole and Hamilton, 2010). The employed microbial consortium greatly feeds on the dissolved carbon source for growth and metabolism, thus resulting in an improved biomass that contributes to increased power output (Angelaalincy et al., 2017). The effect of ionic strength of the anodic chamber has also been found to influence the performance of MFC. An increase in the ionic strength to 400 mM from 100 mM has resulted in 1,330 mW/m2 power density from 720 mW/m2 thereby reducing the internal resistance to 79–161 Ω (Liu et al., 2005). In addition, the flow rate in a MFC has also been found to influence the anodic and cathodic impedence in fuel cells. Aaron et al. (2010) reported that increasing the anodic flow rate decreased the cathodic impedence by 65% however, the anodic impedence remained significantly unaltered. Similarly, with increasing flow conditions, the anode modules produced a power density of 6.0 ± 0.4 Wm−3 which is 1.9 times higher than the control conditions (He et al., 2016b; Table 1). Although a number of factors influence the performance of fuel cells, a genetic approach, which would enhance biofilms production in microorganisms is still considered a promising approach for enhanced fuel cell performance.
Given the role of EPS in EET of microorganisms and its relavence in biofilm formation on electrodes, the prospect of engineering the biofilm for its enhanced adhesion and EET is just the future of the MFCs. S. oneidensis MR-1, a facultative anaerobe is capable of reducing Mn(IV) and Fe(III) oxides and can produce current in microbial fuel cells. The mechanisms employed by S. oneidensis MR-1 for this process have not been fully elucidated. However, several different S. oneidensis MR-1 deletion mutants were made and tested for current production and metal oxide reduction. The results suggested involvement of certain key cytochromes in all of the processes though with varying degrees in each process thus showing a very complex picture of electron transfer to solid and soluble substrates by S. oneidensis MR-1 (Bretschger et al., 2007). The mechanism involved in EET in S. oneidensis MR-1 involves OmcA and MtrC (outer membrane -OM), decaheme c-cyts in direct electron transfer to solid metal oxides and anodes of MFCs, however another member of the genus Shewanella, S. loihica PV-4 showed different mechanism for current generation (mediated electron transfer) (Newton et al., 2009). Another study involving cell surface polysaccharides of Shewanella oneidensis MR-1 demonstrated that the effect of these polysaccharides on not only the cell adhesion to graphite anodes but also the current generation in MFCs, as the electrically non-conductive capsular polysaccharides can interfere with the contact of OM cytochromes to anodes and direct EET via them. Thus, cel surface engineering was prospected as a valuable scheme to generate higher current in bacterial MFC system (Kouzuma et al., 2010). Genetic engineering approaches have been made in a model organism Shewanella oneidensis MR-1where flavin biosynthesis gene cluster ribD-ribC-ribBA-ribE and metal-reducing conduit biosynthesis gene cluster mtrC-mtrA-mtrB were coexpressed in the bacteria and an improved EET capacity in microbial fuel cells with an increase in maximum current density by approximate 110% was seen (Min et al., 2017).
In yet another approach, a synthetic fermenter-exoelectrogen containing a microbial consortium (Escherichia coli-S. oneidensis) was tested to establish a highly electroactive anodic biofilm. Briefly, a synthetic riboflavin pathway from Bacillus subtilis was expressed into E. coli to overproduce flavins in order to facilitate flavin-mediated electron transfer, and a hydrophobic S. oneidensis strain CP2-1-S1 was employed as the exoelectrogen to increase its adhesion to the carbon electrode. The extremely hydrophobic interactions between S. oneidensis and the anode along with the overproduced flavins produced by the recombinant E. coli added an advantage for S. oneidensis over E. coli in the attachment to the anode surface. This rationally engineered anodic biofilm with the modified microbial community profile showed a higher catalytic current (from 0.19 to 1.84 A/m2 at 0 V vs. SHE). The xylose-fed MFC inoculated with this engineered microbial consortium generated a greater power density which was 6.8 times higher than that inoculated with wild type coculture (Yang et al., 2015b).
Similarly, simple surface modifications for enhanced biofilm formation, increased electron transfer rate and higher current density generation from microbial fuel cell (MFC) have also been demonstrated using partial oxidation of carbon felt material by UV/O3 treatment, where the electrochemical studies performed suggested that Shewanella oneidensis MR-1 biofilm formation was improved on UV/O3 treated carbon felt electrodes at an applied potential of −0.3 V vs. Ag/AgCl, where the carbon electrodes exposed to 45 min of UV/O3 treatment provided the best electrochemical results and enhanced bacterial cell attachment (Cornejo et al., 2015). Further, the experimental evidence of a stimulated voltage production of up to 0.3 V in MFC in amendment with 100 nM quinolone signal compared to the control debates the scope of genetic engineering of quorum sensing signaling molecules, thus attributing increased biofilms formation, for enhanced power production in MFCs.
Further, the effect of different operational conditions on biofilm development and nitrification in three moving-bed biofilm reactors (MBBRs) was investigated (Bassin et al., 2012). Organisms such as Shewanella oneidensis and Geobacter sulfurreducans have been studied on the role of the Cytochrome C and pili production in MFCs (Alfonta, 2010). Genetic engineering and gene silencing strategies to explore the role of these appendages have provided clear-cut information on the pathway of EET from bacteria to the anode (Rosenbaum and Angenent, 2009). News reports about research in in United States have reported 50% more fuel production while employing genetically engineered bacteria in a MFC (Hudson, 2013). However, genetic modification or metabolic engineering of microalgae and its putative efficiency in power production has not been reported so far. Still, enhancing the EPS production of the organism through media optimization strategies have been reported to improve the current (mA) generation in microalgae (Angelaalincy et al., 2017). Compared to bacterial system, the algal system is quite complex depicting a large number of genes attributing to various functions. The red alga Porphyridium purpureum has been reported to possess an unusually simple enzyme network containing 19 genes that involve in many critical biosynthetic steps represented by single enzymes. Starch synthase, a glycosyltransferases 5 (GT5) enzyme, has been explored to be involved in priming polysaccharide synthesis apart from its role in chain elongation of amylopectin and production of novel granules (Bhattacharya et al., 2013). The presence of this gene has also been reported in a green microalga, Coelastrella sp. M60 (Karpagam et al., 2018) indicating the presence and importance of the gene in the algae family. Over expression of such genes contributing to polysaccharide production in microalgae has not been reported so far. Hence, cloning and expression of genes contributing to exopolyssacharide production in microalgae needs substantial research and is thereby supposed to be a promising approach for enhanced power production in photosynthetic algal microbial fuel cells (PAMFCs), as the durability of PAMFCs is longer compared to bacterial biofuel cells. Thus, biofilm engineering is a large avenue open for research.
The contribution of microorganisms toward sustainable energy generation, bioremediation and other industrial applications are incredible though a large part of it remains untapped and un-explored. Their ability to coordinate their metabolism upon achieved cellular density is surprising, which can be attributed to different microbial mechanisms among which the EPS bound biofilms of the organisms have a huge share. The composition, morphology, physical properties and thickness of biofilms show a remarkable impact on bioelectricity production. Although, there are numerous applications of biofilms and EPS in specific, their role in MFCs and bioelectricity generation is note- worthy. Many different strategies to engineer biofilm have been explored to harness this metabolism to sustainable energy production have been attempted but, the rate limiting step to this progress is our limited information of the complete metabolism and genetic regulation and the fact that our knowledge about the EET mechanism is limited to the dissimilatory metal- reducing bacteria mainly in the Geobacter spp. and Shewanella spp.; however, some properties of other proteins involved in in the EET need to be explored. Although these two species have contributed much to the MFCs, other exoelectrogens need also to be discovered employed and tapped for future enhancement of MFC supported technologies. Hence, a deeper insight into the biofilm properties and genetic modification of organisms may open new avenues in improving the performance of a MFC.
MA and PV have written the manuscript. RN, MA, GS, BA, and SK actively contributed in writing, editing and improving the manuscript. PV provided support, guidelines and manuscript correction.
The authors thank Department of Science and Technology, Ministry of Science and Technology, New Delhi, India (DST- INSPIRE Fellowship/2014) for funding PV and MA to support this work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Aaron, D., Tsouris, C., Hamilton, C. Y., and Borole, A. P. (2010). Assessment of the effects of flow rate and ionic strength on the performance of an air-cathode microbial fuel cell using electrochemical impedance spectroscopy. Energies 3, 592–606. doi: 10.3390/en3040592
Aelterman, P., Rabaey, K., Clauwaert, P., and Verstraete, W. (2006). Microbial fuel cells for wastewater treatment. Water Sci. Technol. 54:9. doi: 10.2166/wst.2006.702
Agarwal, A., Kahyaoglu, C., and Hansen, D. B. (2012). Characterization of HmqF, a protein involved in the biosynthesis of unsaturated quinolones produced by Burkholderia thailandensis. Biochemistry 51, 1648–1657. doi: 10.1021/bi201625w
Aghababaie, M., Farhadian, M., Jeihanipour, A., and Biria, D. (2015). Effective factors on the performance of microbial fuel cells in wastewater treatment – a review. Environ. Technol. Rev. 4, 71–89. doi: 10.1080/09593330.2015.1077896
Alfonta, L. (2010). Genetically engineered microbial fuel cells. Electroanalysis 22, 822–831. doi: 10.1002/elan.200980001
Angelaalincy, M. J., Balasubramaniem, A. K., Vasantha, V. S., and Varalakshmi, P. (2016). “Microbial fuel cells: a promising alternative energy source,” in Bioenergy Opportunities and Challenges, eds R. Navanietha Krishnaraj and J.-S. Yu (Waretown, NJ: Apple Academic Press Inc.), 63–80.
Angelaalincy, M., Senthilkumar, N., Karpagam, R., Kumar, G. G., Ashokkumar, B., and Varalakshmi, P. (2017). Enhanced Extracellular Polysaccharide Production and Self-Sustainable Electricity Generation for PAMFCs by Scenedesmus sp. SB1. ACS Omega 2, 3754–3765. doi: 10.1021/acsomega.7b00326
Babauta, J., Renslow, R., Lewandowski, Z., and Beyenal, H. (2012). Electrochemically active biofilms: facts and fiction. A review. Biofouling 28, 789–812. doi: 10.1080/08927014.2012.710324
Bassin, J. P., Kleerebezem, R., Rosado, A. S., Van Loosdrecht, M. C., and Dezotti, M. (2012). Effect of different operational conditions on biofilm development, nitrification, and nitrifying microbial population in moving-bed biofilm reactors. Environ. Sci. Technol. 46, 1546–1555. doi: 10.1021/es203356z
Bensaid, S., Ruggeri, B., and Saracco, G. (2015). Development of a photosynthetic microbial electrochemical cell (PMEC) reactor coupled with dark fermentation of organic wastes:medium term perspectives. Energies 8, 399–429. doi: 10.3390/en8010399
Bhattacharya, D., Price, D. C., Chan, C. X., Qiu, H., Rose, N., Ball, S., et al. (2013). Genome of the red alga Porphyridium purpureum. Nat. Commun. 4:1941. doi: 10.1038/ncomms2931
Bond, D. R., and Lovley, D. R. (2003). Electricity Production by Geobacter sulfurreducens Attached to Electrodes Electricity Production by Geobacter sulfurreducens Attached to Electrodes. Appl. Environ. Microbiol. 69, 1548–1555. doi: 10.1128/AEM.69.3.1548-1555.2003
Borole, A. P., and Hamilton, C. Y. (2010). “Energy production from food industry wastewaters using bioelectrochemical cells,” in Emerging Environmental Technologies, Vol. II, ed V. Shah (Dordrecht: Springer), 97–113.
Bradley, R. W., Bombelli, P., Rowden, S. J. L., and Howe, C. J. (2012). Biological photovoltaics: intra- and extra-cellular electron transport by cyanobacteria: figure 1. Biochem. Soc. Trans. 40, 1302–1307. doi: 10.1042/BST20120118
Bretschger, O., Obraztsova, A., Sturm, C. A., Chang, I. S., Gorby, Y. A., Reed, S. B., et al. (2007). Current production and metal oxide reduction by Shewanella Oneidensis MR-1 wild type and mutants. Appl. Environ. Microbiol. 73, 7003–7012. doi: 10.1128/AEM.01087-07
Breuer, M., Rosso, K. M., and Blumberger, J. (2013). Electron flow in multiheme bacterial cytochromes is a balancing act between heme electronic interaction and redox potentials. Proc. Natl. Acad. Sci. U.S.A. 111, 611–616. doi: 10.1073/pnas.1316156111
Byrd, M. S., Sadovskaya, I., Vinogradov, E., Lu, H., Sprinkle, A. B., Richardson, S. H., et al. (2009). Genetic and biochemical analyses of the Pseudomonas aeruginosa Psl exopolysaccharide reveal overlapping roles for polysaccharide synthesis enzymes in Psl and LPS production. Mol. Microbiol. 73, 622–638. doi: 10.1111/j.1365-2958.2009.06795.x
Chan, K.-Y., and Li, C.-Y. V. (2014). Electrochemically Enabled Sustainability: Devices, Materials and Mechanisms for Energy Conversion. CRC Press; Taylor and Francis group.
Chang, I. S., Moon, H., Bretschger, O., Jang, J. K., Park, H., Il Nealson, K. H., et al. (2006). Electrochemically active bacteria (EAB) and mediator-less microbial fuel cells. J. Microbiol. Biotechnol. 16, 163–177. doi: 10.1002/macp.200700627
Chaturvedi, V., and Verma, P. (2016). Microbial fuel cell: a green approach for the utilization of waste for the generation of bioelectricity. Bioresour. Bioprocess. 3:38. doi: 10.1186/s40643-016-0116-6
Chaudhuri, S. K., and Lovley, D. R. (2003). Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat. Biotechnol. 21, 1229–1232. doi: 10.1038/nbt867
Chen, B., Li, F., Liu, N., Ge, F., Xiao, H., and Yang, Y. (2015). Role of extracellular polymeric substances from Chlorella vulgaris in the removal of ammonium and orthophosphate under the stress of cadmium. Bioresour. Technol. 190, 299–306. doi: 10.1016/j.biortech.2015.04.080
Chen, W. (2006). Biological Hydrogen Production by Anaerobic Fermentation. Retrospective Theses and Dissertations,1863, Iowa State University, Ames, IA. Available online at: http://lib.dr.iastate.edu/rtd/1863
Cheng, Q., and Call, D. F. (2016). Hardwiring microbes via direct interspecies electron transfer: mechanisms and applications. Environ. Sci. Process. Impacts 18, 968–980. doi: 10.1039/C6EM00219F
Cheng, S., Liu, H., and Logan, B. E. (2006). Power densities using different cathode catalysts (Pt and CoTMPP) and polymer binders (Nafion and PTFE) in single chamber microbial fuel cells. Environ. Sci. Technol. 40, 364–369. doi: 10.1021/es0512071
Chouler, J., Padgett, G. A., Cameron, P. J., Preuss, K., Titirici, M. M., Ieropoulos, I., et al. (2016). Towards effective small scale microbial fuel cells for energy generation from urine. Electrochim. Acta 192, 89–98. doi: 10.1016/j.electacta.2016.01.112
Christenson, L. (2011). Algal Biofilm Production and Harvesting System for Wastewater Treatment with Biofuels By-Products. All Graduate Theses and Dissertations, 994, UTAH State University, Logan, UT. Available online at: https://digitalcommons.usu.edu/etd/994
Colvin, K. M., Irie, Y., Tart, C. S., Urbano, R., Whitney, J. C., Ryder, C., et al. (2012). The Pel and Psl polysaccharides provide Pseudomonas aeroginosa structural redundance within the biofilm matrix. Environ. Microbiol. 14, 1913–1928. doi: 10.1111/j.1462-2920.2011.02657
Cornejo, J. A., Lopez, C., Babanova, S., Santoro, C., Artyushkova, K., Ista, L., et al. (2015). Surface modification for enhanced biofilm formation and electron transport in shewanella anodes. J. Electrochem. Soc. 162, H597–H603. doi: 10.1149/2.0271509jes
Davis, F., and Higson, S. P. J. (2007). Biofuel cells-recent advances and applications. Biosens. Bioelectron. 22, 1224–1235. doi: 10.1016/j.bios.2006.04.029
Demirbas, A. (2005). Potential applications of renewable energy sources, biomass combustion problems in boiler power systems and combustion related environmental Issues. Progr. Ener. Combust. Sci. 31, 171–192. doi: 10.1016/j.pecs.2005.02.002
Deng, L. F., Li, F. B., Zhou, S. G., Huang, D. Y., and Ni, J. R. (2010). A study of electron-shuttle mechanism in klebsiella pneumoniae based-microbial fuel cells. Chinese Sci. Bull. 55, 99–104. doi: 10.1007/s11434-009-0563-y
Di Domenico, E. G., Petroni, G., Mancini, D., Geri, A., Di Palma, L., and Ascenzioni, F. (2015). Development of electroactive and anaerobic ammonium-oxidizing (Anammox) biofilms from digestate in microbial fuel cells. Biomed. Res. Int. 2015:351014. doi: 10.1155/2015/351014
Diggle, S. P., Winzer, K., Lazdunski, A., Williams, P., and Cámara, M. (2002). Advancing the quorum in Pseudomonas aeruginosa: MvaT and the regulation of N-acylhomoserine lactone production and virulence gene expression. J. Bacteriol. 184, 2576–2586. doi: 10.1128/JB.184.10.2576-2586.2002
Du, Z., Li, H., and Gu, T. (2007). A state of the art review on microbial fuel cells: a promising technology for wastewater treatment and bioenergy. Biotechnol. Adv. 25, 464–482. doi: 10.1016/j.biotechadv.2007.05.004
Eaktasang, N., Kang, C. S., Ryu, S. J., Suma, Y., and Kim, H. S. (2013). Enhanced current production by electroactive biofilm of sulfate-reducing bacteria in the microbial fuel cell. Environ. Eng. Res. 18, 277–281. doi: 10.4491/eer.2013.18.4.277
Flemming, H. C., Neu, T. R., and Wozniak, D. J. (2007). The EPS matrix: the “House of Biofilm Cells.” J. Bacteriol. 189, 7945–7947. doi: 10.1128/JB.00858-07
Franks, A. E. (2015). Microbial electron transport and energy conservation–the foundation for optimizing bioelectrochemical systems. Front. Microbial. 6:575. doi: 10.3389/fmicb.2015.00575
Friedman, L., and Kolter, R. (2004). Two genetic loci produce distinct carbohydrate-rich structural components of the Pseudomonas aeruginosa biofilm matrix two genetic loci produce distinct carbohydrate-rich structural components of the Pseudomonas aeruginosa biofilm matrix. J. Bacteriol. 186, 4457–4465. doi: 10.1128/JB.186.14.4457-4465.2004
Friman, H., Schechter, A., Ioffe, Y., Nitzan, Y., and Cahan, R. (2013). Current production in a microbial fuel cell using a pure culture of Cupriavidus basilensis growing in acetate or phenol as a carbon source. Microb. Biotechnol. 6, 425–434. doi: 10.1111/1751-7915.12026
Gouveia, L., Neves, C., Sebastião, D., Nobre, B. P., and Matos, C. T. (2014). Effect of light on the production of bioelectricity and added-value microalgae biomass in a Photosynthetic Alga Microbial Fuel Cell. Bioresour. Technol. 154, 171–177. doi: 10.1016/j.biortech.2013.12.049
Gude, V. G. (2015). Energy and water autarky of wastewater treatment and power generation systems. Renew. Sustain. Energy Rev. 45, 52–68. doi: 10.1016/j.rser.2015.01.055
He, H., Zhou, M., Yang, J., Hu, Y., and Zhao, Y. (2014). Simultaneous wastewater treatment, electricity generation and biomass production by an immobilized photosynthetic algal microbial fuel cell. Bioprocess Biosyst. Eng. 37, 873–880. doi: 10.1007/s00449-013-1058-4
He, L., Du, P., Chen, Y., Lu, H., Cheng, X., Chang, B., et al. (2016a). Advances in microbial fuel cells for wastewater treatment. Renew. Sustain. Energy Rev. 71, 388–403. doi: 10.1016/j.rser.2016.12.069
He, W., Wallack, M. J., Kim, K., Zhang, X., Yang, W., Zhu, X., et al. (2016b). The effect of fl ow modes and electrode combinations on the performance of a multiple module microbial fuel cell installed at wastewater treatment plant. Water Res. 105, 351–360. doi: 10.1016/j.watres.2016.09.008
Hochkoeppler, A., Ciurli, S., Kofod, P., Venturoli, G., and Zannoni, D. (1997). On the role of cytochrome c8 in photosynthetic electron transfer of the purple non-sulfur bacterium Rhodoferax fermentans. Photosyn. Res. 53, 13–21. doi: 10.1023/A:1005830003198
Hong, B. H., Joe, M. M., Selvakumar, G., Kim, K. Y., Choi, J. H., and Sa, T. M. (2016). Influence of salinity variations on exocellular polysaccharide production, biofilm formation and flocculation in halotolerant bacteria. J. Environ. Biol. 38, 657–664. doi: 10.22438/jeb/38/4/MRN-284
Hudson, P. (2013). Making Fuel From Bacteria: Genetically-Modified Cyanobacteria Could Be More Efficient Than Ethanol. 2–3. Available online at: https://phys.org/news/2013-03-fuel-bacteria-genetically-modified-cyanobacteria-efficient.html
Ishii, S., Shimoyama, T., Hotta, Y., and Watanabe, K. (2008). Characterization of a filamentous biofilm community established in a cellulose-fed microbial fuel cell. BMC Microbiol. 8:6. doi: 10.1186/1471-2180-8-6
Jackson, K. D., Starkey, M., Kremer, S., Parsek, M. R., and Wozniak, D. J. (2004). Identification of psl, a locus encoding a potential exopolysaccharide that is essential for Pseudomonas aeruginosa PAO1 biofilm formation. J. Bacteriol. 186, 4466–4475. doi: 10.1128/JB.186.14.4466-4475.2004
Jadhav, G. S., and Ghangrekar, M. M. (2009). Bioresource technology performance of microbial fuel cell subjected to variation in pH, temperature, external load and substrate concentration. Bioresour. Technol. 100, 717–723. doi: 10.1016/j.biortech.2008.07.041
Jeon, H. J., Seo, K. W., Lee, S. H., Yang, Y. H., Kumaran, R. S., Kim, S., et al. (2012). Production of algal biomass (Chlorella vulgaris) using sediment microbial fuel cells. Bioresour. Technol. 109, 308–311. doi: 10.1016/j.biortech.2011.06.039
Kang, C. S., Eaktasang, N., Kwon, D. Y., and Kim, H. S. (2014). Enhanced current production by Desulfovibrio desulfuricans biofilm in a mediator-less microbial fuel cell. Bioresour. Technol. 165, 27–30. doi: 10.1016/j.biortech.2014.03.148
Karpagam, R., Jawaharraj, K., Ashokkumar, B., Sridhar, J., and Varalakshmi, P. (2018). Unraveling the lipid and pigment biosynthesis in Coelastrella sp. M-60: genomics-enabled transcript profiling. Algal Res. 29, 277–289. doi: 10.1016/j.algal.2017.11.031
Kiely, P. D., Regan, J. M., and Logan, B. E. (2011). The electric picnic: synergistic requirements for exoelectrogenic microbial communities. Curr. Opin. Biotechnol. 22, 378–385. doi: 10.1016/j.copbio.2011.03.003
Korenevsky, A., and Beveridge, T. J. (2007). The surface physicochemistry and adhesiveness of shewanella are affected by their surface polysaccharides. Microbiol. (Reading Engl). 153, 1872–1883. doi: 10.1099/mic.0.2006/003814-0
Kouzuma, A., Meng, X. Y., Kimura, N., Hashimoto, K., and Watanabe, K. (2010). Disruption of the putative cell surface polysaccharide biosynthesis Gene SO3177 in Shewanella oneidensis MR-1 enhances adhesion to electrodes and current generation in microbial fuel cells. Appl. Environ. Microbiol. 76, 4151–4157. doi: 10.1128/AEM.00117-10
Kreft, J. U., and Wimpenny, J. W. (2001). Effect of EPS on biofilm structure and function as revealed by an individual-based model of biofilm growth. Water Sci. Technol. 43, 135–141. doi: 10.2166/wst.2001.0358
Li, W.-W., Yu, H.-Q., and He, Z. (2013). Towards sustainable wastewater treatment by using microbial fuel cells-centered technologies. Energy Environ. Sci. 7, 911–924. doi: 10.1039/C3EE43106A
Liu, H., Cheng, S., and Logan, B. E. (2005). Power generation in fed-batch microbial fuel cells as a function of ionic strength, temperature, and reactor configuration. Environ. Sci. Technol. 39, 5488–5493. doi: 10.1021/es050316c
Liu, H., and Ramnarayanan, R. (2004). Production of electricity during wastewater treatment using a single chamber microbial fuel cell. Environ. Sci. Technol. 38, 2281–2285. doi: 10.1021/es034923g
Liu, Z. D., Du, Z. W., Lian, J., Zhu, X. Y., Li, S. H., and Li, H. R. (2007). Improving energy accumulation of microbial fuel cells by metabolism regulation using Rhodoferax ferrireducens as biocatalyst. Lett. Appl. Microbiol. 44, 393–398. doi: 10.1111/j.1472-765X.2006.02088.x
Lovley, D. R. (2011). Minireview Powering microbes with electricity : direct electron. Environ. Microbial. Rep. 3, 27–35. doi: 10.1111/j.1758-2229.2010.00211.x
Ma, L., Conover, M., Lu, H., Parsek, M. R., Bayles, K., and Wozniak, D. J. (2009). Assembly and development of the Pseudomonas aeruginosa biofilm matrix. PLoS Pathog. 5:e1000354. doi: 10.1371/journal.ppat.1000354
Ma, L., Jackson, K. D., Landry, R. M., Parsek, M. R., and Wozniak, D. J. (2006). Analysis of Pseudomonas aeruginosa conditional psl variants reveals roles for the psl polysaccharide in adhesion and maintaining biofilm structure postattachment. J. Bacteriol. 188, 8213–8221. doi: 10.1128/JB.01202-06
Matsukawa, M., and Greenberg, E. P. (2004). Putative exopolysaccharide synthesis genes influence Pseudomonas aeruginosa biofilm development putative exopolysaccharide synthesis genes influence Pseudomonas aeruginosa biofilm development. J. Bacteriol. 186, 4449–4456. doi: 10.1128/JB.186.14.4449-4456.2004
Min, D., Cheng, L., Zhang, F., Huang, X. N., Li, D. B., Liu, D. F., et al. (2017). Enhancing extracellular electron transfer of shewanella oneidensis MR-1 through coupling improved flavin synthesis and metal-reducing conduit for pollutant degradation. Environ. Sci. Technol. 51, 5082–5089. doi: 10.1021/acs.est.6b04640
Monzon, O., Yang, Y., Li, Q., and Alvarez, P. J. J. (2016). Quorum sensing autoinducers enhance biofilm formation and power production in a hypersaline microbial fuel cell. Biochem. Eng. J. 109, 222–227. doi: 10.1016/j.bej.2016.01.023
Myers, C. R., and Myers, J. M. (1997). Outer membrane cytochromes of Shewanella putrefaciens MR-1: Spectral analysis, and purification of the 83-KDa c-type cytochrome. Biochim. Biophys. Acta Biomembr. 1326, 307–318. doi: 10.1016/S0005-2736(97)00034-5
Nevin, K. P., Kim, B. C., Glaven, R. H., Johnson, J. P., Woodward, T. L., Methé, B. A., et al. (2009). Anode biofilm transcriptomics reveals outer surface components essential for high density current production in Geobacter sulfurreducens fuel cells. PLoS ONE 4:5628. doi: 10.1371/journal.pone.0005628
Newton, G. J., Mori, S., Nakamura, R., Hashimoto, K., and Watanabe, K. (2009). Analyses of current-generating mechanisms of Shewanella loihica PV-4 and Shewanella oneidensis MR-1 in microbial fuel cells. Appl. Environ. Microbiol. 75, 7674–7681. doi: 10.1128/AEM.01142-09
Nocelli, N., Bogino, P. C., Banchio, E., and Giordano, W. (2016). Roles of extracellular polysaccharides and biofilm formation in heavy metal resistance of rhizobia. Mater. (Basel). 9:418. doi: 10.3390/ma9060418
Obasi, L. A., Opara, C. C., and Oji, A. (2012). Performance of cassava starch as a proton exchange membrane in a dual chambered microbial fuel cell. Int. J. Eng. Sci. Technol. 4, 227–238.
Pant, D., Singh, A., Van Bogaert, G., Olsen, S. I., Nigam, P. S., Diels, L., et al. (2012). Bioelectrochemical systems (BES) for sustainable energy production and product recovery from organic wastes and industrial wastewaters. RSC Adv. 2, 1248–1263. doi: 10.1039/c1ra00839k
Panwar, N. L., Kaushik, S. C., and Kothari, S. (2011). Role of renewable energy sources in environmental protection : a review. Renew. Sustain. Energy Rev. 15, 1513–1524. doi: 10.1016/j.rser.2010.11.037
Park, D. H., and Zeikus, J. G. (2003). Improved fuel cell and electrode designs for producing electricity from microbial degradation. Biotechnol. Bioeng. 81, 348–355. doi: 10.1002/bit.10501
Parkash, A., Aziz, S., Abro, M., Soomro, S., and Kousar, A. (2015). Design and fabrication of microbial fuel cell using cow manure for power genration. Sci. Int. 27, 4235–4238.
Patil, S. A., Hägerhäll, C., and Gorton, L. (2012). Electron transfer mechanisms between microorganisms and electrodes in bioelectrochemical systems. Bioanal. Rev. 4, 159–192. doi: 10.1007/11663_2013_2
Permana, D., Rosdianti, D., Ishmayana, S., Rachman, S. D., Putra, H. E., Rahayuningwulan, D., et al. (2015). Preliminary Investigation of Electricity Production Using Dual Chamber Microbial Fuel Cell (DCMFC) with Saccharomyces Cerevisiae as Biocatalyst and Methylene Blue as an Electron Mediator. Proc. Chem. 17, 36–43. doi: 10.1016/j.proche.2015.12.123
Pinto, D. (2016). Electronic Transfer Within a Microbial Fuel Cell. Better Understanding of Experimental and Structural Parameters at the Interface between Electro-Active Bacteria and Carbon-Based Electrodes. Thesis, Material Chemistry, Université Pierre et Marie Curie, Paris VI.
Poddar, S., and Khurana, S. (2011). Geobacter: the electric microbe! efficient microbial fuel cells to generate clean, cheap electricity. Ind. J. Microbiol. 51, 240–241. doi: 10.1007/s12088-011-0180-8
Proft, T., and Baker, E. N. (2009). Pili in Gram-negative and Gram-positive bacteria - Structure, assembly and their role in disease. Cell. Mol. Life Sci. 66, 613–635. doi: 10.1007/s00018-008-8477-4
Qiao, Y., Li, C. M., Bao, S. J., Lu, Z., and Hong, Y. (2008). Direct electrochemistry and electrocatalytic mechanism of evolved Escherichia coli cells in microbial fuel cells. Chem. Commun. 1290–1292. doi: 10.1039/b719955d
Rabaey, K., Boon, N., Siciliano, S. D., Verstraete, W., and Verhaege, M. (2004). Biofuel cells select for microbial consortia that self-mediate electron transfer biofuel cells select for Microbial Consortia that self-mediate electron transfer. Appl. Environ. Microbiol. 70, 5373–5382. doi: 10.1128/AEM.70.9.5373-5382.2004
Read, S. T., Dutta, P., Bond, P. L., Keller, J., and Rabaey, K. (2010). Initial development and structure of biofilms on microbial fuel cell anodes. BMC Microbiol. 10:98. doi: 10.1186/1471-2180-10-98
Reguera, G., Nevin, K. P., Nicoll, J. S., Covalla, S. F., Woodard, T. L., and Lovley, D. R. (2006). Biofilm and nanowire production leads to increased current in Geobacter sulfurreducens fuel cells. Appl. Environ. Microbiol. 72, 7345–7348. doi: 10.1128/AEM.01444-06
Rhoads, A., Beyenal, H., and Lewandowski, Z. (2005). Microbial fuel cell using anaerobic respiration as an anodic reaction and biomineralized manganese as a cathodic reactant. Environ. Sci. Technol. 39, 4666–4671. doi: 10.1021/es048386r
Ringeisen, B. R., Ray, R., and Little, B. (2007). A miniature microbial fuel cell operating with an aerobic anode chamber. J. Power Sourc. 165, 591–597. doi: 10.1016/j.jpowsour.2006.10.026
Rollefson, J. B., Stephen, C. S., Tien, M., and Bond, D. R. (2011). Identification of an Extracellular Polysaccharide Network Essential for Cytochrome Anchoring and Biofilm Formation in. J. Bacteriol. 193, 1023–1033. doi: 10.1128/JB.01092-10
Roller, S. D., Bennetto, H. P., Delaney, G. M., Mason, J. R., Stirling, J. L., and Thurston, C. F. (2008). Electron-transfer coupling in microbial fuel cells: 1. comparison of redox-mediator reduction rates and respiratory rates of bacteria. J. Chem. Technol. Biotechnol. Biotechnol. 34B, 3–12. doi: 10.1002/jctb.280340103
Rosenbaum, M., and Angenent, L. T. (2009). “Genetically modified microorganisms for bioelectrochemical systems,” in Bioelectrochemical Systems: From Extracellular Electron Transfer to Biotechnological Application (Integrated Environmental Technology), eds K. Rabaey, L. Angenent, U. Schröder, and J. Keller (London: IWA Publishing), 101–113.
Santoro, C., Arbizzani, C., Erable, B., and Ieropoulos, I. (2017). Microbial fuel cells: from fundamentals to applications. A review. J. Power Sour. 356, 225–244. doi: 10.1016/j.jpowsour.2017.03.109
Sarjit, A., Tan, S. M., and Dykes, G. A. (2015). Surface modification of materials to encourage beneficial biofilm formation. 2, 404–422. doi: 10.3934/bioeng.2015.4.404
Schröder, U. (2007). Anodic electron transfer mechanisms in microbial fuel cells and their energy efficiency. Phys. Chem. Chem. Phys. 9, 2619–2629. doi: 10.1039/B703627M
Scott, K., and Yu, E. H. (2015). Microbial Electrochemical and Fuel Cells: Fundamentals and Applications. Sawston; Cambridge: Woodhead Publishing.
Seeliger, S., Cord-Ruwisch, R., and Schink, B. (1998). A periplasmic and extracellular c-type cytochrome of Geobacter sulfurreducens acts as a ferric iron reductase and as an electron carrier to other acceptors or to partner bacteria. J. Bacteriol. 180, 3686–3691.
Shi, L., Dong, H., Reguera, G., Beyenal, H., Lu, A., Liu, J., et al. (2016). Extracellular electron transfer mechanisms between microorganisms and minerals. Nat. Publ. Gr. 14, 651–662. doi: 10.1038/nrmicro.2016.93
Singh, S., Dwivedi, C., and Pandey, A. (2016). “Electricity generation in membrane-less single chambered microbial fuel cell,” in 2016 International Conference on Control, Computing, Communication and Materials (ICCCCM) (Allahabad: IEEE), 710–716.
Sun, D., Cheng, S., Wang, A., Li, F., Logan, B. E., and Cen, K. (2015). Temporal-spatial changes in viabilities and electrochemical properties of anode biofilms. Environ. Sci. Technol. 49, 5227–5235. doi: 10.1021/acs.est.5b00175
Sun, D., Chen, J., Huang, H., Liu, W., Ye, Y., and Cheng, S. (2016). The effect of biofilm thickness on electrochemical activity of Geobacter sulfurreducens. Int. J. Hydr. Ener. 41, 16523–16528. doi: 10.1016/j.ijhydene.2016.04.163
Ter Heijne, A., Hamelers, H. V., and Buisman, C. J. (2007). Microbial fuel cell operation with continuous biological ferrous iron oxidation of the catholyte. Environ. Sci. Technol. 41, 4130–4134. doi: 10.1021/es0702824
Tharali, A. D., Sain, N., and Osborne, W. J. (2016). Microbial fuel cells in bioelectricity production. Front. Life Sci. 9, 252–266. doi: 10.1080/21553769.2016.1230787
Toyofuku, M., Inaba, T., Kiyokawa, T., Obana, N., Yawata, Y., and Nomura, N. (2016). Environmental factors that shape biofilm formation. Biosci. Biotechnol. Biochem. 80, 7–12. doi: 10.1080/09168451.2015.1058701
Vasseur, P., Vallet-Gely, I., Soscia, C., Genin, S., and Filloux, A. (2005). The pel genes of the Pseudomonas aeruginosa PAK strain are involved at early and late stages of biofilm formation. Microbiol. (Reading Engl.) 151, 985–997. doi: 10.1099/mic.0.27410-0
Wei, Q., and Ma, L. Z. (2013). Biofilm matrix and its regulation in Pseudomonas aeruginosa. Int. J. Mol. Sci. 14, 20983–21005. doi: 10.3390/ijms141020983
Wrighton, K. C., Thrash, J. C., Melnyk, R. A., Bigi, J. P., Byrne-Bailey, K. G., Remis, J. P., et al. (2011). Evidence for direct electron transfer by a gram-positive bacterium isolated froma microbial fuel cell. Appl. Environ. Microbiol. 77, 7633–7639. doi: 10.1128/AEM.05365-11
Yang, H., Zhou, M., Liu, M., Yang, W., and Gu, T. (2015a). Microbial fuel cells for biosensor applications. Biotechnol. Lett. 37, 2357–2364. doi: 10.1007/s10529-015-1929-7
Yang, Y., Wu, Y., Hu, Y., Cao, Y., Poh, C. L., Cao, B., et al. (2015b). Engineering Electrode-Attached Microbial Consortia for High-Performance Xylose-Fed Microbial Fuel Cell. ACS Catal. 5, 6937–6945. doi: 10.1021/acscatal.5b01733
Yang, Y., Xu, M., Guo, J., and Sun, G. (2012). Bacterial extracellular electron transfer in bioelectrochemical systems. Process Biochem. 47, 1707–1714. doi: 10.1016/j.procbio.2012.07.032
Yang, Z., Pei, H., Hou, Q., Jiang, L., Zhang, L., and Nie, C. (2018). Algal biofilm-assisted microbial fuel cell to enhance domestic wastewater treatment: nutrient, organics removal and bioenergy production. Chem. Eng. J. 332, 277–285. doi: 10.1016/j.cej.2017.09.096
Zhang, L., Zhu, X., Li, J., Liao, Q., and Ye, D. (2011). Biofilm formation and electricity generation of a microbial fuel cell started up under different external resistances. J. Power Sour. 196, 6029–6035. doi: 10.1016/j.jpowsour.2011.04.013
Zhang, Y., Jiang, J., Zhao, Q., Gao, Y., Wang, K., Ding, J., et al. (2017). Accelerating anodic biofilms formation and electron transfer in microbial fuel cells: role of anionic biosurfactants and mechanism. Bioelectrochemistry 117, 48–56. doi: 10.1016/j.bioelechem.2017.06.002
Zhao, F., Harnisch, F., Schröder, U., Scholz, F., Bogdanoff, P., and Herrmann, I. (2005). Application of pyrolysed iron(II) phthalocyanine and CoTMPP based oxygen reduction catalysts as cathode materials in microbial fuel cells. Electrochem. Commun. 7, 1405–1410. doi: 10.1016/j.elecom.2005.09.032
Zhao, F., Harnisch, F., Schröder, U., Scholz, F., Bogdanoff, P., and Herrmann, I. (2006). Challenges and constraints of using oxygen cathodes in microbial fuel cells. Environ. Sci. Technol. 40, 5193–5199. doi: 10.1021/es060332p
Zhao, K., Tseng, B. S., Beckerman, B., Jin, F., Gibiansky, M. L., Harrison, J. J., et al. (2013). Psl trails guide exploration and microcolony formation in Pseudomonas aeruginosa biofilms. Nature 497, 388–391. doi: 10.1038/nature12155
Keywords: microbial fuel cells, extracellular polysaccharides, exoelectrogenic activity, biofilm engineering, electricity generation, cytochrome C, wastewater treatment
Citation: Angelaalincy MJ, Navanietha Krishnaraj R, Shakambari G, Ashokkumar B, Kathiresan S and Varalakshmi P (2018) Biofilm Engineering Approaches for Improving the Performance of Microbial Fuel Cells and Bioelectrochemical Systems. Front. Energy Res. 6:63. doi: 10.3389/fenrg.2018.00063
Received: 02 September 2017; Accepted: 14 June 2018;
Published: 05 July 2018.
Edited by:
Abudukeremu Kadier, National University of Malaysia, MalaysiaReviewed by:
G. Velvizhi, Indian Institute of Chemical Technology (CSIR), IndiaCopyright © 2018 Angelaalincy, Navanietha Krishnaraj, Shakambari, Ashokkumar, Kathiresan and Varalakshmi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Perumal Varalakshmi, dmFyYTUyNzdAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.