- 1Orthopedics, The First People’s Hospital of Taicang, Taicang Affiliated Hospital of Soochow University, Suzhou, Jiangsu, China
- 2Anesthesiology and Surgery, The First People’s Hospital of Taicang, Taicang Affiliated Hospital of Soochow University, Suzhou, Jiangsu, China
Study Design: A Systematic Review and Bayesian Network Meta-Analysis
Objective: To compare the efficacy and safety of abaloparatide (ABA), denosumab (DEN), teriparatide (TER), oral bisphosphonates (OBP), and intravenous bisphosphonates (IBP) in the treatment of male osteoporosis through a network meta-analysis.
Summary of Background Data: Currently, a variety of medications are available for the treatment of male osteoporosis, including abaloparatide, denosumab, teriparatide, and bisphosphonates. These medications are widely applied in male osteoporosis, and existing randomized controlled trials (RCTs) provide strong evidence of their efficacy. However, there is a lack of sufficient systematic comparative studies to guide the choice between these treatments, particularly for specific male osteoporosis populations.
Methods: This systematic review and network meta-analysis (NMA) were conducted strictly in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines and the relevant standards recommended by the Cochrane Collaboration. We performed pairwise meta-analysis using Stata 18.0 software to assess the magnitude of effect sizes and the consistency of findings across studies. For network meta-analysis (NMA), we used R version 4.3.1 along with the gemtc and BUGSnet packages to handle complex multi-treatment comparisons. Using these methods, we were able to comprehensively assess the relative efficacy and safety of different treatment options. All statistical analyses were conducted using Review Manager software (version 5.4), a widely used tool in medical research for meta-analysis, forest plot generation, and bias risk assessment.
Results: Overall, clinical decisions should carefully balance drug efficacy and safety. Although TER performs best in reducing the occurrence of all adverse events, its efficacy in some BMD targets (such as total hip BMD) is relatively lower. In comparison, while OBP has a clear advantage in reducing severe adverse events, its efficacy in some BMD improvements (such as femoral neck BMD) is slightly less. Therefore, clinicians should consider the specific needs of the patient, the treatment goals, and the safety profile of the drug when selecting a medication, particularly for long-term use.
Conclusion: The results indicate that abaloparatide and teriparatide are significantly superior to other drugs in improving lumbar spine and femoral neck BMD, while oral bisphosphonates is the most effective in improving total hip BMD. In terms of safety, teriparatide demonstrates the best performance in all adverse events, and oral bisphosphonates shows a clear advantage in reducing severe adverse events. Future treatment decisions should balance efficacy and safety, with clinical treatment tailored to the individual needs of the patient, including the site of bone loss and sensitivity to adverse events. Future research should explore combination therapies or multi-target strategies to optimize both efficacy and safety.
1 Introduction
Osteoporosis is a disease characterized by reduced bone mineral density and bone fragility, significantly increasing the risk of fractures (1, 2). Although osteoporosis primarily affects women, it also significantly impacts men, particularly in the elderly male population (3). According to epidemiological data, approximately 2 million men in the United States are affected by osteoporosis, and about 1 in 5 white men will experience an osteoporotic fracture during their lifetime (4). With the aging population, the prevalence of osteoporosis in men continues to rise annually (5, 6). The onset of male osteoporosis is often accompanied by a decrease in bone mineral density, especially in men aged 50 and above (7). Although the clinical presentation and treatment strategies for male osteoporosis have distinct features, its pathogenesis is similar to that of female osteoporosis, generally associated with excessive bone resorption or insufficient bone formation (8, 9). Primary osteoporosis refers to osteoporosis without an identifiable underlying disease, whereas secondary osteoporosis is related to other diseases or medication use (10). This study focuses on the treatment of primary male osteoporosis, particularly the treatment strategies in high-risk patients.
Currently, a variety of medications are available for the treatment of male osteoporosis, including abaloparatide, denosumab, teriparatide, and bisphosphonates (11). These medications each have distinct characteristics and have shown effective results in the treatment of male osteoporosis, though their indications, mechanisms, and effects may differ. Bisphosphonates (including both oral and intravenous formulations) remain one of the most widely used therapeutic options in clinical practice. Oral bisphosphonates include alendronate, risedronate, and ibandronate (12, 13), while intravenous bisphosphonates, such as zoledronic acid (14), effectively prevent bone loss by inhibiting bone resorption and demonstrate good efficacy and safety. Abaloparatide, denosumab, and teriparatide are relatively newer treatments used in clinical practice for male osteoporosis, promoting bone mineral density increases and reducing fracture risk through different mechanisms (15–18). This means that, in terms of pharmacological mechanisms, they belong to different classes of drugs overall. These medications are widely applied in male osteoporosis, and existing randomized controlled trials (RCTs) provide strong evidence of their efficacy. However, there is a lack of sufficient systematic comparative studies to guide the choice between these treatments, particularly for specific male osteoporosis populations.
Against this backdrop, this study aims to systematically evaluate and compare the efficacy and safety of abaloparatide (ABA), denosumab (DEN), teriparatide (TER), oral bisphosphonates (OBP), and intravenous bisphosphonates (IBP) in the treatment of male osteoporosis through a network meta-analysis. Specifically, we will examine their effects on bone mineral density improvement, fracture prevention, and adverse events. Through this analysis, we hope to provide an evidence-based, comprehensive treatment guideline to assist clinicians in making more precise decisions in the management of primary male osteoporosis.
2 Materials and methods
2.1 Search strategy
This systematic review and network meta-analysis (NMA) were conducted strictly in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines and the relevant standards recommended by the Cochrane Collaboration (19). These guidelines provide a detailed framework to ensure the transparency, scientific rigor, and consistency of systematic reviews and meta-analyses. The methodology for this review has been pre-registered on the PROSPERO platform with registration number CRD42024623547. For literature collection, we performed comprehensive electronic searches across multiple major databases, including Web of Science, PubMed, and the Cochrane Library, covering all relevant literature from the inception of these databases up to October 2024. To ensure completeness, the specific details and implementation steps of all search strategies are provided in the Supplementary Data. Additionally, to further minimize selection bias and ensure comprehensiveness, we manually searched and cross-checked the references of previously published pairwise meta-analyses.
2.2 Inclusion criteria and study design
This systematic review exclusively incorporates randomized controlled trials (RCTs) for network meta-analysis, while excluding non-original research formats, including case reports, review articles, letters to the editor, conference abstracts, opinion pieces, and study protocols. The study population consists of male patients with primary osteoporosis, and only studies related to primary osteoporosis were included, excluding those involving secondary osteoporosis (osteoporosis caused by gonadal dysfunction, corticosteroid use, cancer, or other systemic diseases).
The efficacy assessment was based on changes in bone mineral density (BMD) at the lumbar spine, femoral neck, and total hip, as well as the percentage change in BMD, to reflect the effects of different therapeutic drugs. Specifically, lumbar spine BMD is measured using dual-energy X-ray absorptiometry (DXA) or similar techniques to assess the mineral content in the lumbar spine, effectively evaluating bone density and strength in the lower spine region (20, 21). Total hip BMD measures the mineral content across the entire hip joint area, including the proximal femur and surrounding structures, providing insights into bone health and strength at the hip (22). It is worth noting that the effects of abaloparatide and teriparatide on total hip BMD may differ from those of bisphosphonate treatment, potentially due to artifact. This is because changes in patient positioning can influence the regional BMD assessed by DXA, leading to measurement variability. Femoral neck BMD measures the mineral content in the femoral neck, a high-risk area for hip fractures, and is significant for predicting fracture risk and bone health (23, 24).
Safety assessments were based on the occurrence of all adverse events (AEs) and serious adverse events (SAEs) (25, 26). All AEs include any negative health responses occurring during clinical trials or treatment, ranging from mild side effects (headache, nausea and local discomfort) to more significant health issues. For example, previous studies have reported upper gastrointestinal discomfort symptoms, such as nausea, vomiting, and epigastric pain, which may result from oral bisphosphonate therapy. In addition, there have been some reports of side effects such as dizziness and injection site reactions associated with teriparatide. Serious adverse events refer to health problems that may pose a life-threatening risk, including fatal events, severe disability, hospitalization, prolonged hospital stay, or even death.
2.3 Data extraction and quality assessment
Data collection was conducted independently by two researchers (XX and XX). During the data extraction process, the following key information was collected: first author, year of publication, study design type, country of origin, detailed information on interventions, sample size, and duration of follow-up. All collected data were organized and entered into an electronic database for subsequent analysis and processing.
For the patient outcome assessment, we primarily focused on the percentage change in bone mineral density (BMD) at the lumbar spine, femoral neck, and total hip to evaluate the efficacy of interventions. These BMD measures reflect critical aspects of bone health and are effective in assessing the impact of interventions on osteoporosis. Furthermore, to comprehensively assess the safety of interventions, we also recorded the occurrence of all adverse events (AEs) and serious adverse events (SAEs) during the treatment period. These data allowed us to evaluate the differences in efficacy and safety between different interventions. For studies with incomplete data, we strictly adhered to the guidance in Section 6.5.2 of the Cochrane Intervention Review Handbook and applied appropriate variance transformation or estimation methods to ensure data completeness and analytical accuracy (27). If the study reported mean differences and P-values, we calculated the standard error of the mean difference between groups using the formula in Section 6.5.2.3 and applied it in subsequent analyses. For studies where variance data were not provided, we assumed a conservative standard deviation of 30 for estimation (27). These steps were executed rigorously following scientific methodological standards to ensure the rigor and reliability of the data analysis.
Finally, to assess the quality of the included studies, we used the Cochrane Collaboration’s recommended bias risk assessment tool for randomized controlled trials (RCTs) (28–30). We performed a rigorous assessment of the risk of bias for each included study utilizing established methodological criteria, encompassing random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, handling of incomplete outcome data, potential selective reporting, and other sources of bias. Based on the evaluation outcomes, each study was categorized as having a low risk, high risk, or unclear risk of bias to ensure transparency and methodological robustness.
It is important to note that in the event of any disputes or inconsistencies in data extraction or quality assessment, a third researcher (XX) intervened to resolve discrepancies through discussion and arbitration, ensuring the consistency and accuracy of the final data.
2.4 Statistical analysis
We performed pairwise meta-analysis using Stata 18.0 software to assess the magnitude of effect sizes and the consistency of findings across studies. For network meta-analysis (NMA), we used R version 4.3.1 along with the gemtc and BUGSnet packages to handle complex multi-treatment comparisons (31–33). Using these methods, we were able to comprehensively assess the relative efficacy and safety of different treatment options. All statistical analyses were conducted using Review Manager software (version 5.4), a widely used tool in medical research for meta-analysis, forest plot generation, and bias risk assessment.
In the data synthesis process, we selected appropriate statistical methods based on the type of data. For categorical variables, we used odds ratios (OR) to assess the relative risk between treatment and control groups, describing the difference in event rates between the two groups. The odds ratio is a commonly used statistic in clinical research that reflects the relative effect of an intervention in preventing a specific event. For continuous variables, we used mean difference (MD) to analyze the effect size between the treatment and control groups, revealing the impact of the intervention on specific physiological parameters such as bone mineral density (34). The mean difference effectively quantifies the changes before and after treatment and between groups, helping to evaluate the actual effects of the intervention.
3 Results
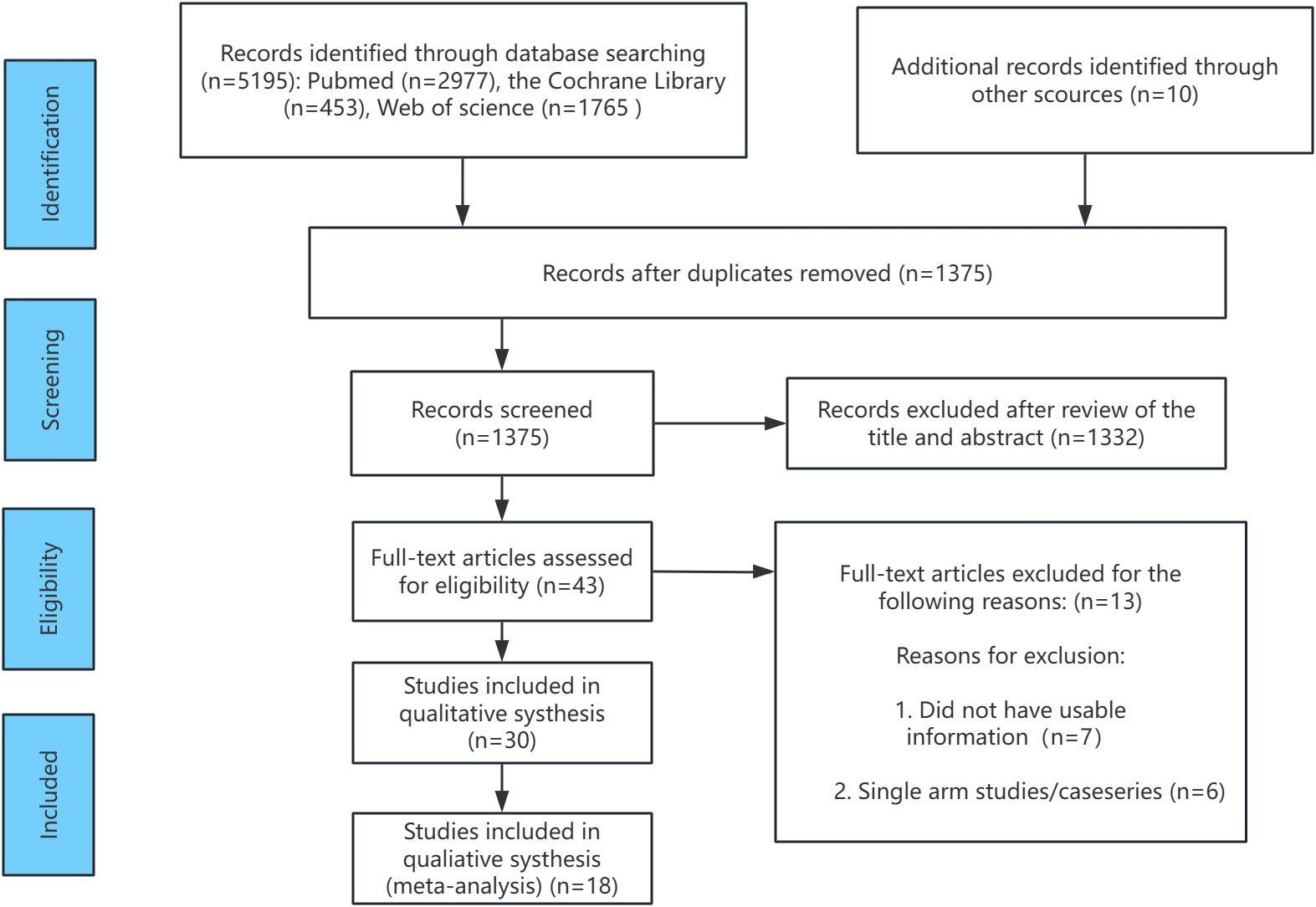
Figure 1 presents the flowchart illustrating the screening process for the included relevant randomized controlled trials (RCTs). During the literature search, a total of 5195 records were identified. After removing duplicates (references that appeared in multiple databases), 1375 unique records remained for further evaluation. Following full-text review and the application of inclusion criteria, several studies with lower relevance to the research topic were excluded. For example, the studies by Ringe in 2006 (35) and 2009 (36) were conducted by the same research team at different time points. To avoid data duplication, the 2006 study was excluded. Studies such as Sambrook 2012 (37), Glüer 2013 (38), Reid 2000 (39), and Wallach 2000 (40) focused on osteoporosis in men treated with high-dose corticosteroids, which was not aligned with the focus of this study, and were therefore excluded. Additionally, studies by Smith 2003 (41) and Fizazi 2011 (42) investigated osteoporosis following estrogen deprivation therapy and testosterone deprivation therapy in prostate cancer patients, respectively. Since the clinical populations in these studies did not meet the inclusion criteria for this research, they were also excluded. Finally, Saag 2019 (43) and Nakamura 2014 (44) were excluded due to insufficient male patient data or unclear gender distinctions. After these exclusions, 18 RCTs involving 4392 participants were included in the analysis. These studies investigated five treatment regimens (oral bisphosphonates, intravenous bisphosphonates, abaloparatide, denosumab, and teriparatide), as well as placebo and alfacalcidol. These studies served as the foundation for the subsequent network meta-analysis.
Figure 2 illustrates the associated network diagram, where each osteoporosis treatment was compared with at least one placebo-controlled trial, and each treatment was directly compared with at least one active drug in the network. Supplementary Table 1 shows the results of the statistical analysis, indicating good consistency across all outcomes, regardless of whether the consistency or inconsistency model was applied and the node-splitting method also did not detect significant inconsistency (P > 0.05). The primary outcomes, including lumbar spine, femoral neck, and total hip bone mineral density, had a follow-up period of 12 months, with fewer studies reporting longer or shorter follow-up durations. To ensure the reliability of the results, we used the follow-up data closest to 12 months for analysis. Supplementary Figure 1 presents the forest plots for all outcomes, and Supplementary Figure 2 displays the network plots for all outcomes. In the network plot, the size of the nodes represents the number of participants in each treatment group, while the thickness of the lines between nodes reflects the number of studies comparing those treatments. Supplementary Figure 3 presents the funnel plots for bias analysis of all outcomes. Visual inspection of the funnel plots revealed no evidence of publication bias among the included studies.
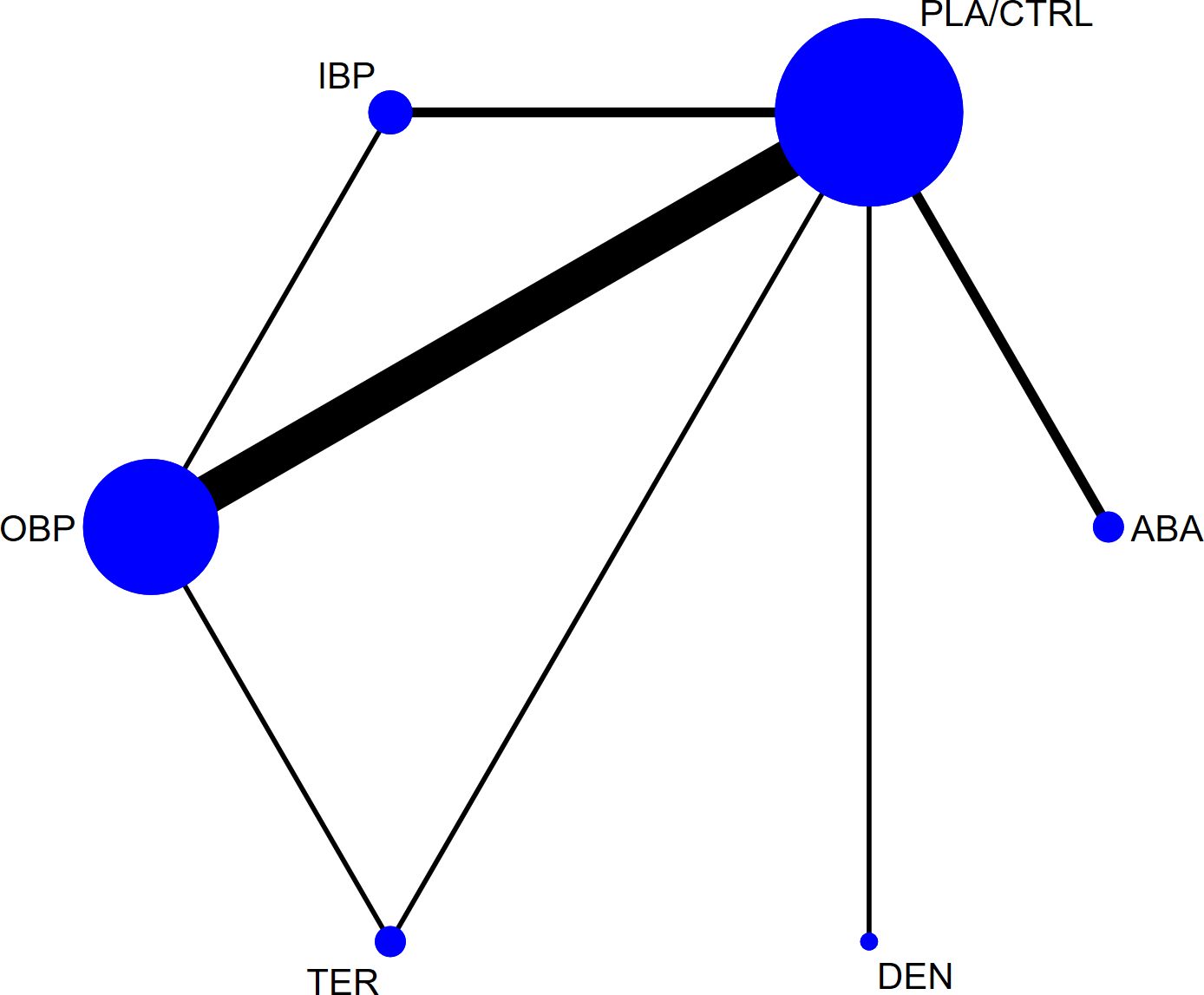
Figure 2. The network plot of all trials (ABA, abaloparatide; DEN, denosumab; TER, teriparatide; OBP, oral bisphosphonates; IBP, intravenous bisphosphonates; PLA/CTRL, placebo/control).
3.1 Study characteristics and study quality
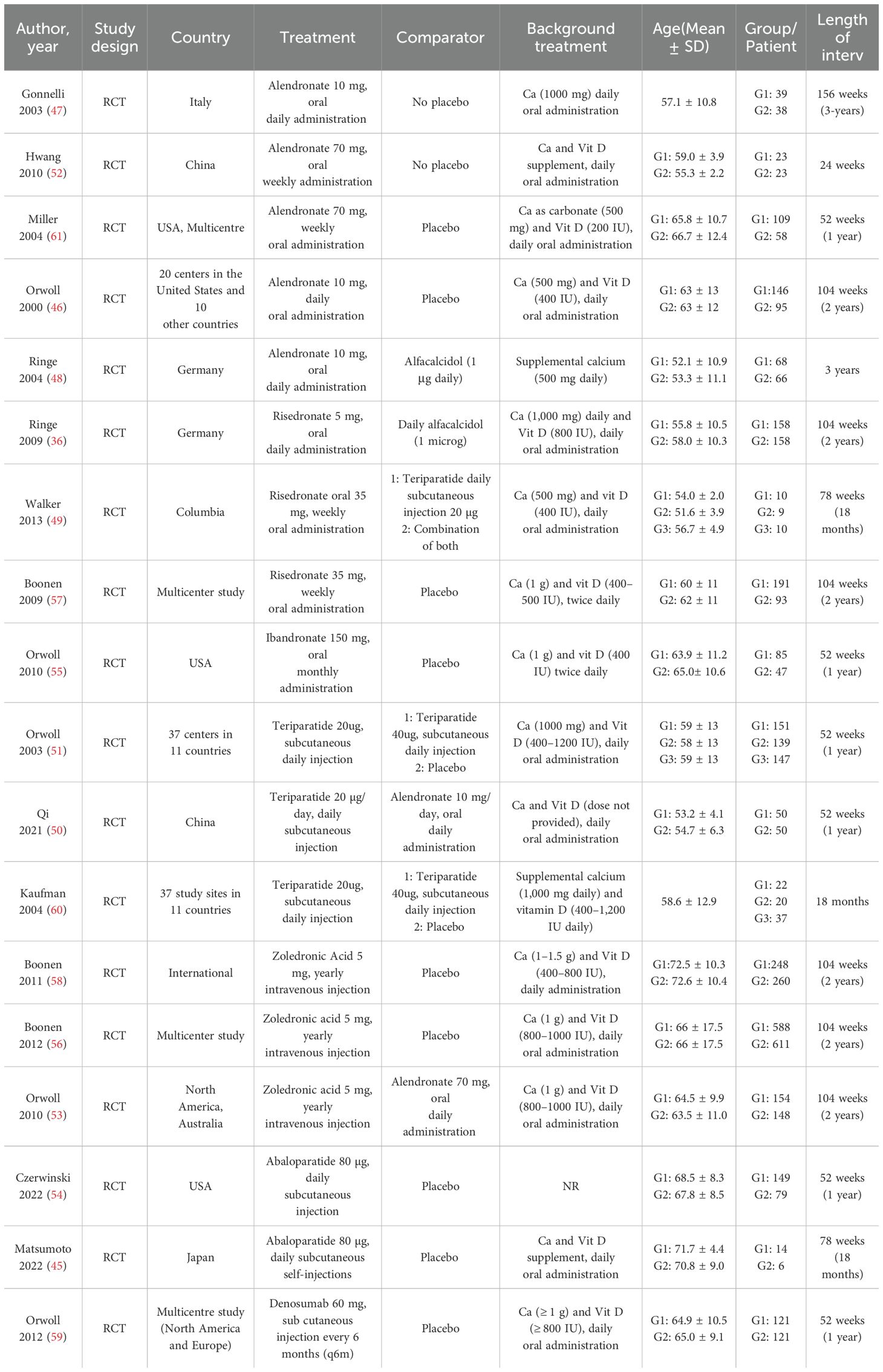
Table 1 summarizes the 18 randomized controlled trials (RCTs) included in the network meta-analysis (NMA) and outlines their key characteristics (36, 45–61). These studies were published between 2000 and 2022, with male participant numbers ranging from 19 to 1199, and study durations spanning from 1 to 3 years. The majority of studies compared treatment regimens with a placebo and/or calcium and vitamin D, while some also included alfacalcidol as a control (PLA/CTRL). Direct comparisons among the four treatment regimens were limited.
Figure 3 shows the risk of bias assessment for the included studies, conducted according to the Cochrane Collaboration guidelines. The assessment primarily focused on the random sequence generation, allocation concealment, and completeness of outcome reporting. Overall, most studies did not fully report the methods for random sequence generation and allocation concealment, particularly the details of the randomization process.

Figure 3. The network plot of all trials.Risk of bias summary for RCTs: Reviewers' judgments about each risk of bias item per included study.
3.2 Lumbar spine BMD
In 12 randomized controlled trials (RCTs) with a total of 2,226 participants, we compared the effects of different treatments on lumbar spine bone mineral density (BMD). The results showed that the ABA and TER treatment groups significantly outperformed other drugs in improving lumbar spine BMD. Figure 4a shows the specific differences between the treatment groups. Further analysis revealed that, aside from ABA and TER, the differences between other treatment groups in lumbar spine BMD were not statistically significant.
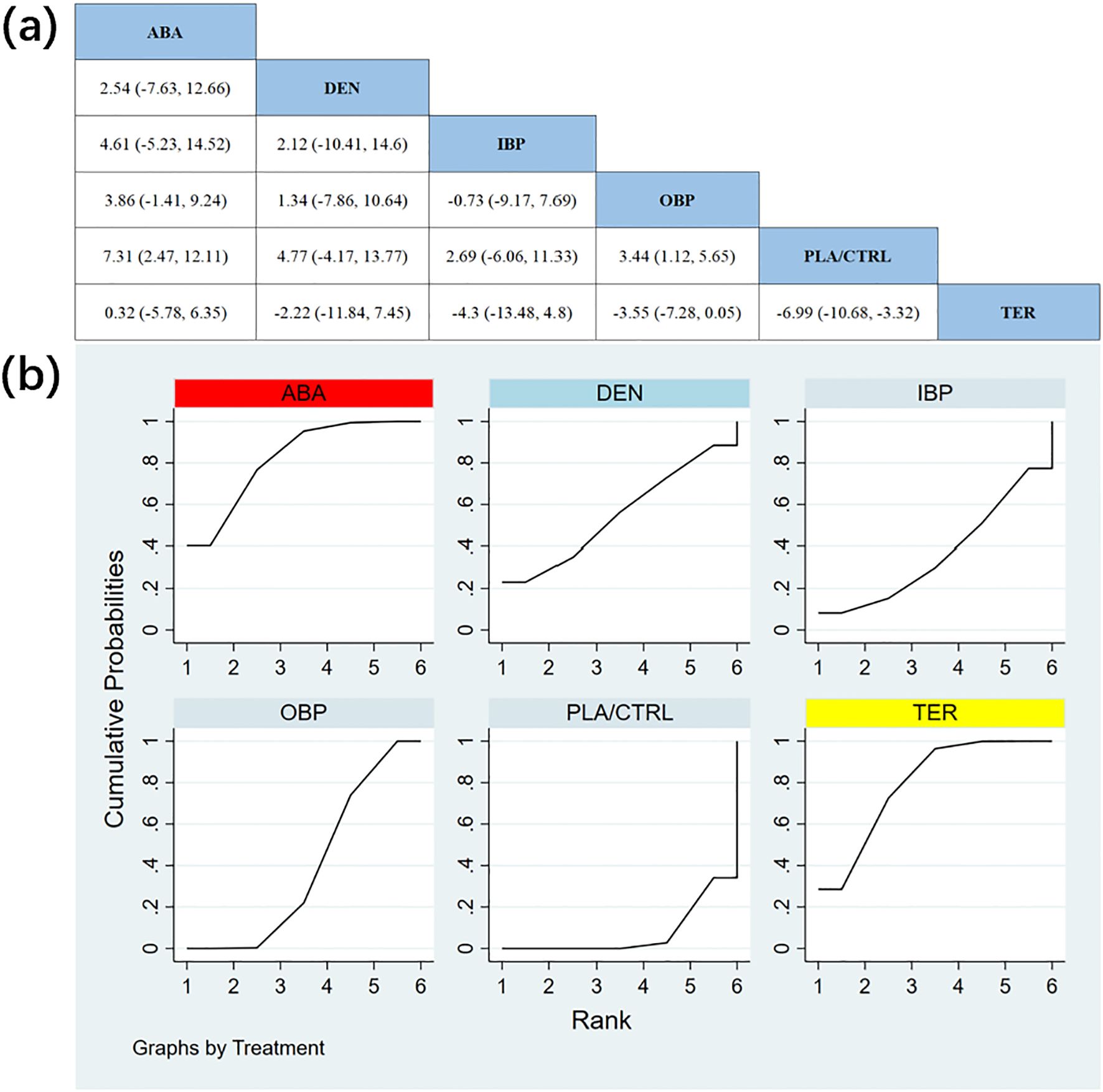
Figure 4. (a) The results of League table for Lumbar spine BMD. (b) Ranking the probability of Lumbar spine BMD percentage change.
To further quantify the effects of different treatments on lumbar spine BMD, we conducted a cumulative ranking curve (SUCRA) analysis. Figure 4b displays the results of lumbar spine BMD improvement, where red represents the highest rank, yellow represents second, and blue represents third. The ranking results revealed significant differences in the effectiveness of various drugs in improving lumbar spine BMD, with higher rankings indicating better treatment outcomes. Based on the SUCRA analysis, the probabilities of improving lumbar spine BMD, from highest to lowest, were as follows: ABA (SUCRA = 82.3%), TER (SUCRA = 79.5%), DEN (SUCRA = 55.2%), OBP (SUCRA = 39.3%), IBP (SUCRA = 36.4%), and PLA/CTRL (SUCRA = 7.4%).
3.3 Total hip BMD
In 11 RCTs with a total of 1,951 participants, we compared the effects of different treatments on total hip BMD. The results showed that the OBP treatment group significantly outperformed other groups in improving total hip BMD. Figure 5a shows the specific differences between the treatment groups. Further analysis revealed that, aside from OBP, the differences between other treatment groups did not reach statistical significance.
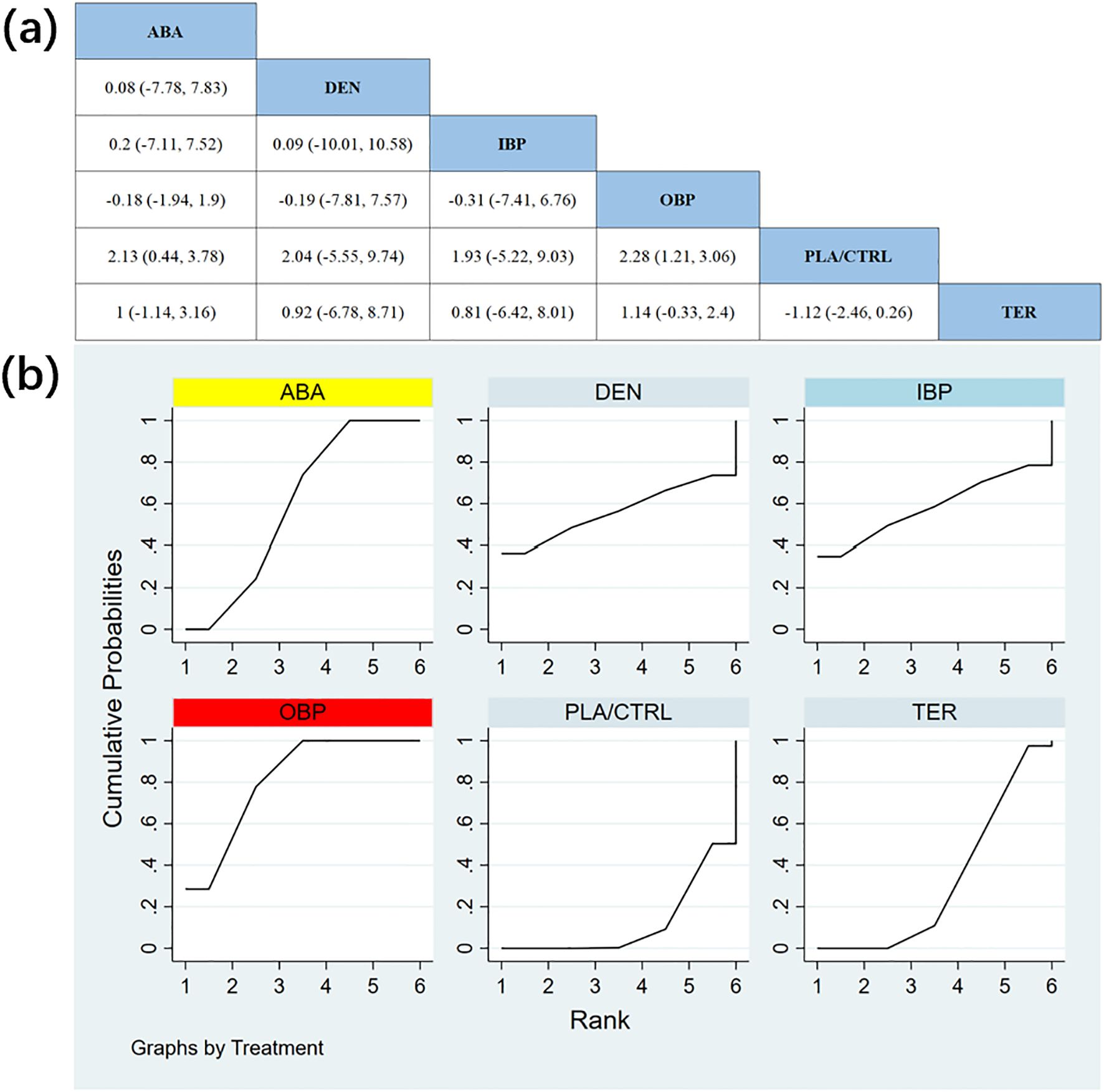
Figure 5. (a) The results of League table for Total hip BMD. (b) Ranking the probability of Total hip BMD percentage change.
To further quantify the effects of different treatments on total hip BMD, we applied the cumulative ranking curve (SUCRA) method. Figure 5b displays the results of total hip BMD improvement. According to the SUCRA analysis, the probabilities of improving total hip BMD, from highest to lowest, were as follows: OBP (SUCRA = 81.3%), ABA (SUCRA = 59.6%), IBP (SUCRA = 58.4%), DEN (SUCRA = 56.3%), TER (SUCRA = 32.5%), and PLA/CTRL (SUCRA = 12.0%).
3.4 Femoral neck BMD
In 12 RCTs with a total of 2,235 participants, we compared the effects of different treatments on femoral neck BMD. The results showed that the TER and ABA treatment groups significantly outperformed other treatment groups in improving femoral neck BMD. Figure 6a shows the specific differences between the treatment groups.
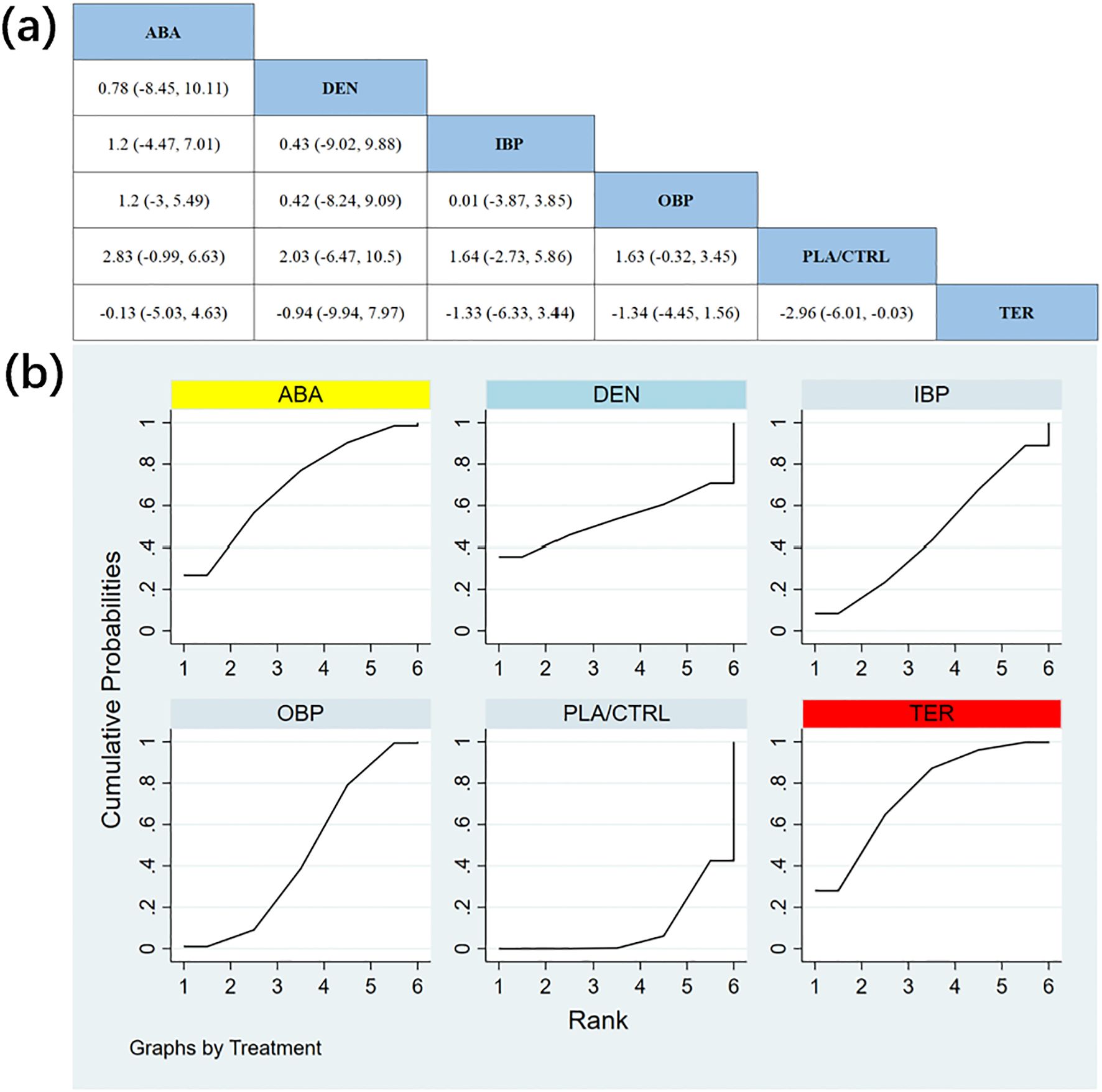
Figure 6. (a) The results of League table for Femoral neck BMD. (b) Ranking the probability of Femoral neck BMD percentage change.
To further quantify the effects of different treatments on femoral neck BMD, we used the cumulative ranking curve (SUCRA) method to rank the treatments. Figure 6b displays the results of femoral neck BMD improvement. Based on the SUCRA analysis, the probabilities of improving femoral neck BMD, from highest to lowest, were as follows: TER (SUCRA = 75.2%), ABA (SUCRA = 69.8%), DEN (SUCRA = 53.3%), IBP (SUCRA = 46.4%), OBP (SUCRA = 45.5%), and PLA/CTRL (SUCRA = 9.8%).
3.5 All adverse events
In 10 RCTs with a total of 3,437 participants, we compared all adverse events (AEs) across different treatments. The results showed that the TER treatment group demonstrated significantly better safety than other treatment groups. Figure 7a shows the specific differences between the treatment groups.

Figure 7. (a) The results of League table for all adverse events. (b) Ranking the probability of all adverse events.
To further quantify the safety of different treatments regarding all adverse events, we used the cumulative ranking curve (SUCRA) method. Figure 7b displays the results of all adverse events. According to the SUCRA analysis, the safety ranking probabilities for all adverse events, from highest to lowest, were as follows: TER (SUCRA = 86.2%), OBP (SUCRA = 63.0%), PLA/CTRL (SUCRA = 53.6%), DEN (SUCRA = 47.8%), ABA (SUCRA = 33.2%), and IBP (SUCRA = 16.2%).
3.6 Serious adverse events
In 8 RCTs with a total of 3,000 participants, we compared serious adverse events (SAEs) across different treatments (TER was excluded due to insufficient data). The results showed that the OBP treatment group demonstrated significantly better safety in terms of SAEs compared to other treatment groups. Figure 8a shows the specific differences between the treatment groups.

Figure 8. (a) The results of League table for serious adverse events. (b) Ranking the probability of serious adverse events.
To further quantify the safety of different treatments regarding serious adverse events, we used the cumulative ranking curve (SUCRA) method. Figure 8b displays the results of serious adverse events. According to the SUCRA analysis, the safety ranking probabilities for serious adverse events, from highest to lowest, were as follows: OBP (SUCRA = 70.2%), IBP (SUCRA = 55.7%), ABA (SUCRA = 44.7%), PLA/CTRL (SUCRA = 41.0%), and DEN (SUCRA = 38.4%).
4 Discussion
This systematic review and network meta-analysis (NMA) provides a comprehensive evaluation of the efficacy and safety of several drugs (ABA, DEN, TER, OBP and IBP) in the treatment of primary osteoporosis in men.
In terms of efficacy, ABA and TER significantly outperform other drugs in improving lumbar spine and femoral neck BMD, while OBP shows the best performance in improving total hip BMD. These results suggest that different drugs or treatments may have distinct mechanisms of action when targeting BMD in different skeletal sites. For example, although ABA and TER show outstanding effects in improving lumbar spine and femoral neck BMD, their impact on total hip BMD is relatively weaker. In contrast, OBP has a significant effect on improving total hip BMD, indicating the selective effects of different drugs on BMD at different sites. This implies that, in the treatment of osteoporosis, the choice of drug should be based on the patient’s specific condition and the sites of BMD change, allowing for more personalized treatment. A tailored treatment strategy could enhance treatment outcomes and clinical satisfaction. It is particularly noteworthy that in the comparison of oral and intravenous bisphosphonates, oral bisphosphonates demonstrate a statistically significant advantage in improving total hip BMD. However, no significant differences in efficacy were observed between oral and intravenous bisphosphonates in terms of lumbar spine and femoral neck BMD.
Regarding safety, TER shows a distinct advantage in the incidence of all adverse events, suggesting that TER not only improves BMD effectively but also has better safety during treatment. This implies that TER could be an ideal choice for long-term therapy, especially for patients requiring prolonged drug treatment. The lower incidence of adverse events significantly improves patient adherence to treatment, thereby enhancing their quality of life. However, while TER excels in reducing all adverse events, OBP shows the most significant advantage in reducing the occurrence of severe adverse events. This suggests that OBP may offer higher safety in preventing serious side effects, particularly for high-risk patients (such as elderly individuals or those with multiple comorbidities), for whom OBP could be a safer treatment option. Additionally, oral bisphosphonates have a safety advantage over intravenous bisphosphonates, especially in reducing the occurrence of severe adverse events.
Overall, clinical decisions should carefully balance drug efficacy and safety. Although TER performs best in reducing the occurrence of all adverse events, its efficacy in some BMD targets (such as total hip BMD) is relatively lower. In comparison, while OBP has a clear advantage in reducing severe adverse events, its efficacy in some BMD improvements (such as femoral neck BMD) is slightly less. Therefore, clinicians should consider the specific needs of the patient, the treatment goals, and the safety profile of the drug when selecting a medication, particularly for long-term use.
Finally, in the comparison of oral versus intravenous bisphosphonates, Louise S. Conwell et al. (62) evaluated the efficacy of bisphosphonates in treating osteoporosis in cystic fibrosis patients through a meta-analysis and found that intravenous bisphosphonates might lead to severe bone pain and flu-like symptoms. This result is consistent with the findings of our network meta-analysis and further supports the greater efficacy and safety of oral bisphosphonates compared to intravenous bisphosphonates in the treatment of primary osteoporosis in men.
In recent years, the incidence of osteoporosis in men has gradually increased, prompting a rise in related clinical research, particularly systematic reviews and network meta-analyses (NMA). Managing osteoporosis in men still faces many challenges, and while existing studies focus on different aspects of drug interventions, fewer studies have addressed the differences in treatment effects between intravenous and oral bisphosphonates. Thus, this study employed a network meta-analysis to comprehensively assess and rank the efficacy and safety of five commonly used drugs in the treatment of male osteoporosis, providing more clinically relevant evidence. This network meta-analysis has several strengths (1): It included 18 studies with a total of 4392 participants, all of which were randomized controlled trials (RCTs), ensuring the reliability and scientific rigor of the data (2); It made a more detailed classification of drugs, dividing bisphosphonates into intravenous and oral categories, further revealing the treatment differences between different formulations and offering more directions for future research (3); The statistical results showed good consistency, reflecting the stability of the methodology, and through indirect comparisons of different treatment regimens, it provided a comprehensive evaluation of drug management in male osteoporosis. However, this study also has some limitations: (1) Some treatment drugs lack direct head-to-head comparisons, which may affect the rigor and reliability of the results and conclusions; (2) Data on severe adverse events for certain drugs (TER) were limited and not included in the analysis, which may impact the comprehensive assessment of drug safety; (3) The studies included in this research spanned a long period (2000–2022), which may lead to differences in study design, patient characteristics, and data collection methods, potentially affecting the quality of the results; (4) Due to the large variety of oral bisphosphonates and the scattered nature of the available data, subgroup analyses were not performed.
Ultimately, to further optimize the treatment outcomes and reduce drug side effects in male osteoporosis, there is a pressing need for more high-quality RCTs. These studies can assist physicians in developing more personalized treatment plans based on the patient’s specific condition, BMD assessment results, and potential risks, thereby achieving the goals of precision medicine and improving the efficacy and safety of clinical treatments.
5 Conclusion
The results indicate that abaloparatide and teriparatide are significantly superior to other drugs in improving lumbar spine and femoral neck BMD, while oral bisphosphonates is the most effective in improving total hip BMD. In terms of safety, teriparatide demonstrates the best performance in all adverse events, and oral bisphosphonates shows a clear advantage in reducing severe adverse events. Future treatment decisions should balance efficacy and safety, with clinical treatment tailored to the individual needs of the patient, including the site of bone loss and sensitivity to adverse events. Future research should explore combination therapies or multi-target strategies to optimize both efficacy and safety.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
LC: Conceptualization, Methodology, Project administration, Writing – original draft, Writing – review & editing. BJ: Conceptualization, Data curation, Software, Writing – original draft, Writing – review & editing. CX: Formal analysis, Investigation, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1558560/full#supplementary-material
Supplementary Table 1 | The results of compareing the deviance information criteria (DIC) between the consistency and inconsistency models.
References
1. Miller PD. Management of severe osteoporosis. Expert Opin pharmacotherapy. (2016) 17:473–88. doi: 10.1517/14656566.2016.1124856
2. Yong EL, Logan S. Menopausal osteoporosis: screening, prevention and treatment. Singapore Med J. (2021) 62:159–66. doi: 10.11622/smedj.2021036
3. Bandeira L, Silva BC, Bilezikian JP. Male osteoporosis. Arch Endocrinol Metab. (2022) 66:739–47. doi: 10.20945/2359-3997000000563
4. Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone mineral research: Off J Am Soc Bone Mineral Res. (2014) 29:2520–6. doi: 10.1002/jbmr.2269
5. Barik S, Kumar V. Male osteoporosis and frailty. Indian J Orthopaedics. (2023) 57:237–44. doi: 10.1007/s43465-023-01027-w
6. Rinonapoli G, Ruggiero C, Meccariello L, Bisaccia M, Ceccarini P, Caraffa A. Osteoporosis in men: A review of an underestimated bone condition. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22042105
7. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone mineral research: Off J Am Soc Bone Mineral Res. (2007) 22:465–75. doi: 10.1359/jbmr.061113
8. Oliveira LG, Guimarães ML. Male osteoporosis. Rev Bras ortopedia. (2010) 45:392–6. doi: 10.1016/s2255-4971(15)30425-0
9. Björnsdottir S, Clarke BL, Mannstadt M, Langdahl BL. Male osteoporosis-what are the causes, diagnostic challenges, and management. Best Pract Res Clin Rheumatol. (2022) 36:101766. doi: 10.1016/j.berh.2022.101766
10. Gennari L, Bilezikian JP. New and developing pharmacotherapy for osteoporosis in men. Expert Opin Pharmacotherapy. (2018) 19:253–64. doi: 10.1080/14656566.2018.1428559
11. Giusti A, Bianchi G. Treatment of primary osteoporosis in men. Clin Interventions Aging. (2015) 10:105–15. doi: 10.2147/cia.S44057
12. Fuggle N, Al-Daghri N, Bock O, Branco J, Bruyère O, Casado E, et al. Novel formulations of oral bisphosphonates in the treatment of osteoporosis. Aging Clin Exp Res. (2022) 34:2625–34. doi: 10.1007/s40520-022-02272-z
13. Conte P, Guarneri V. Safety of intravenous and oral bisphosphonates and compliance with dosing regimens. oncologist. (2004) 9(Suppl 4):28–37. doi: 10.1634/theoncologist.9-90004-28
14. Bishop S, Narayanasamy MJ, Paskins Z, Corp N, Bastounis A, Griffin J, et al. Clinicians’ views of prescribing oral and intravenous bisphosphonates for osteoporosis: A qualitative study. BMC Musculoskeletal Disord. (2023) 24:770. doi: 10.1186/s12891-023-06865-1
15. Brent MB. Abaloparatide: A review of preclinical and clinical studies. Eur J Pharmacol. (2021) 909:174409. doi: 10.1016/j.ejphar.2021.174409
16. Shane E, Shiau S, Recker RR, Lappe JM, Agarwal S, Kamanda-Kosseh M, et al. Denosumab after teriparatide in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab. (2022) 107:e1528–e40. doi: 10.1210/clinem/dgab850
17. Miller PD, Hattersley G, Riis BJ, Williams GC, Lau E, Russo LA, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: A randomized clinical trial. Jama. (2016) 316:722–33. doi: 10.1001/jama.2016.11136
18. Stroup J, Kane MP, Abu-Baker AM. Teriparatide in the treatment of osteoporosis. Am J health-system pharmacy: AJHP: Off J Am Soc Health-System Pharmacists. (2008) 65:532–9. doi: 10.2146/ajhp070171
19. Elsman EBM, Mokkink LB, Terwee CB, Beaton D, Gagnier JJ, Tricco AC, et al. Guideline for reporting systematic reviews of outcome measurement instruments (Omis): prisma-cosmin for omis 2024. J patient-reported outcomes. (2024) 8:64. doi: 10.1186/s41687-024-00727-7
20. Pu HY, Chen Q, Huang K, Wei P. Correlation between forearm bone mineral density measured by dual energy X-ray absorptiometry and hounsfield units value measured by ct in lumbar spine. Z fur Orthopadie und Unfallchirurgie. (2024) 162:247–53. doi: 10.1055/a-1984-0466
21. Davidson S, Vecellio A, Flagstad I, Holton K, Bruzina A, Lender P, et al. Discrepancy between dxa and ct-based assessment of spine bone mineral density. Spine deformity. (2023) 11:677–83. doi: 10.1007/s43390-023-00646-5
22. Bouxsein ML, Eastell R, Lui LY, Wu LA, de Papp AE, Grauer A, et al. Change in bone density and reduction in fracture risk: A meta-regression of published trials. J Bone mineral research: Off J Am Soc Bone Mineral Res. (2019) 34:632–42. doi: 10.1002/jbmr.3641
23. Davenport A. Differences in prevalence of reduced and low bone mineral density between lumbar spine and femoral neck in peritoneal dialysis patients using dual-energy X-ray absorptiometry (Dxa). Peritoneal Dialysis international: J Int Soc Peritoneal Dialysis. (2023) 43:334–8. doi: 10.1177/08968608221146867
24. Kemmler W, Shojaa M, Kohl M, von Stengel S. Effects of different types of exercise on bone mineral density in postmenopausal women: A systematic review and meta-analysis. Calcified Tissue Int. (2020) 107:409–39. doi: 10.1007/s00223-020-00744-w
25. Wu Q, Taboureau O, Audouze K. Development of an adverse drug event network to predict drug toxicity. Curr Res Toxicol. (2020) 1:48–55. doi: 10.1016/j.crtox.2020.06.001
26. Pereira TV, Jüni P, Saadat P, Xing D, Yao L, Bobos P, et al. Viscosupplementation for knee osteoarthritis: systematic review and meta-analysis. BMJ (Clinical Res ed). (2022) 378:e069722. doi: 10.1136/bmj-2022-069722
27. Cashin AG, Folly T, Bagg MK, Wewege MA, Jones MD, Ferraro MC, et al. Efficacy, acceptability, and safety of muscle relaxants for adults with non-specific low back pain: systematic review and meta-analysis. BMJ (Clinical Res ed). (2021) 374:n1446. doi: 10.1136/bmj.n1446
28. Agarwal A, Rochwerg B, Sevransky JE. 21st century evidence: randomized controlled trials versus systematic reviews and meta-analyses. Crit Care Med. (2021) 49:2001–2. doi: 10.1097/ccm.0000000000005343
29. Moher D, Schulz KF, Altman D. The consort statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Jama. (2001) 285:1987–91. doi: 10.1001/jama.285.15.1987
30. Seidler AL, Hunter KE, Cheyne S, Berlin JA, Ghersi D, Askie LM. Prospective meta-analyses and cochrane’s role in embracing next-generation methodologies. Cochrane Database systematic Rev. (2020) 10:Ed000145. doi: 10.1002/14651858.Ed000145
31. Yang F, Wang H, Zou J, Li X, Jin X, Cao Y, et al. Assessing the methodological and reporting quality of network meta-analyses in chinese medicine. Medicine. (2018) 97:e13052. doi: 10.1097/md.0000000000013052
32. Béliveau A, Boyne DJ, Slater J, Brenner D, Arora P. Bugsnet: an R package to facilitate the conduct and reporting of bayesian network meta-analyses. BMC Med Res Method. (2019) 19:196. doi: 10.1186/s12874-019-0829-2
33. Sehmbi H, Retter S, Shah UJ, Nguyen D, Martin J, Uppal V. Epidemiological, methodological, and statistical characteristics of network meta-analysis in anaesthesia: A systematic review. Br J anaesthesia. (2023) 130:272–86. doi: 10.1016/j.bja.2022.08.042
34. van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta-analysis. Res synthesis Methods. (2012) 3:285–99. doi: 10.1002/jrsm.1054
35. Ringe JD, Faber H, Farahmand P, Dorst A. Efficacy of risedronate in men with primary and secondary osteoporosis: results of a 1-year study. Rheumatol Int. (2006) 26:427–31. doi: 10.1007/s00296-005-0004-4
36. Ringe JD, Farahmand P, Faber H, Dorst A. Sustained efficacy of risedronate in men with primary and secondary osteoporosis: results of a 2-year study. Rheumatol Int. (2009) 29:311–5. doi: 10.1007/s00296-008-0689-2
37. Sambrook PN, Roux C, Devogelaer JP, Saag K, Lau CS, Reginster JY, et al. Bisphosphonates and glucocorticoid osteoporosis in men: results of a randomized controlled trial comparing zoledronic acid with risedronate. Bone. (2012) 50:289–95. doi: 10.1016/j.bone.2011.10.024
38. Glüer CC, Marin F, Ringe JD, Hawkins F, Möricke R, Papaioannu N, et al. Comparative effects of teriparatide and risedronate in glucocorticoid-induced osteoporosis in men: 18-month results of the eurogiops trial. J Bone mineral research: Off J Am Soc Bone Mineral Res. (2013) 28:1355–68. doi: 10.1002/jbmr.1870
39. Reid DM, Hughes RA, Laan RF, Sacco-Gibson NA, Wenderoth DH, Adami S, et al. Efficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: A randomized trial. Eur Corticosteroid-Induced Osteoporosis Treat Study. J Bone mineral research: Off J Am Soc Bone Mineral Res. (2000) 15:1006–13. doi: 10.1359/jbmr.2000.15.6.1006
40. Wallach S, Cohen S, Reid DM, Hughes RA, Hosking DJ, Laan RF, et al. Effects of risedronate treatment on bone density and vertebral fracture in patients on corticosteroid therapy. Calcified Tissue Int. (2000) 67:277–85. doi: 10.1007/s002230001146
41. Smith MR, Eastham J, Gleason DM, Shasha D, Tchekmedyian S, Zinner N. Randomized controlled trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer. J Urol. (2003) 169:2008–12. doi: 10.1097/01.ju.0000063820.94994.95
42. Fizazi K, Carducci M, Smith M, Damião R, Brown J, Karsh L, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet (London England). (2011) 377:813–22. doi: 10.1016/s0140-6736(10)62344-6
43. Saag KG, Pannacciulli N, Geusens P, Adachi JD, Messina OD, Morales-Torres J, et al. Denosumab versus risedronate in glucocorticoid-induced osteoporosis: final results of a twenty-four-month randomized, double-blind, double-dummy trial. Arthritis Rheumatol (Hoboken NJ). (2019) 71:1174–84. doi: 10.1002/art.40874
44. Nakamura T, Matsumoto T, Sugimoto T, Hosoi T, Miki T, Gorai I, et al. Clinical trials express: fracture risk reduction with denosumab in Japanese postmenopausal women and men with osteoporosis: denosumab fracture intervention randomized placebo controlled trial (Direct). J Clin Endocrinol Metab. (2014) 99:2599–607. doi: 10.1210/jc.2013-4175
45. Matsumoto T, Sone T, Soen S, Tanaka S, Yamashita A, Inoue T. Abaloparatide increases lumbar spine and hip bmd in Japanese patients with osteoporosis: the phase 3 active-J study. J Clin Endocrinol Metab. (2022) 107:e4222–e31. doi: 10.1210/clinem/dgac486
46. Orwoll E, Ettinger M, Weiss S, Miller P, Kendler D, Graham J, et al. Alendronate for the treatment of osteoporosis in men. New Engl J Med. (2000) 343:604–10. doi: 10.1056/nejm200008313430902
47. Gonnelli S, Cepollaro C, Montagnani A, Bruni D, Caffarelli C, Breschi M, et al. Alendronate treatment in men with primary osteoporosis: A three-year longitudinal study. Calcified Tissue Int. (2003) 73:133–9. doi: 10.1007/s00223-002-1085-7
48. Ringe JD, Dorst A, Faber H, Ibach K. Alendronate treatment of established primary osteoporosis in men: 3-year results of a prospective, comparative, two-arm study. Rheumatol Int. (2004) 24:110–3. doi: 10.1007/s00296-003-0388-y
49. Walker MD, Cusano NE, Sliney J Jr., Romano M, Zhang C, McMahon DJ, et al. Combination therapy with risedronate and teriparatide in male osteoporosis. Endocrine. (2013) 44:237–46. doi: 10.1007/s12020-012-9819-4
50. Qi YJ, Wang WY, Sun WL, Pan QY. Comparative efficacy and safety of alendronate and teriparatide in bone loss reduction and prevention of vertebral fracture in osteoporotic chinese patients. Trop J Pharm Res. (2021) 20:2199–204. doi: 10.4314/tjpr.v20i10.26
51. Orwoll ES, Scheele WH, Paul S, Adami S, Syversen U, Diez-Perez A, et al. The effect of teriparatide [Human parathyroid hormone (1-34)] therapy on bone density in men with osteoporosis. J Bone mineral research: Off J Am Soc Bone Mineral Res. (2003) 18:9–17. doi: 10.1359/jbmr.2003.18.1.9
52. Hwang JS, Liou MJ, Ho C, Lin JD, Huang YY, Wang CJ, et al. The effects of weekly alendronate therapy in Taiwanese males with osteoporosis. J Bone mineral Metab. (2010) 28:328–33. doi: 10.1007/s00774-009-0136-9
53. Orwoll ES, Miller PD, Adachi JD, Brown J, Adler RA, Kendler D, et al. Efficacy and safety of a once-yearly I.V. Infusion of zoledronic acid 5 Mg versus a once-weekly 70-mg oral alendronate in the treatment of male osteoporosis: A randomized, multicenter, double-blind, active-controlled study. J Bone Mineral Research: Off J Am Soc Bone Mineral Res. (2010) 25:2239–50. doi: 10.1002/jbmr.119
54. Czerwinski E, Cardona J, Plebanski R, Recknor C, Vokes T, Saag KG, et al. The efficacy and safety of abaloparatide-sc in men with osteoporosis: A randomized clinical trial. J Bone mineral research: Off J Am Soc Bone Mineral Res. (2022) 37:2435–42. doi: 10.1002/jbmr.4719
55. Orwoll ES, Binkley NC, Lewiecki EM, Gruntmanis U, Fries MA, Dasic G. Efficacy and safety of monthly ibandronate in men with low bone density. Bone. (2010) 46:970–6. doi: 10.1016/j.bone.2009.12.034
56. Boonen S, Reginster JY, Kaufman JM, Lippuner K, Zanchetta J, Langdahl B, et al. Fracture risk and zoledronic acid therapy in men with osteoporosis. New Engl J Med. (2012) 367:1714–23. doi: 10.1056/NEJMoa1204061
57. Boonen S, Orwoll ES, Wenderoth D, Stoner KJ, Eusebio R, Delmas PD. Once-weekly risedronate in men with osteoporosis: results of a 2-year, placebo-controlled, double-blind, multicenter study. J Bone mineral research: Off J Am Soc Bone Mineral Res. (2009) 24:719–25. doi: 10.1359/jbmr.081214
58. Boonen S, Orwoll E, Magaziner J, Colón-Emeric CS, Adachi JD, Bucci-Rechtweg C, et al. Once-yearly zoledronic acid in older men compared with women with recent hip fracture. J Am Geriatrics Soc. (2011) 59:2084–90. doi: 10.1111/j.1532-5415.2011.03666.x
59. Orwoll E, Teglbjærg CS, Langdahl BL, Chapurlat R, Czerwinski E, Kendler DL, et al. A randomized, placebo-controlled study of the effects of denosumab for the treatment of men with low bone mineral density. J Clin Endocrinol Metab. (2012) 97:3161–9. doi: 10.1210/jc.2012-1569
60. Kaufman JM, Orwoll E, Goemaere S, San Martin J, Hossain A, Dalsky GP, et al. Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: treatment and discontinuation of therapy. Osteoporosis international: J established as result cooperation between Eur Foundation Osteoporosis Natl Osteoporosis Foundation USA. (2005) 16:510–6. doi: 10.1007/s00198-004-1713-3
61. Miller PD, Schnitzer T, Emkey R, Orwoll E, Rosen C, Ettinger M, et al. Weekly oral alendronic acid in male osteoporosis. Clin Drug Invest. (2004) 24:333–41. doi: 10.2165/00044011-200424060-00003
Keywords: abaloparatide, denosumab, teriparatide, bisphosphonates, male osteoporosis
Citation: Chen L, Ji B and Xia C (2025) Efficacy and safety of abaloparatide, denosumab, teriparatide, oral bisphosphonates, and intravenous bisphosphonates in the treatment of male osteoporosis: a systematic review and Bayesian network meta-analysis. Front. Endocrinol. 16:1558560. doi: 10.3389/fendo.2025.1558560
Received: 10 January 2025; Accepted: 28 March 2025;
Published: 16 April 2025.
Edited by:
Jeonghoon Ha, The Catholic University of Korea, Republic of KoreaReviewed by:
Gustavo Roberto Cointry, National University of Rosario, ArgentinaAmirala Bakhshian Nik, Medical College of Wisconsin, United States
Copyright © 2025 Chen, Ji and Xia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cong Xia, MTMxOTQzMTI5ODVAMTYzLmNvbQ==
 Liangshi Chen
Liangshi Chen Bomei Ji2
Bomei Ji2