
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 11 February 2025
Sec. Clinical Diabetes
Volume 16 - 2025 | https://doi.org/10.3389/fendo.2025.1542946
This article is part of the Research Topic World Diabetes Day 2024: Exploring Mechanisms, Innovations, and Holistic Approaches in Diabetes Care View all 3 articles
 Md Faruque Pathan1
Md Faruque Pathan1 Nazma Akter2
Nazma Akter2 Marufa Mustari3
Marufa Mustari3 M. Saifuddin4
M. Saifuddin4 Mirza Sharifuzzaman4
Mirza Sharifuzzaman4 Mohammad Motiur Rahman5
Mohammad Motiur Rahman5 Mohammed Ripon6
Mohammed Ripon6 S. M. Mohiuddin7
S. M. Mohiuddin7 A. B. M. Kamrul-Hasan8
A. B. M. Kamrul-Hasan8 Mohammad Abdul Hannan9
Mohammad Abdul Hannan9 Muhammad Shah Alam10
Muhammad Shah Alam10 Samira Mahjabeen3
Samira Mahjabeen3 Faria Afsana11
Faria Afsana11 Muhammed Abu Bakar11
Muhammed Abu Bakar11 Tahniyah Haq3
Tahniyah Haq3 Afsar Ahammed12
Afsar Ahammed12 Samir Kumar Talukder13
Samir Kumar Talukder13 Sourav Sarkar14
Sourav Sarkar14 Shahjada Selim3*†
Shahjada Selim3*†Background: Management of type 2 diabetes mellitus (T2DM) during Ramadan fasting presents unique challenges due to prolonged fasting periods, irregular meal schedules, and altered medication timing, potentially impacting glycemic control. Ertugliflozin, a sodium-glucose co-transporter 2 (SGLT2) inhibitor, has been shown to improve glycemic control in T2DM effectively. However, the effectiveness of ertugliflozin during Ramadan fasting, a period with unique glycemic challenges, has not been studied extensively.
Methods: This study was a multicenter, real-life experience study involving 1373 adult patients with known T2DM for at least one year, an HbA1c level of less than 10%, and who intended to fast during Ramadan. Participants were divided into two groups: the Ertu group (n=703), consisting of patients who had been on a stable dose of ertugliflozin for at least three months before Ramadan, and the non-Ertu group (n=670), which included patients receiving other oral antihyperglycemic drugs (OADs) except ertugliflozin. Patients attended a baseline visit one month before the first day of Ramadan and a follow-up visit within one month after the last day of Ramadan. Both visits included history taking, physical examinations, and laboratory tests. The primary endpoints were changes in HbA1c levels, body weight, body mass index (BMI), and the incidence of hypoglycemia during Ramadan fasting.
Results: The mean age of the study participants was 50.37 ± 11.14 (SD) years, with 40.6% male and 58.7% female. Patients receiving ertugliflozin showed significant reduction in HbA1C (-0.65 ± 0.67% vs. -0.22 ± 0.64%, p<0.001), body weight (-1.24 ± 2.58 kg vs. -0.36 ± 3.41 kg, p<0.001), and BMI (-0.48 ± 1.03 kg/m² vs. -0.11 ± 1.33 kg/m², p<0.001) compared to the non-Ertu group. Hypoglycemia was reported in 0.3% of the ertugliflozin group and 0.7% of the other group, with comparable adverse events (p=.23; ≥0.05), indicating a favorable safety profile for ertugliflozin during fasting.
Conclusion: This study demonstrates that ertugliflozin is effective and safe for patients with T2DM during Ramadan fasting.
Fasting during the holy month of Ramadan is an essential component of regular spiritual practice for adult Muslims, requiring abstinence from food and drink for 12 to 20 hours a day. For individuals with diabetes mellitus (DM), this significant alteration of meal times may lead to adverse health consequences including hypoglycemia, hyperglycemia, dehydration, etc. (1–5). Many Muslim patients with type 2 diabetes mellitus (T2DM) choose to fast each year, despite medical advice cautioning against fasting (4–6). Ongoing anti-diabetic medication may also increase potential risks for adverse health conditions if careful attention to choose anti-diabetic agents followed by adjustments in dose and timing is not implemented (7).
A number of studies have been conducted focusing on the efficacy and safety of antidiabetic agents (such as metformin, sulphonyleureas, DPP-4 inhibitors, GLP-1 agonists, and SGLT2 inhibitors) (8–11). DPP-4 inhibitors, metformin, and GLP-1 agonists are generally safer than sulphonylureas and insulin during fasting, with a lower risk of hypoglycemia (12–19). However, recent guidelines have prioritized the use of sodium-glucose co-transporter 2 inhibitors (SGLT2i) for managing diabetes during Ramadan fasting considering their benefits including weight management, cardiovascular benefits, renal protection, and flexibility in dosing, though caution is advised regarding potential hypoglycemia when combined with insulin or sulphonylureas (20, 21). SGLT2 inhibitors, including canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin, have demonstrated effective glycemic control in patients with type 2 diabetes mellitus (T2DM), both as monotherapy and as add-on therapy, along with additional benefits such as weight loss and blood pressure reduction (22, 23). Ertugliflozin, the newest member of this class, received global approval in 2017 as part of a comprehensive treatment plan alongside standard diet and exercise (24). With a daily single-dose oral administration schedule, ertugliflozin alone or in combination has shown benefits in reducing cardiorenal risk, including a 10% reduction in hospitalization for heart failure and approximately a 30% reduction in the progression of kidney disease (25–30). Consequently, ertugliflozin provides a convenient dosing option during Ramadan fasting, offering effective glycemic control and additional health benefits, including weight loss, blood pressure reduction, cardiovascular protection, and preservation of renal function (31). However, due to the risk of dehydration, several guidelines recommend caution in the use of SGLT2 inhibitors for individuals at risk of volume depletion or those taking angiotensin-converting enzyme inhibitors (ACE-I) and diuretics (32).
Several clinical trials and studies have assessed the safety and efficacy of SGLT2 inhibitors during the holy month of Ramadan, reporting low risks for hypoglycemia, dehydration, postural hypotension, diabetic ketoacidosis, or genitourinary infections with these drugs (8, 11, 33–38). Trial on empagliflozin found showed a significantly low frequency of hypoglycemia (16%) compared to the standard treatment group (32%) concluding stating this drug with a lower risk of hypoglycemia symptoms and higher tolerability (36). A trial on canagliflozin showed 92% of patients experienced no hypoglycemic events during Ramadan, maintaining a consistent safety profile (38). A study on dapagliflozin found that 3.4% of patients reported symptomatic hypoglycemia compared to 19.2% in the sulphonyleurea group, resulting in a 76% relative risk reduction for hypoglycemia in patients taking dapagliflozin during the 4th week of Ramadan (35).
The effectiveness of ertugliflozin during Ramadan fasting remains underexplored, particularly in real-world settings where patient characteristics, adherence to fasting practices, and dietary habits can vary widely. Current studies have primarily focused on other SGLT2 inhibitors, leaving a gap in understanding how ertugliflozin specifically affects glycemic control and safety outcomes for Muslim patients with T2DM during fasting. With the rising incidence of diabetes, especially in Bangladesh where Muslims are predominant, research on diabetes management during Ramadan is crucial. A similar study conducted in Bangladesh evaluated the efficacy and safety of empagliflozin in patients with T2DM during Ramadan and observed significant improvements in HbA1c and weight reduction, with no serious adverse events reported (34). This study, conducted in a population with similar ethnic and cultural lifestyles, demonstrated that empagliflozin was both effective and safe for use during fasting periods. These findings provide a useful reference point for further studies on ertugliflozin in similar contexts. Thus, the ErtuRamadan study assessed the effectiveness of ertugliflozin among T2DM patients who fast during Ramadan. The results will help healthcare providers create targeted, evidence-based treatment plans and enhance decision-making for Muslim T2DM patients observing fast.
ErtuRamadan study was a real-life experience study conducted over a period of five months (January 2024 to May 2024). Fifteen specialized centers of different divisions of Bangladesh dedicated to the treatment of diabetes mellitus were considered study sites.
Muslim patients aged ≥ 18 years with T2DM for at least 1 year, an HbA1c <10%, who intended to observe Ramadan fasting, and who had been on stable doses of ertugliflozin for at least 3 months before Ramadan (either as monotherapy or in combination with other anti-hyperglycemic agents) were approached for inclusion in this study. Patients with any acute condition, and taking insulin or any other SGLT2 inhibitors within 3 months prior to include in the study, having contraindication for ertugliflozin were excluded from this study.
Participants were enrolled in two groups based on whether they were receiving ertugliflozin as OAD or not. The patients receiving ertugliflozin along with or without other OADs were categorized as the Ertu group. The patients receiving standard care with any other OADs were categorized as non-Ertu group. Patients receiving insulin were excluded from this study.
A total of 1373 patients were included in final analysis where the Ertu group included 703 patients and non-Ertu group included 670 patients (Figure 1). As this study was designed as a real-world observational study, the treatment allocation (Ertugliflozin vs. other OHA) was conducted based on the physician’s clinical judgment. No matching for baseline glycemic status was performed, as the aim was to evaluate the effectiveness of Ertugliflozin in routine clinical practice.
Throughout the month of Ramadan, all patients in the Ertu group continued their ertugliflozin treatment, while those in the non-Ertu group were not initiated ertugliflozin or any other SGLT2 inhibitors. All participants were required to attend a screening and enrollment visit three months prior to Ramadan, as well as baseline and follow-up visits before within 1 month, during, and after 1 month of Ramadan for clinical assessment and data collection. Informed written consent was obtained from each patient before enrollment.
The primary end point of the study was the change from baseline (pre-Ramadan assessment) in HbA1c level. Secondary end points included the proportion of patients achieving the desired glycemic control (HbA1c<7.0%), changes from baseline in fasting plasma glucose, post-prandial plasma glucose, systolic and diastolic blood pressure, eGFR, serum creatinine, serum sodium (Na) and potassium (K) and incidence of other adverse events (AEs) related to ertugliflozin (urinary tract infections, genital mycotic infection, and osmotic diuresis–related AEs) or incidents of other illness not related to ertugliflozin during Ramadan.
Data were collected using a semi-structured case record form, which included relevant information regarding sociodemographic profiles, anthropometric measurements, and clinical information from the enrolled patients. Each participant underwent a comprehensive history taking, physical examination, and necessary laboratory investigations.
During the study, patients maintained a logbook for 30 days to record their blood glucose levels, instances of hypoglycemia (symptomatic or reported), adverse events (symptomatic or reported), number of fasting days, and any changes in medication. They were instructed to utilize their logbooks to report any hypoglycemic events immediately. In cases of reported hypoglycemia, adjustments to medications were made as necessary based on regular assessments conducted from the logbook entries.
After Ramadan, patients were evaluated for changes in body weight and relevant clinical investigations. The logbooks and adverse event forms were collected during this final post-Ramadan visit. Ongoing communication via telephone was also established to monitor patients’ conditions effectively.
Measurements taken included body weight, height, blood pressure, HbA1c, fasting and post-prandial glucose levels, creatinine, sodium (Na), potassium (K), alanine aminotransferase (ALT), and estimated glomerular filtration rate (eGFR), both before and after Ramadan. All data were documented in separate case record forms (CRF).
Body mass index (BMI): BMI was calculated by dividing the participant’s weight in kilograms by the square of their height in meters, applying the formula kg/m². According to the Asia-Pacific classification defined by the Western Pacific Regional Office of the WHO, BMI categories were as follows: underweight (<18.50 kg/m²), normal (18.50–22.99 kg/m²), overweight (23.00–24.99 kg/m²), and obese (≥25 kg/m²) (39).
Hypoglycemia: Hypoglycemia was defined as plasma glucose levels below 70 mg/dL (<3.9 mmol/L). Severe hypoglycemia was described as episodes involving altered mental or physical functioning necessitating external assistance for recovery. Hypoglycemic episodes were categorized into: symptomatic (with signs such as dizziness, blurred vision, palpitations, nausea, sweating, confusion, tremors, or intense hunger, regardless of biochemical confirmation), biochemically confirmed (self-monitored blood glucose <3.9 mmol/L, with or without symptoms), and severe episodes (requiring external help, or associated with seizures or loss of consciousness) (40).
Volume depletion: This condition was defined as a significant reduction in extracellular fluid volume due to sustained salt and fluid loss exceeding intake. Clinical manifestations included symptoms such as hypotension, orthostatic hypotension, postural dizziness, dehydration, syncope, or presyncope (41).
Urinary tract infection (UTI): A UTI was identified as an infection affecting any component of the urinary system, including the kidneys, ureters, bladder, or urethra, with most cases involving the lower urinary tract (42).
Hypertension: Hypertension is defined as a systolic blood pressure (SBP) of ≥140 mmHg and/or a diastolic blood pressure (DBP) of ≥90 mmHg, measured on at least two separate occasions, or current use of antihypertensive medication by registered physician (43, 44).
Dyslipidemia: Dyslipidemia is defined as abnormal levels of lipids in the blood, typically characterized by elevated total cholesterol (≥200 mg/dL), LDL cholesterol (≥140 mg/dL), triglycerides (≥150 mg/dL), or low HDL cholesterol (<40 mg/dL) (45).
Chronic kidney disease (CKD): CKD is defined as a gradual loss of kidney function over time. It is diagnosed when there is evidence of kidney damage or an estimated glomerular filtration rate (eGFR) below 60 mL/min/1.73 m² for at least three months (46).
Hyponatremia: A serum sodium level of less than 135 milliequivalents per liter (mEq/L) was defined as hyponatremia (47).
Hypokalemia: A serum potassium level of less than 3.5 milliequivalents per liter (mEq/L) was defined as hypokalemia (48).
estimated Glomerular Filtration Rate (eGFR): eGFR is determined using the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation, which incorporates serum creatinine, age and gender (49).
The formula used is:
● Scr: Serum creatinine (measured in mg/dL).
● κ: 0.7 for females and 0.9 for males.
● α: -0.329 for females and -0.411 for males.
● Age: The patient’s age in years.
Data analysis was conducted with the statistical software SPSS version 25.0. Descriptive statistics was used to describe the baseline characteristics of the study patients. Continuous variables were presented by mean and standard deviation and the categorical variables were presented by frequency and percentages. Association between categorical variables were determined using Chi-square test and Fishers’ exact test according to the applicability. The difference between continuous variables of two groups was determined by an independent student t-test. The difference of continuous variables between pre-Ramadan and post-Ramadan assessment were determined using paired t-test. Statistical significance was considered with a p value of less than 05.
The study protocol was reviewed and approved by the institutional review board (IRB) of Bangabandhu Sheikh Mujib Medical University (BSMMU/2023/4977).
A total of 1373 patients were included in ErtuRamadan study, 703 patients were receiving ertugliflozin (Ertu group) and 670 were receiving any other anti-hyperglycemic agents than ertugliflozin (non-Ertu group). Demographic and biochemical characteristics of patients at baseline were mostly similar between the two groups except for duration of DM and body weight.
The average age of the participants was 50 years, with 40.6% male and 58.7% female. Notably, the duration of diabetes was significantly longer in the ertugliflozin group compared to the non-Ertu (p <0.05). In terms of body weight, patients in the ertugliflozin group had a higher average compared to those in the non-Ertu group (p <0.05). Regarding comorbidities, both groups exhibited high rates of hypertension and dyslipidemia, but the ertugliflozin group had a significantly higher prevalence of fatty liver disease and coronary artery disease (p <0.05) (Table 1A).
Glycemic control was poorer in the Ertu group, with an average HbA1c of 8.16% compared to 7.79% in the non-Ertu group (p <0.05). The estimated glomerular filtration rate (eGFR) and serum creatinine level was comparable across both groups. Serum ALT was also similar in both groups. Regarding serum electrolytes, serum Na was similar in both groups but serum K was lower in patients of non-Ertu group (p<.05) (Table 1B).
A significantly greater proportion of patients in the non-Ertu group were on sulphonyleureas (62.8% vs. 43.3%) and metformin (74% vs. 52.3%) compared to the Ertu group (p < 0.001). However, the use of DPP4 inhibitors (32.6% vs. 32.4%) and thiazolidinediones (32.6% vs. 32.4%) was similar across both groups (Figure 2).
Both the ertugliflozin and non-ertugliflozin groups showed significant improvements in glycemic control after Ramadan (p<0.001). In the ertugliflozin group, the proportion of patients achieving good glycemic control (HbA1c<7%) increased from 9.8% to 25.1%, while in the non-ertugliflozin group, it rose from 17.8% to 25.7% (Figure 3).
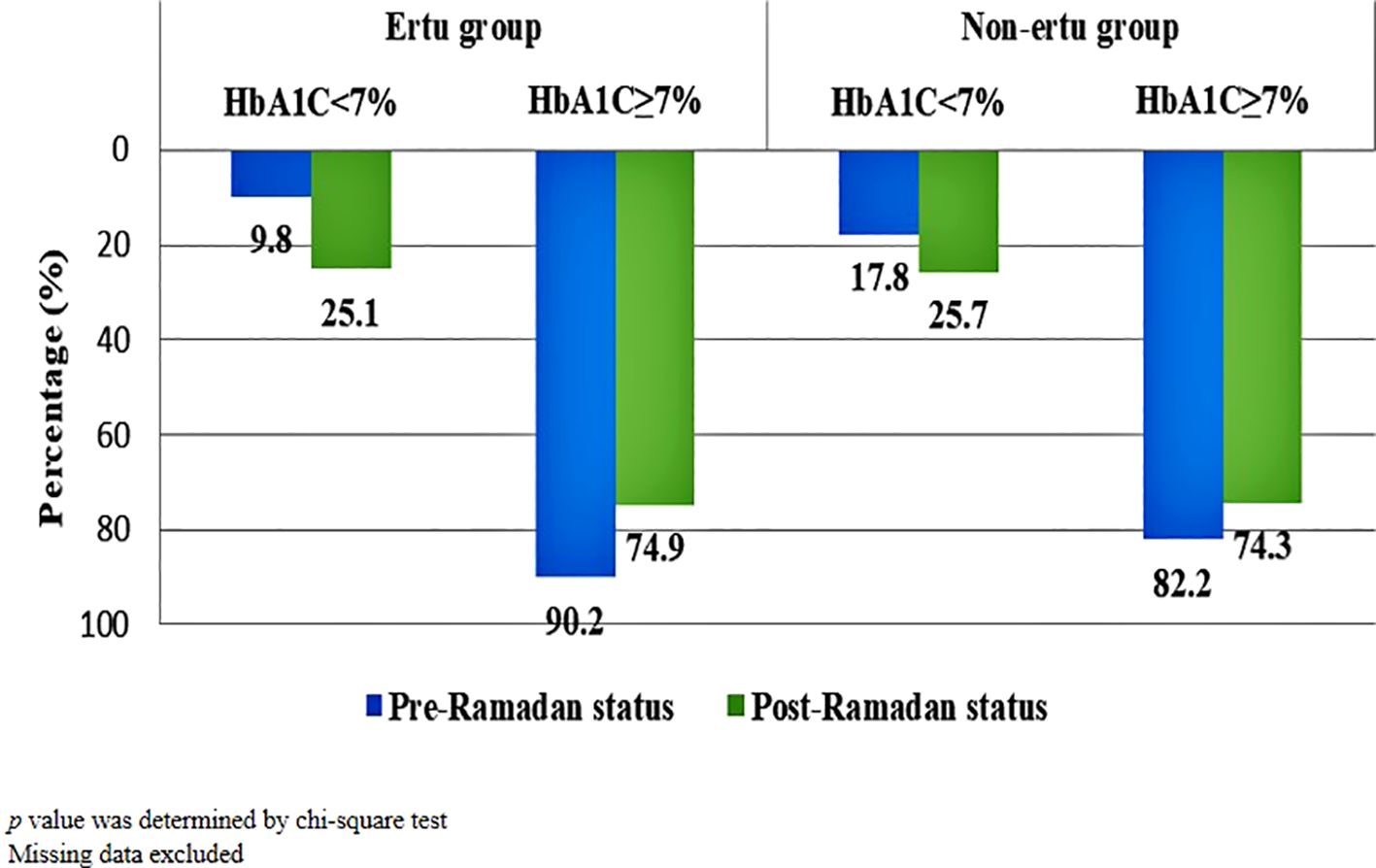
Figure 3. Pre-Ramadan and post-Ramadan glycemic control (HbA1C) status of study participants (n=1373).
The ertugliflozin group demonstrated a more pronounced improvement in glycemic control compared to the non-ertugliflozin group (Figure 4).
HbA1c was significantly reduced from pre-Ramadan to post-Ramadan in both groups. Similarly, fasting plasma glucose (FPG) levels and postprandial plasma glucose (PPG) were significantly reduced in both groups. In terms of serum electrolytes, serum Na levels significantly decreased in both groups irrespective of diuretics or ACE inhibitors medication (p < 0.001). SerumK levels also showed slight significant reduction in the Ertu group, while the non-Ertu group saw an increase with statistical significance (Table 2).

Table 2. Pre- and post-Ramadan glycemic parameters and serum electrolytes in study participants (n=1373).
In the ertugliflozin group, patients experienced significant reductions in both body weight and BMI compared to the non-Ertu group (p < 0.05). Glycemic control was also notably better in the ertugliflozin group, with significant improvements observed in HbA1c, fasting plasma glucose, and post-prandial plasma glucose levels (p < 0.05 for all comparisons). Both systolic and diastolic blood pressure reductions were more pronounced in the ertugliflozin group than in the non-Ertu group (p < 0.05). Additionally, serum sodium and potassium levels showed significant decreases in the ertugliflozin group, while these levels increased in the non-Ertu group (p < 0.05). However, no significant differences were observed in the changes in estimated glomerular filtration rate (eGFR) between the two groups (Table 3).
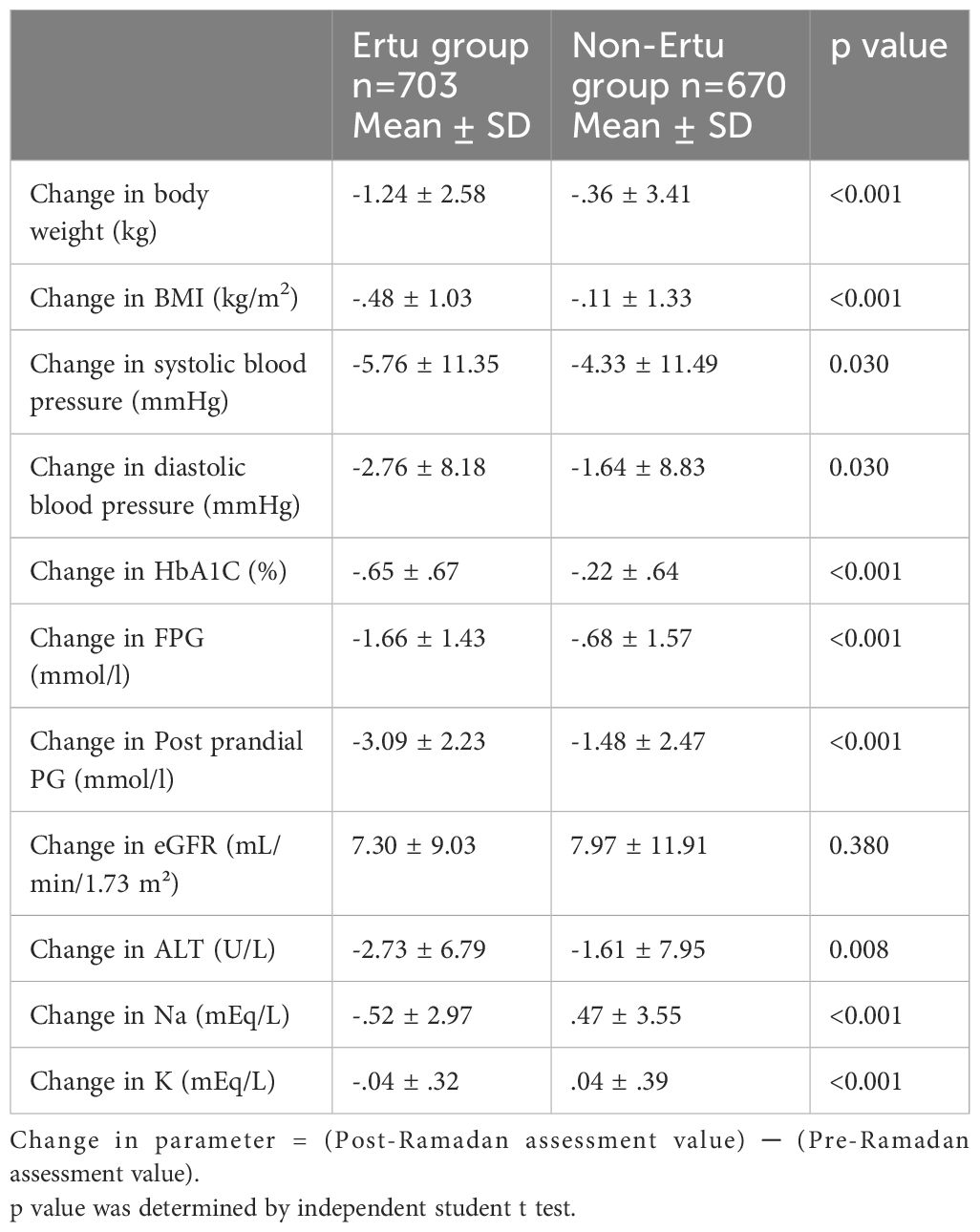
Table 3. Change in blood glucose parameter, body weight, BMI, blood pressure, eGFR, and other biochemical values from baseline at post-Ramadan visit among study participants (n=1373).
In the ertugliflozin group, significant reductions in BMI were observed in the overweight and obese categories, with a notable decrease post-Ramadan. The normal-weight and underweight categories did not show significant changes. In contrast, the non-ertugliflozin group showed minimal changes in BMI across all categories, except for obese category (Table 4).
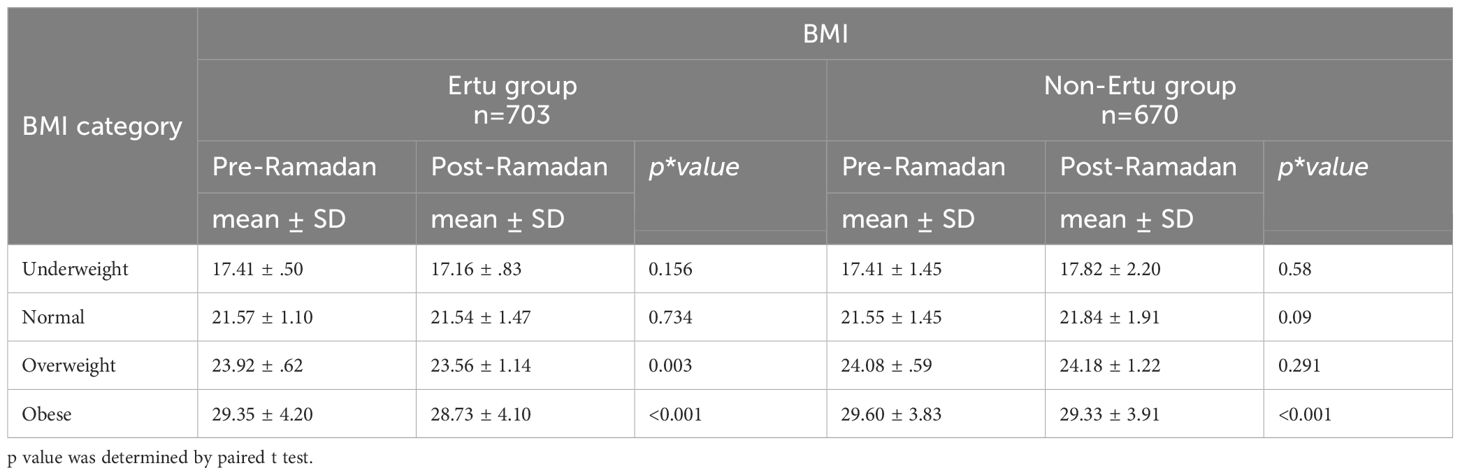
Table 4. Pre- and post-Ramadan BMI of the study participants across different BMI categories (n=1373).
In both groups, both patients who received and didn’t receive sulphonylureas showed statistically significant changes in BMI after Ramadan (p<001). But patients not using sulphonylureas had a more pronounced decrease in BMI post-Ramadan compared to those using sulphonylureas, with the Ertu group showing more consistent reductions than the non-Ertu group. p value was determined by paired t test (Figure 5).
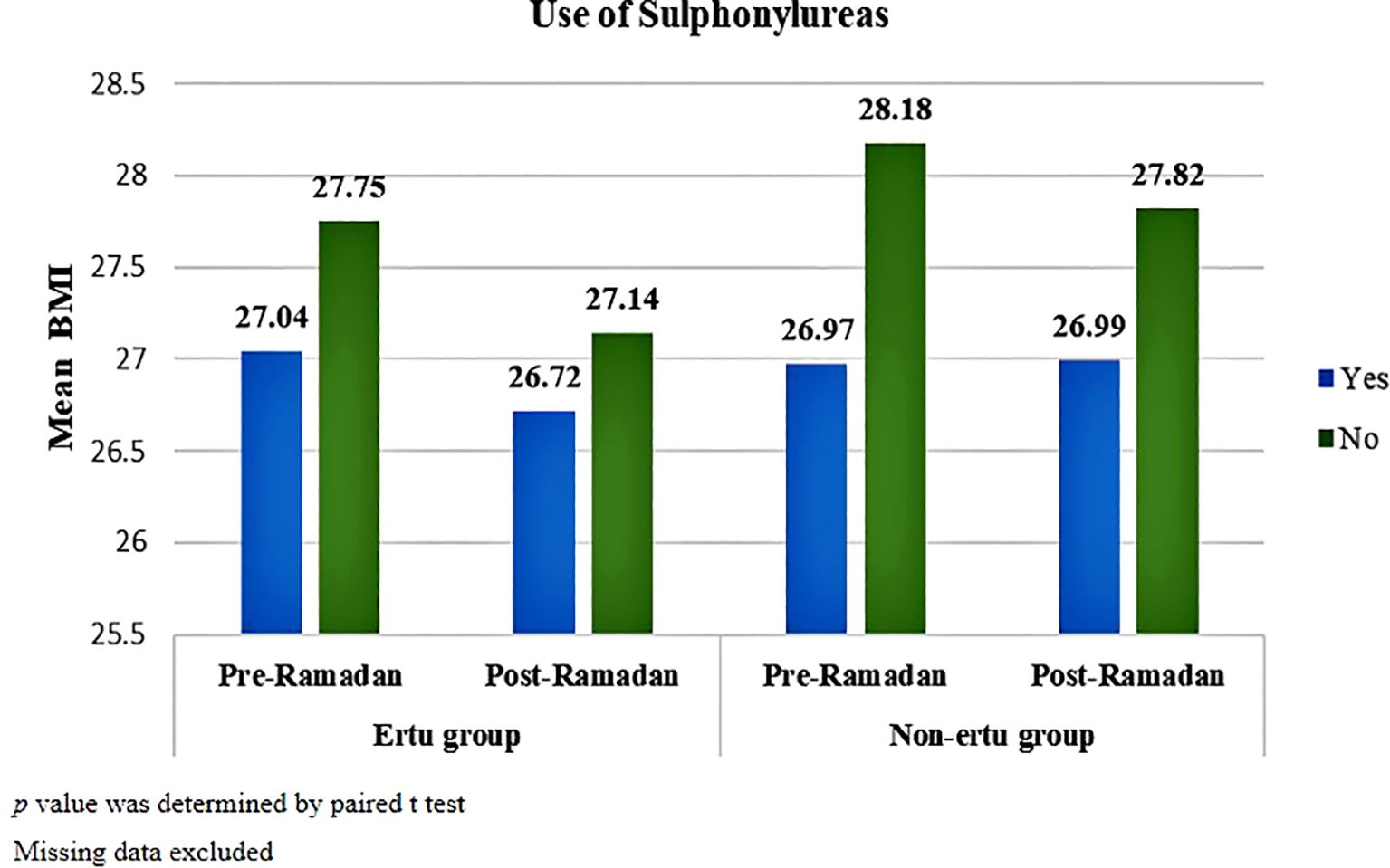
Figure 5. Pre-and post-Ramadan BMI in study participants stratified by use of sulphonylureas (n=1373).
Significant reductions in HbA1c were observed in most BMI categories for both groups. In the Ertu group, normal-weight, overweight, and obese patients had significant decrease of BMI while underweight patients showed a non-significant reduction. In the non-Ertu group, all BMI categories, including underweight patients, experienced significant HbA1c reductions post-Ramadan. Overall, the Ertu group showed more pronounced decreases in HbA1c across most categories (Table 5).
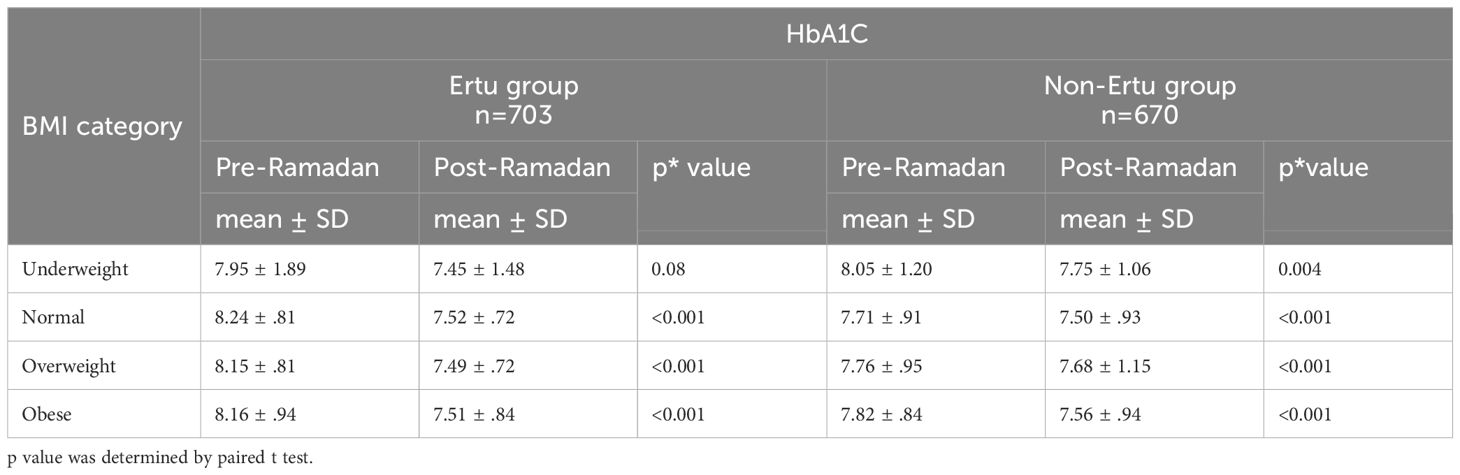
Table 5. Pre- and post-Ramadan HbA1Camong the study participants across different BMI categories (n=1373).
Hypoglycemia was reported in 0.3% patients of Ertu group and 0.7% patients of non-Ertu group. Incidents of urinary tract infections, abdominal pain, and dehydration were low and not statistically different between groups. Overall, adverse events were comparable (p≥0.05) (Table 6).
Ertugliflozin, an SGLT2 inhibitor, has demonstrated considerable efficacy in the management of type 2 diabetes mellitus (T2DM) in previous trials (25–30). Its’ insulin-independent mechanism leads to significant glycosuria and osmotic diuresis, resulting in improved glycemic control (50). Additionally, ertugliflozin contributes to the reduction of cardio-renal risks in patients with T2DM (51). However, concerns about the safety and efficacy of SGLT2 inhibitors during prolonged Ramadan fasting prompted this clinical trial to assess the effectiveness of ertugliflozin in T2DM patients observing Ramadan in Bangladesh (32). This study observed effective glycemic control and comparable safety in patients receiving ertugliflozin (Ertu group) as monotherapy or add-on with other OAD.
At baseline, patients of both arms exhibited similar demographic characteristics. The mean age of participants was 50 years which aligns with previous national studies (52–54). A higher proportion of female participants were observed with a male: female ratio of 1:1.44 which aligns with previous research on T2DM patients in Bangladesh (34, 52). However, it contrasts with other studies in the country where male participants generally outnumber females (55, 56), potentially due to cultural and social factors. Most participants were urban residents. The average BMI was 26 which lies upper than normal value. Moreover, the majority of the patients were presented with a considerable burden of comorbidities while hypertension and dyslipidemia were the most common. These findings are consistent with existing literature indicating that individuals with T2DM often present with multiple health challenges that complicate their management during periods of fasting (57–60).
In baseline or pre-Ramadan assessment, only 9.8% of patients in the Ertu group and 17.9% patients in non-Ertu group were presented with a controlled glycemic status (<7%) with an overall mean of 7.98 ± .91 (SD)%. Previous studies conducted in Bangladesh reported nearly similar mean HbA1C (34, 61). The mean HbA1C was 8.16 ± .91 (SD)% and 7.79 ± .87 (SD)% in Ertu group and non-Ertu group accordingly where HbA1C was statistically higher in Ertu group. The higher baseline HbA1c and lower number of patients with glycemic control observed in the Ertu group reflects a poorer initial glycemic control, likely due to longer diabetes duration and associated comorbidities. The post-Ramadan assessment demonstrated that patients of both arms experienced a significant reduction in HbA1C levels. Patients with good glycemic control were statistically increased in post-Ramadan assessment to 25.1% in the Ertu group and 25.7% in non-Ertu group. These findings coordinate with previous studies where Ramadan fasting and ongoing management leads to a statistically significant reduction in blood glucose levels of T2DM patients (62–64). The change in HbA1C from pre-Ramadan assessment to post-Ramadan assessment is significantly higher in the ertugliflozin arm compared to control arm (-.65 ± .67% Vs -.22 ± .64%). This finding underscores ertugliflozin’s effectiveness in glycemic control during fasting. Previous study conducted in Bangladesh focusing on the role of empagliflozin, another SGLT2 inhibitor in T2DM patients observing Ramadan fasting was reported similar findings (34). Other SGLT2 inhibitors including canagliflozin, dapagliflozin and empagliflozin also showed significant improvement in glycemic status during Ramadan fasting (11, 35, 36, 65). An overall reduction in body weight and BMI was observed which was significantly pronounced in the ertugliflozin group, highlighting its role in weight management for overweight individuals with T2DM. Similar findings were reported by canagliflozin and empagliflozin (34, 65). But another study reported weight and BMI reduction in Ramadan fasting regardless of whether patients were on SGLT2 inhibitors or not (37).
However, electrolyte changes were also observed, particularly in the ertugliflozin group. Serum sodium and potassium levels decreased significantly post-Ramadan in the ertugliflozin group, with sodium reducing from 138.56 to 138.08 mEq/L and potassium from 3.93 to 3.88 mEq/L. In contrast, the non-ertugliflozin group showed a slight increase in potassium levels while sodium levels also decreased but to a lesser extent. These findings highlight the diuretic effect of ertugliflozin, which may contribute to mild electrolyte imbalances, emphasizing the need for careful monitoring of electrolytes during fasting periods to prevent dehydration and related complications.
In the present study, comparable rates of hypoglycemic episodes were observed in patients taking ertugliflozin and those on other anti-hyperglycemic agents (0.3% vs. 0.7%). These findings align with previous research indicating that SGLT2 inhibitors are generally associated with fewer hypoglycemic events compared to traditional therapies (11, 35, 36, 65). The few hypoglycemic events were also mild symptomatic in nature. Patients were instructed to record events immediately, enabling healthcare providers to monitor and respond effectively. No severe hypoglycemic events requiring extensive medical intervention were reported. All cases were successfully managed through standard home-based interventions and no dose or medication adjustment or alteration was required. Furthermore, the study revealed no statistically significant differences in the rates of dehydration between the groups, indicating that ertugliflozin can be safely administered during fasting periods. The risk of volume depletion is particularly pertinent during Ramadan fasting, as prolonged periods without food or water may lead to dehydration, postural hypotension, and dizziness. However, our study did not observe significant adverse effects related to volume depletion in the ertugliflozin group.
The study was not beyond limitations. The first limitation was that this study excluded patients receiving insulin. A large number of patients use insulin along with or without SGLT2 inhibitors during Ramadan fasting, highlighting the need for further study on those receiving insulin. Another limitation was that we could not consider the dose and duration of the other OADs and stratify accordingly. Another limitation was that there was a possibility of recall bias regarding self-reported symptoms.
These findings of this study support the use of ertugliflozin as an effective and safe option for managing T2DM in patients observing Ramadan fasting. By demonstrating significant improvements in glycemic control and a favorable safety profile, ertugliflozin offers a treatment option for Muslim individuals with T2DM during Ramadan. Further research is warranted to confirm these findings and explore the long-term safety and efficacy of SGLT2 inhibitors during prolonged fasting periods.
In individuals with T2DM fasting during Ramadan, the use of Ertugliflozin is safe, effective, and well-tolerated. Patients experienced significant improvements in glycemic control, with a notable reduction in HbA1c levels. Additionally, reductions in body weight and BMI were also more pronounced in the ertugliflozin group compared to non-ertugliflozin users. However, a thorough pre-Ramadan assessment and education, including hydration advice, are essential followed by careful monitoring is essential. Further structured clinical trials should be conducted to support this finding.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The study was approved by the Institutional Review Board of Bangabandhu Sheikh Mujib Medical University (BSMMU/2023/4977). The studies were conducted in accordance with the local legislation and institutional requirements. Informed written consent was obtained from all the participants involved in the study.
MP: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing, Resources, Software, Validation. NA: Conceptualization, Formal analysis, Methodology, Resources, Supervision, Writing – review & editing, Data curation, Software, Validation, Visualization. MM: Conceptualization, Formal analysis, Methodology, Software, Supervision, Validation, Visualization, Investigation, Project administration, Writing – review & editing. MSa: Conceptualization, Investigation, Methodology, Project administration, Software, Data curation, Funding acquisition, Resources, Writing – review & editing. MSh: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Formal analysis, Writing – review & editing. MMR: Conceptualization, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. MR: Conceptualization, Project administration, Software, Supervision, Visualization, Data curation, Formal analysis, Writing – review & editing. SM: Conceptualization, Project administration, Software, Supervision, Visualization, Methodology, Validation, Writing – review & editing. AK: Conceptualization, Methodology, Project administration, Validation, Data curation, Formal analysis, Funding acquisition, Writing – review & editing. MH: Conceptualization, Formal analysis, Project administration, Validation, Investigation, Software, Supervision, Writing – review & editing. MA: Conceptualization, Formal analysis, Project administration, Supervision, Validation, Data curation, Funding acquisition, Visualization, Writing – review & editing. SM: Conceptualization, Data curation, Formal analysis, Project administration, Validation, Visualization, Investigation, Methodology, Software, Writing – review & editing. FA: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Supervision, Visualization, Writing – review & editing. MB: Conceptualization, Data curation, Funding acquisition, Investigation, Project administration, Resources, Supervision, Validation, Writing – review & editing. TH: Conceptualization, Funding acquisition, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – review & editing. AA: Conceptualization, Data curation, Funding acquisition, Methodology, Resources, Software, Validation, Visualization, Writing – review & editing. ST: Conceptualization, Data curation, Funding acquisition, Investigation, Resources, Software, Supervision, Visualization, Writing – review & editing. SRS: Conceptualization, Formal analysis, Funding acquisition, Investigation, Project administration, Resources, Visualization, Data curation, Methodology, Software, Supervision, Validation, Writing – review & editing. SJS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Funding acquisition, Resources, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors would like to express their sincere gratitude to Pi Research & Development Center, Dhaka, Bangladesh (www.pirdc.org), for their help in manuscript revision and editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ahmedani MY, Haque MS, Basit A, Fawwad A, Alvi SF. Ramadan Prospective Diabetes Study: the role of drug dosage and timing alteration, active glucose monitoring and patient education. Diabetes Med. (2012) 29:709–15. doi: 10.1111/j.1464-5491.2011.03563.x
2. Hassanein M, Bravis V, Hui E, Devendra D. Ramadan-focused education and awareness in type 2 diabetes. Diabetologia. (2009) 52:367–8. doi: 10.1007/s00125-008-1220-8
3. Patel NR, Kennedy A, Blickem C, Rogers A, Reeves D, Chew-Graham C. Having diabetes and having to fast: a qualitative study of british Muslims with Diabetes. Heal Expect. (2015) 18:1698–708. doi: 10.1111/hex.2015.18.issue-5
4. Jabbar A, Hassanein M, Beshyah SA, Boye KS, Yu M, Babineaux SM. CREED study: Hypoglycaemia during Ramadan in individuals with Type 2 diabetes mellitus from three continents. Diabetes Res Clin Pract. (2017) 132:19–26. doi: 10.1016/j.diabres.2017.07.014
5. Salti I, Bénard E, Detournay B, Bianchi-Biscay M, Le Brigand C, Voinet C, et al. A population-based study of diabetes and its characteristics during the fasting month of Ramadan in 13 countries: results of the epidemiology of diabetes and Ramadan 1422/2001 (EPIDIAR) study. Diabetes Care. (2004) 27:2306–11. doi: 10.2337/diacare.27.10.2306
6. Gad H, Al-Muhannadi H, Purra H, Mussleman P, Malik RA. The effect of Ramadan focused education on patients with type 2 diabetes: A systematic review and meta-analysis. Diabetes Res Clin Pract. (2020) 162:108122. doi: 10.1016/j.diabres.2020.108122
7. Hassanein M, Afandi B, Ahmedani MY, Alamoudi RM, Alawadi F, Bajaj HS, et al. Diabetes and ramadan: Practical guidelines 2021. Diabetes Res Clin Pract. (2022) 185:109185. doi: 10.1016/j.diabres.2021.109185
8. Ahmed I, Raja UY, Wahab MU, Rehman T, Ishtiaq O, Aamir AH, et al. Efficacy and safety of combination of empagliflozin and metformin with combination of sitagliptin and metformin during Ramadan: an observational study. BMC Endocr Disord. (2022) 22:1–7. doi: 10.1186/s12902-022-01168-3
9. Aziz KM. Fasting during Ramadan: Efficacy, safety, and patient acceptability of vildagliptin in diabetic patients. Diabetes Metab Syndr Obes. (2015) 8:207–11. doi: 10.2147/DMSO.S54683
10. Brady EM, Davies MJ, Gray LJ, Saeed MA, Smith D, Hanif W, et al. metformin in patients with established type 2 diabetes during Ramadan: the Treat 4 Ramadan Trial original article. Diabetes Obes Metab. (2014) 527–36. doi: 10.1111/dom.12249
11. Bashier A, Khalifa AA, Abdelgadir EI, Al Saeed MA, Al Qaysi AA, Ali Bayati MB, et al. Safety of sodium-glucose cotransporter 2 inhibitors (SGLT2-I) during the month of ramadan in muslim patients with type 2 diabetes. Oman Med J. (2018) 33:104–10. doi: 10.5001/omj.2018.21
12. Loh HH, Yee A, Loh HS, Sukor N, Kamaruddin NA. Comparative studies of dipeptidyl peptidase 4 inhibitor vs sulphonylurea among Muslim Type 2 diabetes patients who fast in the month of Ramadan: A systematic review and meta-analysis. Prim Care Diabetes. (2016) 10:210–9. doi: 10.1016/j.pcd.2015.09.001
13. Gray LJ, Dales J, Brady EM, Khunti K, Hanif W DM. Safety and effectiveness of non-insulin glucose-lowering agents in the treatment of people with type 2 diabetes who observe Ramadan: a systematic review and meta-analysis. Diabetes Obes Metab. (2015) 17:639–48. doi: 10.1111/dom.2015.17.issue-7
14. Aravind SR, Tayeb KA, Ismail SB, Shehadeh N, Kaddaha G, Liu R, et al. Ramadan Study Group Hypoglycaemia in sulphonylurea-treated subjects with type 2 diabetes undergoing Ramadan fasting: a five-country observational study. Curr Med Res Opin. (2011) 27:1237–42. doi: 10.1185/03007995.2011.578245
15. Ibrahim M, Davies MJ, Ahmad E, Annabi FA, Eckel RH, Ba-Essa EM, et al. Recommendations for management of diabetes during Ramadan: update 2020, applying the principles of the ADA/EASD consensus. BMJ Open Diabetes Res Care. (2020) 8:1–14. doi: 10.1136/bmjdrc-2020-001248
16. Brady EM, Davies MJ, Gray LJ, Saeed MA, Smith D, Hanif W, et al. A randomized controlled trial comparing the GLP-1 receptor agonist liraglutide to a sulphonylurea as add on to metformin in patients with established type 2 diabetes during Ramadan: the Treat 4 Ramadan Trial. Diabetes Obes Metab. (2014) 16:527–36. doi: 10.1111/dom.12249
17. Azar ST, Echtay A, Wan Bebakar WM, Al Araj S, Berrah A, Omar M, et al. Efficacy and safety of liraglutide compared to sulphonylurea during Ramadan in patients with type 2 diabetes (LIRA-Ramadan): a randomized trial. Diabetes Obes Metab. (2016) 18:1025–33. doi: 10.1111/dom.2016.18.issue-10
18. Khalifa AA, El Rashid AO, Bashier AMK. Safety and efficacy of liraglutide as an add-on therapy to pre-existing anti-diabetic regimens during Ramadan, a prospective observational trial. J Diabetes Metab Disord. (2015) 6:590. doi: 10.4172/2155-6156.1000590
19. Pan C, Yang W, Barona JP, Wang Y, Niggli M, Mohideen P, et al. Comparison of vildagliptin and acarbose monotherapy in patients with Type 2 diabetes: a 24-week, double-blind, randomized trial. Diabetes Med. (2008) 25:435–41. doi: 10.1111/j.1464-5491.2008.02391.x
20. American Diabetes Association Professional Practice Committee. Summary of revisions: standards of care in diabetes-2024. Diabetes Care. (2024) 47:S5–10. doi: 10.2337/dc24-SREV
21. Qaseem A, Obley AJ, Shamliyan T, Hicks LA, Harrod CS, Crandall CJ, et al. Newer pharmacologic treatments in adults with type 2 diabetes: A clinical guideline from the American college of physicians. Ann Intern Med. (2024) 177:658–66. doi: 10.7326/M23-2788
22. Solini A. Role of SGLT2 inhibitors in the treatment of type 2 diabetes mellitus. Acta Diabetol. (2016) 16:1–8. doi: 10.1007/s00592-016-0856-y
23. Marx N, McGuire DK. Sodium-glucose cotransporter-2 inhibition for the reduction of cardiovascular events in high-risk patients with diabetes mellitus. Eur Hear J. (2016) 37:3192–200. doi: 10.1093/eurheartj/ehw110
24. Markham A. Ertugliflozin: first global approval. Drugs. (2018) 78:513–9. doi: 10.1007/s40265-018-0878-6
25. Terra SG, Focht K, Davies M, Frias J, Derosa G, Darekar A, et al. efficacy and safety study of ertugliflozin monotherapy in people with type 2 diabetes mellitus inadequately controlled with diet and exercise alone. Diabetes Obes Metab. (2017) 19:721–8. doi: 10.1111/dom.2017.19.issue-5
26. Aronson R, Frias J, Goldman A, Darekar A, Lauring B, Terra SG. Longterm efficacy and safety of ertugliflozin monotherapy in patients with inadequately controlled T2DM despite diet and exercise: VERTIS MONO extension study. Diabetes Obes Metab. (2018) 20:1452–60. doi: 10.1111/dom.2018.20.issue-6
27. Miller S, Krumins T, Zhou H, Huyck S, Johnson J, Golm G, et al. Ertugliflozin and sitagliptin co-initiation in patients with type 2 diabetes: the VERTIS SITA randomized study. Diabetes Ther. (2018) 9:253–68. doi: 10.1007/s13300-017-0358-0
28. Rosenstock J, Frias J, Páll D, Charbonnel B, Pascu R, Saur D, et al. Effect of ertugliflozin on glucose control, body weight, blood pressure and bone density in type 2 diabetes mellitus inadequately controlled on metformin monotherapy (VERTIS MET). Diabetes Obes Metab. (2018) 20:520–9. doi: 10.1111/dom.2018.20.issue-3
29. Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. (2020) 383:1425–35. doi: 10.1056/NEJMoa2004967
30. Pratley RE, Cannon CP, Cherney DZI, Cosentino F, McGuire DK, Essex MN, et al. Cardiorenal outcomes, kidney function, and other safety outcomes with ertugliflozin in older adults with type 2 diabetes (VERTIS CV): secondary analyses from a randomised, double-blind trial. Lancet Heal Longev. (2023) 4:e143–54. doi: 10.1016/S2666-7568(23)00032-6
31. Tootee A, Larijan B. Ramadan fasting and diabetes, latest evidence and technological advancements: 2021 update. J Diabetes Metab Disord. (2021) 20:1085–91. doi: 10.1007/s40200-021-00806-2
32. Bajaj HS, Abouhassan T, Ahsan MR, Arnaout A, Hassanein M HR. Diabetes Canada position statement for people with types 1 and 2 diabetes who fast during Ramadan. Can J Diabetes. (2019) 43:3–12. doi: 10.1016/j.jcjd.2018.04.007
33. Sheikh A, Das B, Sattar S, Islam N. Safety of sodium-glucose cotransporter 2 inhibitors (SGLT2i) during the month of Ramadan in patients with type 2 diabetes mellitus in Pakistani population—an observational study from a tertiary care center in Karachi. Endocrine. (2023) 80:64–70. doi: 10.1007/s12020-022-03290-7
34. Pathan MF, Akter N, Selim S, Saifuddin M, Qureshi NK, Kamrul-Hasan AB, et al. Efficacy and safety of empagliflozin in patients with type 2 diabetes mellitus fasting during Ramadan: A real-world study from Bangladesh. Diabetes Metab Syndr Obes. (2022) 15:4011–21. doi: 10.2147/DMSO.S380544
35. Wan Seman WJ, Kori N, Rajoo S, Othman H, Mohd Noor N, Wahab NA, et al. Switching from sulphonylurea to a sodium-glucose cotransporter2 inhibitor in the fasting month of Ramadan is associated with a reduction in hypoglycaemia. Diabetes Obes Metab. (2016) 18:628–32. doi: 10.1111/dom.2016.18.issue-6
36. Samkari MM, Neda’a SB, Alhajaji R, Ahmed ME, Al Raddadi A, Bahget AK, et al. Safety and tolerability of empagliflozin use during the holy month of Ramadan by fasting patients with type 2 diabetes: a prospective cohort study. Saudi Pharm J. (2023) 31:972–8. doi: 10.1016/j.jsps.2023.04.022
37. Shao Y, Lim GJ, Chua CL, Wong YF, Yeoh EC, Low SK, et al. The effect of Ramadan fasting and continuing sodium-glucose co-transporter-2 (SGLT2) inhibitor use on ketonemia, blood pressure and renal function in Muslim patients with type 2 diabetes. Diabetes Res Clin Pr. (2018) 142:85–91. doi: 10.1016/j.diabres.2018.05.022
38. Hassanein M, Echtay A, Hassoun A, Alarouj M, Afandi B, Poladian R, et al. Tolerability of canagliflozin in patients with type 2 diabetes mellitus fasting during Ramadan: Results of the Canagliflozin in Ramadan Tolerance Observational Study (CRATOS). Int J Clin Pract. (2017) 71:1–9. doi: 10.1111/ijcp.2017.71.issue-10
39. World Health Organization. Regional Office for the Western Pacific. The Asia-Pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia (2000). Available at: https://iris.who.int/handle/10665/206936 (Accessed December 10, 2024).
40. Seaquist ER, Anderson J, Childs B, Cryer P, Dagogo-Jack S, Fish L, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. (2013) 36:1314–95. doi: 10.2337/dc12-2480
41. Asim M, Alkadi MM, Asim H, Ghaffar A. Dehydration and volume depletion: How to handle the misconceptions. World J Nephrol. (2019) 8:23–32. doi: 10.5527/wjn.v8.i1.23
42. Bilsen MP, Jongeneel RMH, Schneeberger C, Platteel TN, van Nieuwkoop C, Mody L, et al. Definitions of urinary tract infection in current research: A systematic review. Open Forum Infect Dis. (2023) 1:10. doi: 10.1093/ofid/ofad332
43. Directorate General of Health Services, National Heart Foundation Hospital and Research Institute, World Health Organization. National guidelines for management of hypertension in Bangladesh (2013). Available online at: https://t.ly/Jgngl (Accessed December 10, 2024).
44. Rahman MA, Halder HR, Yadav UN, Mistry SK. Prevalence of and factors associated with hypertension according to JNC 7 and ACC/AHA 2017 guidelines in Bangladesh. Sci Rep. (2021) 11:1–10. doi: 10.1038/s41598-021-94947-2
45. Ali N, Samadder M, Kathak RR, Islam F. Prevalence and factors associated with dyslipidemia in Bangladeshi adults. PloS One. (2023) 18:1–13. doi: 10.1371/journal.pone.0280672
46. Banik S, Ghosh A. Prevalence of chronic kidney disease in Bangladesh: a systematic review and meta-analysis. Int Urol Nephrol. (2021) 53:713–8. doi: 10.1007/s11255-020-02597-6
47. Monnerat S, Atila C, Refardt J, Christ-Crain M. Prevalence of admission hyponatremia in patients with diabetes treated with and without an SGLT2 inhibitor. J Endocr Soc. (2023) 9:7. doi: 10.1210/jendso/bvad011
48. Coregliano-Ring L, Goia-Nishide K, Rangel ÉB. Hypokalemia in diabetes mellitus setting. Medicina (B Aires). (2022) 58:431. doi: 10.3390/medicina58030431
49. Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604. doi: 10.7326/0003-4819-150-9-200905050-00006
50. Nguyen VK, White JR. Overview of ertugliflozin. Clin Diabetes. (2019) 37:176–8. doi: 10.2337/cd18-0097
51. Cheng Q, Zou S, Feng C, Xu C, Zhao Y, Shi X, et al. Effect of ertugliflozin on renal function and cardiovascular outcomes in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Med (United States). (2023) 102:E33198. doi: 10.1097/MD.0000000000033198
52. Chowdhury MAB, Islam M, Rahman J, Uddin MJ, Haque MR. Diabetes among adults in Bangladesh: changes in prevalence and risk factors between two cross-sectional surveys. BMJ Open. (2022) 12:1–9. doi: 10.1136/bmjopen-2021-055044
53. Kamrul-Hasan ABM, Hannan MA, Asaduzzaman M, Rahman MM, Alam MS, Amin MN, et al. Prevalence and predictors of diabetes distress among adults with type 2 diabetes mellitus: a facility-based cross-sectional study of Bangladesh. BMC Endocr Disord. (2022) 22:28. doi: 10.1186/s12902-022-00938-3
54. Amin MF, Bhowmik B, Rouf R, Khan MI, Tasnim SA, Afsana F, et al. Assessment of quality of life and its determinants in type-2 diabetes patients using the WHOQOL-BREF instrument in Bangladesh. BMC Endocr Disord. (2022) 22:162. doi: 10.1186/s12902-022-01072-w
55. Afroz A, Alam K, Ali L, Karim A, Alramadan MJ, Habib SH, et al. Type 2 diabetes mellitus in Bangladesh: A prevalence based cost-of-illness study. BMC Health Serv Res. (2019) 19:1–12. doi: 10.1186/s12913-019-4440-3
56. Akhtar S, Nasir JA, Sarwar A, Nasr N, Javed A, Majeed R, et al. Prevalence of diabetes and pre-diabetes in Bangladesh: a systematic review and meta-analysis. BMJ Open. (2020) 10:e036086. doi: 10.1136/bmjopen-2019-036086
57. Shuvo SD, Hossen MT, Riazuddin M, Hossain MS, Mazumdar S, Parvin R, et al. Prevalence of comorbidities and its associated factors among type-2 diabetes patients: a hospital-based study in Jashore District, Bangladesh. BMJ Open. (2023) 13:e076261. doi: 10.1136/bmjopen-2023-076261
58. Banik PC, Mondal R, Hussain A. Overweight and obesity among the urban and rural type 2 diabetic subjects in Bangladesh. J Xiangya Med. (2020) 5:37–7. doi: 10.21037/jxym-20-96
59. Kamrul-Hasan ABM, Talukder SK, Kabir MA, Mustari M, Un Nabi MM, Gaffar AJ, et al. Comparison of fasting and random lipid profiles among subjects with type 2 diabetes mellitus: an outpatient-based cross-sectional study in Bangladesh. Diabetol Metab Syndr. (2023) 15:139. doi: 10.1186/s13098-023-01120-y
60. Selim S, Alam MS, Talukder SK, Kabir ML, Gaffar AJ, Kabir MA, et al. Status of lipid control in Bangladeshi subjects with type 2 diabetes mellitus on lipid-lowering drugs: a multicenter, facility-based, cross-sectional study. BMC Endocr Disord. (2023) 23:1–9. doi: 10.1186/s12902-023-01522-z
61. Kamrul-Hasan ABM, Alam MS, Kabir MA, Chowdhury SR, Hannan MA, Chowdhury EUR, et al. Risk stratification using the 2021 IDF-DAR risk calculator and fasting experience of Bangladeshi subjects with type 2 diabetes in Ramadan: The DAR-BAN study. J Clin Transl Endocrinol. (2023) 31:100315. doi: 10.1016/j.jcte.2023.100315
62. Bener A, Al-Hamaq-Aoa A, Öztürk M, Çatan F, Haris PI, Rajput Ku ÖA. Effect of ramadan fasting on glycemic control and other essential variables in diabetic patients. Ann Afr Med. (2018) 17:196–202. doi: 10.4103/aam.aam_63_17
63. Sahin SB, Ayaz T, Ozyurt N, Ilkkilic K, Kirvar A, Sezgin H, et al. The impact of fasting during Ramadan on the glycemic control of patients with type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. (2013) 121:531–4. doi: 10.1055/s-0033-1347247
64. Ismail NA, Olaide Raji H, Abd Wahab N, Mustafa N, Kamaruddin NA, Abdul Jamil M, et al. Glycemic control among pregnant diabetic women on insulin who fasted during Ramadan. Iran J Med Sci. (2011) 36:254–9.
65. Hassanein MM, Echtay A, Hassoun A, Alarouj M, Afandi B, Poladian R, et al. Tolerability of canagliflozin in patients with type 2 diabetes mellitus fasting during Ramadan: Results of the Canagliflozin in Ramadan Tolerance Observational Study (CRATOS). Int J Clin Pract. (2018) 142:85–91. doi: 10.1111/ijcp.12991
Keywords: diabetes mellitus, Ramadan, fasting, ertugliflozin, SGLT2 inhibitors, safety, efficacy
Citation: Pathan MF, Akter N, Mustari M, Saifuddin M, Sharifuzzaman M, Rahman MM, Ripon M, Mohiuddin SM, Kamrul-Hasan ABM, Hannan MA, Alam MS, Mahjabeen S, Afsana F, Bakar MA, Haq T, Ahammed A, Talukder SK, Sarkar S and Selim S (2025) Effectiveness of ertugliflozin during Ramadan fasting in patients with type 2 diabetes mellitus: a real-world study (ErtuRamadan study). Front. Endocrinol. 16:1542946. doi: 10.3389/fendo.2025.1542946
Received: 10 December 2024; Accepted: 22 January 2025;
Published: 11 February 2025.
Edited by:
Åke Sjöholm, Gävle Hospital, SwedenReviewed by:
Khadija Hafidh, Dubai Health Authority, United Arab EmiratesCopyright © 2025 Pathan, Akter, Mustari, Saifuddin, Sharifuzzaman, Rahman, Ripon, Mohiuddin, Kamrul-Hasan, Hannan, Alam, Mahjabeen, Afsana, Bakar, Haq, Ahammed, Talukder, Sarkar and Selim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shahjada Selim, c2VsaW1zaGFoamFkYUBnbWFpbC5jb20=
†ORCID: Shahjada Selim, orcid.org/0000-0001-7749-3542
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.