
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol. , 28 January 2025
Sec. Thyroid Endocrinology
Volume 16 - 2025 | https://doi.org/10.3389/fendo.2025.1533169
 Mohammadamin Parsaei1†
Mohammadamin Parsaei1† Mohadese Dashtkoohi2†
Mohadese Dashtkoohi2† Elahe Amirkhalili3
Elahe Amirkhalili3 Mohammadreza Chashmyazdan4
Mohammadreza Chashmyazdan4 Tim I. M. Korevaar5,6*
Tim I. M. Korevaar5,6* Pantea Nazeri1*
Pantea Nazeri1*Background: Maternal thyroid hormones play a vital role in fetal development, and imbalances can lead to adverse outcomes. Iron deficiency may impair thyroid function due to iron’s essential role in iodine oxidation during thyroid hormone synthesis. This review examines the relationship between various indicators of maternal iron status and thyroid function during pregnancy.
Methods: We conducted a systematic search in MEDLINE/PubMed, Web of Science, Embase, Scopus, and the Cochrane Library for studies published up to 2023. Meta-analyses determined pooled thyroid hormone levels in patients with and without iron deficiency, using serum ferritin (cut-off = 30 µg/L) and hemoglobin (cut-off = 11 g/dL). Meta-regression analyses examined linear relationships between iron status indicators and thyroid hormones.
Results: Forty-seven studies involving 53,152 pregnant women were included. Meta-analysis showed no significant difference in thyroid-stimulating hormone, free T4, or total T4 when considering serum ferritin levels in iron-deficient versus iron-sufficient individuals. However, regarding hemoglobin levels, iron deficiency was associated with higher thyroid-stimulating hormone (2.31 mIU/L vs. 1.75 mIU/L) and lower free T4 (10.7 pmol/L vs. 13.3 pmol/L), but not total T4. Meta-regression revealed no significant associations between serum ferritin and thyroid hormones. Conversely, maternal hemoglobin levels were inversely associated with thyroid-stimulating hormone (P-value = 0.009) and directly associated with free T4 (P-value < 0.001), with no significant link to total T4.
Conclusions: Maternal hemoglobin levels are more strongly correlated with thyroid function than serum ferritin levels. This suggests that monitoring hemoglobin could enhance the early detection and management of thyroid dysfunction during pregnancy.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO, identifier CRD4202451820.
Thyroid dysfunction is a common endocrine disorder during pregnancy, affecting approximately 2.5% of pregnancies (1). Various thyroid disorders can develop or worsen during pregnancy, potentially leading to adverse outcomes for both the mother and the fetus (2). These changes are primarily due to altered thyroid-stimulating hormone (TSH) and thyroid-binding protein levels, influenced by elevated maternal estrogen levels (3–5). For instance, maternal hypothyroidism is associated with increased risks of adverse fetal outcomes such as preeclampsia, low birth weight, and intellectual impairments. Conversely, hyperthyroidism can lead to fetal complications including tachycardia, growth restriction, prematurity, and stillbirths (6–8). Therefore, early detection and treatment of thyroid disorders in pregnancy are crucial.
Iron deficiency (ID), characterized by reduced extracellular iron in the bone marrow and serum ferritin (SF), is recognized as the most prevalent nutritional deficiency (9). Pregnant women are particularly susceptible to ID and its more severe form, ID anemia, due to the increased iron demands associated with expanded blood volume to support maternal physiological functions and fetal development (10). It is suggested that ID can adversely affect thyroid hormone synthesis due to its critical role in intracellular oxygen delivery within thyroid tissue, thereby disrupting related metabolic pathways (11). Iron is a component of thyroid peroxidase (TPO), which is essential for thyroid hormone biosynthesis (12). TPO catalyzes the oxidation of iodine, a process activated by TSH. Thus, ID may hinder TPO activity and thyroid metabolism, reducing thyroid hormone production (13).
While several studies have investigated the relationship between thyroid function and ID during pregnancy, this association remains inadequately established (14, 15). A systematic review of eight articles indicated that ID is associated with elevated levels of TSH and reduced levels of free thyroxine (FT4), as well as an increased prevalence of subclinical and overt hypothyroidism in pregnant women (9). Nonetheless, more research is needed to explore the relationship between maternal iron status indicators beyond SF and thyroid function during pregnancy (16, 17). For example, Hb is one of the most robust indicators of body iron status, and its association with maternal thyroid function has been studied extensively (6, 17). However, a comprehensive overview is still lacking.
This study aims to systematically review the association between various indicators of maternal iron status and thyroid function in pregnant women. We expect that the findings will provide valuable insights into the relationship between iron status and thyroid function during pregnancy. These insights could potentially inform guidelines for diagnosing and managing pregnancy-induced thyroid disorders by taking maternal iron status into account.
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (18) and is registered in the International Prospective Register of Systematic Reviews (PROSPERO) under the registration code CRD42024518203. The research question for this systematic review was formulated using the Participants, Intervention, Comparators, Outcomes, Study design (PICOS) framework (19), as follows:
Population (P): Healthy pregnant women;
Intervention (I): Maternal iron status;
Comparators (C): ID vs. Iron sufficiency;
Outcomes (O): Maternal thyroid function parameters and thyroid disorders;
Study Design (S): Observational studies, i.e., prospective and retrospective cohorts, cross-sectional, and case-control studies.
We conducted an extensive literature search across multiple databases, including MEDLINE/PubMed, Web of Science, Embase, Scopus, and the Cochrane Library, covering publications up to 2023. Our search employed key terms such as ‘pregnancy,’ ‘pregnant women,’ ‘iron status,’ ‘iron deficiency,’ ‘iron markers,’ ‘iron levels,’ ‘thyroid function parameters,’ ‘thyroid hormones,’ ‘thyroid gland,’ and ‘thyroid disorders.’ Additionally, we performed a manual search by reviewing the reference lists of original articles and relevant reviews.
Following the searches, two independent investigators (MP and PN) meticulously screened the titles and abstracts of studies to exclude those that did not meet the eligibility criteria. Subsequently, they conducted a thorough review of the full texts of potentially relevant studies to determine their inclusion in the systematic review. The inclusion criteria encompassed the following aspects: 1) human studies, 2) healthy pregnant women as participants, 3) studies reporting iron status using at least one indicator, and 4) studies providing data on various thyroid parameters and frequency of thyroid disorders. Notably, we excluded studies involving pregnant women who were supplemented with iron/folic acid, as well as those with pregnancy complications.
In the case of included studies, we utilized a standardized form specifically designed for this review to extract relevant data. The extracted information included details such as the first author, year of publication, study country, the number of pregnant women investigated, pregnancy timing, iron status indicators, thyroid tests, and any potential correlations between maternal iron status and thyroid function parameters or thyroid disorders during pregnancy. To ensure accuracy, the extracted data underwent cross-checking, and any discrepancies were resolved through discussion or consultation with a third investigator (MD).
We evaluated the quality of studies using the Newcastle-Ottawa Scale (20). Each study was assessed across the following domains: 1) sample population selection [scored on a scale of 0–4], 2) comparability of subjects in different outcome groups [scored on a scale of 0–3], and 3) appropriate outcome assessment [scored on a scale of 0–3]. Based on their Newcastle-Ottawa scores, studies were categorized as very good (9–10 points), good (7–8 points), satisfactory (5–6 points), or unsatisfactory (0–4 points).
In this study, thyroid function in pregnant women was evaluated using TSH, free T4 (FT4), and total T4 (TT4). The means and standard deviations (SDs) of these continuous variables were extracted from each included study for the meta-analysis. When mean and SD values were not explicitly reported, they were estimated from available medians, interquartile ranges (IQRs), ranges, or 95% confidence intervals (CIs) using established formulas (21, 22).
Data analysis was performed using Comprehensive Meta-Analysis (CMA) software version 2.2.064, with statistical significance set at a P-value < 0.05 and a 95% CI. Since most of the included studies assessed the association between SF or Hb levels and thyroid hormones, and only a few studies have investigated the role of other iron status indicators in relation to thyroid function, we focused on these two indicators in this meta-analysis. Meta-analyses were conducted to calculate the pooled thyroid hormone levels in pregnant women based on their Hb and SF levels. For studies examining SF, a cut-off of 30 µg/L was used to distinguish between iron sufficiency (above 30 µg/L) and ID (below 30 µg/L) (23). For studies reporting Hb levels, pregnant women were categorized into two groups with Hb levels above 11 mg/dl (indicating iron sufficiency) and below 11 mg/dl (indicating ID) (24). Heterogeneity was evaluated using Cochrane’s Q statistic and the I² index, with I² values exceeding 50% considered indicative of substantial heterogeneity. In the presence of significant heterogeneity, a random-effects model was applied to calculate pooled effects; otherwise, a fixed-effects model was planned. Forest plots were generated to visually represent the pooled mean and 95% CI of thyroid hormones in different subgroups of iron status. We evaluated the presence of publication bias by both Egger’s regression asymmetry test in addition to funnel plot (25). Additionally, in relevant cases, we used the trim and fill method to address publication bias. Corrected results were reported after trimming if they significantly differed from the results where publication bias was present (26).
Furthermore, a meta-regression model was applied to investigate the linear relationships between iron status indicators (SF and Hb) and the three thyroid hormones (TSH, FT4, and TT4) during pregnancy. For this purpose, CMA software version 2.2.064 was utilized, with statistical significance set at a p-value < 0.05. The analysis employed mixed-effects regression using the method of moments. This approach enabled the examination of the relationship between study-level covariates and effect sizes across the included studies. The meta-regression output included pooled β, standard errors (SEs), and P-values for the slope.
The systematic search yielded a total of 1,037 studies. After excluding 963 studies during the title and abstract screening process, 74 studies underwent full-text screening and were assessed for eligibility. Of these, 19 studies were excluded due to the inclusion of women with pregnancy-related complications, 7 were excluded due to incomplete data, and 1 was excluded due to iron and folic acid supplementation. Consequently, 47 studies successfully met the inclusion criteria and were systematically reviewed. Figure 1 provides detailed information regarding the study selection process for this review.
The geographical distribution of the reviewed studies is as follows: Eastern Asia (n=15), Southern Asia (n=14), Europe (n=9), the Middle East (n=6), Africa (n=1), North America (n=1), and South America (n=1). Most studies employed a cross-sectional design (n=31) (6, 12, 16, 27–54), while 13 studies utilized a prospective cohort design (17, 55–66) and three employed a retrospective cohort design (67–69). Sample sizes across the studies ranged from 28 to 14,043 pregnant women, culminating in a total of 53,152 patients included in this systematic review. Twenty studies focused on women in their first trimester of pregnancy (6, 12, 16, 29–36, 39, 45–47, 49, 53, 58, 60, 69), with four (38, 43, 50, 63) and seven studies (27, 37, 40, 52, 55, 61, 62) exclusively examining women in their second and third trimesters, respectively. Additionally, four studies included women in both the first and second trimesters (17, 41, 56, 68), two included women in the first and third trimesters (44, 48), and one study included women in both the second and third trimesters (66). Furthermore, nine studies encompassed women from all three trimesters of pregnancy (28, 42, 51, 54, 57, 59, 64, 65, 67).
The reported measures of iron status across the reviewed studies included: hemoglobin (Hb) (n=34), SF (n = 29), serum iron (SI) (n=8), mean corpuscular volume (MCV) (n=7), red blood cell (RBC) count (n=6), mean corpuscular hemoglobin (MCH) (n=5), transferrin receptor (TfR) (n=5), mean corpuscular hemoglobin concentration (MCHC) (n=4), hematocrit (Hct) (n=3), total iron-binding capacity (TIBC) (n=3), body iron store (BIS) (n=2), total body iron (TBI) (n=2), transferrin (Tf) (n=2), transferrin saturation (TS) (n=2), dietary intake recall (DIR) (n=1), erythropoietin (EP) (n=1), packed cell volume (PCV) (n=1), and urinary iron-binding capacity (UIBC) (n=1). The distribution of reported thyroid function measures included: TSH (n=45), FT4 (n=37), free T3 (FT3) (n=17), TT4 (n=16), anti-thyroid peroxidase antibody (TPO-Ab) (n=15), total T3 (TT3), anti-thyroglobulin antibody (Tg-Ab) (n=7), thyroglobulin (Tg) (n=3), thyroid volumes assessed via sonographic examination (n=2), and reverse T3 (r-T3) (n=1). More detailed information from each included study is provided in Table 1.
The quality assessment results of the included studies are presented in Table 2. A total of 32 studies were deemed eligible for inclusion in further meta-analyses. Four studies were classified as having very good overall quality (29, 32, 48, 58), employing well-designed and mature study designs and reporting styles. Additionally, 23 studies demonstrated good overall quality (6, 12, 16, 28, 30, 33, 34, 36, 38–40, 43–45, 49, 52–54, 56, 59, 61, 66, 70), while five studies were rated as having satisfactory overall quality (41, 42, 46, 47, 51).
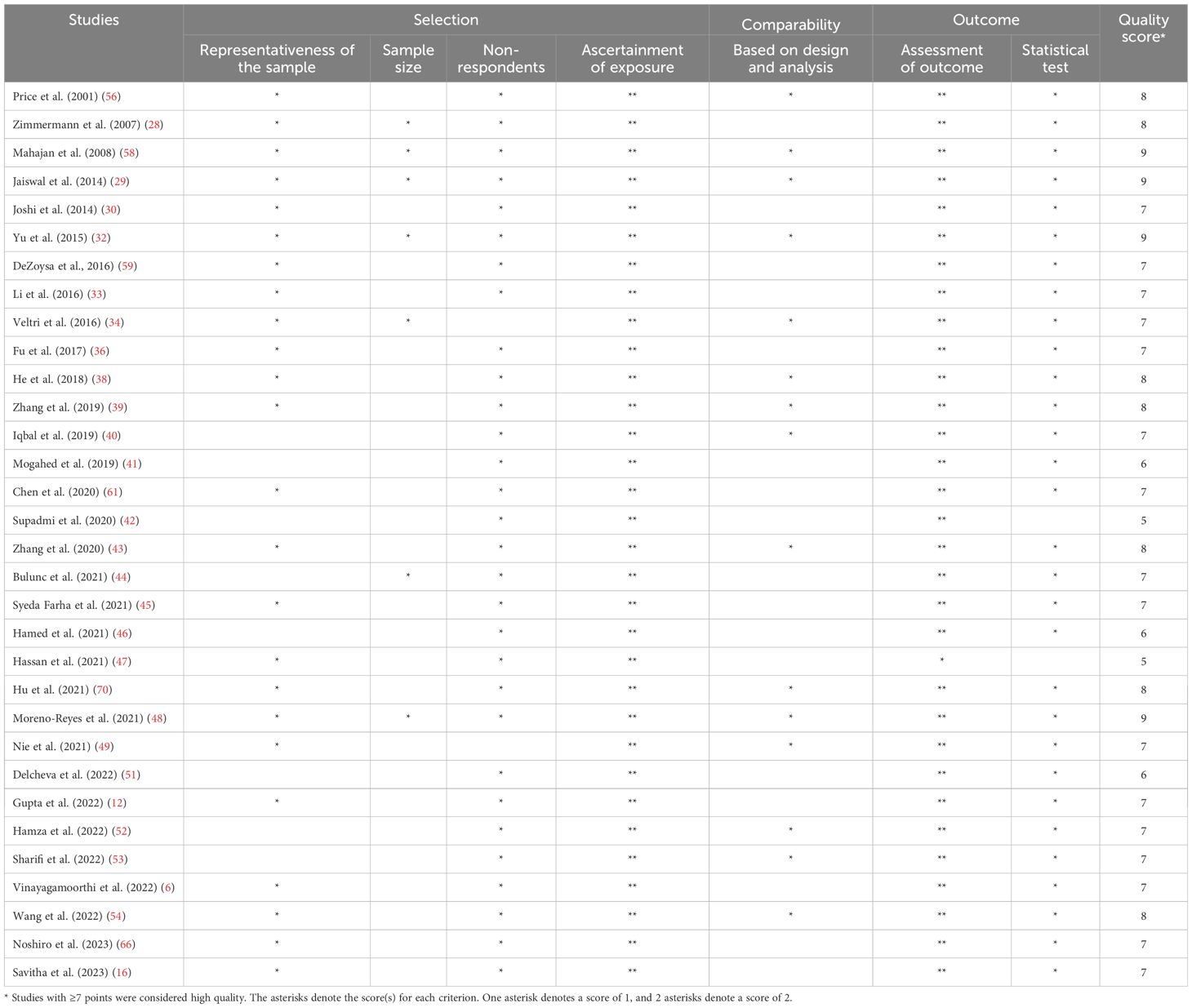
Table 2. Risk of bias assessment of the included studies based on the Newcastle-Ottawa Quality Assessment Scale (adapted for cross-sectional studies).
Figures 2A, B present the pooled mean and 95% CI of TSH levels in pregnant women with and without ID, based on SF concentrations. These plots indicated substantial heterogeneity between the study-specific estimates (test for heterogeneity: I² = 97.8% and 99.4%, respectively, P < 0.001 for both), necessitating the use of a random-effects model for a more appropriate estimate. No publication bias was detected between these two subgroups. The mean TSH levels in pregnant women with ID (SF < 30 μg/L) (12, 16, 28, 33, 34, 36, 39–42, 47, 48, 51, 54, 56, 59, 66) was 1.73 mIU/L (95% CI: 1.62-1.84 mIU/L), while in pregnant women without ID (SF > 30 μg/L) (6, 16, 32–34, 36, 38, 39, 43, 46, 54, 56, 58, 59) it was 1.84 mIU/L (95% CI: 1.70-1.97 mIU/L). Comparing the 95% CIs, no significant difference was found in TSH levels between pregnant women with and without ID.
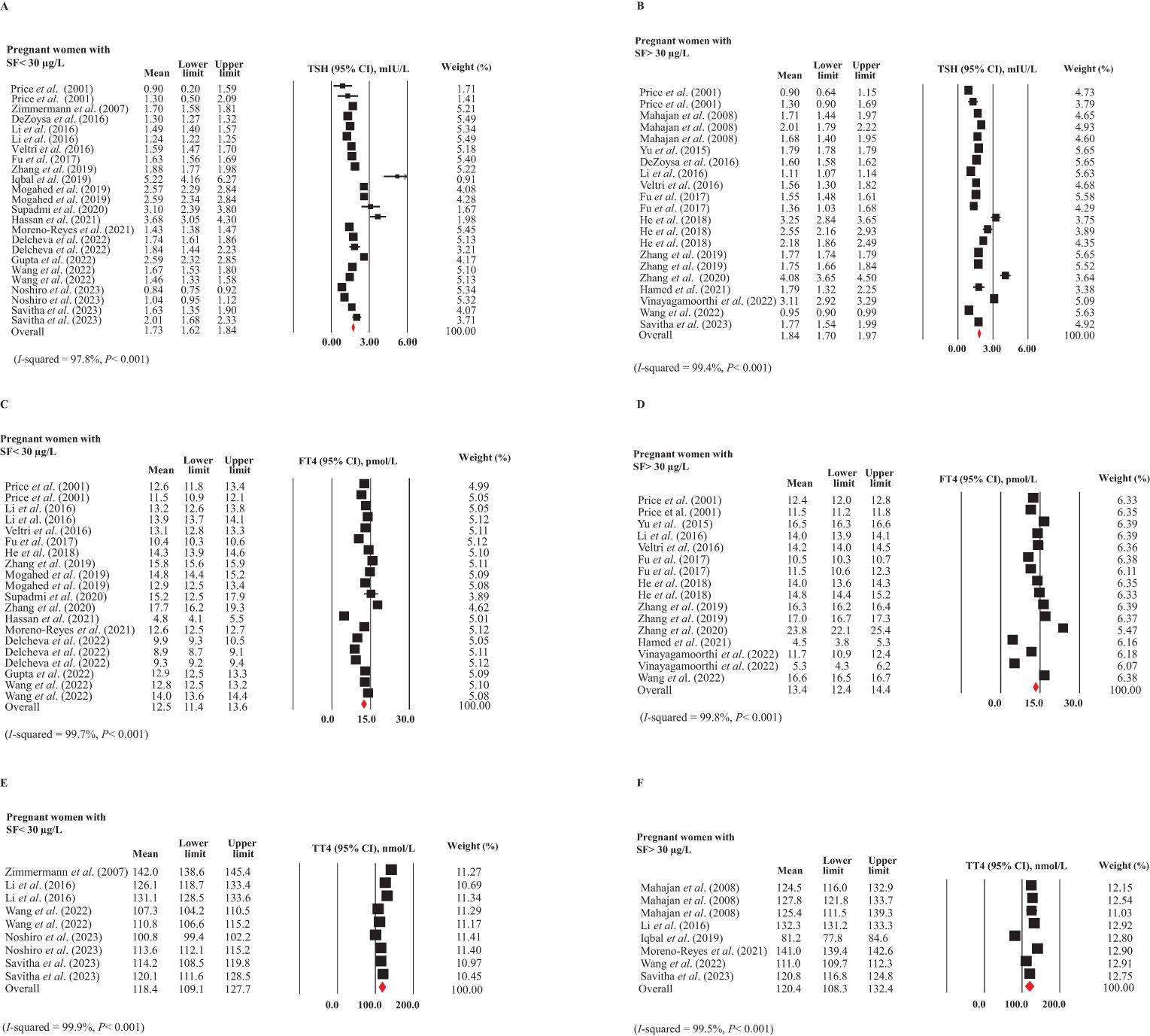
Figure 2. Forest plots for meta-analyses estimating the pooled thyroid hormones in pregnant women with (SF < 30 µg/L) and without (SF > 30 µg/L) ID. (A) TSH in ID, (B) TSH in non-ID, (C) FT4 in ID, (D) FT4 in non-ID, (E) TT4 in ID, and (F) TT4 in non-ID.
In Figures 2C, D, the forest plots show the mean and 95% CIs for FT4 levels in pregnant women with ID (12, 33, 34, 36, 38, 39, 41–43, 47, 48, 51, 54, 56) and without ID (6, 32–34, 36, 38, 39, 43, 46, 54, 56), along with the pooled estimates. The studies included for women with and without ID exhibited substantial heterogeneity (I² = 99.7% and 99.8%, respectively, P < 0.001 for both). There was no publication bias in these subgroups. The mean FT4 levels in pregnant women with ID were 12.5 pmol/L (95% CI: 11.4-13.6 pmol/L), while in those without ID, the levels were 13.4 pmol/L (95% CI: 12.4-14.4 pmol/L). Comparing the 95% CIs indicated that these differences were not statistically significant.
The pooled TT4 (95% CI) of pregnant women with (16, 28, 33, 54, 66) and without ID (16, 33, 40, 48, 54, 58) are given in Figures 2E, F. The studies included for women with and without ID showed substantial heterogeneity (I2 = 99.9 and 99.5%, respectively, P <0.001 for both). We observed no publication bias in these subgroups. There was no significant difference in TT4 levels between pregnant women with ID (118.4 nmol/L, 95% CI: 109.1-127.7 nmol/L) and those without ID (120.4 nmol/L, 95% CI: 108.3-132.4 nmol/L).
The funnel plots for publication bias assessment are provided in the Supplementary Materials.
In Figures 3A, B, the forest plots depict studies with mean values and 95% CIs, as well as the pooled estimates for the mean TSH in pregnant women with (12, 16, 30, 33, 40, 45, 47, 49, 54, 58) and without ID (6, 16, 28, 29, 33, 36, 41, 44, 46, 48, 49, 51, 53, 54, 58, 59, 61, 70) based on Hb concentration. The plots showed substantial heterogeneity among included studies (I2 = 92.5 and 99.4%, respectively, P<0.001 in both), necessitating the use of a random-effects model. The mean TSH levels were higher in pregnant women with ID (Hb < 11 g/dL) compared to those without ID (Hb > 11 g/dL). A comparison of the 95% CIs revealed a significant difference between these values [2.31 mIU/L (95% CI: 2.01-2.61 mIU/L) vs. 1.75 mIU/L (95% CI: 1.62-1.87 mIU/L)]. After applying the trim and fill method to correct for publication bias in the subgroup of pregnant women without ID (P = 0.004), the mean TSH level in women without ID was adjusted to 1.67 mIU/L (95% CI: 1.54-1.79 mIU/L). This difference between the two subgroups remained statistically significant even after bias correction.
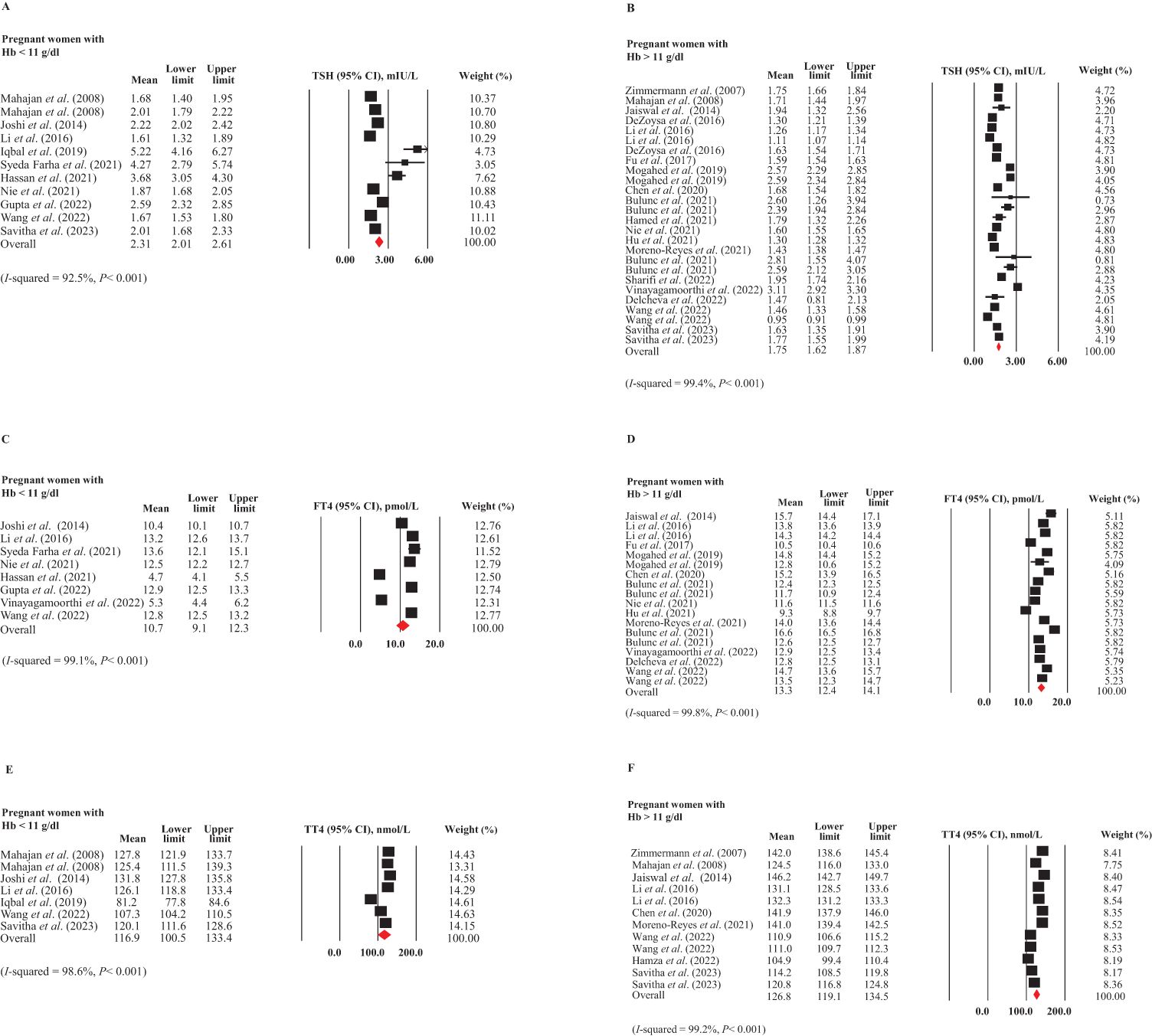
Figure 3. Forest plots for meta-analyses estimating the pooled thyroid hormones in pregnant women with (Hb < 11 g/dL) and without (Hb > 11 g/dL) ID. (A) TSH in ID, (B) TSH in non-ID, (C) FT4 in ID, (D) FT4 in non-ID, (E) TT4 in ID, and (F) TT4 in non-ID.
Regarding FT4 levels, due to substantial heterogeneity among the included studies (I² = 92.5% and 99.4%, respectively, P < 0.001 for both), a random-effects model was employed for the analysis. As depicted in Figures 3C, D, the weighted FT4 levels (95% CI) were 10.7 pmol/L (95% CI: 9.1-12.3 pmol/L) in pregnant women with ID (6, 12, 30, 33, 45, 47, 49, 54) and 13.3 pmol/L (95% CI: 12.4-14.1 pmol/L) in those without ID (6, 29, 33, 36, 41, 44, 48, 49, 51, 54, 61, 70), indicating a significant difference between the two subgroups. There was no publication bias in these subgroups.
As illustrated in Figures 3E, F, substantial heterogeneity was observed between the two subgroups of studies (I² = 98.6% and 99.2%, respectively, P < 0.001 for both). The mean values and 95% CIs for TT4 levels were 116.9 nmol/L (95% CI: 100.5-133.4 nmol/L) in pregnant women with ID (16, 30, 33, 40, 54, 58) and 126.8 nmol/L (95% CI: 119.1-134.5 nmol/L) in those without ID (16, 28, 29, 33, 48, 52, 54, 58, 61). However, this difference was not statistically significant. No publication bias was detected in these two groups.
The funnel plots for publication bias assessment are provided in the Supplementary Materials.
The meta-regression analyses indicated no significant associations between maternal SF and TSH (P = 0.081), FT4 (P = 0.246), or TT4 (P = 0.524) levels during pregnancy (Figure 4A). As shown in Figure 4B, there was a significant inverse association between maternal Hb and TSH levels (pooled β (SE) = -0.119 (0.046), P = 0.009). However, a significant positive association was observed between Hb and FT4 concentrations (pooled β (SE) = 1.395 (0.255), P < 0.001). No significant association was found between Hb and TT4 levels in pregnant women (P = 0.419). Detailed results of the meta-regression analyses are presented in Table 3.
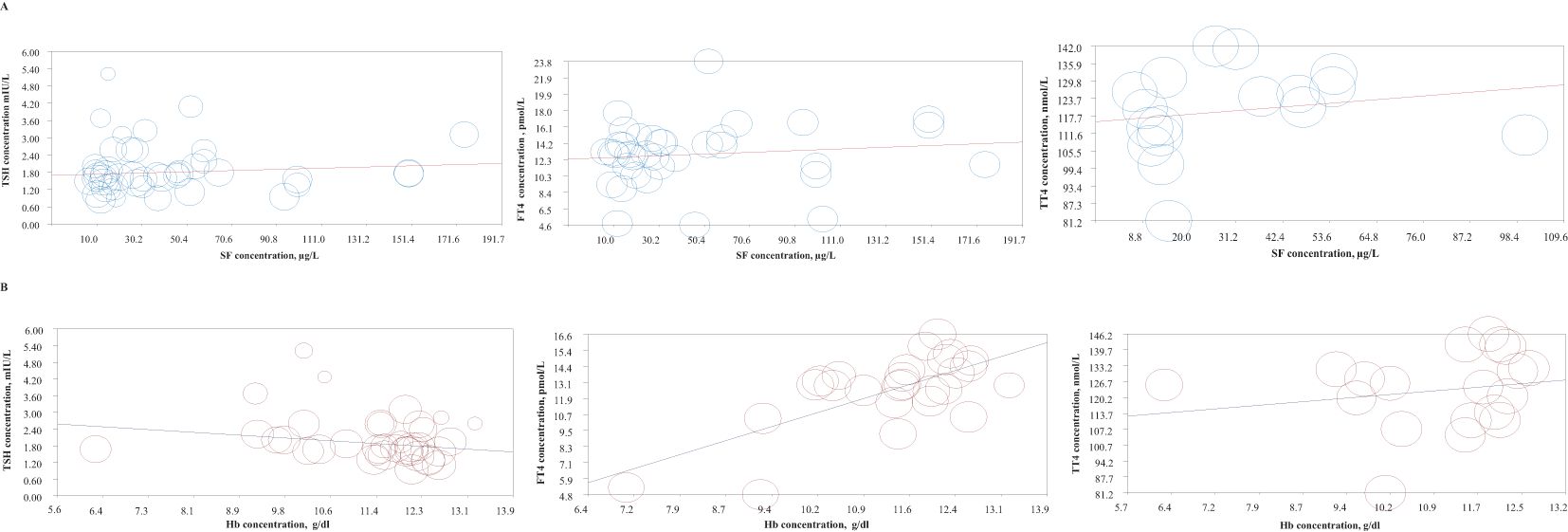
Figure 4. Meta-regression plots examining the association between thyroid hormone levels (TSH, FT4, and TT4) with (A) SF levels, and (B) Hb levels during pregnancy.
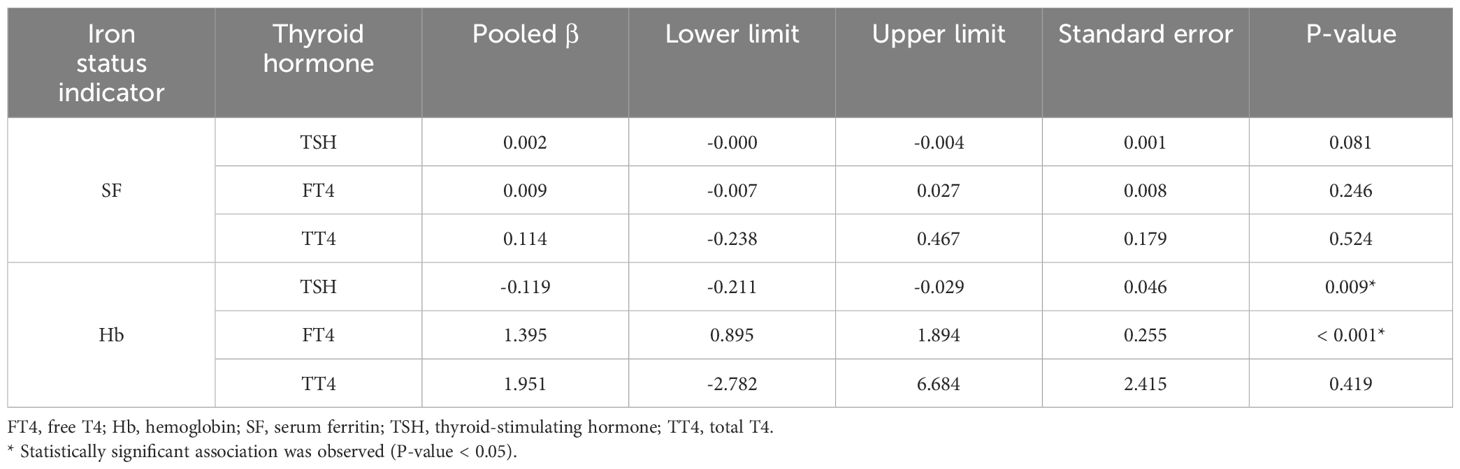
Table 3. Results of the meta-regression analyses on the association of SF and Hb levels with thyroid hormone levels during pregnancy.
Furthermore, a graphical abstract for our review findings is provided in Figure 5.
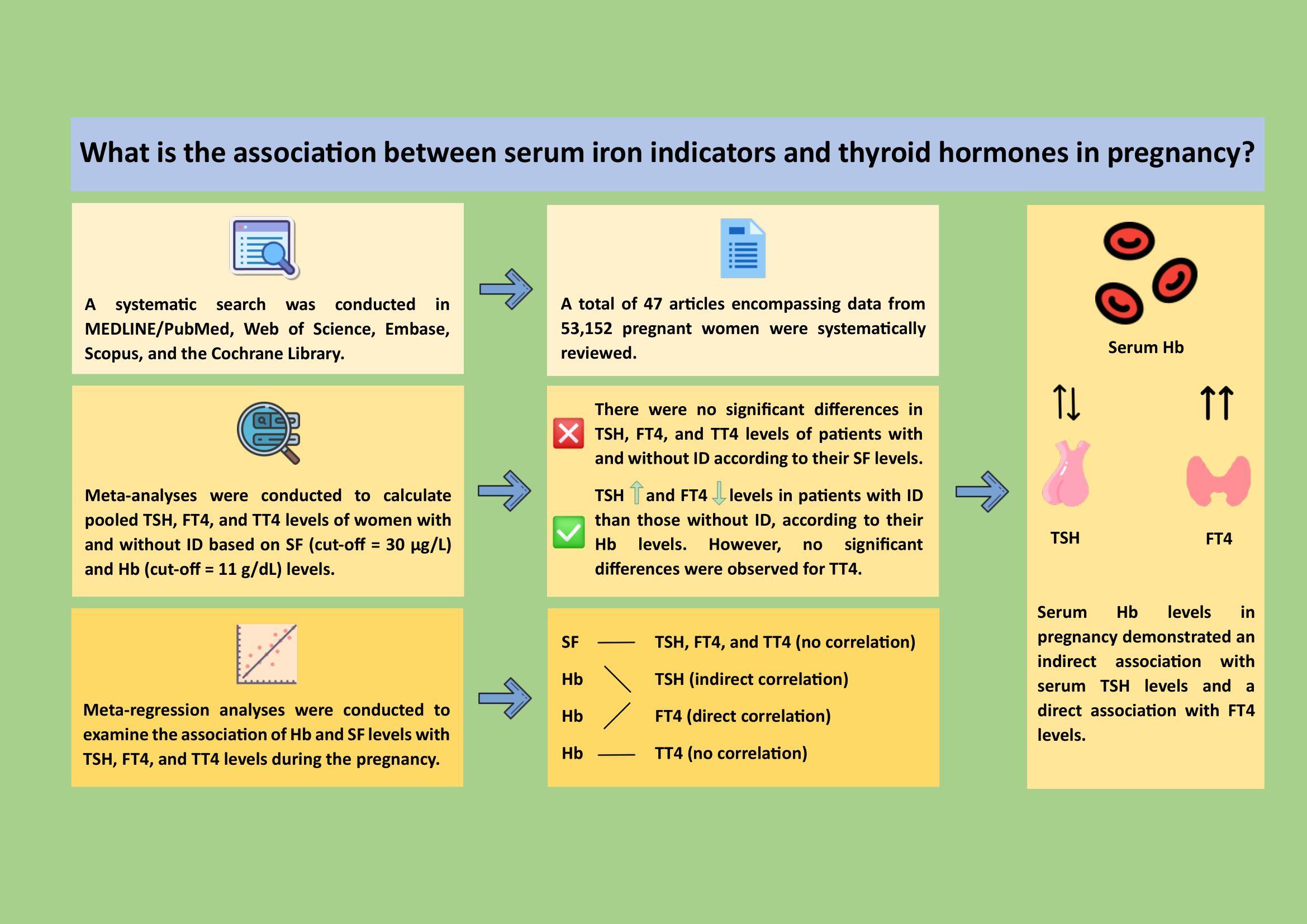
Figure 5. (Graphical abstract): A systematic review of studies from MEDLINE/PubMed, Web of Science, Embase, Scopus, and Cochrane Library up to 2023 was performed. Meta-analyses assessed pooled thyroid hormone levels with serum ferritin (SF, cut-off = 30 µg/L) and hemoglobin (Hb, cut-off = 11 g/dL). Forty-seven studies with 53,152 pregnant women were included. No significant differences in TSH, FT4, or TT4 were found for SF levels. However, iron deficiency was linked to higher TSH (2.31 vs. 1.75 mIU/L) and lower FT4 (10.7 vs. 13.3 pmol/L) but not TT4. Meta-regression showed maternal Hb levels had significant associations with TSH and FT4, but not TT4. These results suggested that monitoring maternal serum Hb may improve early detection and management of thyroid dysfunction during pregnancy (Icons by icons8.com). FT4, free T4; Hb, hemoglobin; ID, iron deficiency; SF, serum ferritin; TSH, thyroid-stimulating hormone; TT4, total T4.
Our meta-analyses revealed that defining ID based on Hb levels in pregnant women was associated with a higher likelihood of thyroid dysfunction, evidenced by elevated TSH levels and reduced FT4 concentrations, compared to those with sufficient iron status. However, no significant differences were observed in TT4 levels. In contrast, when maternal SF levels were used as the marker of ID, no significant differences in TSH, FT4, or TT4 levels were observed between pregnant women with and without ID. These findings were further corroborated by our meta-regression analyses, which demonstrated stronger associations between Hb levels and various thyroid hormones compared to SF.
Iron is a crucial trace element, essential for intracellular oxygen transport and the proper functioning of various enzymes (71). Given iron’s significant role in intracellular oxygen delivery and its involvement as a component of the TPO enzyme, which catalyzes iodine oxidation—the initial step in thyroid hormone production—it is plausible that reduced SI levels and related indicators may be significantly associated with thyroid dysfunction (12). In this regard, previous research highlighted the association between the iron profile and thyroid function. Previous research has underscored the relationship between iron status and thyroid hormone levels. A large cross-sectional study involving 42,162 participants demonstrated that individuals with hypothyroid or hyperthyroid function were more likely to have anemia. Furthermore, baseline thyroid dysfunction was associated with an increased likelihood of developing anemia during follow-up (72). Another study, conducted among 2,356 participants in the United States, revealed an inverse relationship between iron status and thyroid autoimmunity in reproductive-aged women (72). Specifically, each unit increase in SI was linked to a 43% reduction in the risk of Hashimoto’s thyroiditis (73). Additionally, SI was found to negatively correlate with TPO-Ab and exhibit a non-linear association with Tg-Ab level (73). Moreover, a recent meta-analysis of ten cross-sectional studies further highlighted this interplay, showing that patients with ID had significantly lower levels of TSH, FT4, and FT3 compared to those without ID (74). This suggests a potential connection between iron deficiency, thyroid function, and thyroid autoimmunity, particularly in certain patient groups. Notably, the meta-analysis emphasized a stronger association between these factors in pregnant women (74), underscoring the need for a deeper understanding of the interactions between iron metabolism and thyroid function in this population.
Our findings indicated a significant association between maternal iron status, specifically Hb levels, with thyroid function during pregnancy. Interestingly, we observed that serum Hb levels are a superior predictor of thyroid function during pregnancy compared to SF levels. Although previous research has acknowledged serum Hb as a widely available and cost-effective indicator of iron status, it has also highlighted its significant limitations, particularly its reduced sensitivity and specificity in detecting ID (75). On the other hand, SF has traditionally been regarded as a more specific and reliable indicator of iron status, as it provides insights into the size of iron stores in the body. However, the utility of SF as an iron status marker may be compromised during life stages such as pregnancy, where iron stores are physiologically depleted (75). Moreover, SF measures the amount of stored iron rather than the oxygen-carrying capacity of blood, which is directly reflected by Hb levels (76, 77). Given the critical role of iron in the oxygenation processes involved in thyroid hormone synthesis (78–80), it is rational to observe a stronger association between Hb levels and thyroid function compared to SF levels This suggests that during pregnancy, when iron demands are heightened, Hb may serve as a more direct and relevant marker for assessing thyroid function.
This review possesses several key strengths that distinguish it from other studies examining the association of serum Hb and SF levels with thyroid function during pregnancy (9, 74). First, we conducted an extensive and systematic search for eligible studies that provided data on at least one iron indicator and one thyroid function indicator, allowing us to offer a comprehensive overview of the associations and comparisons among these indicators. Furthermore, we performed multiple meta-analyses to precisely assess the relationships between thyroid function indicators (TSH, FT4, and TT4) and the two prominent iron status indicators, Hb and SF. Our comprehensive search strategy enabled us to incorporate data from a large cohort of pregnant women, which enhances the generalizability of our findings to broader populations. This also allowed us to compare the pooled values and 95% CIs of TSH, FT4, and TT4 levels between women with and without ID. Additionally, by pooling data from all eligible studies, we conducted an in-depth meta-regression analysis, providing robust insights into the relationship between different iron status indicators and thyroid function tests during pregnancy, as well as their predictive capacities.
However, several limitations of this review should be acknowledged. A substantial proportion of the included studies were conducted in Eastern and Southern Asia, which may constrain the geographical generalizability of our findings. Additionally, the variation in study designs and measurement methods among the included studies resulted in significant heterogeneity across all meta-analyses. Furthermore, the populations studied encompassed patients from different trimesters of pregnancy, introducing additional variability. Consequently, further research is needed to obtain more reliable and definitive results regarding the association between maternal iron status and thyroid function, as well as to clarify the specific relationships and diagnostic values of iron indicators for thyroid function during each trimester of pregnancy.
In conclusion, this study established a significant association between maternal iron status and thyroid function during pregnancy, with serum Hb levels demonstrating stronger associations with thyroid indicators—specifically, an inverse relationship with TSH and direct relationships with FT4—compared to SF. These findings suggest that monitoring maternal Hb levels, which is a readily available and cost-effective test, can provide valuable insights into the potential risk of thyroid dysfunction during pregnancy. Early detection of such risks enables timely diagnostic and therapeutic interventions, which are crucial for preventing the serious adverse effects of thyroid dysfunction on both mothers and fetuses.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
MP: Data curation, Investigation, Visualization, Writing – original draft. MD: Investigation, Writing – original draft. EA: Formal analysis, Software, Writing – original draft. MC: Resources, Writing – review & editing. TK: Methodology, Supervision, Writing – review & editing. PN: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by a financial grant from the Tehran University of Medical Sciences, Tehran, Iran (grant number 1401-04-418-63477).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1533169/full#supplementary-material
BIS, body iron store; CI, confidence intervals; CMA, Comprehensive Meta-Analysis; DIR, dietary intake recall; EP, erythropoietin; FT3, free T3; FT4, free thyroxine; Hb, hemoglobin; Hct, hematocrit; ID, iron deficiency; IQR, interquartile ranges; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; PCV, packed cell volume; PICOS, Participants, Intervention, Comparators, Outcomes, Study design; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses; PROSPERO, International Prospective Register of Systematic Reviews; RBC, red blood cell; r-T3, reverse T3 (r-T3); SD, standard deviations; SE, standard errors; SF, serum ferritin; SI, serum iron; TBI, total body iron; Tf, transferrin; TfR, transferrin receptor; Tg, thyroglobulin; Tg-Ab, anti-thyroglobulin antibody; TIBC, total iron-binding capacity; TPO, thyroid peroxidase; TPO-Ab, anti-thyroid peroxidase antibody; TS, transferrin saturation; TSH, thyroid-stimulating hormone; TT3, total T3; TT4, total T4; UIBC, urinary iron-binding capacity.
1. LeBeau SO, Mandel SJ. Thyroid disorders during pregnancy. Endocrinol Metab Clin North Am. (2006) 35:117–36, vii. doi: 10.1016/j.ecl.2005.09.009
2. Ramprasad M, Bhattacharyya SS, Bhattacharyya A. Thyroid disorders in pregnancy. Indian J Endocrinol Metab. (2012) 16:S167–70. doi: 10.4103/2230-8210.104031
3. Tudosa R, Vartej P, Horhoianu I, Ghica C, Mateescu S, Dumitrache I. Maternal and fetal complications of the hypothyroidism-related pregnancy. Maedica. (2010) 5(2):116.
4. Shan ZY, Chen YY, Teng WP, Yu XH, Li CY, Zhou WW, et al. A study for maternal thyroid hormone deficiency during the first half of pregnancy in China. Eur J Clin Invest. (2009) 39(1):37–42. doi: 10.1111/j.1365-2362.2008.02055.x
5. Pop VJ, Vulsma T. Maternal hypothyroxinaemia during (early) gestation. Lancet. (2005) 365:1604–6. doi: 10.1016/S0140-6736(05)66489-6
6. Vinayagamoorthi R, Dhiman P, Kollipaka R, Sabita P, Hemavathy V. Association of hypothyroidism with low serum ferritin levels and iron-deficiency anemia during the first trimester of pregnancy. Cureus J Med Sci. (2022) 14(8):e28307. doi: 10.7759/cureus.28307
7. Brent GA. The debate over thyroid-function screening in pregnancy. N Engl J Med. (2012) 366:562–3. doi: 10.1056/NEJMe1112591
8. Moleti M, Di Mauro M, Sturniolo G, Russo M, Vermiglio F. Hyperthyroidism in the pregnant woman: Maternal and fetal aspects. J Clin Transl Endocrinol. (2019) 16:100190. doi: 10.1016/j.jcte.2019.100190
9. Luo J, Wang X, Yuan L, Guo L. Iron deficiency, a risk factor of thyroid disorders in reproductive-age and pregnant women: A systematic review and meta-analysis. Front Endocrinol (Lausanne). (2021) 12:629831. doi: 10.3389/fendo.2021.629831
10. Scholl TO. Iron status during pregnancy: setting the stage for mother and infant. Am J Clin Nutr. (2005) 81:1218S–22S. doi: 10.1093/ajcn/81.5.1218
11. Hess SY, Zimmermann MB, Arnold M, Langhans W, Hurrell RF. Iron deficiency anemia reduces thyroid peroxidase activity in rats. J Nutr. (2002) 132(7):1951–5. doi: 10.1093/jn/132.7.1951
12. Gupta N, Narayan A, Tonk RS, Gupta SK, Narayan A. Study of relationship between iron deficiency and thyroid function in pregnant females. Cureus. (2022) 14(12):e32411. doi: 10.7759/cureus.32411
13. Zimmermann MB, Köhrle J. The impact of iron and selenium deficiencies on iodine and thyroid metabolism: biochemistry and relevance to public health. Thyroid. (2002) 12:867–78. doi: 10.1089/105072502761016494
14. Zimmermann MB. The influence of iron status on iodine utilization and thyroid function. Annu Rev Nutr. (2006) 26:367–89. doi: 10.1146/annurev.nutr.26.061505.111236
15. Beard JL, Brigham DE, Kelley SK, Green MH. Plasma thyroid hormone kinetics are altered in iron-deficient rats. J Nutr. (1998) 128(8):1401–8. doi: 10.1093/jn/128.8.1401
16. Savitha V, Venugopal B, Preethi GA, Basavangowdappa H Subba Rao VM, et al. Testing the tormenting TRIO: A study of thyroid autoimmunity, iron deficiency and thyroid diseases in the first trimester of pregnancy. J Indian Acad Clin Med. (2023) 24:12–6.
17. Jain A, Ahirwar A, Dwivedi S, Rath RS. Prevalence and complications of subclinical and overt hypothyroidism in pregnancy at North Indian tertiary care center. Indian J Community Med. (2023) 48(2):285–90. doi: 10.4103/ijcm.ijcm_242_22
18. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. (2021) 372:n71. doi: 10.1136/bmj.n71
19. Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S. PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res. (2014) 14:579. doi: 10.1186/s12913-014-0579-0
20. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
21. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. (2018) 27(6):1785–805. doi: 10.1177/0962280216669183
22. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Method. (2014) 14:1–13. doi: 10.1186/1471-2288-14-135
23. Guyatt GH, Oxman AD, Ali M, Willan A, McIlroy W, Patterson C. Laboratory diagnosis of iron-deficiency anemia: an overview. J Gen Intern Med. (1992) 7(2):145–53. doi: 10.1007/BF02598003
24. Raut AK, Hiwale KM. Iron deficiency anemia in pregnancy. Cureus. (2022) 14:e28918. doi: 10.7759/cureus.28918
25. Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. Bmj. (2006) 333(7568):597–600. doi: 10.1136/bmj.333.7568.597
26. Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56(6):455–63. doi: 10.1111/j.0006-341X.2000.00455.x
27. Pathak P, Kapil U, Kapoor SK, Saxena R, Kumar A, Gupta N, et al. Prevalence of multiple micronutrient deficiencies amongst pregnant women in a rural area of Haryana. Indian J Pediatr. (2004) 71(11):1007–14. doi: 10.1007/BF02828117
28. Zimmermann MB, Burgi H, Hurrell RF. Iron deficiency predicts poor maternal thyroid status during pregnancy. J Clin Endocrinol Metab. (2007) 92:3436–40. doi: 10.1210/jc.2007-1082
29. Jaiswal N, Melse-Boonstra A, Thomas T, Basavaraj C, Sharma SK, Srinivasan K, et al. High prevalence of maternal hypothyroidism despite adequate iodine status in Indian pregnant women in the first trimester. Thyroid. (2014) 24(9):1419–29. doi: 10.1089/thy.2014.0071
30. Joshi K. Early gestation screening of pregnant women for iodine deficiency disorders and iron deficiency in urban centre in Vadodara, Gujarat, India. J Dev origins Health Dis. (2014) 5:63–8. doi: 10.1017/S2040174413000470
31. Refaat B. Prevalence of pregnancy induced thyroid dysfunction and the characteristics of the associated anaemia in primigravida Saudi women during the first trimester: A cross-sectional study. Gazzetta Med Italiana Archivio per le Sci Mediche. (2014) 173:567–78.
32. Yu X, Shan Z, Li C, Mao J, Wang W, Xie X, et al. Iron deficiency, an independent risk factor for isolated hypothyroxinemia in pregnant and nonpregnant women of childbearing age in China. J Clin Endocrinol Metab. (2015) 100(4):1594–601. doi: 10.1210/jc.2014-3887
33. Li S, Gao X, Wei Y, Zhu G, Yang C. The relationship between iron deficiency and thyroid function in Chinese women during early pregnancy. J Nutr Sci Vitaminol (Tokyo). (2016) 62(6):397–401. doi: 10.3177/jnsv.62.397
34. Veltri F, Decaillet S, Kleynen P, Grabczan L, Belhomme J, Rozenberg S, et al. Prevalence of thyroid autoimmunity and dysfunction in women with iron deficiency during early pregnancy: is it altered? Eur J Endocrinol. (2016) 175(3):191–9. doi: 10.1530/EJE-16-0288
35. Baghel M, Batra J, Thimmaraju KV, Itagappa M, Modala S, Baghel JS. Thyroid status, iron, folic acid and vitamin B12 levels in pregnancy. IOSR J Dent Med Sci. (2017) 16:01–3. doi: 10.9790/0853-1603060103
36. Fu J, Yang A, Zhao J, Zhu Y, Gu Y, Xu Y, et al. The relationship between iron level and thyroid function during the first trimester of pregnancy: A cross-sectional study in Wuxi, China. J Trace Elements Med Biol. (2017) 43:148–52. doi: 10.1016/j.jtemb.2017.01.004
37. Jiskani SA, Muneer S, Atzaz N. Iron deficiency anemia during pregnancy predicts poor maternal thyroid status. (2017) 2(2):13–16.
38. He L, Shen C, Zhang Y, Chen Z, Ding H, Liu J, et al. Evaluation of serum ferritin and thyroid function in the second trimester of pregnancy. Endocr J. (2018) 65(1):75–82. doi: 10.1507/endocrj.EJ17-0253
39. Zhang HY, Teng XC, Shan ZY, Wang ZJ, Li CY, Yu XH, et al. Association between iron deficiency and prevalence of thyroid autoimmunity in pregnant and non-pregnant women of childbearing age: a cross-sectional study. Chin Med J (Engl). (2019) 132(18):2143–9. doi: 10.1097/CM9.0000000000000409
40. Iqbal S, Rust P, Weitensfelder L, Ali I, Kundi M, Moshammer H, et al. Iron and iodine status in pregnant women from a developing country and its relation to pregnancy outcomes. Int J Environ Res Public Health. (2019) 16(22):4414. doi: 10.3390/ijerph16224414
41. Mogahed MM, El Sayed Amer E, El-Awady MA. Maternal thyroid status and its relation to ferritin and vitamin B12 in Saudi pregnant women. Egypt J Internal Med. (2019) 31:129–35. doi: 10.4103/ejim.ejim_107_18
42. Supadmi S, Kusrini I, Kusumawardani HD. The influence of iron depletion and chronic energy deficiency on the risk of hypothyroidism in pregnant women living in iodine deficiency disorders endemic areas in Badegan Ponorogo district east Java, Indonesia. J Nutr Sci Vitaminol. (2020) 66:S456–62. doi: 10.3177/jnsv.66.S456
43. Zhang Y, Huang X, Chen Z, Yang Q, Li X, Zhang R, et al. Iron deficiency, a risk factor for thyroid autoimmunity during second trimester of pregnancy in China. Endocr Pract. (2020) 26(6):595–603. doi: 10.4158/EP-2019-0220
44. Bulunc NH, Yildiz E. The relationship between biochemical and hemoglobin results and quality index scores of the mediterranean diet of pregnant women in the first and the third trimester. Prog Nutr. (2021) 23(4):e2021197. doi: 10.23751/pn.v23i4.11333
45. Syeda Farha S, Urooj A. Urinary Iodine Concentration as an indicator of Iodine status and its Correlation with the Thyroid hormones and Hemoglobin levels in first trimester pregnant women-An Exploratory Study. Curr Res Nutr Food Sci. (2021) 9:791–9. doi: 10.12944/CRNFSJ.9.3.07
46. Hamed RM, Nori W, Hussein ZA. Can serum ferritin predict thyroid performance in the first trimester? J Pak Med Assoc. (2021) 71:S3–s7.
47. Hassan N, Yousfani S, Sheikh N, Memon R, Sheeraz S, Sultana F, et al. Frequency of thyroid disorder with iron deficiency anemia in pregnancy. Pakistan J Med Health Sci. (2021) 15:1466–8.
48. Moreno-Reyes R, Corvilain B, Daelemans C, Wolff F, Fuentes Peña C, Vandevijvere S. Iron deficiency is a risk factor for thyroid dysfunction during pregnancy: A population-based study in Belgium. Thyroid. (2021) 31(12):1868–77. doi: 10.1089/thy.2021.0286
49. Nie GY, Wang R, Liu P, Li M, Sun DJ. Mild anemia may affect thyroid function in pregnant Chinese women during the first trimester. Front Endocrinol. (2021) 12:772917. doi: 10.3389/fendo.2021.772917
50. Wu WX, Lu J, Ruan X, Ma C, Lu W, Luo Y, et al. Maternal essential metals, thyroid hormones, and fetal growth: Association and mediation analyses in Chinese pregnant women. J Trace Elements Med Biol. (2021) 68:126809. doi: 10.1016/j.jtemb.2021.126809
51. Delcheva G, Maneva A, Deneva T, Bivolarska A. Association between iron and thyroid status in pregnant women. J IMAB - Annu Proceed (Scientific Papers). (2022) 28:4194–201. doi: 10.5272/jimab.2022281.4194
52. Hamza MA. Lymphocytes Prediction of Homeostasis Model Assessment of Beta-cells Function (HOMA-B) and C-peptide Level during Pregnancy: New Insight into Beta-cells Proliferation and Insulin Sensitivity. Baghdad Sci J. (2022) 19:821–8. doi: 10.21123/bsj.2022.19.4.0821
53. Sharifi N, Ebrahimiyan A, Mardani F, Erfanpoor S, Rahmani R. The relationship between complete blood count parameters with thyroid stimulating hormone levels in pregnant women. Iranian J Obstet Gynecol Infertil. (2022) 25:77–82.
54. Wang F, Zhang Y, Yuan Z, Li Y, Liu S, Zeng X, et al. The association between iron status and thyroid hormone levels during pregnancy. J Trace Elem Med Biol. (2022) 74:127047. doi: 10.1016/j.jtemb.2022.127047
55. Geissler C, Margen S, Calloway DH. Lactation and pregnancy in Iran. III. Hormonal factors Am J Clin Nutr. (1979) 32:1097–111. doi: 10.1093/ajcn/32.5.1097
56. Price A, Obel O, Cresswell J, Catch I, Rutter S, Barik S, et al. Comparison of thyroid function in pregnant and non-pregnant Asian and western Caucasian women. Clin Chim Acta. (2001) 308(1–2):91–8. doi: 10.1016/S0009-8981(01)00470-3
57. Larsson A, Palm M, Hansson LO, Axelsson O. Reference values for clinical chemistry tests during normal pregnancy. BJOG: Int J Obstet Gynaecol. (2008) 115(7):874–81. doi: 10.1111/j.1471-0528.2008.01709.x
58. Mahajan S, Aalinkeel R, Shah P, Singh S, Gupta N, Kochupillai N. Nutritional anaemia dysregulates endocrine control of fetal growth. Br J Nutr. (2008) 100(2):408–17. doi: 10.1017/S000711450889438X
59. DeZoysa G. Effect of iodine and iron status during pregnancy on maternal and neonatal thyroid functions: A prospective cohort study in Bope-Poddala health division. (2016) 4.
60. Rosario PW, Oliveira LFF, Calsolari MR. Maternal hypothyroxinemia in the first trimester of gestation and association with obstetric and neonatal outcomes and iron deficiency: a prospective Brazilian study. Arch Endocrinol Metab. (2018) 62:332–6. doi: 10.20945/2359-3997000000043
61. Chen HM, Kuo FC, Chen CC, Wu CF, Sun CW, Chen ML, et al. New trimester-specific reference intervals for clinical biochemical tests in Taiwanese pregnant women-cohort of TMICS. PloS One. (2020) 15(12):e0243761. doi: 10.1371/journal.pone.0243761
62. Mahadik K, Choudhary P, Roy PK. Study of thyroid function in pregnancy, its feto-maternal outcome; a prospective observational study. BMC Pregnancy Childbirth. (2020) 20. doi: 10.1186/s12884-020-03448-z
63. Novakovic TR, Dolicanin ZC, Babic GM, Djordjevic NZ. The maternal leucocytes in thrombophilia and hypothyroidism and their influence on fetal cells. Serbian J Exp Clin Res. (2020) 21:217–23. doi: 10.2478/sjecr-2018-0022
64. Chowdhury MD, Ghose S, Mohan A. Relationship between thyroid disorder and iron deficiency anaemia in pregnancy. (2021) 7(2):163–67. doi: 10.21276/obgyn.2021.7.2.10
65. Lisowska-Myjak B, Strawa A, Zborowska H, Jakimiuk A, Skarżyńska E. Associations between the thyroid panel and serum protein concentrations across pregnancy. Sci Rep. (2021) 11(1):15970. doi: 10.1038/s41598-021-94358-3
66. Noshiro K, Umazume T, Inubashiri M, Tamura M, Hosaka M, Watari H. Association between Edinburgh postnatal depression scale and serum levels of ketone bodies and vitamin D, thyroid function, and iron metabolism. Nutrients. (2023) 15(3):768. doi: 10.3390/nu15030768
67. Teng X, Shan Z, Li C, Yu X, Mao J, Wang W, et al. Iron deficiency may predict greater risk for hypothyroxinemia: A retrospective cohort study of pregnant women in China. Thyroid. (2018) 28(8):968–75. doi: 10.1089/thy.2017.0491
68. Hua J, Shen J, Zhang J, Zhou Y, Du W, Williams GJ. The association between COVID-19 pandemic and maternal isolated hypothyroxinemia in first and second trimesters. Psychoneuroendocrinology. (2021) 128:105210. doi: 10.1016/j.psyneuen.2021.105210
69. Zhu P, Chu R, Pan S, Lai X, Ran J, Li X. Impact of TPOAb-negative maternal subclinical hypothyroidism in early pregnancy on adverse pregnancy outcomes. Ther Adv Endocrinol Metab. (2021) 12:20420188211054690. doi: 10.1177/20420188211054690
70. Hu L, Pei Y, Luo X, Wen L, Xiao H, Liu J, et al. A multivariate modeling method for the prediction of low fetal fraction before noninvasive prenatal testing. Sci Prog. (2021) 104(4):368504211052359. doi: 10.1177/00368504211052359
71. Georgieff MK. Iron deficiency in pregnancy. Am J obstet gynecol. (2020) 223:516–24. doi: 10.1016/j.ajog.2020.03.006
72. Wopereis MK, Du Puy RS, Van Heemst D, Walsh JP, Bremner A, Bakker SJ, et al. The relation between thyroid function and anemia: A pooled analysis of individual participant data. J Clin Endocrinol Metab. (2018) 103(10):3658–67. doi: 10.1210/jc.2018-00481
73. Zhang L, Li Y, Yang L, Luo Z, Wu Z, Wang J, et al. Inverse association between serum iron levels and Hashimoto’s thyroiditis in United States females of reproductive age: analysis of the NHANES 2007-2012. Front Nutr. (2024) 11:1410538. doi: 10.3389/fnut.2024.1410538
74. Garofalo V, Condorelli RA, Cannarella R, Aversa A, Calogero AE, La Vignera S. Relationship between iron deficiency and thyroid function: A systematic review and meta-analysis. Nutrients. (2023) 15(22):4790. doi: 10.3390/nu15224790
75. Lynch S. The rationale for selecting and standardizing iron status indicators. Geneva Switzerland: World Health Organ. (2012), 56–62.
76. Otto JM, Montgomery HE, Richards T. Haemoglobin concentration and mass as determinants of exercise performance and of surgical outcome. Extrem Physiol Med. (2013) 2:33. doi: 10.1186/2046-7648-2-33
77. Knovich MA, Storey JA, Coffman LG, Torti SV, Torti FM. Ferritin for the clinician. Blood Rev. (2009) 23(3):95–104. doi: 10.1016/j.blre.2008.08.001
78. Ozer A, Bruick R. Non-heme dioxygenases: cellular sensors and regulators jelly rolled into one? Natural Chem Biol. (2007) 3:144–53. doi: 10.1038/nchembio863
79. Rouault TA. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Natural Chem Biol. (2006) 2:406–14. doi: 10.1038/nchembio807
Keywords: hemoglobin, iron, pregnancy, serum ferritin, thyroid hormones
Citation: Parsaei M, Dashtkoohi M, Amirkhalili E, Chashmyazdan M, Korevaar TIM and Nazeri P (2025) Association of iron status indicators with thyroid hormone concentrations during pregnancy: a systematic review and meta-analysis. Front. Endocrinol. 16:1533169. doi: 10.3389/fendo.2025.1533169
Received: 23 November 2024; Accepted: 06 January 2025;
Published: 28 January 2025.
Edited by:
Miloš Žarković, University of Belgrade, SerbiaReviewed by:
Mirjana D. Stojkovic, University of Belgrade, SerbiaCopyright © 2025 Parsaei, Dashtkoohi, Amirkhalili, Chashmyazdan, Korevaar and Nazeri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pantea Nazeri, bmF6ZXJpLnBhbnRlYUBnbWFpbC5jb20=; Tim I. M. Korevaar, dC5rb3JldmFhckBlcmFzbXVzbWMubmw=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.