
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 21 February 2025
Sec. Clinical Diabetes
Volume 16 - 2025 | https://doi.org/10.3389/fendo.2025.1529447
Aims: To investigate the effect of early childhood infections and antibiotic use on the risk of type 1 diabetes in a general population cohort.
Research Design and Methods: The All Babies In Southeast Sweden (ABIS) cohort followed 16 428 children from birth. Questionnaires collected at 1 year (n=11 093), 3 years (n=8 890) and 5 years of age (n=7 445) included data on infections and antibiotic use and were validated against national registers. After a mean follow-up of 25 years, 168 individuals have been diagnosed with type 1 diabetes (1.0% of the original cohort, aged 1-24.5 years).
Results: There were few significant differences in type or frequency of early childhood infections or antibiotic use between cases with type 1 diabetes and the reference group (remaining individuals who did not develop type 1 diabetes) after adjusting for sex, heredity and socioeconomic status. A small number of type 1 diabetes children (4.8% compared to 0.8% of the reference group) reported six or more episodes of gastroenteritis in the 1-3-year age group, resulting in an adjusted odds ratio (aOR) of 8.21; 95% CI 2.70-25.01, p<0.001. Cases of type 1 diabetes with an increased genetic risk (n=91) reported fewer episodes of the common cold between 1 and 3 years of age compared to the reference group (aOR 0.27; 0.13-0.58, p<0.001). Individuals with type 1 diabetes without risk-associated HLA alleles (n=14) reported a higher frequency of pneumonia in the 1–3- and 3–5-year age group (aOR 26.08; 6.29-108.17, p<0.001 and aOR 35.63; 4.10-309.96, p=0.001 respectively), and had more viral and total infections registered in the National Patient Register from 0-5 years (aOR 5.72; 1.59-20.57, p=0.008 and aOR 18.71; 1.95-179.55, p=0.01).
Conclusions: Childhood infections could increase the risk of developing type 1 diabetes in a small group of individuals without risk-associated HLA alleles, but this was not seen in the majority with HLA-risk. More research is required for this overlooked population, including screening and prevention trials. The association to frequent gastrointestinal infections in the first years of life needs to be reproduced in other studies to be confirmed.
The incidence of type 1 diabetes has increased greatly over the past 40-50 years (1), with a peak in global incidence around 10-14 years of age (2). The genetic risk of type 1 diabetes is mainly due to HLA class II genotypes but the incidence of type 1 diabetes in individuals without high HLA-risk has increased (3), and populations of similar genetic backgrounds like Finland and Russian Karelia have shown an age-adjusted six-fold incidence gradient (4).
Several large, prospective cohort studies designed to study environmental risk factors have not found any conclusive evidence, but early exposures like diet (5) and psychosocial stress (6–8) have been suggested. Accumulating evidence points towards a possible interaction between early childhood infections and risk of type 1 diabetes. Prospective studies of mainly genetically at-risk children have found evidence of respiratory tract infections caused by enteroviruses (9, 10) and more recently SARS-CoV-2 (11) preceding onset of overt type 1 diabetes. Gastroenteritis requiring hospitalization during the first 18 months of life has also been reported as a risk factor (12). Viruses have been isolated from the pancreas of patients with new-onset type 1 diabetes (13, 14). We have previously found that respiratory tract infection in early pregnancy increases the risk of childhood type 1 diabetes in the general population (15), and enterovirus infection during pregnancy has been linked to increased risk of childhood-onset type 1 diabetes (16) indicating development of autoimmunity after exposure to viral antigens in utero. The “fertile field” hypothesis suggests that repeated infections in childhood create a proinflammatory cytokine environment that could activate autoreactive T-cells in genetically predisposed individuals (17).
The gut microbiome plays an important role in immune system maturation, and the interaction between potential environmental risk factors like diet, breastfeeding (18) and antibiotic use (19), and the activation of autoreactive immune cells through the important barrier function of the gut and the microbiome composition itself is complex (20). In fact, antibiotics could be an important mediator between environmental risks and autoimmunity as it is both associated with alterations in a healthy gut microbiome, as well as a marker for primarily bacterial infections. For instance, early-life exposures to antibiotic treatment has been linked to other diseases like juvenile idiopathic arthritis (21), asthma (22), and neurodevelopmental disorders like autism and ADHD (23). The “hygiene hypothesis” proposes that a decreased microbial load in the environment could trigger the maturing immune system to overreact to otherwise harmless microorganisms (24), while simultaneously decreasing the herd immunity for other potentially harmful microbes (25). Increased hygiene also affects the gut microbiota (26). In a recent study, we found an association between gut microbiome composition in early childhood and development of type 1 diabetes later in life (27), and we have previously described an association between genetic risk for autoimmunity and microbiome composition at 1 year of age (28). As most observational studies only include children with an increased genetic risk, knowledge about individuals without risk-associated HLA alleles is lacking. We hypothesize that gastrointestinal infections and use of antibiotics early in life might play a special role for later development of type 1 diabetes, and that a possible association between childhood infection and type 1 diabetes could be modified by genetic risk of autoimmunity.
The aim of this study was therefore to investigate if type and frequency of childhood infections, especially gastrointestinal infections (“gastroenteritis”), and antibiotic use could affect the risk of type 1 diabetes in a general non-selected population cohort.
All data was derived from the large prospective population-based cohort study All Babies in Southeast Sweden (ABIS). ABIS included children born in Southeast Sweden from October 1st 1997 to October 1st 1999 and they have been followed from birth until adulthood. At birth, the parents of 78.6% of newborn children chose to participate (17 055 of 21 700), of whom we had useful questionnaires from 16 428. In addition to extensive questionnaires, biological samples were collected at each follow-up. See flowchart for details (Figure 1). Details about the study layout have been published elsewhere (29); see www.abis-studien.se.
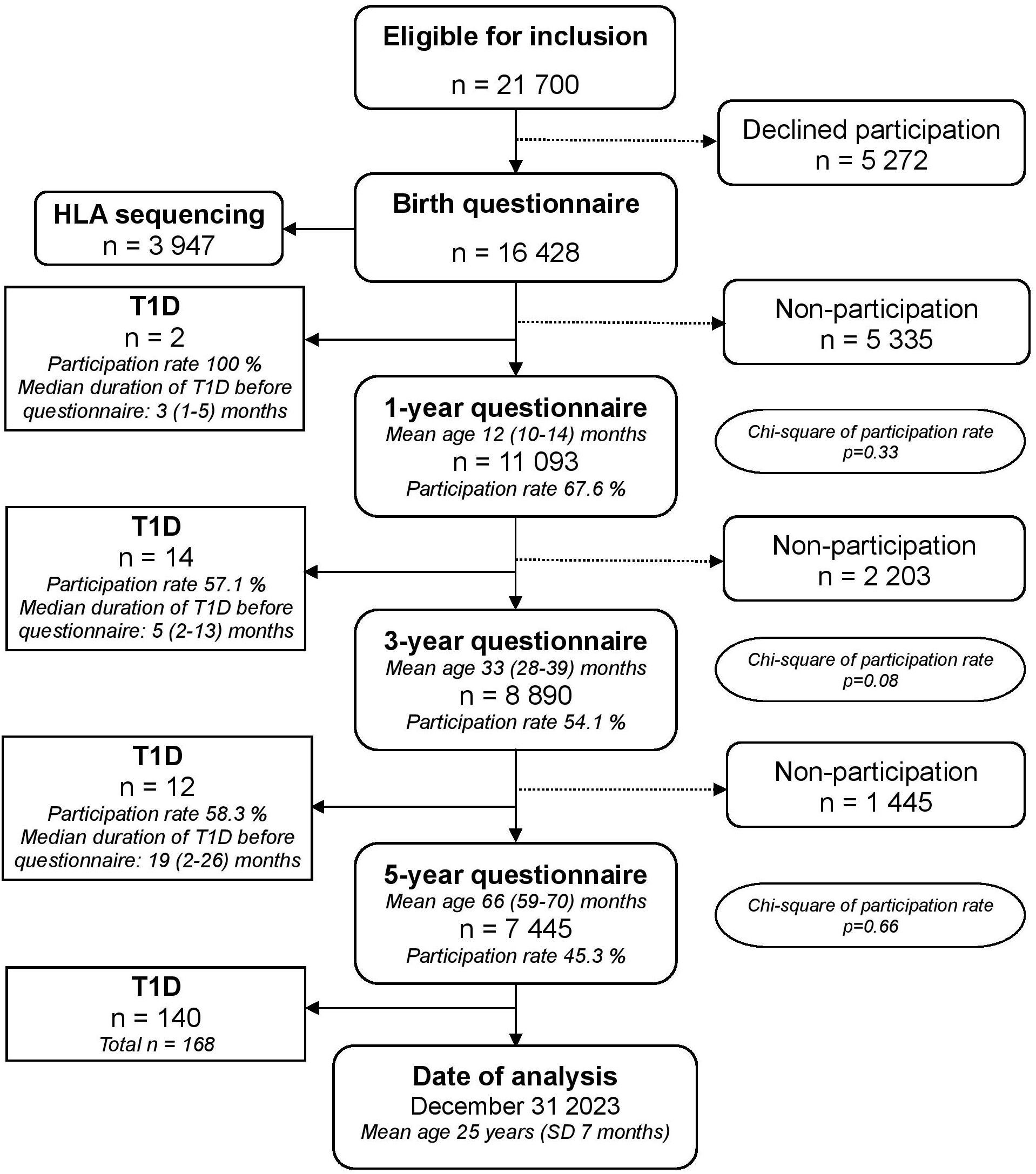
Figure 1. Flow chart of the ABIS birth cohort and participation in the 1-, 3- and 5-year questionnaires. All families participating in the birth questionnaire were invited to participate in all subsequent follow-ups, regardless of participation in previous questionnaires. Mean age at each questionnaire include 95 % confidence intervals. Median duration of type 1 diabetes before participation in the following questionnaire include range in months. Chi-square tests were used to compare participation rates for type 1 diabetes and reference group for each questionnaire, p-values for each questionnaire are included in the flow chart.
For this study, questionnaires collected at 1, 3 and 5 years of age were used. Each questionnaire retrospectively covered the period from the previous questionnaire. The questionnaires were answered by the child’s parents or caregivers and included, but was not restricted to, questions on diet, infections, drugs, physical activity, psychosocial situation, living situation and other exposures early in life. Before participating in the study, carers were informed of the aim to investigate environmental risk factors for immune-mediated diseases, but not of specific hypotheses about frequency of infections and type 1 diabetes.
Carers reported frequency of infections occurring during the period between two questionnaires in intervals of “none”, “1-2”, “3-5” or “6 or more”. Data on specific age at each infection, severity and duration of infections or associated symptoms (i.e. fever) were not available for analysis.
Beside data on infections from the questionnaires, The Swedish National Patient Register (NPR) was used to collect data on infections registered from hospital admissions and specialized outpatient health care visits from birth to 5 years of age for all children enrolled in ABIS. These visits were initiated by the families themselves and represent acute infections. The NPR data was restricted to date of visit and ICD-10 diagnostic codes; information on duration and associated symptoms were not available in the NPR. The NPR does not include data from primary health care visits. The ICD-10 diagnostic codes were used to manually categorize infections into respiratory tract infections, gastroenteritis, urinary tract infection, and unspecified viral infections. All infections from the questionnaires and the NPR were further categorized into either bacterial or viral according to most likely infectious agent, i.e. influenza was categorized as viral while pneumonia, acute otitis media and urinary tract infection were categorized as bacterial. Gastroenteritis was categorized as viral unless otherwise stated in the ICD-10 code. As the reported frequency of the common cold was extremely high for all age groups (>99%), this infection was not included in the analysis of viral infections from the questionnaires. The ICD-10 codes were reviewed to exclude duplicates registered for the same infection dating within 14 days of the first code, i.e. a tonsillitis with a follow-up visit registered within a few days with a similar diagnostic code would count as the same respiratory tract infection both visits. Initial analyses of data from the NPR were performed by combining all registered infections in each category as “1 or more registered infections” compared to “No infections”. If possible, infections were then binned into 3 categories: “No registered infections”, “1-2 registered infections” and “3 or more registered infections”.
A diagnosis of type 1 diabetes was identified via the Swedish National Patient Register (NPR) and The Swedish Childhood Diabetes Registry (SWEDIABKIDS) and validated by the Swedish National Drug Prescription Register for prescription of insulin. Age in months at onset of diabetes was calculated based on date of birth and date of diagnosis as registered in the NPR, SWEDIABKIDS, and/or date of first insulin prescription.
Genetic risk of type 1 diabetes was determined by HLA genotyping and categorized according to presence of common European HLA-DR-DQ haplotypes associated with the risk of autoimmunity (30). Increased or high genetic risk was defined as the presence of one or two risk-associated HLA haplotypes: (DR3)-DQA1*05-DQB1*02 with no protective haplotypes, and/or (DR4)-DQA1*03-DQB1*0302 with or without protective haplotypes. A neutral or low genetic risk was defined as the presence of (DR3)-DQA1*05-DQB1*02 in combination with one of the protective haplotypes or the absence of any risk-associated haplotypes with one or two protective haplotypes: (DR15)-DQB1*0602, (DR13)-DQB1*0603, (DR5)-DQA1*05-DQB1*0301 and (DR7)-DQA1*03-DQB1-0303.
The data was stored in a common database and statistically analyzed using the SPSS 29.0 program (IBM Corp., Armonk, NY: USA).
An adjusted odds ratio (aOR) and 95% confidence intervals (CI) were estimated for each explanatory variable by multiple logistic regression analysis, adjusting for interaction with sex, family history of type 1 diabetes and socioeconomic status. Socioeconomic status (SES) defined as maternal education level at birth was included as confounder due to previously reported associations with type 1 diabetes in this cohort (31). Basic characteristics of the cohort were analyzed using Chi2 tests.
The primary hypothesis tested was whether exposure to infections or antibiotics during early childhood affected the risk of type 1 diabetes in the general population. The initial analyses were based on age (birth to 1 year, 1 to 3 years, 3 to 5 years), type of infection, likely infectious agent (virus or bacteria) and use of antibiotics. Secondary analyses were performed based on HLA risk group, age at onset of type 1 diabetes, and sex, as boys have previously been described as more susceptible to infections. A cut-off age of 10.5 years for girls and 11.5 years for boys was chosen to distinguish onset of type 1 diabetes from before and after the start of puberty (32), as exposure to early childhood infections is hypothesized to have a greater impact on the progress to overt disease in younger children. In the subgroup analyses based on sex, the variable “sex” was removed as a confounder from the logistic regression. To adjust for multiple testing, the Benjamini & Hochberg procedure was performed to determine significance using a false discovery rate (FDR) of 0.05. Infections reported after onset of type 1 diabetes were excluded. In cases where questionnaires were returned within ≤3 months after diagnosis (n=5), reported infections from that questionnaire were still included in the analyses due to the inability to pinpoint exact timing of infections in relation to onset of disease, and risk of losing valuable information about the period preceding overt type 1 diabetes.
Written and oral information about the project was given to the parents already during pregnancy, and then repeated at the maternity ward after birth and at the well-baby checkups. Participating families were also offered the opportunity to watch a video about the study. Informed consent was given when the parents or guardians returned the questionnaires at birth, 1, 3 or 5 years or delivered biological samples.
The ABIS project was approved by the Research Ethics Committees of the Faculty of Health Science at the University of Linköping, Linköping, Sweden (Dnr-96-287, Dnr-99-321 and Dnr-03-092) and the Medical Faculty at the University of Lund, Lund, Sweden (LU 83-97). Linking the ABIS registers to the NPR was approved by the Research Ethics Committee in Linköping (Dnr-03-513 and Dnr-2018/380-32).
The datasets generated and/or analyzed during the current study are available from the last author on reasonable request after ethical approval.
As of December 31st 2023, 168 individuals from the original cohort had developed type 1 diabetes (1.0%), 56.0% males and 44.0% females compared to 51.8% males and 48.2% females in the reference group without type 1 diabetes. With a mean follow-up of 25 years 2 months (SD 7 months), 168 cases of type 1 diabetes correspond to an incidence rate of 40.9/100 000 individuals/year. HLA genotyping data was available for 3947 study participants. In the HLA-typed group with type 1 diabetes (n=105), 86.7% were categorized as having a high or increased genetic risk, compared to 38.3% of the reference group (p<0.001). Having a first-degree relative with type 1 diabetes was associated with an 11-fold increased risk of developing type 1 diabetes (OR 11.47; 95% CI 6.78-19.40, p<0.001). Low and intermediate maternal education at birth was associated with an increased risk of type 1 diabetes compared to high maternal education, defined as International Standard Classification of Education (ISCED) level I-IV compared to level V-VII (Table 1).
Age at diagnosis of type 1 diabetes ranged from 12 months to 24 years 6 months, with a mean age at diagnosis of 12 years 3 months. Mean age at diagnosis differs almost 1.5 years between females (11 years, 5 months) and males (12 years, 10 months) due to a large overrepresentation of males with an onset of type 1 diabetes after 17 years (75% males). Having a neutral or decreased genetic risk of type 1 diabetes was associated with a mean age at diagnosis 3.5 years later than those with a high or increased genetic risk (13 years, 9 months compared to 10 years, 2 months, Table 2).
The common cold was by far the most common infection in all age categories with no difference in frequency between cases of type 1 diabetes and the reference group (Table 3). The other respiratory tract infections analyzed (otitis media, tonsillitis, pneumonia, influenza) did not differ between the groups (Supplementary Table 1). Infections primarily interpreted as either bacterial or viral were reported similarly for both cases of type 1 diabetes and the reference group for all age groups, and there were no significant differences in reported number of antibiotic treatments between the groups (Table 3, Figure 2).
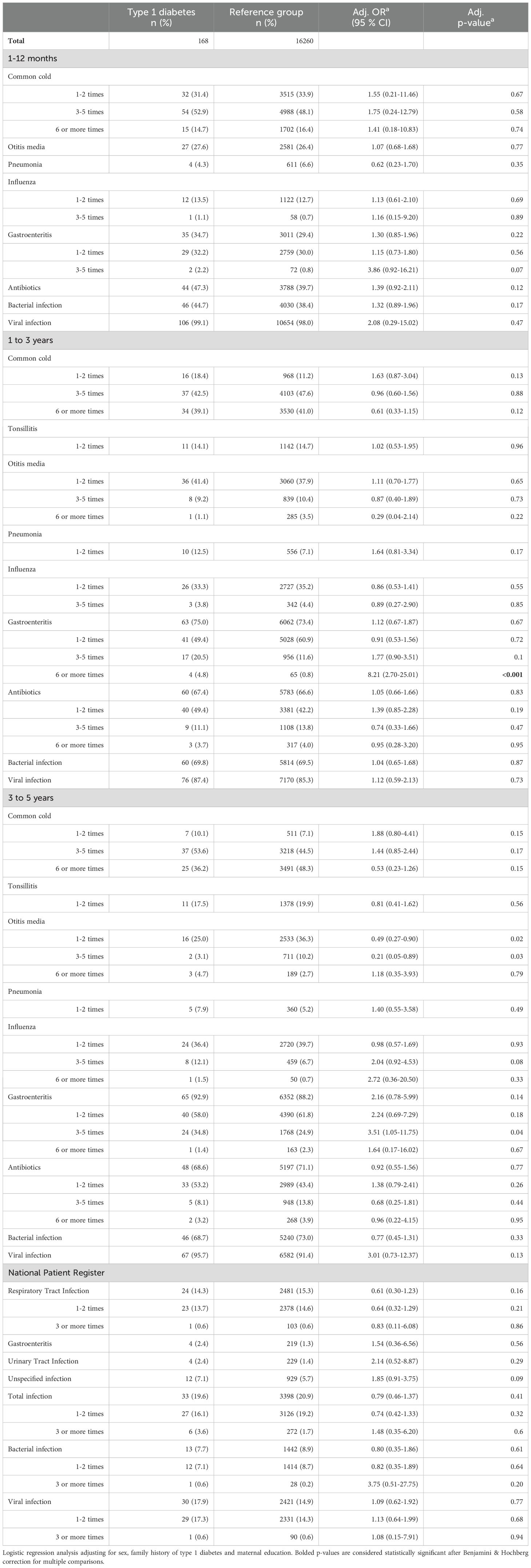
Table 3. Infections during the first 5 years of life in individuals with type 1 diabetes compared to a reference group without type 1 diabetes.
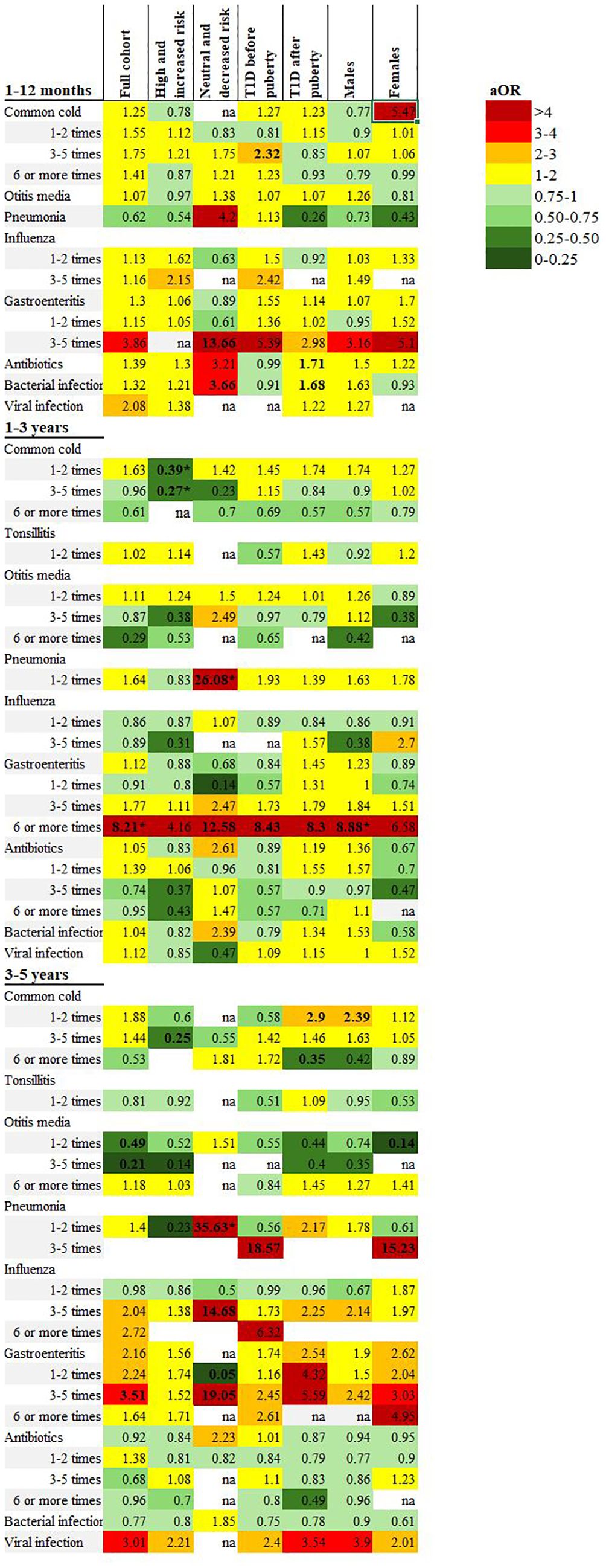
Figure 2. Heatmap illustrating adjusted odds ratios of associations between early childhood infections reported in the 1-, 3- and 5-year questionnaires and subgroup analyses including the full cohort, high and increased genetic risk, neutral and decreased genetic risk, onset of type 1 diabetes before and after puberty and males/females. All included analyses were adjusted for sex, family history of type 1 diabetes and maternal education. Bolded text indicates CI below/above 1. *Statistically significant after Benjamini & Hochberg correction for multiple comparisons.
Six or more episodes of gastroenteritis in the 1-3-year age group was reported for 4.8% of children with type 1 diabetes compared to 0.8% of the reference group (adjusted odds ratio (aOR) 8.21; 2.70-25.01, p<0.001). Of the children with a diagnosis of type 1 diabetes before five years of age (n=26), half (n=13) had reported one or more episodes of gastroenteritis after the onset of diabetes. These episodes were excluded from the analyses.
Most recorded infections from the NPR in the first five years of life were categorized as respiratory tract infections (66.8%), with unspecified viral infections the second most common (22.1%). The total number of registered infections was lower than the reported number of infections in the questionnaires. Only 20.9% of ABIS children had one or more registered infections from ages 0-5 years in the NPR. Of these, 14.9% had infections categorized as viral and 8.9% had infections categorized as bacterial. There was no statistically significant difference in frequency of any type of infection between the ages of 0-5 for the cohort (Table 3, Figure 3).
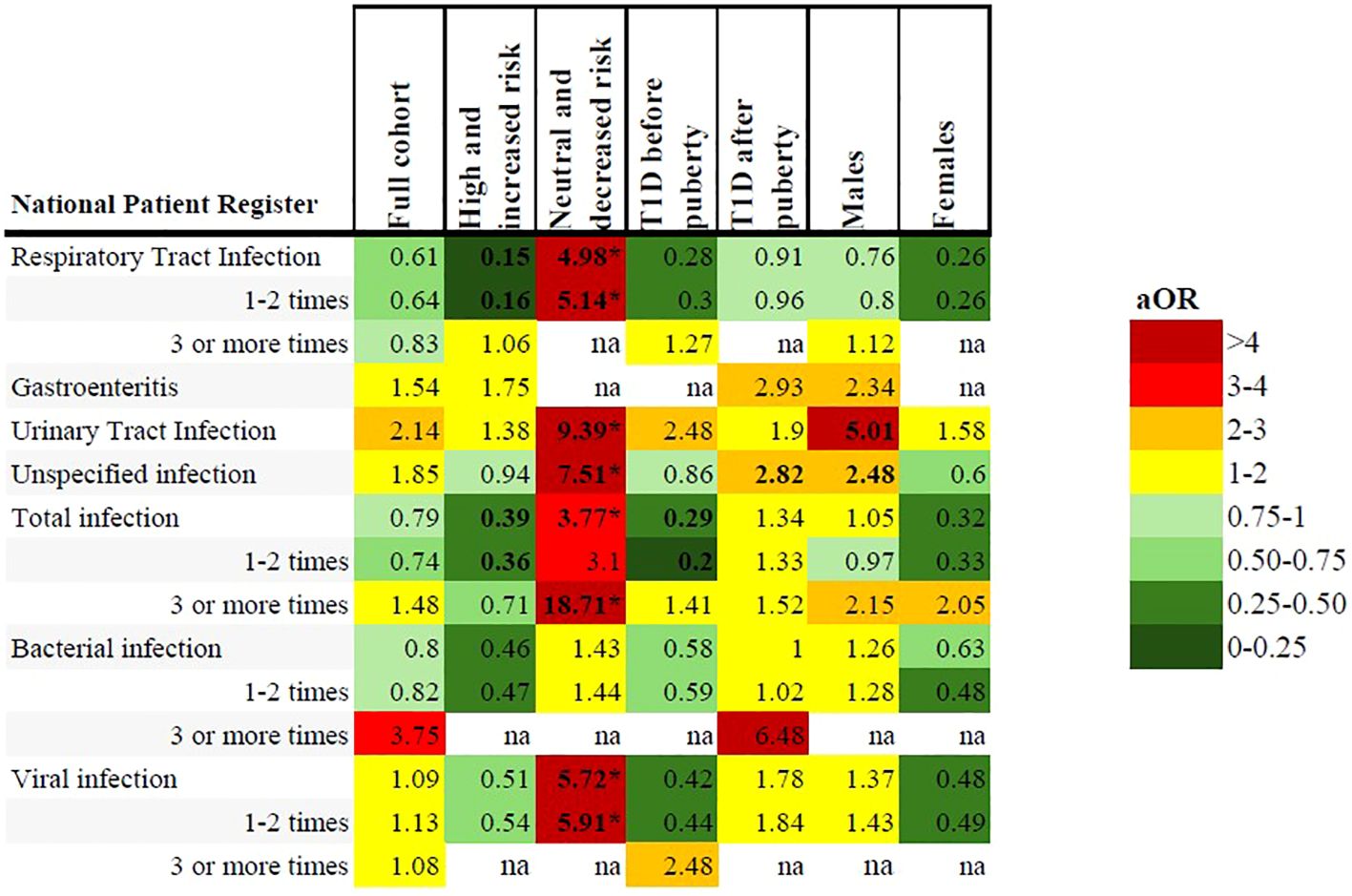
Figure 3. Heatmap illustrating adjusted odds ratios of associations between early childhood infections registered in the NPR and subgroup analyses including the full cohort, high and increased genetic risk, neutral and decreased genetic risk, onset of type 1 diabetes before and after puberty and males/females. All included analyses were adjusted for sex, family history of type 1 diabetes and maternal education. Bolded text indicates CI below/above 1. *Statistically significant after Benjamini & Hochberg correction for multiple comparisons.
Type 1 diabetes cases with a high or increased genetic risk (n=91, 86.7%) had significantly fewer episodes of the common cold compared to the reference group with the same genetic risk (n=1470, 38.3%). In the 1-3-year age group, 3-5 episodes of the common cold was reported for 31.3% of children with type 1 diabetes compared to 44.5% of the reference group (aOR 0.27; 0.13-0.58, p<0.001). There were no other significant associations with infections from the questionnaires.
In the NPR, respiratory tract infections were registered less frequently in the type 1 diabetes group (11.0% compared to 16.2% of the reference group), but this was not statistically significant after correcting for multiple comparisons, (Table 4, Figures 2 and 3 and Supplementary Table 2).
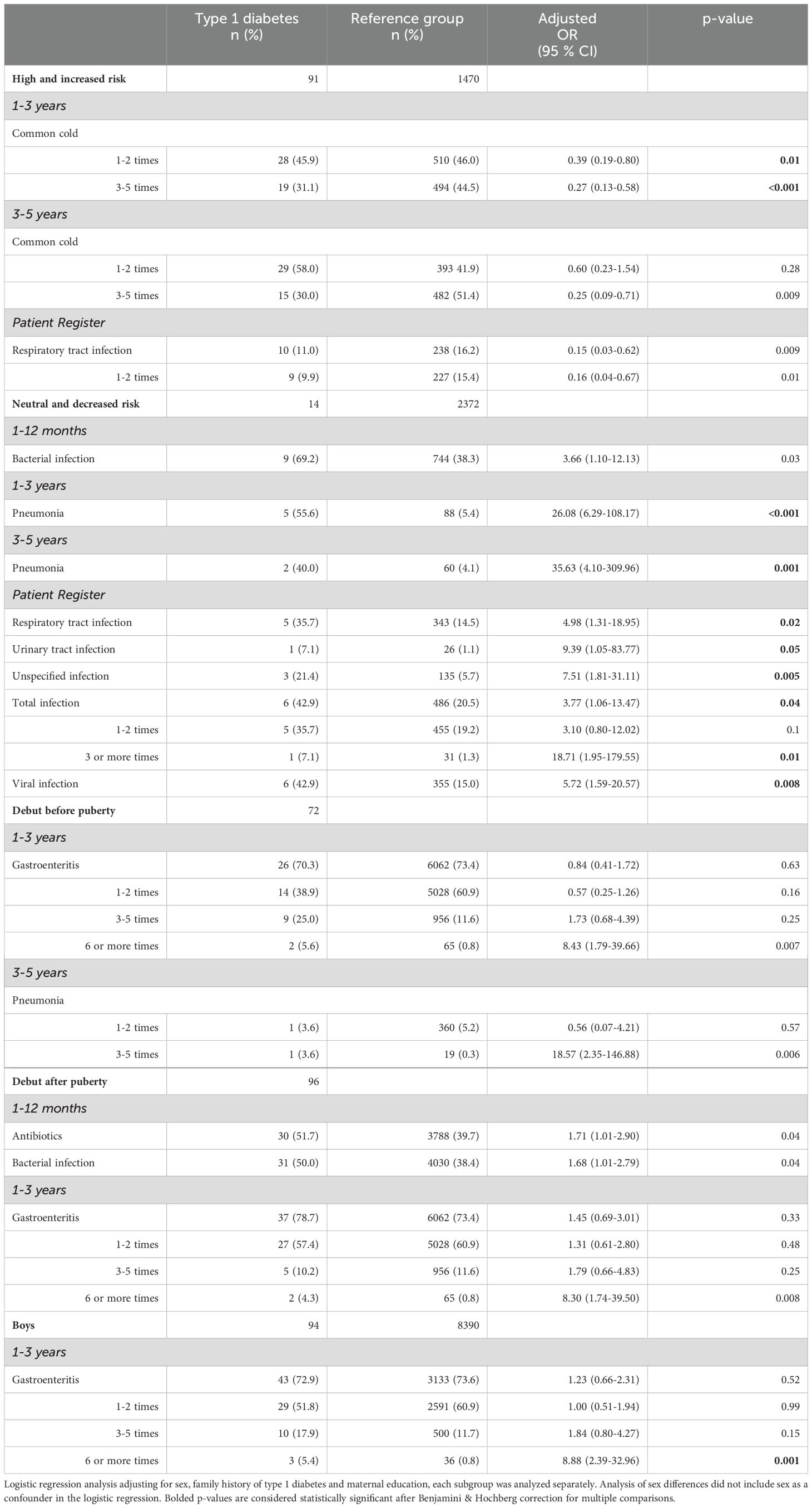
Table 4. Significant associations with infections in children with a diagnosis of type 1 diabetes from the subgroup analyses based on genetic risk of autoimmunity, onset of type 1 diabetes before or after puberty and sex.
In the type 1 diabetes group, 14 individuals had a neutral or decreased genetic risk of autoimmunity (13.3%), compared to 2372 of the reference group (61.7%). Pneumonia was reported more frequently for the type 1 diabetes group in all three age categories, statistically significant in the 1-3- and 3-5-year age group (aOR 26.08; 6.29-108.17, p<0.001, and 35.63; 4.10-309.96, p=0.001, respectively). In the 1-12-month age group, both antibiotics and bacterial infections were more frequently reported in the type 1 diabetes group (69.2% compared to 39.3%), though this association was not statistically significant.
In the NPR, children with type 1 diabetes had significantly more registered respiratory tract infections, urinary tract infections, unspecified infections, viral infections and total infections compared to the reference group. A total of three or more infections was associated with an aOR of 18.71 (1.95-179.55), p=0.01 (Table 4, Figures 2, 3, Supplementary Table 3).
A diagnosis of type 1 diabetes before puberty (n=72) was associated with both a self-reported six or more episodes of gastroenteritis in the 1-3 year age group (aOR 8.43; 1.79-39.66, p=0.007), and 3-5 episodes of pneumonia in the 3-5 year age group (aOR 18.57; 2.35-146.88, p=0.006) but neither association was significant after correcting for multiple comparisons and are likely due to small sample sizes. There were no other statistically significant differences in either the questionnaires nor the NPR for any other type of infection or antibiotic treatment (Table 3, Figures 2, 3, Supplementary Table 4).
Cases with an onset of type 1 diabetes after puberty (n=96) had an association with six or more episodes of gastroenteritis in the 1-3-year age group (aOR 8.30; 1.74-39.50, p=0.008), but this association was not significant after correcting for multiple comparisons. Antibiotics and infections categorized as bacterial were more frequently reported for the type 1 diabetes group (51.7% vs 39.7%, and 50.0% vs 38.4% respectively) in the 0-1-year age group, but this difference was also not statistically significant (Table 4, Figure 2, Supplementary Table 5).
There were no statistically significant associations with any registered infections in the NPR (Figure 3, Supplementary Table 5).
Males with type 1 diabetes (n=94) had a significant association with six or more episodes of gastroenteritis in the 1-3-year age group (reported for 5.4% of males with type 1 diabetes compared to 0.8% of reference males, aOR 8.88; 2.39-32.96, p=0.001). There were no significant associations with any other types of infection in either the questionnaires or the NPR (Table 4, Figures 2, 3, Supplementary Table 6).
Females with type 1 diabetes (n=74) had no significant association with any infection in the questionnaires or the NPR (Supplementary Table 7).
In general, there were no significant differences in type or frequency of reported infections in the ABIS questionnaires between cases with type 1 diabetes and the reference group without type 1 diabetes during the observed period of 0-5 years of age. However, in the 1-3-year age group, a reported six or more episodes of gastroenteritis were significantly associated with type 1 diabetes. This correlates with earlier findings of the importance of the microbiome at one year of age (27) and could represent a pathophysiological mechanism linking gastrointestinal infections and autoimmunity through an altered gut microbiome in early childhood. Instances of gastroenteritis reported after a diagnosis of type 1 diabetes were excluded from the analyses, mitigating the risk of a false association caused by an increased susceptibility of infections in patients with dysglycemia. It is important to note that only 4 children with type 1 diabetes reported 6 or more episodes of gastroenteritis in this age group. To determine the significance of this association, these results need to be replicated in a larger cohort of children with type 1 diabetes. Notably, there were no significant differences in number of antibiotic treatments between the groups for any age category, contradicting the theory of transient gut dysbiosis as an important promotor of the autoimmune process for this cohort. These findings align with results from another questionnaire-based study (12) as well as data from a prospective cohort of genetically at-risk children (33). Other register-based studies have found evidence of antibiotic treatments, especially broad-spectrum (19) or antibiotics prescribed for respiratory tract infections (34), in the first 1-2 years of life to increase the risk of childhood type 1 diabetes in children delivered by cesarean section but not in vaginally delivered children, implying a vulnerability to the gut-altering effects of antibiotics in only certain circumstances. Unfortunately, the analyses in this cohort did not specify use of broad-spectrum or narrow-spectrum antibiotics, and data from the Swedish Drug Register was not available during this period.
Some individuals with type 1 diabetes have no risk-associated HLA alleles, and environmental triggers initiating the autoimmune process in these cases are arguably more consequential. This was reflected in a later diabetes debut compared to children with a high genetic risk. In this group, both viral and bacterial infections, like pneumonia, were significantly associated with type 1 diabetes. The autoimmune process in these children is probably dependent on a series of environmental triggers as proposed by the “fertile field” hypothesis, and the unselected population in the ABIS cohort is uniquely suited to study these associations. This low-risk cohort is an often-overlooked population, as they are not usually included in screening programs or prevention trials, and they appear to have a different sensitivity to childhood environmental exposures, like infections, than high-risk children. More research is needed to confirm these associations, but the results imply that screening programs to detect early stages of type 1 diabetes could benefit from a general population design as opposed to screening only first-degree relatives of type 1 diabetes individuals. Individuals without risk-associated HLA alleles might even respond better to certain preventative therapies than high-risk individuals due to their decreased susceptibility to autoimmunity.
The subgroup analysis of high and increased risk individuals yields few significant associations between infections and development of type 1 diabetes. The reference group reported more episodes of the common cold than the type 1 diabetes group, in favor of the hygiene hypothesis (24) and contrary to results from the TEDDY study linking respiratory tract infections in early childhood to development of autoimmunity in genetically at-risk children (17). As the common cold is one of the most prevalent infections in early childhood, this could represent a decreased viral load negatively affecting the self-tolerance of the developing immune system (35). The TEDDY study has also reported bidirectional associations between gastrointestinal infections in early childhood and islet autoimmunity, where timing of the infection before or after 1 year of age either increased or decreased the risk of autoimmunity up to 10 years (36). As detection of islet autoantibodies was not included in this study, a similar analysis could not be performed for this cohort. It is possible that the timing of the questionnaires was too infrequent to detect significant associations in this group.
Despite the theory that early childhood infections could have a greater impact on the risk of developing type 1 diabetes before puberty, the results were similar for both onset before and after puberty. After adjusting for multiple testing, there were no differences in reported number or type of infections or antibiotics compared to the reference group.
Males with frequent episodes of gastroenteritis in early childhood had an increased risk of type 1 diabetes like the overall cohort, while females with type 1 diabetes had no significant associations with gastroenteritis or any other infections in any age group. Epidemiological studies have found more robust immune responses, decreased prevalence of certain persistent viruses and decreased viral loads in females compared to males (37). These differences are partly attributed to X-linked immune-associated genes (37).
ABIS is a prospective birth cohort including the general population. The aim was to study immune-mediated diseases, not just type 1 diabetes, eliminating the risk of selection bias. Participating families were not informed of any prior hypotheses or specific areas of study. The aim and design of this study will inherently result in multiple testing and risk of false discovery. An attempt to mitigate this was made by including possible confounders in the logistic regression analyses and performing a Benjamini & Hochberg procedure to determine statistical significance.
Self-reported data could suffer from recall bias, with an increased risk of under-reporting in cases of acute illness such as infections compared to chronic conditions (38). The relatively long intervals of approximately two years between questionnaires could amplify this risk. In particular, minor illnesses might be under-reported. It is possible that family constellation, age and number of siblings, cultural differences and coexisting chronic diseases might affect a carer’s likelihood to recall and report infections. However, the participation rate between families of children with type 1 diabetes and the reference group was similar for all age groups, and excluding infections registered after a diagnosis of type 1 diabetes eliminates the effect of systematic under-reporting for either group (or recall bias). In addition, the reported occurrence of minor viral infections like the common cold was consistently over 90% for all age groups, suggesting that the effect of under-reporting was likely small.
The self-reported data in the ABIS questionnaires was intended to cover all infectious episodes, including minor self-limiting infections, allowing for analysis of associations between childhood infections and type 1 diabetes that would not be possible using only medical records. Meanwhile, the NPR includes data from hospital admissions and specialized outpatient visits, likely underestimating the occurrence of minor infections but includes almost all major infections and complements the data from the ABIS questionnaires. Excluding infections registered after a diagnosis of type 1 diabetes mitigates the risk of reverse causation.
Methodologically, it would have been preferable to perform a Cox regression analysis of the entire period from 0-5 years to investigate the effect of a total infectious load during early childhood, however the assumption of proportional hazards over time was not met in this study. The lack of data on exact age, duration and associated symptoms of each infection reported in the questionnaires, in addition to unevenness in the extent of missing data between questionnaires, prohibited the use of a Cox regression analysis with time-dependent variables or other sensitivity analyses. For these reasons, a separate logistic regression analysis of each time period was performed instead. This approach yielded more comparisons, which might result in lack of significance after correcting for FDR using the Benjamini-Hochberg method. However, the small sample sizes, especially in subgroup analyses, warrants cautious interpretation and the risk of a type I error was deemed larger than the risk of a type II error.
The outcome of type 1 diabetes was determined by a clinical diagnosis requiring both symptoms of hyperglycemia and laboratory confirmation. This is considered a late stage of the disease, preceded by months or years of islet autoimmunity. It was not possible to distinguish whether early childhood infections had an effect on onset of islet autoimmunity or progression to overt type 1 diabetes in this study, but the study period of 0-5 years coincides with the peak age of seroconversion (39) and only a small proportion of cases progressed to manifest type 1 diabetes during the study period.
The greatest strength of using a general population cohort is the inclusion of individuals not followed in other high-risk cohorts, allowing for greater generalizability and the possibility to study the often-neglected group of type 1 diabetes cases without risk-associated HLA alleles. While this group is small, the diagnosis of type 1 diabetes in these individuals is just as life-altering as for anyone else, and they are rarely included in observational or prevention studies.
Type and frequency of reported infections in the ABIS questionnaires was similar between both cases with type 1 diabetes and the reference group for all ages 0-5 years. Based on our results, infections and antibiotic use in early childhood do not seem to have a major impact in developing manifest type 1 diabetes in the general population, but individuals without risk-associated HLA alleles could be more sensitive to the effects of childhood infections. The association to frequent episodes of gastroenteritis in early childhood needs to be reproduced in other studies to be confirmed.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Research Ethics Committees of the Faculty of Health Science at the University of Linköping, Linköping, Sweden (Dnr-96-287, Dnr-99-321 and Dnr-03-092) and the Medical Faculty at the University of Lund, Lund, Sweden (LU 83-97). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
MB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. JW: Conceptualization, Methodology, Project administration, Resources, Supervision, Writing – review & editing. JL: Conceptualization, Data curation, Funding acquisition, Project administration, Resources, Supervision, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from Barndiabetesfonden (Swedish Child Diabetes Foundation Swedish Research Council, Grant/Award Number: K2005-72X-11242-11A and K2008-69X-20826-01-4); Medical Research Council of Southeast Sweden (FORSS); JDRF Wallenberg Foundation, Grant/Award Number: K 98-99D-12813-01A. ALF-grants and funding from Clinical research project for resident doctors in Region Östergotland, and Joanna Cocozza Foundation. The study sponsors were not involved in the design of the study; the collection, analysis and interpretation of data; writing the report; and did not impose any restrictions regarding the publication of the report.
Special thanks to all participating families in the ABIS study and the staff at the Well Baby clinics.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1529447/full#supplementary-material
1. Hummel K, McFann KK, Realsen J, Messer LH, Klingensmith GJ, Chase HP. The increasing onset of type 1 diabetes in children. J Pediatrics. (2012) 161:652–7.e1. doi: 10.1016/j.jpeds.2012.03.061
2. Gregory GA, Robinson TIG, Linklater SE, Wang F, Colagiuri S, de Beaufort C, et al. Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: a modelling study. Lancet Diabetes endocrinology. (2022) 10:741–60. doi: 10.1016/S2213-8587(22)00218-2
3. Fourlanos S, Varney MD, Tait BD, Morahan G, Honeyman MC, Colman PG, et al. The rising incidence of type 1 diabetes is accounted for by cases with lower-risk human leukocyte antigen genotypes. Diabetes Care. (2008) 31:1546–9. doi: 10.2337/dc08-0239
4. Kondrashova A, Reunanen A, Romanov A, Karvonen A, Viskari H, Vesikari T, et al. A six-fold gradient in the incidence of type 1 diabetes at the eastern border of Finland. Ann Med. (2005) 37:67–72. doi: 10.1080/07853890410018952
5. Frederiksen B, Kroehl M, Lamb MM, Seifert J, Barriga K, Eisenbarth GS, et al. Infant exposures and development of type 1 diabetes mellitus: The Diabetes Autoimmunity Study in the Young (DAISY). JAMA pediatrics. (2013) 167:808–15. doi: 10.1001/jamapediatrics.2013.317
6. Sepa A, Frodi A, Ludvigsson J. Mothers’ experiences of serious life events increase the risk of diabetes-related autoimmunity in their children. Diabetes Care. (2005) 28:2394–9. doi: 10.2337/diacare.28.10.2394
7. Rewers M, Ludvigsson J. Environmental risk factors for type 1 diabetes. Lancet (London England). (2016) 387:2340–8. doi: 10.1016/S0140-6736(16)30507-4
8. Nygren M, Carstensen J, Koch F, Ludvigsson J, Frostell A. Experience of a serious life event increases the risk for childhood type 1 diabetes: the ABIS population-based prospective cohort study. Diabetologia. (2015) 58:1188–97. doi: 10.1007/s00125-015-3555-2
9. Carré A, Vecchio F, Flodström-Tullberg M, You S, Mallone R. Coxsackievirus and type 1 diabetes: diabetogenic mechanisms and implications for prevention. Endocrine Rev. (2023) 44:737–51. doi: 10.1210/endrev/bnad007
10. Wang K, Ye F, Chen Y, Xu J, Zhao Y, Wang Y, et al. Association between enterovirus infection and type 1 diabetes risk: A meta-analysis of 38 case-control studies. Front endocrinology. (2021) 12:706964. doi: 10.3389/fendo.2021.706964
11. Wang Y, Guo H, Wang G, Zhai J, Du B. COVID-19 as a trigger for type 1 diabetes. J Clin Endocrinol Metab. (2023) 108:2176–83. doi: 10.1210/clinem/dgad165
12. Tapia G, Stordal K, Marild K, Kahrs CR, Skrivarhaug T, Njolstad PR, et al. Antibiotics, acetaminophen and infections during prenatal and early life in relation to type 1 diabetes. Int J Epidemiol. (2018) 47:1538–48. doi: 10.1093/ije/dyy092
13. Yoon JW, Austin M, Onodera T, Notkins AL. Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. New Engl J Med. (1979) 300:1173–9. doi: 10.1056/NEJM197905243002102
14. Oikarinen S, Krogvold L, Edwin B, Buanes T, Korsgren O, Laiho JE, et al. Characterisation of enterovirus RNA detected in the pancreas and other specimens of live patients with newly diagnosed type 1 diabetes in the DiViD study. Diabetologia. (2021) 64(11):2491–501. doi: 10.1007/s00125-021-05525-0
15. Bélteky M, Wahlberg J, Ludvigsson J. Maternal respiratory infections in early pregnancy increases the risk of type 1 diabetes. Pediatr diabetes. (2020) 21:1193–201. doi: 10.1111/pedi.13075
16. Allen DW, Kim KW, Rawlinson WD, Craig ME. Maternal virus infections in pregnancy and type 1 diabetes in their offspring: Systematic review and meta-analysis of observational studies. Rev Med virology. (2018) 28:e1974. doi: 10.1002/rmv.v28.3
17. Lonnrot M, Lynch KF, Elding Larsson H, Lernmark A, Rewers MJ, Torn C, et al. Respiratory infections are temporally associated with initiation of type 1 diabetes autoimmunity: the TEDDY study. Diabetologia. (2017) 60:1931–40. doi: 10.1007/s00125-017-4365-5
18. Wahlberg J, Vaarala O, Ludvigsson J. Dietary risk factors for the emergence of type 1 diabetes-related autoantibodies in 2½-year-old Swedish children. Br J Nutr. (2006) 95:603–8. doi: 10.1079/BJN20051676
19. Clausen TD, Bergholt T, Bouaziz O, Arpi M, Eriksson F, Rasmussen S, et al. Broad-spectrum antibiotic treatment and subsequent childhood type 1 diabetes: A nationwide danish cohort study. PloS One. (2016) 11:e0161654. doi: 10.1371/journal.pone.0161654
20. Tamburini S, Shen N, Wu HC, Clemente JC. The microbiome in early life: implications for health outcomes. Nat Med. (2016) 22:713–22. doi: 10.1038/nm.4142
21. Kindgren E, Ludvigsson J. Infections and antibiotics during fetal life and childhood and their relationship to juvenile idiopathic arthritis: a prospective cohort study. Pediatr Rheumatol Online J. (2021) 19:145. doi: 10.1186/s12969-021-00611-4
22. Murk W, Risnes KR, Bracken MB. Prenatal or early-life exposure to antibiotics and risk of childhood asthma: A systematic review. Pediatrics. (2011) 127:1125–38. doi: 10.1542/peds.2010-2092
23. Ahrens AP, Hyötyläinen T, Petrone JR, Igelström K, George CD, Garrett TJ, et al. Infant microbes and metabolites point to childhood neurodevelopmental disorders. Cell. (2024) 187:1853–73.e15. doi: 10.1016/j.cell.2024.02.035
24. Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. (2010) 464:1293–300. doi: 10.1038/nature08933
25. Ludvigsson J. Why diabetes incidence increases—a unifying theory. Ann New York Acad Sci. (2006) 1079:374–82. doi: 10.1196/annals.1375.058
26. Rook GA, Brunet LR. Microbes, immunoregulation, and the gut. Gut. (2005) 54:317–20. doi: 10.1136/gut.2004.053785
27. Bélteky M, Milletich PL, Ahrens AP, Triplett EW, Ludvigsson J. Infant gut microbiome composition correlated with type 1 diabetes acquisition in the general population: the ABIS study. Diabetologia. (2023) 66:1116–28. doi: 10.1007/s00125-023-05895-7
28. Russell JT, Roesch LFW, Ördberg M, Ilonen J, Atkinson MA, Schatz DA, et al. Genetic risk for autoimmunity is associated with distinct changes in the human gut microbiome. Nat Commun. (2019) 10:3621. doi: 10.1038/s41467-019-11460-x
29. Ludvigsson J, Ludvigsson M, Sepa A. Screening for prediabetes in the general child population: maternal attitude to participation. Pediatr diabetes. (2001) 2:170–4. doi: 10.1034/j.1399-5448.2001.20405.x
30. Ilonen J, Kiviniemi M, Lempainen J, Simell O, Toppari J, Veijola R, et al. Genetic susceptibility to type 1 diabetes in childhood - estimation of HLA class II associated disease risk and class II effect in various phases of islet autoimmunity. Pediatr diabetes. (2016) 17 Suppl 22:8–16. doi: 10.1111/pedi.12327
31. White PA, Faresjö T, Jones MP, Ludvigsson J. Low maternal education increases the risk of Type 1 Diabetes, but not other autoimmune diseases: a mediating role of childhood BMI and exposure to serious life events. Sci Rep. (2023) 13:6166. doi: 10.1038/s41598-023-32869-x
32. Brix N, Ernst A, Lauridsen LLB, Parner E, Støvring H, Olsen J, et al. Timing of puberty in boys and girls: A population-based study. Paediatric perinatal Epidemiol. (2019) 33:70–8. doi: 10.1111/ppe.2019.33.issue-1
33. Kemppainen KM, Vehik K, Lynch KF, Larsson HE, Canepa RJ, Simell V, et al. Association between early-life antibiotic use and the risk of islet or celiac disease autoimmunity. JAMA Pediatr. (2017) 171:1217–25. doi: 10.1001/jamapediatrics.2017.2905
34. Wernroth ML, Fall K, Svennblad B, Ludvigsson JF, Sjolander A, Almqvist C, et al. Early childhood antibiotic treatment for otitis media and other respiratory tract infections is associated with risk of type 1 diabetes: A nationwide register-based study with sibling analysis. Diabetes Care. (2020) 43(5):991–9. doi: 10.2337/dc19-1162
35. Wang L, Wang FS, Gershwin ME. Human autoimmune diseases: a comprehensive update. J Internal Med. (2015) 278:369–95. doi: 10.1111/joim.2015.278.issue-4
36. Lönnrot M, Lynch KF, Rewers M, Lernmark Å, Vehik K, Akolkar B, et al. Gastrointestinal infections modulate the risk for insulin autoantibodies as the first-appearing autoantibody in the TEDDY study. Diabetes Care. (2023) 46:1908–15. doi: 10.2337/dc23-0518
37. Wilkinson NM, Chen HC, Lechner MG, Su MA. Sex differences in immunity. Annu Rev Immunol. (2022) 40:75–94. doi: 10.1146/annurev-immunol-101320-125133
38. Bonaventure A, Kane E, Simpson J, Roman E. Maternal infections and medications in pregnancy: how does self-report compare to medical records in childhood cancer case-control studies? Int J Epidemiol. (2023) 52:1187–96. doi: 10.1093/ije/dyad019
Keywords: childhood environmental factors, gastroenteritis, infections, sex differences, type 1 diabetes
Citation: Bélteky M, Wahlberg J and Ludvigsson J (2025) Infections and antibiotic use in early childhood have limited importance in developing manifest type 1 diabetes – The ABIS cohort study. Front. Endocrinol. 16:1529447. doi: 10.3389/fendo.2025.1529447
Received: 16 November 2024; Accepted: 30 January 2025;
Published: 21 February 2025.
Edited by:
Wen-hong Li, University of Texas Southwestern Medical Center, United StatesReviewed by:
Tiago Jeronimo Dos Santos, University of Almeria, SpainCopyright © 2025 Bélteky, Wahlberg and Ludvigsson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Malin Bélteky, bWFsaW4uYmVsdGVreUBsaXUuc2U=
†These authors have contributed equally to this work
‡ORCID: Malin Bélteky, orcid.org/0000-0002-4015-7075
Jeanette Wahlberg, orcid.org/0000-0003-4061-6830
Johnny Ludvigsson, orcid.org/0000-0003-1695-5234
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.