
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 17 February 2025
Sec. Cardiovascular Endocrinology
Volume 16 - 2025 | https://doi.org/10.3389/fendo.2025.1525815
This article is part of the Research Topic Re-visiting Risk Factors for Cardiometabolic Diseases: Towards a New Epidemiological Frontier View all 23 articles
Background: The cardiometabolic index (CMI) is a new comprehensive measure that reflects visceral obesity and metabolic function. This study aimed to examine associations between CMI and adult mortality from all causes and specific causes, as well as gender differences, using the National Health and Nutrition Examination Survey (NHANES) database.
Methods: We included 37,539 adult participants with complete data from the 1999-2018 NHANES database. We categorized the participants according to gender and constructed three models to investigate the relationship between CMI and the outcome variables. These were analyzed using Kaplan-Meier curve analysis, COX proportional risk models, and restricted cubic spline (RCS).
Results: Baseline characteristics showed that among both male and female participants, those who died exhibited higher levels of CMI compared to those who survived. Kaplan-Meier curves showed an increasing trend in all-cause and specific mortality with increasing follow-up time. When CMI was categorized according to quartiles (Q1-Q4), the probability of survival was lower in the Q4 group compared to Q1. We found no gender differences between all three mortality rates. In COX regression analyses, all-cause, cardiovascular, and diabetes mortality were significantly higher in Q4 in the whole population and female participants, whereas no significant differences were identified among male participants. The RCS showed a nonlinear positive correlation in diabetes mortality for females and a linear positive correlation in all-cause and cardiovascular mortality. As for males, CMI was positively and nonlinearly associated with all-cause and diabetes mortality. Besides, there is no statistically significant correlation for males in cardiovascular mortality.
Conclusion: There were gender differences in the correlation between CMI and all-cause mortality, cardiovascular mortality, and diabetes mortality in the adult population. The findings indicated that adult females with elevated CMI levels were at an elevated risk of mortality from all causes, cardiovascular disease, and diabetes. At the same time, there were no significant associations in adult males.
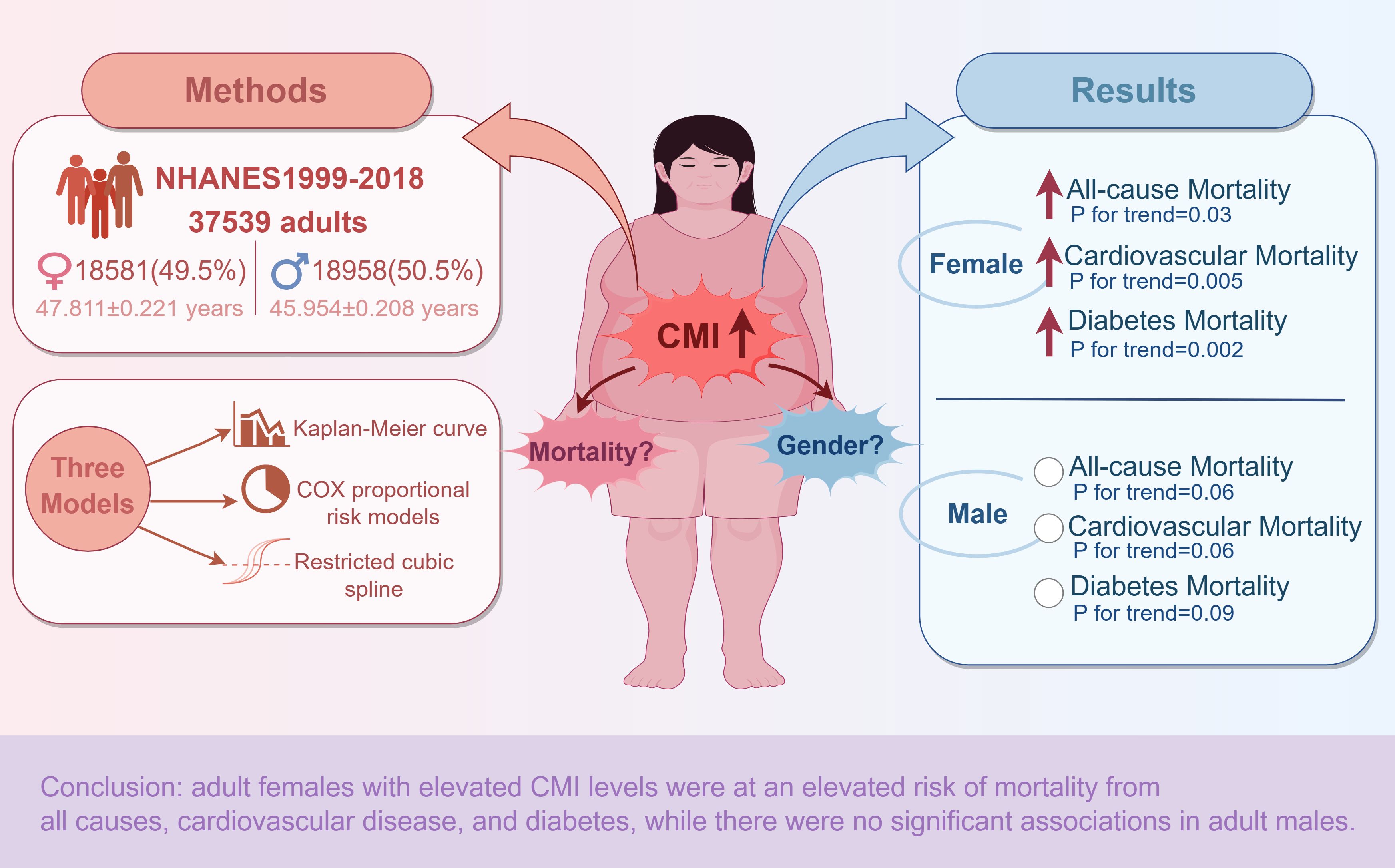
Graphical Abstract. Overview of the methodology and main results of the study of gender differences in the association between CMI and all-cause and specific mortality. Red arrows indicate statistically significant elevations. White circles indicate no statistical significance. Materials provided by FigDraw (www.figdraw.com).
Cardiometabolic diseases are a series of metabolic dysfunctions, including cardiovascular diseases and diabetes mellitus. Among adults worldwide, they constitute one of the primary causes of morbidity and mortality (1, 2). Effective identification and active control of cardiometabolic diseases in their early stages represent a crucial determinant of disease development and prognosis. The development of cardiometabolic diseases is most closely related to obesity (3, 4). In the past, we usually used body mass index (BMI) to define obesity (5). However, this approach cannot determine the proportion of fat in the body composition and thus has many limitations (6). In recent years, a growing number of people with normal-weight obesity (NWO) has prompted a surge in research interest in the health implications of visceral obesity (7).
Visceral obesity is directly related to cardiometabolic diseases (8), and many new measures have emerged for assessing visceral obesity. Wakabayashi et al. (9) were the first to propose CMI, which is derived from a combination of lipids and obesity parameters and can be calculated to detect cardiometabolic risk in humans simply and effectively, providing a comprehensive assessment of cardiometabolic risk (10–12).
In recent years, relevant studies have found that CMI may be a marker for predicting renal function (13), depression (14), impaired fasting glucose, insulin resistance, type 2 diabetes mellitus (15), non-alcoholic fatty liver disease and fibrosis (16), biological aging (17), endometriosis (18, 19), and erectile dysfunction (20) in older adults. Regarding the effect of CMI on mortality, recent studies have shown that elevated CMI is positively associated with all-cause mortality in middle-aged and older adults and is partially mediated by inflammation (21, 22). Moreover, in the general population, CMI has an L-shaped nonlinear association with all-cause mortality (23).
Based on the above research, we have three research gaps. Firstly, we focused on adults over 18, the leading group who develop cardiometabolic diseases. Secondly, because CMI is associated with metabolic diseases, we focused on the correlation between CMI and all-cause, cardiovascular, and diabetes mortality. To our knowledge, this is the first study to include the correlation between CMI and diabetes mortality. Finally, there are differences in whole-body lipid metabolism between men and women, and trends in body fat accumulation in women are associated with an increased risk of metabolic-related diseases (24), so we further explored sex differences between CMI and different mortality correlations.
A cross-sectional survey of U.S. citizens’ health and nutritional status, NHANES, was conducted in 1999 and contains a wide range of demographic, economic, nutritional, and health data (25). NHANES employs a sophisticated, four-stage sampling design, utilizing a stratified, multistage probability sampling approach to ensure the sample is representative of the entire nation (26). Fifteen different sites are selected each year from a sampling frame of all U.S. counties, from which it chooses a nationally representative sample of comprising approximately 5,000 individuals (27). Written informed consent and ethical review by the National Centre for Health Statistics (NCHS) Ethics Review Committee were obtained from all participants in the NHANES study.
In this study, we selected 101,316 participants who participated in the NHANES survey from 1999 to 2018 and linked them to NDI data to obtain information on participant follow-up. After excluding those who failed to meet the inclusion criteria, the study finally included 37539 participants. The flowchart in Figure 1 illustrates the process for screening the inclusion population.
CMI, as an exposure variable in this study, was derived by calculation from blood indicators and body data measured by professionals in a mobile examination van. The formula is as follows (9): CMI=TG(mg/dl)/HDL(mg/dl)×Waist(cm)/Height(cm).
The outcome variables in this research included all-cause mortality and two specific mortality rates. The specific mortality rates included cardiovascular mortality and diabetes mortality. We obtained mortality-related data from the NDI Death Certificate Record (www.cdc.gov/nchs/data-linkage/mortality-public.htm), which is currently updated until December 31, 2019 (28). Combining NHANES and NDI-related data provides comprehensive information on participant deaths, including time of death and cause of death, among other comprehensive information. Based on the fact that the study period is 1999-2018, we used the person-months from the date of interview to the date of death or the end of the period of death (PERMTH_INT) to calculate the follow-up time in this study. People can obtain specific information from the official website mentioned above. Specifically, the codes I00-09, I11, I13, I20-51, and I60-I69 identify cardiovascular mortality, while the codes E10-E14 identify diabetes mortality in the International Classification of Diseases (ICD)-10.
The covariates in the study consisted primarily of demographic characteristics of gender (male, female), age (years), race (Mexican American, non-Hispanic white, non-Hispanic black, other Hispanic, and other race), marriage (married, never married, separated, divorced, widowed, and living with partner), household income (Poverty Income Ratio, PIR), and educational qualification (less than 9th grade, 9-11th grade, high school graduate, some college, and college graduate or above). Other covariates included body mass index (BMI, kg/m2), alcohol intake (never, light/moderate, heavy, former), and smoking status (never, current, former). Laboratory tests included triglycerides (TG, mg/dl), total cholesterol (TC, mg/dl), high-density lipoprotein (HDL, mg/dl), and glomerular filtration rate (eGFR, mL/min/1.73 m2). A questionnaire determined coronary heart disease (CHD) diagnosis based on self-reported responses. A diagnosis of diabetes mellitus(DM) is the fulfillment of any of the following conditions: (1) doctor told you have diabetes, (2) glycohemoglobin HbA1c (%) >= 6.5, (3) fasting glucose (mmol/l) >= 7.0, (4) random blood glucose (mmol/l) >= 11.1, (5) two-hour OGTT blood glucose (mmol/l) >= 11.1, (6) Use of diabetes medication or insulin. The diagnosis of hypertension is based on the following criteria: (1) self-reported hypertension, (2) mean systolic blood pressure (SBP) ≥140 mmHg and/or mean diastolic blood pressure (DBP) ≥90 mmHg, and (3) use of antihypertensive medications. The diagnosis of hyperlipidemia is fulfilling the following conditions: (1) diagnosis of hypertriglyceridemia (TG>=150mg/dL), (2) diagnosis of hypercholesterolemia: (i) TC>=200mg/dL, (ii) low-density lipoprotein cholesterol (LDL-C) >=130mg/dL (iii) HDL<40mg/dL in men and <50mg/dL in women, and (3) current taking lipid-lowering drugs. The official NHANES website provides specific technical information regarding covariate determination.
Every analysis in this research utilized the R language (version 4.3.0) and achieved statistical significance with a two-tailed p-value below 0.05. We divided participants into four groups according to their CMI quartiles (Q1-Q4), and the lowest quartile was considered the baseline. We divided the population based on gender and CMI quartiles to investigate the relationship between CMI and all-cause and specific mortality. The weights of the analyses were adjusted to prevent oversampling. We used weighted means (95% confidence intervals [CIs]) and weighted percentages (95% confidence intervals) to describe continuous and categorical variables, respectively (29). To assess gender differences in the relationship between CMI and outcome variables, we devised three weighted COX regression models, with model 1 unadjusted for confounders, model 2 adjusted for age and ethnicity, and model 3 adjusted for age, race, marital status, smoking status, alcohol use, eGFR, and TC.
In addition, the RCS was employed with three nodes (5th, 50th, and 95th percentiles) to assess dose-response patterns between CMI and all-cause and specific mortality. We generated Kaplan-Meier survival curves for various mortality rates based on CMI quartiles and survival times.
We included 37,539 participants over ten consecutive survey cycles from 1999 to 2018. Tables 1, 2 demonstrate the baseline characteristics of participants by CMI quartile (Q1-Q4) by gender, with a mean age of 45.954 for male participants and 47.811 for female participants. In Q1-Q4, there were notable disparities (p < 0.05) between males and females in age, gender, race, marriage, education, poverty level, BMI, smoking, alcohol consumption, CHD, DM, hypertension, hyperlipidemia, height, waist circumference, total cholesterol, triglycerides, HDL, and glomerular filtration rate. Supplementary Table S1 demonstrates the baseline characteristics of participants by survival status and shows that those who died exhibited higher levels of CMI compared with those who survived.
Throughout the monitoring phase, participants experienced 5600 deaths due to any cause, comprising 1765 from cardiovascular causes and 200 from diabetes. As shown in Figure 2, we performed Kaplan-Meier analysis. The results showed that CMI differed significantly in predicting all-cause, cardiovascular, and diabetes mortality between Q1-Q4 groups in the total population, females and males. All three mortality rates showed an increasing trend with increasing follow-up time, and we found no gender differences. Nevertheless, the differences were more pronounced in the female than in the male group.
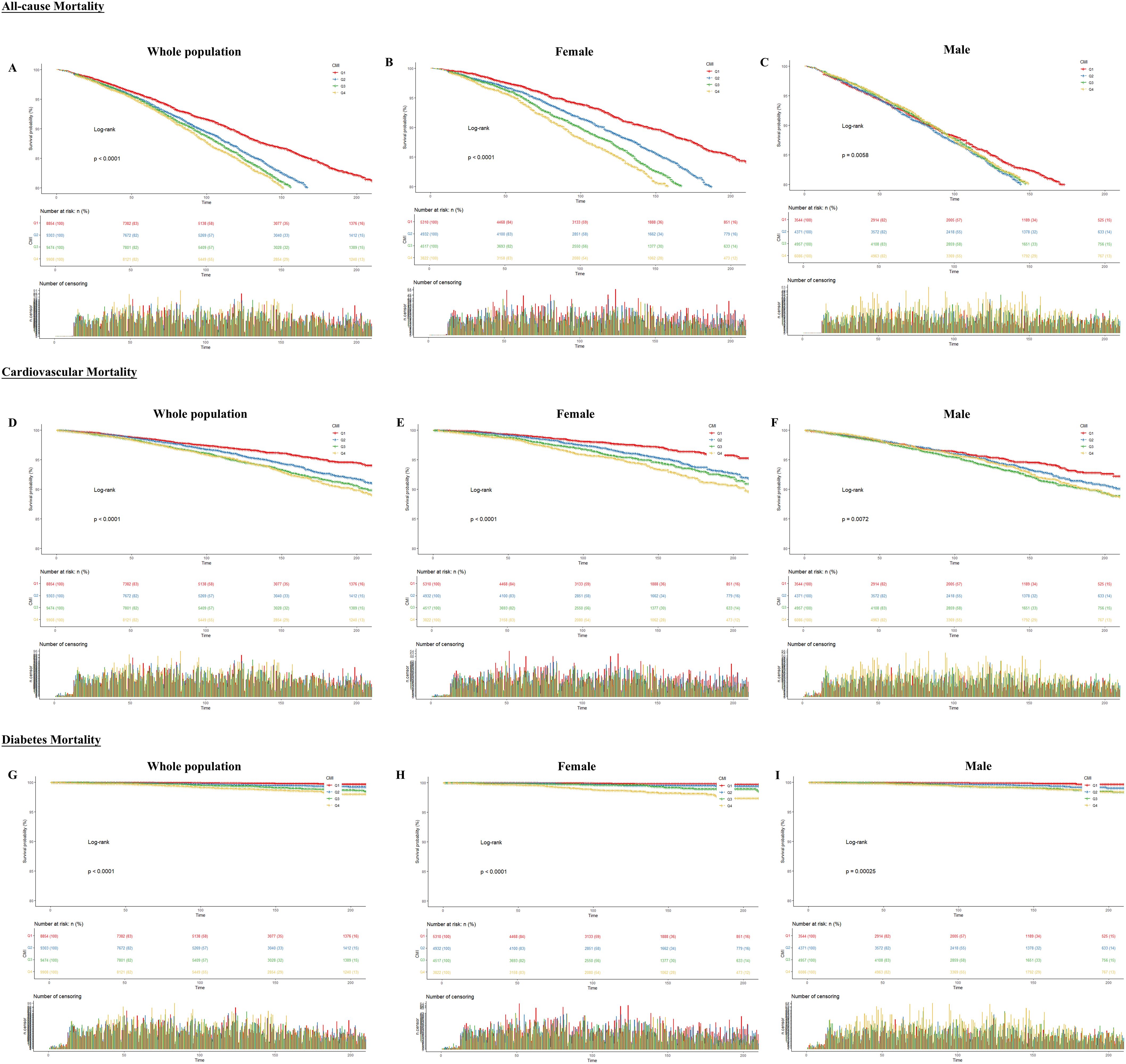
Figure 2. Kaplan-Meier curves between CMI and all-cause and specific mortality rates. The X-axis indicates follow-up time (months); the Y-axis indicates survival probability. (A) All-cause mortality for the whole population (B) All-cause mortality for females (C) All-cause mortality for males (D) Cardiovascular mortality for the whole population (E) Cardiovascular mortality for females (F) Cardiovascular mortality for males (G) Diabetes mortality for the whole population (H) Diabetes mortality for females (I) Diabetes mortality for males.
Tables 3–5 show the results of the COX regression analyses between CMI and outcomes, respectively. When CMI was a continuous variable, CMI in model 3 was significantly correlated with increased mortality rates due to all causes, cardiovascular and diabetes in the whole population (all-cause: HR: 1.03, 95%CI: 1.02-1.05, P<0.0001; cardiovascular: HR: 1.04, 95%CI: 1.02-1.05, P <0.0001; diabetes: HR: 1.11, 95%CI: 1.09-1.14, P<0.0001) and participants of both genders (all-cause: male: HR: 1.03, 95%CI: 1.01-1.04, P<0.001; female: HR: 1.05, 95%CI: 1.02-1.07, P<0.001. cardiovascular: male: HR: 1.03, 95%CI: 1.01-1.05, P=0.01; female: HR: 1.05, 95%CI: 1.03-1.08, P<0.0001. diabetes: male: HR: 1.09, 95%CI: 1.06-1.12, P<0.0001; female: HR: 1.15, 95%CI: 1.09-1.22, P<0.0001). As a categorical variable based on quartiles, CMI demonstrated a notable rise in mortality rates for all causes, cardiovascular disease, and diabetes in Q4 compared with Q1 in the whole population (all-cause: P for trend=0.005; cardiovascular: P for trend<0.001; diabetes: P for trend<0.001) and female participants (all-cause: P for trend=0.03; cardiovascular: P for trend= 0.005; diabetes: P for trend=0.002). In contrast, male participants had no significant difference (all-cause: P for trend=0.06; cardiovascular: P for trend=0.06; diabetes: P for trend=0.09).
We assessed the RCS using model 3 to investigate further the correlation between CMI and mortality rates (Figure 3), which showed that in the total population, CMI and all-cause mortality were nonlinearly related (P for nonlinear = 0.0276), as well as diabetes mortality (P for nonlinearity = 0.001). Cardiovascular mortality was linearly related (P for nonlinear = 0.1272). All three mortality rates increased with increasing CMI. Across sexes, females showed a linear response relationship in all-cause mortality (P for nonlinear = 0.4645) and cardiovascular mortality (P for nonlinear = 0.7686) and a nonlinear response relationship in diabetes mortality (P for nonlinear < 0.001); males showed a nonlinear response relationship in all-cause mortality (P for nonlinear = 0.0324) and diabetes mortality (P for nonlinear = 0.0168), but there was no statistically significant relationship with cardiovascular mortality (P for overall = 0.1367).
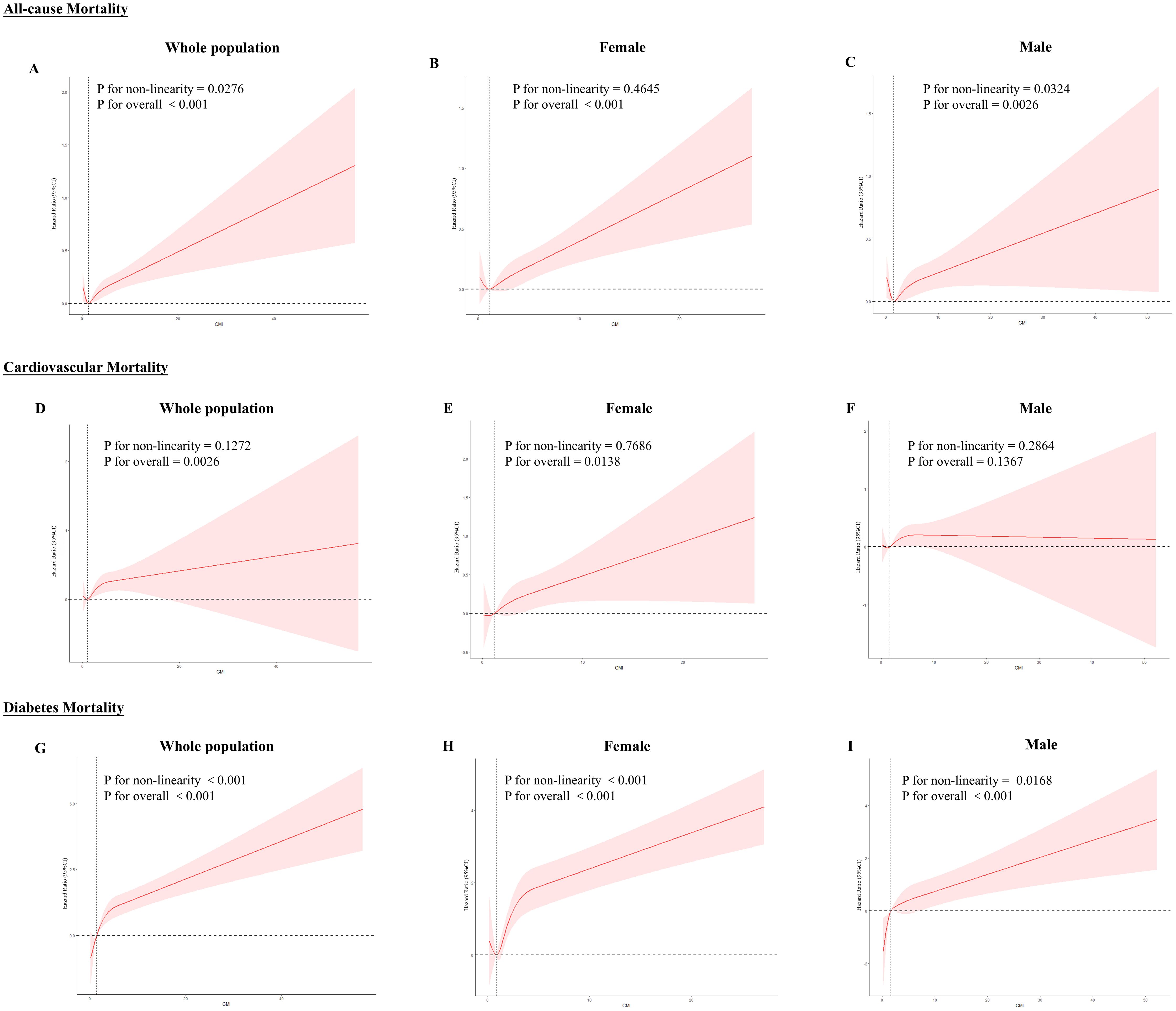
Figure 3. RCS curves between CMI and all-cause and specific mortality rates. Red lines represent the estimated hazard ratio, and the shaded red area corresponds to the 95% confidence intervals. (A) All-cause mortality for the whole population (B) All-cause mortality for females (C) All-cause mortality for males (D) Cardiovascular mortality for the whole population (E) Cardiovascular mortality for females (F) Cardiovascular mortality for males (G) Diabetes mortality for the whole population (H) Diabetes mortality for females (I) Diabetes mortality for males.
Using NHANES data, we conducted a cohort study of 37,539 participants. The results showed significant gender differences between CMI and all-cause, cardiovascular, and diabetes mortality among the general adult population in the United States. In women, we found that CMI independently predicted mortality from all causes, cardiovascular diseases, and diabetes, but this association did not hold for men. Moreover, we further explored the trend changes by Kaplan-Meier and RCS curves. It is the first study to address gender differences in the correlation between CMI and mortality in the general adult population.
Researchers initially used CMI, a relatively new measure of obesity and lipid levels, to screen for diabetes and obesity (9). Subsequently, many related studies have shown a correlation between CMI and an increased risk of developing diabetes, with Zha et al. (30) finding a nonlinear positive relationship between CMI and diabetes risk in Japanese adults and Qiu et al. (31) finding the same positive association in a study of middle-aged and older adults in China. In addition, studies have also found associations between CMI and a variety of metabolic disorders, such as CVD. For example, Cai et al. (32) found that CMI was positively associated with the risk of new-onset CVD in patients with hypertension and obstructive sleep apnea. The above studies suggest that CMI can not only be used to assess diabetes but is also significantly associated with metabolism-related diseases. It is consistent with this study’s finding that CMI is significantly and positively associated with all-cause, cardiovascular, and diabetes mortality in the U.S. adult population.
Our study population was the general adult population, and we further analyzed that there was a significant correlation between CMI and all-cause mortality, cardiovascular mortality, and diabetes mortality in females. We tried to explain the reasons for the emergence of this gender difference.
Based on previous reports, it appears that cardiovascular and diabetic disease risk differs by gender. In older American populations, white men have a higher risk of obesity, whereas white women have a higher risk of hypertension and diabetes (33). Similarly, in Asian populations, research in a Japanese population showed a stronger relationship between obesity and cardiometabolic risk factors in women (34) and a greater cardiometabolic risk of glucose and dyslipidemia in women than in men (35). Shi et al. (36) found that higher AIP levels in women significantly increase the risk of developing prediabetes and diabetes. In women, impaired glucose tolerance (IGT) is more prevalent than in men, and obesity-associated type 2 diabetes mellitus is associated with significant gender and regional differences (37). The above findings suggest that gender differences in cardiovascular-related and diabetes-related diseases are more common among populations in various regions. Few studies have now reported sex differences between CMI and cardiovascular and diabetes-related diseases. Elevated CMI is associated with eccentric and centripetal left ventricular hypertrophy in females compared with males (38). It suggests that CMI is likely to be a marker for explaining the adverse cardiovascular effects of central obesity (39, 40). Moreover, the correlation between CMI and diabetes mellitus is more pronounced among female patients (9, 15). The above result is in line with the findings in the current study.
Gender differences in CMI reflecting adverse cardiometabolic outcomes may be related to the characteristics of body fat distribution in different gender populations. The specific mechanisms may be related to sex hormones, adipocyte characteristics, and the adipose microenvironment. Premenopausal women usually have more subcutaneous adipose tissue (SAT). In contrast, men have more trunk and visceral adipose tissue (VAT), and this characteristic shifts after menopause, with an increase in the distribution of trunk and VAT, the development of centripetal obesity, and an increase in waist circumference in women compared with that in men, which dramatically increases the risk of diabetes mellitus and cardiovascular disease in women (41–44). The CMI measures the lipid profile of the body, and the CMI is an indicator of cardiometabolic disease risk in women. While CMI is a comprehensive indicator for detecting dyslipidemia and abdominal obesity, it can reflect VAT distribution well. It appears to be more relevant to women in predicting adverse outcomes in cardiometabolic-related diseases. At the same time, we should also be concerned about the impact of obesity on adverse outcomes in other pre-existing conditions. The clinical phenotype of obesity is similar to that of hypertrophic cardiomyopathy (HCM) (45). Relevant studies have shown that among patients with HCM, women are older than men and have a higher risk of HCM-related complications and death (46, 47). Obesity is also a significant risk factor for increased rates of adverse events in patients with conditions such as stroke (48), thromboembolic disease (49), and atrial fibrillation (50).
In addition, an imbalance in female sex hormones significantly increases the risk of cardiometabolic issues. Sex hormones may influence the distribution of adipose molecules. Estrogen may affect fat distribution through its effect on adrenergic receptor distribution in long-lasting adipocytes. Testosterone may also play a specific role in adipose tissue distribution through its impact on lipoprotein lipase (LPL) activity and rate-limiting fatty acid uptake (51). Sex hormones also influence adipose cycling factors (e.g., leptin and lipocalin), which are directly related to the accumulation of SAT (52). The absence of positive results in males in this study may be related to the role of androgens. Androgens are involved in regulating lipid metabolism and may inhibit fat deposition (53). Therefore, high androgen levels in men may protect against lipid metabolism-related diseases.
Intrinsic properties of adipocytes may also account for gender differences in fat distribution and function. Adipocytes from SAT and VAT have different physiological properties, with STA adipocytes undergoing hyperplasia and hypertrophy, whereas VAT adipocytes experience hypertrophy only (54). Researchers have linked this intracellular difference to distinct patterns of gene expression. In addition to the intrinsic properties of adipocytes, differences in the extrinsic microenvironment and extracellular matrix can also lead to gender differences in adipose distribution, which is related to the regulation of the activation of adipocyte precursors that drive adipocyte proliferation (55).
In summary, the results of our study indicate that CMI is significantly linked to an increased risk of all-cause mortality, cardiovascular mortality, and diabetes mortality among adult women. It suggests that we may be able to judge the prognostic risk by the change of CMI value in clinical practice, especially for patients with cardiac disease, diabetes, and other underlying diseases. It is also important to pay attention to the influence of gender differences on disease prognosis. We recommended that adult women with high CMI values regularly engage in moderate exercise and pay attention to the management of lipids and waist circumference in order to reduce the risk of adverse prognosis of cardiometabolic-related diseases (56). More generally, it is worthwhile for medical personnel to consider whether there is a need to formulate different treatment standards and protocols according to gender in clinical diagnosis and treatment. At the same time, it is also necessary to pay attention to the screening of the relevant population. Clinical staff can apply new electrocardiography, echocardiography, and cardiac CT imaging to better screen high-risk groups (57–59) and reduce or delay the occurrence of cardiometabolic-related adverse outcomes.
However, this study has some limitations: (i) Since this study only examined the U.S. population, more studies on other races are required for generalizability. (ii) Human reporting factors may affect the accuracy of NHANES data on causes of death, population classification, and death certificates. (iii) We considered potential confounders, but other factors may remain unconsidered.
In our study, we found that CMI was significantly positively associated with all-cause mortality, cardiovascular mortality, and diabetes mortality in adult females in the United States but not in males. According to the findings, it is plausible that CMI can be employed as a predictive indicator for mortality, particularly in relation to all-cause mortality, cardiovascular-specific mortality, and diabetes mortality among adult females.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by The Research Ethics Review Board at the National Center for Health Statistics (NCHS). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
TL: Conceptualization, Data curation, Formal analysis, Software, Writing – original draft, Writing – review & editing. HaZ: Formal analysis, Software, Writing – review & editing. HuZ: Conceptualization, Formal analysis, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by Three-year Action Plan of the Major Clinical Research Program of Shanghai Shenkang Medical Development Center (No. SHDC2020CR1053B and No. SHDC2020CR6012-003).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1525815/full#supplementary-material
1. Tahir UA, Gerszten RE. Molecular biomarkers for cardiometabolic disease: risk assessment in young individuals. Circ Res. (2023) 132:1663–73. doi: 10.1161/CIRCRESAHA.123.322000
2. Guo F, Moellering DR, Garvey WT. The progression of cardiometabolic disease: validation of a new cardiometabolic disease staging system applicable to obesity. Obesity. (2014) 22:110–8. doi: 10.1002/oby.20585
3. GBD 2015 Obesity Collaborators, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. (2017) 377:13–27. doi: 10.1056/NEJMoa1614362
4. Powell-Wiley TM, Poirier P, Burke LE, Després JP, Gordon-Larsen P, Lavie CJ, et al. Obesity and cardiovascular disease: A scientific statement from the American heart association. Circulation. (2021) 143:e984–e1010. doi: 10.1161/CIR.0000000000000973
5. Apovian CM. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care. (2016) 22:s176–85.
6. Rothman KJ. BMI-related errors in the measurement of obesity. Int J Obes. (2008) 32:S56–9. doi: 10.1038/ijo.2008.87
7. Oliveros E, Somers VK, Sochor O, Goel K, Lopez-Jimenez F. The concept of normal weight obesity. Prog Cardiovasc Dis. (2014) 56:426–33. doi: 10.1016/j.pcad.2013.10.003
8. Mohammadian Khonsari N, Khashayar P, Shahrestanaki E, Kelishadi R, Mohammadpoor Nami S, Heidari-Beni M, et al. Normal weight obesity and cardiometabolic risk factors: A systematic review and meta-analysis. Front Endocrinol (Lausanne). (2022) 13:857930. doi: 10.3389/fendo.2022.857930
9. Wakabayashi I, Daimon T. The “cardiometabolic index” as a new marker determined by adiposity and blood lipids for discrimination of diabetes mellitus. Clin Chim Acta. (2015) 438:274–8. doi: 10.1016/j.cca.2014.08.042
10. Shi WR, Wang HY, Chen S, Guo XF, Li Z, Sun YX. Estimate of prevalent diabetes from cardiometabolic index in general Chinese population: a community-based study. Lipids Health Dis. (2018) 17:236. doi: 10.1186/s12944-018-0886-2
11. Wang H, Chen Y, Sun G, Jia P, Qian H, Sun Y. Validity of cardiometabolic index, lipid accumulation product, and body adiposity index in predicting the risk of hypertension in Chinese population. Postgrad Med. (2018) 130:325–33. doi: 10.1080/00325481.2018.1444901
12. Wang HY, Shi WR, Yi X, Wang SZ, Luan SY, Sun YX. Value of reduced glomerular filtration rate assessment with cardiometabolic index: insights from a population-based Chinese cohort. BMC Nephrol. (2018) 19:294. doi: 10.1186/s12882-018-1098-8
13. Miao M, Deng X, Wang Z, Jiang D, Lai S, Yu S, et al. Cardiometabolic index is associated with urinary albumin excretion and renal function in aged person over 60: Data from NHANES 2011-2018. Int J Cardiol. (2023) 384:76–81. doi: 10.1016/j.ijcard.2023.04.017
14. Cheng L, Wu Q, Wang S. Cardiometabolic index is associated with increased depression: A population-based study. J Affect Disord. (2024) 348:259–64. doi: 10.1016/j.jad.2023.12.073
15. Song J, Li Y, Zhu J, Liang J, Xue S, Zhu Z. Non-linear associations of cardiometabolic index with insulin resistance, impaired fasting glucose, and type 2 diabetes among US adults: a cross-sectional study. Front Endocrinol (Lausanne). (2024) 15:1341828. doi: 10.3389/fendo.2024.1341828
16. Yan L, Hu X, Wu S, Cui C, Zhao S. Association between the cardiometabolic index and NAFLD and fibrosis. Sci Rep. (2024) 14:13194. doi: 10.1038/s41598-024-64034-3
17. Sun M, Bao S. Association between cardiometabolic index and biological aging in the US population: evidence from NHANES 2015-2020. Front Aging Neurosci. (2024) 16:1507035. doi: 10.3389/fnagi.2024.1507035
18. Hou J, Chen W, Wang R, Huang X, Cao X, Wang X. Relationship between Cardiometabolic index and endometriosis in a US nationally representative sample: results from NHANES 1999-2006. Front Endocrinol. (2024) 15:1450965. doi: 10.3389/fendo.2024.1450965
19. Wang J, Wang B, Liu T, Shang J, Gu X, Zhang T, et al. Association between cardiometabolic Index (CMI) and endometriosis: a cross-sectional study on NHANES. Lipids Health Dis. (2024) 23:328. doi: 10.1186/s12944-024-02314-7
20. Zhang Y, Wu X, Liu G, Feng X, Zhang W, Jiang H, et al. Association between cardiometabolic index and erectile dysfunction among US adults: a cross-sectional analysis of the National Health and Nutrition Examination Survey 2001-2004. Int J Impot Res. (2024) 36:422–9. doi: 10.1038/s41443-023-00801-6
21. Xu B, Wu Q, La R, Lu L, Abdu FA, Yin G, et al. Is systemic inflammation a missing link between cardiometabolic index with mortality? Evidence from a large population-based study. Cardiovasc Diabetol. (2024) 23:212. doi: 10.1186/s12933-024-02251-w
22. Zhu M, Jin H, Yin Y, Xu Y, Zhu Y. Association of cardiometabolic index with all-cause and cardiovascular mortality among middle-aged and elderly populations. Sci Rep. (2025) 15:681. doi: 10.1038/s41598-024-83914-2
23. Liu M, Wang C, Liu R, Wang Y, Wei B. Association between cardiometabolic index and all-cause and cause-specific mortality among the general population: NHANES 1999-2018. Lipids Health Dis. (2024) 23:425. doi: 10.1186/s12944-024-02408-2
24. Williams CM. Lipid metabolism in women. Proc Nutr Soc. (2004) 63:153–60. doi: 10.1079/PNS2003314
25. Patel CJ, Pho N, McDuffie M, Easton-Marks J, Kothari C, Kohane IS, et al. A database of human exposomes and phenomes from the US National Health and Nutrition Examination Survey. Sci Data. (2016) 3:160096. doi: 10.1038/sdata.2016.96
26. Rothwell CJ, Madans JH, Porter KS. National health and nutrition examination survey: sample design, 2011-2014. Vital Health Stat 2. (2014) 162:1–33.
27. Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National health and nutrition examination survey: plan and operations, 1999-2010. Vital Health Stat Ser 1 Programs Collect Proced. (2013) 56):1–37.
28. Brämer GR. International statistical classification of diseases and related health problems. Tenth revision World Health Stat Q. (1988) 41:32–6.
29. Chen TC, Parker JD, Clark J, Shin HC, Rammon JR, Burt VL. National health and nutrition examination survey: estimation procedures, 2011-2014. Vital Health Stat 2. (2018) 177):1–26.
30. Zha F, Cao C, Hong M, Hou H, Zhang Q, Tang B, et al. The nonlinear correlation between the cardiometabolic index and the risk of diabetes: A retrospective Japanese cohort study. Front Endocrinol (Lausanne). (2023) 14:1120277. doi: 10.3389/fendo.2023.1120277
31. Qiu Y, Yi Q, Li S, Sun W, Ren Z, Shen Y, et al. Transition of cardiometabolic status and the risk of type 2 diabetes mellitus among middle-aged and older Chinese: A national cohort study. J Diabetes Investig. (2022) 13:1426–37. doi: 10.1111/jdi.13805
32. Cai X, Hu J, Wen W, Wang J, Wang M, Liu S, et al. Associations of the cardiometabolic index with the risk of cardiovascular disease in patients with hypertension and obstructive sleep apnea: results of a longitudinal cohort study. Oxid Med Cell Longev. (2022) 2022:4914791. doi: 10.1155/2022/4914791
33. Yu H, Armstrong N, Pavela G, Kaiser K. Sex and race differences in obesity-related genetic susceptibility and risk of cardiometabolic disease in older US adults. JAMA Netw Open. (2023) 6:e2347171. doi: 10.1001/jamanetworkopen.2023.47171
34. Wakabayashi I. Age-dependent influence of gender on the association between obesity and a cluster of cardiometabolic risk factors. Gend Med. (2012) 9:267–77. doi: 10.1016/j.genm.2012.05.004
35. He H, Ni Y, Chen J, Zhao Z, Zhong J, Liu D, et al. Sex difference in cardiometabolic risk profile and adiponectin expression in subjects with visceral fat obesity. Transl Res. (2010) 155:71–7. doi: 10.1016/j.trsl.2009.08.003
36. Shi Y, Wen M. Sex-specific differences in the effect of the atherogenic index of plasma on prediabetes and diabetes in the NHANES 2011-2018 population. Cardiovasc Diabetol. (2023) 22:19. doi: 10.1186/s12933-023-01740-8
37. Kautzky-Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev. (2016) 37:278–316. doi: 10.1210/er.2015-1137
38. Wang H, Sun Y, Li Z, Guo X, Chen S, Ye N, et al. Gender-specific contribution of cardiometabolic index and lipid accumulation product to left ventricular geometry change in general population of rural China. BMC Cardiovasc Disord. (2018) 18:62. doi: 10.1186/s12872-018-0798-0
39. Shah RV, Abbasi SA, Heydari B, Rickers C, Jacobs DR Jr, Wang L, et al. Insulin resistance, subclinical left ventricular remodeling, and the obesity paradox: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. (2013) 61:1698–706. doi: 10.1016/j.jacc.2013.01.053
40. Kronmal RA, Smith VE, O’Leary DH, Polak JF, Gardin JM, Manolio TA. Carotid artery measures are strongly associated with left ventricular mass in older adults (a report from the Cardiovascular Health Study). Am J Cardiol. (1996) 77:628–33. doi: 10.1016/s0002-9149(97)89319-8
41. Wannamethee SG, Papacosta O, Whincup PH, Carson C, Thomas MC, Lawlor DA, et al. Assessing prediction of diabetes in older adults using different adiposity measures: a 7 year prospective study in 6,923 older men and women. Diabetologia. (2010) 53:890–8. doi: 10.1007/s00125-010-1670-7
42. Meisinger C, Döring A, Thorand B, Heier M, Löwel H. Body fat distribution and risk of type 2 diabetes in the general population: are there differences between men and women? The MONICA/KORA Augsburg cohort study. Am J Clin Nutr. (2006) 84:483–9. doi: 10.1093/ajcn/84.3.483
43. Schulze MB, Heidemann C, Schienkiewitz A, Bergmann MM, Hoffmann K, Boeing H. Comparison of anthropometric characteristics in predicting the incidence of type 2 diabetes in the EPIC-Potsdam study. Diabetes Care. (2006) 29:1921–3. doi: 10.2337/dc06-0895
44. Garcia M, Mulvagh SL, Merz CN, Buring JE, Manson JE. Cardiovascular disease in women: clinical perspectives. Circ Res. (2016) 118:1273–93. doi: 10.1161/CIRCRESAHA.116.307547
45. Zaromytidou M, Savvatis K. The weight of obesity in hypertrophic cardiomyopathy. Clin Med. (2023) 23:357–63. doi: 10.7861/clinmed.2023-0194
46. Ghiselli L, Marchi A, Fumagalli C, Maurizi N, Oddo A, Pieri F, et al. Sex-related differences in exercise performance and outcome of patients with hypertrophic cardiomyopathy. Eur J Prev Cardiol. (2020) 27:1821–31. doi: 10.1177/2047487319886961
47. Buongiorno AL, Blandino A, Bianchi F, Masi AS, Pierri A, Mabritto B, et al. Effectiveness of 2014 ESC HCM-Risk-SCD score in prediction of appropriate implantable-cardioverter-defibrillator shocks. J Cardiovasc Med. (2023) 24:313. doi: 10.2459/JCM.0000000000001458
48. Mahadevan A, Vasavada A, Reyaz N, Rajarajan S, Babu K, Sundaram DM, et al. Bariatric surgery association with risk of recurrent stroke hospitalization among older stroke survivors with obesity: A national inpatient sample study (2016–2019). Obes Pillars. (2024) 12:100126. doi: 10.1016/j.obpill.2024.100126
49. Koskinas KC, Van Craenenbroeck EM, Antoniades C, Blüher M, Gorter TM, Hanssen H, et al. Obesity and cardiovascular disease: an ESC clinical consensus statement. Eur J Prev Cardiol. (2024) 00:1–37. doi: 10.1093/eurjpc/zwae279
50. Sha R, Baines O, Hayes A, Tompkins K, Kalla M, Holmes AP, et al. Impact of obesity on atrial fibrillation pathogenesis and treatment options. J Am Heart Assoc. (2024) 13:e032277. doi: 10.1161/JAHA.123.032277
51. Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol. (2015) 402:113–9. doi: 10.1016/j.mce.2014.11.029
52. Neeland IJ, Ayers CR, Rohatgi AK, Turer AT, Berry JD, Das SR, et al. Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obes (Silver Spring). (2013) 21:E439–47. doi: 10.1002/oby.20135
53. Vermeulen A, Goemaere S, Kaufman JM. Testosterone, body composition and aging. J Endocrinol Invest. (1999) 22:110–6.
54. Bloor ID, Symonds ME. Sexual dimorphism in white and brown adipose tissue with obesity and inflammation. Horm Behav. (2014) 66:95–103. doi: 10.1016/j.yhbeh.2014.02.007
55. Jeffery E, Wing A, Holtrup B, Sebo Z, Kaplan JL, Saavedra-Peña R, et al. The adipose tissue microenvironment regulates depot-specific adipogenesis in obesity. Cell Metab. (2016) 24:142–50. doi: 10.1016/j.cmet.2016.05.012
56. Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. (2007) 297:1465–77. doi: 10.1001/jama.297.13.1465
57. Efremidis M, Vlachos K, Kyriakopoulou M, Mililis P, Martin CA, Bazoukis G, et al. The RV1-V3 transition ratio: A novel electrocardiographic criterion for the differentiation of right versus left outflow tract premature ventricular complexes. Heart Rhythm O2. (2021) 2:521–8. doi: 10.1016/j.hroo.2021.07.009
58. Najaf Zadeh S, Malagutti P, Sartore L, Madhkour R, Berto MB, Gräni C, et al. Prognostic value of advanced echocardiography in patients with ischemic heart disease: A comprehensive review. Echocardiography. (2025) 42:e70065. doi: 10.1111/echo.70065
Keywords: cardiometabolic index, all-cause mortality, specific mortality, gender differences, NHANES
Citation: Li T, Zhou H and Zhou H (2025) Gender differences in the relationship between cardiometabolic index and all-cause and specific mortality in the United States adults: a national study. Front. Endocrinol. 16:1525815. doi: 10.3389/fendo.2025.1525815
Received: 10 November 2024; Accepted: 24 January 2025;
Published: 17 February 2025.
Edited by:
Linwei Tian, The University of Hong Kong, Hong Kong SAR, ChinaCopyright © 2025 Li, Zhou and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua Zhou, emhvdWh1YUBzaHV0Y20uZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.