- 1Department of Cardiovascular Medicine, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, China
- 2Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, PA, United States
Background: With a focus on metabolism-related cardiovascular diseases, the triglyceride-glucose (TyG) index has been used as a surrogate marker of insulin resistance in the prognosis of coronary heart disease. However, the prognostic role of the TyG index in patients with elevated triglycerides, still requires further research. This study aimed to investigate the association between the TyG index and Major Adverse Cardiac and Cerebrovascular Events (MACCE) in patients with hypertriglyceridemia undergoing drug-eluting stent percutaneous coronary intervention (DES-PCI).
Methods: Out of 2250 patients, 813 with hypertriglyceridemia who underwent DES-PCI were retrospectively analyzed. MACCE was regarded as the primary endpoint. Kaplan–Meier (KM) curves were used to evaluate the association between the TyG index and different endpoints. Restricted cubic spline (RCS) analysis was used to examine the relation between the TyG index and MACCE. Subgroup analysis was conducted to further evaluate the interaction between the TyG index and subgroup indicators.
Results: Cox regression analysis identified the TyG index as an independent predictor of MACCE (hazard ratio [HR] 1.53, 95% confidence interval [CI] 1.15–2.04, P = 0.004). Receiver operating characteristic (ROC) analysis determined 9.19 as the cutoff value of TyG index. The Kaplan–Meier curve indicated that patients with a TyG index > 9.19 had higher risks of MACCE (HR 2.23, 95% CI 1.35–3.67, P = 0.002), MACE (HR 2.38, 95% CI 1.39–4.09, P = 0.002), unplanned repeat revascularization (HR 2.05, 95% CI 1.02–4.09, P = 0.043) and all-cause death (HR 3.31, 95%CI 1.15–9.47, P = 0.026) than those of patients with a low TyG index. RCS analysis revealed a linear relation between the TyG index and MACCE risk (P for nonlinearity = 0.879, P for overall trend = 0.044).
Conclusions: This study demonstrated that a high TyG index is associated with an increased risk of MACCE, suggesting that the TyG index may serve as a valuable prognostic marker in patients with hypertriglyceridemia undergoing DES-PCI.
Introduction
Atherosclerotic cardiovascular disease is the primary cause of chronic heart disease, resulting in continuous clinical and economic burdens. Although the low-density lipoprotein cholesterol (LDL-C) has been the primary target of treatment for decades, the effect of triglycerides (TGs) on residual lipoprotein risk remains controversial (1). Hypertriglyceridemia is characterized by an elevated plasma TG level exceeding 150 mg/dL (1.7 mmol/L). Mild-to-moderate hypertriglyceridemia is mainly associated with the development of atherosclerotic plaques, even leading to pancreatic dysfunction (2). The incidence of elevated TG levels and hypertriglyceridemia in the Chinese population has been rising annually (3). According to the DYSIS-China National Cross-Sectional Study, even after initiating treatment with cholesterol-lowering medications, such as statins, at least 40% of patients exhibit persistently elevated TG levels (4).
The TyG index has been found to correlate with many metabolism-related diseases, such as non-alcoholic fatty liver disease, stroke and kidney injury. Insulin resistance (IR), which involves impaired glucose absorption and utilization, plays an important role in the development of metabolic syndrome and cardiovascular disease (5). The hyper insulinemic-euglycemic clamp (HIEC) technique is the gold standard for quantifying IR. The HIEC technique uses the simultaneous infusion of exogenous insulin and glucose to increase plasma insulin levels while maintaining blood glucose at basal homeostasis levels to evaluate insulin sensitivity but is limited because of its cost and complexity. The homeostasis model assessment of IR (HOMA-IR) technique offers similar results to HIEC within a specific range. However, when islet cells are reduced or fail, accurate quantification of IR cannot be performed (6). The TyG index, a non-insulin-based marker, offers a more cost-effective alternative by combining fasting blood glucose and TG measurements (7). The TyG index has demonstrated a stronger association with diabetes in Korean individuals who were not underweight or obese than in those with HOMA-IR (8). A comparison of the TyG index, TG/high-density lipoprotein cholesterol (HDL-C) ratio levels in patients who underwent PCI at Fu Wai hospital showed that the TyG index is a valuable non-insulin-based parameter for metabolic evaluation in patients undergoing PCI (9). A largescale real-world study across five continents found that the TyG index was closely related to future cardiac-cerebral vascular diseases and diabetes (10). Despite these findings, the relation between the TyG index and hypertriglyceridemia requires further exploration. Our study aimed to investigate the relation between the TyG index and Major Adverse Cardiac and Cerebrovascular Events (MACCE) in patients with hypertriglyceridemia undergoing drug-eluting stent percutaneous coronary intervention (DES-PCI), addressing the gap in studies regarding the TyG index in lipid metabolism disorders. These findings may provide a valuable reference for lipid-lowering treatment post-DES-PCI and offer new evidence for the application of the TyG index in patients with coronary artery disease (CAD).
Materials and methods
Study population
This was a single-center, retrospective, observational cohort study. Data on consecutive patients who underwent DES-PCI were obtained from the Hospital Information System Database at the First Hospital Affiliated of Xi’an Jiaotong University, between June 2013 and September 2016. Hypertriglyceridemia was defined as fasting plasma TG ≥ 1.7 mmol/L. The inclusion criteria were as follows: (1) age ≥ 18 years; (2) diagnosed with CAD; and (3) having undergone PCI and DES implantation. The exclusion criteria are shown in Figure 1. Of the 2250 patients, 813 were ultimately enrolled in the study after excluding patients with plasma TG levels < 1.7 mmol/L (n = 1319) and those who lacked lipid or glucose test results (n = 63). Patient lost to follow-up (n = 45) were also excluded. Patients were classified into two groups based on the occurrence of MACCE during the follow-up period: the MACCE (n = 100) and no-MACCE groups (n = 713).
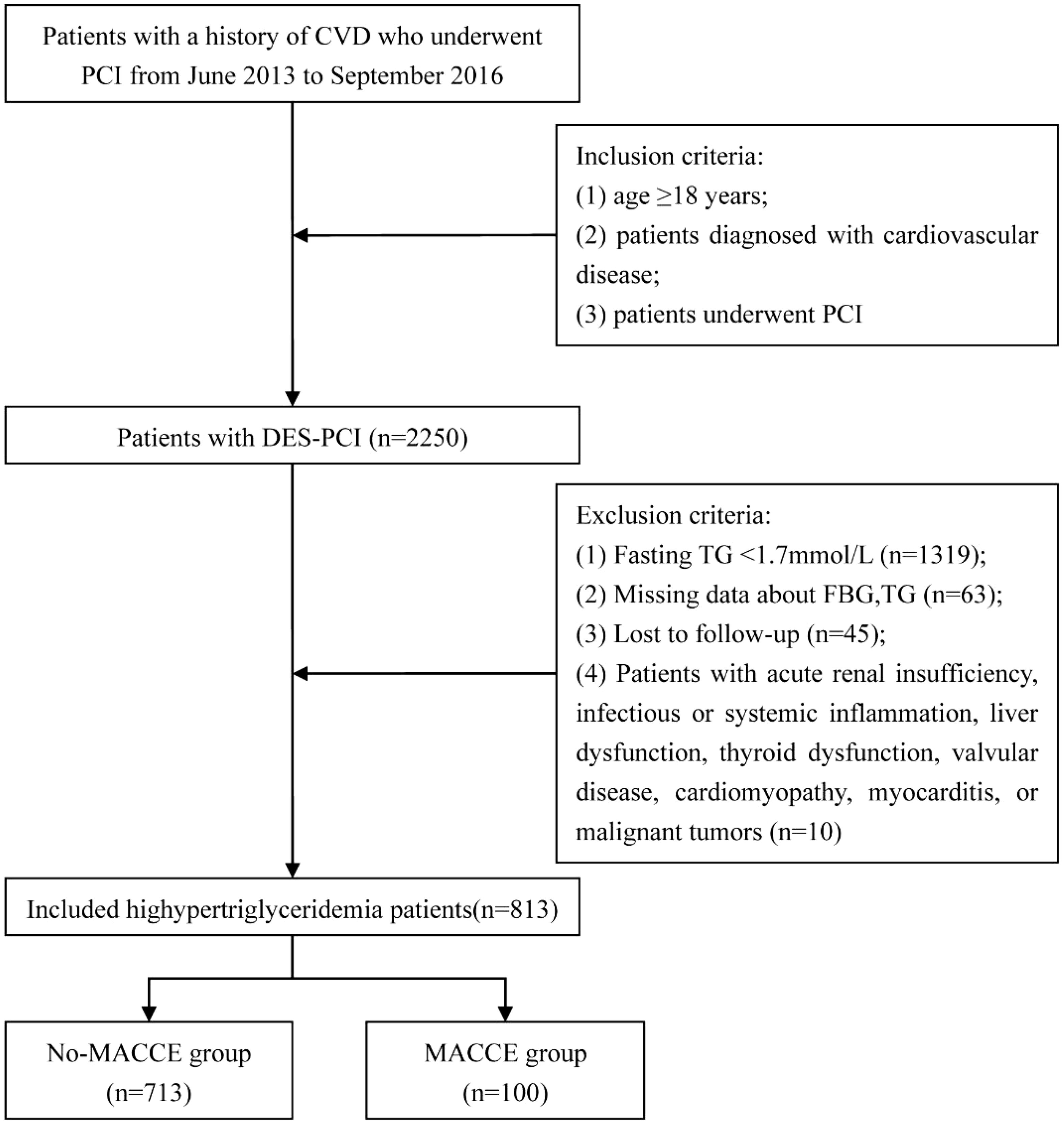
Figure 1. Enrollment flow chart. DES-PCI, drug-eluting stent percutaneous coronary intervention; FBG, fasting blood glucose; TG, triglyceride; MACCE, major adverse cardiac and cerebrovascular events; TyG, triglyceride-glucose index.
Data collection and definitions
Demographic characteristics, clinical history, family history of CAD, laboratory indicators, and angiographic data, were obtained from the electronic medical record system of the First Affiliated Hospital of Xi’an Jiaotong University. The demographic characteristics included age, sex, baseline blood pressure, and heart rate. Patient history included smoking, drinking, established type 2 diabetes mellitus (T2DM), hypertension, stroke, previous myocardial infarction, and dyslipidemia. T2DM was defined by the following criteria: FPG levels ≥ 7.0 mmol/L, random blood glucose levels ≥ 11.1 mmol/L, 2- h plasma glucose levels after an oral glucose tolerance test (OGTT) ≥ 11.1 mmol/L, or the utilization of insulin or oral hypoglycemic agents. Dyslipidemia was characterized using the ICD-10 code E78 along with the use of lipid-lowering medications or a total cholesterol level ≥ 240 mg/dL. Hypertension was defined as systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg, or treatment with antihypertensive drugs. Laboratory tests included white blood cell count, platelets, red blood cell count, hemoglobin, total cholesterol, TG, LDL-C, HDL-C, serum creatinine, fasting blood glucose, and glycated hemoglobin A1c (HbA1c). These tests were performed according to standardized procedure and assay systems. Blood samples were collected before PCI. The TyG index was calculated as ln [fasting TG (mg/dL) × fasting glucose (mg/dL)/2].
Percutaneous coronary intervention
DES-PCI, which refers to DES implantation into the target vessel, was performed by experienced operators following standard procedures. All patients received aspirin (300 mg), ticagrelor (180 mg), or clopidogrel (300 mg) before the procedure, and aspirin (100 mg QD), ticagrelor (90 mg BID), or clopidogrel (75 mg QD) after the procedure. Coronary angiography and PCI were performed according to standard protocols. Angiographic results were recorded by the cardiac catheterization laboratory, and every PCI report was reviewed by at least two independent cardiologists.
Follow-up and endpoints
Follow-ups were conducted by doctors, either in outpatient settings or via telephone, during the follow-up period (every 30 d, then every 6 months for up to 6 years). The primary endpoint was a composite of the first occurrence of all MACCEs within the 66-month follow-up duration. The primary endpoint was MACCE, defined as follows: (1) all-cause death, (2) nonfatal myocardial infarction, (3) nonfatal stroke, and (4) unplanned repeat revascularization. The other outcomes were major adverse cardiovascular events (MACE), unplanned repeat revascularization, and all-cause death.
Statistical analysis
Statistical analyses were performed using R version 4.2.1. All tests were two-tailed, with statistical significance set at a P-value < 0.05. Continuous variables are expressed as medians (interquartile range), whereas categorical variables are reported as frequencies and percentages. Receiver operating characteristic (ROC) curves were generated to compare the TyG index with fasting blood glucose, TG, LDL-C, pro–brain natriuretic peptide (pro-BNP), and HbA1c levels to predict MACCE in patients with hypertriglyceridemia. The Kaplan–Meier curve tracked survival based on different endpoints in patients with a high or low TyG index. Cox regression analysis demonstrated an independent association between the TyG index and MACCE occurrence. Subgroup analyses were conducted based on age, sex, hypertension, T2DM, multi-vessel disease, and laboratory measurements.
Results
Baseline characteristics
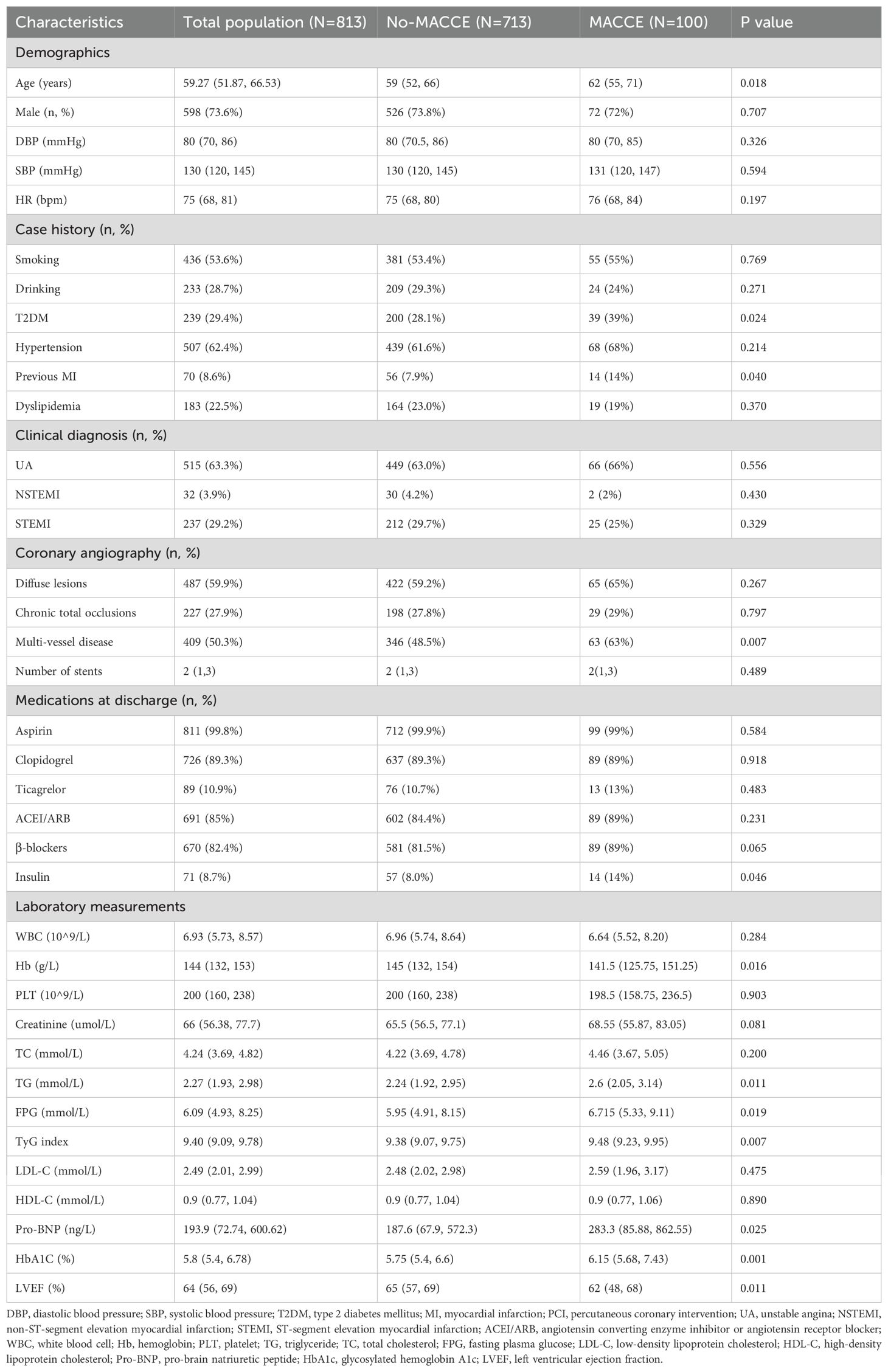
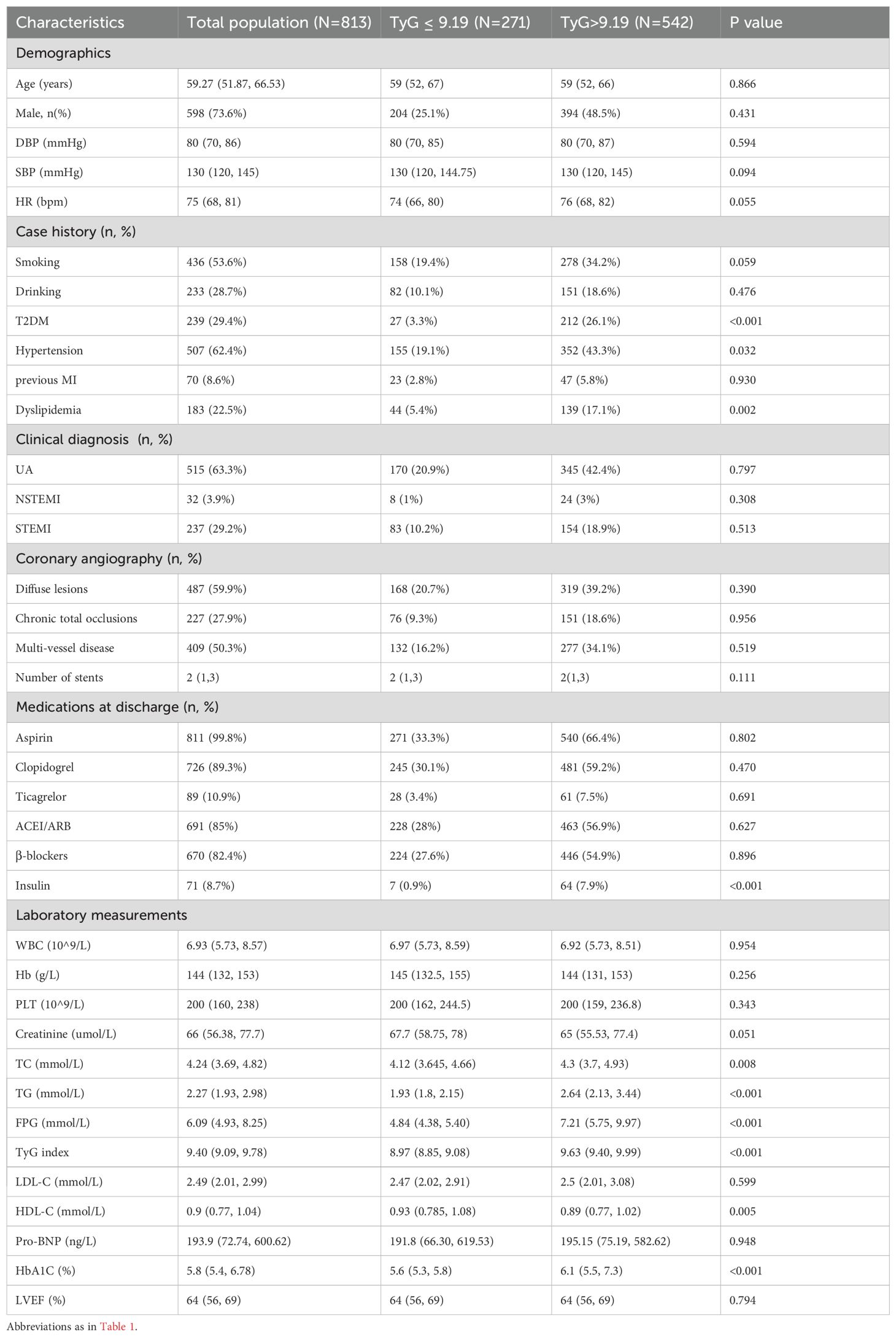
Ultimately, 813 participants were included in the study. The median follow-up period was 66 months (5.5 years). Among these patients, 100 (12.3%) experienced MACCE within 1 year whereas 713 did not (Table 1). The average age of the participants was 59 years, and 73.6% were men. Participants who experienced MACCE had high proportions of previous myocardial infraction, multivessel- CAD, and insulin use (all P < 0.05). Additionally, patients with MACCE had elevated levels of FPG, TG, BNP, and HbA1c, as well as significant differences in left ventricular ejection fraction (LVEF) and hemoglobin (all P < 0.05). Based on the cut-off value of the ROC analysis in Supplementary Figure S1, patients were divided into two groups: TyG < 9.19 and TyG > 9.19 (Table 2). Patients with a TyG index > 9.19 were more likely to have hypertension, T2DM, and dyslipidemia (P < 0.05). Regarding laboratory measurements, patients with a high TyG index tended to have high plasma TG, total cholesterol, FPG, HDL-C, and HbA1C levels(all P < 0.05).
Association between the TyG index and endpoint events
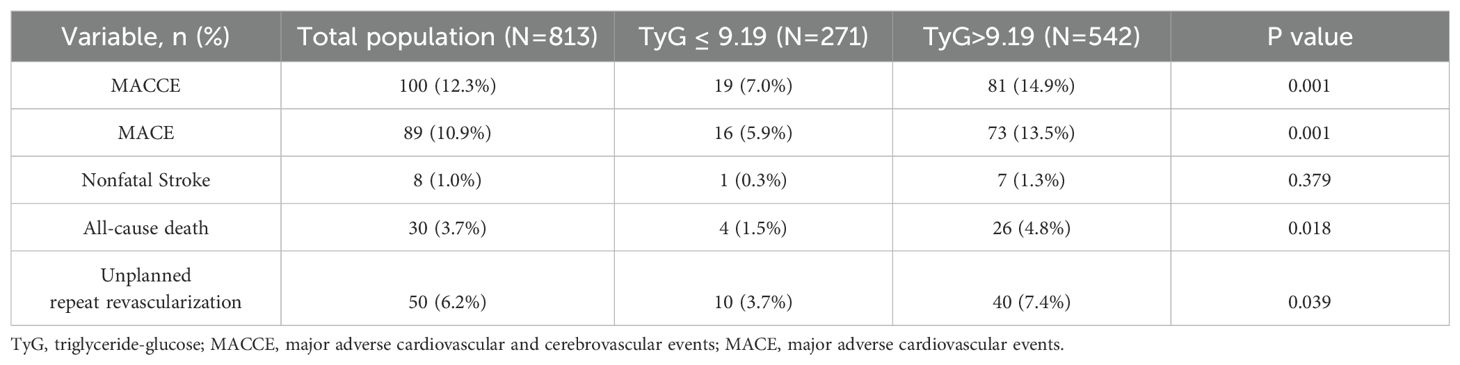
During the maximum follow-up period of 66 months, 100 (12.3%) patients developed MACCE, including 30 (3.7%) all-cause deaths, 12 (1.5%) nonfatal myocardial infarction, 8 (1.0%) nonfatal strokes, and 50 (6.2%) unplanned repeat revascularizations. Compared with those in the low TyG index group, the rates of MACCE (14.9% vs. 7.0%, P < 0.001), MACE (13.5% vs. 5.9%, P < 0.001), all-cause death (4.8% vs. 1.5%, P = 0.018), and unplanned repeat revascularization (7.4% vs. 3.7%, P = 0.039) were significantly higher in the high TyG index group. However, the rates of nonfatal stroke were similar between the two groups (Table 3).
Kaplan–Meier curves showed that individuals with a high TyG index were more likely to develop MACCE (log-rank P = 0.002) and MACE (log-rank P = 0.002) than the patients in the low TyG index group (Figure 2). This increased incidence was primarily attributable to the growing risk of all-cause mortality (log-rank P = 0.026). Additionally, patients in the high TyG index group were more likely to experience unplanned repeat revascularization than those in the low TyG index group (log-rank P = 0.043).
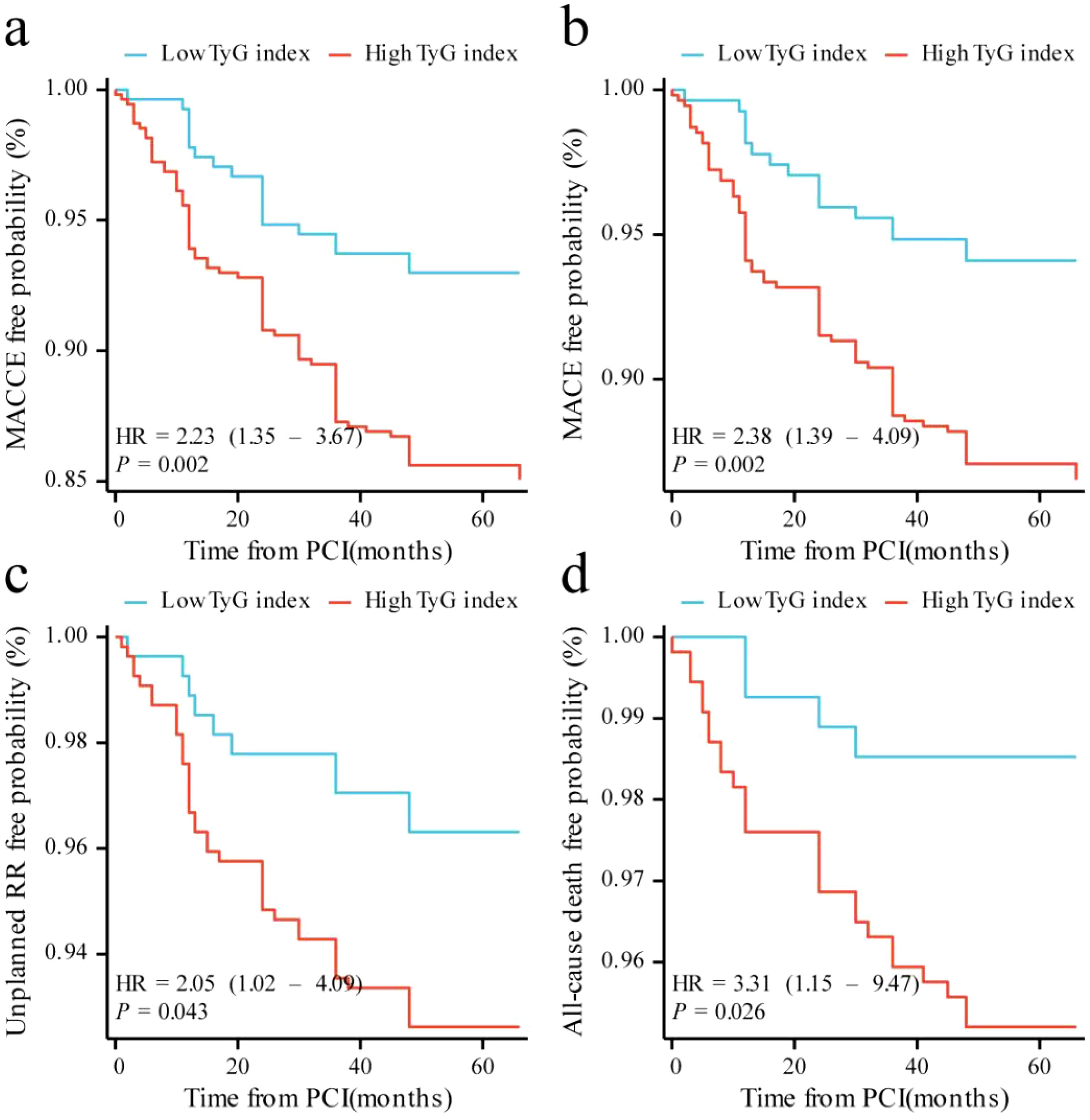
Figure 2. Kaplan–Meier curves for different endpoints: (a) MACCE; (b) MACE; (c) unplanned repeat revascularization; (d) all-cause death. MACCE, major adverse cardiac and cerebrovascular events; MACE, major adverse cardiovascular events; RR, repeat revascularization.
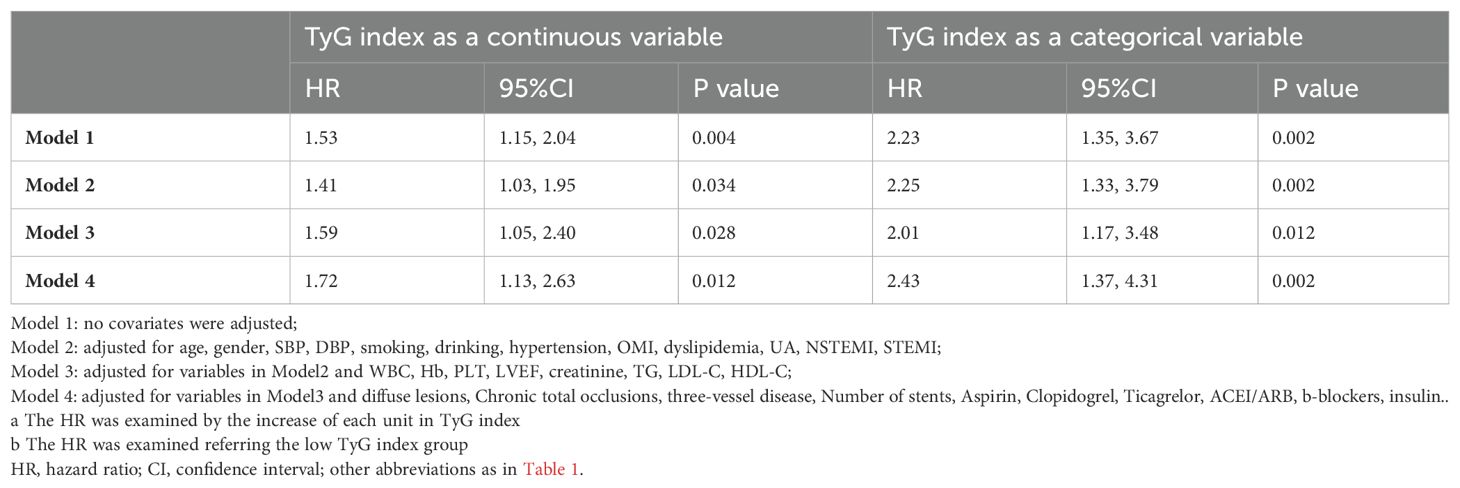
Table 4 presents the predictive value of TyG index for MACCE in different Cox proportional hazards models. Variables were chosen because they included statistically significant factors (P < 0.1) in univariate Cox regression analyses (Supplementary Table S1), as well as factors that might influence clinical outcomes. The TyG index was significantly associated with MACCE before adjustments for any variable (HR, 1.53; 95% CI 1.15–2.04; P = 0.004), with the high TyG index group at elevated risk (HR, 2.23; 95% CI 1.35–3.67; P = 0.002). After adjustment for general information, history, diagnosis (including age, sex, SBP, DBP, smoking, drinking, hypertension, previous MI, dyslipidemia, UA, NSTEMI, and STEMI) in Model 2, the TyG index remained an independent predictor for MACCE (HR, 1.41; 95% CI 1.03–1.95; P = 0.034). After adjustment for variables in Model 2 and key serologic test results (including WBC, Hb, PLT, LVEF, creatinine, TG, LDL-C, and HDL-C) in Model 3, a high TyG index remained significant for the occurrence of MACCE events (HR, 2.01; 95% CI 1.17–3.48; P = 0.012). After adjustment for variables in Model 3, procedure diagnoses and postoperative medications (including diffuse lesions, chronic total occlusions, three-vessel disease, number of stents, aspirin, clopidogrel, ticagrelor, ACEI/ARB, β-blockers, and insulin) in Model 4, the risk of MACCE in the high TyG group was 2.43 times higher than in patients with a TyG index < 9.19 (Model 4: HR, 2.43; 95% CI 1.37–4.31; P = 0.002). With the adjustment in Model 4, restricted cubic spline analysis depicted the relation between the TyG index and MACCE risk (Figure 3; P for nonlinearity = 0.879, P for overall trend = 0.044).
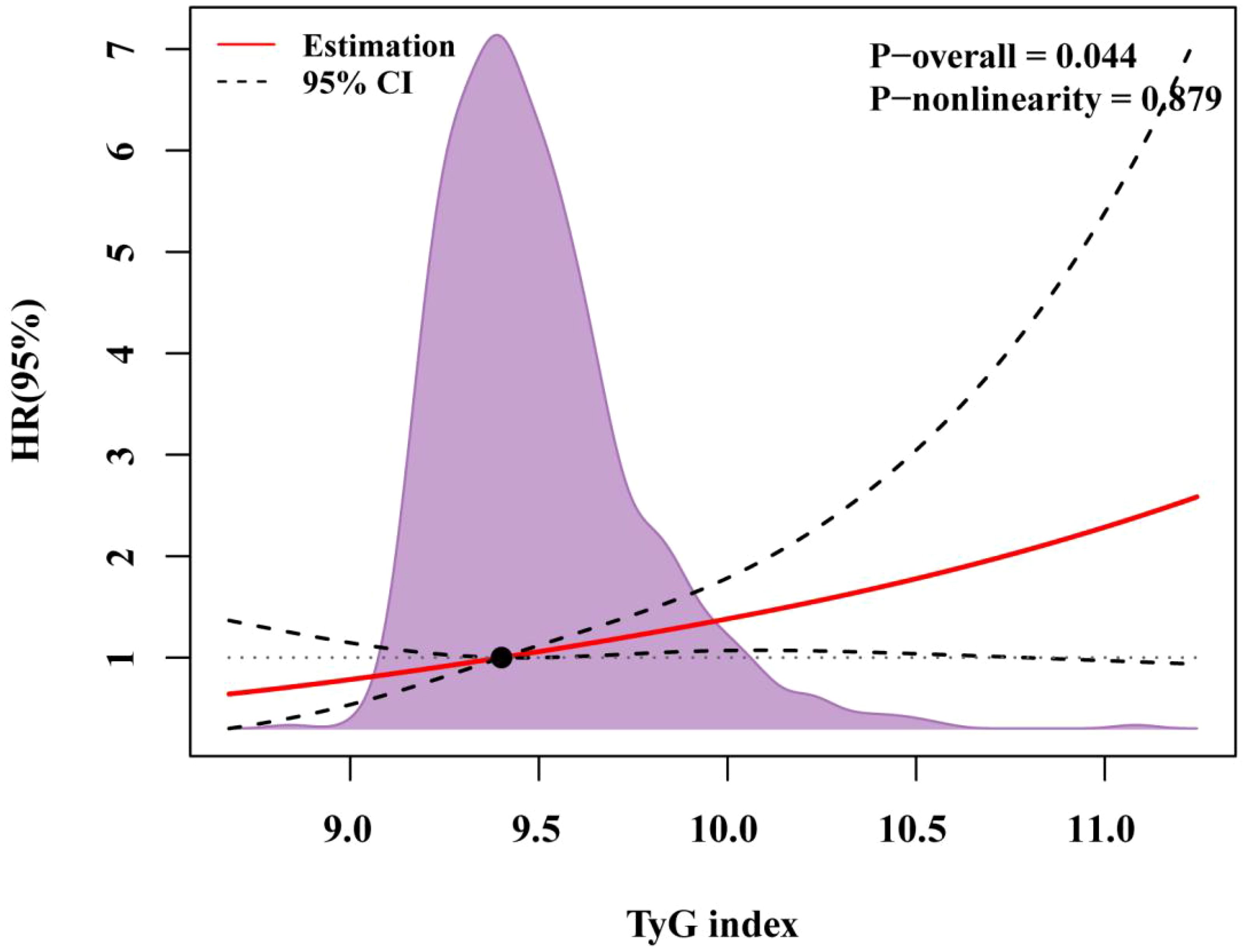
Figure 3. RCS regression for the adjusted dose–response relationship between the TyG index and MACCE. Data of the TyG index for repeat revascularization was fitted with a linear regression model using restricted cubic spines with three knots at the 5th, 50th, and 95th percentiles of the TyG index. Y-axis represents the hazardous ratio, and the dashed lines are 95% confidence intervals. OR, odds ratio.
Subgroup analysis
Subgroup analysis evaluated the influence of the TyG index as a continuous variable on MACCE in Model 4 based on age, sex, hypertension, T2DM, LDL-C, LVEF, and creatinine levels. As shown in Figure 4, no significant interaction was observed among the subgroups (P > 0.05). However, it was observed that in older men with hypertension and multivessel CAD (indicated by coronary angiography), LVEF ≥ 50%, LDL-C < 2.6 mmol/L, and creatinine ≥ 70 µmol/L, the incidence of MACCE was more likely to increase (P < 0.1). In patients undergoing elective PCI surgery, which were diagnosed with NSTEMI or UA, the incidence of MACCE was more likely to increase with the increase of TyG index (P < 0.1).
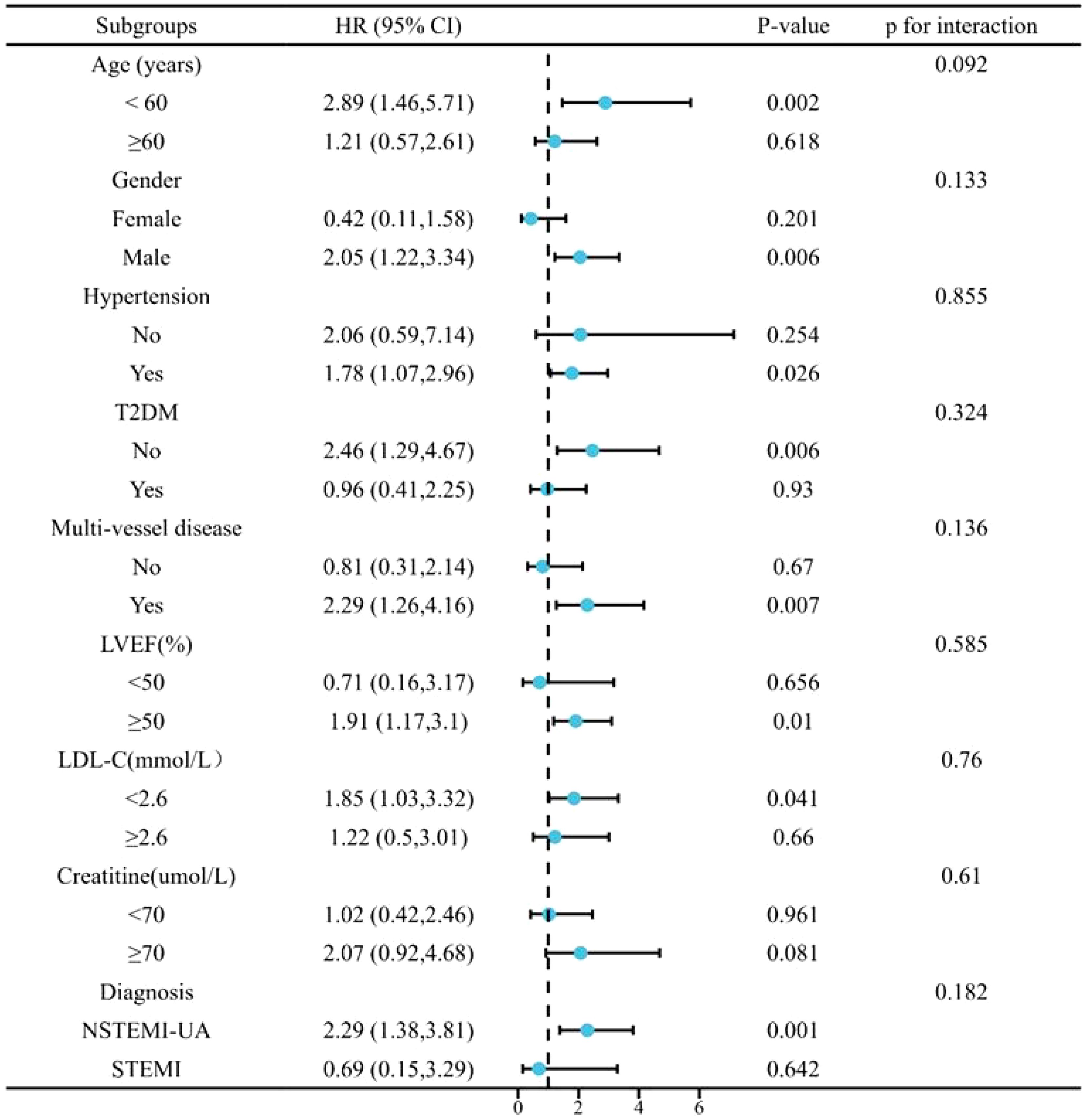
Figure 4. Subgroup analysis for the impact of the TyG index on MACCE. MACCE, major adverse cardiac and cerebrovascular events; TyG, triglyceride-glucose.
Discussion
In our retrospective study, we identified a correlation between the TyG index and MACCE in patients with hypertriglyceridemia undergoing DES-PCI. The major findings were as follows: (1) the TyG index could independently predict MACCE, with its significance remaining after adjusting for all covariates; (2) the risk of MACCE increased with an increase in the TyG index; and (3) patients with a TyG index > 9.19 exhibited higher occurrences of MACCE, MACE, unplanned repeat revascularization, and all-cause death compared with those of patients with a TyG index < 9.19. Our findings suggest the potential value of the TyG index in patients with hypertriglyceridemia who underwent DES-PCI.
The relation between hypercholesterolemia and atherosclerosis is well established, and LDLC lowering therapy serve as the foundation for reducing the risk of atherosclerotic cardiovascular disease (11). However, even with adequate LDL-C control, a significant residual cardiovascular risk remains, with hypertriglyceridemia being one such risk factor (12). Plasma TG levels fluctuate considerably with daily meals. Individuals with underlying lipid or glucose metabolism dysfunction, such as T2DM or obesity, tend to experience prolonged hypertriglyceridemia compared with those without metabolic dysfunction (13). Long-term hypertriglyceridemia can lead to multiple metabolic abnormalities, including hyperglycemia, oxidative stress, and elevated levels of coagulation factors (14).
The TyG index, which incorporates blood glucose and TG levels, is a simple and convenient method for evaluating the metabolic status. As a systemic metabolic dysfunction, IR is considered significantly related to CVD and T2DM (15). Compared to the HIEC and HOMA-IR methods, the TyG index is easier to access through a single blood sample. Zou analyzed the TyG index in different obesity phenotypes and normal body mass index (BMI), categories and demonstrated the prognostic value of the TyG index in metabolic dysfunction (16). Additionally, the concept of cardiovascular-kidney-metabolic syndrome has recently been proposed because of the interplay between metabolic, renal, and cardiovascular diseases (17). Recent prospective studies have indicated that the TyG index can independently predict the incidence of chronic kidney disease in a non-diabetic population (18). By comparing various factors associated with MACCE in patients with CVD after PCI, one study identified the TyG index as a non-insulin-based marker for risk assessment (9). Thus, the TyG index serves as a surrogate marker of metabolic status and may predict outcomes in the CVD population.
Although the TyG index has demonstrated good predictive efficacy in different populations, few studies have focused on patients with hypertriglyceridemia undergoing DES-PCI. In our study, we enrolled 813 patients with elevated TG levels who underwent coronary stent implantation. Patients who experienced MACCE had high TyG and HbA1c levels (Table 1). The baseline TyG index, stratified by a cutoff of 9.19, indicated that patients with a high TyG index were more susceptible to hypertension and diabetes (Table 2). When comparing the TyG index to FPG or HbA1c in predicting MACCE previous studies have suggested that it is more predictive of cardiovascular events in patients with ACS (19). However, these conclusions remain controversial. The ROC curve showed no distinct differences in the predictive effect of the TyG index and HbA1c, LDL-C, or BNP (Supplementary Table S1). These differences may be attributed to the effects of hypoglycemic and lipid-lowering treatments. Additionally, some indicators may have been influenced by non-fasting conditions.
ROC analysis indicated that the cutoff value for predicting MACCE in our patients with hypertriglyceridemia was 9.19. This cutoff value also had predictive value for endpoints, such as MACE, unplanned repeat revascularization, and all-cause death (P < 0.05). Differences in ethnic groups and clinical characteristics may account for variations in the TyG index cutoff values. In previous studies, the cutoff value of the TyG index based on the ROC method ranged between 8.5 and 9 (20). Because our population consists of patients with high TG levels, the cutoff value of the TyG index was higher than that of the general population. In the baseline dataset (Table 2), we observed that patients with a TyG index > 9.19 had higher systolic blood pressure and heart rates, as well as more frequent histories of smoking, comorbid hypertension, or diabetes, than those of patients with a TyG index < 9.19. Regarding medications, more patients received insulin treatment. As a high TyG index reflects the state of IR, it provides a reference for selecting follow-up treatments (including insulin sensitizers) (21).
The TyG index was correlated with the risk of MACCE, and nonlinearity could not be proved (nonlinear P = 0.879, P overall = 0.044), which is consistent with published research (22). An observational study of post-PCI patients with T2DM and non-ST-segment elevation myocardial infarction found that an increase in the TyG index elevated the risk of MACCE, independent of other risk factors (23). Similar findings have been observed in patients with chronic coronary syndromes, regardless of whether they had T2DM (24, 25). These results suggest that even small increases in the TyG index can be clinically significant, emphasizing the importance of monitoring and managing TyG levels in patients to mitigate MACCE risk.
The subgroup analysis in Figure 4 showed that patients with non-T2DM, who may not be suitable for insulin-based measures, had a higher risk of MACCE events with a high TyG index. A high TyG index was also associated with the incidence of MACCE incident in men (< 60 years old), patients with hypertension, non-T2DM, multivessel-CAD, LVEF ≥ 50%, LDL < 2.6 mmol/L, or creatinine ≥ 70 µmol/L (P < 0.1). Recent findings have highlighted the predictive value of the TyG index in chronic kidney disease (26). TyG monitoring warrants further surveillance and intervention in patients with hypertriglyceridemia with those typical.
These findings may aid in identifying high-risk patients, optimizing secondary prevention, and adjusting therapeutic approaches for patients with CVD and lipid metabolism disorders. Further research is needed to verify our findings and uncover the underlying mechanisms between TGs and cardiovascular disease.
Study limitations
This study had some limitations. First, it was a single-center retrospective study based on a relatively small sample size, which was the first to apply the TyG index to the prognosis of patients with hypertriglyceridemia undergoing DES-PCI. Second, the TyG index was calculated on admission, and follow-up examinations require further evaluation. Plasma TG levels may also be affected by other treatments. Moreover, the study lacked relevant data on other indicators of metabolic disorders, such as waist circumference, BMI. Further research on the longitudinal TyG fluctuations due to the effects of different drugs is ongoing. Finally, our results need to be verified in multicenter prospective cohort studies.
Conclusions
Our study is the first to apply the TyG index to patients with hypertriglyceridemia undergoing DES-PCI. In this population, an increase in the TyG index was associated with an elevated risk of MACCE after adjusting for other variables. Patients with a high TyG index (> 9.19) had a higher risk of MACE, unplanned repeat revascularization, and all-cause death than that of patients with a low TyG index.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Clinical Research Ethics Committee of the First Affiliated Teaching Hospital, School of Medicine(SOM), Xi’an Jiaotong University. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from gifted from another research group. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
YW: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. YL: Data curation, Formal Analysis, Investigation, Resources, Writing – original draft. SG: Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing. ZZ: Data curation, Formal Analysis, Investigation, Resources, Writing – original draft. JB: Supervision, Validation, Visualization, Writing – review & editing. BL: Conceptualization, Formal Analysis, Methodology, Resources, Software, Writing – original draft. RF: Conceptualization, Methodology, Software, Visualization, Writing – original draft. NG: Conceptualization, Funding acquisition, Investigation, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (Grant No. 82474212) and Key research and development program of Shaanxi (No. 2023-YBSF-361).
Acknowledgments
We gratefully acknowledge all the investigators and patients who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1519895/full#supplementary-material
Abbreviations
TyG, Triglyceride-glucose; MACCE, Major adverse cardiac and cerebrovascular events; MACEs, Major adverse cardiovascular events; ROC, Receiver operating characteristic; DES-PCI, Drug-eluting stent percutaneous coronary intervention; IR, Insulin resistance; CVDs; Cardiovascular diseases; HOMA-IR: Homeostasis model assessment of insulin resistance; HIEC, Hyper insulinemic-euglycemic clamp; T2DM, Type 2 diabetes mellitus; PCI, Percutaneous coronary intervention; TG, Triglyceride; FPG, Fasting plasma glucose; LDL-C, Low-density lipoprotein cholesterol; HDL-C, High-density lipoprotein cholesterol; Pro-BNP, Pro-brain natriuretic peptide; HbA1c, Glycosylated hemoglobin A1c; LVEF, Left ventricular ejection fraction; HR, Hazard ratio; CI, confidence interval.
References
1. Mourikis P, Zako S, Dannenberg L, Nia AM, Heinen Y, Busch L, et al. Lipid lowering therapy in cardiovascular disease: From myth to molecular reality. Pharmacol Ther. (2020) 213:107592. doi: 10.1016/j.pharmthera.2020.107592
2. Sascău R, Clement A, Radu R, Prisacariu C, Stătescu C. Triglyceride-rich lipoproteins and their remnants as silent promoters of atherosclerotic cardiovascular disease and other metabolic disorders: A review. Nutrients. (2021) 13:1774. doi: 10.3390/nu13061774
3. Hu SS. Cardiovascular risk factors in China. J Geriatr Cardiol. (2024) 21:153–99. doi: 10.26599/1671-5411.2023.06.001
4. Zhao S, Wang Y, Mu Y, Yu B, Ye P, Yan X, et al. Prevalence of dyslipidaemia in patients treated with lipid-lowering agents in China: results of the DYSlipidemia International Study (DYSIS). Atherosclerosis. (2014) 235:463–9. doi: 10.1016/j.atherosclerosis.2014.05.916
5. Minh HV, Tien HA, Sinh CT, Thang DC, Chen CH, Tay JC, et al. Assessment of preferred methods to measure insulin resistance in Asian patients with hypertension. J Clin Hypertens (Greenwich). (2021) 23:529–37. doi: 10.1111/jch.14155
6. Kuroe A, Fukushima M, Usami M, Ikeda M, Nakai Y, Taniguchi A, et al. Impaired beta-cell function and insulin sensitivity in Japanese subjects with normal glucose tolerance. Diabetes Res Clin Pract. (2003) 59:71–7. doi: 10.1016/S0168-8227(02)00177-8
7. Chang WT, Liu CC, Huang YT, Wu JY, Tsai WW, Hung KC, et al. Diagnostic efficacy of the triglyceride-glucose index in the prediction of contrast-induced nephropathy following percutaneous coronary intervention. Front Endocrinol (Lausanne). (2023) 14:1282675. doi: 10.3389/fendo.2023.1282675
8. Kim B, Kim GM, Huh U, Lee J, Kim E. Association of HOMA-IR versus tyG index with diabetes in individuals without underweight or obesity. Healthc (Basel). (2024) 12:2458. doi: 10.3390/healthcare12232458
9. He J, Song C, Yuan S, Bian X, Lin Z, Yang M, et al. Triglyceride-glucose index as a suitable non-insulin-based insulin resistance marker to predict cardiovascular events in patients undergoing complex coronary artery intervention: a large-scale cohort study. Cardiovasc Diabetol. (2024) 23:15. doi: 10.1186/s12933-023-02110-0
10. Lopez-Jaramillo P, Gomez-Arbelaez D, Martinez-Bello D, Abat MEM, Alhabib KF, Avezum Á, et al. Association of the triglyceride glucose index as a measure of insulin resistance with mortality and cardiovascular disease in populations from five continents (PURE study): a prospective cohort study. Lancet Healthy Longev. (2023) 4:e23–33. doi: 10.1016/S2666-7568(22)00247-1
11. Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. (2005) 366:1267–78. doi: 10.1016/S0140-6736(05)67394-1
12. Crea F. Residual lipidic risk beyond low-density lipoprotein cholesterol: new challenges and opportunities. Eur Heart J. (2023) 44:3935–8. doi: 10.1093/eurheartj/ehad671
13. Borén J, Taskinen MR, Björnson E, Packard CJ. Metabolism of triglyceride-rich lipoproteins in health and dyslipidaemia. Nat Rev Cardiol. (2022) 19:577–92. doi: 10.1038/s41569-022-00676-y
14. Raji OE, Kyeremah EB, Sears DD, St-Onge MP, Makarem N. Chrononutrition and cardiometabolic health: an overview of epidemiological evidence and key future research directions. Nutrients. (2024) 16:2332. doi: 10.3390/nu16142332
15. Lee EY, Yang HK, Lee J, Kang B, Yang Y, Lee SH, et al. Triglyceride glucose index, a marker of insulin resistance, is associated with coronary artery stenosis in asymptomatic subjects with type 2 diabetes. Lipids Health Dis. (2016) 15:155. doi: 10.1186/s12944-016-0324-2
16. Zou S, Yang C, Shen R, Wei X, Gong J, Pan Y, et al. Association between the triglyceride-glucose index and the incidence of diabetes in people with different phenotypes of obesity: A retrospective study. Front Endocrinol (Lausanne). (2021) 12:784616. doi: 10.3389/fendo.2021.784616
17. Ndumele CE, Rangaswami J, Chow SL, Neeland IJ, Tuttle KR, Khan SS, et al. Cardiovascular-kidney-metabolic health: A presidential advisory from the American heart association. Circulation. (2023) 148:1606–35. doi: 10.1161/CIR.0000000000001184
18. Chen N, Ma LL, Zhang Y, Chu X, Dong J, Yan YX. Association of long-term triglyceride-glucose index patterns with the incidence of chronic kidney disease among non-diabetic population: evidence from a functional community cohort. Cardiovasc Diabetol. (2024) 23:7. doi: 10.1186/s12933-023-02098-7
19. Hu C, Zhang J, Liu J, Liu Y, Gao A, Zhu Y, et al. Discordance between the triglyceride glucose index and fasting plasma glucose or HbA1C in patients with acute coronary syndrome undergoing percutaneous coronary intervention predicts cardiovascular events: a cohort study from China. Cardiovasc Diabetol. (2020) 19:116. doi: 10.1186/s12933-020-01091-8
20. Tahapary DL, Pratisthita LB, Fitri NA, Marcella C, Wafa S, Kurniawan F, et al. Challenges in the diagnosis of insulin resistance: Focusing on the role of HOMA-IR and Tryglyceride/glucose index. Diabetes Metab Syndr. (2022) 16:102581. doi: 10.1016/j.dsx.2022.102581
21. Lee MJ, Bae JH, Khang AR, Yi D, Yun MS, Kang YH. Triglyceride-glucose index predicts type 2 diabetes mellitus more effectively than oral glucose tolerance test-derived insulin sensitivity and secretion markers. Diabetes Res Clin Pract. (2024) 210:111640. doi: 10.1016/j.diabres.2024.111640
22. Guo X, Shen R, Yan S, Su Y, Ma L. Triglyceride-glucose index for predicting repeat revascularization and in-stent restenosis in patients with chronic coronary syndrome undergoing percutaneous coronary intervention. Cardiovasc Diabetol. (2023) 22:43. doi: 10.1186/s12933-023-01779-7
23. Zhao Q, Zhang TY, Cheng YJ, Ma Y, Xu YK, Yang JQ, et al. Impacts of triglyceride-glucose index on prognosis of patients with type 2 diabetes mellitus and non-ST-segment elevation acute coronary syndrome: results from an observational cohort study in China. Cardiovasc Diabetol. (2020) 19:108. doi: 10.1186/s12933-020-01086-5
24. Tao S, Yu L, Li J, Xie Z, Huang L, Yang D, et al. Prognostic value of triglyceride-glucose index in patients with chronic coronary syndrome undergoing percutaneous coronary intervention. Cardiovasc Diabetol. (2023) 22:322. doi: 10.1186/s12933-023-02060-7
25. Zhang Y, Ding X, Hua B, Liu Q, Gao H, Chen H, et al. Predictive effect of triglyceride−glucose index on clinical events in patients with type 2 diabetes mellitus and acute myocardial infarction: results from an observational cohort study in China. Cardiovasc Diabetol. (2021) 20:43. doi: 10.1186/s12933-021-01236-3
Keywords: triglyceride-glucose index, triglycerides, coronary artery disease, percutaneous coronary intervention, hypertriglyceridemia
Citation: Wang Y, Lu Y, Gao S, Zhong Z, Bao JY, Liu B, Fan R and Guo N (2025) Association between high triglyceride-glucose index and MACCE in hypertriglyceridemia patients undergoing percutaneous coronary intervention. Front. Endocrinol. 16:1519895. doi: 10.3389/fendo.2025.1519895
Received: 30 October 2024; Accepted: 27 March 2025;
Published: 14 April 2025.
Edited by:
Linwei Tian, The University of Hong Kong, Hong Kong SAR, ChinaReviewed by:
Marat V. Ezhov, Ministry of Health of the Russian Federation, RussiaJerzy Beltowski, Medical University of Lublin, Poland
Brendon Pearce, Stellenbosch University, South Africa
Copyright © 2025 Wang, Lu, Gao, Zhong, Bao, Liu, Fan and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Guo, bmd1b21kQG1haWwueGp0dS5lZHUuY24=
 Yichuan Wang1
Yichuan Wang1 Ning Guo
Ning Guo