- 1Division of Gynecological Endocrinology and Reproductive Medicine, Women’s University Hospital, Inselspital Bern, University of Bern, Bern, Switzerland
- 2Division of Reproductive Endocrinology and Infertility, University Hospital Basel, Basel, Switzerland
- 3Department of Gynecology and Obstetrics, Hospital Wolhusen, Luzern, Switzerland
- 4Department of Gynecology and Obstetrics, Bern University Hospital, University of Bern, Bern, Switzerland
- 5Medical Library, University Library of Bern, University of Bern, Bern, Switzerland
Background: Cesarean sections are becoming more common worldwide. One of the long-term complications of cesarean section is a cesarean scar defect or isthmocele. The presence of isthmocele is associated with infertility.
Objectives: This systematic review and meta-analysis examined the effect of laparoscopic isthmocele repair on the reproductive outcomes of patients with and without infertility.
Search strategy: We searched MEDLINE, EMBASE, and the Cochrane CENTRAL databases in April 2024.
Selection criteria: The study included cohort studies, case-control studies, and case series reporting reproductive outcomes after laparoscopic isthmocele repair among women with or without diagnosed infertility.
Data collection and analysis: The meta-analysis examined rates of live birth, pregnancy, and miscarriage.
Main results: The search identified 866 records and 17 articles were included. Clinical pregnancy rates after isthmocele resection were 62% (95% confidence interval (CI) 54-69%) in women with infertility, compared to 33% (95% CI: 16-57%) in women without infertility and 36% in women with unknown fertility status (36%, 95% CI: 21–55%). Live birth rates were 72% (95% CI: 54–85%) among those with infertility, 78% (95% CI: 46–94%) among those without infertility, and 61% (95% CI: 42–77%) with unknown fertility status. Women with and without infertility had low miscarriage rates of 10% (95% CI: 6–16%) and 7% (95% CI: 3–18%), respectively. The prevalence of co-existing endometriosis was 29% (95% CI: 22–37%). The statistical heterogeneity of the studies ranged from 0 to 86%.
Conclusions: Laparoscopic isthmocele repair has demonstrated the potential to improve reproductive outcomes, specifically in cases where infertility is linked to isthmocele-related factors, such as challenges during embryo transfer or impaired implantation. However, further well-designed multicenter trials must confirm these findings and provide stronger evidence.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier (CRD42024548864).
Introduction
The prevalence of cesarean deliveries is rising at an alarming rate worldwide (1). The World Health Organization (WHO) has determined that the optimal rate for cesarean delivery is 15% (2, 3), but a global rate of 29% is predicted for 2030. This increase is primarily attributed to the expansion of indications for primary cesarean delivery and a notable decline in vaginal deliveries following a previous cesarean delivery (4).
This increasing cesarean rate is associated with a corresponding increase in short- and long-term complications (5–7). One of the long-term complications is the formation of a cesarean scar defect, which is also referred to as an isthmocele. The European Niche Taskforce has formally defined a cesarean scar defect as a groove in the uterine myometrium of at least 2 mm at the site of the cesarean scar according to transvaginal ultrasound (8, 9). The incidence of Isthmocele is as high as 70% among women who have previously undergone a cesarean section. Approximately 30% of these women experience symptoms (10).
Isthmocele can result in abnormal uterine bleeding, pelvic pain, and ectopic pregnancy (11–13). Furthermore, postmenstrual spotting is associated with Isthmocele volume and is inversely related to residual myometrial thickness (14–16).
One of the most relevant consequences is the risk of infertility. Several studies have shown a decrease of 15-40% in pregnancy and live birth rates following cesarean sections (17–19). In the case of isthmocele, pregnancy, and live birth rates have been reported to be as low as 20%-30%, depending on the size of the defect and whether surgical correction has been attempted (20). Vissers et al. presented potential mechanisms for infertility associated with isthmoceles: Random damage to the environment for sperm penetration and implantation may occur, and intrauterine fluid (mucus or blood) related to the isthmocele may accumulate, potentially hindering implantation. Furthermore, changes in immunobiology or increased inflammation may arise, and distorted uterine contractility may result from fibrosis or disruption of the myometrial layer at the site of the isthmocele, which acts as a physical barrier to embryo transfer and implantation (21).
In recent years, surgical techniques have been developed to treat symptomatic isthmoceles, including laparoscopic excision, resectoscopic, vaginal, and laparotomy repair. Women with isthmocele-associated infertility should be treated individually with a multidisciplinary approach. It has been suggested that isthmocele repair may have a beneficial effect on secondary infertility after cesarean section (22). However, there are no general guidelines for the treatment of isthmocele in cases of infertility.
Laparoscopic surgery offers the additional advantage of diagnosing and treating other potential causes of infertility concurrently (23, 24). In cases where coexisting endometriosis is present, the affected tissue can be resected during the same surgical procedure (25). In some cases, laparoscopy is combined with hysteroscopy, thereby enhancing the visibility of the isthmocele (25, 26).
Currently, there is no conclusive evidence in support of the use of these surgical techniques for reproductive purposes. Therefore, this systematic review and meta-analysis aims to evaluate the results of laparoscopic correction of isthmocele among women with and without infertility and to analyze its impact on reproductive outcomes.
Materials and methods
Registration of protocols
The study protocol was registered under the Prospective International Registry of Systematic Reviews, PROSPERO (registry number CRD42024548864). The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used (27).
Search strategy
A systematic literature search was conducted using the Medline, Embase, and Cochrane CENTRAL databases in April 2024. An initial MEDLINE search strategy was developed by a medical information specialist and tested with a list of basic references. After refinement and querying, complex search strategies were established for each information source based on database-specific controlled vocabulary (thesaurus terms/subject headings) and text words. Synonyms, acronyms, and similar terms were included in the text word search. The search was limited to publications from 1946 to the present. The search terms included “isthmocele”, “niche”, “cesarean section”, “laparoscopic repair”, “Rendez-vous”, and “fertility and pregnancy outcome”. We incorporated respective thesaurus terms and used synonyms, acronyms, and similar terms for all concepts in the text word search. Animal-only studies were excluded from the MEDLINE and Embase searches using a double negative search strategy based on Ovid “humans only” filters. The detailed final search strategies are presented as a Supplementary File (S1). In addition to searching the electronic databases, reference lists and bibliographies of relevant publications were checked for relevant studies. All identified citations were imported into Covidence and duplicates were removed automatically (28).
Inclusion and exclusion criteria
Investigators AV, JG, and VV independently assessed studies for inclusion using the Covidence software (Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia, www.covidence.org) (29). The eligibility was based on original articles revealing information on reproductive outcomes in patients with or without infertility after laparoscopic resection of isthmocele. Studies that included therapeutic interventions with vaginal techniques or hysteroscopic or repair by laparotomy, as well as studies with an inadequate design or based on animals were excluded.
Data extraction
The extracted data were abstracted and reviewed in detail by three investigators (AV, JG, and VV) independently. Primary variables of interest included study population characteristics such as patient age, cause and duration of infertility, niche size, pre- and post-intervention RMT, duration of follow-up, presence of endometriosis, and reproductive outcomes (conception method, clinical pregnancy rate, miscarriage rate, and live birth rate). Disagreements were discussed and resolved by consensus.
Selected groups
The general focus of this study was on women with a desire to become pregnant and a diagnosis of an isthmocele. The population was divided into three groups. The first group comprised women with infertility. Infertility is internationally defined by the World Health Organization (WHO) as the inability to conceive after 12 months of regular, unprotected sexual intercourse. This definition was adopted for its global reach and for representing a well-established clinical and epidemiological standard, taking into account the discrepancies among the clinical guidelines of the American Society for Reproductive Medicine (ASRM), the National Institute for Health and Care Excellence (NICE), and ESHRE (30–32). The second group comprised women who wanted to conceive but did not have infertility, and the third group comprised women with reported results and unknown fertility status.
Outcomes
Only studies that assess one or more of the following reproductive outcomes were included (32): clinical pregnancy (CP), miscarriage (MC), and live birth (LB). Clinical pregnancy was defined as pregnancy documented by ultrasound with a gestational sac in the uterus. Miscarriage was defined as the spontaneous loss of a fetus before 20 weeks of gestation, and live birth was defined as a delivery that resulted in a live newborn.
Quality assessment
The Newcastle-Ottawa Scale (NOS) was used for the quality assessment of each study (33). Three parameters were considered for each study: subject selection (0-4 stars), comparability (0-2 stars), and study outcome (0-3 stars). The scoring was as follows: Good quality (= 3 or 4 stars in the selection domain AND 1 or 2 stars in the comparability domain AND 2 or 3 stars in the outcome/exposure domain), fair quality (= 2 stars in the selection domain AND 1 or 2 stars in the comparability domain AND 2 or 3 stars in the outcome/exposure domain), and poor quality (= 0 or 1 star in the selection domain OR 0 stars in the comparability domain OR 0 or 1 stars in the outcome/exposure domain). All included studies were reviewed by AV and VV Independently to assess the risk of bias. Disagreements were resolved by consensus.
Data synthesis
The primary outcome of our systematic review was the reproductive outcomes (CP, M, LB) after laparoscopic isthmocele repair. For the pooled ORs, statistical analyses were performed with the “metaphor” function of the R software (R Core Team, Vienna, Austria, 2013). Heterogeneity was examined using Cohen’s Q statistic and the I2 statistic. In the presence of high heterogeneity, random-effects models were used.
Results
Results of the systematic review
A total of 3685 citations were identified from searching the databases. Seventy-eight studies remained after screening the abstracts and full text of the study topic. However, we excluded 61 of these studies for failing to meet our pre-specified inclusion criteria. Therefore, 17 articles were included in the systematic review (Figure 1).
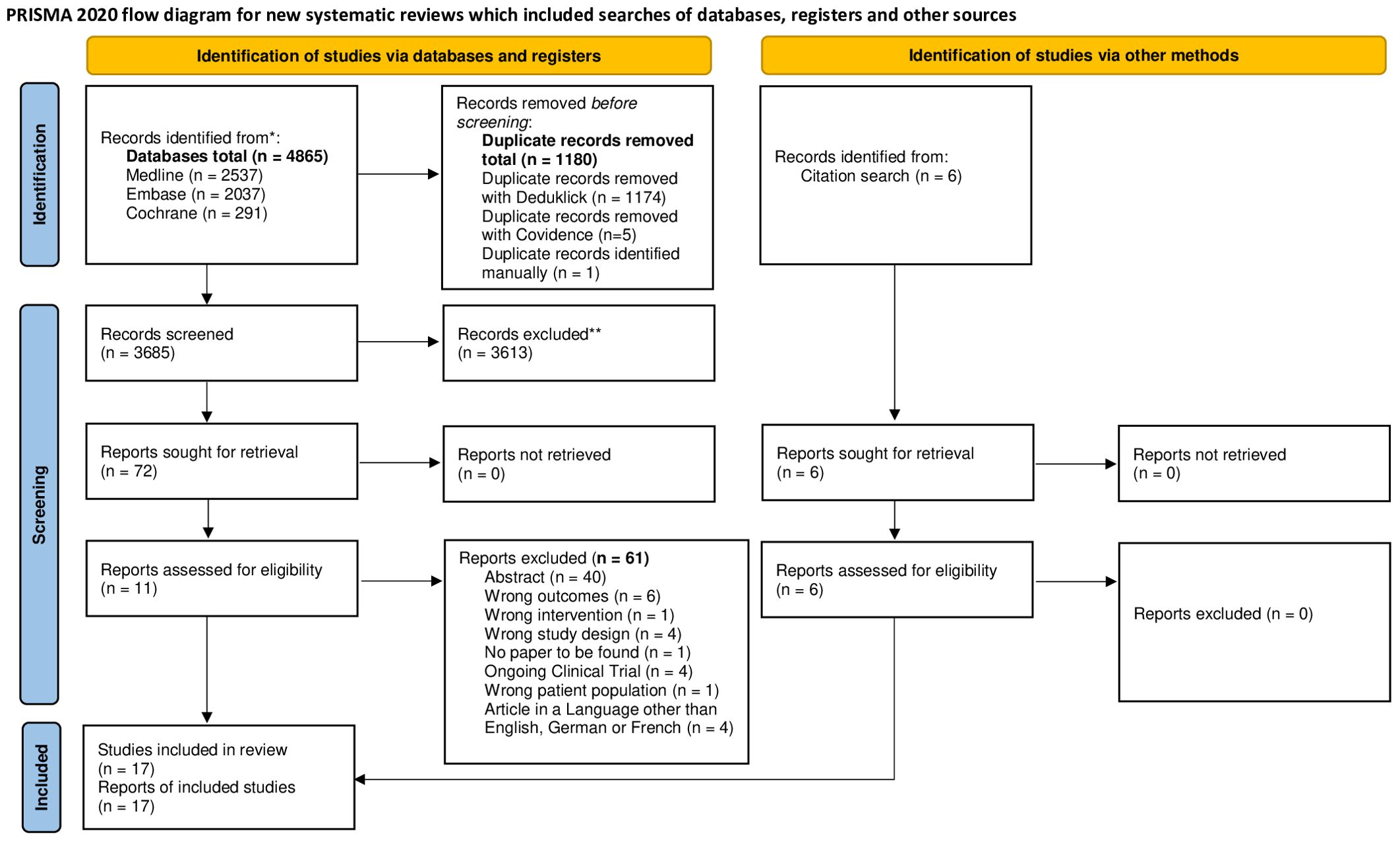
Figure 1. PRISMA flow diagram. FLOWCHART of the literature search and selection process. *Consider, if feasible to do so, reporting the number of records identified from each database or register searched (rather than the total number across all databases/registers). **If automation tools were used, indicate how many records were excluded by a human and how many were excluded by automation tools. Source: Page MJ, et al. BMJ 2021;372:n71. doi: 10.1136/bmj.n71. This work is licensed under CC BY 4.0. To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/.
Study characteristics
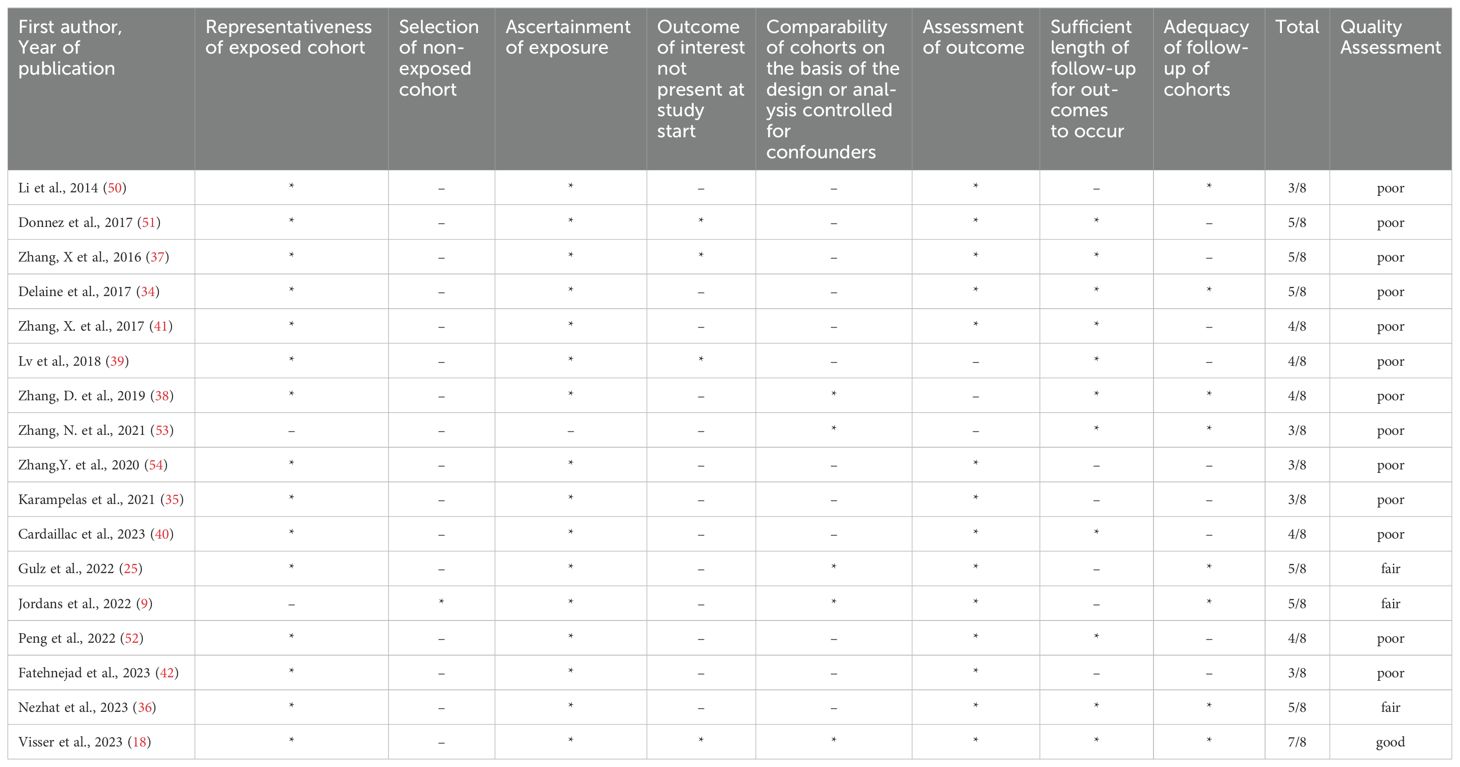
The characteristics of the study populations are summarized in Table 1. The included studies were retrospective (n = 14) and prospective (n = 3). They were conducted in different regions, including Europe (n = 8), America (n = 1), and Asia (n = 8). In total, 866 women were included in the review, of whom 309 (35.6%) (17 studies) were eligible for meta-analysis. The sample sizes of the studies varied considerably, ranging from 9 to 146 patients.
We identified one good-quality study (18). The methodological quality of the majority of these studies was rated as either poor (n = 13) or fair (n = 3), mainly due to the lack of a comparison group (Table 2).
The included studies vary in design, population size, diagnostic methods, and outcome measures, highlighting the heterogeneity in isthmocele repair literature. The sample sizes range from small case series (n=9) (34) to larger prospective cohort studies (n=133) (18), reflecting different levels of statistical power. Study populations also differ, with some focusing exclusively on laparoscopic repair (23, 35) and others incorporating combined laparoscopic and hysteroscopy approaches (36, 37).
Of the included articles, 7 studies comprised infertile women with isthmocele (206 women). Five (18, 23, 25, 38, 39) studies described reproductive outcomes in women with a niche without a diagnosis of infertility (118 women) and with unknown fertility status (522 women). Eleven of the studies included a combination of women with and without infertility. Of the 8 studies reporting women with infertility, 2 reported infertility definition or duration. Only 3 studies explicitly examined cases under infertility treatment (Table 3). Importantly, only some studies included patients undergoing ART, with notable variations in ART rates (18, 36). Reproductive outcomes among women depending on their fertility status after isthmocele repair surgery are shown in Tables 3, 4.
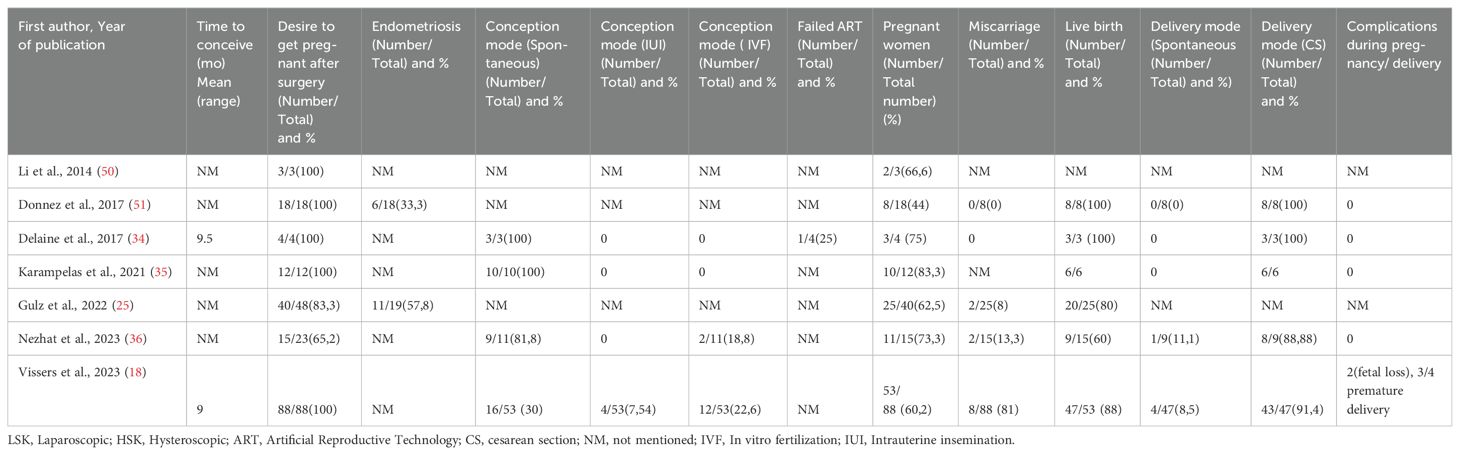
Table 3. Summary results of the included studies: Reproductive outcomes in women with infertility after laparoscopic isthmocele repair.
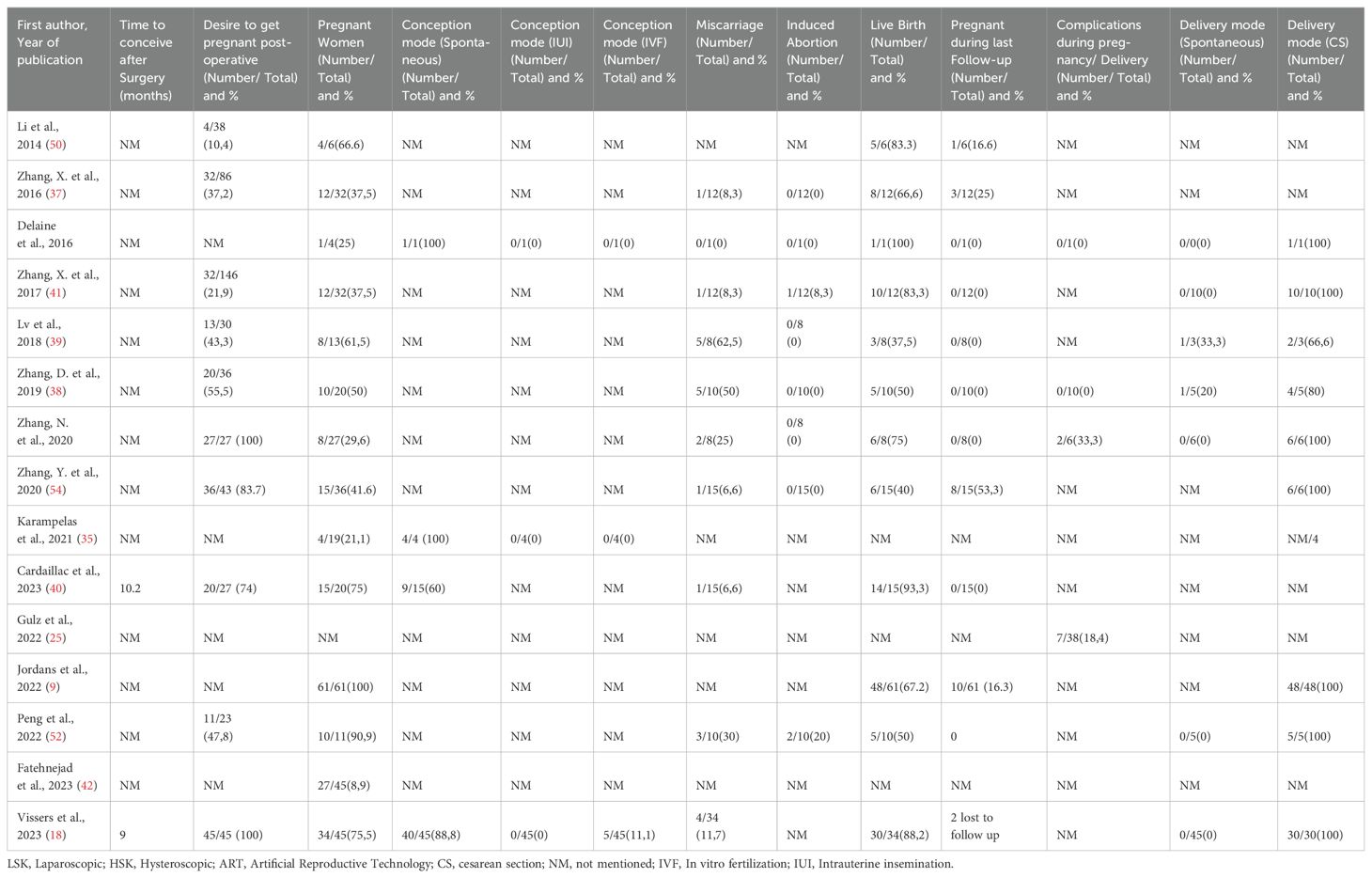
Table 4. Summary results of the included studies: Reproductive outcomes in women without infertility and unknown fertility after laparoscopic isthmocele repair.
Preoperative diagnostic methods varied, with most studies utilizing transvaginal sonography (TVS), while some included additional imaging techniques such as MRI and saline infusion sonohysterography (23, 34, 36). Differences in niche size assessment and residual myometrial thickness (RMT) measurement further complicate comparisons across studies. Some studies reported significant improvements in RMT post-surgery (38, 40), whereas others did not assess this outcome.
Residual myometrial thickness ranged from 2.5 mm to 5 mm in all women who underwent laparoscopic niche repair. The majority of studies have indicated that laparoscopic repair is the optimal approach when myometrial thickness is less than 3 mm, as this reduces the risk of perforating the bladder with the hysteroscopic approach. One study (41) has already established the indication for laparoscopic repair of the niche starting from a myometrium thickness of 5 mm. In three studies (25, 40, 42) the laparoscopic niche repair was conducted due to a cesarean scar pregnancy involving a total of 25 women. The reproductive outcomes of these cases were not described separately.
Endometriosis was reported in several studies, with prevalence rates ranging from 21% to 44% ( (18, 24, 25, 34, 36), suggesting a potential association between isthmocele and endometriosis. However, few studies accounted for its impact on fertility outcomes, leaving gaps in understanding its role in reproductive prognosis. Two studies described findings of iatrogenic adenomyosis in the uterine scar tissue. Gulz et al. reported an overall prevalence of endometriosis of 26.5% (25) with a predominance of peritoneal endometriosis (63%). It is noteworthy that 11% of patients (n=9) exhibited iatrogenic adenomyosis in the uterine scar tissue. The presence of iatrogenic adenomyosis was associated with the co-existence of extrauterine endometriosis (25). Donnez et al. described adenomyosis in the uterine scar with a prevalence of 21% (23).
Results of the meta-analysis
A meta-analysis of 17 studies comprising 309 women was conducted to evaluate reproductive outcomes in women after laparoscopic niche resection (Figure 1). CPR was calculated considering all women who underwent isthmocele repair; LBR was estimated using the population of women who achieved pregnancy as the reference denominator.
Reproductive outcomes after laparoscopic isthmocele repair
15 studies were eligible for inclusion in the analysis of CP, 12 in the analysis of MC, and 14 in the analysis of LB. After isthmocele repair, CP occurred in 44% (95% CI: 32-56%), MC in 15% (95% CI: 9-24%) and LB in 72% (95% CI, 61%–81%) (Figure 2) The heterogeneity test revealed significant heterogeneity among the studies I2 = 85, p < 0.01, I2 = 49, p < 0.01 and I2 = 60, p < 0.01 respectively.
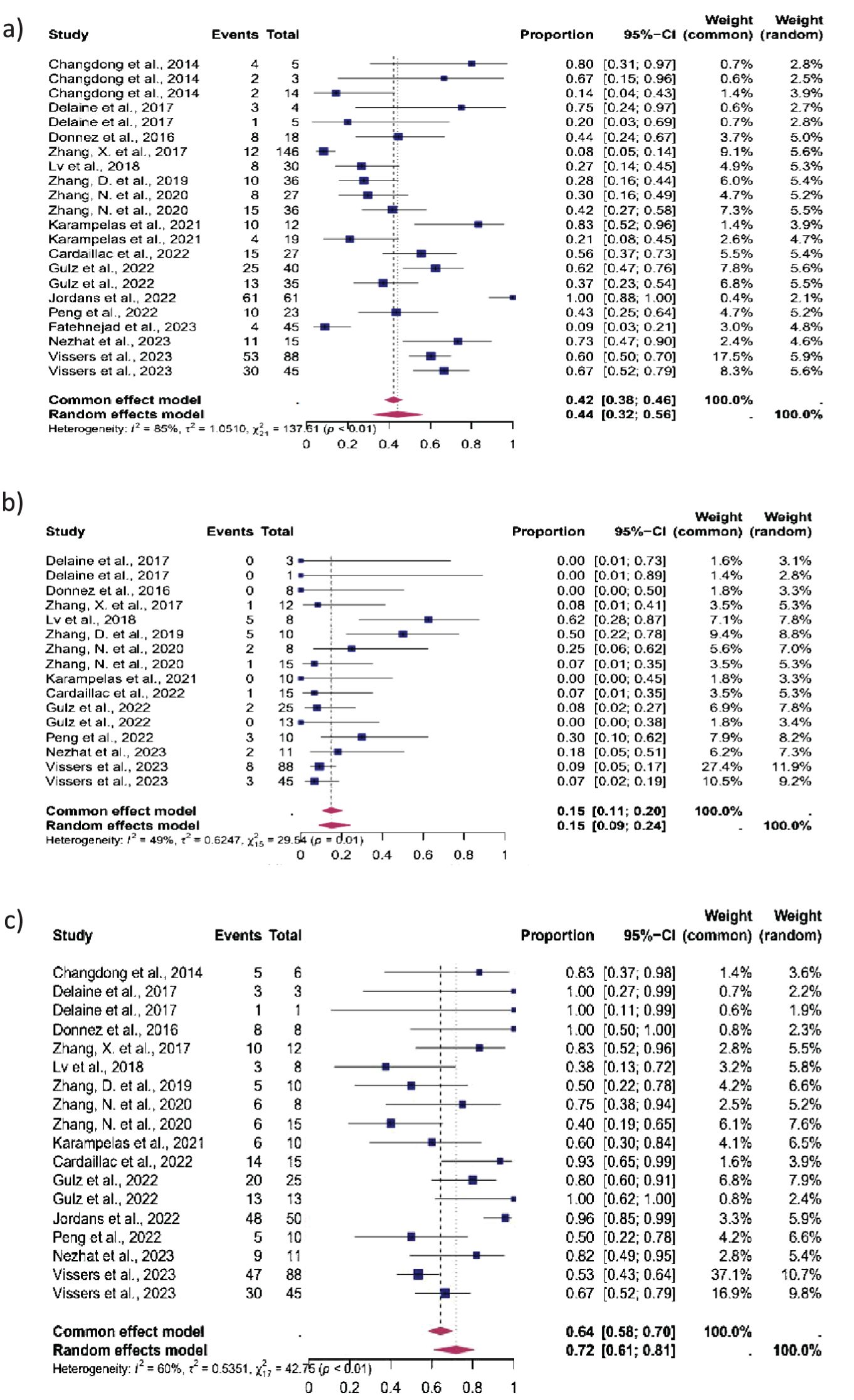
Figure 2. Pooled overall prevalence of the effect of laparoscopic isthmocele repair reproductive outcomes. Forest plot of proportions and 95% confidence intervals (CI) for studies evaluating the prevalence of reproductive outcomes in women who underwent laparoscopic repair of the isthmocele. Blue squares for each study indicate the proportion, the size of the boxes indicates the weight of the study, and the horizontal lines indicate the 95% CI. The data in bold and pink diamond represent the pooled prevalence for post-treatment infertility and 95% CI. Overall estimates are shown in the fixed- and random-effect models. This pooled overall prevalence analysis evaluates three key variables: (a) clinical pregnancy rate, (b) miscarriage rate, and (c) live birth rate.
Subgroup analysis
The reproductive outcomes were stratified according to the fertility status (women with infertility, without infertility, and unknown fertility status) (Figures 3–5).
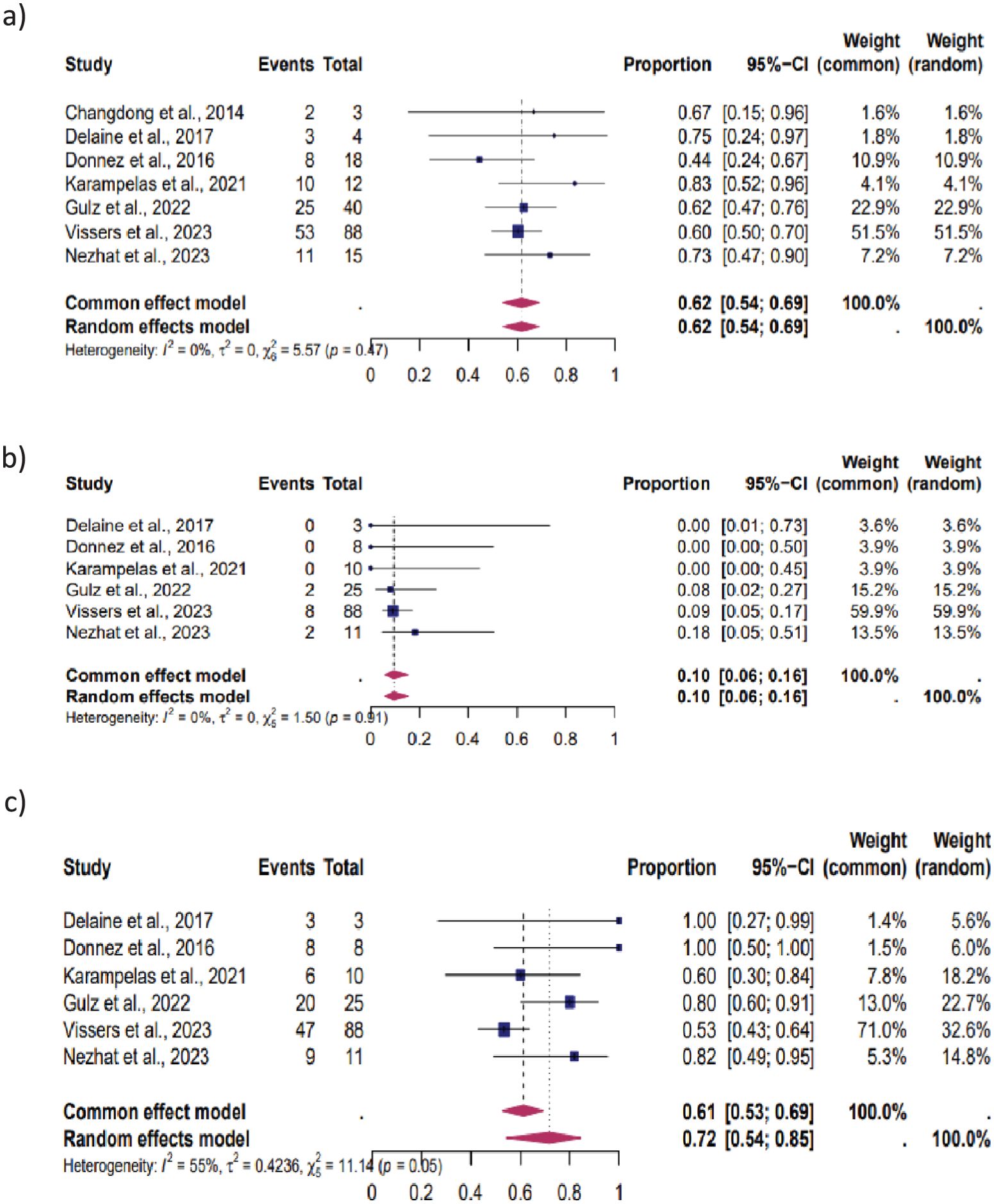
Figure 3. Pooled overall prevalence of the effect of laparoscopic isthmocele repair on reproductive outcomes in women with infertility. For details, see the legend of Figure 2. This pooled overall prevalence analysis evaluates three key variables: (a) clinical pregnancy rate, (b) miscarriage rate, and (c) live birth rate.
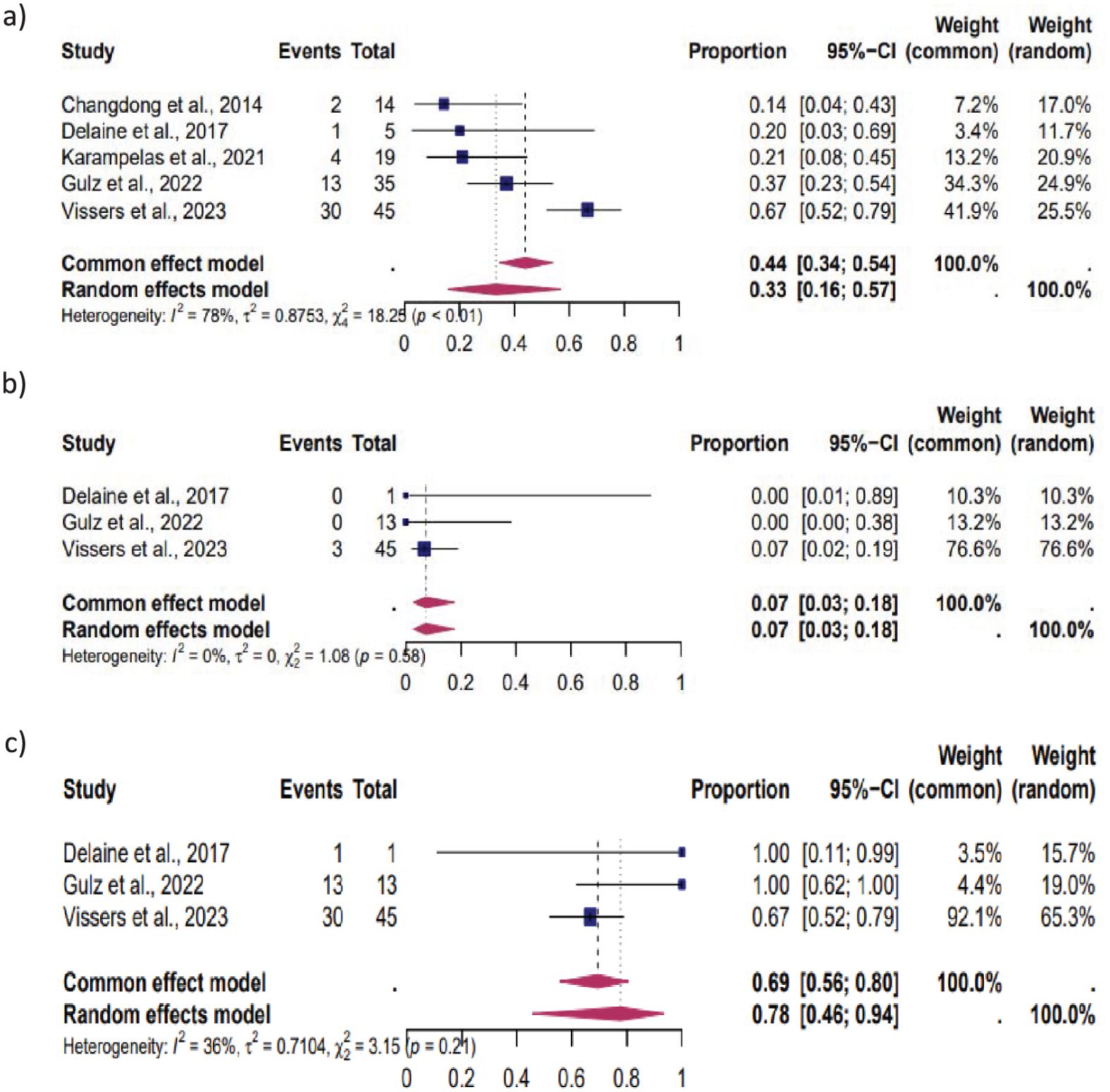
Figure 4. Pooled overall prevalence of the effect of laparoscopic isthmocele repair on reproductive outcome in women without infertility. For details, see the legend of Figure 2. This pooled overall prevalence analysis evaluates three key variables: (a) clinical pregnancy rate, (b) miscarriage rate, and (c) live birth rate.
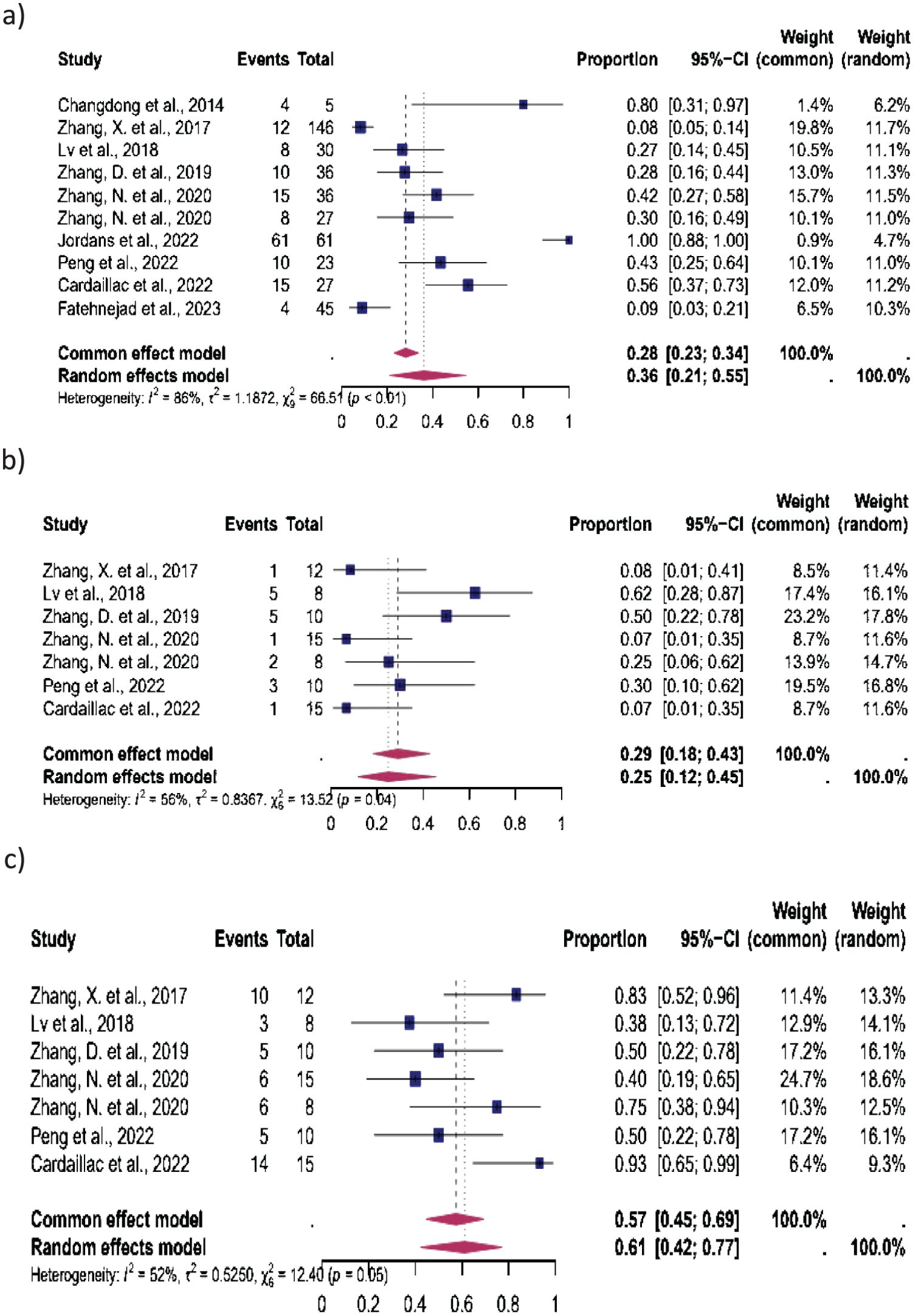
Figure 5. Pooled overall prevalence of the effect of laparoscopic isthmocele repair of the reproductive outcome in women with unknown fertility status. For details, see the legend of Figure 2. This pooled overall prevalence analysis evaluates three key variables: (a) clinical pregnancy rate, (b) miscarriage rate, and (c) live birth rate.
Women with infertility: Seven studies were eligible for inclusion in this subgroup analysis: CP was observed in 62% (95% CI, 54%–69%), MC in 10% (95% CI, 6%–16%) and LB in 72% (95% CI, 54%–85%). The heterogeneity test revealed significant heterogeneity among the studies I2 = 0, p < 0.01, I2 = 0, p < 0.01 and I2 = 55, p < 0.01 respectively (Figure 3).
Women without infertility: Five studies were included in this subgroup analysis, which focused on women without infertility. The results showed that 33% (95% CI, 16%–57%) of these women experienced CP, 7% (95% CI, 3%–18%) MC, and 78% (95% CI, 46%–94%) LB. The heterogeneity test revealed significant heterogeneity among the studies for CP (I2 = 78, p < 0.01), MC (I2 = 0, p < 0.01), and LB (I2 = 36, p < 0.01) (Figure 4).
Women with unknown fertility status: Ten studies were included in this subgroup analysis. CP occurred in 36% (95% CI, 21%–55%), MC in 25% (95% CI, 12%–45%), and LB in 61% (95% CI, 42%–77%). The heterogeneity test revealed significant heterogeneity among the studies, with I2 = 86, p < 0.01 for CP, I2 = 56, p < 0.01 for MC, and I2 = 52, p < 0.01 for LB (Figure 5).
Prevalence of endometriosis during isthmocele repair
Five studies were eligible for the analysis of the pooled prevalence of endometriosis diagnosed during isthmocele repair. The analysis showed an overall prevalence of endometriosis of 29% (95% CI, 22%-37%). The heterogeneity test showed low heterogeneity between studies I2 = 32, p < 0.01 (Figure 6).
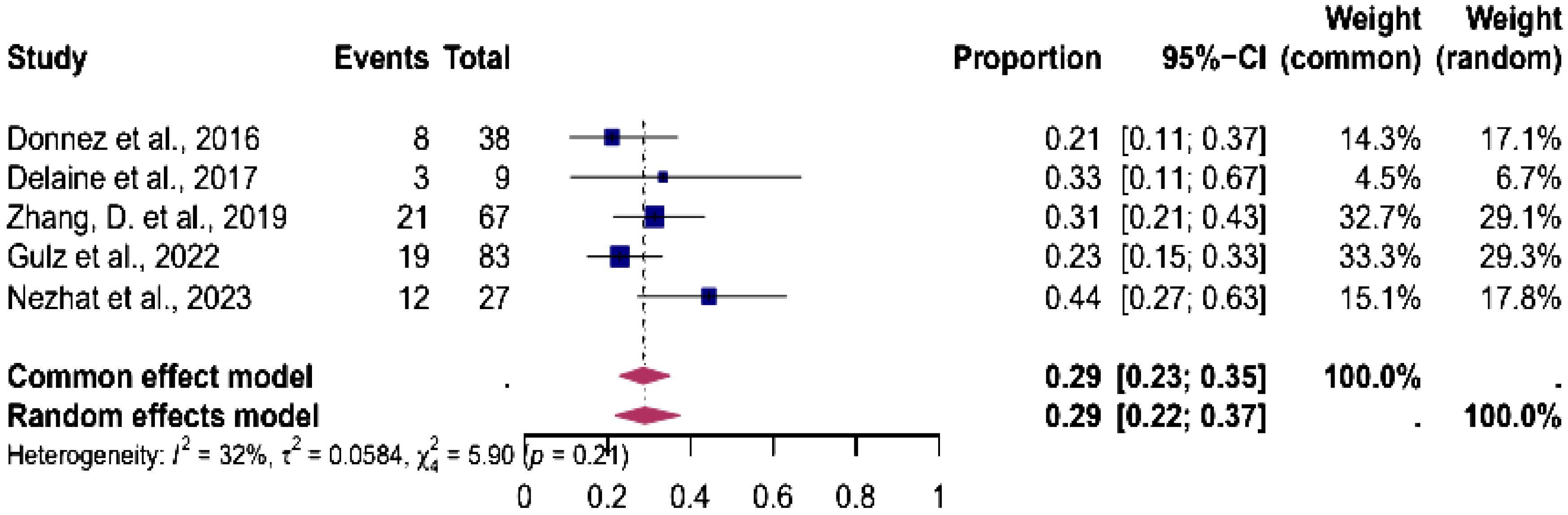
Figure 6. Pooled overall prevalence of endometriosis in women during laparoscopic isthmocele repair. Forest plot of proportions and 95% confidence intervals (CI) for studies evaluating the prevalence of endometriosis in women undergoing laparoscopic isthmocele repair. Blue squares for each study indicate the proportion, the size of the boxes indicates the weight of the study, and the horizontal lines indicate the 95% CI. The data in bold and pink diamond represent the pooled prevalence for post-treatment infertility and 95% CI. Overall estimates are shown in the fixed- and random-effect models. This pooled overall prevalence analysis evaluates three key variables: (a) clinical pregnancy rate, (b) miscarriage rate, and (c) live birth rate.
Discussion
Main findings
The aim of this study was to evaluate the effect of laparoscopic isthmocele repair on reproductive outcomes. Given the recent increase in cesarean section rates and potential long-term complications, considerable attention has been given to evaluating whether surgery has the potential to improve reproductive outcomes among women with isthmoceles.
Our review revealed the positive effect of laparoscopic isthmocele resection on reproductive outcomes, with the following key findings: First, 44% of women experienced CP after isthmocele repair, while LB was notably high at 72%, with all pregnant women as the reference denominator. Second, women with infertility had the highest rates of CP (62%; 95% confidence interval (CI): 54–69%) and LB (72%; 95% CI: 54–85%) compared to women without infertility (CP: 33%, 95% CI: 16–57%/LB: 78%, 95% CI: 46–94%) and women with unknown fertility status (CP: 36%, 95% CI: 21–55%/LB: 61%, 95% CI: 42–77%). Third, the prevalence of endometriosis at surgery was 29% (95% CI: 22–37%).
Strengths and limitations
Although our study strictly followed the recommendations to provide high-quality evidence summaries, some limitations are evident. First, most of the included studies were based on retrospective data, resulting in high statistical heterogeneity. Second, there was a lack of data regarding inconsistency, poor description of other relevant causes of infertility, no information on the time until pregnancy, and a lack of data regarding fertilization methods. Third, another limitation of the included studies is that they did not exclusively include women with secondary infertility, but also women who presented with difficulties in embryo transfer or had other bleeding disturbances. This broader patient population may introduce variability in the results and limit the ability to generalize the findings specifically to women with secondary infertility. Thus, we could not perform sub-analyses of cases requiring ART. Fourth, some studies did not justify the choice of treatment, and there was a paucity of information regarding the cesarean scar defect or magnetic resonance imaging following surgery.
Interpretation
Infertility with isthmocele was significantly higher than without (66% vs 46%; p=0.03) (43). Complete niche resection reduces the incidence of infertility-related complications such as postmenstrual bleeding and chronic endometritis. This may be explained by the prevention of blood accumulation, which is associated with disturbed cervical mucus quality, sperm transport, and the uterine microbiota, potentially interfering with the delicate process of embryo implantation (21, 23).
One mechanism that could explain the association between isthmocele and infertility, particularly in patients undergoing ART, is the alteration of the endometrial environment due to hemorrhagic disturbances. Residual and abnormal bleeding caused by the isthmocele creates an environment that is less receptive to embryo implantation, as it interferes with the synchronization between the endometrial phase and embryo transfer. Furthermore, the presence of isthmocele may complicate embryo transfer, further exacerbating the challenges for achieving successful implantation. This disruption affects the quality and stability of the endometrium, significantly compromising reproductive outcomes (21). Therefore, our results suggest that laparoscopic repair of the isthmocele may improve reproductive outcomes by reducing the factors previously described.
Our study revealed that isthmocele repair led to a 44% CPR and a 72% LBR (having all pregnant women as the denominator), suggesting that while implantation may be initially impaired, pregnancy maintenance improves significantly. Endometrial alterations, including residual bleeding and structural anomalies, likely create a less receptive environment for embryo implantation (21). These findings highlight the potential benefits of isthmocele repair in improving reproductive outcomes, particularly by addressing factors that interfere with early implantation and gestational progression (44, 45).
In a recent systematic review and meta-analysis (22), no clear differences were found in the prevalence of CP, MC, and LB between treatment options (laparoscopic suturing and knotting, hysteroscopy, laparotomy, and vaginal approach) (22). In our meta-analysis, however, we found that the prevalence of LB was higher in the infertility group than among women without infertility. One interpretation of these results could be that among women with infertility, the isthmocele may contribute to fertility problems, so the treatment of the niche may have more impact. In addition, the procedure results in enhanced passage and anatomical suitability for embryo transfer, and it also permits the potential for performing endometriosis resection, which is also related to infertility (25).
However, as laparoscopic surgical repair is a non-standardized treatment, the quality of studies on this topic is heterogeneous. Only one included study had good quality (18). The study cohorts had mixed populations and a lack of information on fertility history or the need for any fertility treatment, especially the use of assisted reproductive techniques. Therefore, a sub-analysis of the group of patients receiving assisted reproductive treatment could not be performed.
There is a complete lack of randomized controlled trials comparing the effect of isthmocele repair on reproductive outcome parameters with control groups without surgical intervention. So far, the LAPRES trial (Dutch Trial Register (ref. no. NL6350 http://www.trialregister.nl). Is the only registered trial in which patients with infertility have undergone laparoscopic repair. This is a randomized, unblinded, controlled trial involving 200 infertile women with a 2-year follow-up (46).
Reproductive outcome analysis in infertile patients after isthmocele repair, as performed in our study, is currently the only strategy to assess the impact of surgical interventions on reproductive outcomes. Notably, none of the selected studies specifically reported on the mode of conception of subsequent pregnancy among women with previous failed ART before surgery. Nezhat et al. (36) and Vissers et al. (18) included patients undergoing ART, a key factor in evaluating the impact of isthmocele repair on reproductive outcomes. However, neither study specified the mode of conception in women with prior ART failures before surgery, making it difficult to determine whether improved outcomes were due to the procedure itself or ART. Further research with robust methodological design is needed to control for these factors and clarify the association between surgical intervention and reproductive outcomes.
Five studies advised patients to wait at least three months before attempting conception after surgery. Conversely, 2 studies suggest a waiting period of 1 year before trying again (25, 38). Only the study by Vissers et al. (18), included recommendations for postoperative care, which entailed the administration of contraceptives for 6 months following the procedure. This was deemed necessary to allow for sufficient postoperative time for uterine healing. Notably, none of the studies identified any perioperative complications, especially a lack of uterine dehiscence described after other isthmocele interventions. None of the studies mentioned postoperative complications.
A key aspect of the surgical approach is that laparoscopy has the added advantage of simultaneous diagnosis and treatment of other potential causes of infertility (24). Endometriosis is often a co-morbidity and can cause infertility, chronic inflammation, and anatomical changes due to adhesions. The role of endometriosis as a risk factor for isthmocele is still not fully established, but emerging evidence suggests a potential association between the two conditions. Endometriosis could impair post-cesarean wound healing, leading to defective scar formation and increasing the risk of isthmocele. This may occur through chronic inflammation, fibrosis, altered immune responses, and abnormal endometrial remodeling, all of which are known to affect tissue repair. Proposed mechanisms include altered endometrial receptivity, intrauterine fluid accumulation, disrupted uterine contractility, and a higher prevalence of recurrent implantation failure (21, 36, 47).
Endometriosis was found in 27% of patients with isthmocele who underwent laparoscopic resection in a retrospective study by Gulz et al. (25). The findings of the Gulz et al., 2022 study, which reported a 27% prevalence of endometriosis, align closely with our results, indicating a 29% prevalence and further supporting the association between isthmocele and endometriosis (25). The presence of endometrial glands or stromal tissue within the scar was found in 21-27% of cases in two other studies (23, 25, 48). A deeper understanding of this association is essential to optimize treatment strategies and improve patient outcomes. Therefore, endometriosis can be resected during the same procedure. This suggests a potential association between isthmocele and endometriosis, warranting further research to clarify the underlying mechanisms and clinical implications of this relationship.
Considering the availability of different treatment options and the lack of clinical guidelines on this issue, consideration should be given to ultrasound of the residual myometrial thickness, the presence of other pathologies (e.g., endometriosis, adhesions, tubal obstruction, etc.), as well as the patient’s symptoms before deciding on a management approach. If surgical treatment is indicated, the choice between a hysteroscopic resection and laparoscopic or vaginal repair should be based on factors such as the residual myometrial thickness and the skills of the surgeon (49). If the residual myometrial thickness is less than 3 mm, a laparoscopic or vaginal repair technique should be used. The defect is completely removed, and the myometrium is reattached with sutures.
Furthermore, Verberkt et al. recommended that future studies should examine the effects of uterine-niche-related surgery (22): Important topics include structured evaluation of all causes of infertility, cesarean scar defect measurement before and after surgery, structured follow-up for at least 2 years, detailed information on duration of interest, previous fertility treatments or about conception mode, and sample size powered for pregnancy rate. Considering these recommendations and interdisciplinary work between surgeons, obstetricians, and reproductive medicine, the underlying role of infertility and its outcomes in terms of reproduction can be identified.
Conclusion
Laparoscopic repair of isthmoceles is associated with good reproductive outcomes, suggesting this intervention is effective. Women with a history of infertility may benefit. However, further Randomized Controlled Trials are required to provide robust evidence to support this hypothesis.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
AV: Conceptualization, Data curation, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. JG: Conceptualization, Data curation, Methodology, Supervision, Writing – review & editing. VV: Data curation, Methodology, Software, Writing – review & editing. JP: Data curation, Formal analysis, Methodology, Writing – review & editing. MG: Methodology, Supervision, Writing – review & editing. TK: Conceptualization, Data curation, Investigation, Methodology, Software, Writing – review & editing. MM: Validation, Writing – review & editing. Mv: Conceptualization, Funding acquisition, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The Institute Biochimique SA, Lugano, Switzerland, supported the study with an unrestricted grant, which did not play any role in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1507482/full#supplementary-material
Supplementary Table 1 | Database Search Strategies. A systematic literature search in Medline, Embase, and Cochrane CENTRAL.
References
1. Betran AP, Ye J, Moller A-B, Souza JP, Zhang J. Trends and projections of caesarean section rates: global and regional estimates. BMJ Glob Health. (2021) 6:e005671. doi: 10.1136/bmjgh-2021-005671
2. World Health Organization Human Reproduction Programme, 10 April 2015. WHO Statement on caesarean section rates. Reprod Health Matters. (2015) 23:149–50. doi: 10.1016/j.rhm.2015.07.007
3. WHO. Caesarean section rates continue to rise, amid growing inequalities in access(2021). Available online at: https://www.who.int/news/item/16-06-2021-caesarean-section-rates-continue-to-rise-amid-growing-inequalities-in-access (Accessed June 16, 2021).
4. Mylonas I, Friese K. Indications for and risks of elective cesarean section. Dtsch Arztebl Int. (2015) 112:489–95. doi: 10.3238/arztebl.2015.0489
5. Clark EAS, Silver RM. Long-term maternal morbidity associated with repeat cesarean delivery. Am J Obstet Gynecol. (2011) 205:S2–10. doi: 10.1016/j.ajog.2011.09.028
6. Cao D, Chen L. Effect of previous caesarean section on reproductive and pregnancy outcomes after assisted reproductive technology: A systematic review and meta−analysis. Exp Ther Med. (2024) 28:284. doi: 10.3892/etm.2024.12572
7. Diaz SD, Jones JE, Seryakov M, Mann WJ. Uterine rupture and dehiscence: ten-year review and case-control study. South Med J. (2002) 95:431–5. doi: 10.1097/00007611-200295040-00012
8. Jordans IPM, de Leeuw RA, Stegwee SI, Amso NN, Barri-Soldevila PN, van den Bosch T, et al. Sonographic examination of uterine niche in non-pregnant women: a modified Delphi procedure. Ultrasound Obstet Gynecol. (2019) 53:107–15. doi: 10.1002/uog.19049
9. Jordans IPM, Verberkt C, De Leeuw RA, Bilardo CM, Van Den Bosch T, Bourne T, et al. Definition and sonographic reporting system for Cesarean scar pregnancy in early gestation: modified Delphi method. Ultrasound Obstet Gynecol. (2022) 59:437–49. doi: 10.1002/uog.24815
10. Iannone P, Nencini G, Bonaccorsi G, Martinello R, Pontrelli G, Scioscia M, et al. Isthmocele: from risk factors to management. Rev Bras Ginecol Obstet. (2019) 41:44–52. doi: 10.1055/s-0038-1676109
11. Tsuji S, Nobuta Y, Hanada T, Takebayashi A, Inatomi A, Takahashi A, et al. Prevalence, definition, and etiology of cesarean scar defect and treatment of cesarean scar disorder: A narrative review. Reprod Med Biol. (2023) 22:e12532. doi: 10.1002/rmb2.12532
12. Borges LM, Scapinelli A, De Baptista Depes D, Lippi UG, Coelho Lopes RG. Findings in patients with postmenstrual spotting with prior cesarean section. J Minimally Invasive Gynecology. (2010) 17:361–4. doi: 10.1016/j.jmig.2010.02.007
13. Tower AM, Frishman GN. Cesarean scar defects: an underrecognized cause of abnormal uterine bleeding and other gynecologic complications. J Minim Invasive Gynecol. (2013) 20:562–72. doi: 10.1016/j.jmig.2013.03.008
14. Bij de Vaate AJM, van der Voet LF, Naji O, Witmer M, Veersema S, Brölmann H a. M, et al. Prevalence, potential risk factors for development and symptoms related to the presence of uterine niches following Cesarean section: systematic review. Ultrasound Obstet Gynecol. (2014) 43:372–82. doi: 10.1002/uog.13199
15. Mohr-Sasson A, Dadon T, Brandt A, Shats M, Axcelrod M, Meyer R, et al. The association between uterine scar defect (niche) and the presence of symptoms. Reprod BioMedicine Online. (2023) 47:103221. doi: 10.1016/j.rbmo.2023.04.012
16. Wang J, Pang Q, Wei W, Cheng L, Huang F, Cao Y, et al. Definition of large niche after Cesarean section based on prediction of postmenstrual spotting: Chinese cohort study in non-pregnant women. Ultrasound Obstet Gyne. (2022) 59:450–6. doi: 10.1002/uog.24817
17. Gurol-Urganci I, Bou-Antoun S, Lim CP, Cromwell DA, Mahmood TA, Templeton A, et al. Impact of Caesarean section on subsequent fertility: a systematic review and meta-analysis. Hum Reprod. (2013) 28:1943–52. doi: 10.1093/humrep/det130
18. Vissers J, Hehenkamp WJK, Brölmann HAM, Lambalk CB, Huirne JAF. Reproductive outcomes after laparoscopic resection of symptomatic niches in uterine cesarean scars: Long-term follow-up on the prospective LAPNICHE study. Acta Obstet Gynecol Scand. (2023) 102:1643–52. doi: 10.1111/aogs.14647
19. Ohashi M, Tsuji S, Kasahara K, Oe R, Tateoka Y, Murakami T. Influence of cesarean section on postpartum fertility and dysmenorrhea: A retrospective cohort study in Japan. Womens Health Rep (New Rochelle). (2024) 5:22–9. doi: 10.1089/whr.2023.0109
20. Vitagliano A, Cicinelli E, Viganò P, Sorgente G, Nicolì P, Busnelli A, et al. Isthmocele, not cesarean section per se, reduces in vitro fertilization success: a systematic review and meta-analysis of over 10,000 embryo transfer cycles. Fertility Sterility. (2024) 121:299–313. doi: 10.1016/j.fertnstert.2023.11.007
21. Vissers J, Hehenkamp W, Lambalk CB, Huirne JA. Post-Caesarean section niche-related impaired fertility: hypothetical mechanisms. Hum Reprod. (2020) 35:1484–94. doi: 10.1093/humrep/deaa094
22. Verberkt C, Klein Meuleman SJM, Ket JCF, Van Wely M, Bouwsma E, Huirne JAF. Fertility and pregnancy outcomes after a uterine niche resection in women with and without infertility: a systematic review and meta-analysis. F&S Rev. (2022) 3:174–89. doi: 10.1016/j.xfnr.2022.05.003
23. Donnez O, Donnez J, Orellana R, Dolmans M-M. Gynecological and obstetrical outcomes after laparoscopic repair of a cesarean scar defect in a series of 38 women. Fertil Steril. (2017) 107:289–296.e2. doi: 10.1016/j.fertnstert.2016.09.033
24. Donnez O. Cesarean scar defects: management of an iatrogenic pathology whose prevalence has dramatically increased. Fertility Sterility. (2020) 113:704–16. doi: 10.1016/j.fertnstert.2020.01.037
25. Gulz M, Imboden S, Nirgianakis K, Siegenthaler F, Rau TT, Mueller MD. Endometriosis and isthmocele: common or rare? JCM. (2022) 11:1158. doi: 10.3390/jcm11051158
26. Nirgianakis K, Oehler R, Mueller M. The Rendez-vous technique for treatment of caesarean scar defects: a novel combined endoscopic approach. Surg Endosc. (2016) 30:770–1. doi: 10.1007/s00464-015-4226-6
27. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
28. Borissov N, Haas Q, Minder B, Kopp-Heim D, von Gernler M, Janka H, et al. Reducing systematic review burden using Deduklick: a novel, automated, reliable, and explainable deduplication algorithm to foster medical research. Syst Rev. (2022) 11:172. doi: 10.1186/s13643-022-02045-9
29. Van der Mierden S, Tsaioun K, Bleich A, Leenaars CHC. Software tools for literature screening in systematic reviews in biomedical research. ALTEX. (2019) 36:508–17. doi: 10.14573/altex.1902131
30. O’Flynn N. Assessment and treatment for people with fertility problems: NICE guideline. Br J Gen Pract. (2014) 64:50–1. doi: 10.3399/bjgp14X676609
31. Turesheva A, Aimagambetova G, Ukybassova T, Marat A, Kanabekova P, Kaldygulova L, et al. Recurrent Pregnancy Loss Etiology, Risk Factors, Diagnosis, and Management. Fresh Look into a Full Box. J Clin Med. (2023) 12:4074. doi: 10.3390/jcm12124074
32. Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, De Mouzon J, Sokol R, et al. The international glossary on infertility and fertility care, 2017†‡§. Hum Reprod. (2017) 32:1786–801. doi: 10.1093/humrep/dex234
33. Wells G, Shea S, O’Connell D, Peterson J, Welch V, Losos M. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. (2009). Available online at: http://www.evidencebasedpublichealth.de/download/Newcastle_Ottowa_Scale_Pope_Bruce.pdf (Accessed February 4, 2025).
34. Delaine M, Lecointre L, Akladios CY, Hummel M, Host A, Garbin O. Prise en charge cœlioscopique des isthmocèles – Étude d’une série de 9 cas. Gynécologie Obstétrique Fertilité Sénologie. (2017) 45:262–8. doi: 10.1016/j.gofs.2017.03.007
35. Karampelas S, Salem Wehbe G, De Landsheere L, Badr DA, Tebache L, Nisolle M. Laparoscopic Isthmocele Repair: Efficacy and Benefits before and after Subsequent Cesarean Section. JCM. (2021) 10:5785. doi: 10.3390/jcm10245785
36. Nezhat C, Zaghi B, Baek K, Nezhat A, Nezhat F, Lindheim S, et al. Outcomes of laparoscopic cesarean scar defect repair: retrospective and observational study. J Clin Med. (2023) 12:3720. doi: 10.3390/jcm12113720
37. Zhang X, Yang M, Wang Q, Chen J, Ding J, Hua K. Prospective evaluation of five methods used to treat cesarean scar defects. Intl J Gynecology Obste. (2016) 134:336–9. doi: 10.1016/j.ijgo.2016.04.011
38. Zhang D, Liang S, Zhu L. Comparison of transvaginal repair versus laparoscopic repair of lower-segment cesarean scar defects. Int J Gynaecol Obstet. (2019) 145:199–204. doi: 10.1002/ijgo.12797
39. Lv B, Xie X, Liu C, Lin Y. Laparoscopic combined with hysteroscopic repair or operative hysteroscopy in the treatment of symptomatic cesarean-induced diverticulum. Med Sci (Paris). (2018) 34 Focus issue F1:47–51. doi: 10.1051/medsci/201834f109
40. Cardaillac C, Salmon C, Vaucel E, Gueudry P, Lavoue V, Nyangoh Timoh K, et al. Robot-assisted laparoscopy repair of uterine isthmocele: A two-center observational study. Intl J Gynecology Obste. (2023) 160:244–8. doi: 10.1002/ijgo.14319
41. Zhang X, Wu C, Yang M, Xu H, He Y, Ding J, et al. Laparoscopic repair of cesarean section scar defect and the surgical outcome in 146 patients. Int J Clin Exp Med. (2017) 10:4408–16.
42. Fatehnejad M, Hadizadeh A, Tayebi A, Ayati A, Marjani N, Gheshlaghi P, et al. Assessment of the clinical outcomes and complications of hysteroscopic and laparoscopic approaches in the treatment of symptomatic isthmocele: An observational study. Intl J Gynecology Obste. (2023) 163:965–71. doi: 10.1002/ijgo.14926
43. Nobuta Y, Tsuji S, Kitazawa J, Hanada T, Nakamura A, Zen R, et al. Decreased fertility in women with cesarean scar syndrome is associated with chronic inflammation in the uterine cavity. Tohoku J Exp Med. (2022) 258:237–42. doi: 10.1620/tjem.2022.J082
44. Asoglu MR, Celik C, Ozturk E, Cavkaytar S, Bahceci M. Impact of isthmocele on assisted reproductive treatment outcomes: an age-matched retrospective study. J Minimally Invasive Gynecology. (2021) 28:1113–20. doi: 10.1016/j.jmig.2020.10.002
45. Baldini GM, Lot D, Malvasi A, Di Nanni D, Laganà AS, Angelucci C, et al. Isthmocele and infertility. JCM. (2024) 13:2192. doi: 10.3390/jcm13082192
46. Vissers J, Klein Meuleman SJM, de Leeuw RA, van Eekelen R, Groenman FA, Mol BW, et al. Effectiveness of laparoscopic niche resection versus expectant management in patients with unexplained infertility and a large uterine caesarean scar defect (uterine niche): protocol for a randomised controlled trial (the LAPRES study). BMJ Open. (2023) 13:e070950. doi: 10.1136/bmjopen-2022-070950
47. Vidal A, Bora C, Von Holzen J, Gulz M, Obmann VC, Pape J, et al. Cine-MRI for quantifying uterine peristalsis: A systematic review and meta-analysis. JCM. (2025) 14:1021. doi: 10.3390/jcm14031021
48. Tanimura S, Funamoto H, Hosono T, Shitano Y, Nakashima M, Ametani Y, et al. New diagnostic criteria and operative strategy for cesarean scar syndrome: Endoscopic repair for secondary infertility caused by cesarean scar defect. J Obstet Gynaecol. (2015) 41:1363–9. doi: 10.1111/jog.12738
49. Chang Y, Tsai EM, Long CY, Lee CL, Kay N. Resectoscopic treatment combined with sonohysterographic evaluation of women with postmenstrual bleeding as a result of previous cesarean delivery scar defects. Am J Obstet Gynecol. (2009) 200:370.e1–4. doi: 10.1016/j.ajog.2008.11.038
50. Li C, Guo Y, Liu Y, Cheng J, Zhang W. Hysteroscopic and laparoscopic management of uterine defects on previous cesarean delivery scars. J Perinat Med. (2014) 42(3):363–70. doi: 10.1515/jpm-2013-0081
51. Donnez O, Donnez J, Orellana R, Dolmans MM. Gynecological and obstetrical outcomes after laparoscopic repair of a cesarean scar defect in a series of 38 women. Fertil Steril. (2017) 107(1):289–96.e2. doi: 10.1016/j.fertnstert.2016.09.033
52. Peng C, Huang Y, Lu Y, Zhou Y. Comparison of the Efficacy of Two Laparoscopic Surgical Procedures Combined with Hysteroscopic Incision in the Treatment of Cesarean Scar Diverticulum. J Invest Surg. (2022) 35(1):225–30. doi: 10.1080/08941939.2020.1830319. Erratum in: J Invest Surg. 2022 Jan;35(1):231-232. doi: 10.1080/08941939.2020.1836692
53. Zhang NN, Wang GW, Yang Q. Endoscopic Treatment of Previous Cesarean Scar Defect in Women with Postmenstrual Bleeding: A Retrospective Cohort Study. J Invest Surg. (2021) 34(10):1147–55. doi: 10.1080/08941939.2020.1766161. Erratum in: J Invest Surg. 2021 34(10):1156-1157. doi: 10.1080/08941939.2020.1770378
Keywords: Cesarean section scar defect, isthmocele, laparoscopic niche resection, reproductive outcomes, Cesarean section
Citation: Vidal A, Geiger J, Vinayahalingam V, Pape J, Gulz M, Karrer T, Mueller MD and von Wolff M (2025) High live birth rates after laparoscopic isthmocele repair in infertility: a systematic review and meta-analysis. Front. Endocrinol. 16:1507482. doi: 10.3389/fendo.2025.1507482
Received: 07 October 2024; Accepted: 04 March 2025;
Published: 15 April 2025.
Edited by:
Jan I. Olofsson, Karolinska Institutet (KI), SwedenReviewed by:
Evangelia Elenis, Uppsala University Hospital, SwedenMilan Milenkovic, University of Gothenburg, Sweden
Copyright © 2025 Vidal, Geiger, Vinayahalingam, Pape, Gulz, Karrer, Mueller and von Wolff. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Angela Vidal, YW5nZWxhLnZpZGFsQGluc2VsLmNo
 Angela Vidal
Angela Vidal James Geiger
James Geiger Vithusha Vinayahalingam
Vithusha Vinayahalingam Janna Pape1
Janna Pape1 Tanya Karrer
Tanya Karrer Michael D. Mueller
Michael D. Mueller Michael von Wolff
Michael von Wolff