
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol. , 28 February 2025
Sec. Thyroid Endocrinology
Volume 16 - 2025 | https://doi.org/10.3389/fendo.2025.1505085
Background: Previous studies have identified a close relationship between ghrelin levels and thyroid disease. Ghrelin levels were lower in patients with hyperthyroidism compared with healthy individuals, and increased after treatment. However, other studies have reported inconsistent results. As such, the association between ghrelin and thyroid disease remains controversial.
Methods: A literature search of the Web of Science, Wiley Online Library, Embase, and PubMed databases was performed. The title or abstract search term “thyroid” was used in combination with “ghrelin”. Meta-analysis results are reported as standardized mean difference with corresponding 95% confidence interval (CI).
Results: Twenty-three studies were included in this meta-analysis. Ghrelin levels in patients with hyperthyroidism were significantly lower than those in healthy individuals (SMD: -1.03, 95% CI [-1.75, 0.32]), but significantly higher after effective treatment (SMD: 0.77, 95% CI [0.03, 1.51]). Ghrelin levels were higher, but not significantly, in patients with hypothyroidism compared with healthy controls (SMD: 0.48, 95% CI [-0.13, 1.08]).
Conclusions: This systematic review is the first to evaluate the relationship between ghrelin and thyroid disease. Determining the role of ghrelin in thyroid disease will significantly contribute to understand of symptom or pathomechanism.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42024591501.
The thyroid is among the most important endocrine glands in the human body. Its main role is to synthesize and secrete thyroid hormones, which impact growth, metabolism, and development. Thyroid hormones can affect the nervous, circulatory, digestive, reproductive, and other systems, and play a role in regulating human energy metabolism. Thyroid dysfunction is the most common thyroid disease (1). Searching for mutual relationships between other hormones and the thyroid hormones is important for a better understanding of symptom or pathomechanism.
Ghrelin is an endogenous polypeptide composed of 28 amino acids, first identified in the gastric mucosa of rats by Kojima et al. in 1999. Ghrelin is the endogenous ligand of the growth hormone secretagogue receptor (GHS-R). Ghrelin has the highest concentration in the stomach and is expressed in the intestine, hypothalamus, pituitary, thyroid, heart, and other tissues, laying the foundation for its wide range of physiological effects. Ghrelin promotes the release of growth hormone, enhances appetite, regulates fat metabolism, protects the vascular endothelium, and improves cardiac function (2). Studies have shown that ghrelin can alter the morphology of thyroid-stimulating hormone-secreting cells and decrease serum thyroid-stimulating hormone levels when injected into the lateral cerebral ventricles of rats (3). Patients with thyroid dysfunction exhibit a variety of abnormalities, such as changes in thyroid hormone levels and autoimmune disorders, which are often accompanied by abnormal energy metabolism of glucose and lipids. In recent years, changes in ghrelin levels in this state have gradually become a “hot topic” of research. Riis et al. found that ghrelin levels were significantly lower in patients with hyperthyroidism than those in healthy individuals, and were significantly increased after treatment (4). Gjedde et al. reported a significant increase in ghrelin levels of patients with hypothyroidism than healthy controls and ghrelin levels was significant decreased after treatment of hypothyroidism (5). However, the results of other similar studies were inconsistent with those of earlier investigations (6, 7). The ghrelin levels of hyperthyroidism and hypothyroidism were not significant change in some studies. Therefore, the relationship between ghrelin levels and thyroid disease remains controversial. Whether changes in ghrelin levels are related to thyroid disease remains to be confirmed. As such, the present meta-analysis aimed to systematically and comprehensively evaluate the relationship between ghrelin levels and thyroid disease.
A literature search of the Web of Science, Wiley Online Library, PubMed, and Embase databases was performed. The search scope included studies investigating the relationship between ghrelin and patients with thyroid disease, published up to October 2024. The title or abstract search terms “ghrelin” was used in combination with “thyroid”. Eligible studies were restricted to those published in English. The reference lists of the retrieved studies were manually searched to identify additional, potentially eligible studies. The present study was registered in the PROSPERO Database (CRD42024591501). All requisite items reported for systematic reviews and meta-analyses are listed in the Supplementary Table S1.
Meta-analysis was performed on studies fulfilling the following criteria: sufficient data regarding ghrelin levels in patients with thyroid disease and healthy controls; case-control or cohort design; and publication language in English.
Duplicate studies, those with insufficient data, samples without serum and plasma data, animal studies, meta-analyses, reviews, meeting summaries, case reports, and editorials were excluded.
Two investigators independently reviewed the titles and abstracts of the retrieved studies according to predetermined inclusion and exclusion criteria. Disagreements regarding study selection during the review process were resolved through consensus discussion based on an established standard. If consensus could not be reached, a third investigator was invited to participate in the final decision as to whether the study fulfilled the inclusion criteria. Data extracted from the selected studies included the following: first author; publication year; region; design; sample; and disease.
The Cochrane Collaboration recommends the Newcastle-Ottawa Scale (NOS) as a tool for assessing bias in observational studies (8). The NOS was used as an evaluation standard, and two researchers were based on the research content of the case-control studies and cohort studies, the methodological quality of independent evaluation, including selection of the research objective, comparability between groups, and outcome measures. An NOS score between 0 and 9 was calculated, with ≥ 6 points considered to be a high-quality study; the higher the score, the higher the quality of the study.
Bias was also assessed with the ROBINS-I (risk of bias in non-randomized studies-of interventions) tool for nonrandomized studies. The ROBINS-I tool views each study as an attempt to emulate a hypothetical pragmatic randomized trial and assesses seven domains through which bias might be introduced: bias due to confounding, bias in the selection of participants into the study, bias in classification of interventions, bias due to deviations from intended interventions, bias due to missing data, bias in the measurement of outcomes, and bias in the selection of the reported result. The judgments within each domain carry forward to an overall risk of bias of: ‘Low’, ‘Moderate’, ‘Serious’, ‘Critical’ or ‘No information’ (9).
Results are expressed as standardized mean difference (SMD) with corresponding 95% confidence interval (CI). Heterogeneity was assessed according to I2 and P values, as follows: I2 < 50%, P > 0.1 represented low heterogeneity, and a fixed effects model was used for analysis; I2 ≥ 50% and P ≤ 0.1 represented high heterogeneity, and a random effects model was used for analysis. Sensitivity analyses were performed on the sources of heterogeneity in the outcome indicators. Egger’s test was used to assess publication bias. Differences with P < 0.05 were considered to be statistically significant. Statistical analysis was performed using Stata Release 12.0 (StataCorp LLC, College Station, TX, USA).
A total of 853 relevant studies were retrieved from the Web of Science, Wiley Online Library, PubMed, and Embase databases, of which 499 duplicates were excluded, and 278 were excluded after reading titles and abstracts. An additional 53 studies were excluded after reading the full texts; ultimately, therefore, 23 studies comprising 1089 patients with thyroid disease and 693 controls were included in the meta-analysis (4–7, 10–28). A flow diagram illustrating the study selection process is presented in Figure 1. The characteristics of the included studies are summarized in Table 1. All 23 studies included in this meta-analysis fulfilled the criteria for NOS categories of selection, comparability, and exposure. The ROBINS-I assessment of study bias for included studies was presented in Supplementary Table S2.
Ghrelin levels were significantly lower in patients with hyperthyroidism than those in healthy individuals (SMD: -1.03, 95% CI [-1.75, 0.32]). Forest plots of the results are presented in Figure 2. Ghrelin levels of patients with hyperthyroidism were significantly higher after effective treatment (SMD: 0.77, 95% CI [0.03, 1.51]) (Figure 3). Ghrelin levels were not significantly higher in patients with hypothyroidism than in healthy controls (SMD: 0.48, 95% CI [-0.13, 1.08]) (Figure 4). And there was also no significant difference of ghrelin levels in patients with hypothyroidism after effective treatment (SMD: -0.33, 95% CI [-1.23, 0.57]) (Figure 5). Four studies about euthyroid patients with Hashimoto’s thyroiditis and three studies about thyroid carcinomas, there were both no significant difference of ghrelin levels compared to the healthy controls (SMD: -0.52, 95% CI [-0.82, 1.85] and SMD: 0.36, 95% CI [-1.03, 1.76]).
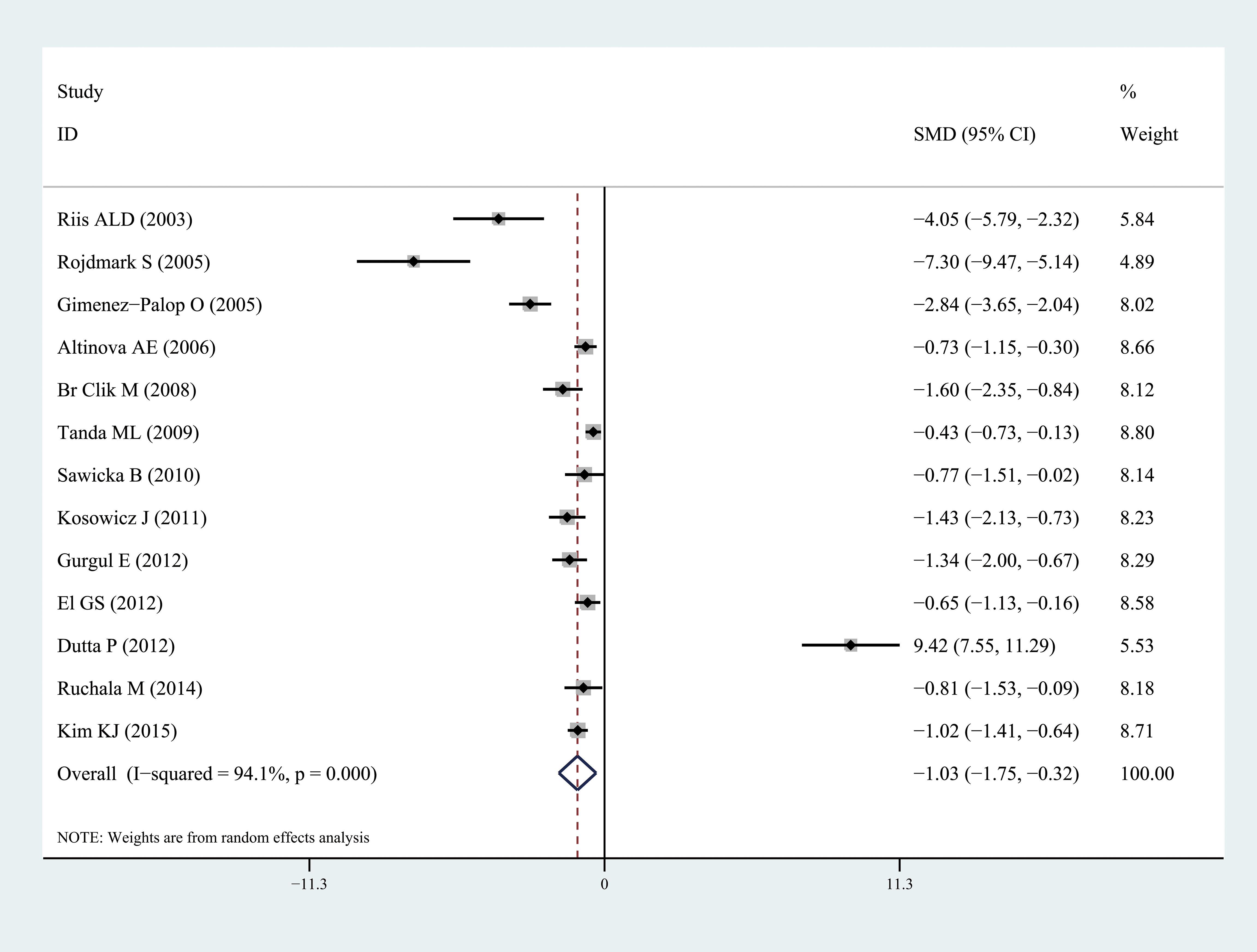
Figure 2. Forest plots of ghrelin level in patients with hyperthyroidism compared to healthy individuals. Diamond represents the pooled SMDs at 95% CI. SMD, standardized mean difference; CI, confidence interval.
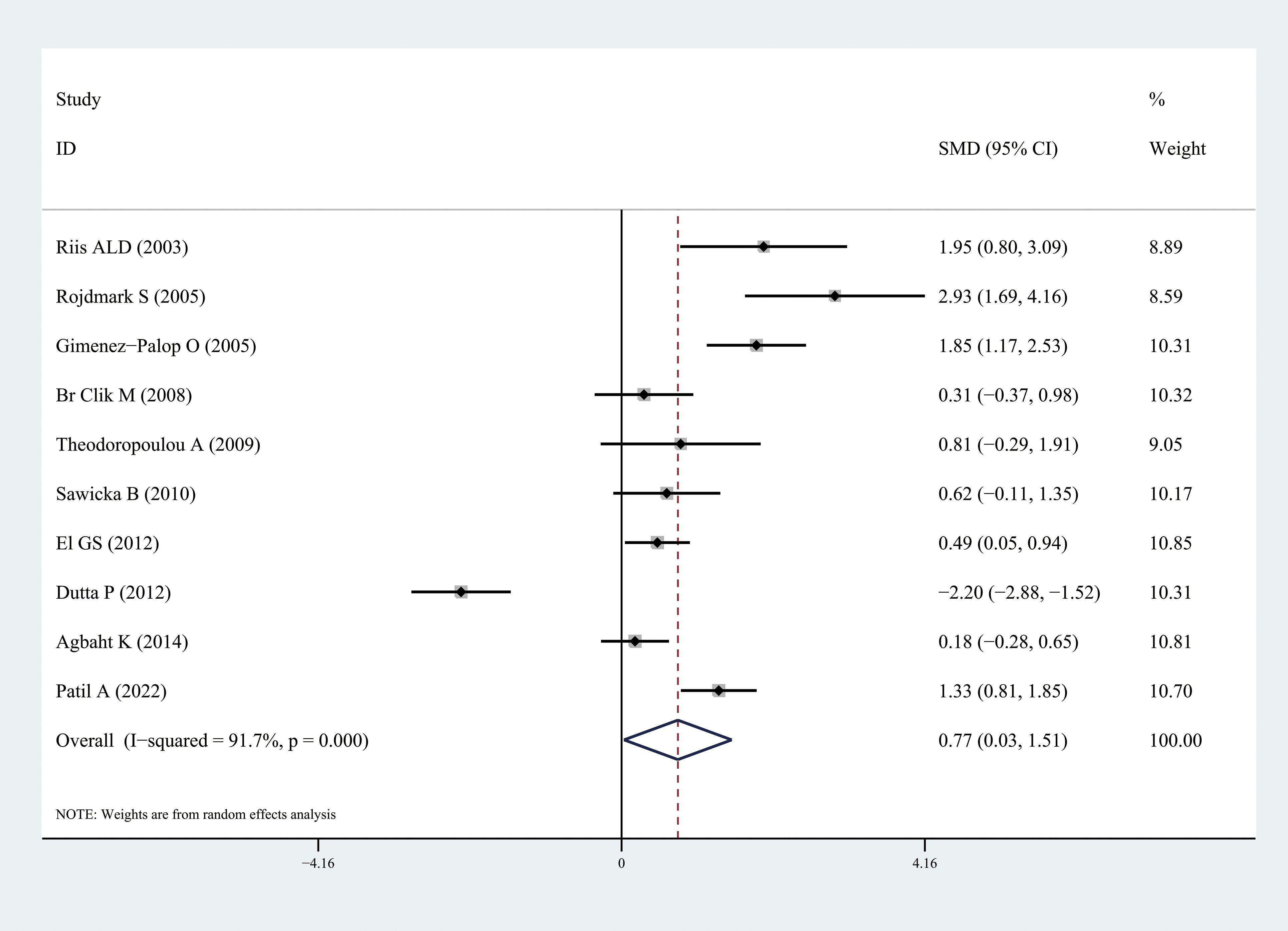
Figure 3. Forest plots of ghrelin level in patients with hyperthyroidism after treatment compared to before. Diamond represents the pooled SMDs at 95% CI. SMD, standardized mean difference; CI, confidence interval.
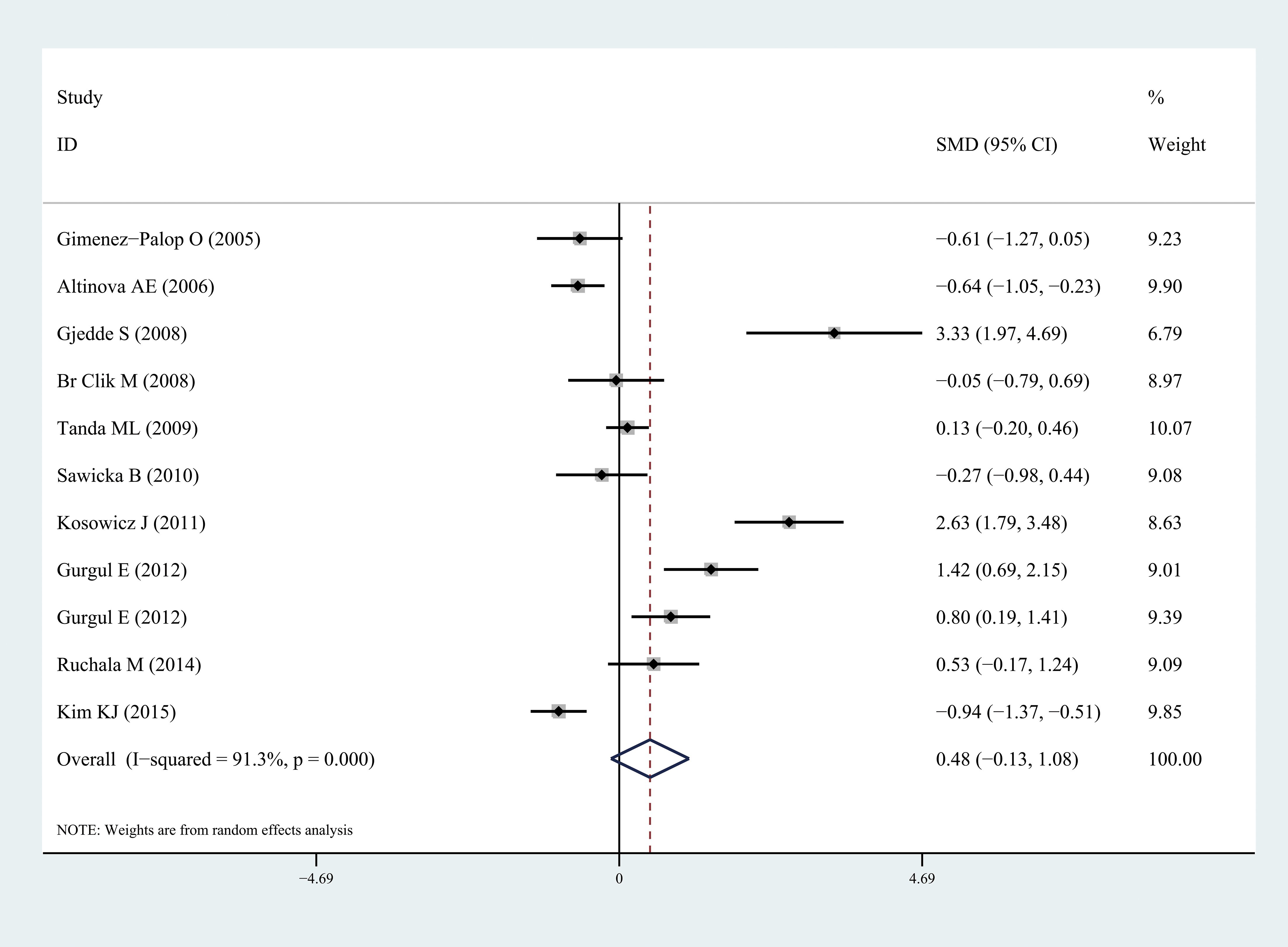
Figure 4. Forest plots of ghrelin level in patients with hypothyroidism compared to healthy individuals. Diamond represents the pooled SMDs at 95% CI. SMD, standardized mean difference; CI, confidence interval.
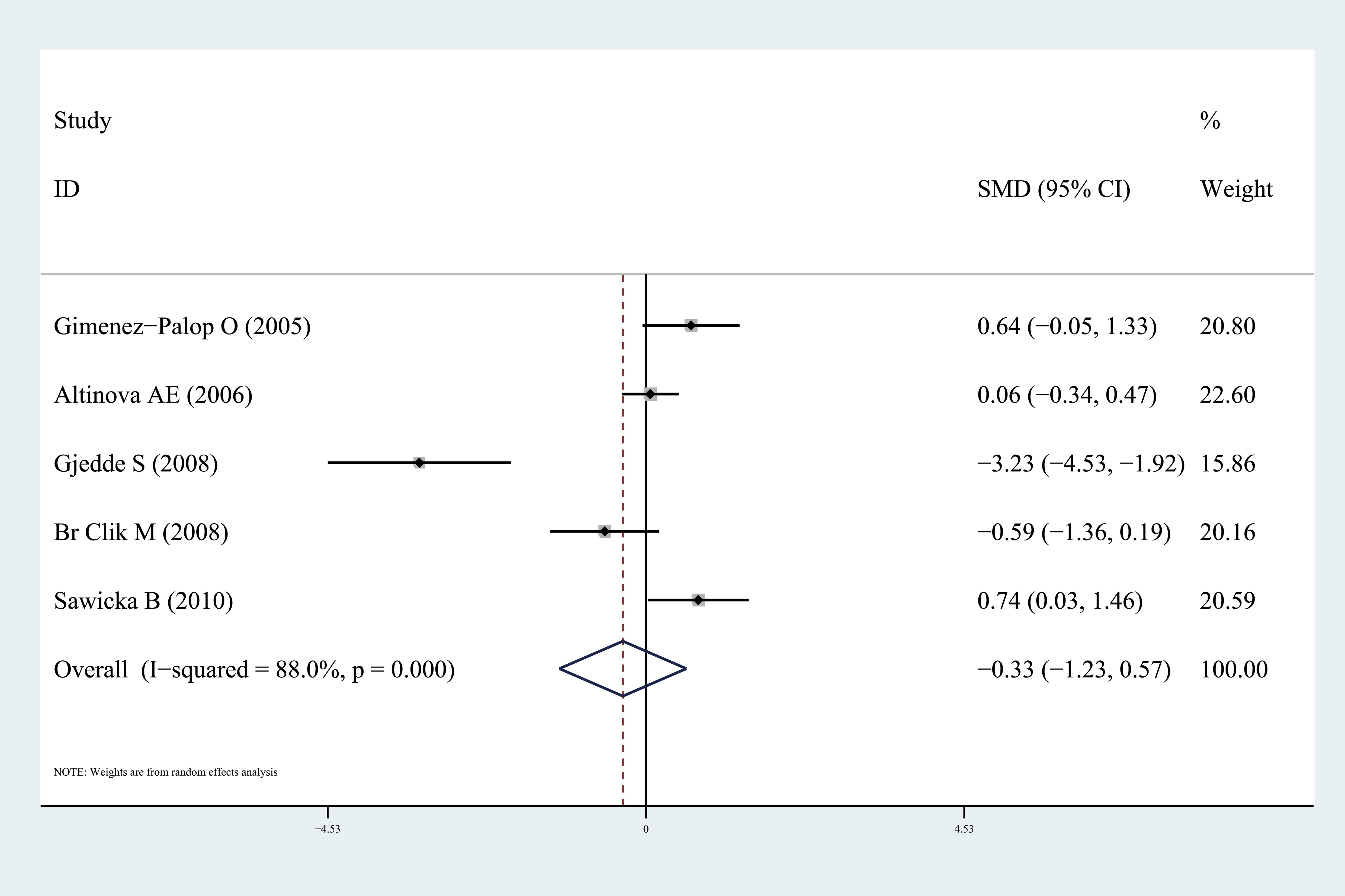
Figure 5. Forest plots of ghrelin level in patients with hypothyroidism after treatment compared to before. Diamond represents the pooled SMDs at 95% CI. SMD, standardized mean difference; CI, confidence interval.
To ensure the reliability of the results, the calculations were recalculated by stepwise elimination of ≥ 1 studies and sensitivity analysis using a random-effects model. The results of sensitivity analysis were presented in Supplementary Figures S1–S4. A thorough and comprehensive database search was performed. Tests for the presence of publication bias in the results of systematic reviews were performed. Egger’s tests were used to assess overall publication bias in the included studies, with no bias observed in any of the included studies.
This systematic review is the first comprehensive evaluation of ghrelin levels in patients with thyroid disease. Twenty-three independent studies were included in this meta-analysis. We concluded that ghrelin levels were significantly lower in patients with hyperthyroidism than in healthy controls, and ghrelin levels of patients with hyperthyroidism were significantly higher after treatment. Ghrelin levels were higher—but not significantly—in patients with hypothyroidism than those in healthy controls.
Ghrelin is an endogenous growth hormone-releasing peptide, so it is a ligand of GHS-R. When combined with pituitary GHS-R, it strongly promotes the release of GH. Ghrelin is mainly synthesized in type X/A cells in the fundus of the stomach, and approximately 60%–70% of circulating ghrelin is derived from the stomach, whereas most of the remainder is derived from the small intestine (2). Ghrelin can promote the secretion of gastric acid, enhance gastric motility, stimulate appetite, increase food intake, increase the absorption of nutrients, especially lipids, promote adipogenesis, affect endocrine function of the pancreas and glucose metabolism, and regulate energy balance (29). Ghrelin production depends on food intake: it increases in the fasting state and decreases after meals; ghrelin levels negatively correlate with body mass: its concentration increases in malnutrition and decreases in obesity. Ghrelin promotes the secretion of adrenocorticotropic hormone, glucocorticoids, prolactin, and other hormones. Raghay et al. detected the distribution of ghrelin in human and rat thyroid tissues using immunohistochemical techniques and found that ghrelin was secreted by thyroid C cells in human and rat thyroid tissues (30). A higher ghrelin were observed in children with higher normal TSH concentration than those in children with lower normal TSH (31).
Increased appetite and weight loss are common among patients with hyperthyroidism. Increased ghrelin secretion can also increase appetite; however, it has been found that ghrelin levels in patients with hyperthyroidism are lower than those in healthy controls, suggesting that hyperthyroidism-induced increase in food intake is not mediated by ghrelin. The same conclusion was reached in animal experiments. Caminos et al. found that the messenger RNA (mRNA) expression of ghrelin in rats with hyperthyroidism was significantly lower than that in the healthy control group (32). Correspondingly, serum ghrelin level was also significantly lower than that in the healthy control group. Most studies have shown that hyperinsulinemia and hyperglycemia inhibit ghrelin secretion. A study by Soriano-Guillen reported that plasma ghrelin levels in 28 obese children who underwent an oral glucose tolerance test were significantly decreased, and when plasma glucose concentration reached its maximum, plasma ghrelin concentration was at its lowest (33). Leonetti et al. found that the hyperinsulinemic state of 35 obese subjects who underwent a hyperinsulinemic euglycemic clamp test exhibited reduced plasma ghrelin levels after continuous insulin infusion (34). Thyroxine promotes intestinal glucose absorption, accelerates the oxidative utilization of glucose and hepatic glycogenolysis, affects insulin secretion and action, and causes insulin resistance by reducing insulin secretion and peripheral insulin sensitivity. Moreover, several clinical studies confirmed that patients with hyperthyroidism have increased Homeostatic Model Assessment for Insulin Resistance (i.e., “HOMA-IR”) indices. Tong et al. confirmed that ghrelin inhibits glucose-induced insulin release and consumption by injecting healthy individuals with exogenous ghrelin (35).
Thyroid hormones have multiple effects on cholesterol levels. They promote cholesterol metabolism and affect cholesterol synthesis and transport. Patients with hyperthyroidism often exhibit clinical manifestations such as decreased blood lipid levels. Because ghrelin function is predicated on binding to lipoproteins, a drop in blood lipid levels can cause a corresponding decrease in ghrelin levels (36). In addition, elevated thyroid hormone levels stimulate the renin-angiotensin-aldosterone system, leading to increased renal blood flow and glomerular filtration rates. This also causes ghrelin to be metabolized at a faster rate, thereby reducing its concentration in the blood. Additionally, it is known that hyperthyroidism is associated with increased activity of the sympathetic nervous system and with abnormalities in the growth hormone/insulin-like growth factor 1 axis, which may affect glucose homeostasis, insulin sensitivity, and ghrelin levels (37). In general, these mechanisms lead to a decrease in blood ghrelin levels in patients with hyperthyroidism, which is a compensatory change in the body. In addition, ghrelin levels have been found to be significantly increased in patients with hyperthyroidism after effective treatment. Therefore, ghrelin levels may also be an effective indicator of hyperthyroidism treatment in the future.
In this study, ghrelin levels in patients with hypothyroidism were higher than those in healthy individuals; however, the difference was not statistically significant. There is a close relationship between hypothyroidism and hyperlipidemia. Thyroid hormones can affect the synthesis and degradation of cholesterol. The synthesis and degradation of lipids in patients with hypothyroidism are decreased, resulting in an increase in blood lipid concentration, which leads to an increase in ghrelin levels. In addition, the rate of metabolism is reduced in patients with hypothyroidism. Increased ghrelin levels may also be a compensatory response to change in appetite and intake caused by decreased thyroid hormone levels. These pathophysiological events may lead to changes in ghrelin levels.
Kanamoto et al. used polymerase chain reaction and immunocytochemical techniques to demonstrate that both the human thyroid follicular carcinoma cell line and the TT cell line of medullary thyroid carcinoma synthesize and secrete ghrelin in vitro (38). The TT cell line of medullary thyroid carcinoma synthesized more ghrelin than the TT cell line of thyroid follicular carcinoma. The N-PAP cell line and ARO cell line derived from undifferentiated thyroid carcinoma also produced ghrelin, and the treatment of these cell lines with ghrelin at a concentration of 100 nmol/L to 1 μmol/L exhibited a dose-dependent inhibition of cell proliferation. Because thyroid carcinomas is associated with insulin resistance, ghrelin levels in patients with thyroid carcinomas were analyzed. The results of 3 related studies demonstrated no significant difference in ghrelin levels between patients with thyroid carcinomas and healthy individuals; however, more research is needed to determine the relationship between them.
Hattori et al. first reported the presence of GHS-R in immune cells. Subsequent studies have revealed that immune cells express ghrelin as well as ghrelin receptors. Expression of ghrelin mRNA has been observed in normal human T lymphocytes, B lymphocytes, and neutrophils (39). In some inflammatory diseases, such as inflammatory bowel disease, ankylosing spondylitis, and sepsis, circulating ghrelin levels are significantly increased and are correlated with disease status (40). Several studies have analyzed changes in ghrelin levels in patients with Hashimoto’s thyroiditis with normal thyroid function and found no significant difference from the normal population. However, due to the small number of samples included, it was difficult to draw definitive conclusions. Ghrelin can significantly inhibit the production of inflammatory cytokines by inflammatory cells, such as interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)-α. IL-1α, interferon-gamma and TNF-α can down-regulate sodium-iodine symporter and affect thyroid hormone synthesis (41). These results suggest that ghrelin plays a role in the pathogenesis of autoimmune thyroid disease and may be used to control the inflammatory response caused by lymphocytes during the treatment of this disease.
The present study had some limitations. First, the methods used to measure ghrelin levels varied among studies. Graves’ disease is the most common cause of hyperthyroidism, whereas Hashimoto’s thyroiditis and thyroid surgery are the most common causes of hypothyroidism. Different causes of hypothyroidism can affect ghrelin levels. Third, studies investigating patients with euthyroid Hashimoto’s thyroiditis, thyroid carcinomas, and ghrelin levels are limited. Therefore, more experimental studies about treatment of thyroid disease by ghrelin is needed. As such, the results of the present meta-analysis should be interpreted with caution.
This systematic review is the first to evaluate the relationship between ghrelin and thyroid disease. Ghrelin levels were significantly lower in patients with hyperthyroidism. Determining the role of ghrelin in thyroid disease will significantly contribute to understand of symptom or pathomechanism.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
CX: Formal Analysis, Methodology, Project administration, Writing – original draft. JY: Data curation, Formal Analysis, Validation, Writing – original draft. HL: Funding acquisition, Software, Supervision, Writing – original draft. XS: Conceptualization, Data curation, Writing – original draft. HW: Conceptualization, Methodology, Project administration, Writing – original draft.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was financially supported by the Science Foundation of Suzhou (grant No. SLJ2022010).
We would like to thank Editage (www.editage.cn) for English language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1505085/full#supplementary-material
Supplementary Figure 1 | The sensitivity analysis results of ghrelin level in patients with hyperthyroidism compared to healthy individuals.
Supplementary Figure 2 | The sensitivity analysis results of ghrelin level in patients with hyperthyroidism after treatment compared to before.
Supplementary Figure 3 | The sensitivity analysis results of ghrelin level in patients with hypothyroidism compared to healthy individuals.
Supplementary Figure 4 | The sensitivity analysis results of ghrelin level in patients with hypothyroidism after treatment compared to before.
1. Acosta GJ, Singh ON, Brito JP. Epidemiologic changes in thyroid disease. Curr Opin Endocrinol Diabetes Obes. (2024) 31:184–90. doi: 10.1097/MED.0000000000000877
2. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. NATURE. (1999) 402:656–60. doi: 10.1038/45230
3. Sosic-Jurjevic B, Stevanovic D, Milosevic V, Sekulic M, Starcevic V. Central ghrelin affects pituitary-thyroid axis: histomorphological and hormonal study in rats. NEUROENDOCRINOLOGY. (2009) 89:327–36. doi: 10.1159/000188603
4. Riis ALD, Hansen TK, Møller N, Weeke J, Jørgensen JOL. Hyperthyroidism is associated with suppressed circulating ghrelin levels. J Clin Endocrinol Metab. (2003) 88:853–7. doi: 10.1210/jc.2002-021302
5. Gjedde S, Vestergaard ET, Gormsen LC, Riis ALD, Rungby J, Møller N, et al. Serum ghrelin levels are increased in hypothyroid patients and become normalized by l-thyroxine treatment. J Clin Endocrinol Metab. (2008) 93:2277–80. doi: 10.1210/jc.2007-2619
6. Dutta P, Bhansali A, Walia R, Khandelwal N, Das S, Masoodi SR. Weight homeostasis & its modulators in hyperthyroidism before & after treatment with carbimazole. Indian J Med Res (New Delhi India: 1994). (2012) 136:242–8.
7. Altinova AE, Toruner F, Karakoc A, Yetkin I, Ayvaz G, Cakir N, et al. Serum Ghrelin Levels in patients with Hashimoto’s thyroiditis. Thyroid (New York N.Y.). (2006) 16:1259. doi: 10.1089/thy.2006.16.1259
8. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non randomised Studies in Meta-Analyses. Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed 2014 Aug 5).
9. Sterne JA, Hernán MA, Reeves BC, Savovi´c J, Berkman ND, Viswanathan M, et al. Robins-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. (2016) 355:i4919. doi: 10.1136/bmj.i4919
10. Röjdmark S, Calissendorff J, Danielsson O, Brismar K. Hunger-satiety signals in patients with Graves’ thyrotoxicosis before, during, and after long-term pharmacological treatment. ENDOCRINE. (2005) 27:55–61. doi: 10.1385/ENDO:27:1:055
11. Morpurgo PS, Cappiello V, Verga U, Vicentini L, Vaghi I, Lauri E, et al. Ghrelin in human medullary thyroid carcinomas. Clin Endocrinol (Oxf). (2005) 63:437–41. doi: 10.1111/j.1365-2265.2005.02360.x
12. Gimeínez-Palop O, Gimeínez-Peírez G, Mauricio D, Berlanga E, Potau N, Vilardell C, et al. Circulating ghrelin in thyroid dysfunction is related to insulin resistance and not to hunger, food intake or anthropometric changes. Eur J Endocrinol. (2005) 153:73–9. doi: 10.1530/eje.1.01934
13. Altınova AE, Törüner FB, Aktürk M, Elbeğ Ş, Yetkin İ, Çakır N, et al. Reduced serum acylated ghrelin levels in patients with hyperthyroidism. Hormone Res. (2006) 65:295–9. doi: 10.1159/000092603
14. Br Clik M, Marcisz CA, Giebel S, Orze A. Serum leptin and ghrelin levels in premenopausal women with stable body mass index during treatment of thyroid dysfunction. Thyroid (New York N.Y.). (2008) 18:545–50. doi: 10.1089/thy.2007.0300
15. Tanda ML, Lombardi V, Genovesi M, Ultimieri F, Lai A, Gandolfo M, et al. Plasma total and acylated Ghrelin concentrations in patients with clinical and subclinical thyroid dysfunction. J Endocrinol Invest. (2009) 32:74–8. doi: 10.1007/BF03345683
16. Theodoropoulou A, Psyrogiannis A, Metallinos IC, Habeos I, Vgenakis AG, Kyriazopoulou V. Ghrelin response to oral glucose load in hyperthyroidism, before and after treatment with antithyroid drugs. J Endocrinol Invest. (2009) 32:94–7. doi: 10.1007/BF03345693
17. Sawicka B, Bossowski A, Szalecki M, Wysoka J, Koput A, Zelazowska-Rutkowska B, et al. Relationship between metabolic parameters and thyroid hormones and the level of gastric peptides in children with autoimmune thyroid diseases. J Pediatr Endocrinol metabolism: JPEM. (2010) 23:345. doi: 10.1515/jpem.2010.055
18. Kosowicz J, Baumann-Antczak A, Ruchala M, Gryczynska M, Gurgul E, Sowinski J. Thyroid hormones affect plasma ghrelin and obestatin levels. Horm Metab Res. (2011) 43:121–5. doi: 10.1055/s-0030-1269853
19. El GS, El KF, Mousa AA, Omar AA. Plasma levels of resistin and ghrelin before and after treatment in patients with hyperthyroidism. Endocr Pract. (2012) 18:376–81. doi: 10.4158/EP11130.OR
20. Gurgul E, Ruchała M, Kosowicz J, Zamysłowska H, Wrotkowska E, Moczko J, et al. Ghrelin and obestatin in thyroid dysfunction. ENDOKRYNOL Pol. (2012) 63:456–62.
21. Ağbaht K, Erdogan MF, Emral R, Baskal N, Güllü S. Circulating glucagon to ghrelin ratio as a determinant of insulin resistance in hyperthyroidism. ENDOCRINE. (2014) 45:106–13. doi: 10.1007/s12020-013-9951-9
22. Biyikli HH, Arduc A, Isik S, Ozuguz U, Caner S, Dogru F, et al. Assessing the relationship between serum ghrelin levels and metabolic parameters and autoimmunity in patients with euthyroid hashimoto’s thyroiditis. Endocr Pract. (2014) 20:818–24. doi: 10.4158/EP13469.OR
23. Malandrino N, Miceli A, Leggio L, Mingrone G, Capristo E. High ghrelin levels in post-treatment euthyroid patients with Hashimoto’s thyroiditis: a case-control preliminary study. Exp Clin Endocr Diabetes. (2014) 122:540. doi: 10.1055/s-0034-1376965
24. Ruchala M, Gurgul E, Stangierski A, Wrotkowska E, Moczko J. Individual plasma ghrelin changes in the same patients in hyperthyroid, hypothyroid and euthyroid state. Peptides (New York N.Y.: 1980). (2014) 51:31–4. doi: 10.1016/j.peptides.2013.10.018
25. Kim KJ, Kim BY, Mok JO, Kim CH, Kang SK, Jung CH. Serum concentrations of ghrelin and leptin according to thyroid hormone condition, and their correlations with insulin resistance. Endocrinol Metab (Seoul). (2015) 30:318–25. doi: 10.3803/EnM.2015.30.3.318
26. Ucan B, Sahin M, Kizilgul M, Ozbek M, Ozdemir S, Calıskan M, et al. Serum ghrelin levels in papillary thyroid carcinoma. Arch Endocrinol Metab. (2017) 61:464–9. doi: 10.1590/2359-3997000000290
27. Mele C, Samà MT, Bisoffi AA, Caputo M, Bullara V, Mai S, et al. Circulating adipokines and metabolic setting in differentiated thyroid cancer. Endocr CONNECT. (2019) 8:997–1006. doi: 10.1530/EC-19-0262
28. Patil A, Vaikkakara S, Dasari MD, Ganta S, Sachan A, Vinapamula KS. Mediators of energy homeostasis in hyperthyroidism. Arch Endocrinol Metab. (2022) 66:808–14. doi: 10.20945/2359-3997000000511
29. Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, et al. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. ENDOCRINOLOGY. (2000) 141:4255–61. doi: 10.1210/endo.141.11.7757
30. Raghay K, Garcia-Caballero T, Nogueiras R, Morel G, Beiras A, Dieguez C, et al. Ghrelin localization in rat and human thyroid and parathyroid glands and tumours. HISTOCHEM Cell Biol. (2006) 125:239–46. doi: 10.1007/s00418-005-0044-6
31. Adamczewska K, Adamczewski Z, Lupinska A, Lewinski A, Stawerska R. Strong positive correlation between TSH and ghrelin in euthyroid non-growth hormone-deficient children with short stature. MOLECULES. (2020) 25:3912. doi: 10.3390/molecules25173912
32. Caminos JE, Seoane LM, Tovar SA, Casanueva FF, Dieguez C. Influence of thyroid status and growth hormone deficiency on ghrelin. Eur J Endocrinol. (2002) 147:159–63. doi: 10.1530/eje.0.1470159
33. Soriano-Guillen L, Barrios V, Martos G, Chowen JA, Campos-Barros A, Argente J. Effect of oral glucose administration on ghrelin levels in obese children. Eur J Endocrinol. (2004) 151:119–21. doi: 10.1530/eje.0.1510119
34. Leonetti F, Iacobellis G, Ribaudo MC, Zappaterreno A, Tiberti C, Iannucci CV, et al. Acute insulin infusion decreases plasma ghrelin levels in uncomplicated obesity. Regul Pept. (2004) 122:179–83. doi: 10.1016/j.regpep.2004.06.014
35. Tong J, Prigeon RL, Davis HW, Bidlingmaier M, Kahn SE, Cummings DE, et al. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. DIABETES. (2010) 59:2145–51. doi: 10.2337/db10-0504
36. Theander-Carrillo C, Wiedmer P, Cettour-Rose P, Nogueiras R, Perez-Tilve D, Pfluger P, et al. Ghrelin action in the brain controls adipocyte metabolism. J Clin Invest. (2006) 116:1983–93. doi: 10.1172/JCI25811
37. Loeb JN. Metabolic changes in thyrotoxicosis. In: Braveman LE, Utiger RD, editors. Werner and ingbar’s the thyroid: A fundamental and clinical text, 7th ed. Lippincott Williams & Wilkins, Philadelphia, PA (1996). p. 687–93.
38. Kanamoto N, Akamizu T, Hosoda H, Hataya Y, Ariyasu H, Takaya K, et al. Substantial production of ghrelin by a human medullary thyroid carcinoma cell line. J Clin Endocrinol Metab. (2001) 86:4984–90. doi: 10.1210/jcem.86.10.7891
39. Hattori N, Saito T, Yagyu T, Jiang BH, Kitagawa K, Inagaki CGH. GH receptor, GH secretagogue receptor, and ghrelin expression in human T cells, B cells, and neutrophils. J Clin Endocrinol Metab. (2001) 86:4284–91. doi: 10.1210/jcem.86.9.7866
40. Karmiris K, Koutroubakis IE, Xidakis C, Polychronaki M, Voudouri T, Kouroumalis EA. Circulating levels of leptin, adiponectin, resistin, and ghrelin in inflammatory bowel disease. Inflammation BOWEL Dis. (2006) 12:100–5. doi: 10.1097/01.MIB.0000200345.38837.46
Keywords: ghrelin, thyroid disease, hyperthyroidism, hypothyroidism, meta - analysis
Citation: Xin C, Yao J, Li H, Sun X and Wang H (2025) Relationship between ghrelin and thyroid disease: a meta-analysis. Front. Endocrinol. 16:1505085. doi: 10.3389/fendo.2025.1505085
Received: 02 October 2024; Accepted: 13 February 2025;
Published: 28 February 2025.
Edited by:
Joseph V Martin, Rutgers University Camden, United StatesReviewed by:
Laiba Arshad, Forman Christian College, PakistanCopyright © 2025 Xin, Yao, Li, Sun and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huijuan Wang, wanghuijuan8@163.com; Xin Sun, sunxin77@126.com
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.