
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 06 March 2025
Sec. Cancer Endocrinology
Volume 16 - 2025 | https://doi.org/10.3389/fendo.2025.1504266
 Yang Zhou1‡
Yang Zhou1‡ Yongbing Sun1‡
Yongbing Sun1‡ Qi Qiao1‡
Qi Qiao1‡ Xin Qi2
Xin Qi2 Xinbei Lin1
Xinbei Lin1 Yawei Du3
Yawei Du3 Ao Liu1
Ao Liu1 Jing Zhou4‡
Jing Zhou4‡ Xue Lv5
Xue Lv5 Zhonglin Li1
Zhonglin Li1 Xiaoling Wu6
Xiaoling Wu6 Zhi Zou1
Zhi Zou1 Michael Zhang7†
Michael Zhang7† Jiadong Zhu8
Jiadong Zhu8 Feifei Shang8
Feifei Shang8 Hao Li9
Hao Li9 Yongli Li8*
Yongli Li8*Background: Limited research has explored the effect of high-density lipoprotein cholesterol (HDL-C) on lung cancer’s seven autoantibodies (7-AABs). This study investigated the association between serum HDL-C and 7-AABs among 5,574 Chinese adults aged ≥ 18 years from January 2018 to December 2023.
Methods: This cross-sectional study utilized physical examination data from the Department of Health Management at Henan Provincial People’s Hospital. The associations between HDL-C and autoantibodies, such as tumor protein 53(P53), SRY-box containing gene 2 (SOX2), and ATP-dependent RNA helicase 4-5 (GBU4-5), were modeled using a restricted cubic spline logistic regression model.
Results: After the adjustment for factors, such as age and body mass index, the binary logistic regression model showed distinct correlations between serum HDL-C levels and autoantibodies, including P53, SOX2, and 7-AABs. Restricted cubic spline logistic regression analysis indicated that the increased level of serum HDL-C was associated with a decreased risk of positive P53 (all participants: HDL-C: 1.227–1.366 mmol/L, P HDL-C=0.028), SOX2 (all participants: HDL-C ≥ 1.227 mmol/L, P HDL-C =0.021; all women: HDL-C ≥ 1.224 mmol/L, P HDL-C=0.037), GBU4-5 (all women: HDL-C ≥ 1.269 mmol/L, P HDL-C=0.039), and 7-AABs (all women: HDL-C ≥ 1.224 mmol/L, P HDL-C=0.015). In women, HDL-C levels between 1.163 and 1.224 mmol/L correlated with an increased risk of positive 7-AABs test results.
Conclusions: Elevated HDL-C levels exhibited an independent association with a reduced risk of positivity for 7-AABs of lung cancer, especially in the female physical examination population. These findings suggest that high HDL-C levels may play a role in hindering lung cancer development with gender differences. However, further confirmation is still needed in the future.
Lung cancer is a prevalent and important cause of mortality worldwide (1, 2). The incidence of lung cancer has been rapidly increasing, and 3.6 million cases are predicted for 2040 (3). In China, lung cancer is the most common malignant tumor in males and has the highest mortality rate in men and women. This condition poses a serious health threat to the population (4). During lung cancer development, tumor tissues produce lung cancer-related proteins, which stimulate the immune system to produce highly specific autoantibodies. These autoantibodies can serve as biomarkers for lung cancer (5). In a large-scale clinical study focusing on the Chinese population, Ren et al. demonstrated the clinical utility of a set of autoantibodies known as seven autoantibodies (7-AABs) for early lung cancer screening (6). These autoantibodies include tumor protein 53 (p53), G antigen 7 (GAGE7), protein-encoding gene product 9.5 (PGP9.5), cancer/testis-associated antigen (CAGE), melanoma antigen A1 (MAGEA1), SRY-box containing gene 2 (SOX2), and ATP-dependent RNA helicase 4-5 (GBU4-5). Several studies have reported a diagnostic specificity for lung cancer as high as 90% (7). The China Food and Drug Administration has recognized the 7-AABs spectrum as the sole autoantibody spectrum for early lung cancer screening and diagnosis. Numerous studies have explored the correlation between metabolic syndrome (MS) and lung and other cancers (8, 9). High-density lipoprotein cholesterol (HDL-C), a component of MS (10), also correlates with the progression of various cancers (11, 12). Crudele et al. observed that low HDL levels can be used as a novel marker to predict hepatocellular carcinoma development in patients with liver fibrosis (13). ŞAHIN et al. reported that HDL levels were lower in the lung cancer metastasis group compared with the healthy group (14). A retrospective observational study conducted by Luo et al. revealed that high HDL-C levels in lung cancer patients prior to chemotherapy were independent predictors of a long disease-free survival. This condition suggests that monitoring HDL-C fluctuations can be a valuable predictor for lung cancer and blood lipid levels should be monitored long-term for effective tumor management (15). Lin et al. confirmed a significant inverse association between HDL-C levels and the risk of lung carcinoma (16). HDL-C may regulate cancer development by influencing proliferative and inflammatory pathways, which leads to a favorable anticancer state (17). HDL-C is closely related to lung cancer progression, which can be aid in identifying and monitoring high-risk patients (18). Elevated HDL levels can reduce oxidative stress and proinflammatory molecule levels in cancer cells and the tumor microenvironment (TME) (19).
However, no research explored the association between 7-AABs and serum HDL-C levels among the Chinese population. The effect of fluctuations in serum HDL-C levels on the screening results of 7-AABs should be explored to provide new ideas for their joint detection to improve the efficiency of lung cancer screening and realize the early detection and diagnosis of lung cancer in clinical practice. Therefore, in this study, we assumed that an association exists between 7-AABs and HDL-C levels. The homogeneous sample in this study aided in elucidating the association between HDL-C and lung cancer autoantibodies among Chinese adults and the findings may provide guidance for the use of HDL-C as an early screening indicator for lung cancer, which may compensate for the shortcomings of the clinical lung cancer screening process.
This research aimed to reveal the potential correlations between HDL-C and 7-AABs and analyze epidemiological data to elucidate such association. By investigating the influence of serum HDL-C on the detection results for 7-AABs, this study sought to provide new insights and evidence for the anticancer effects and lung cancer screening effects of HDL-C.
Data were obtained from the physical examination database of the medical examination center at Henan Provincial People’s Hospital from 2018 to 2023. The inclusion criteria for this study comprised the following: (1) adult medical examination personnel over 18 years of age, (2) complete blood biochemical examination information, and (3) complete general demographic information. The exclusion criteria included the following: (1) a long-term smoking history of 30 pack years (20, 21); (2) long-term use of lipid-lowering drugs; (3) a history of metabolic disorder diseases, endocrine diseases, or various malignant tumors. Trained personnel conducted face-to-face interviews for the collection of basic data, including age, sex, metabolic indicators, medical history, and medication history. Initially, 7,257 participants were included, but 1,683 were subsequently excluded due to incomplete blood biochemical index data. Ultimately, the study incorporated 5,574 participants (Figure 1).
Before the survey, all study participants underwent uniform training to ensure the accuracy and reliability of data acquisition. The participants’ underlying data were collected through a standardized questionnaire, which included information on their medical history related to malignant tumors, liver and kidney diseases, thyroid disorders, and the intake of lipid metabolism-regulating drugs. After the participants have completed the survey questionnaires, the data were collated, checked, and verified.
① After a 12 h fasting period, professional medical staff measured the height, weight, and blood pressure of the participants, who were wearing light clothing and had removed their shoes, in the morning. Each measurement was performed twice and averaged to minimize errors. ② Body mass index (BMI) was calculated as weight divided by height squared (kg/m2).
① FBG detection: All subjects fasted for 8 h or more before fasting venous blood samples were collected to avoid heavy drinking and eating. ② Blood lipid tests: A total of 5 mL venous blood was obtained from the participants and sent to the laboratory for analysis of triglyceride (TG) and HDL-C levels. Blood lipid and glucose levels were measured using an Olympus® AU 5400 automatic biochemical analyzer (Olympus Corporation, Japan, Shizuoka). Other indexes were estimated through conventional examination techniques.
The diagnostic criteria for MS were referred to the International Diabetes Federation, the American Heart Association and the American Heart Association, Lung and Blood Institute in 2009 (22): ① obesity: BMI ≥ 25 kg/m2; ② hyperglycemia: FBG ≥ 5.6 mmol/L; ③ raised blood pressure: systolic blood pressure (SBP) ≥ 130 mmHg and (or) diastolic blood pressure (DBP) ≥ 85 mmHg; ④ TG ≥ 1.7 m mol/L; ⑤ HDL-C< 0.9 mmol/L for the males and <1.0 mmol/L for the female subjects. MS was diagnosed in three or more cases with the above risk factors.
1. Serum Specimen Collection: A total of 3 mL peripheral venous blood was collected from the subjects’ elbow vein after a 12 h fast. Blood was collected in a vacuum blood collection tube, which was then centrifuged at 3,000 × g for 10 min, after standing at room temperature for 30 min. The serum was separated and frozen at -80°C in a refrigerator.
2. Reagents and Instruments: The detection reagent was from a kit for the detection of seven types of autoantibodies =(Hangzhou Kaibaoluo Biotechnology Co., Ltd) was used. An ST-360 microplate reader from Shanghai Kehua Experimental System Co., Ltd. was employed.
3. Methods: Indirect enzyme-linked immunosorbent assay was performed to determine the concentrations of 7-AABs in serum samples. The detection process followed the procedures described in literature: Tumor-associated antigen (TAA) proteins were immobilized in microwells (50 mL/well) by a streptavidin tag. Serum samples diluted over [1∶109] with phosphate buffer were added to the microwells to bind the autoantibodies to the respective TAAs. Horseradish peroxidase-labeled antihuman IgG was added to each well to bind the autoantibodies. Serum autoantibody concentrations were measured using a standard curve of the serial dilution of positive antibodies (6).
4. Interpretation Method of Test Results: The 7-AABs detection kits provided positive cutoff values for each antibody. A positive result for a specific antibody was considered when a single-antibody detection value is higher than its corresponding cutoff value. If one or more antibodies showed positive results, the overall findings for the 7-AABs were considered positive (6).
In this study, the 7-AABs score was the dependent variable and the HDL-C level the independent variable. The covariates included age, BMI, SBP, DBP, FBG, and TG.
Statistical analysis was conducted using SPSS (version 27.0, SPSS, Chicago, IL, USA), Origin (version 2022b, OriginLab, USA), and R (version 4.3.3, The R Foundation) software. The normality of data was assessed via the Kolmogorov−Smirnov test. Continuous variables were presented as mean ± standard deviation (x ± s) or median M (Q1, Q3), depending on their distribution. Wilcoxon Mann−Whitney test and two-tailed Student’s t-test were used to analyze nonnormally and normally distributed data, respectively. A correlation heatmap among the five diagnostic indexes of MS, including serum HDL-C and the 7-AABs of lung cancer, was generated using Origin software. Multivariate logistic regression analysis was performed on the above indicators using R software, and a classification forest plot was created for visual representation. The restricted cubic spline logistic regression model was utilized to identify linear or nonlinear associations between the 7-AABs and HDL-C levels in the physical examination population. Objective calculations were performed to determine the cutoff value for the correlation between the 7-AABs concentration and HDL-C levels. A two-tailed P <0.05 was considered statistically significant.
A total of 5,574 adult physical examination personnel, including 2,942 males and 2,632 females, were selected to participate in this study. Serum HDL-C levels were used to determine the characteristics of male and female participants, with different cutoffs for males (<0.9 and ≥0.9 mmol/L) and females (< 1.0 and ≥ 1.0 mmol/L) (22). As shown in Table 1, male participants with HDL-C levels < 0.9 mmol/L exhibited higher MS indicators (BMI, SBP, TG, and FBG), MS detection rates, and lung cancer autoantibody indicators (PGP9.5 and SOX2) compared with those in the HDL-C ≥ 0.9 mmol/L group. In the female cohort, participants with HDL-C levels < 1.0 mmol/L had higher age, MS indicators (BMI, SBP, TG, and FBG), and MS detection rates, No significant difference was observed in the 7-AABs between the two groups (Table 2).
Pearson correlation analysis revealed the following trends: Among all participants, HDL-C was negatively correlated with PGP9.5 and SOX2. HDL-C was negatively correlated with P53, PGP9.5, and SOX2 in the male cohort and with SOX2 in the female cohort. Overall, HDL-C exhibited a significant negative correlation with SOX2, regardless of gender (P < 0.01) (Figure 2).
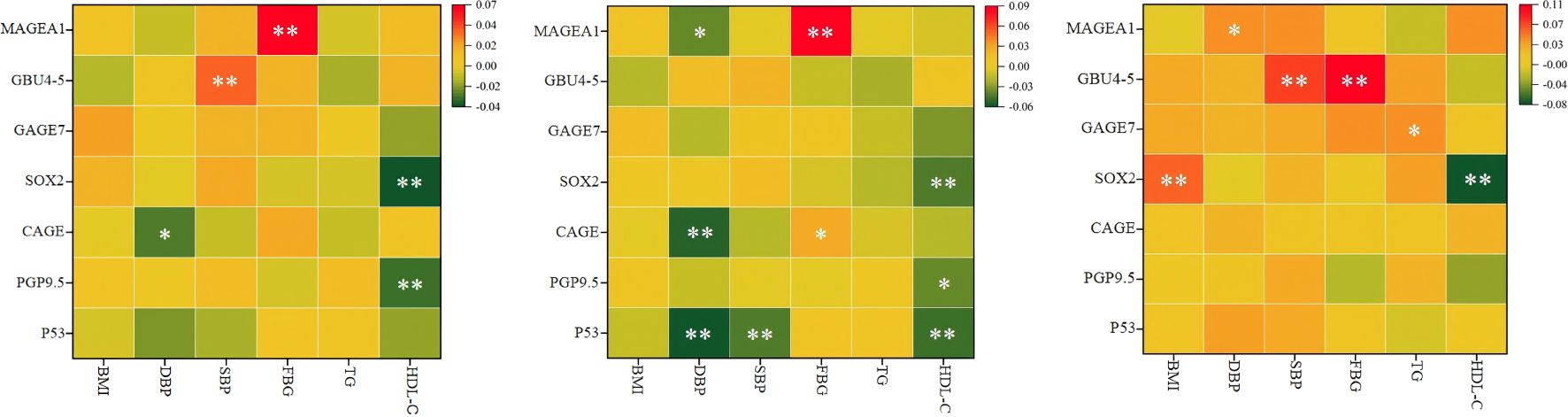
Figure 2. Pearson correlation analysis results of MS related indicators and 7- AABs. (A) for all, (B) for male, (C) for female. HDL-C, high-density lipoprotein cholesterol; BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure; FBG, fasting blood glucose; TG, triglycerides; MS, Metabolic syndrome;p53, tumor protein 53; PGP9.5, protein gene product 9.5;CAGE, cancer/testis‑associated antigen; SOX2, SRY‑box containing gene 2; GAGE7, G antigen 7; GBU4‑5, ATP‑dependent RNA helicase; MAGE A1, melanoma antigen A1; 7 - AABs, 7-autoantibodies; n, number of subjects; %, weighted percentage; *P < 0.05, **P < 0.01.
After the adjustment for age, sex, BMI, and various MS indicators, such as DBP, SBP, FBG, and TG, the research demonstrated a significant decrease in the risk of positive results for the combined 7-AABs test with the increase in the HDL-C levels (OR = 0.675, 95% confidence interval (CI): 0.483–0.944, P = 0.022). Similarly, the risk of positive P53 and SOX2 test results significantly decreased with increased HDL-C levels (OR = 0.314, 95% CI: 0.111–0.885, P = 0.028; OR = 0.396, 95% CI: 0.203–0.772, P = 0.007). However, HDL-C showed no effect of on the detection of PGP9.5 (OR = 0.549, 95% CI: 0.222–1.356, P = 0.194), CAGE (OR = 1.123, 95% CI: 0.461–2.734, P = 0.799), GAGE7 (OR = 1.149, 95% CI: 0.526–2.509, P = 0.728), GBU4-5 (OR = 0.600, 95% CI: 0.353–1.020, P = 0.059), or MAGEA1 (OR = 4.126, 95% CI: 0.958–17.762, P = 0.057). Table 3 and Figure 3 provide additional details.
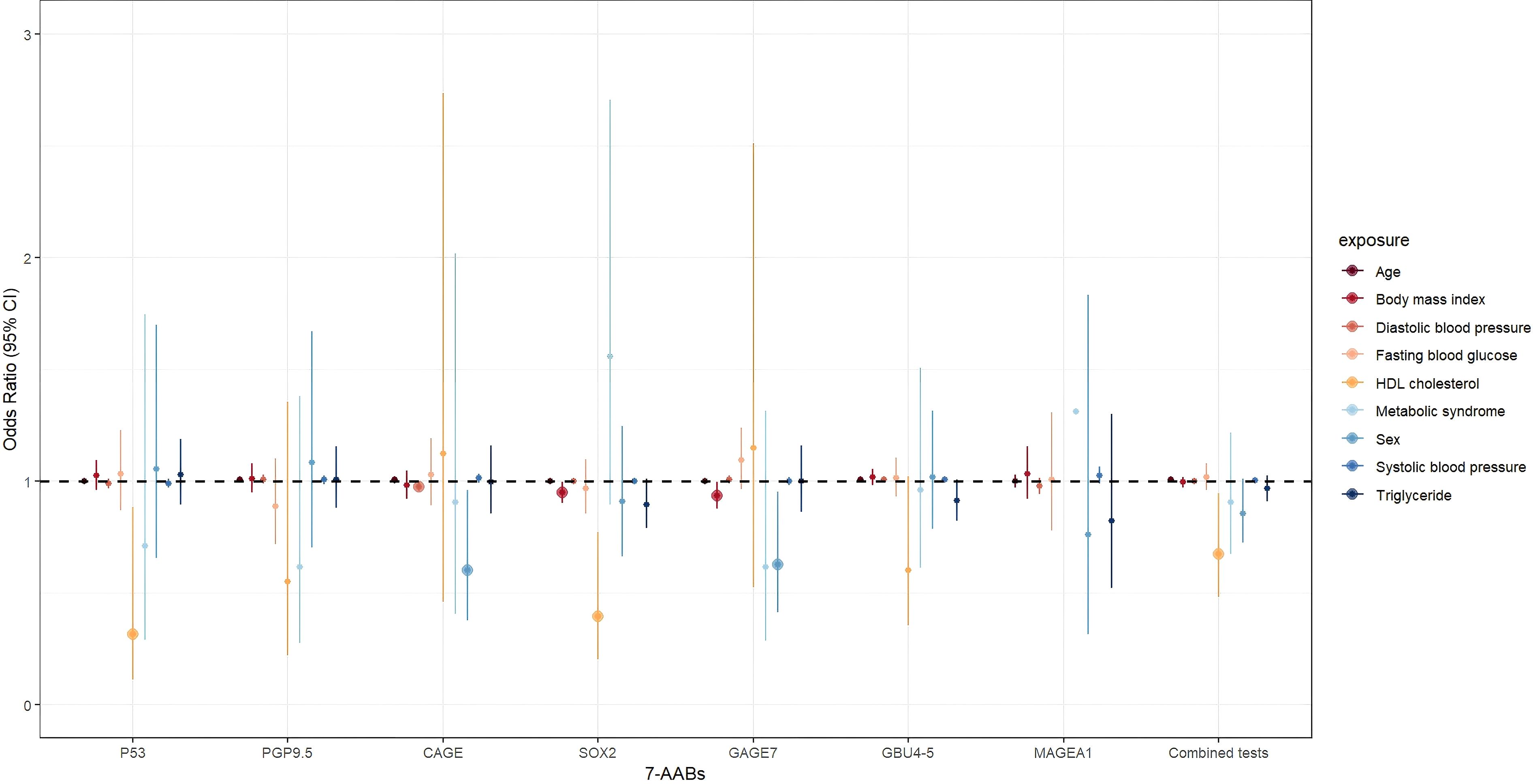
Figure 3. The association between MS and general indicators and 7-AABs combined test results in all participants. exposure: Age; Sex; BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure; FBG, fasting blood glucose; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; MS, Metabolic syndrome.
To present the analysis findings in a more visual manner, we conducted restricted cubic spline regression, and the results are displayed in Figures 4 and 5. After the adjustment for age, BMI, DBP, SBP, FBG, TG, and MS, the overall results showed a significant linear association between the hazard of positive P53 detection and HDL-C levels (P HDL-C=0.028, P Nonlinear=0.185). Specifically, the HDL-C levels between 0 and 1.227 mmol/L correlated with an increased risk of positive P53 in the tests. However, the HDL-C levels between 1.227 and 1.366 mmol/L correlated with a decreased risk of positive P53 (Figure 4A1). No linear nor nonlinear associations were found between HDL-C and P53 levels in the male (P HDL-C = 0.155, P Nonlinear = 0.133; Figure 4A2) and female (P HDL-C = 0.127, P Nonlinear = 0.758; Figure 4A3) cohorts. Figure 4B1 confirms a significant linear association between HDL-C levels and the hazard of positive SOX2 test results (P HDL-C = 0.021, P Nonlinear = 0.265). Specifically, at the serum HDL-C level greater than 1.227 mmol/L, the risk of positive SOX2 test results decreased with the increase in HDL-C levels. However, no association was found between the HDL-C levels and SOX2 test results in the male (P HDL-C = 0.444, P Nonlinear = 0.625; Figure 4B2) and female (P HDL-C = 0.037, P Nonlinear = 0.232; Figure 4B3) cohorts.
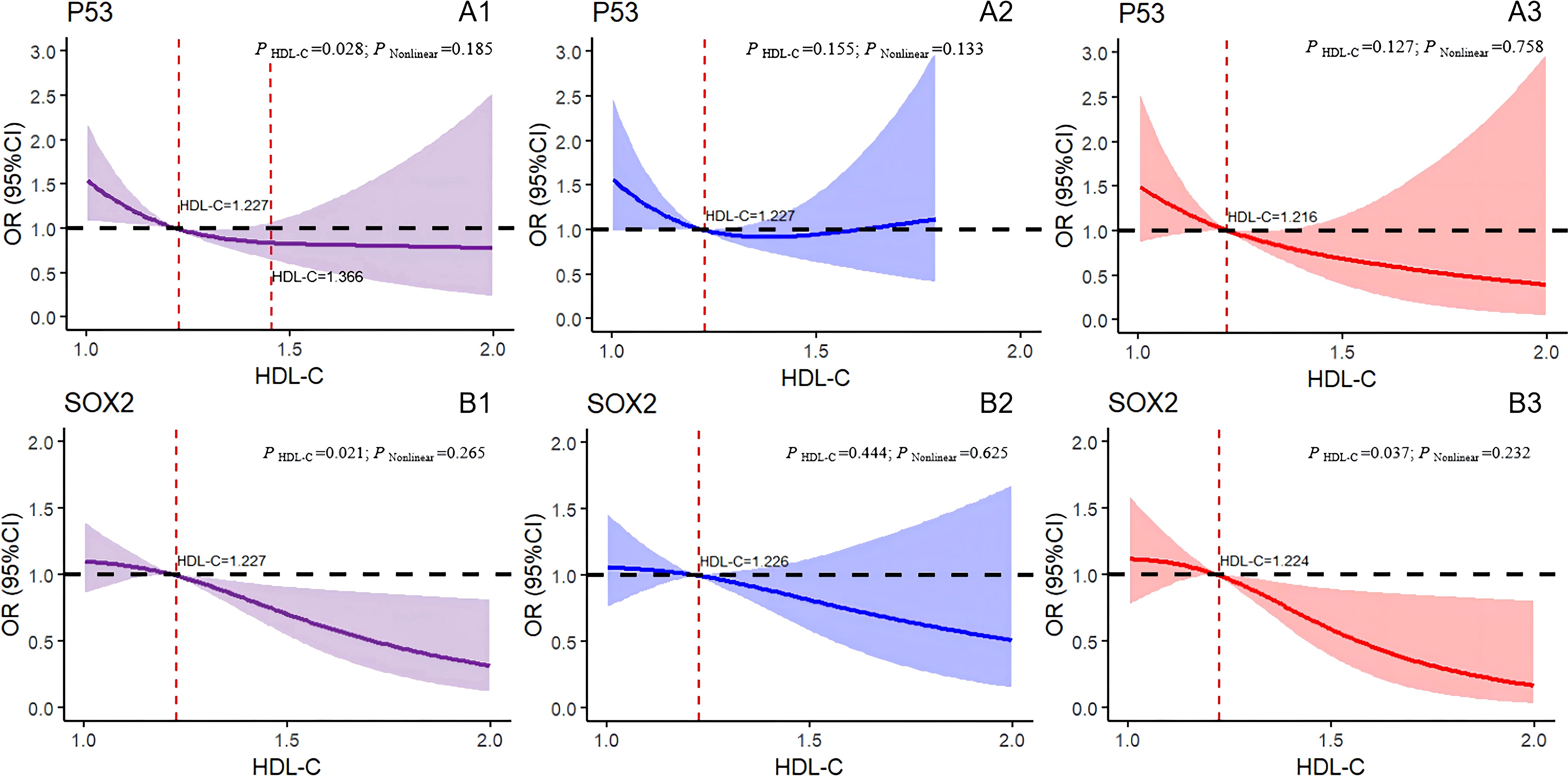
Figure 4. Logistic regression models with cubic natural spline analysis of HDL-C on a continuous scale and odds of positive 7-AABs. (A1–A3) for P53, (B1–B3) for SOX2. Multivariate regression models, adjusted for age, BMI, DBP, SBP, FBG,TG, and MS. Odds ratios are indicated by solid lines, and 95% CIs are represented by shaded areas (the shaded area located above the black dashed line refers to the promotion of positive risk occurrence; that located below the black dotted line denotes the obstructed positive risk occurrence; shaded areas spanning the black dashed line mean no statistical significance). P Nonlinear, P for nonlinearity. Purple for all participants; blue for all males; red for all females. P53, tumor protein 53; SOX2, SRY-box containing gene 2; HDL-C, high-density lipoprotein cholesterol.
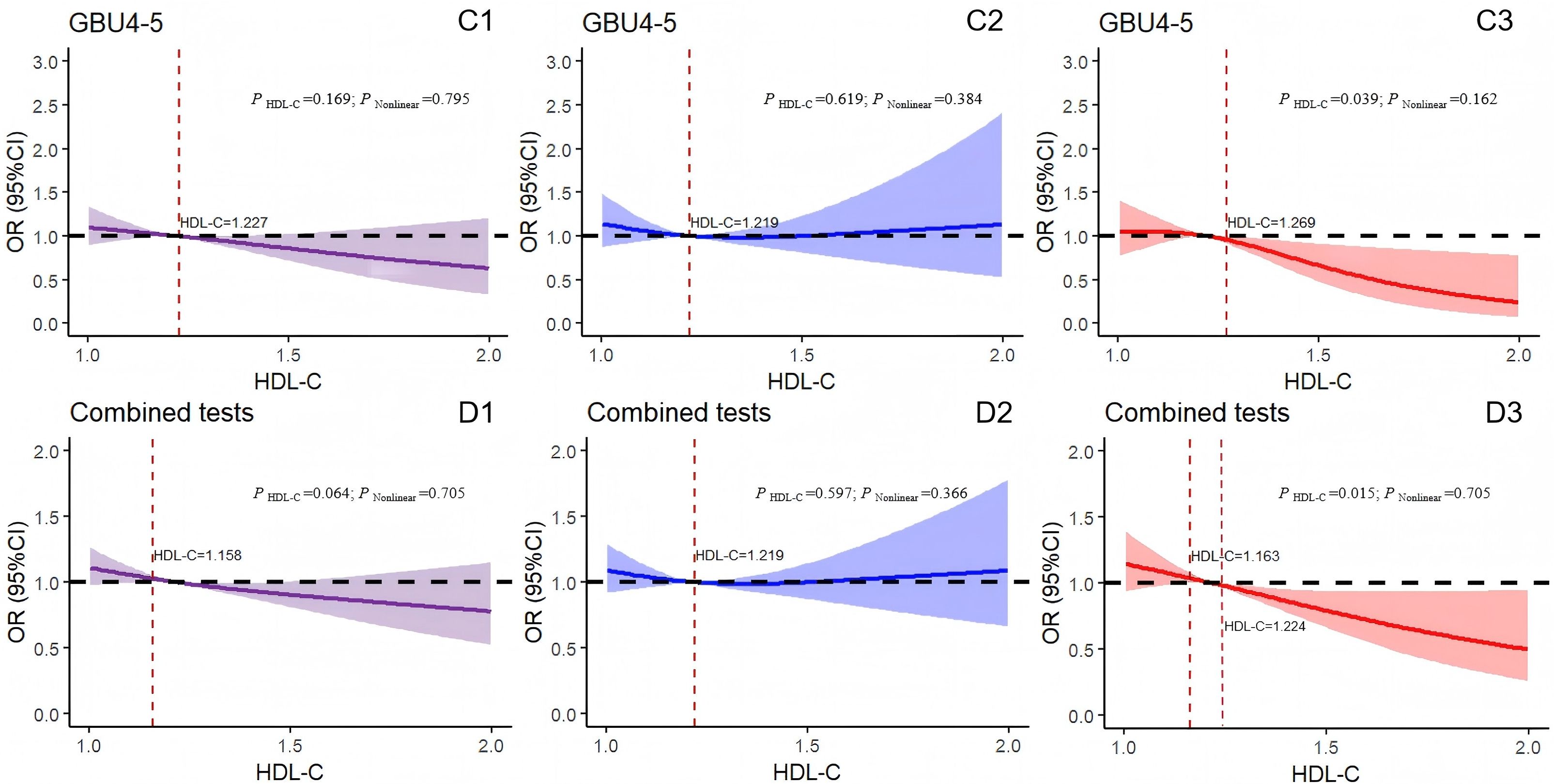
Figure 5. Logistic regression models with cubic natural spline analysis of HDL-C on a continuous scale and odds of positive 7-AABs. (C1–C3) for GBU4-5 and (D1–D3) for 7-AABs combined tests. Multivariate regression models adjusted for age, BMI, DBP, SBP, FBG, TG, and MS. Odds ratios are indicated by solid lines and 95% CIs by shaded areas (the shaded area located above the black dashed line represents the promotion of positive risk occurrence; that located below the black dotted line denotes the obstructed positive risk occurrence; shaded areas spanning the black dashed line indicate no statistical significance). P Nonlinear, P for nonlinearity. Purple for all participants; blue for all males; red for all females. GBU4-5, ATP-dependent RNA helicase; combined tests: 7-AABs combined tests; HDL-C, high-density lipoprotein cholesterol.
Figure 5C1 indicates the absence of linear or nonlinear associations between the results of the GBU 4-5 test and HDL-C levels (P HDL-C = 0.169, P Nonlinear = 0.795). Similarly, the GBU 4-5 test results and HDL-C levels in the male group showed no association (P HDL-C = 0.619, P Nonlinear = 0.384; Figure 5C2). By contrast, in the female group, when HDL-C exceeded 1.269 mmol/L, the risk of positive GBU 4-5 test results decreased with the increase in HDL-C levels (P HDL-C = 0.039, P Nonlinear = 0.162; Figure 5C3). Furthermore, the concentration of HDL-C in the total participants displayed no correlation with the 7-AABs joint detection results (P Nonlinear = 0.705, r = 0.064) (Figure 5D1). Similarly, in the male group (Figure 5D2), no linear nor nonlinear correlation was observed between the HDL-C levels and 7-AABs combined detection results (P HDL-C = 0.597, P Nonlinear = 0.366). By contrast, in the female group (Figure 5D3), a significant association was detected between the HDL-C levels and 7-AABs combined detection results (P HDL-C = 0.015, P Nonlinear = 0.705). Specifically, the HDL-C levels between 1.163 and 1.224 mmol/L correlated with an increased risk of positive 7-AABs test results. However, when the concentration of HDL-C exceeded 1.224 mmol/L, the risk of positive 7-AABs combined test results decreased with the increase in HDL-C levels. In addition, no linear nor nonlinear associations were recorded between the serum HDL-C levels and detection results for PGP9.5, CAGE, GAGE7, GBU 4-5, or MAGEA1.
This research aimed to explore the correlation between the serum HDL-C and detection results of 7-AABs in a physical examination population. Highly homogeneous samples (n = 5,574) aged ≥ 18 years and who underwent physical examination within six years were included in this study. After the adjustment for variables, such as age, BMI, etc., certain concentrations of HDL-C were associated with a reduced risk of positive test results for P53, SOX2, GBU 4-5, and the 7-AABs. Notably, in women, the HDL-C levels between 1.163 and 1.224 mmol/L correlated with an increased risk of positive 7-AABs test results. However, this phenomenon was not observed in the male group. Thus, this research provides evidence of a sex-specific association between the 7-AABs and serum HDL-C in a Chinese physical examination population, independent of age, BMI, etc.
Metabolic reprogramming is an important indicator of cancer (23). Lipid metabolism plays a crucial role in this process because the resulting signaling molecules can modify the TME and stimulate tumor cell proliferation, invasion, and metastasis (24). In addition, HDL-C can regulate antitumor immunity (25), promote T cell aggregation, and increase the influx of macrophages into the TME (26). Lung cancer, which is responsible for the majority of cancerrelated deaths worldwide, is often diagnosed at advanced stages, when it is incurable (5). Current biological and epidemiological evidence increasingly supports the idea that reduced levels of HDL-C contribute to a high risk of lung cancer (14, 17). Multiple molecular abnormalities can be detected throughout tumor development (27). The immune system of lung cancer patients releases specific autoantibodies against TAAs through the production of high-affinity T cells, which can serve as biomarkers. These autoantibodies are more stable than the antigens themselves; they exhibit higher levels and remain detectable in the serum for many years (28). Furthermore, these autoantibodies can be detected during the asymptomatic phase of lung cancer (29). However, the fundamental association and interaction between serum HDL-C and the 7-AABs remain unexplored domestically and internationally.
In this study, prior to adjustment for age, BMI, and metabolism-related confounding factors, serum HDL-C showed a negative correlation with PGP9.5 and SOX2 in all participants. HDL-C was negatively correlated with P53, PGP9.5, and SOX2 in men and negatively correlated with SOX2 in women. PGP9.5 is a ubiquitin hydrolase found in neurons and neuroendocrine cells. This enzyme can be abnormally expressed and secreted in lung cancer cells, which leads to humoral reactions. Elevated PGP9.5 expression is associated with lung cancer development and is prevalent in lung cancer cell lines (30). The P53 gene is one of the earliest discovered anti-oncogenes (31). During its mutation under the influence of various factors, the resulting P53 protein promotes tumor cell survival, inhibits apoptosis, and leads to cancer development (32). This mutation also induces the production of p53 antibodies in the blood (33). Mutant p53 is correlated with a high incidence of cell-in-cell structures in lung adenocarcinoma, which is a common feature and a crucial predictor of poor prognosis and disease recurrence (34). SOX2 is a transcription factor associated with sex-determining genes. This factor possesses an HMG-specific domain. SOX2 is often overexpressed in squamous cell carcinoma and small-cell lung cancer (35). Furthermore, it serves as an independent predictor of poor prognosis in lung adenocarcinoma (36). This study revealed that serum HDL-C concentration was inversely associated with the levels of PGP9.5, P53, SOX2, and other autoantibodies. The underlying mechanism may involve immune regulation and the capability of HDL-C to suppress inflammation (37) and its inhibition of tumor cell proliferation or growth (38).
In regard to the association between malignant tumor hazards and HDL-C levels, extensive research has consistently shown an inverse correlation between HDL-C and cancer hazards (39). Dessi et al. revealed significantly lower levels of HDL-C in a lung cancer cohort compared with a healthy control group (40). After adjusting for confounding factors, such as age, BMI, DBP, SBP, FBG, TG, and MS, this research also demonstrated a distinctly negative correlation between the detection of 7-AABs and HDL-C in the female cohort. The risk of positive test results for SOX2, GBU4-5, and 7-AABs gradually decreased when the serum HDL-C levels increased to ≥1.2 mmol/L. Numerous studies have proven the connection between cancer occurrence and development and chronic inflammation. Low levels of serum HDL-C may indicate a reduced ability to resist inflammation and consequently promote the advancement of lung cancer (41). The results of this study suggest that increased serum HDL-C levels may contribute to tumor suppression through the enhancement of the anti-inflammatory properties of HDL-C (42). This reduction in the body’s inflammatory response can potentially delay the progression of lung cancer and subsequently decrease the levels of autoantibodies. However, this study did not observe any effect of serum HDL-C on any autoantibody test results in the cohort of men. According to Luo et al., P53, PGP 9.5, SOX 2, GAGE 7, and GBU 4-5 levels do not differ in gender (35). The reason may be that differences in sexual habits and hormone secretion between men and women affect the level of HDL-C. Usually, the normal range of HDL-C is higher in women than in men. Moreover, serum HDL-C is speculated to be more sensitive to the early monitoring of lung cancer autoantibodies in women than in men.
However, the increase in serum HDL-C levels do not necessarily equate to improved outcomes. Zhong et al. reported that the lowest hazard of malignant tumor-related death was associated with HDL-C levels between 64–68 mg/dL (12). The lowest hazard was observed at 56–66 mg/dL in males and at 66–73 mg/dL in females. Compared with the HDL-C level of 66 mg/dL, each increase or decrease of 10 mg/dL in the HDL-C level was associated with risk ratios of cancer death of 1.02 and 1.11, respectively. Furthermore, some Mendelian randomized studies have failed to establish a definitive association between cancer hazards, including those of lung cancer, and HDL-C (43, 44). By contrast, one research suggested that high HDL-C levels were associated with an increased risk of breast cancer (44). The present research also revealed a similar phenomenon. Among all participants, the HDL-C levels between 0 and 1.227 mmol/L correlated with an increased risk of positive P53 test findings. However, when the HDL-C was ≥ 1.366 mmol/L, no difference was observed in P53 detection. In the female cohort, HDL-C levels between 1.163 and 1.224 mmol/L correlated with an increased risk of positive 7-AABs in the tests. Moreover, this study failed to reveal any association among CAGE, GAGE 7, MAGEA1, and HDL-C with or without adjusting for confounding factors, such as age and sex. These antibodies are speculated to be less sensitive as those such as P53, which may indicate a high false negative rate in some lung cancer-related antibody profiles (45) and may be related to whether marital status, socioeconomic factors, or dietary habits were excluded in the study; further research is still needed in the future. This work is the first to identify the varying effects of serum HDL-C levels on lung cancer autoantibodies at different levels. Therefore, it holds immense significance and has the potential to establish HDL-C as an early monitoring indicator for lung cancer. The combined detection of 7-AABs and HDL-C (HDL-C≥1.224 mmol/L, P = 0.015) may improve the sensitivity and specificity of lung cancer screening, and its diagnostic efficacy is expected to be higher than that of pure antibody detection.
The study participants were carefully selected to ensure the applicability of the findings to a broader population. In addition, through the utilization of advanced statistical analysis methods, such as multivariate logistic regression analysis and restricted cubic spline logistic regression model, this study revealed a detailed dose–response association between serum HDL-C and 7-AABs. The study’s robustness and interpretability were enhanced by adjusting for covariates, such as age. However, this research has limitations. First, as a cross-sectional study, the establishment of a causal association between serum HDL-C and 7-AABs levels was challenging, and the effect of HDL-C on 7-AABs requires further validation via longitudinal studies. Second, this research disregarded certain demographic indicators, such as marital status, occupation, and educational level. Lastly, the data used were all obtained from the same physical examination department, which limits the generalizability of the results. In the future, a multicenter follow-up study involving multiple hospitals will be conducted to verify the generalizability of the findings of this work. Therefore, the conclusions of this research must be validated through comparative analysis in the context of multiple healthcare institutions.
The findings strongly support the complex correlations between serum HDL-C and 7-AABs. The results indicate that high levels of HDL-C are highly associated with a reduced risk of positive 7-AABs detection, which suggests that HDL-C holds potential as an effective biomarker for the assessment of an individual’s cancer risk. Based on these observations, future research should include prospective cohort studies to further validate the feasibility of using HDL-C as a predictive bioindicator for cancer potential to provide a scientific basis for the early prevention and treatment of cancer.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the Ethics Committee of Henan Provincial People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
YZ: Data curation, Methodology, Writing – original draft. YS: Methodology, Writing – review & editing. QQ: Data curation, Methodology, Writing – review & editing. XQ: Data curation, Writing – review & editing. XBL: Methodology, Writing – review & editing. YD: Data curation, Writing – review & editing. AL: Data curation, Writing – review & editing. JZ: Data curation, Writing – review & editing. XL: Data curation, Writing – review & editing. ZL: Methodology, Writing – review & editing. XW: Data curation, Writing – review & editing. ZZ: Data curation, Writing – review & editing. MZ: Methodology, Writing – review & editing. JZ: Methodology, Writing – review & editing. FS: Methodology, Writing – review & editing. HL: Writing – review & editing. YL: Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Central Plains Science and Technology Innovation leading talent Program (244200510016); National Key R&D Program of China (2022YFC2010001); Key scientific research project of Henan University (25A310026); Medical Science and Technology Research Project of Henan Province (SBGJ202302011, LHGJ20210054, LHGJ20240050); Henan Provincial Science and Technology Tackling Program Project Funding (242102311018, 242102311121, 222102310283).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
HDL-C, high-density lipoprotein cholesterol; BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure; FBG, fasting blood glucose; TG, triglyceride; MS, metabolic syndrome; p53, tumor protein 53; PGP9.5, protein-encoding gene product 9.5; CAGE, cancer/testis-associated antigen; SOX2, SRY-box containing gene 2; GAGE7, G antigen 7; GBU4-5, ATP-dependent RNA helicase; MAGE A1, melanoma antigen A1; 7-AABs, 7-autoantibodies; CFDA, China Food and Drug Administration; DFS, disease-free survival; TME, tumor microenvironment; ELISA, enzyme-linked immunosorbent assay; SCLC, small cell lung cancer.
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. (2022) 72:7–3. doi: 10.3322/caac.21708
2. La Salvia A, Rossi A, Galetta D, Gobbini E, De Luca E, Novello S, et al. Intercalated chemotherapy and epidermal growth factor receptor inhibitors for patients with advanced non–small-cell lung cancer: A systematic review and meta-analysis. Clin Lung Cancer. (2017) 18:23–33.e1. doi: 10.1016/j.cllc.2016.08.006
3. International Agency for Research on Cancer, World Health Organization. Global cancer observatory. International Agency for Research on Cancer website (Accessed January 4, 2023).
4. Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). (2022) 135:584–90. doi: 10.1097/CM9.0000000000002108
5. Broodman I, Lindemans J, van Sten J, Bischoff R, Luider T. Serum protein markers for the early detection of lung cancer: A focus on autoantibodies. J Proteome Res. (2017) 16:3–13. doi: 10.1021/acs.jproteome.6b00559
6. Ren S, Zhang S, Jiang T, He Y, Ma Z, Cai H, et al. Early detection of lung cancer by using an autoantibody panel in Chinese population. Oncoimmunology. (2017) 7:e1384108. doi: 10.1080/2162402X.2017.1384108
7. Du Q, Yu R, Wang H, Yan D, Yuan Q, Ma Y, et al. Significance of tumor-associated autoantibodies in the early diagnosis of lung cancer. Clin Respir J. (2018) 12:2020–8. doi: 10.1111/crj.12769
8. Li M, Cao SM, Dimou N, Wu L, Li JB, Yang J. Association of metabolic syndrome with risk of lung cancer: A population-based prospective cohort study. Chest. (2024) 165:213–23. doi: 10.1016/j.chest.2023.08.003
9. López-López Á, Godzien J, Soldevilla B, Gradillas A, López-Gonzálvez Á, Lens-Pardo A, et al. Oxidized lipids in the metabolic profiling of neuroendocrine tumors - Analytical challenges and biological implications. J Chromatogr A. (2020) 1625:461233. doi: 10.1016/j.chroma.2020.461233
10. Sun Y, Qi X, Wang X, Lin X, Zhou Y, Du Y, et al. Association between high-density lipoprotein cholesterol and lumbar bone mineral density in Chinese: a large cross-sectional study. Lipids Health Dis. (2024) 23:27. doi: 10.1186/s12944-024-02023-1
11. Liu T, Zhou T, Luo F, Yang Y, Zhao S, Huang Y, et al. Clinical significance of kinetics of low-density lipoprotein cholesterol and its prognostic value in limited stage small cell lung cancer patients. Cancer Control. (2021) 28:10732748211028257. doi: 10.1177/10732748211028257
12. Zhong GC, Huang SQ, Peng Y, Wan L, Wu YQ, Hu TY, et al. HDL-C is associated with mortality from all causes, cardiovascular disease and cancer in a J-shaped dose-response fashion: a pooled analysis of 37 prospective cohort studies. Eur J Prev Cardiol. (2020) 27:1187–203. doi: 10.1177/2047487320914756
13. Crudele L, De Matteis C, Piccinin E, Gadaleta RM, Cariello M, Di Buduo E, et al. Low HDL-cholesterol levels predict hepatocellular carcinoma development in individuals with liver fibrosis. JHEP Rep. (2022) 5:100627. doi: 10.1016/j.jhepr.2022.100627
14. Şahin F, Aslan A. Relationship between inflammatory and biological markers and lung cancer. J Clin Med. (2018) 7:160. doi: 10.3390/jcm7070160
15. Luo F, Zeng KM, Cao JX, Zhou T, Lin SX, Ma WJ, et al. Predictive value of a reduction in the level of high-density lipoprotein-cholesterol in patients with non-small-cell lung cancer undergoing radical resection and adjuvant chemotherapy: a retrospective observational study. Lipids Health Dis. (2021) 20:109. doi: 10.1186/s12944-021-01538-1
16. Lin X, Lu L, Liu L, Wei S, He Y, Chang J, et al. Blood lipids profile and lung cancer risk in a meta-analysis of prospective cohort studies. J Clin Lipidol. (2017) 11:1073–81. doi: 10.1016/j.jacl.2017.05.004
17. Pirro M, Ricciuti B, Rader DJ, Catapano AL, Sahebkar A, Banach M. High density lipoprotein cholesterol and cancer: Marker or causative? Prog Lipid Res. (2018) 71:54–69. doi: 10.1016/j.plipres.2018.06.001
18. Li Z, Xu J, Feng W, Ma Z, Wu Y, Zhu T, et al. The clinical significance of preoperative serum triglyceride, high-density lipoprotein, and low-density lipoprotein levels in lung squamous cell carcinoma. Sci Rep. (2022) 12:16828. doi: 10.1038/s41598-022-18589-8
19. Ossoli A, Wolska A, Remaley AT, Gomaraschi M. High-density lipoproteins: A promising tool against cancer. Biochim Biophys Acta Mol Cell Biol Lipids. (2022) 1867:159068. doi: 10.1016/j.bbalip.2021.159068
20. de Koning HJ, Meza R, Plevritis SK, ten Haaf K, Munshi VN, Jeon J, et al. Benefits and harms of computed tomography lung cancer screening strategies: a comparative modeling study for the U.S. Preventive Services Task Force. Ann Intern Med. (2014) 160:311–20. doi: 10.7326/M13-2316
21. Humphrey LL, Deffebach M, Pappas M, Baumann C, Artis K, Mitchell JP, et al. Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive services task force recommendation. Ann Intern Med. (2013) 159:411–20. doi: 10.7326/0003-4819-159-6-201309170-00690
22. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. (2009) 120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644
23. Li Z, Kang Y. Lipid metabolism fuels cancer’s spread. Cell Metab. (2017) 25:228–30. doi: 10.1016/j.cmet.2017.01.016
24. Snaebjornsson MT, Janaki-Raman S, Schulze A. Greasing the wheels of the cancer machine: the role of lipid metabolism in cancer. Cell Metab. (2020) 31:62–76. doi: 10.1016/j.cmet.2019.11.010
25. Wang Y, Sun XQ, Lin HC, Wang DS, Wang ZQ, Shao Q, et al. Correlation between immune signature and high-density lipoprotein cholesterol level in stage II/III colorectal cancer. Cancer Med. (2019) 8:1209–17. doi: 10.1002/cam4.1987
26. Zamanian-Daryoush M, DiDonato JA, Apolipoprotein A-I. Cancer. Front Pharmacol. (2015) 6:265. doi: 10.3389/fphar.2015.00265
27. La Salvia A, Meyer ML, Hirsch FR, Kerr KM, Landi L, Tsao MS, et al. Rediscovering immunohistochemistry in lung cancer. Crit Rev Oncol Hematol. (2024) 200:104401. doi: 10.1016/j.critrevonc.2024.104401
28. Anderson KS, LaBaer J. The sentinel within: exploiting the immune system for cancer biomarkers. J Proteome Res. (2005) 4:1123–33. doi: 10.1021/pr0500814
29. Chapman CJ, Murray A, McElveen JE, Sahin U, Luxemburger U, Türeci O, et al. Autoantibodies in lung cancer: possibilities for early detection and subsequent cure. Thorax. (2008) 63:228–33. doi: 10.1136/thx.2007.083592
30. Li S, Ma Y, Xiong Y, Zhang P, Wang X, Wang Y, et al. Five tumor-associated autoantibodies expression levels in serum predict lung cancer and associate with poor outcome. Transl Cancer Res. (2019) 8:1364–73. doi: 10.21037/tcr.2019.07.25
31. Teras LR, Gapstur SM, Maliniak ML, Jacobs EJ, Gansler T, Michel A, et al. Prediagnostic antibodies to serum p53 and subsequent colorectal cancer. Cancer Epidemiol Biomarkers Prev. (2018) 27:219–23. doi: 10.1158/1055-9965.EPI-17-0407
32. Jin S, Cao S, Li J, Meng Q, Wang C, Yao L, et al. Cancer/testis antigens (CTAs) expression in resected lung cancer. Onco Targets Ther. (2018) 11:4491–9. doi: 10.2147/OTT.S159491
33. Mattioni M, Soddu S, Prodosmo A, Visca P, Conti S, Alessandrini G, et al. Prognostic role of serum p53 antibodies in lung cancer. BMC Cancer. (2015) 15:148. doi: 10.1186/s12885-015-1174-4
34. Izumchenko E, Chang X, Brait M, Fertig E, Kagohara LT, Bedi A, et al. Targeted sequencing reveals clonal genetic changes in the progression of early lung neoplasms and paired circulating DNA. Nat Commun. (2015) 6:8258. doi: 10.1038/ncomms9258
35. Luo B, Mao G, Ma H, Chen S. The role of seven autoantibodies in lung cancer diagnosis. J Thorac Dis. (2021) 13:3660–8. doi: 10.21037/jtd-21-835
36. Maier S, Wilbertz T, Braun M, Scheble V, Reischl M, Mikut R, et al. SOX2 amplification is a common event in squamous cell carcinomas of different organ sites. Hum Pathol. (2011) 42:1078–88. doi: 10.1016/j.humpath.2010.11.010
37. Madsen CM, Varbo A, Nordestgaard BG. Low HDL cholesterol and high risk of autoimmune disease: two population-based cohort studies including 117341 individuals. Clin Chem. (2019) 65:644–52. doi: 10.1373/clinchem.2018.299636
38. Cruz PM, Mo H, McConathy WJ, Sabnis N, Lacko AG. The role of cholesterol metabolism and cholesterol transport in carcinogenesis: a review of scientific findings, relevant to future cancer therapeutics. Front Pharmacol. (2013) 4:119. doi: 10.3389/fphar.2013.00119
39. Nderitu P, Bosco C, Garmo H, Holmberg L, Malmström H, Hammar N, et al. The association between individual metabolic syndrome components, primary liver cancer and cirrhosis: A study in the Swedish AMORIS cohort. Int J Cancer. (2017) 141:1148–60. doi: 10.1002/ijc.30818
40. Chi PD, Liu W, Chen H, Zhang JP, Lin Y, Zheng X, et al. High-density lipoprotein cholesterol is a favorable prognostic factor and negatively correlated with C-reactive protein level in non-small cell lung carcinoma. PloS One. (2014) 9:e91080. doi: 10.1371/journal.pone.0091080
41. Xu C, Zhou L, Lu L, Chen T, Wei S, Lin X, et al. Inflammation has a role in urethane-induced lung cancer in C57BL/6J mice. Mol Med Rep. (2016) 14:3323–8. doi: 10.3892/mmr.2016.5661
42. Pedersen KM, Çolak Y, Bojesen SE, Nordestgaard BG. Low high-density lipoprotein and increased risk of several cancers: 2 population-based cohort studies including 116,728 individuals. J Hematol Oncol. (2020) 13:129. doi: 10.1186/s13045-020-00963-6
43. Bull CJ, Bonilla C, Holly JM, Perks CM, Cybulski C, Cannon-Albright L, et al. Blood lipids and prostate cancer: a Mendelian randomization analysis. Cancer Med. (2016) 5:1125–36. doi: 10.1002/cam4.695
44. Nowak C, Ärnlöv J. A Mendelian randomization study of the effects of blood lipids on breast cancer risk. Nat Commun. (2018) 9:3957. doi: 10.1038/s41467-018-06467-9
Keywords: HDL-C, 7-AABs, lung cancer, Chinese adults, physical examinations
Citation: Zhou Y, Sun Y, Qiao Q, Qi X, Lin X, Du Y, Liu A, Zhou J, Lv X, Li Z, Wu X, Zou Z, Zhang M, Zhu J, Shang F, Li H and Li Y (2025) Association between high-density lipoprotein cholesterol and 7-autoantibodies: a study on physical examination data from 2018 to 2023. Front. Endocrinol. 16:1504266. doi: 10.3389/fendo.2025.1504266
Received: 01 October 2024; Accepted: 20 January 2025;
Published: 06 March 2025.
Edited by:
Marica Cariello, University of Bari Aldo Moro, ItalyReviewed by:
Anna La Salvia, National Institute of Health (ISS), ItalyCopyright © 2025 Zhou, Sun, Qiao, Qi, Lin, Du, Liu, Zhou, Lv, Li, Wu, Zou, Zhang, Zhu, Shang, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongli Li, c2h5bGl5b25nbGlAMTI2LmNvbQ==
†Present address: Michael Zhang, Sevenoaks Health Management Center, Canada-Canada Institute of Health Engineering, University of Manitoba, Winnipeg, MB, Canada
‡These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.