
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Endocrinol. , 31 January 2025
Sec. Reproduction
Volume 16 - 2025 | https://doi.org/10.3389/fendo.2025.1501110
A correction has been applied to this article in:
Corrigendum: Gestational diabetes mellitus and polycystic ovary syndrome, a position statement from EGOI-PCOS
 Paola Quaresima1
Paola Quaresima1 Samuel H. Myers2
Samuel H. Myers2 Basilio Pintaudi3,4
Basilio Pintaudi3,4 Rosario D’Anna3,5
Rosario D’Anna3,5 Michele Morelli6
Michele Morelli6 Vittorio Unfer3,7*
Vittorio Unfer3,7*Gestational diabetes mellitus is a worldwide health issue in pregnancy, posing a threat to both mother and child. One of the major risk factors for the development of gestational diabetes mellitus is polycystic ovary syndrome, primarily due to the biochemical hyperandrogenism and metabolic issues, commonly observed in these patients. In recent years, the Expert Group on Inositol in Basic and Clinical Research and on PCOS (EGOI-PCOS) has sought to better understand the pathogenesis behind polycystic ovary syndrome, in order to accurately diagnose and treat patients according to their individual needs. Through the scope of polycystic ovary syndrome, this position paper examines the characteristics of both conditions, and underlying biological mechanisms, before moving on to common treatment strategies to avoid or treat gestational diabetes mellitus in women with polycystic ovary syndrome.
Gestational diabetes mellitus (GDM), is currently the most common medical complication of pregnancy (1). The reported GDM prevalence varies substantially worldwide, ranging from 7.1% in North America and the Caribbean to 27.6% in North Africa and the Middle East, with a lack of uniformity in the screening standards and diagnostic criteria, presenting a challenge for prevalence studies (2). GDM poses a health risk to pregnant women as it is associated with adverse pregnancy outcomes such as pre-eclampsia, polyhydramnios, shoulder dystocia, fetal macrosomia, neonatal hypoglycemia, and in extreme cases, perinatal mortality (1). The actual GDM screening strategies varies across the world, from a universal screening approach, to selected population screens targeted at those women considered to be at risk for GDM, such as women with PCOS. Both conditions are associated with metabolic alterations, with insulin resistance being observed in a majority of PCOS patients. This shared clinical feature, predisposes women with PCOS to develop GDM during pregnancy.
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in females of reproductive age, affecting 10-13% of women worldwide (3, 4). As PCOS can present with a varied range of clinical symptoms, it is important to have clear criteria and advice for patients and physicians alike to help guide diagnosis and treatment. Consequently, several diagnostic criteria have been published regarding the identification of PCOS, for the past 20 years, the most applied of these has been the Rotterdam Criteria. These criteria describe PCOS as a condition with at least two out of three of the following: biochemical and/or clinical hyperandrogenism, oligo/anovulation, and polycystic ovarian morphology (5), consequently dividing the patient cohort into four phenotypes:
● Phenotype A: biochemical and/or clinical hyperandrogenism, oligo/anovulation, and polycystic ovarian morphology.
● Phenotype B: biochemical and/or clinical hyperandrogenism, oligo/anovulation.
● Phenotype C: biochemical and/or clinical hyperandrogenism, polycystic ovarian morphology.
● Phenotype D: oligo/anovulation, and polycystic ovarian morphology.
Of note, three of these phenotypes contain hyperandrogenism, with one phenotype (D) being non-hyperandrogenic. This difference has been a point of contention with numerous societies such as the AE-PCOS and the EGOI-PCOS arguing that the non-hyperandrogenic subgroup represents a separate condition (6, 7). Women with PCOS typically suffer from insulin resistance in addition to other metabolic issues; however, it is notable that this is more commonly observed in hyperandrogenic PCOS patients. Consequently, it may be argued that these metabolic alterations in these patients are a key part of the pathogenesis of the condition (8). Despite insulin resistance’s crucial role in PCOS, it was not included as a diagnostic in the most recent iteration of the international clinical guidelines (9). In 2023, the EGOI-PCOS laid out the EGOI-PCOS criteria, which reclassified hyperandrogenic PCOS as endocrine-metabolic syndrome (EMS), including insulin resistance as a fundamental part of the diagnosis. For the patients with phenotype D, the name multi-follicular ovarian syndrome (MFOD) was proposed, with these patients being formally separated from EMS due to them not presenting with hyperandrogenism nor insulin resistance (10).
Considering the significant association between GDM and PCOS, this article describes the link between these conditions and how this differs between patient groups, in order to discuss therapeutic approaches that can be utilized in the treatment of both conditions, with the aim of aiding patient care.
An association between PCOS and GDM has been established in the literature with PCOS thought to be a major causal factor for GDM (11). In detail, GDM develops in approximately 40% of pregnancies in women with PCOS (12). This is evidenced in a recent meta-analysis Qiu et al. demonstrated that PCOS was associated with an increased risk of GDM via a random-effects model (OR 2.02, 95% CI: 1.74–2.34, p < 0.0001) (13). In a further meta-analysis of 33 studies and a sample size of 92,819, PCOS was significantly associated with an increased risk of GDM (OR 1.51, 95% CI:1.17–1.94) (14). Interestingly, the prevalence of GDM changes depending on the presented PCOS phenotype, those characterized by the presence of hyperandrogenism and oligomenorrhea, such as A and B, and C (EMS) were at a significantly increased risk of developing diabetes during pregnancy in comparison to phenotype D (or MFOD according to EGOI-PCOS) (15). Furthermore, in a multicenter cohort study maternal complications were significantly higher in women with hyperandrogenic PCOS (adjusted OR 2.67, 3.47-8.87) versus their normoandrogenic counterparts (1.60, 0.74-3.49) (16).
Accordingly, the most recent guidelines an OGTT should be performed in all women without pre-existing diabetes, when planning pregnancy or seeking fertility treatment due to the high risk of hyperglycemia and associated comorbidities during pregnancy (9).
As previously stated, the EGOI-PCOS has proposed a formal reclassification of PCOS, with a separation of typically insulin resistant-hyperandrogenic PCOS patients, and typically normoandrogenic PCOS patients who do not typically demonstrate insulin dysfunction. The redivision of these patient groups aims to improve patient care, as current therapeutic approaches, namely the use of oral contraceptives and insulin sensitizers, are not applicable to the normoandrogenic cohort. Consequently, normoandrogenic PCOS patients lack suitable therapeutic options, and it is hoped that the EGOI-PCOS reclassification will encourage further research into appropriate treatments for this patient group. As it pertains to GDM, women with phenotype D (or MFOD according to EGOI-PCOS classification) have a reduced risk of GDM and thus pregnancy advice regarding GDM should be tailored accordingly.
Women with PCOS are characterized by multiple well-known risk factors for GDM, with the most common of these risk factors being elevated BMI (33-88% of women with PCOS BMI >25 kg/m2) (17). Irregular menstrual patterns are also an independent risk factor for the development of GDM (18). While the specific rationale for this observation is not well understood, it is hypothesized that irregular menstrual cycles could be an indicator of a hormonal and metabolic imbalance, which is thought to play a role in GDM (19). Other associated risk factors for GDM include an elevated free androgen index (FAI), as SHBG is negatively associated with further risk of GDM (20).
Familial history of type 2 diabetes represents a significant risk factor for the development of GDM, as evidenced by a cohort study including 1129 pregnant women with first- or second-degree relatives with T2DM. Women with first-degree relatives and/or women with second-degree relatives with T2DM demonstrated a significant higher prevalence of GDM (26.6%, 26.3%, and 33.3% respectively) versus negative controls (15.9%) (21).
Insulin resistance, an integral part of GDM, is associated with other common endocrine disorders such as thyroid dysfunction. A retrospective study of 662 pregnant women, 412 of which had GDM, demonstrated that women with GDM had a significantly higher concentration of TSH, in addition to a high FT3:FT4 ratio (22). Consequently, these biomarkers may have potential in the future to identify increased risk of GDM in patients seeking pregnancy. A similar relationship is observed in PCOS patients where that the incidence of hypothyroidism is higher (11–14%) compared with healthy controls (1–2%) (23). Moreover, GDM, hypothyroidism and PCOS share common metabolic symptoms or comorbidities including insulin resistance, dyslipidemia, and obesity, which may explain the correlation between PCOS, thyroid dysfunction, and GDM incidence.
Numerous societies and organizations have attempted to standardize diagnosis and screening methods to identify GDM. The World Health Organization (WHO) defines GDM as “any level of the early of first detection of glucose intolerance in pregnancy (24), and set out diagnostic and screening criteria in 1991, recommending the use of an 2h 75g OGTT as the standard diagnostic test with a fasting and 2h threshold of 126 and 140 mg/dl respectively (25). The OGTT test typically is performed between the 24th and 28th week of pregnancy. Alongside the WHO criteria, the International Association of Diabetes in Pregnancy Study Groups (IADPSG) criteria represent the most globally applied diagnostic criteria (26). Much like the WHO criteria, the IADPSG recommend the use of a 2h 75g OGTT but with the following cutoffs (Fasting glucose: 95 mg/dl, 1h 180 mg/dl, 2h 153 mg/dl). In 2013 the WHO updated the 1999 guidelines bringing them into line with the IADPSG recommendations (27). It should be noted that the IADPSG criteria are not routinely used in the United States and Canada, as the American Diabetes Association recommends the use of a 100g 3h OGTT (28). Additionally, the UK does not follow the IADPSG guidelines, instead following National Institute for Health and Care Excellence (NICE) guidelines, which recommend the use of a 2h OGTT (Fasting glucose 101 mg/dl, 2h 140mg/dl). The NICE guidelines differ from the recommendations from other societies through the advocation for selective risk-factor-based testing (29). Risk factors considered by the NICE guidelines include BMI >30, prior macrosomic baby weight of >4.5kg, previous incidence of GDM, family history of diabetes, an ethnicity based factors (30). The diagnostic and screening criteria are summarized in Table 1.
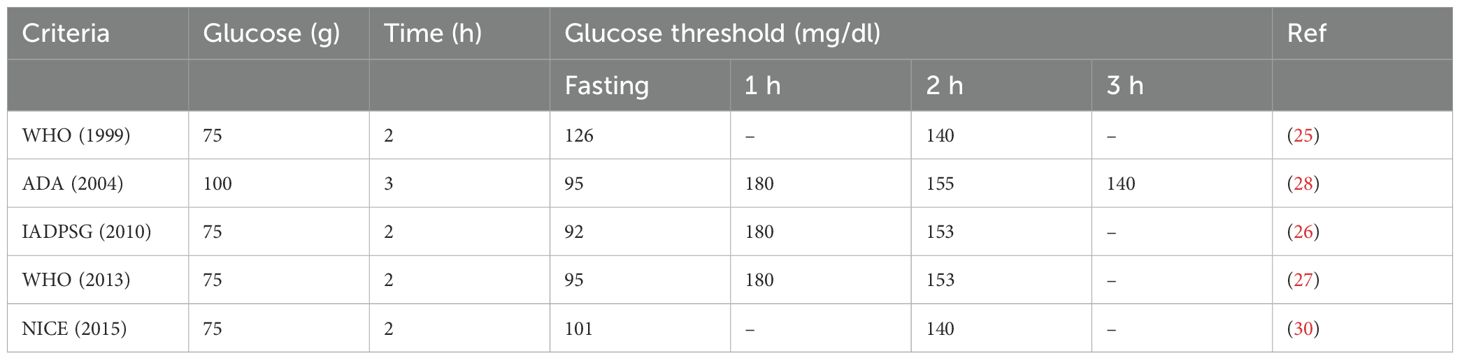
Table 1. Summary of diagnostic and screening criteria for GDM- adapted from Choudhury et al. (26).
As PCOS increases the risk of development of GDM, screening protocols must reflect the specific needs of these women. The 2023 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome advised an OGTT in women with PCOS and without pre-existing diabetes prior to planning pregnancy or seeking fertility treatment (31). If this OGTT is not performed, an OGTT should be offered at the first prenatal visit, in addition to another test between the 24th and 28th week of gestation. This advice is in line with the current recommendation of EGOI-PCOS. To the best of our knowledge, the benefits of early screening in PCOS populations have not been studied; however, some conclusions may be drawn from studies investigating the effect of early screening in GDM patients. In detail, hyperglycemia at the first prenatal visit is a known predictor of GDM as observed in a study by Zhu et al. which underlined the importance of measuring fasting plasma glucose at this visit (32). Furthermore, a retrospective cohort study conducted by Liu et al, demonstrated that while the measurement of fasting plasma glucose at the first prenatal visit was not associated with lipid concentrations in mid-pregnancy, it was associated with numerous fetal outcomes such as birthweight, head circumference, and shoulder circumference (33). Considering the prevalence of hyperglycemia in PCOS, the above findings support the implementation of early screening protocols in PCOS patients.
PCOS is commonly associated with insulin resistance (IR), a term used to describe a decrease in the cellular response to insulin signaling which subsequently induces an increase in insulin secretion (34). During insulin resistance in PCOS, higher insulin levels lead to the reduced liver synthesis and reduced secretion of SHBG. A reduction of SHBG levels results in higher bioavailable testosterone, which is associated with the impairment islets of Langerhans function, thereby impairing pancreatic function which leads to further IR (35). Consequently, increased systemic insulin levels result in an increase in insulin-dependent androgen overproduction within ovarian theca cells, thereby creating a vicious circle where IR leads to hyperandrogenism and vice versa (36).
In order to facilitate proper growth of the fetus, correct nutrient flow across the placenta must be regulated during pregnancy. Transport of glucose across the placenta is performed by facilitated transport facilitated by glucose transporters (GLUTs) and is dependent upon fetal and maternal systemic concentrations (37). In the later stages of pregnancy, the fetal need for glucose increases, thus glucose transport is increased, which has the potential to lower maternal glucose levels. Consequently, maternal physiological endocrine changes occur, increasing maternal insulin resistance and hepatic glucose production (38). However, this process may result in the development of GDM in cases where pancreatic β-cell function is insufficient to overcome not only the physiological IR associated with pregnancy, but IR caused by metabolic risk factors such as those commonly presented in PCOS (39).
From a molecular viewpoint, GDM acts primarily on three different tissues: placenta, adipose, and skeletal muscle (40). Within the placenta, GDM induces an increase in mTOR, NFKB, and TLKR3 signaling, in addition to endoplasmic reticulum (ER) stress; meanwhile, PI3K, SIRT, AMPK and PPAR signaling is reduced. Within the adipose tissue GSK3, NOD, PI3K, and ER stress are increased, while AMPK and PPAR signaling are decreased. Lastly within skeletal muscle AMPK, PI3K, and SIRT signaling are decreased, while ER stress and GSK3 signaling are increased. The net result of these molecular alterations are placental and endothelial disruption, increased inflammation and an increased level of insulin resistance within the mother, which can go on to cause long and short-term effects within the offspring. Endothelial disruption, inflammation, and insulin resistance are hallmarks of PCOS, which can worsen GDM, providing an explanation for why PCOS patients are at risk of developing GDM.
The characteristic PCOS features, obesity, IR, and hyperandrogenism have significant implications for both short and long-term pregnancy outcomes. Evidence suggests that women with PCOS may be at greater risk for various obstetric complications, aside from GDM, such as hypertensive disorders of pregnancy, premature birth, induction of labor and cesarean delivery (41, 42). In detail, a meta-metanalysis of 63 studies observed that women with PCOS were more likely to have gestational hypertension (29 studies, OR: 2.58, 95% CI: 1.95‐3.41), preeclampsia (26 studies, OR: 1.87, 95% CI: 1.55‐2.25), induction of labor (5 studies, OR: 1.87, 95% CI: 1.55‐2.25), and caesarean section (25 studies, OR: 1.37, 95% CI: 1.21‐1.56) (11). Moreover, the presence of PCOS together with GDM leads to a 2.4-fold increase in risk of hypertension and a 2-fold increase in risk of preeclampsia compared to GDM alone (43). Furthermore, in a retrospective population study over a period of 11 years conducted by Mills et al, an increased risk of GDM was observed women with PCOS vs the control group (aOR 2.19, 95% CI 2.02–2.37), and women within the PCOS group were also more likely to develop chronic hypertension (aOR 1.38, 95% CI 1.27–1.50, P < 0.001), gestational hypertension (aOR 1.47, 95% CI 1.31–1.64), and preeclampsia (aOR 1.29, 95% CI 1.14–1.45) (44).
GDM and PCOS pose significant risks to the fetus in addition to the mother. This was apparent in a case-controlled study that demonstrated that neonatal hypoglycemia risk is increased in patients with both GDM and PCOS compared to those with GDM alone, with an incidence of 17% and 5% respectively between the two groups (43). Moreover, among women with PCOS, the odds of developing neonatal jaundice and respiratory complications were significantly higher compared to the non-PCOS control group (45). Of note, while the majority of evidence from the literature indicates that neonates of mothers with GDM and PCOS were more likely to be of a lower birthweight compared to neonates from mothers with GDM alone, others described opposite results, with higher birthweight and macrosomia (46).
In addition, women with GDM have an increased risk of developing metabolic syndrome, notably, this effect is also observed in offspring of women with GDM compared to offspring not exposed to GDM in utero (47). This risk is further compounded by the increased prevalence metabolic syndrome observed in PCOS patients (48).
Considering these increased risks to both mother and child, medical care must be tailored to suit the individual needs of these women. As lifestyle changes represent the first recommendation in both conditions, appropriate diet and exercise counseling should be made available to the patient, with diet and exercise plans in addition to frequent follow-ups sessions to ensure compliance. Moreover, as pregnancies in women with GDM and/or PCOS are associated with higher degree of pregnancy complications, psychological support should be made available. A systematic review of 44 studies, observed that women with GDM had increased rates of anxiety or depression during pregnancy. Furthermore, the presence of anxiety or depression during pregnancy increased the incidence of GDM suggesting a cyclic effect (49). As PCOS is also associated with mental health issues (50), appropriate psychological intervention in these women may lower GDM incidence or reduce the impact of GDM in these women. Lastly women with PCOS should be informed of the risk of developing GDM when planning contraception or undergoing fertility care.
The primary goal of GDM treatment is the prevention of excessive fetal growth and GDM related pregnancy complications. According to the “Pedersen hypothesis” increased maternal glycaemia directly associates with both excessive fetal growth and other unwanted complications associated with GDM (51). Consequently, the primary goal of GDM management and treatment is to achieve glycemic control, therefore lifestyle modifications are routinely initiated following diagnosis (52). It is recommended that pregnant women with or at risk of GDM consume a hypoglycemic diet including three main meals and 2–4 snacks, comprising of ≥175 g of carbohydrates daily, in order to insure appropriate fetal growth and cerebral development (53). Exercise also represents a core component of lifestyle modification, aerobic and resistance exercise at a moderate intensity, a minimum of three times a week for 30-60 min is recommended for women with/or at risk of GDM (54). In total, lifestyle changes have been demonstrated to enable up to 80% women to reach their glycemic targets (52).
While lifestyle changes are sufficient for the majority of patients, insulin therapy represents a valid approach when glycemic control cannot be established. Despite this, no clear indications exist to guide when a patient should begin insulin therapy. Typically, insulin therapy is typically initiated when glucose levels are between 5.2 and 5.6 mmol/L during fasting and 6.6-7.9mmol/L during at the second or third trimester of pregnancy (17). The basal insulins approved for use in pregnancy are the neutral protamine Hagedorn (NPH), an intermediate-acting insulin, and the long-acting insulin analog detemir (55). The short-acting insulin analogs aspart, lispro, and the long-acting detemir are approved by the U.S. Food and Drug Administration (FDA) as category B drugs and are commonly used in pregnancy (56), and have been demonstrated to mirror the endogenous pattern of insulin secretion. The long-acting insulin analogs glargine and degludec have not received official authorization for use in pregnancy; however, several observational studies exploring the use of insulin glargine in pregnancy report a similar safety profile compared to NPH insulin (57). In addition, in patients who are accustomed to the use of glucose monitoring devices continuous rapid acting insulin analogues may be employed, typically in women who have progressed to GDM or suffer from type-1 diabetes mellitus (58). Clinical benefits from insulin therapy include reduced incidence of macrosomia, lower cranial thoracic circumference ratio and a reduced incidence of caesarean sections. Benefits of insulin therapy depend on the type of insulin used with long-acting insulin resulting in a higher incidence of macrosomia vs short acting insulin treatments (59). It is important to consider the associated risks when prescribing insulin therapy, which include of hypertensive disorder in pregnancy, gestational weight gain, labor induction, impaired placental insulin signaling, and reduced birthweight (58).
Oral glucose lowering medications, such as metformin, have been studied in women with GDM due to their demonstrated worth in related diseases such as type 2 diabetes (60). Metformin primarily functions by suppressing hepatic glucose production, leading to a reduction in fasting plasma glucose levels (61). Metformin is routinely prescribed in women with PCOS who suffer from metabolic abnormalities. In a recent meta-analysis of 32 RCTs, use of metformin in patients with PCOS resulted in significant reductions to BMI, HOMA, and fasting glucose levels, with a moderate certainty of evidence (62). However, these metabolic changes were coupled with a significant increase in mild gastrointestinal adverse effects compared to the placebo. Regarding hormonal changes, in the same meta-analysis total testosterone was reduced in the metformin group versus placebo, albeit with a very low certainty.
Concerning metformin use during pregnancy, according to a recent meta-analysis, metformin administration showed mixed results in pregnant women with PCOS as it was associated with a reduced preterm delivery risk; however, metformin use was also associated with a larger neonatal head circumference (63). Moreover, a large population-based cohort study revealed that metformin supplementation in women with PCOS reduced the risks preeclampsia, gestational diabetes, cesarean section, and preterm birth in comparison to the control PCOS group; however, metformin supplementation in women without PCOS may increase the risk of obesity in offspring (64). Furthermore, metformin is associated with gastrointestinal adverse effects which may not be tolerable for some patients (65). It should be noted that metformin crosses the placenta, and maternal and fetal concentrations have been demonstrated to be comparable; however, no evidence of teratogenicity nor short term adverse neonatal outcomes has been reported, while long term affects are still unclear (66).
The use of metformin and insulin was compared by Wu et al. in a meta-analysis which compared 24 randomized controlled trials (RCTs) involving 4934 patients with GDM. Compared to insulin, metformin demonstrated a significantly reduced risk of preeclampsia, induction of labor, cesarean delivery, macrosomia, neonatal intensive care unit admission, neonatal hypoglycemia, and large for gestational age (67). Despite the promising results of metformin vs insulin in GDM, further study is required to measure the effect of GDM in PCOS patients in comparison to insulin therapy (68). A recent study on two open label RCTs investigated the benefits of metformin vs insulin in terms of post birth outcomes. The offspring from the original cohorts of the prior studies were followed up after 9 years, following this period no difference was observed in terms of offspring growth and glucose metabolism; however, the lipid profile in the metformin group was significantly improved over the insulin cohort (69). A recent metanalysis investigated the effect of metformin and insulin treatment during pregnancy on the cardiometabolic outcomes in offspring. This meta-analysis conducted by Rawat et al. consisted of five RCT studies (metformin 409 children, insulin 434 children). The metformin group demonstrated significantly higher fat free mass, and lower triglyceride and plasma glucose levels between the ages of 5 and 9 than the insulin group (70). Aside from these observations, no other cardiometabolic differences could be identified between the two groups. In cases where glycemic control cannot be achieved, patients with GDM taking metformin are recommended insulin therapy, occurring in approximately a third of patients (71).
In terms of its tolerability, metformin is generally regarded to have an acceptable safety profile. The most common adverse effect of metformin treatment is gastrointestinal effects, which may render metformin intolerable for some patient groups (65). Metformin is contraindicated in patients with severe renal impairment, due to its association with lactic acidosis which may occur in a minority of patients. Due to this concern metformin has received a black-box warning from the FDA regarding lactic acidosis (72). Consequently, these safety concerns should be considered when prescribing metformin in pregnant individuals.
Inositol is a naturally occurring polyol which is present in numerous food sources including cereals, legumes, seeds, and nuts (73). In nature the two most common stereoisomers of inositol are myo-inositol (MI) and D-chiro-inositol, these molecules function as second messengers of various endocrine signals including follicle stimulating hormone (FSH), thyroid stimulating hormone (TSH) and insulin (74). Under insulin stimulation, MI is converted to DCI via a unidirectional tissue-specific epimerase, maintaining a balance between DCI and MI, ensuring normal metabolic function (74). Inositol is involved in various steps of the insulin signaling pathway and can act as an insulin sensitizer (75). In detail, MI ameliorates IR by encouraging the translocation of GLUT4 to the plasma membrane to increase glucose uptake. Similar to the action of metformin, this function of MI against IR reduces insulin-dependent androgen signaling and can counteract hyperandrogenism in women with PCOS (76). Moreover, MI demonstrates non-inferiority against metformin in treating PCOS across most measurable outcomes. In a recent meta-analysis of 26 RCTs by Greff et al., endocrine-metabolic outcomes such as changes to BMI, free testosterone, total testosterone, androstenedione, and glucose levels in addition to AUC were equivalent between MI and metformin (77). Moreover, in the same analysis MI supplementation was associated with a normal menstrual cycle and increased sex hormone binding globulin level (SHBG) levels versus placebo.
Numerous studies have evaluated the use of MI in PCOS patients at risk with or who have developed GDM. A retrospective study in pregnant women with PCOS demonstrated that MI supplementation halved the risk of GDM in comparison to the control group (78). Moreover, in the same study MI was well tolerated throughout pregnancy with no adverse effects reported for the fetus or the mother. A recent meta-analysis evaluated patients undergoing MI supplementation in comparison to routine treatment across four RCTs. In total, MI supplementation lead to a significantly reduced requirement for insulin treatment and HOMA; however, no difference was observed in birth weight, incidence of cesarean section or the requirement for administration to the neonatal intensive care unit (79). An earlier meta-analysis compared the results of 7 RCTs investigating the use of inositol (either MI or a combination of MI/DCI) in the prevention of GDM (73). In the analyzed trials, use of 4g of MI/day reduced the incidence of GDM, plasma glucose levels as measured by OGTT and 2h OGTT. Furthermore, MI supplementation reduced several secondary outcomes such as the need for insulin treatment and reduced the incidence of preterm delivery and neonatal hypoglycemia. It should be noted that the combination of MI (1.1g) and DCI (27.6 mg) was equivalent to the control group across all measured parameters, suggesting the use of MI alone is more effective and may represent a potential therapeutic in the GDM disease space. Further studies are required to fully evaluate the use of MI in GDM. In a meta-analysis of six RCTs including 887 women, MI supplementation was observed to potentially reduce the risk of GDM (RR 0.54; CI [0.30, 0.96]); however, the certainty of evidence was classified as low to very low (80). Furthermore, the same meta-analysis identified no adverse outcomes due to MI supplementation, and a reduction of preterm delivery and pregnancy induced hypertension; however, once more the strength of evidence was classified as low to very low. Furthermore, to the best of our knowledge no meta-analyses have addressed the use of myo-inositol in patients with both PCOS and GDM demonstrating an urgent need for further study.
In terms of its safety profile, MI is well-tolerated and is included in the list of generally recognized as safe (GRAS) food additives. Gastrointestinal effects are only observed in very high doses of MI (over 12g/day), which are excessive of what is typically recommended (81). Considering these factors MI does not have any contraindications.
As noted above, treatment options for GDM primarily consist of lifestyle adjustments, in addition to insulin therapy when required. Furthermore, insulin sensitizers such as metformin and inositol, have potential in reducing the need or dose of insulin therapy; however, the long-term implications of metformin are yet to be discovered. Once pregnancy has been achieved this treatment plan is also recommended for patients with both GDM and PCOS. Prior to conception PCOS patients may be recommended therapies aimed at treating hyperandrogenism and restoring menstrual cyclicity. Clomiphene and more recently letrozole are routinely employed to induce ovulation; however, once pregnancy is achieved this therapy is stopped (82). Patients with PCOS frequently develop signs of clinical hyperandrogenism such as hirsutism, acne, and alopecia. Cosmetic treatments such as laser hair removal can be continued during pregnancy, as may topical anti-androgenic creams with the exception of hydroquinone and tretinoin which may have increased systematic absorption rates (83).
Women with PCOS are a higher risk of developing gestational diabetes during pregnancy. It is, however, important to contextualize this potential risk, as hyperandrogenic PCOS phenotypes are known to be a higher risk group than normoandrogenic PCOS patients. The characteristic hormonal and metabolic profile of hyperandrogenic PCOS patients includes insulin resistance, and an elevated FAI, both of which are risk factors for the development of GDM. Women with PCOS are at further risk of other obstetric complications including hypertensive disorders, premature birth, induction of labor and cesarean delivery.
Due to the elevated risk of developing GDM, an OGTT is advised in all women with PCOS and without pre-existing diabetes, when planning pregnancy or seeking fertility treatment. If not performed prior to conception, an OGTT may be performed at the first antenatal visit or at least prior to 24-28 weeks gestation. In the incidence of a GDM diagnosis, healthy diet and physical activity should be promptly recommended, and if glycemic targets are not reached insulin treatment should be considered as a first line therapy. In addition, pharmacological insulin sensitizers such as metformin may have merit; however, potential concerns remain regarding long term adverse effects in the offspring. As an alternative to metformin, MI has shown potential for GDM prevention in PCOS populations.
The connection between PCOS and pregnancy complications, such as GDM, have been well described within the literature. Described below are some of the key research questions that merit further research according to the EGOI-PCOS:
● As a society, the EGOI-PCOS are seeking to reclassify PCOS into EMS and MFOD; however, it is yet to be known whether this reclassification would affect the association with GDM between both groups.
● Is there a difference in how the subgroups of PCOS (i.e. hyperandrogenic vs non-hyperandrogenic) respond to therapies designed to treat GDM.
● Further larger powered studies are required to evaluate the efficacy of insulin sensitizers in GDM treatments. Furthermore, the long-term effects of metformin in offspring should be studied.
● The benefits of early screening for GDM in PCOS patients in terms of pregnancy outcomes should be investigated further.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
PQ: Conceptualization, Writing – original draft. SM: Writing – original draft, Writing – review & editing. BP: Writing – review & editing. RD: Writing – review & editing. MM: Supervision, Writing – review & editing. VU: Conceptualization, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
SM and VU are employees of Lo.Li Pharma s.r.l,
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Nakshine VS, Jogdand SD. A comprehensive review of gestational diabetes mellitus: impacts on maternal health, fetal development, childhood outcomes, and long-term treatment strategies. Cureus. (2023) 15:e47500. doi: 10.7759/cureus.47500
2. Wang H, Li N, Chivese T, Werfalli M, Sun H, Yuen L, et al. Idf diabetes atlas: estimation of global and regional gestational diabetes mellitus prevalence for 2021 by international association of diabetes in pregnancy study group&X2019;S criteria. Diabetes Res Clin Pract. (2022) 183:109050. doi: 10.1016/j.diabres.2021.109050
3. Yu O, Christ JP, Schulze-Rath R, Covey J, Kelley A, Grafton J, et al. Incidence, prevalence, and trends in polycystic ovary syndrome diagnosis: A United States population-based study from 2006 to 2019. Am J Obstet Gynecol. (2023) 229:39.e1–.e12. doi: 10.1016/j.ajog.2023.04.010
4. Unfer V, Kandaraki E, Pkhaladze L, Roseff S, Vazquez-Levin MH, Laganà AS, et al. When one size does not fit all: reconsidering pcos etiology, diagnosis, clinical subgroups, and subgroup-specific treatments. Endocr Metab Sci. (2024) 14:100159. doi: 10.1016/j.endmts.2024.100159
5. The Rotterdam ESHRE/ASRM‐sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (Pcos). Hum Reprod. (2004) 19:41–7. doi: 10.1093/humrep/deh098
6. Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. The androgen excess and pcos society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. (2009) 91:456–88. doi: 10.1016/j.fertnstert.2008.06.035
7. Myers SH, Russo M, Dinicola S, Forte G, Unfer V. Questioning pcos phenotypes for reclassification and tailored therapy. Trends Endocrinol Metab. (2023) 34:694–703. doi: 10.1016/j.tem.2023.08.005
8. de Zegher F, Ibáñez L. Leader vs follower in the tango of polycystic ovary syndrome: insulin resistance vs androgen excess. Acta Obstet Gynecol Scandinavica. (2024) 103:1680–1. doi: 10.1111/aogs.14802
9. Teede HJ, Tay CT, Laven J, Dokras A, Moran LJ, Piltonen TT, et al. Recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. (2023) 120:767–93. doi: 10.1016/j.fertnstert.2023.07.025
10. Myers SH, Forte G, Unfer V. Has the name pcos run its course? Arch Gynecol Obstet. (2024) 310:1761–2. doi: 10.1007/s00404-024-07571-6
11. Bahri Khomami M, Joham AE, Boyle JA, Piltonen T, Silagy M, Arora C, et al. Increased maternal pregnancy complications in polycystic ovary syndrome appear to be independent of obesity-a systematic review, meta-analysis, and meta-regression. Obes Rev. (2019) 20:659–74. doi: 10.1111/obr.12829
12. Choudhury AA, Rajeswari VD. Polycystic ovary syndrome (Pcos) increases the risk of subsequent gestational diabetes mellitus (Gdm): A novel therapeutic perspective. Life Sci. (2022) 310:121069. doi: 10.1016/j.lfs.2022.121069
13. Qiu Y, Zhang X, Ni Y. Association between polycystic ovarian syndrome and risk of gestational diabetes mellitus: A meta-analysis. Gynecol Obstet Invest. (2022) 87:150–8. doi: 10.1159/000521728
14. Ban M, Sun Y, Chen X, Zhou X, Zhang Y, Cui L. Association between maternal polycystic ovarian syndrome undergoing assisted reproductive technology and pregnancy complications and neonatal outcomes: A systematic review and meta-analysis. J Ovarian Res. (2024) 17:6. doi: 10.1186/s13048-023-01331-x
15. Dehghani Firoozabadi A, Dehghani Firouzabadi R, Eftekhar M, Sadat Tabatabaei Bafghi A, Shamsi F. Maternal and neonatal outcomes among pregnant women with different polycystic ovary syndrome phenotypes: A cross-sectional study. Int J Reprod BioMed. (2020) 18:339–46. doi: 10.18502/ijrm.v13i5.7154
16. de Wilde MA, Lamain-de-Ruiter M, Veltman-Verhulst SM, Kwee A, Laven JS, Lambalk CB, et al. Increased rates of complications in singleton pregnancies of women previously diagnosed with polycystic ovary syndrome predominantly in the hyperandrogenic phenotype. Fertil Steril. (2017) 108:333–40. doi: 10.1016/j.fertnstert.2017.06.015
17. Barber TM. Why are women with polycystic ovary syndrome obese? Br Med Bull. (2022) 143:4–15. doi: 10.1093/bmb/ldac007
18. Yu T, Wu D, Cao Y, Zhai J. Association between menstrual patterns and adverse pregnancy outcomes in patients with polycystic ovary syndrome. Front Endocrinol. (2021) 12:740377. doi: 10.3389/fendo.2021.740377
19. Wang Y-X, Wang S, Mitsunami M, Manson JE, Rich-Edwards JW, Wang L, et al. Pre-pregnancy menstrual cycle regularity and length and the risk of gestational diabetes mellitus: prospective cohort study. Diabetologia. (2021) 64:2415–24. doi: 10.1007/s00125-021-05531-2
20. West S, Ollila M-M, Franks S, Piltonen T, Jokelainen J, Nevalainen J, et al. Overweight, obesity and hyperandrogenemia are associated with gestational diabetes mellitus: A follow-up cohort study. Acta Obstet Gynecol Scandinavica. (2020) 99:1311–9. doi: 10.1111/aogs.13883
21. Monod C, Kotzaeridi G, Linder T, Eppel D, Rosicky I, Filippi V, et al. Prevalence of gestational diabetes mellitus in women with a family history of type 2 diabetes in first- and second-degree relatives. Acta Diabetol. (2023) 60:345–51. doi: 10.1007/s00592-022-02011-w
22. Yanachkova V, Kamenov Z. The relationship between thyroid dysfunction during pregnancy and gestational diabetes mellitus. Endokrynol Polska. (2021) 72:226–31. doi: 10.5603/EP.a2021.0016
23. Fan H, Ren Q, Sheng Z, Deng G, Li L. The role of the thyroid in polycystic ovary syndrome. Front Endocrinol. (2023) 14:1242050. doi: 10.3389/fendo.2023.1242050
24. Macaulay S, Dunger DB, Norris SA. Gestational diabetes mellitus in Africa: A systematic review. PloS One. (2014) 9:e97871. doi: 10.1371/journal.pone.0097871
25. Organization WH. World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications, 1999. Geneva: World Health Organization (1999). Available at: https://www.paho.org/en/documents/who-definition-diagnosis-and-classification-diabetes-mellitus-and-its-complications-1999 (Accessed September 01, 2024).
26. Choudhury AA, Devi Rajeswari V. Gestational diabetes mellitus - a metabolic and reproductive disorder. Biomed Pharmacother. (2021) 143:112183. doi: 10.1016/j.biopha.2021.112183
27. Shareef M, Saleh L, van den Meiracker AH, Visser W. The impact of implementing the who-2013 criteria for gestational diabetes mellitus on its prevalence and pregnancy outcomes: A comparison of the who-1999 and who-2013 diagnostic thresholds. Eur J Obstet Gynecol Reprod Biol. (2020) 246:14–8. doi: 10.1016/j.ejogrb.2019.12.013
28. Association AD. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2020. Diabetes Care. (2019) 43:S14–31. doi: 10.2337/dc20-S002
29. McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers. (2019) 5:47. doi: 10.1038/s41572-019-0098-8
30. National Institute for Health and Care Excellence. Diabetes in Pregnancy: Management from Preconception to the Postnatal Period (2015). Available online at: https://www.nice.org.uk/guidance/ng3/resources (Accessed September 01, 2024).
31. Teede HJ, Tay CT, Laven JJE, Dokras A, Moran LJ, Piltonen TT, et al. Recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome. J Clin Endocrinol Metab. (2023) 108:2447–69. doi: 10.1210/clinem/dgad463
32. Zhu WW, Yang HX, Wei YM, Yan J, Wang ZL, Li XL, et al. Evaluation of the value of fasting plasma glucose in the first prenatal visit to diagnose gestational diabetes mellitus in China. Diabetes Care. (2013) 36:586–90. doi: 10.2337/dc12-1157
33. Liu B, Geng H, Yang J, Zhang Y, Deng L, Chen W, et al. Early pregnancy fasting plasma glucose and lipid concentrations in pregnancy and association to offspring size: A retrospective cohort study. BMC Pregnancy Childbirth. (2016) 16:56. doi: 10.1186/s12884-016-0846-7
34. Li M, Chi X, Wang Y, Setrerrahmane S, Xie W, Xu H. Trends in insulin resistance: insights into mechanisms and therapeutic strategy. Signal Transduct Target Ther. (2022) 7:216. doi: 10.1038/s41392-022-01073-0
35. Xu W, Morford J, Mauvais-Jarvis F. Emerging role of testosterone in pancreatic B Cell function and insulin secretion. J Endocrinol. (2019) 240:R97–R105. doi: 10.1530/joe-18-0573
36. Shorakae S, Ranasinha S, Abell S, Lambert G, Lambert E, de Courten B, et al. Inter-related effects of insulin resistance, hyperandrogenism, sympathetic dysfunction and chronic inflammation in pcos. Clin Endocrinol. (2018) 89:628–33. doi: 10.1111/cen.13808
37. Novakovic B, Gordon L, Robinson WP, Desoye G, Saffery R. Glucose as a fetal nutrient: dynamic regulation of several glucose transporter genes by DNA methylation in the human placenta across gestation. J Nutr Biochem. (2013) 24:282–8. doi: 10.1016/j.jnutbio.2012.06.006
38. Parrettini S, Caroli A, Torlone E. Nutrition and metabolic adaptations in physiological and complicated pregnancy: focus on obesity and gestational diabetes. Front Endocrinol. (2020) 11:611929. doi: 10.3389/fendo.2020.611929
39. Plows JF, Stanley JL, Baker PN, Reynolds CM, Vickers MH. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. (2018) 19:3342. doi: 10.3390/ijms19113342
40. Nguyen-Ngo C, Jayabalan N, Salomon C, Lappas M. Molecular pathways disrupted by gestational diabetes mellitus. J Mol Endocrinol. (2019) 63:R51–72. doi: 10.1530/jme-18-0274
41. Schneider D, Gonzalez JR, Yamamoto M, Yang J, Lo JC. The association of polycystic ovary syndrome and gestational hypertensive disorders in a diverse community-based cohort. J Pregnancy. (2019) 2019:9847057. doi: 10.1155/2019/9847057
42. Valgeirsdottir H, Sundström Poromaa I, Kunovac Kallak T, Vanky E, Akhter T, Roos N, et al. Polycystic ovary syndrome and extremely preterm birth: A nationwide register-based study. PloS One. (2021) 16:e0246743. doi: 10.1371/journal.pone.0246743
43. Aktun HL, Yorgunlar B, Acet M, Aygun BK, Karaca N. The effects of polycystic ovary syndrome on gestational diabetes mellitus. Gynecol Endocrinol. (2016) 32:139–42. doi: 10.3109/09513590.2015.1101438
44. Mills G, Badeghiesh A, Suarthana E, Baghlaf H, Dahan MH. Polycystic ovary syndrome as an independent risk factor for gestational diabetes and hypertensive disorders of pregnancy: A population-based study on 9.1 million pregnancies. Hum Reprod. (2020) 35:1666–74. doi: 10.1093/humrep/deaa099
45. Rees DA, Jenkins-Jones S, Morgan CL. Contemporary reproductive outcomes for patients with polycystic ovary syndrome: A retrospective observational study. J Clin Endocrinol Metab. (2016) 101:1664–72. doi: 10.1210/jc.2015-2682
46. Slouha E, Alvarez VC, Gates KM, Ankrah NMN, Clunes LA, Kollias TF. Gestational diabetes mellitus in the setting of polycystic ovarian syndrome: A systematic review. Cureus. (2023) 15:e50725. doi: 10.7759/cureus.50725
47. Pathirana MM, Lassi ZS, Ali A, Arstall MA, Roberts CT, Andraweera PH. Association between metabolic syndrome and gestational diabetes mellitus in women and their children: A systematic review and meta-analysis. Endocrine. (2020) 71:310–20. doi: 10.1007/s12020-020-02492-1
48. Chen W, Pang Y. Metabolic syndrome and pcos: pathogenesis and the role of metabolites. Metabolites. (2021) 11:869. doi: 10.3390/metabo11120869
49. OuYang H, Chen B, Abdulrahman AM, Li L, Wu N. Associations between gestational diabetes and anxiety or depression: A systematic review. J Diabetes Res. (2021) 2021:9959779. doi: 10.1155/2021/9959779
50. Wang G, Liu X, Zhu S, Lei J. Experience of mental health in women with polycystic ovary syndrome: A descriptive phenomenological study. J Psychosom Obstet Gynecol. (2023) 44:2218987. doi: 10.1080/0167482X.2023.2218987
51. Alejandro EU, Mamerto TP, Chung G, Villavieja A, Gaus NL, Morgan E, et al. Gestational diabetes mellitus: A harbinger of the vicious cycle of diabetes. Int J Mol Sci. (2020) 21:5003. doi: 10.3390/ijms21145003
52. Le DC, Vu TB, Tran TN, Nguyen TL, Nguyen TB, Nguyen DC, et al. The effectiveness of lifestyle changes in glycemic control among pregnant women with gestational diabetes mellitus. Medicina (Kaunas). (2023) 59:1587. doi: 10.3390/medicina59091587
53. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 15. Management of diabetes in pregnancy: standards of care in diabetes—2023. Diabetes Care. (2022) 46:S254–S66. doi: 10.2337/dc23-S015
54. Yang X, Han R, Xiang Z, Li H, Zhao Q, Chen L, et al. Clinical practice guidelines on physical activity and exercise for pregnant women with gestational diabetes mellitus: A systematic review. Int J Nurs Pract. (2023) 29: e13141. doi: 10.1111/ijn.13141
55. Lambert K, Holt RI. The use of insulin analogues in pregnancy. Diabetes Obes Metab. (2013) 15:888–900. doi: 10.1111/dom.12098
56. Toledano Y, Hadar E, Hod M. Safety of insulin analogues as compared with human insulin in pregnancy. Expert Opin Drug Saf. (2016) 15:963–73. doi: 10.1080/14740338.2016.1182153
57. Jethwani P, Saboo B, Jethwani L, Chawla R, Maheshwari A, Agarwal S, et al. Use of insulin glargine during pregnancy: A review. Diabetes Metab Syndr: Clin Res Rev. (2021) 15:379–84. doi: 10.1016/j.dsx.2021.01.012
58. Subiabre M, Silva L, Toledo F, Paublo M, López MA, Boric MP, et al. Insulin therapy and its consequences for the mother, foetus, and newborn in gestational diabetes mellitus. Biochim Biophys Acta (BBA) - Mol Basis Dis. (2018) 1864:2949–56. doi: 10.1016/j.bbadis.2018.06.005
59. Pöyhönen-Alho M, Teramo K, Kaaja R. Treatment of gestational diabetes with short- or long-acting insulin and neonatal outcome: A pilot study. Acta Obstet Gynecol Scandinavica. (2002) 81:258–9. doi: 10.1034/j.1600-0412.2002.810312.x
60. Priya G, Kalra S. Metformin in the management of diabetes during pregnancy and lactation. Drugs Context. (2018) 7:212523. doi: 10.7573/dic.212523
61. Foretz M, Guigas B, Viollet B. Metformin: update on mechanisms of action and repurposing potential. Nat Rev Endocrinol. (2023) 19:460–76. doi: 10.1038/s41574-023-00833-4
62. Melin J, Forslund M, Alesi S, Piltonen T, Romualdi D, Spritzer PM, et al. The impact of metformin with or without lifestyle modification versus placebo on polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled trials. Eur J Endocrinol. (2023) 189:S38–64. doi: 10.1093/ejendo/lvad098
63. Cao Q, Hu Y, Fu J, Huang X, Wu L, Zhang J, et al. Gestational metformin administration in women with polycystic ovary syndrome: A systematic review and meta-analysis of randomized control studies. J Obstet Gynaecol Res. (2021) 47:4148–57. doi: 10.1111/jog.v47.12
64. Fornes R, Simin J, Nguyen MH, Cruz G, Crisosto N, van der Schaaf M, et al. Pregnancy, perinatal and childhood outcomes in women with and without polycystic ovary syndrome and metformin during pregnancy: A nationwide population-based study. Reprod Biol Endocrinol. (2022) 20:30. doi: 10.1186/s12958-022-00905-6
65. Nabrdalik K, Skonieczna-Żydecka K, Irlik K, Hendel M, Kwiendacz H, Łoniewski I, et al. Gastrointestinal adverse events of metformin treatment in patients with type 2 diabetes mellitus: A systematic review, meta-analysis and meta-regression of randomized controlled trials. Front Endocrinol. (2022) 13:975912. doi: 10.3389/fendo.2022.975912
66. Toft JH, Økland I. Metformin use in pregnancy: what about long-term effects in offspring? Acta Obstet Gynecol Scandinavica. (2024) 103:1238–41. doi: 10.1111/aogs.14878
67. Wu R, Zhang Q, Li Z. A meta-analysis of metformin and insulin on maternal outcome and neonatal outcome in patients with gestational diabetes mellitus. J Maternal-Fetal Neonatal Med. (2024) 37:2295809. doi: 10.1080/14767058.2023.2295809
68. Tocci V, Mirabelli M, Salatino A, Sicilia L, Giuliano S, Brunetti FS, et al. Metformin in gestational diabetes mellitus: to use or not to use, that is the question. Pharmaceuticals. (2023) 16:1318. doi: 10.3390/ph16091318
69. Paavilainen E, Tertti K, Nikkinen H, Veijola R, Vääräsmäki M, Loo B-M, et al. Metformin versus insulin therapy for gestational diabetes: effects on offspring anthropometrics and metabolism at the age of 9 Years: A follow-up study of two open-label, randomized controlled trials. Diabetes Obes Metab. (2022) 24:402–10. doi: 10.1111/dom.14589
70. Rawat D, Gupta Y, Yadav AK, Tembhre MK, Das P, Bakkireddy S, et al. Cardiometabolic outcomes in offspring of women treated with metformin versus insulin for gestational diabetes: A systematic review and meta-analysis. Diabetes Metab Syndr: Clin Res Rev. (2024) 18:103134. doi: 10.1016/j.dsx.2024.103134
71. Balsells M, García-Patterson A, Solà I, Roqué M, Gich I, Corcoy R. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: A systematic review and meta-analysis. Bmj. (2015) 350:h102. doi: 10.1136/bmj.h102
72. Corcoran C, Jacobs TF. Metformin. Statpearls. Treasure Island (FL: StatPearls Publishing LLC (2025).
73. Wei J, Yan J, Yang H. Inositol nutritional supplementation for the prevention of gestational diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Nutrients. (2022) 14:2831. doi: 10.3390/nu14142831
74. Milewska EM, Czyzyk A, Meczekalski B, Genazzani AD. Inositol and human reproduction. From cellular metabolism to clinical use. Gynecol Endocrinol. (2016) 32:690–5. doi: 10.1080/09513590.2016.1188282
75. Cabrera-Cruz H, Oróstica L, Plaza-Parrochia F, Torres-Pinto I, Romero C, Vega M. The insulin-sensitizing mechanism of myo-inositol is associated with ampk activation and glut-4 expression in human endometrial cells exposed to a pcos environment. Am J Physiology-Endocrinol Metab. (2020) 318:E237–e48. doi: 10.1152/ajpendo.00162.2019
76. Fedeli V, Catizone A, Querqui A, Unfer V, Bizzarri M. The role of inositols in the hyperandrogenic phenotypes of pcos: A re-reading of Larner’s results. Int J Mol Sci. (2023) 24:6296. doi: 10.3390/ijms24076296
77. Greff D, Juhász AE, Váncsa S, Váradi A, Sipos Z, Szinte J, et al. Inositol is an effective and safe treatment in polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled trials. Reprod Biol Endocrinol. (2023) 21:10. doi: 10.1186/s12958-023-01055-z
78. D’Anna R, Di Benedetto V, Rizzo P, Raffone E, Interdonato ML, Corrado F, et al. Myo-inositol may prevent gestational diabetes in pcos women. Gynecol Endocrinol. (2012) 28:440–2. doi: 10.3109/09513590.2011.633665
79. Chen H, Xiong J, Li Z, Chen Y, Zhang M, Chen D, et al. Influence of myo-inositol on metabolic status for gestational diabetes: A meta-analysis of randomized controlled trials. J Maternal-Fetal Neonatal Med. (2024) 37:2228450. doi: 10.1080/14767058.2023.2228450
80. Factor PA, Corpuz H. The efficacy and safety of myo-inositol supplementation for the prevention of gestational diabetes mellitus in overweight and obese pregnant women: A systematic review and meta-analysis. J ASEAN Fed Endocr Societies. (2023) 38:102–12. doi: 10.15605/jafes.038.02.11
81. Carlomagno G, Unfer V. Inositol safety: clinical evidences. Eur Rev Med Pharmacol Sci. (2011) 15:931–6.
82. Liu Z, Geng Y, Huang Y, Hu R, Li F, Song Y, et al. Letrozole compared with clomiphene citrate for polycystic ovarian syndrome: A systematic review and meta-analysis. Obstet Gynecol. (2023) 141:523–34. doi: 10.1097/aog.0000000000005070
Keywords: gestational diabetes mellitus, polycystic ovary syndrome, insulin, metformin, myo-inositol
Citation: Quaresima P, Myers SH, Pintaudi B, D’Anna R, Morelli M and Unfer V (2025) Gestational diabetes mellitus and polycystic ovary syndrome, a position statement from EGOI-PCOS. Front. Endocrinol. 16:1501110. doi: 10.3389/fendo.2025.1501110
Received: 24 September 2024; Accepted: 15 January 2025;
Published: 31 January 2025.
Edited by:
Zhiqin Bu, Zhengzhou University, ChinaCopyright © 2025 Quaresima, Myers, Pintaudi, D’Anna, Morelli and Unfer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vittorio Unfer, dnVuZmVyQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.