
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 19 February 2025
Sec. Cardiovascular Endocrinology
Volume 16 - 2025 | https://doi.org/10.3389/fendo.2025.1478640
Background: Despite extensive research on the epidemiological risk factors linking diabetes and carotid plaques, evidence regarding sex-specific differences remains scarce. This study aims to investigate the gender differences in the association between fasting blood glucose levels and the risk of carotid plaques among cardiovascular high-risk populations.
Methods: This study used cross-sectional data from a large prospective cohort study. From 2019 to 2020, fasting blood glucose and carotid ultrasound examinations were conducted on high-risk cardiovascular populations in the pilot area. The independent variable was fasting blood glucose, and the dependent variable was the presence or absence of carotid plaques. A multivariable logistic regression model was used to calculate risk ratios. Generalized additive models combined with curve fitting were employed to assess the nonlinear relationship between fasting blood glucose levels and carotid plaques in the overall population and different genders.
Results: This study included 1,063 cardiovascular high-risk patients. In the fully adjusted model, fasting blood glucose levels in men were positively associated with carotid plaques (OR = 1.21, 95% CI: 1.02-1.45, p = 0.0312). In women, fasting blood glucose levels were also positively associated with the risk of carotid plaque formation (OR = 1.15, 95% CI: 0.95-1.39). The generalized additive model results showed a linear relationship between fasting blood glucose levels and carotid plaques in the overall population. Stratified results indicated a linear relationship in men, while a nonlinear relationship was observed in women.
Conclusion: There is an association between fasting blood glucose levels and carotid plaque, with an increased risk of carotid plaque as fasting blood glucose rises. Moreover, this relationship differs between sexes.
Carotid plaque formation is closely related to atherosclerosis. The growth of carotid plaques is often accompanied by plaque rupture (1, 2), playing a critical role in the development of adverse cardiovascular events such as ischemic stroke (3–5). Carotid ultrasound is commonly used as a screening tool for subclinical atherosclerosis, providing an assessment of cardiovascular disease risk independent of traditional risk factors (6, 7). However, the development of carotid plaques usually occurs without noticeable clinical symptoms for a long period before the onset of stroke.
The microvascular and macrovascular complications of diabetes are considered the origin of atherosclerosis (8). In people with diabetes, the risk of atherosclerotic cardiovascular disease increases by two to three times (9, 10). The main mechanism is the endothelial injury-inflammation response theory. High blood glucose induces oxidative stress and increases advanced glycation end products, causing arterial wall inflammation and endothelial dysfunction, leading to the accumulation of fatty streaks and fibrous plaques in the arteries (11, 12). From 2021 to 2045, the prevalence of diabetes is expected to increase from 10% to 12%, with the global affected population rising from 537 million to 783 million (9).
Blood glucose levels are a modifiable factor in the formation of carotid plaques. Additionally, early diagnosis of prediabetes and the establishment of healthy habits can help prevent the progression of diabetes and reduce cardiovascular risk. Currently, there is some controversy regarding the evidence on the relationship between blood glucose levels and carotid plaques (13–15). A study of 1,475 individuals found a significant association between glucose status and the occurrence of carotid plaques and stenosis (14). However, another meta-analysis found no significant association between impaired glucose tolerance and the incidence of carotid plaques in women (16). We hypothesized that the relationship between fasting blood glucose levels and carotid plaques differs between sexes. Therefore, this study utilized cross-sectional data from cardiovascular high-risk populations identified through community screening to investigate sex-specific differences in this association.
The cross-sectional study population was derived from high-risk cardiovascular individuals identified in the initial screening of the Zhejiang Province Wenzhou pilot of the large-scale prospective study (China PEACE Million Persons Project), established by the National Center for Cardiovascular Diseases of China in 2014 (17). The China PEACE Million Persons Project has been conducted continuously across China since 2014, and by 2023, the project has expanded to 383 pilots nationwide, screening millions of individuals (18). The inclusion criteria for the initial screening population in this study were individuals born between January 1, 1948, and December 31, 1988 (aged 35-75 years), who were permanent residents of the pilot project area, defined as having resided in the project area for at least six months in the 12 months prior to screening, and who voluntarily participated in the cardiovascular high-risk screening and signed an informed consent form.
During the initial screening process, project staff assessed cardiovascular disease risk by conducting preliminary inquiries about cardiovascular health, physical examinations, and rapid tests for blood glucose and lipids to identify high-risk individuals.
The diagnostic criteria for identifying high-risk cardiovascular patients are as follows: individuals meeting any one of the four criteria listed below are classified as high-risk.
1. History of myocardial infarction or stroke (ischemic or hemorrhagic), or undergoing Percutaneous Coronary Intervention (PCI) or Coronary Artery Bypass Grafting (CABG).
2. Systolic blood pressure (SBP) ≥160 mmHg or diastolic blood pressure (DBP) ≥100 mmHg.
3. Low density lipoprotein cholesterol (LDL-C) ≥160 mg/dL (4.14 mmol/L) or high density lipoprotein cholesterol (HDL-C) <30 mg/dL (0.78 mmol/L).
4. Cardiovascular risk was assessed based on the 2008 WHO guidelines for cardiovascular risk assessment and management (19), which utilize risk prediction charts considering factors such as age, sex, systolic blood pressure (measured twice and averaged, in mmHg), smoking status (current smokers or those who quit within the past year are considered smokers), diabetes status (previously diagnosed diabetes, use of hypoglycemic medications, or insulin injections), and total cholesterol (TC, mmol/L). If the ten-year cardiovascular disease risk is ≥20%, the individual is classified as high-risk.
Trained surveyors conducted baseline assessments using a structured questionnaire for all high-risk individuals, while physical examinations, laboratory tests, and ultrasound examinations were performed by experienced clinicians. The questionnaire included demographic variables (birthdate, sex, occupation, educational level, and marital status), family history of diseases (diabetes, cardiovascular diseases, cerebrovascular diseases, and dyslipidemia), self-reported cardiovascular risk factors (smoking habits, alcohol consumption, hypertension), and medication history. Additionally, menopausal status and age at menopause were assessed for female participants.
Physical examinations included measurements of height, weight, and waist circumference. Blood pressure measurements were taken using an electronic sphygmomanometer (HEM7211; Omron, Japan) calibrated by the metrology department. Subjects were seated for 5 minutes before measurement, avoiding any noticeable positional changes or emotional disturbances. Resting blood pressure was recorded on the right upper arm while the subject was seated. Blood pressure was measured twice with a 5-minute interval. If the difference between the two systolic measurements was greater than 10 mmHg, a third measurement was taken. The average of the two systolic (SBP) and diastolic (DBP) measurements was used.
Fasting venous blood samples were collected using yellow-top vacuum blood collection tubes. Within 2 hours of collection, the whole blood samples were centrifuged at 1000-1200 g for 10-15 minutes at 4°C using a refrigerated centrifuge to separate the serum. All measurements were performed using the ROCHE c701 biochemical analyzer (ROCHE, Germany). Routine biochemical indicators included triglycerides (TG), total cholesterol (TC), fasting blood glucose, low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C).
Carotid arteries were assessed using a 7.5 MHz probe (Sonosite Micromaxx Ultrasound, Sonosite Inc, Bothell, WA, USA) for Doppler ultrasound. The patient was positioned supine with the neck rotated to the opposite side of the examination. Images were obtained from the distal wall of the common carotid artery, near the bifurcation, at three different angles, each covering a 1 cm segment.
Hypertension was defined as systolic blood pressure (SBP) ≥140 mmHg, diastolic blood pressure (DBP) ≥90 mmHg, self-reported history of hypertension, or current use of antihypertensive medications (20). Diabetes was defined as a fasting blood glucose level ≥7.0 mmol/L, any self-reported history of diabetes, or current use of diabetes medications (21). Alcohol consumption was classified as none, light drinking (1-2 drinks/day), moderate drinking (3-4 drinks/day), or heavy drinking (>5 drinks/day) according to definitions from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) (22). Smoking status was classified based on survey information into three categories: non-smoker, former smoker, and current smoker (23). Female participants were categorized as postmenopausal or premenopausal based on the absence or presence of menstruation within the 12 months prior to recruitment (24, 25). Additionally, participants were further stratified into two groups based on the duration of menopause. We redefined patients’ education levels as binary variables: “high school or above” and “below high school”. Carotid plaque was diagnosed as local intimal thickening >1 mm or thickening >50% of the surrounding intima-media thickness (IMT).
Based on the WHO diabetes diagnostic criteria (26), we categorized the study participants into three groups: normal fasting glucose (NFG) for fasting blood glucose levels ≤6.0 mmol/L, impaired fasting glucose (IFG) for fasting blood glucose levels >6.0 and <7.0 mmol/L, and high fasting glucose (HFG) for fasting blood glucose levels ≥7.0 mmol/L.
Continuous variables are expressed as means ± standard deviations or medians (interquartile ranges), while categorical variables are presented as frequencies or percentages. For continuous variables with a normal distribution, one-way ANOVA was used for between-group comparisons, while the Kruskal-Wallis test was used for non-normally distributed variables. Categorical variables were compared between groups using the chi-square test or Fisher’s exact test. Univariate logistic regression was used to examine the relationship between each variable and carotid plaque, while multivariate logistic regression models were used to calculate the odds ratio (OR) of fasting blood glucose levels for carotid plaque. Following the STROBE guidelines, we presented the results of the unadjusted, minimally adjusted, and fully adjusted multivariate regression models. Adjustment adequacy was determined based on whether the change in odds ratio was less than 10% when covariances were added to the model (27) and by the clinical interpretation between indicators. Trend tests were conducted for the mean fasting blood glucose levels in each group, treated as continuous variables.
The statistical analyses of this study were performed via R, version 4.2.0 (R Foundation) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA). The level of statistical significance was set at P < 0.05.
A total of 6,105 residents participated in the initial screening, with 1,582 identified as high-risk cardiovascular patients. Among them, 478 lacked carotid ultrasound or blood test results, resulting in 1,063 patients included in the study (Figure 1). Of the total population, 445 were male (41.86%) and 618 were female (58.14%). Baseline clinical and biochemical characteristics of the participants are shown in Table 1. The NFG group consisted of 619 individuals (234 males and 385 females), the IFG group had 243 individuals (107 males and 136 females), and the HFG group included 201 individuals (104 males and 97 females). Among males, there were significant differences between fasting blood glucose level groups in terms of weight, BMI, waist circumference, ALT, AST, TG, smoking status, and use of lipid-lowering medications (P < 0.05). Among females, significant differences were observed between fasting blood glucose level groups in age, weight, BMI, waist circumference, ALT, prevalence of carotid plaques and hypertension, menopausal status and age at menopause, and use of lipid-lowering medications (P < 0.05).
The results of the univariate analysis are shown in Table 2. The univariate analysis revealed that in males, factors associated with carotid plaques included age, SBP, AST/ALT, hypertension, current smoking status, and use of antihypertensive medications. In female participants, significant associations with increased risk of carotid plaques were found with age, marital status, education degree, family income, SBP, urea, fasting blood glucose levels, triglycerides, hypertension, diabetes, menopausal status and age at menopause, and the use of antihypertensive, antidiabetic, lipid-lowering, and antiplatelet medications.
We used multivariate logistic regression analysis to assess the association between fasting blood glucose levels and carotid plaques (Table 3). In the male population, the unadjusted model showed that fasting blood glucose levels were not significantly associated with the formation of carotid plaques (OR = 1.07, 95% CI: 0.94-1.22; P = 0.288). In the partially adjusted model (adjusting for age and BMI) and the fully adjusted model (adjusting for age, BMI, sex, marital status, education level, family income, smoking and drinking status, systolic and diastolic blood pressure, heart rate, ALT, AST, cholesterol, triglycerides, urea, and use of lipid-lowering medication), significant associations were observed: partially adjusted model (OR = 1.20, 95% CI: 1.03-1.40; P = 0.021) and fully adjusted model (OR = 1.21, 95% CI: 1.02-1.45; P = 0.031). In the female population, the unadjusted model showed a significant association between fasting blood glucose levels and arterial plaques (OR = 1.32, 95% CI: 1.15-1.53; p < 0.001). However, in the partially adjusted and fully adjusted models, the association between fasting blood glucose levels and plaque formation weakened, with ORs of 1.16 and 1.19, respectively. Among the different fasting blood glucose level groups, using the NFG group as a reference, the risk of carotid plaques was significantly higher in the HFG group (p-values < 0.05). In the HFG group, based on Model II, each one-unit increase in glucose was associated with an 88% higher risk of carotid plaques.
We constructed fitting curves using a generalized additive model to analyze the nonlinear relationship between fasting blood glucose levels and carotid artery plaques in the overall population and across different genders (Figure 2). In the overall population, we found a linear relationship between fasting blood glucose levels and carotid artery plaques after adjusting for age, BMI, gender, marital status, education level, family income, smoking and alcohol consumption, systolic and diastolic blood pressure, heart rate, ALT, AST, cholesterol, triglycerides, urea, and lipid-lowering medication use. After stratifying by gender, we observed that the relationship between fasting blood glucose levels and carotid artery plaques remains linear in men but is nonlinear in women. Among females, stratified curve fitting was performed based on menopausal status and duration of menopause (Supplementary Figure S1). After adjusting for the same covariates, a differential association between glucose levels and carotid plaque risk was observed between postmenopausal and premenopausal women. Similarly, differences were also noted across groups with varying durations of menopause.
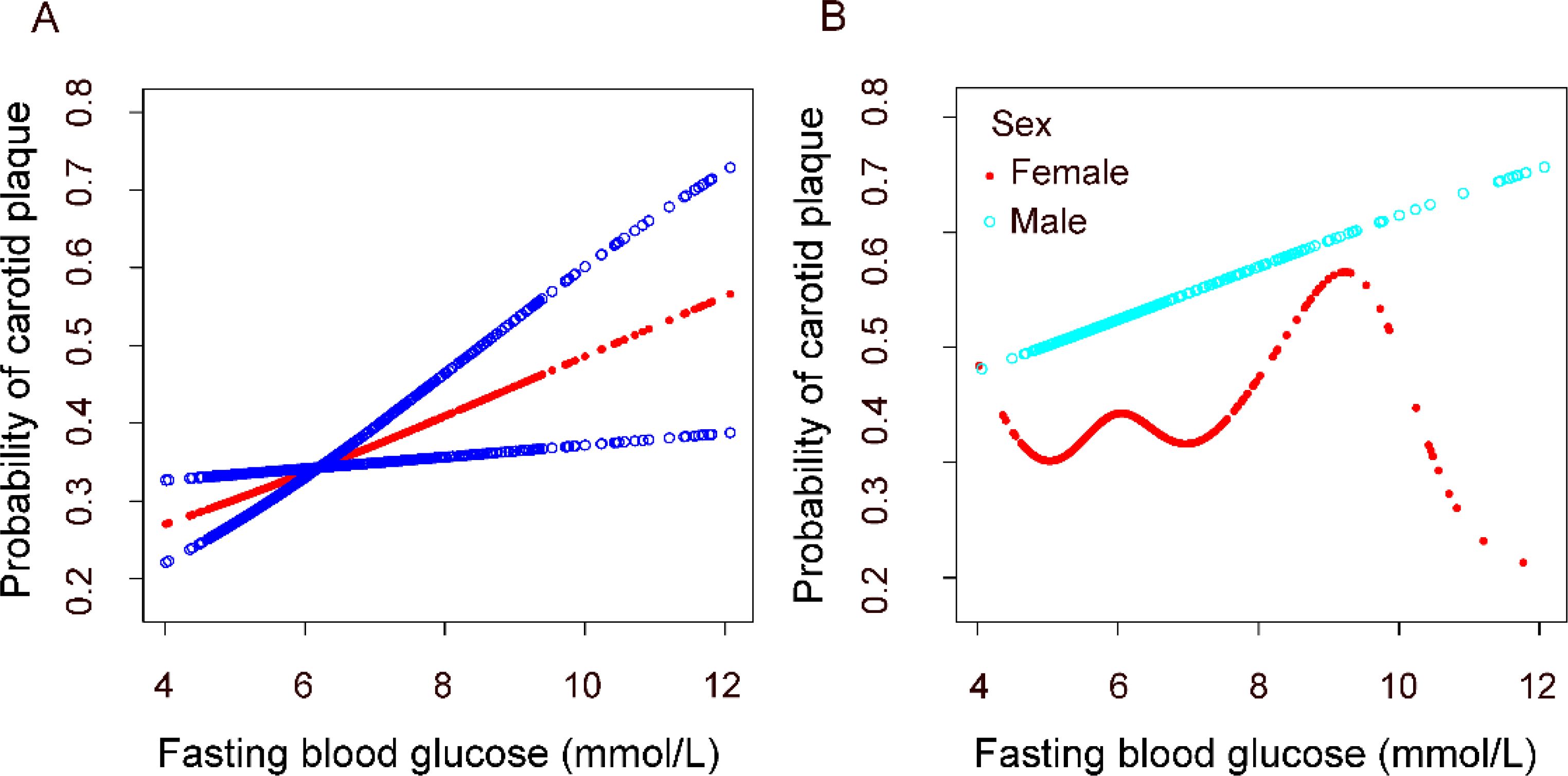
Figure 2. Association between fasting plasma glucose and carotid plaque. (A) in the general population. (B) in different gender groups. All analyses were adjusted for confounding factors including Age; BMI; Family income; Education degree; Marital status; Smoking; Alcohol; SBPUBP: Heart rate; LDL-C; Urea; Antilipemic medication.
This cross-sectional survey of a community population revealed that higher fasting blood glucose levels were associated with an increased risk of carotid plaques in individuals at high cardiovascular risk. Further analysis indicated that the association between glucose levels and carotid plaque risk varied by sex. To our knowledge, this is the first study to report a sex-specific relationship between fasting glucose levels and carotid plaques. Moreover, our findings demonstrate that males are at a higher risk of carotid plaques compared to females. Postmenopausal women have a higher risk than premenopausal women, and women with longer menopausal durations exhibit a greater risk than those with shorter durations.
Numerous studies have investigated the relationship between fasting blood glucose levels and carotid artery plaques. Mostaza et al. (14) found that the prevalence of carotid atherosclerosis, assessed by the presence of carotid artery plaques, significantly increases as blood glucose status progresses from normal to prediabetes and diabetes among participants aged 45-74,but no sex differences were found according to diabetes diagnosis by fasting glucose. The study by Adeleke O. Fowokan (13) demonstrated that glucose could predict the size of carotid plaques and highlighted differences among ethnic groups. In contrast, our study revealed that the relationship between glucose levels and carotid plaques varies by sex. A cohort study conducted by Salvador Jr. et al. (28) identified a positive association between fasting blood glucose and the presence of carotid plaques, consistent with our findings. Our study further revealed sex-specific differences in the risk association between fasting blood glucose and carotid plaques. Some studies have reported contrary results. G. Brohall (16) conducted a meta-analysis of women aged 64 and found no significant association between impaired glucose tolerance and the incidence of carotid artery plaques. The author hypothesized that hormone replacement therapy (HRT) and cardiovascular medications might have influenced the results, though this hypothesis was not confirmed. In our study, cardiovascular-related medications were included as covariates in the analysis. We still observed an association between fasting blood glucose levels and carotid plaque risk in females, indicating that the relationship between fasting blood glucose and carotid plaques is independent of cardiovascular medication use.
The mechanisms underlying the relationship between blood glucose levels and carotid artery plaques remain unclear. Research has shown that high blood glucose mimics the effects of inflammatory mediators such as NF-kB, leading to endothelial cell activation and dysfunction, which are early stages in the development of atherosclerosis (11). Our study found that the relationship between fasting blood glucose levels and carotid artery plaques differs between men and women. A possible explanation is the presence of estrogen and its protective effect against coronary artery atherosclerosis (CAA). Studies have confirmed that estrogen significantly reduces the incidence of coronary artery atherosclerosis by improving vascular function and regulating lipid metabolism (29). The protective effects of estrogen on the vasculature may explain why women are less affected by high blood glucose-induced atherosclerosis. To test these hypotheses, we conducted stratified curve analyses based on menopausal status and duration of menopause in female participants. The results showed that postmenopausal women had a higher risk of carotid plaques compared to premenopausal women. Furthermore, women with longer menopausal durations were at greater risk than those with shorter durations.
This study has several limitations. First, as a cross-sectional study, we could only identify associations but could not establish causality. Additionally, participant selection was based on voluntary enrollment from residents at the project site. Although the refusal rate was low, selection bias cannot be completely ruled out. Moreover, as an observational study, the influence of numerous confounding factors cannot be excluded. To address this, we adjusted for various potential confounders in multivariable regression models. Menopausal status and duration, used as proxies for estrogen changes, were included in the analysis; however, these variables do not directly demonstrate the role of estrogen. Future research should further investigate the potential roles of factors such as inflammation and plasma corticosteroid levels in the development of carotid plaques. Our data are derived from middle-aged and elderly participants in a Chinese community, which may limit the generalizability of our findings to other populations.
Our study demonstrates that fasting blood glucose levels are associated with the occurrence of carotid plaques in high-risk cardiovascular populations. Higher fasting blood glucose levels correlate with an increased risk of carotid plaques. Furthermore, this relationship differs between sexes.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by The Central Ethics Committee of the National Center for Cardiovascular Diseases and the Ethics Committee of Wenzhou People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
MX: Data curation, Formal analysis, Resources, Validation, Visualization, Writing – original draft. KX: Conceptualization, Data curation, Methodology, Writing – review & editing. WL: Conceptualization, Methodology, Writing – review & editing. RS: Conceptualization, Methodology, Writing – review & editing. SY: Data curation, Resources, Supervision, Visualization, Writing – review & editing. XC: Funding acquisition, Project administration, Resources, Visualization, Writing – review & editing. YL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – review & editing, Writing – original draft.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by Zhejiang Provincial Health Commission(2021KY1079) and Wenzhou Science and Technology Bureau (Y20210964).
The authors thank the field investigators for their contribution and the participants for their cooperation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1478640/full#supplementary-material
1. Bengtsson E, Hultman K, Edsfeldt A, Persson A, Nitulescu M, Nilsson J, et al. CD163+ macrophages are associated with a vulnerable plaque phenotype in human carotid plaques. Sci Rep. (2020) 10:14362. doi: 10.1038/s41598-020-71110-x
2. Zhao Y, Gu Y, Liu Y, Guo Z. Evaluation of the correlation between distribution location and vulnerability of carotid plaque in patients with transient ischemic attack. Vasc Health Risk Manage. (2024) 20:77–87. doi: 10.2147/vhrm.S447418
3. Takaya N, Yuan C, Chu B, Saam T, Underbill H, Cai J, et al. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: A prospective assessment with MRI - Initial results. Stroke. (2006) 37:818–23. doi: 10.1161/01.STR.0000204638.91099.91
4. Joh JH, Cho S. Cardiovascular risk of carotid atherosclerosis: global consensus beyond societal guidelines. Lancet Global Health. (2020) 8:e625–6. doi: 10.1016/s2214-109x(20)30132-7
5. Raggi P, Stein JH. Carotid intima-media thickness should not be referred to as subclinical atherosclerosis: A recommended update to the editorial policy at Atherosclerosis. Atherosclerosis. (2020) 312:119–20. doi: 10.1016/j.atherosclerosis.2020.09.015
6. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. (2006) 3:e442. doi: 10.1371/journal.pmed.0030442
7. Song P, Fang Z, Wang H, Cai Y, Rahimi K, Zhu Y, et al. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: a systematic review, meta-analysis, and modelling study. Lancet Global Health. (2020) 8:e721–9. doi: 10.1016/s2214-109x(20)30117-0
8. Chawla A, Chawla R, Jaggi S. Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian J Endocrinol Metab. (2016) 20:546–51. doi: 10.4103/2230-8210.183480
9. Booth GL, Kapral MK, Fung K, Tu JV. Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: a population-based retrospective cohort study. Lancet (London England). (2006) 368:29–36. doi: 10.1016/s0140-6736(06)68967-8
10. Preis SR, Pencina MJ, Hwang SJ, D’Agostino RB, Savage PJ, Levy D, et al. Trends in cardiovascular disease risk factors in individuals with and without diabetes mellitus in the Framingham Heart Study. Circulation. (2009) 120:212–20. doi: 10.1161/circulationaha.108.846519
11. Funk SD, Yurdagul A Jr., Orr AW. Hyperglycemia and endothelial dysfunction in atherosclerosis: lessons from type 1 diabetes. Int J Vasc Med. (2012) 2012:569654. doi: 10.1155/2012/569654
12. Puri R, Kataoka Y, Uno K, Nicholls SJ. The distinctive nature of atherosclerotic vascular disease in diabetes: pathophysiological and morphological insights. Curr Diabetes Rep. (2012) 12:280–5. doi: 10.1007/s11892-012-0270-y
13. Fowokan AO, Lesser IA, Humphries KH, Mancini JGB, Lear SA. The predictive relationship between baseline insulin and glucose with subclinical carotid atherosclerosis after 5 years in a multi-ethnic cohort. Atherosclerosis. (2017) 257:146–51. doi: 10.1016/j.atherosclerosis.2016.12.013
14. Mostaza JM, Lahoz C, Salinero-Fort MA, de-Burgos-Lunar C, Laguna F, Estirado E, et al. Carotid atherosclerosis severity in relation to glycemic status: a cross-sectional population study. Atherosclerosis. (2015) 242:377–82. doi: 10.1016/j.atherosclerosis.2015.07.028
15. Savic ZN, Djordjevic PB, Ilic MM, Popovic SS, Dimitrijevic-Sreckovic V, Canovic FM, et al. Carotid artery plaque in patients with disorders of glucose regulation. Acta chirurgica Iugoslavica. (2008) 55:43–6. doi: 10.2298/ACI0801043S
16. Brohall G, Schmidt C, Behre CJ, Hulthe J, Wikstrand J, Fagerberg B. Association between impaired glucose tolerance and carotid atherosclerosis: A study in 64-year-old women and a meta-analysis. Nutrition Metab Cardiovasc Diseases. (2009) 19:327–33. doi: 10.1016/j.numecd.2008.02.002
17. Lu J, Xuan S, Downing NS, Wu C, Li L, Krumholz HM, et al. Protocol for the China PEACE (Patient-centered evaluative assessment of cardiac events) million persons project pilot. BMJ Open. (2016) 6:e010200. doi: 10.1136/bmjopen-2015-010200
18. Lu J, Lu Y, Wang X, Li X, Linderman GC, Wu C, et al. Prevalence, awareness, treatment, and control of hypertension in China: data from 1·7 million adults in a population-based screening study (China PEACE Million Persons Project). Lancet. (2017) 390:2549–58. doi: 10.1016/S0140-6736(17)32478-9
19. Mendis S, Lindholm LH, Mancia G, Whitworth J, Alderman M, Lim S, et al. World Health Organization (WHO) and International Society of Hypertension (ISH) risk prediction charts: assessment of cardiovascular risk for prevention and control of cardiovascular disease in low and middle-income countries. J hypertension. (2007) 25:1578–82. doi: 10.1097/HJH.0b013e3282861fd3
20. Zheng R, Mao Y. Triglyceride and glucose (TyG) index as a predictor of incident hypertension: a 9-year longitudinal population-based study. Lipids Health Dis. (2017) 16:175. doi: 10.1186/s12944-017-0562-y
21. Hassan W, Saquib J, Khatri M, Kazmi SK, Kotak S, Hassan H, et al. Short- and long-term cardiovascular outcomes in insulin-treated versus non-insulin-treated diabetes mellitus patients after percutaneous coronary intervention: A systematic review and meta-analysis. Indian Heart J. (2022) 74:13–21. doi: 10.1016/j.ihj.2021.12.004
22. Avitabile E, Díaz A, Montironi C, Pérez-Guasch M, Gratacós-Ginès J, Hernández-Évole H, et al. Adding inflammatory markers and refining national institute on alcohol abuse and alcoholism criteria improve diagnostic accuracy for alcohol-associated hepatitis. Clin Gastroenterol Hepatol. (2023) 21:3080–3088.e9. doi: 10.1016/j.cgh.2023.03.023
23. Zhang Y, Wu Z, Li X, Wei J, Zhang Q, Wang J. Association between the triglyceride-glucose index and carotid plaque incidence: a longitudinal study. Cardiovasc Diabetol. (2022) 21:244. doi: 10.1186/s12933-022-01683-6
24. Clavel-Chapelon F. Differential effects of reproductive factors on the risk of pre- and postmenopausal breast cancer. Results from a large cohort of French women. Br J Cancer. (2002) 86:723–7. doi: 10.1038/sj.bjc.6600124
25. Dam V, van der Schouw YT, Onland-Moret NC, Groenwold RHH, Peters SAE, Burgess S, et al. Association of menopausal characteristics and risk of coronary heart disease: a pan-European case-cohort analysis. Int J Epidemiol. (2019) 48:1275–85. doi: 10.1093/ije/dyz016
26. van Herpt TTW, Ligthart S, Leening MJG, van Hoek M, Lieverse AG, Ikram MA, et al. Lifetime risk to progress from pre-diabetes to type 2 diabetes among women and men: comparison between American Diabetes Association and World Health Organization diagnostic criteria. BMJ Open Diabetes Res Care. (2020) 8:e001529. doi: 10.1136/bmjdrc-2020-001529
27. Kernan WN, Viscoli CM, Brass LM, Broderick JP, Brott T, Feldmann E, et al. Phenylpropanolamine and the risk of hemorrhagic stroke. New Engl J Med. (2000) 343:1826–32. doi: 10.1056/nejm200012213432501
28. Salvador D Jr., Liv P, Norberg M, Pahud de Mortanges A, Saner H, Glisic M, et al. Changes in fasting plasma glucose and subclinical atherosclerosis: A cohort study from VIPVIZA trial. Atherosclerosis. (2024) 394:117326. doi: 10.1016/j.atherosclerosis.2023.117326
Keywords: fasting plasma glucose, carotid plaque, sex differences, cross sectional study, logistic regression
Citation: Xu M, Xu K, Lin W, Sun R, Yan S, Chen X and Lin Y (2025) Sex-specific differences in the relationship between fasting plasma glucose and carotid plaque in a cardiovascular high-risk population: a cross-sectional study. Front. Endocrinol. 16:1478640. doi: 10.3389/fendo.2025.1478640
Received: 10 August 2024; Accepted: 31 January 2025;
Published: 19 February 2025.
Edited by:
Luciana Venturini Rossoni, University of São Paulo, BrazilReviewed by:
Rolando Juan Jose Ramirez, University of Akron, United StatesCopyright © 2025 Xu, Xu, Lin, Sun, Yan, Chen and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuzhan Lin, MTAxNjA5OTA0N0BxcS5jb20=; Xiaoshu Chen, Y2hlbjE5OTYwMTZAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.