- 1Hepatobiliary Pancreatic Surgery, Banan Hospital Affiliated of Chongqing Medical University, Chongqing, China
- 2Xiangya School of Medicine, Central South University, Changsha, China
- 3College of Education, Wenzhou University, Wenzhou, China
Background: This investigation explores the worldwide impact of diabetes burden associated with air pollution, drawing on data from the Global Burden of Disease Study 2021.
Method: The influence of air pollution on diabetes burden was analyzed at global, regional, and national levels. The study considered variations across age groups and genders and explored the relationship between disease impact and the Socio-Demographic Index (SDI). Additionally, an ARIMA model was employed to predict the future incidence of diabetes burden related to air pollution until 2050.
Result: In 2021, approximately 281.91 thousand fatalities and 12.90 million disability-adjusted life years were attributed to diabetes burden due to air pollution, featuring an age-standardized mortality rate (ASMR) of 3.3234 (95% UI, 1.9549–4.6634) and an age-standardized DALY rate (ASDR) of 148.9167 (95% UI, 86.5013–224.9116) per 100,000 individuals. There was a noticeable escalation in the disease burden over the period studied. The most severe effects were noted in individuals aged 60 and above. The data also revealed a higher disease burden among males. Forecasting suggests that while low SDI regions might see a decrease in death rates, lower-middle SDI areas could face an increase in standardized mortality rates. On a national scale, Russia, Mexico, and several African nations are predicted to experience rising diabetes burden attributable to air pollution mortality rates and age-standardized mortality rates from now to 2050. South Asia and Africa are anticipated to witness substantial growth in age-standardized death rates compared to other areas.
Conclusion: The results provide essential insights for developing preventive strategies for diabetes burden and measures to mitigate air pollution.
1 Introduction
Diabetes, a persistent medical condition, interferes with how the body processes food into energy. It often emerges in individuals with obesity, which impairs glucose management and heightens insulin resistance, elevating diabetes risk (1). Approximately 90% of type 2 diabetes cases are linked to excess weight or obesity, and the prevalence of obesity-induced impaired glucose tolerance is projected to climb to 420 million globally by 2025 (2). The simultaneous presence of diabetes and obesity, known as diabesity, can intensify cardiometabolic disorders and is emerging as a significant global health challenge amid the obesity crisis (3). Understanding the modifiable risk factors is essential for the large-scale control and prevention of diabesity.
Environmental hazards, particularly fine particulate matter (PM2.5), pose significant global health risks. Research indicates that PM2.5 exposure correlates with higher risks of both obesity (4, 5) and diabetes (6, 7). Yet, the impact of PM2.5 on diabesity remains unexplored. Animal studies suggest that PM2.5 exposure can trigger insulin resistance and systemic inflammation (8, 9), leading to obesity (10) and disrupted glucose tolerance, changes in adipokine levels, and mitochondrial dysfunction, eventually causing diabetes (11). Recent findings from the UK Biobank also demonstrate a more pronounced link between PM2.5 exposure and diabetes among obese individuals. Despite this, global studies specifically addressing the impact of air pollution on diabetes mellitus are lacking.
While well-established risk factors like obesity, lack of physical activity, and hereditary traits are recognized (12, 13), emerging evidence suggests a critical impact of environmental contributors, particularly air pollution, on the development of type 2 diabetes mellitus (T2DM). A comprehensive review and meta-analysis involving over 1.7 million individuals from 16 different studies has identified a robust link between sustained exposure to fine particulate matter (PM2.5) and a heightened risk of T2DM. Notably, an increase of 10 µg/m3 in PM2.5 levels corresponds to a 9% rise in the incidence of T2DM (14, 15). This highlights the urgency of exploring the underlying mechanisms connecting air pollution and T2DM, and the imperative to devise effective preventive measures.
However, previous studies didn’t analyze the overall disease burden of diabetes burden attributable to air pollution. Thus, this study assesses how air pollution affects the global burden of diabetes burden by analyzing mortality and disability-adjusted life years (DALYs) trends across various demographics and socio-economic indicators from 1990 to 2021. Future trends are also projected using the autoregressive integrated moving average (ARIMA) model, supported by earlier studies (12, 13). These insights aim to support the prevention of diabetes burden and the management of environmental pollution.
2 Method
2.1 Study population
Our research utilized data from the Global Burden of Disease Study (GBD) 2021, available at http://ghdx.healthdata.org/gbd-results-tool. The GBD 2021 encompasses a comprehensive assessment of 369 diseases and injuries, along with 87 risk factors, spanning 204 countries from 1990 to 2021 (14).
The methodologies adopted to evaluate the diabetes burden are described in external references (15, 16). This summary outlines the methods employed in the GBD 2021. Data on diabetes mellitus mortality were sourced from vital registration systems, surveillance systems, and verbal autopsy reports. Adjustments were made to this vital registration data to enhance precision and to correct data deficiencies and misclassification issues (17). These corrections were incorporated into the Cause of Death Ensemble model (CODEm), which generated localized, annual estimates of diabetes-related deaths by age and gender (16, 18). A comparative risk assessment was also conducted to identify principal risk factors for diabetes mellitus. The population attributable fraction (PAF) was computed to assess the extent of air pollution’s contribution to the diabetes burden. The estimates of diabetes mellitus mortality and disability-adjusted life years (DALYs) related to air pollution were derived by applying these specific PAFs to the mortality and DALY statistics by location, year, age, and gender (14). DALYs represent an aggregate measure of disease burden, integrating years of life lost due to premature death (YLLs) and years lived with disability (YLDs). YLLs were calculated by multiplying the number of deaths due to lower respiratory infections (LRIs) within each age group by the group’s residual life expectancy. YLDs were calculated by multiplying the prevalence of LRIs by the disability weights adjusted for severity (17). The Socio-Demographic Index (SDI), ranging from 0 (lowest) to 100 (highest), was determined based on factors such as the total fertility rate for those under 25 years old (TFU25), average educational level for those over 15 (EDU15+), and adjusted per capita income. Based on the SDI, the 204 nations and territories were classified into five categories: low SDI, low-middle SDI, middle SDI, high-middle SDI, and high SDI (17).
2.2 Statical analysis
Age-standardized rates (ASR) were employed to adjust mortality and DALY figures for countries with diverse age structures and demographic profiles. We applied a linear regression to the natural logarithm of these rates, expressed as y = α+βx+ϵ, where x denotes the year, and y is the natural log of the rate. The estimated annual percentage change (EAPC) was calculated using the formula 100 * (e^β − 1), accompanied by a 95% confidence interval (95% CI). An upward trend in ASR was determined if both the EAPC and the lower limit of the 95% CI were positive, whereas a decline was noted if the EAPC and the upper limit of the 95% CI were negative. Stability in ASR was inferred if neither condition was met (19, 20). The correlation between ASR and the Socio-demographic Index (SDI) was investigated using a Gaussian process regression model with Loess smoothing, assessed via Spearman rank correlation tests (19, 21). Decomposition analysis was performed to assess the effects of aging, population growth, and epidemiological changes on DALY variations from 1990 to 2021, as elaborated in earlier studies (22).
Moreover, the ARIMA (Autoregressive Integrated Moving Average) model was utilized to examine the effects of air pollution on diabetes mellitus trends and to project these trends globally, regionally, and nationally from 2020 to 2050. In the ARIMA model configuration (p, d, q), ‘AR’ indicates the autoregressive part with p representing the number of lag observations included in the model; ‘MA’ denotes the moving average segment with q indicating the number of lag forecast errors; and d specifies the degree of differencing required for data stabilization (23). The model’s selection was refined based on the Akaike Information Criterion (AIC) and the Bayesian Information Criterion (BIC).
Uncertainty intervals (UI) of 95% were determined for all calculated figures (24). The rates were presented per 100,000 population. Data management, analysis, and visualization tasks were executed using R software, version 4.3.2 (25–29).
3 Result
3.1 Spatiotemporal patterns of diabetes burden attributable to air pollution
In 2021, air pollution was responsible for roughly 281.91 thousand deaths and 12.90 million disability-adjusted life years (DALYs) related to diabetes mellitus, featuring an age-standardized mortality rate (ASMR) of 3.3234 (95% UI, 1.9549–4.6634) and an age-standardized DALY rate (ASDR) of 148.9167 (95% UI, 86.5013–224.9116) per 100,000 individuals. Over the past three decades, there has been a marked rise in the diabetes burden attributable to air pollution (Table 1, Supplementary Table S1).
In terms of the Socio-demographic Index (SDI), while high SDI and high-middle SDI regions have experienced a decrease in the diabetes burden due to air pollution, other regions have observed an increase. Additionally, an increase in the overall burden was noted across all SDI regions (Table 1, Supplementary Table S1, Figure 1
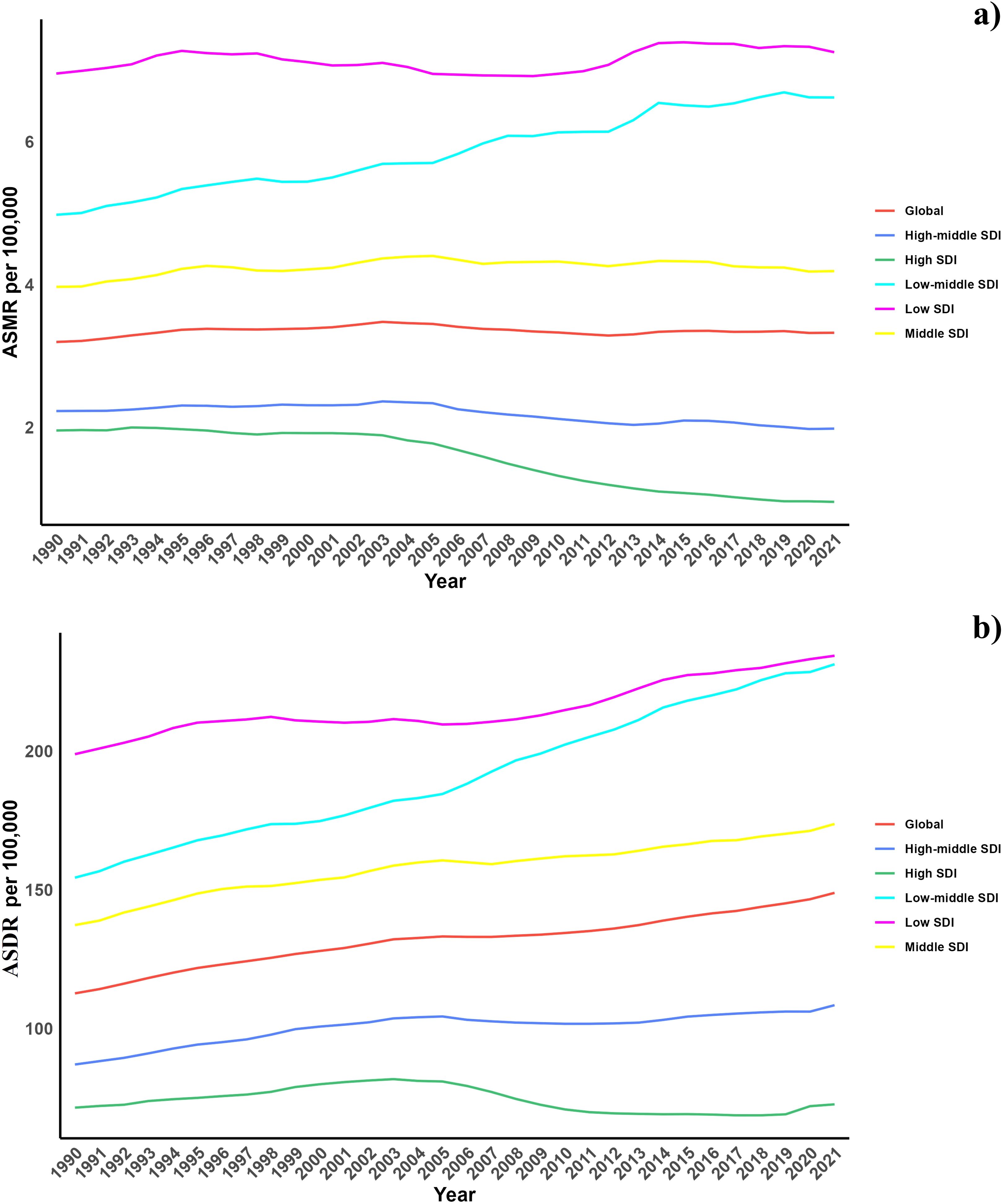
Figure 1. (a) Temporal trends of ASMR of diabetes mellitus attributable to air pollution from 1990 to 2021 in different SDI regions. (b) Temporal trends of ASDR of diabetes mellitus attributable to air pollution from 1990 to 2021 in different SDI regions.
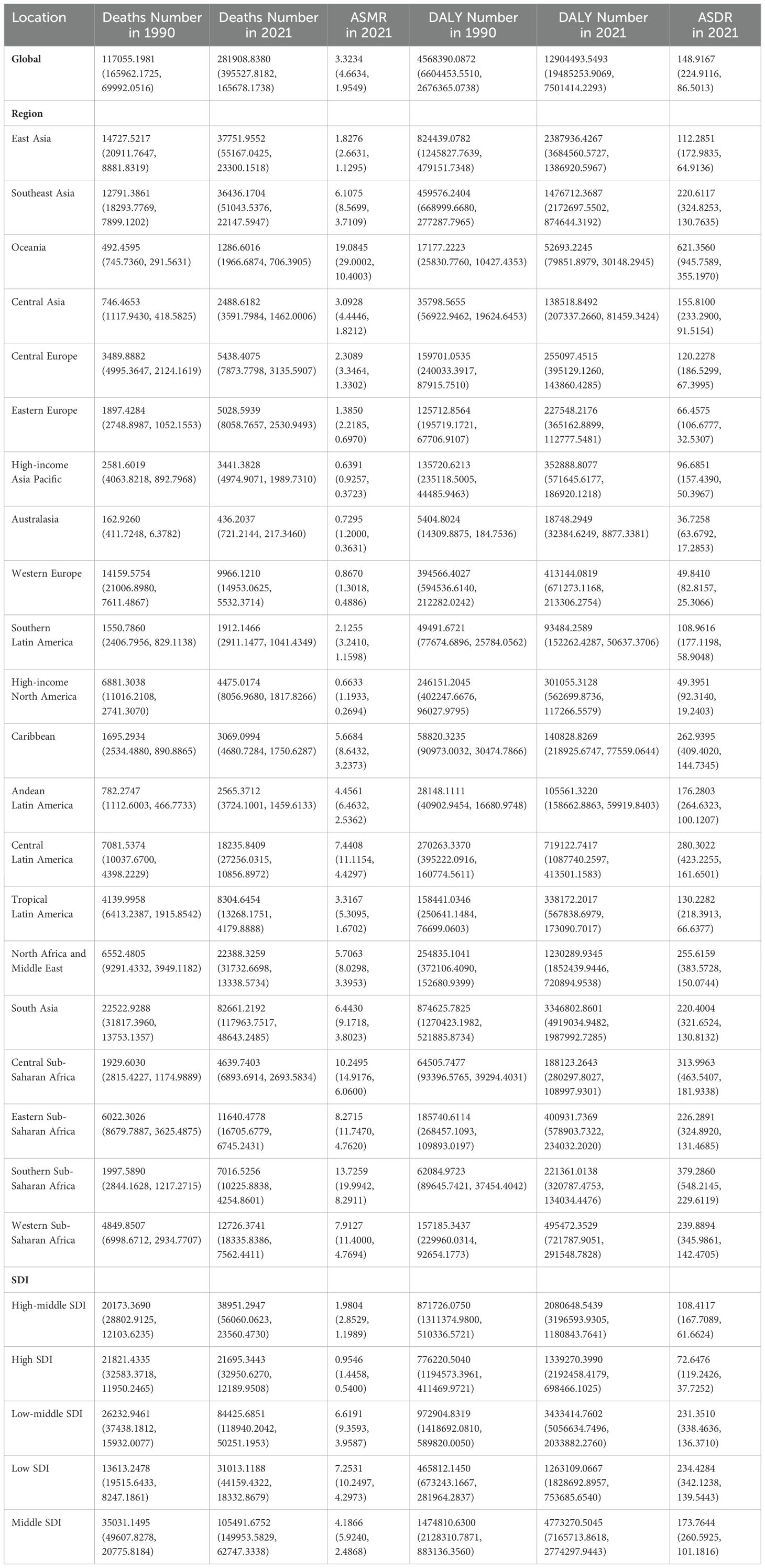
Table 1. Global and regional deaths and DALYs of diabetes mellitus attributable to air pollution in 1990 and 2021 in 27 global regions.
Region-wise, East Asia, Southeast Asia, and South Asia registered the highest impact with the most deaths and DALYs, whereas Oceania, Central Asia, and Australasia recorded the lowest. Conversely, in terms of ASMR and ASDR, Oceania, Central Sub-Saharan Africa, and Southern Sub-Saharan Africa demonstrated the highest burden (Table 1).
At the national level, in 2021, there was significant variability in ASMR across countries, with populous nations like China and India showing the highest rates, reflecting major health challenges linked to environmental and public health issues (Figure 2). From 1990 to 2021, some countries, especially in Western Europe and North America, saw a decline in ASMR, indicating improvements in health metrics. However, countries such as Honduras and Libya experienced increases, primarily due to PM2.5 pollution and other environmental concerns, underscoring unique regional health challenges that differ from the global trend toward better health outcomes (Table 1, Supplementary Table S1, Figure 3). The geographical and chronological trends of ASDR mirrored those of ASMR.
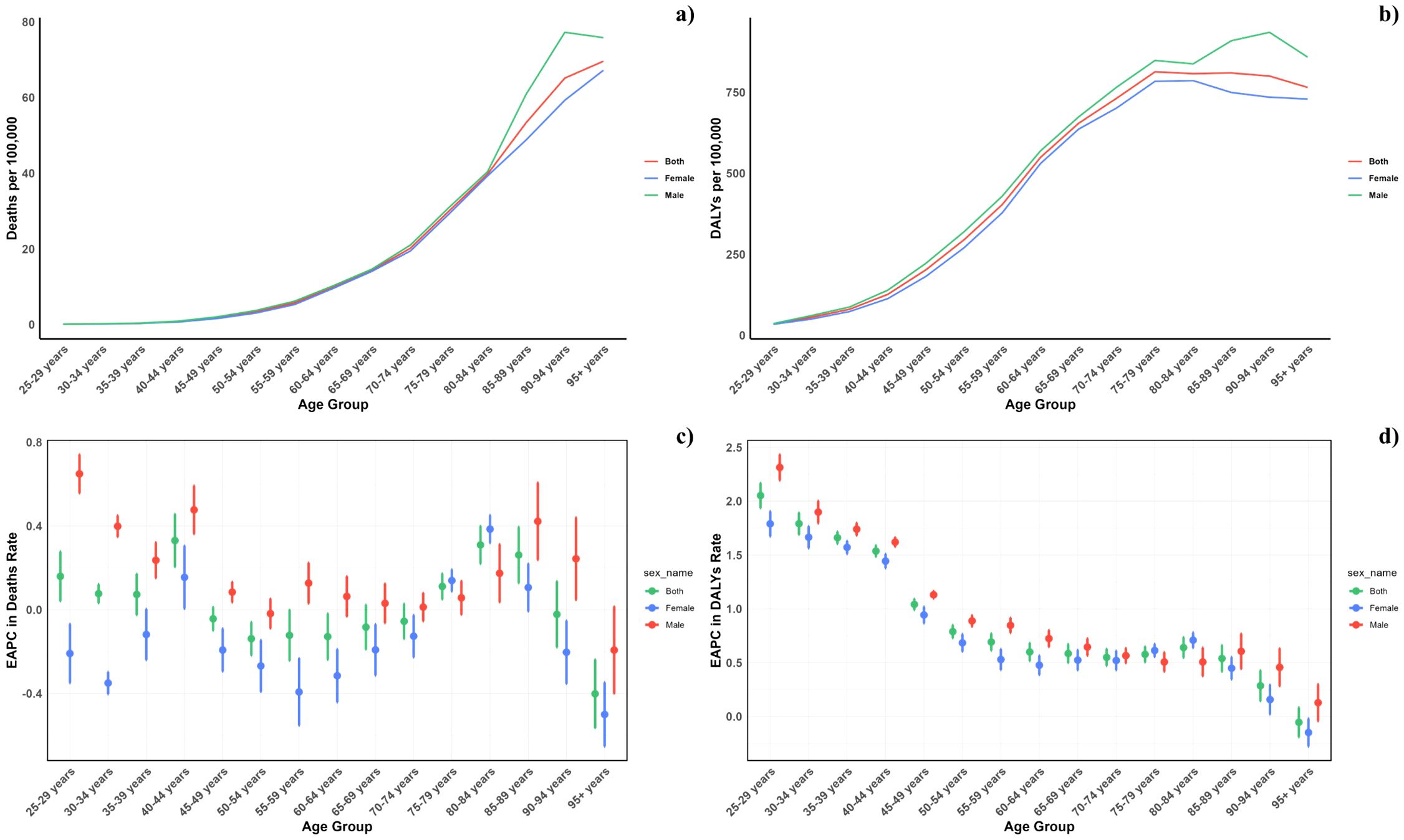
Figure 2. Age–specific rates of global deaths (a) and DALYs (b) of diabetes mellitus attributable to air pollution, by sex, in 2021 and the corresponding EAPC of global deaths (c) and DALYs (d) from 1990 to 2021.
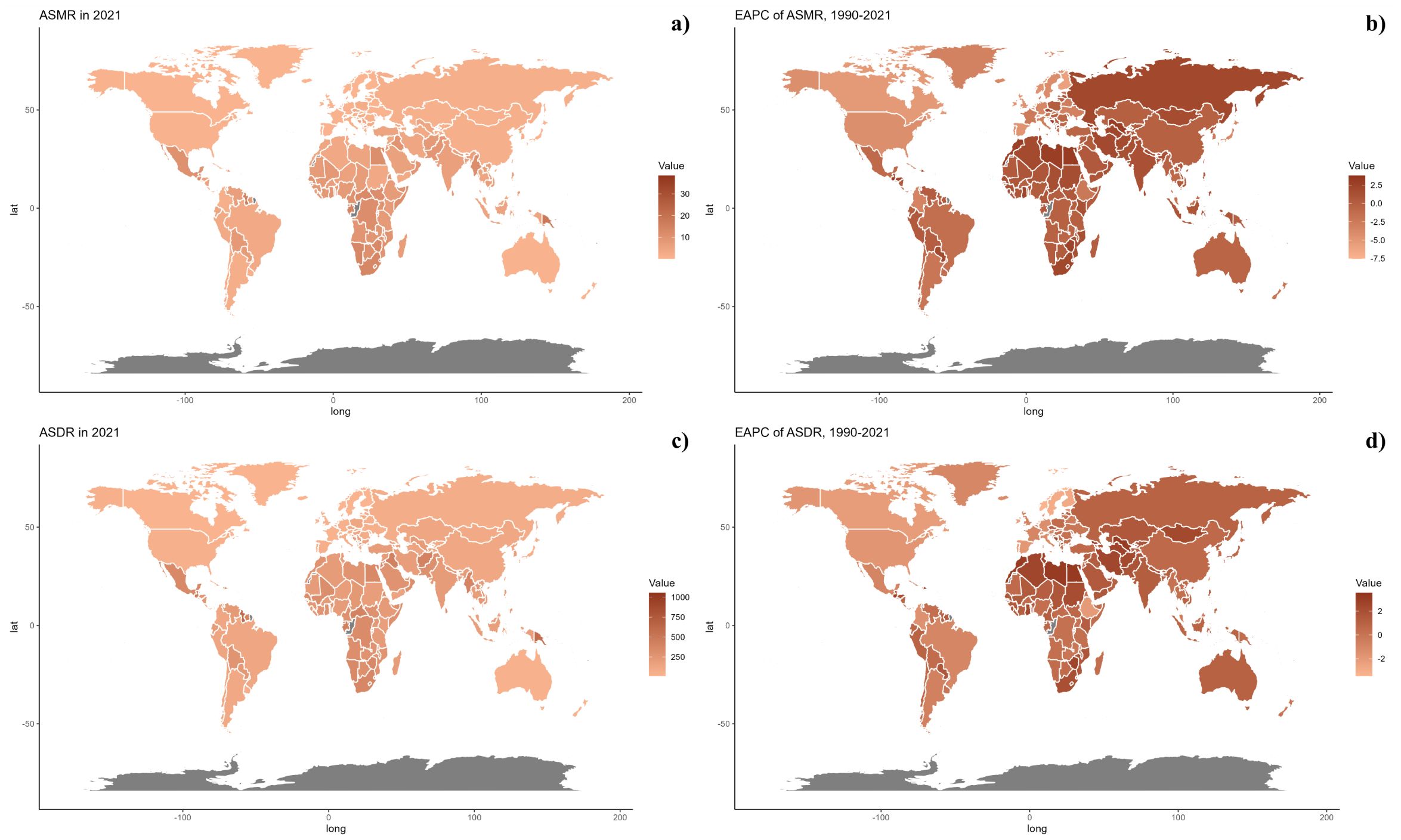
Figure 3. (a) Age-specific rates of global deaths of diabetes mellitus attributable to air pollution, by sex, in 2021. (b) Age-specific rates of global DALYs (b) of diabetes mellitus attributable to air pollution, by sex, in 2021. (c) Corresponding EAPC of global deaths from 1990 to 2021. (d) Corresponding EAPC of global DALYs (d) from 1990 to 2021.
3.2 Age and sex patterns
Figure 2 illustrates the age-specific global mortality and DALY rates for diabetes mellitus in 2021, along with changes from 1990. These rates reveal a J-shaped pattern, indicating an increase in mortality and DALYs among individuals below 60, with a significant surge observed in the 60+ age groups. Across all age brackets, males demonstrated consistently higher mortality rates from air pollution than females. Similarly, the DALY rates associated with air pollution were elevated for all age groups. In every SDI region, males exhibited higher mortality and DALY rates, with this gender difference being consistent across the regions (Figure 4).
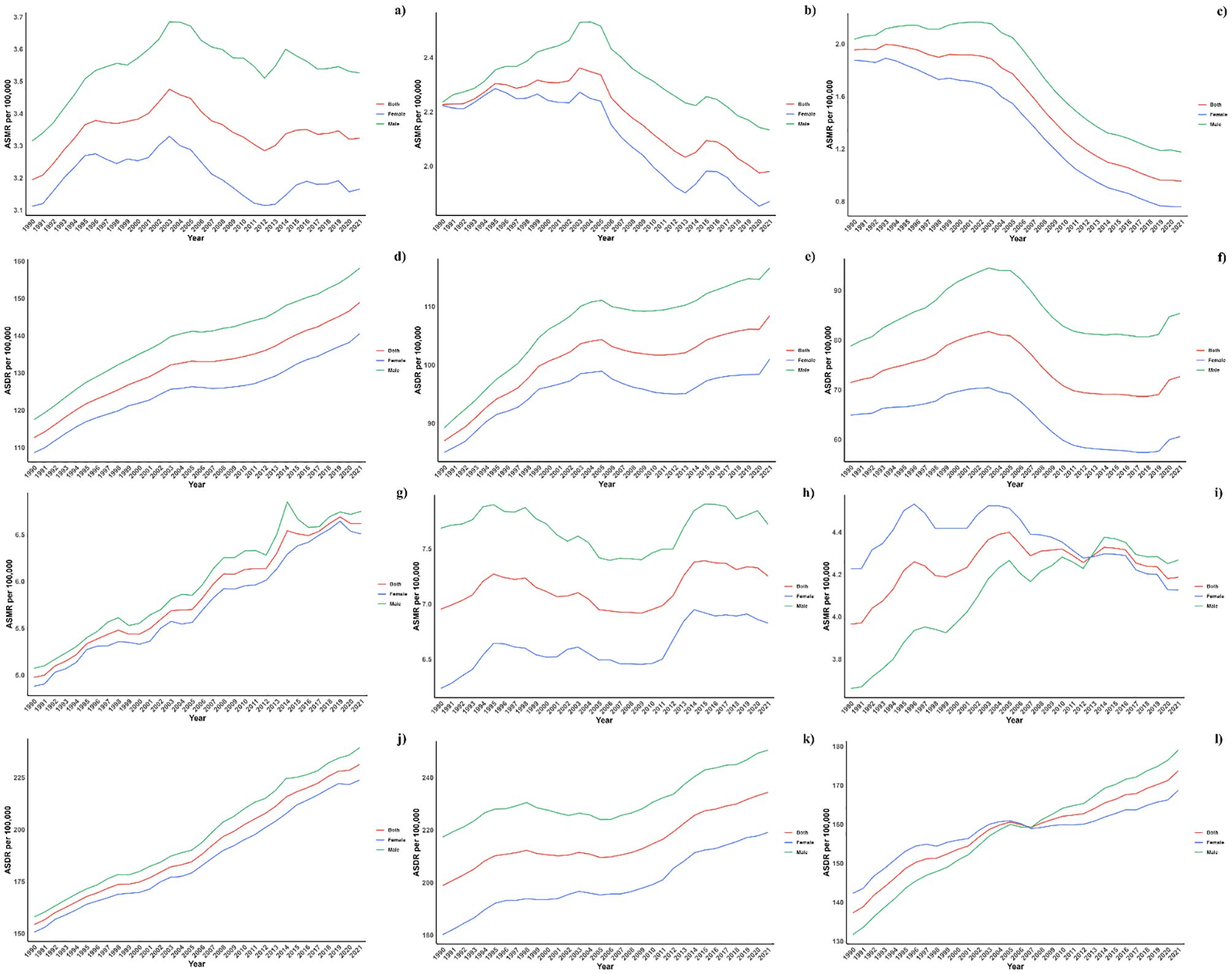
Figure 4. (a) ASMR in diabetes mellitus Burden Attributable to Air Pollution across Global Regions. (b) ASMR in diabetes mellitus Burden Attributable to Air Pollution across High-middle SDI Regions. (c) ASMR in diabetes mellitus Burden Attributable to Air Pollution across High SDI Regions. (d) ASMR in diabetes mellitus Burden Attributable to Air Pollution across Low-middle SDI Regions. (e) ASMR in diabetes mellitus Burden Attributable to Air Pollution across Low SDI Regions. (f) ASMR in diabetes mellitus Burden Attributable to Air Pollution across Middle SDI Regions. (g) ASDR in diabetes mellitus Burden Attributable to Air Pollution across Global Regions. (h) ASDR in diabetes mellitus Burden Attributable to Air Pollution across High-middle SDI Regions. (i) ASDR in diabetes mellitus Burden Attributable to Air Pollution across High SDI Regions. (j) ASDR in diabetes mellitus Burden Attributable to Air Pollution across Low-middle SDI Regions. (k) ASDR in diabetes mellitus Burden Attributable to Air Pollution across Low SDI Regions. (l) ASDR in diabetes mellitus Burden Attributable to Air Pollution across Middle SDI Regions.
3.3 Association with the socio-demographic index
Figure 5 presents a comparison of the observed and forecasted age-standardized DALY (ASDR) and mortality rates (ASMR) due to air pollution, juxtaposed with Socio-Demographic Index (SDI) values at both regional and national levels from 1990 to 2021. A negative correlation was observed between ASDR and SDI, indicating a reduction in burden with increasing SDI. Areas such as Oceania, southern sub-Saharan Africa, and central Latin America recorded higher ASDR than anticipated during this timeframe. Moreover, the observed ASDR figures were higher than projected in western sub-Saharan Africa and Australia. The trends for observed versus projected ASMR based on SDI at regional levels were consistent with the ASDR results. Figure 5 also illustrates the observed versus projected ASDR and ASMR at the national level for 2021, revealing a similar inverse relationship between these rates and SDI, both regionally and nationally.
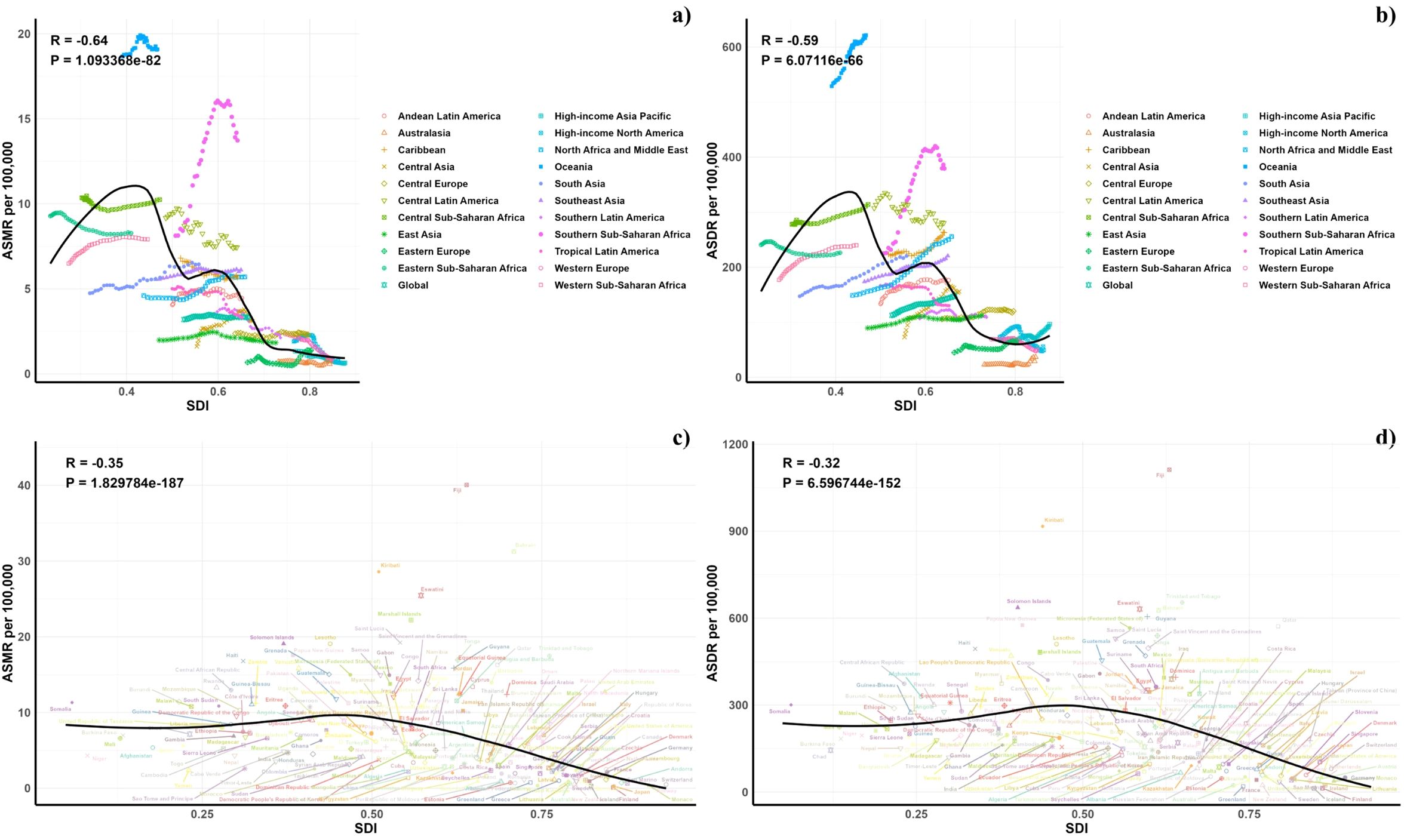
Figure 5. Correlations between ASMR (a, c) and ASDR (b, d) of diabetes mellitus attributable to air pollution and SDI at the regional level (a, b) and the national level (c, d).
3.4 Forecasts for the mortality, DALYs rate, ASMR and ASDR of diabetes burden attributable to air pollution in global (2022–2050)
Projections for mortality rates, DALY rates, ASMR, and ASDR related to CVDs due to air pollution are detailed in Figures 6–8. Regionally, the mortality rate is expected to decline only in the low SDI region. However, age-standardized assessments indicate that the low-middle SDI region is likely to see an increase. For DALY rates and ASDR, all SDI regions, with the exception of the high SDI, are anticipated to experience significant increases in the future (Figure 6).
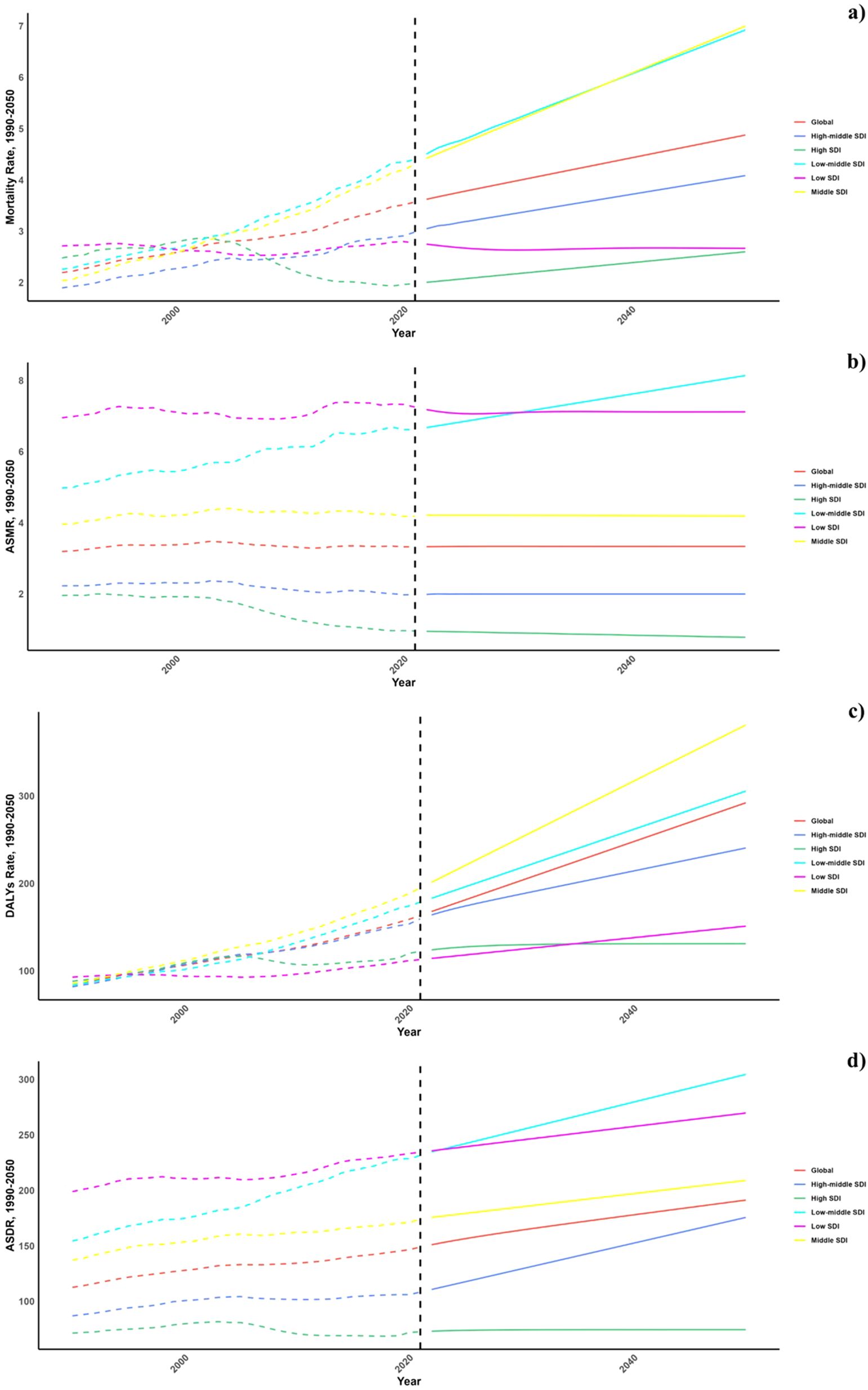
Figure 6. Estimated Trends of Mortality Rate (a), DALYs Rate (b), ASMR (c) and ASDR (d), 1990–2050 at the Regional Level.
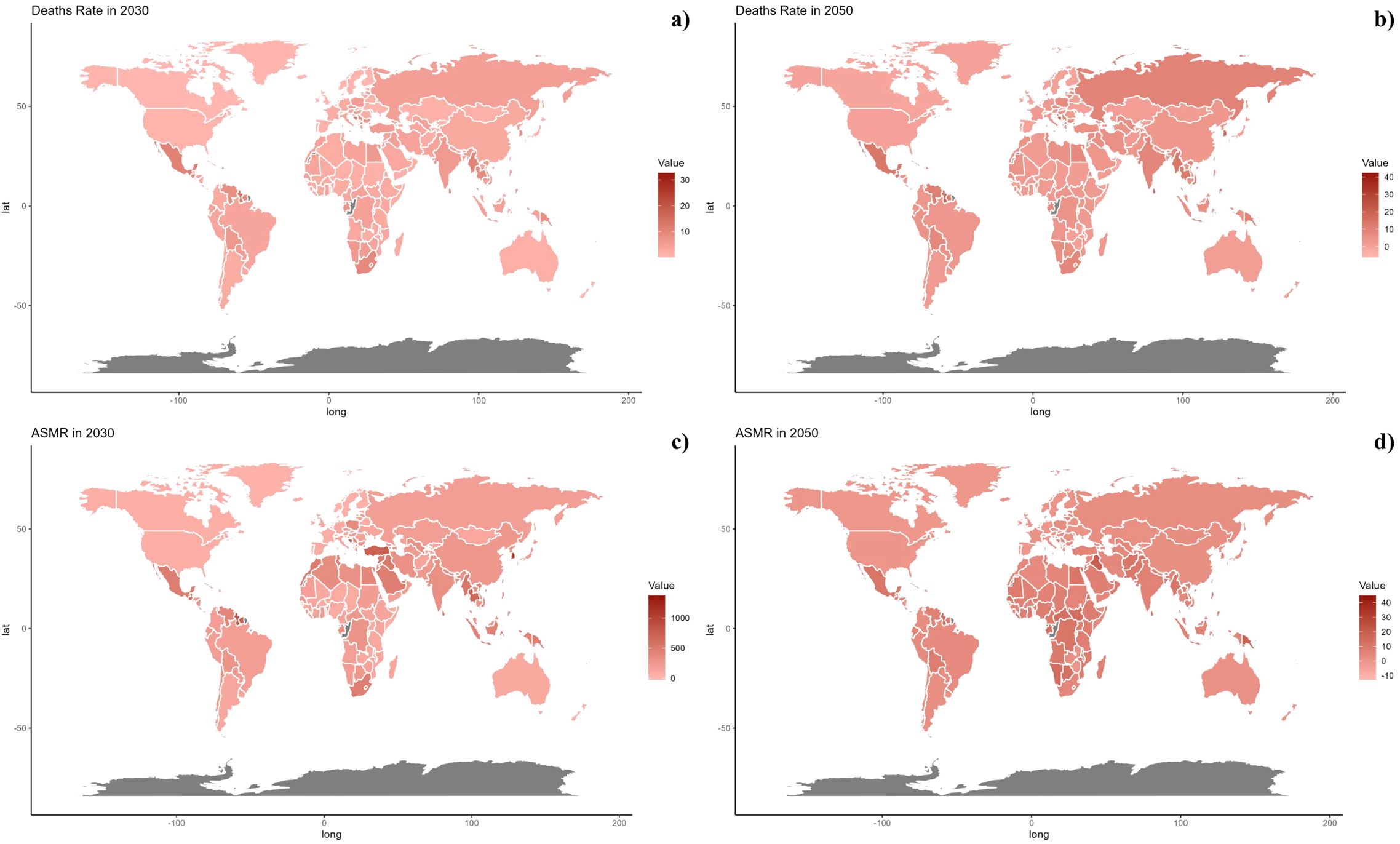
Figure 7. Estimated Trends of Mortality Rate (a, b) and ASMR (c, d) in 2030 (a, c) and 2050 (b, d) at the National Level.
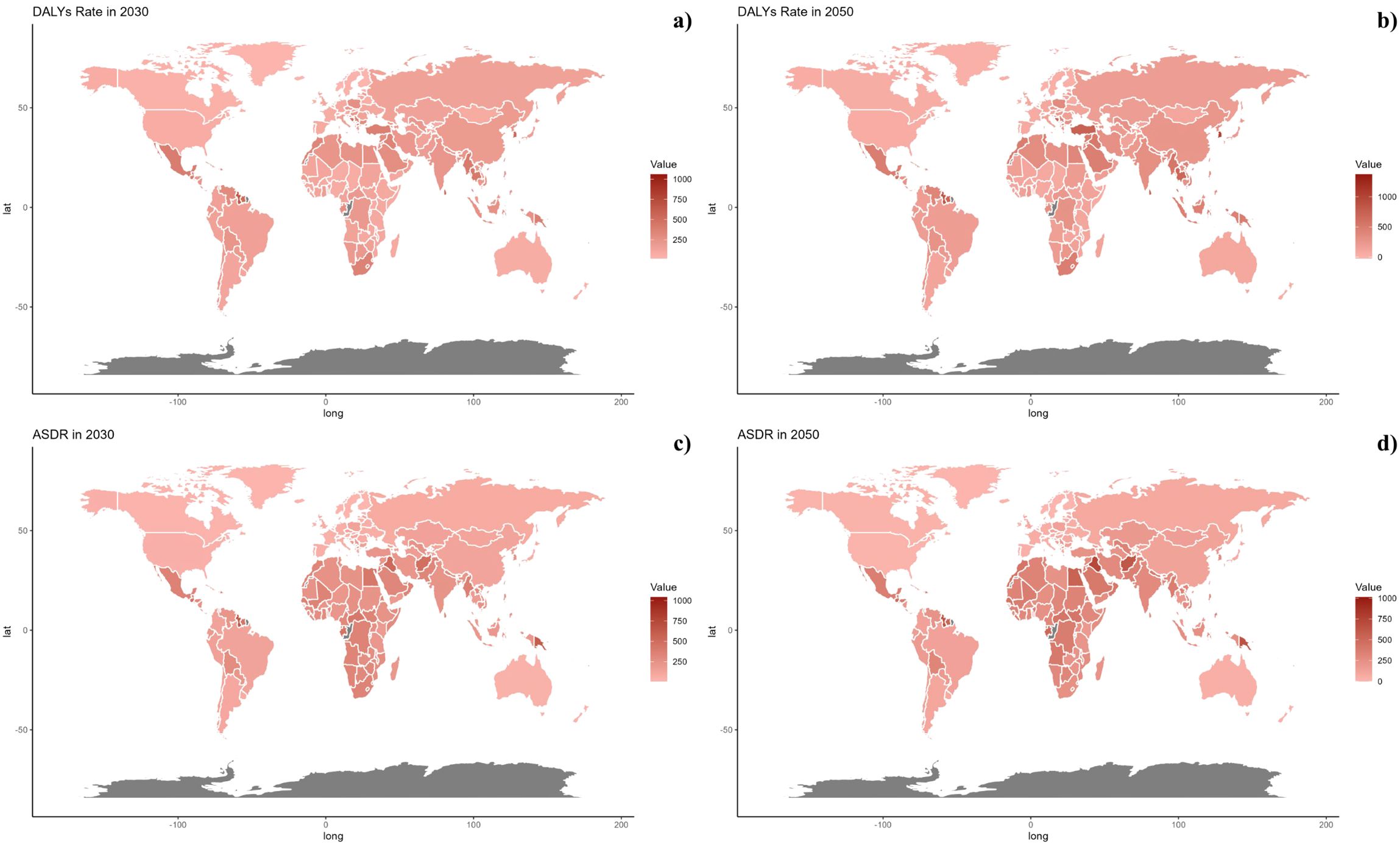
Figure 8. Estimated Trends of DALYs Rate (a, b) and ASDR (c, d) in 2030 (a, c) and 2050 (b, d) at the National Level.
On a national scale, the patterns are expected to stay stable in both 2030 and 2050. However, the projected death rates and ASMR from diabetes mellitus due to air pollution are notably higher in Russia, Mexico, and most African countries compared to others for both 2030 and 2050. In terms of ASDR, nations in South Asia and Africa are expected to witness more significant increases than other countries (Figures 7, 8).
4 Discussion
This study examined the diabetes burden worldwide resulting from air pollution between 1990 and 2021, identifying a significant upward trend during this timeframe. The most impacted demographic included individuals over the age of 60, with males showing higher rates. Projections indicate that while mortality rates might decrease in regions with a low Social Development Index (SDI), standardized mortality rates are expected to rise in lower-middle SDI areas. Moreover, diabetes mortality and age-standardized death rates associated with air pollution are projected to remain elevated in Russia, Mexico, and many African nations, with a notably sharper increase in age-standardized mortality rates expected in South Asia and Africa compared to other regions. To our knowledge, this research is the first to thoroughly assess diabetes burden from air pollution.
In 2017, PM2.5 exposure contributed to 4.58 million deaths and 142.52 million DALYs worldwide (30). While age-standardized rates (ASRs) of deaths and DALYs saw a decline, the decrease was modest on a global scale, with certain regions like Latin America, Africa, South Asia, the Middle East, and Oceania experiencing an increase in the absolute burden as the global population rose from 5.3 billion in 1990 to 7.6 billion in 2017. With rapid industrialization and urbanization, the health impact of ambient PM2.5 in low- and middle-income areas has become increasingly apparent. Concurrently, Cookstove Intervention Programs expanded, reducing household PM2.5 burdens in developing nations, though only 60% of the global population had access to clean fuels (31–34). Significantly, five Asian nations (India, China, Pakistan, Indonesia, and Bangladesh) represented the highest burdens, accounting for over 50% of all deaths and DALYs related to PM2.5.
According to the World Health Organization, as economic conditions and sanitation improved, non-communicable diseases (NCDs) represented over 60% of the global disease burden and contributed to more than 70% of deaths in 2017 (35). Major diseases linked to PM2.5 were among the top 10 causes of death worldwide in 2016 (36). The leading three conditions for PM2.5-associated DALYs were ischemic heart disease (IHD), cerebrovascular disease, and lower respiratory infections (LRI), followed by chronic obstructive pulmonary disease (COPD), diabetes, and lung cancer, all of which ranked in the top 20 causes of global DALYs (37). Our findings indicated an increase in deaths and DALYs from PM2.5-related NCDs particularly among those over 70, while the 14-69 age group largely contributed to DALYs in conditions like stroke, IHD, and diabetes, posing significant challenges for public health and socio-economic development by impacting the labor force.
Studies have shown that economic growth has often led to substantial environmental pollution which adversely affects glucose metabolism, promoting insulin resistance and Type 2 Diabetes Mellitus (T2DM, 39). Meta-analyses have established a positive association between significant air pollutants (like PM2.5, PM10, NO2, and NOX) and T2DM (38). From 2000 to 2015, PM2.5 levels escalated from 31.2 μg/m3 to 97.0 μg/m3 in 15 Chinese provinces and regions (39). Additionally, research has linked a 10 μg/m3 increase in PM2.5 exposure to a 10% heightened risk of developing T2DM (40). Consequently, there is an urgent need to address the issue of diabetes mellitus caused by air pollution.
Furthermore, our studies have shown that East Asia, Southeast Asia, and South Asia bear the greatest burden, with the highest numbers of deaths and DALYs, highlighting that densely populated and rapidly industrializing countries like India and China face disproportionately high disease burdens from PM2.5, exacerbated by significant rural-urban economic disparities and uneven public health resources. Between 2000 and 2010, health welfare costs and losses in labor productivity due to air pollution accounted for 6.5% of China’s GDP annually, and 8.5% of India’s GDP in 2013, while Bangladesh incurs around $6.5 billion annually in related costs. In response, the Chinese government implemented the Air Pollution Prevention and Control Action Plan, revised the Law on the Prevention and Control of Atmospheric Pollution, and took steps such as switching to natural gas for heating, regulating power plants, and limiting vehicle emissions (41–43). Similarly, the Indian government initiated the National Clean Air Program aimed at enhancing air quality monitoring, boosting pollution control capabilities, and reducing PM2.5 and PM10 levels by 20–30% by 2024. These proactive measures toward eco–friendly development are expected to mitigate public health losses from PM2.5, enhance economic dynamism through improved labor productivity and energy efficiency, and assist these countries in breaking out of detrimental cycles toward sustainable development.
Air pollution, especially PM2.5, ozone (O₃), and nitrogen oxides (NOx), can reduce vitamin D synthesis by blocking UVB radiation necessary for its production in the skin. This effect is particularly pronounced in highly polluted urban areas, where low UV exposure contributes to higher rates of vitamin D deficiency. Additionally, pollution–induced inflammation and oxidative stress can impair insulin sensitivity, increasing the risk of diabetes. Both reduced vitamin D levels and increased systemic inflammation from air pollutants may synergistically elevate the risk of type 2 diabetes, highlighting the need for effective environmental and health interventions (44).
Building on our findings, actionable policy recommendations are essential to mitigate the diabetes burden attributable to air pollution. Governments should prioritize stricter air quality regulations and promote cleaner energy alternatives to reduce particulate matter exposure, particularly in high–risk regions. Integrating air pollution mitigation strategies with existing public health programs targeting diabetes prevention could amplify their impact. Additionally, improving urban planning to decrease air pollution in densely populated areas and raising public awareness about the health risks associated with air pollution are critical. Future policies should also emphasize tailored interventions for vulnerable populations, such as the elderly and individuals in lower SDI regions.
In the context of our study findings, the recent public health measures and policy developments in countries like China and India provide insightful examples. China’s Air Pollution Prevention and Control Action Plan and India’s National Clean Air Program (NCAP) aim to significantly reduce PM2.5 and PM10 levels. These initiatives demonstrate a proactive approach to mitigating air pollution and its associated health impacts, including diabetes mellitus. By incorporating these national efforts into our discussion, we can better illustrate the potential for public health policies to influence and possibly reduce the burden of diseases exacerbated by environmental pollutants (45–47).
Our research encounters several notable constraints. Primarily, there is a deficiency of primary data from less developed areas, particularly in Sub–Saharan Africa, where assessments are predominantly based on mathematical modeling, leading to wide ranges of uncertainty. Additionally, air pollution comprises a complex mixture of various components, each possessing unique physicochemical properties and toxic effects that vary significantly across different regions and seasons.
5 Conclusion
This study offers an in–depth analysis of the global burden of diabetes mellitus attributed to air pollution from 1990 to 2021, observing a significant increase over the years. Individuals aged over 60 were the most impacted, with men facing a greater burden. Looking ahead, only regions with a low Socio–Demographic Index (SDI) are expected to see a decrease in mortality rates, whereas those in lower–middle SDI regions will likely witness an increase in standardized mortality rates. On a national scale, countries such as Russia, Mexico, and most of Africa are anticipated to continue experiencing elevated diabetes mortality rates and ASMR due to air pollution up to 2030 and 2050. Both South Asia and Africa are projected to encounter substantial rises in ASDR compared to other areas.
This underscores the urgent necessity for policymakers to devise and refine preventive measures tailored to specific populations to reduce the diabetes burden associated with air pollution in the future.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
QM: Formal analysis, Writing – original draft. XioZ: Data curation, Investigation, Writing – original draft. XinZ: Visualization, Writing – original draft. YK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1475822/full#supplementary-material
References
1. Lingvay I, Sumithran P, Cohen RV, le Roux CW. Obesity management as a primary treatment goal for type 2 diabetes: time to reframe the conversation. Lancet. (2022) 399:394–405. doi: 10.1016/S0140–6736(21)01919–X
2. Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world–a growing challenge. N Engl J Med. (2007) 356:213–5. doi: 10.1056/NEJMp068177
3. Ng ACT, Delgado V, Borlaug BA, Bax JJ. Diabesity: the combined burden of obesity and diabetes on heart disease and the role of imaging. Nat Rev Cardiol. (2021) 18:291–304. doi: 10.1038/s41569–020–00465–5
4. Li Q, Liu F, Huang K, Liang F, Shen C, Liao J, et al. Physical activity, long–term fine particulate matter exposure and type 2 diabetes incidence: A prospective cohort study. Chronic Dis Transl Med. (2024) 10:205–15. doi: 10.1002/cdt3.128
5. Liu M, Tang W, Zhang Y, Wang Y, Kangzhuo B, Li Y, et al. Urban–rural differences in the association between long–term exposure to ambient air pollution and obesity in China. Environ Res. (2021) 201:111597. doi: 10.1016/j.envres.2021.111597
6. Chen S, Liu D, Huang L, Guo C, Gao X, Xu Z, et al. Global associations between long–term exposure to PM2.5 constituents and health: A systematic review and meta–analysis of cohort studies. J Hazard Mater. (2024) 474:134715. doi: 10.1016/j.jhazmat.2024.134715
7. Li X, Wang M, Song Y, Ma H, Zhou T, Liang Z, et al. Obesity and the relation between joint exposure to ambient air pollutants and incident type 2 diabetes: A cohort study in UK Biobank. PloS Med. (2021) 18:e1003767. doi: 10.1371/journal.pmed.1003767
8. Chen H, Burnett RT, Kwong JC, Villeneuve PJ, Goldberg MS, Brook RD, et al. Risk of incident diabetes in relation to long–term exposure to fine particulate matter in Ontario, Canada. Environ Health Perspect. (2013) 121:804–10. doi: 10.1289/ehp.1205958
9. Hotamisligil GS. Inflammation and metabolic disorders. Nature. (2006) 444:860–7. doi: 10.1038/nature05485
10. Sun Q, Yue P, Deiuliis JA, Lumeng CN, Kampfrath T, Mikolaj MB, et al. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet–induced obesity. Circulation. (2009) 119:538–46. doi: 10.1161/CIRCULATIONAHA.108.799015
11. Si H, Gao T, Yang J, Zhu J, Han Y, Li C, et al. Multi–omics reveals hypertrophy of adipose tissue and lipid metabolism disorder via mitochondria in young mice under real–ambient exposure to air pollution. Front Pharmacol. (2023) 14:1122615. doi: 10.3389/fphar.2023.1122615
12. Hassan F, Hatah E, Chong WW, Ali AM. Impact of attendance to a pharmacist–managed medication adherence clinic on glycemic control and risk factors for non–completion among persons with type 2 diabetes mellitus in selangor, Malaysia. Ther Clin Risk Manage. (2024) 20:495–503. doi: 10.2147/TCRM.S442026
13. Khobrani FM, Alzahrani AM, Binmahfoodh DS, Hemedy RA, Abbas SI. Risk factors and diagnostic performance of predictors as a screening technique for gestational diabetes mellitus: a retrospective cross–sectional study. Ann Med Surg (Lond). (2024) 86:4384–8. doi: 10.1097/MS9.0000000000002247
14. Liang W, Zhu H, Xu J, Zhao Z, Zhou L, Zhu Q, et al. Ambient air pollution and gestational diabetes mellitus: An updated systematic review and meta–analysis. Ecotoxicol Environ Saf. (2023) 255:114802. doi: 10.1016/j.ecoenv.2023.114802
15. Goldney J, Henson J, Edwardson CL, Khunti K, Davies MJ, Yates T. Long–term ambient air pollution exposure and prospective change in sedentary behaviour and physical activity in individuals at risk of type 2 diabetes in the UK. J Public Health (Oxf). (2024) 46:e32–42. doi: 10.1093/pubmed/fdad263
16. Safiri S, Mahmoodpoor A, Kolahi AA, Nejadghaderi SA, Sullman MJM, Mansournia MA, et al. Global burden of lower respiratory infections during the last three decades. Front Public Health. (2023) 10:1028525. doi: 10.3389/fpubh.2022.1028525
17. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/S0140–6736(20)30925–9
18. Foreman KJ, Lozano R, Lopez AD, Murray CJ. Modeling causes of death: an integrated approach using CODEm. Popul Health Metr. (2012) 10:1. doi: 10.1186/1478–7954–10–1
19. Jin X, Ren J, Li R, Gao Y, Zhang H, Li J, et al. Global burden of upper respiratory infections in 204 countries and territories, from 1990 to 2019. EClinicalMedicine. (2021) 37:100986. doi: 10.1016/j.eclinm.2021.100986
20. Lu H, Tan Z, Liu Z, Wang L, Wang Y, Suo C, et al. Spatiotemporal trends in stroke burden and mortality attributable to household air pollution from solid fuels in 204 countries and territories from 1990 to 2019. Sci Total Environ. (2021) 775:145839. doi: 10.1016/j.scitotenv.2021.145839
21. Safiri S, Kolahi AA, Hoy D, Buchbinder R, Mansournia MA, Bettampadi D, et al. Global, regional, and national burden of neck pain in the general population, 1990–2017: systematic analysis of the Global Burden of Disease Study 2017. BMJ. (2020) 368:m791. doi: 10.1136/bmj.m791
22. Hu J, Ke R, Teixeira W, Dong Y, Ding R, Yang J, et al. Global, regional, and national burden of CKD due to glomerulonephritis from 1990 to 2019: A systematic analysis from the global burden of disease study 2019. Clin J Am Soc Nephrol. (2023) 18:60–71. doi: 10.2215/CJN.0000000000000017
23. Zhu B, Wang Y, Zhou W, Jin S, Shen Z, Zhang H, et al. Trend dynamics of gout prevalence among the Chinese population, 1990–2019: A joinpoint and age–period–cohort analysis. Front Public Health. (2022) 10:1008598. doi: 10.3389/fpubh.2022.1008598
24. Mao Q, Zhu X, Zhang X, Kong Y. Effect of air pollution on the global burden of cardiovascular diseases and forecasting future trends of the related metrics: a systematic analysis from the Global Burden of Disease Study 2021. Front Med (Lausanne). (2024) 11:1472996. doi: 10.3389/fmed.2024.1472996
25. Mao Q, Zhu X, Zhang X, Kong Y. Triglyceride–glucose Index and Its combination with obesity indicators mediating the association between 2–hydroxyfluorene and the prevalence of cardiovascular disease: Evidence from the NHANES (2005–2018). Ecotoxicol Environ Saf. (2024) 287:117283. doi: 10.1016/j.ecoenv.2024.117283
26. Mao Q, Zhu X, Kong Y. Sleep duration mediates the association between heavy metals and the prevalence of depression: an integrated approach from the NHANES (2005–2020). Front Psychiatry. (2024) :1455896. doi: 10.3389/fpsyt.2024.1455896
27. Kong Y, Zhu X, Yang Y, Xu H, Ma L, Zuo Y. Knowledge, attitudes, practice, and public health education demand regarding PARI prevention: a cross–sectional study among Chinese undergraduates. Front Public Health. (2024) 12:1387789. doi: 10.3389/fpubh.2024.1387789
28. Kong Y, Xu H, Li C, Yang Y, Zhu X, Zuo Y. Construction of PARI public health education programs for Chinese undergraduates: a Delphi study. Front Public Health. (2024) 12:1390011. doi: 10.3389/fpubh.2024.1390011
29. Cheng Z, Kong Y, Yang W, Xu H, Tang D, Zuo Y. Association between serum copper and blood glucose: a mediation analysis of inflammation indicators in the NHANES (2011–2016). Front Public Health. (2024) 12:1401347. doi: 10.3389/fpubh.2024.1401347
30. Bu X, Xie Z, Liu J, Wei L, Wang X, Chen M, et al. Global PM2.5–attributable health burden from 1990 to 2017: Estimates from the Global Burden of disease study 2017. Environ Res. (2021) 197:111123. doi: 10.1016/j.envres.2021.111123
31. Hystad P, Larkin A, Rangarajan S, AlHabib KF, Avezum Á, Calik KBT, et al. Associations of outdoor fine particulate air pollution and cardiovascular disease in 157 436 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet Planetary Health. (2020) 4(6):e235–45. doi: 10.1016/S2542-5196(20)30103-0
32. Arku RE, Birch A, Shupler M, Yusuf S, Hystad P, Brauer M. Characterizing exposure to household air pollution within the Prospective Urban Rural Epidemiology (PURE) study. Environ Int. (2018) 114:307–17. doi: 10.1016/j.envint.2018.02.033
33. Qingsong M, Xiao R, Yang W, Wang X, Kong YZ. Global burden of pneumoconiosis attributable to occupational particulate matter, gasses, and fumes from 1990~2021 and forecasting the future trends: a population-based study. Front Public Health. (2025) 12:1494942. doi: 10.3389/fpubh.2024.1494942
34. WHO, Chowdhury S. Dey World population prospects: the 2017 revision. Environ Int. (2016) 91:283–90.
35. WHO. World population prospects: the 2017 revision . Available online at: http://www.who.int/phe/health_topics/outdoorair/databases/cities/en/.
36. WHO Global Health. Estimates 2016: disease burden by cause, age, sex, by country and by region, 2000–2016. Geneva: World Health Organization (2018).
37. Global, regional, and national disability–adjusted life–years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. (2017) 390:1260–344.
38. Balti EV, Echouffo–Tcheugui JB, Yako YY, Kengne AP. Air pollution and risk of type 2 diabetes mellitus: a systematic review and meta–analysis. Diabetes Res Clin Pract. (2014) 106:161–72. doi: 10.1016/j.diabres.2014.08.010
39. Huang K, Liang F, Yang X, Liu F, Li J, Xiao Q, et al. Long term exposure to ambient fine particulate matter and incidence of stroke: prospective cohort study from the China–PAR project. BMJ. (2019) 367:l6720. doi: 10.1136/bmj.l6720
40. Eze IC, Hemkens LG, Bucher HC, Hoffmann B, Schindler C, Künzli N, et al. Association between ambient air pollution and diabetes mellitus in Europe and North America: systematic review and meta–analysis. Environ Health Perspect. (2015) 123:381–9. doi: 10.1289/ehp.1307823
41. Miller L, Xu X. Ambient PM2.5 human health effects—findings in China and research directions. Atmosphere. (2018) 9:424. doi: 10.3390/atmos9110424
42. Zou B, Li S, Lin Y, Wang B, Cao S, et al. Efforts in reducing air pollution exposure risk in China: state versus individuals. Environ Int. (2020) 137:105504. doi: 10.1016/j.envint.2020.105504
43. Yue H, He C, Huang Q, Yin D, Bryan BA. Stronger policy required to substantially reduce deaths from PM2.5 pollution in China. Nat Commun. (2020) 11(1):1462. doi: 10.1038/s41467-020-15319-4
44. Trifan DF, Tirla AG, Moldovan AF, Moș C, Bodog F, Maghiar TT, et al. Can vitamin D levels alter the effectiveness of short–term facelift interventions? Healthcare (Basel). (2023) 11:1490. doi: 10.3390/healthcare11101490
45. Sasso FC, Pafundi PC, Caturano A, Galiero R, Vetrano E, Nevola R, et al. Impact of direct acting antivirals (DAAs) on cardiovascular events in HCV cohort with pre–diabetes. Nutr Metab Cardiovasc Dis. (2021) 31:2345–53. doi: 10.1016/j.numecd.2021.04.016
46. Sasso FC, Pafundi PC, Gelso A, Bono V, Costagliola C, Marfella R, et al. Relationship between albuminuric CKD and diabetic retinopathy in a real–world setting of type 2 diabetes: Findings from No blind study. Nutr Metab Cardiovasc Dis. (2019) 29:923–30. doi: 10.1016/j.numecd.2019.05.065
Keywords: diabetes, air pollution, mortality forecasting, epidemiology, disease burden
Citation: Mao Q, Zhu X, Zhang X and Kong Y (2025) Diabetes burden attributable to air pollution from 1990~2021 and the future trends: a population-based study. Front. Endocrinol. 16:1475822. doi: 10.3389/fendo.2025.1475822
Received: 04 August 2024; Accepted: 07 March 2025;
Published: 08 April 2025.
Edited by:
Pedro M. Baptista, Health Research Institute of Aragon (IIS Aragon), SpainReviewed by:
Luca Rinaldi, University of Molise, ItalyCosmin Mihai Vesa, University of Oradea, Romania
Jiaman Xu, Boston University, United States
Farhad Saravani, Tehran University of Medical Sciences, Iran
Copyright © 2025 Mao, Zhu, Zhang and Kong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuzhe Kong, Y3N1eXV6aGVrb25nQGZveG1haWwuY29t
 Qingsong Mao
Qingsong Mao Xiaoyi Zhu2
Xiaoyi Zhu2 Yuzhe Kong
Yuzhe Kong