
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 27 January 2025
Sec. Reproduction
Volume 16 - 2025 | https://doi.org/10.3389/fendo.2025.1460976
This article is part of the Research Topic Lifestyle and Environmental Factors and Human Fertility View all 16 articles
 Miaoxin Chen1†
Miaoxin Chen1† Qiaoyu Chen1†
Qiaoyu Chen1† Gengze Liao2†
Gengze Liao2† Chunyan Sun1
Chunyan Sun1 Cong Liu3
Cong Liu3 Xia Meng3
Xia Meng3 Wentao Li4
Wentao Li4 Andong Qiu1
Andong Qiu1 Orhan Bukulmez5
Orhan Bukulmez5 Haidong Kan3
Haidong Kan3 Feng Wang2
Feng Wang2 Lap Ah Tse2*
Lap Ah Tse2* Xiaoming Teng1*
Xiaoming Teng1*Background: Excessive exposure to PM2.5 can be detrimental to reproductive health. The objective of this study was to investigate the potential associations between ambient PM2.5 exposure during different periods and negative pregnancy outcomes, such as miscarriage and preterm birth, in patients who underwent assisted reproductive technology (ART).
Methods: This retrospective cohort study examined the outcomes of 2,839 infertile women aged ≤ 45 years who underwent their first fresh or frozen-thawed embryo transfer at the Shanghai First Maternity and Infant Hospital between April 2016 and December 2019. Satellite data were used to determine the daily average levels of PM2.5, and exposure was categorized as excessive if it exceeded the WHO’s interim target 2 level of 50 µg/m3. The analysis was conducted separately for seven different periods. Our study used multinomial logistic regression models to explore the potential associations between PM2.5 exposure and adverse pregnancy outcomes. Sensitivity analysis was conducted by excluding women who underwent blastocyst transfer.
Results: Daily PM2.5 exposure exceeding the threshold (50 µg/m3) was associated with an increased risk of miscarriage during the period after confirmation of clinical pregnancy or biochemical pregnancy, with adjusted odds ratios (AORs) of 2.22 (95% CI 1.75-2.81) and 2.23 (95% CI 1.68-2.96), respectively. Moreover, for each increase of 10 µg/m3 above the threshold for PM2.5, there was a 46% elevated risk of preterm birth (AOR = 1.46, 95% CI 1.09-1.94) during the period after the confirmation of clinical pregnancy and a 61% elevated risk of preterm birth (AOR = 1.61, 95% CI 1.16-2.23) during the period after the confirmation of biochemical pregnancy. Our stratified analyses revealed that women with an endometrial thickness <11 mm or who underwent frozen embryo transfer were more vulnerable to PM2.5 exposure, leading to higher rates of preterm birth.
Conclusion: Excessive PM2.5 exposure after biochemical pregnancy or clinical pregnancy was associated with increased risks of preterm birth and miscarriage among women who underwent ART.
Ambient fine particulate matter with an aerodynamic diameter ≤ 2.5 μm (PM2.5) poses a health threat to populations worldwide and has detrimental effects on multiple organs and systems, including the reproductive system (1, 2). In addition, physiological changes during pregnancy make pregnant women particularly susceptible to the negative health impacts of PM2.5 (3). Previous research has indicated a link between PM2.5 and potential risks to fecundability and fertility (4, 5), as well as unfavorable pregnancy outcomes such as miscarriage (6), fetal growth restriction (7), low birth weight, and preterm birth (3, 8, 9). However, cohort studies regarding PM2.5 exposure and pregnancy outcomes were mainly conducted in women who conceived naturally (2, 7, 8). However, there is a gap in knowledge concerning the potential link between PM2.5 exposure and adverse pregnancy outcomes among women undergoing ART treatment.
The estimated rate of infertility among couples of reproductive age in China is as high as 25%, which exceeds the global average of 15% (10). An increasing number of infertile couples are undergoing ART treatments to conceive and have children, with in vitro fertilization (IVF) being the most commonly utilized method (11). Previous studies have demonstrated that women who conceive through IVF may be more vulnerable to ambient air pollution, including PM2.5 exposure, than women who conceive naturally (12), with a decreased probability of oocyte yield, clinical pregnancy and live birth among women with high ambient air pollution during pregnancy (11, 13–21). Limited research has been conducted on the potential link between PM2.5 exposure and adverse pregnancy outcomes, including preterm birth and pregnancy loss, in women undergoing ART.
Preterm birth and pregnancy loss, including miscarriage and biochemical pregnancy loss, are major adverse pregnancy outcomes of IVF treatment for patients who have a positive human chorionic gonadotropin (hCG) test (22). Nevertheless, there is a lack of research on the relationship between exposure to PM2.5 and preterm birth and/or pregnancy loss in women undergoing IVF, and the findings are inconclusive (12, 19, 23). In a prospective study of women in the United States, chronic daily PM2.5 exposure (mean concentration of 8.8 μg/m3) from the date of the first positive hCG test until the date of pregnancy loss or live birth was not significantly associated with pregnancy loss (19). The lack of a significant association might be due to the lower concentration of PM2.5 exposure, which was within the normal level of the WHO’s 24-hour air quality guidelines (PM2.5 < 15 μg/m3) (19, 24). In contrast, another prospective cohort study in China showed a significant increase in the likelihood of biochemical pregnancy loss for every 10 μg/m3 increase in PM2.5 exposure from the time of hCG testing to 30 days after embryo transfer. The average daily PM2.5 exposure of patients in this study was 45.4 μg/m3 (25). The available evidence on the link between preterm birth and excessive PM2.5 exposure is scarce. A retrospective study in Hangzhou of China, revealed that daily PM2.5 exposure at a mean level of 36.0 μg/m3 was significantly associated with an increased risk of preterm birth in all periods of pregnancy (from 85 days before oocyte retrieval to delivery outcome) among the ART population (23). However, a national cohort study in China reported that an increased risk of preterm birth in newborns conceived by ART was only linked to excessive PM2.5 exposure (median 55.0 μg/m3) during the third trimester (12).
Therefore, the current understanding of the association between PM2.5 exposure and adverse pregnancy outcomes, such as preterm birth and pregnancy loss, in women undergoing IVF is incomplete and uncertain. Moreover, there is insufficient evidence to determine which stage of pregnancy after IVF treatment is most susceptible to adverse pregnancy outcomes resulting from high levels of PM2.5 exposure. Our study aimed to investigate the effects of PM2.5 exposure on adverse pregnancy outcomes (i.e., pregnancy loss and preterm birth) between different pregnancy periods among women who underwent ART.
This was a retrospective cohort study. The subjects of this study were women residing in Shanghai who were transferred their first fresh or frozen embryos at the Centre for Assisted Reproduction of Shanghai First Maternity and Infant Hospital from April 2016 to December 2019. The following patients who underwent ART at our center were not included in this study: (1) were aged more than 45 years, (2) used donor semen, (3) underwent preimplantation genetic testing, (4) exhibited oocyte maturation in vitro, (5) had an endometrial thinness <8 mm with fresh embryo transfer, and (6) had an endometrial thinness <7 mm with frozen embryo transfer. Figure 1 shows the flowchart outlining the recruitment and follow-up of the study participants. This study received approval from the Research Ethics Committee of Shanghai First Maternity and Infant Hospital (KS22298).
The center followed a standard operating procedure (SOP) to administer controlled ovarian stimulation (COS) treatment to all participants. The details of the COS protocol were described previously (21). ART procedures, including semen preparation, conventional IVF or intracytoplasmic sperm injection (ICSI) processes, embryo culture, and assessment, were performed as described previously (26). According to the Center’s SOP, women who underwent fresh embryo transfer received one or two high-quality embryos three or five days after oocyte retrieval with the guidance of transabdominal ultrasound. Luteal phase support was initiated on the day of oocyte retrieval (27). The procedures for endometrial preparation and luteal phase support in frozen-thawed embryo transfer have been previously described (21). Women with a positive hCG test continued to receive luteal-phase support until ten weeks of pregnancy.
The presence of biochemical pregnancy was determined by a serum hCG level exceeding 10 mIU/ml two weeks after embryo transfer (16). The term biochemical pregnancy loss refers to an early pregnancy loss that does not develop into a clinical pregnancy (28). All pregnant women were followed up until delivery or miscarriage. Clinical pregnancy was indicated upon the identification of a gestational sac through ultrasound four weeks after embryo transfer. Miscarriage was defined as the spontaneous loss of an intrauterine pregnancy before the 20th week of gestation (29). Live birth was defined as the delivery of one or more living infants with a gestational age of 20 weeks or more or a birth weight exceeding 1,000 g (30). Delivery between 20 and 37 weeks of gestation was classified as preterm birth, while delivery at 37 weeks of gestation or later was considered full-term birth (31).
As previously described, we retrieved covariate data from medical records at the Centre, which included information on the age of the females, age of the males, body mass index (BMI), address, level of education, employment status, duration and causes of infertility, COS method, duration of ovarian stimulation, dosage of gonadotropin stimulation used, progesterone level on the trigger day, total number of oocytes retrieved, method used for fertilization, thickness of the endometrial lining on the day the embryo was transferred, year and season of embryo transfer, number of transferred embryos and whether the transferred embryos were fresh or frozen (21).
As shown in Figure 2, we used 7 periods of IVF treatment and pregnancy to study the effects of daily exposure to PM2.5 on the outcomes of IVF therapy. The periods were as follows: period 1 included the time between the 3 months prior to oocyte retrieval and gonadotropin-induced ovarian stimulation; period 2 included the time between gonadotropin-induced ovarian stimulation and oocyte retrieval; period 3 included the total 3 months preceding oocyte retrieval; period 4 included the time from embryo transfer to the time of serum hCG testing; period 5 followed from the hCG serum test to the time of ultrasound testing to confirm intrauterine pregnancy; period 6 included the time of ultrasound testing to the time of delivery or miscarriage; and finally, period 7 included the whole period between the hCG serum testing stage and the time of delivery or miscarriage.
Following an established method (31, 32), we used a satellite-based model with a spatial resolution of 1x1 km to estimate the daily concentrations of PM2.5 during each of the seven periods studied. This was done utilizing a random forest algorithm where the PM2.5 measurements of >1,500 ground-based monitoring sites between 2013 and 2019 were used as the dependent variable, and the multiangle implementation of atmospheric correction (MAIAC) aerosol optical depth (AOD) retrieval data were used as the independent variables. We also utilized additional predictors, including population density, land use data, meteorological variables, and the Modern-Era Retrospective Analysis for Research and Applications (MERRA-2) PM2.5 products. In terms of missing AOD data, we approached this issue through a gap-filling method where estimated PM2.5 data were generated by combining findings from models with and without AOD data. Each participant had their daily mean PM2.5 concentration assigned by connecting the 1x1 km grid data to their residential address geocodes (32).
One-way ANOVA was utilized to assess variations among continuous variables that followed a normal distribution, while the Kruskal−Wallis test was used for continuous variables with a skewed distribution. The chi-square test and Fisher’s exact test were applied to assess the categorical variables between different IVF/ICSI outcomes or time periods.
The average daily PM2.5 concentration among our participants across the study period was 36.9 µg/m3, which is close to the current suggested Air Quality Guidelines (AQGs) 24-hour interim target 3 (i.e., 37.5 µg/m3) (33)). We also plotted a series of model deviances for the association between the average PM2.5 concentration and IVF outcomes and found that the minimum deviance values were greater than 40 µg/m3 (Supplementary Figure 1). Therefore, we used the 24-hour interim target 2 (50 µg/m3) (34) as a threshold for excessive PM2.5 exposure (24).
Multinomial logistic regression analysis was conducted to determine the adjusted odds ratios (AORs) and their corresponding 95% confidence intervals (95% CIs) for the associations between PM2.5 exposure and pregnancy outcomes among women receiving infertility treatments. PM2.5 was included in the models as a continuous variable representing concentrations exceeding 50 μg/m³, and the AORs represented the change in odds of adverse outcomes per 10 μg/m³ increase in PM2.5 above this threshold. To address potential confounding factors, we adjusted for both known and potential variables in our multinomial regression models, as these variables have been identified as potential risk factors for IVF pregnancy outcomes (35, 36). The previous study have suggested that clinical pregnancy and live birth rates declined with each millimeter below 8 mm in fresh IVF-ET and below 7 mm in frozen-ET (37). To validate the estimates, we performed sensitivity analyses by excluding women who underwent blastocyst transfer. R statistical software version 3.6.3, with the ‘nnet’ package used in multinomial logistic regression, was used for conducting the statistical analyses. A two-tailed p value of less than 0.05 was considered to indicate statistical significance.
A total of 2,839 women whose mean age was 32.7 ± 3.9 years were included in the final data analysis. The majority (69.1%) of participants were treated with fresh embryo transfer, while 30.9% received frozen embryo transfer. Table 1 displays the demographic and clinical characteristics of the participants using descriptive statistics. According to pregnancy outcomes, participants were classified into five groups: full-term birth (34.0% of cases), preterm birth (5.9% of cases), miscarriage (6.4% of cases), biochemical pregnancy loss (6.5% of cases), and nonpregnancy (47.0% of cases).
The daily PM2.5 concentrations of the participants during different periods are shown in Table 2. There were significant differences in the average daily PM2.5 concentration among women with distinct pregnancy outcomes during Period 4 (from embryo transfer to the serum hCG test), Period 6 (after confirmation of clinical pregnancy), and Period 7 (after confirmation of biochemical pregnancy). The results displayed in Table 3 demonstrate a significant correlation between each 10 µg/m3 increase in PM2.5 above the threshold of 50 µg/m3 and increased risk of miscarriage (AOR = 2.22; 95% CI: 1.75-2.81 for Period 6; AOR = 2.23; 95% CI: 1.68-2.96 for Period 7), using full-term birth as the reference group (Table 3). In addition, excessive PM2.5 pollution above the 50 µg/m3 threshold was associated with a 46% increase in the risk of preterm birth (AOR = 1.46, 95% CI: 1.09-1.94) during period 6 and a 61% increase in the risk of preterm birth (AOR = 1.61, 95% CI: 1.16-2.23) during period 7.
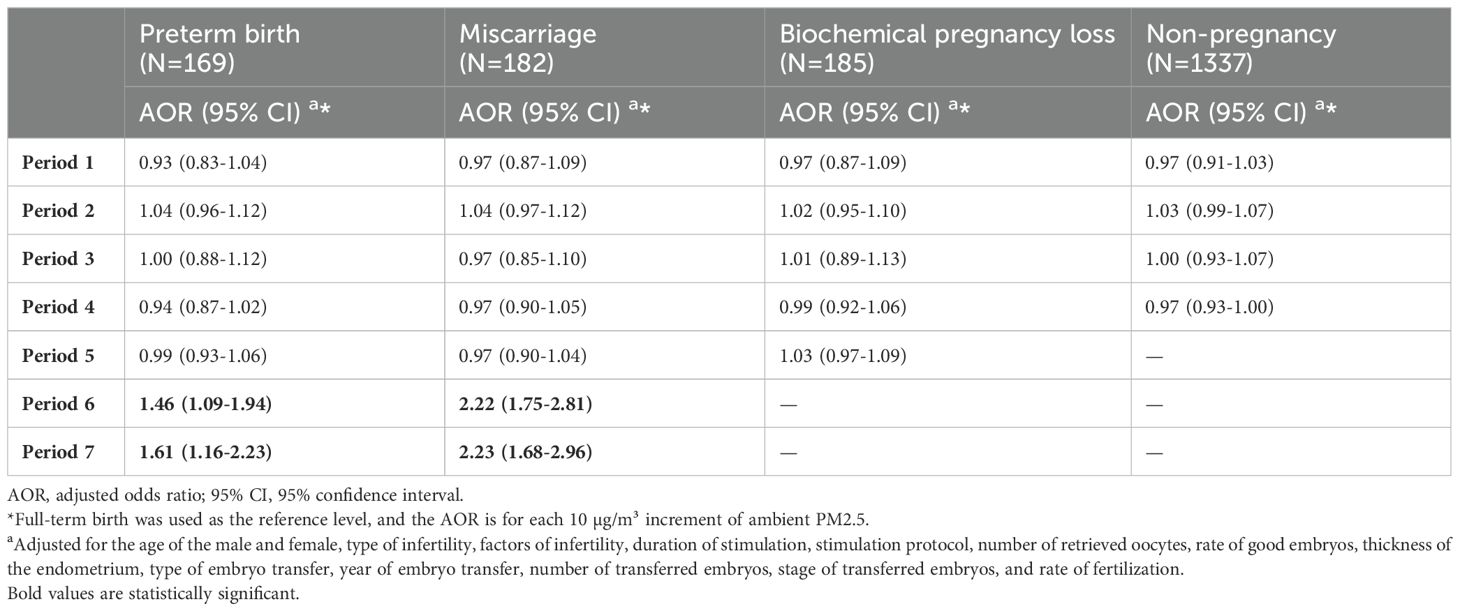
Table 3. Associations of daily PM2.5 above the WHO interim target 2 in different periods with IVF outcomes among participants in Shanghai.
We examined whether the estimated associations between PM2.5 and preterm birth and miscarriage differed among subgroups in Period 7. As shown in Figure 3, the estimated association with preterm birth varied by endometrial thickness and type of embryo transfer. Women with an endometrial thickness <11 mm or frozen embryo transfer were more susceptible to the adverse effects of a 10 µg/m3 increase in PM2.5 above the threshold on preterm birth rates; however, there was no significant variation in the association between excessive PM2.5 exposure and miscarriage among these subgroups for Period 7. The association remained almost unchanged in the sensitivity analyses (Supplementary Table 1).
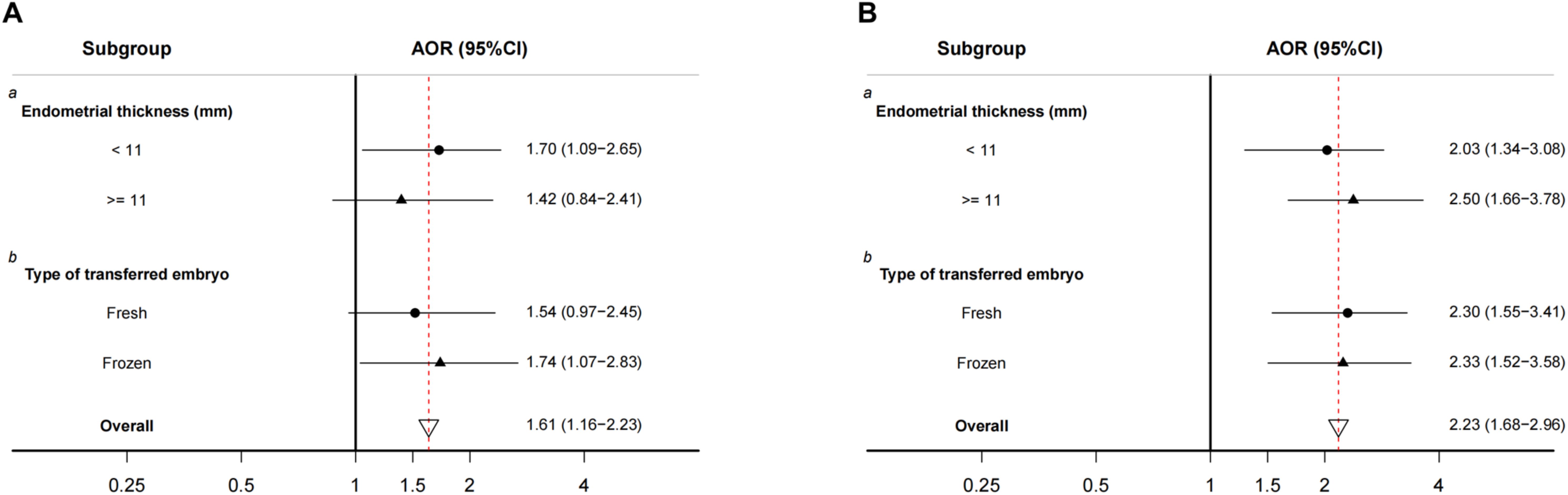
Figure 3. Stratified analyses of associations between daily PM2.5 above the WHO interim target 2 in period 7, (A) preterm birth and (B) miscarriage. AOR, adjusted odds ratio; 95% CI, 95% confidence interval. The full-term birth group was used as a reference. AOR for each 10 µg/m³ increment of ambient PM2.5. a Adjusted for the age of male and female, type of infertility, factors of infertility, duration of stimulation, stimulation protocol, number of retrieved oocytes, rate of good embryos, type of embryo transfer, year of embryo transfer, number of transferred embryos, stage of transferred embryos, and rate of fertilization. b Adjusted for the age of the male and female, type of infertility, factors of infertility, duration of stimulation, stimulation protocol, number of retrieved oocytes, rate of good embryos, thickness of the endometrium, year of embryo transfer, number of transferred embryos, stage of transferred embryos, and rate of fertilization.
The findings of our study suggest a significant association between maternal exposure to high levels of ambient PM2.5 (above 50 µg/m3) after confirmation of biochemical pregnancy or clinical pregnancy and increased risks of miscarriage and preterm birth in women who underwent ART treatment. Moreover, a significant association between PM2.5 exposure and miscarriage was observed in all subgroups, covering diverse endometrial thickness ranges and types of embryo transfer, whereas the detrimental effects on preterm birth were more pronounced among women with endometrial thickness <11 mm or who underwent frozen embryo transfer.
The relationships between exposure to PM2.5 and adverse pregnancy outcomes, such as preterm birth and pregnancy loss, among women undergoing IVF treatment are still not fully understood. The existing research presents varying findings, with some studies suggesting a potential association between PM2.5 exposure and adverse outcomes, while others do not find significant correlations (12, 19, 23, 25). Factors such as the concentration and duration of PM2.5 exposure, as well as geographical location, may influence the outcomes observed. Studies conducted in areas with higher levels of PM2.5 pollution tend to show a stronger association with adverse pregnancy outcomes (13, 23, 25).
Only a few population-based studies have examined the effects of maternal exposure to PM2.5 on miscarriage (38). A retrospective cohort study conducted in a naturally conceived population demonstrated that miscarriage was associated with maternal acute exposure to ambient PM2.5 during the four weeks after conception (38). Furthermore, a significant relationship was observed between PM2.5 and fetal death caused by miscarriage (39). However, the effect of PM2.5 exposure in the ART population on the incidence of miscarriage is unclear. We found that ambient PM2.5 exposure after the confirmation of biochemical or clinical pregnancy was associated with a greater risk of miscarriage in infertile women undergoing IVF cycles. In contrast, two multicenter retrospective cohort studies in the USA and China suggested that PM2.5 was not associated with a decreased risk of live birth or increased risk of pregnancy loss in women who underwent ART (18, 20). The discrepancy in the literature may be due to differences in study design (exposure at patients’ address or IVF unit), air pollution monitoring mode (monitoring station or different validated models), different IVF cycle protocols among the centers, PM2.5 concentrations and organic composition (high or low in different regions), and demographic characteristics of the study population.
The global number of preterm births associated with PM2.5 was estimated to be approximately 2.7 million in 2010 (40). Preterm birth is a major contributor to perinatal and early neonatal mortality and has been linked to adverse long-term health consequences, including cognitive, immunological, neurodevelopmental, and cardiovascular diseases (3). With the development of infertility treatment, the goal is to help infertile couples obtain healthy babies, not just live births. Therefore, it is crucial to investigate and understand the effects of PM2.5 on preterm birth among the population undergoing IVF treatment. A study in the USA reported that a 10% decrease in PM2.5 levels nationwide in 2008 contributed to a reduction of 5,016 preterm births, potentially reducing government expenditures by hundreds of millions of dollars annually, and that when additional health expenditures for preterm birth offspring in later years are taken into account, the savings in health finances could exceed $1 billion (41). Furthermore, a national cohort study in mainland China demonstrated that women who underwent IVF treatment had a greater risk of preterm birth due to PM2.5 exposure during the third trimester than women who conceived naturally (12). Although the local government has implemented stringent policies to reduce PM2.5 levels, exposure to unhealthy levels of PM2.5 is common for Shanghai residents (42). In this study, we found that a high level of PM2.5 exposure above the WHO interim target 2 (50 µg/m3) during pregnancy was significantly related to increased odds of preterm birth following the IVF cycle. However, Shi et al. reported a significant link between PM2.5 exposure and a greater rate of preterm birth within 85 days before oocyte retrieval, from the onset of gonadotropin administration until oocyte retrieval, throughout the first trimester of pregnancy, and throughout the entire IVF pregnancy (23). A potential explanation for this might be that the percentage of two embryos transferred in their study was as high as 84.5% (58% in our study), and the preterm birth rate in their study was much greater than that in our study (9.25% versus 5.95%). Previous research has shown an association between embryo transfer and an increased risk of preterm birth (43, 44). Consequently, transferring multiple embryos as an independent risk factor for preterm birth may influence the outcomes of PM2.5 exposure.
Stratified analyses provide additional evidence that excessive PM2.5 exposure over the threshold (i.e., 50 µg/m3) may act together with other biological factors (e.g., endometrial thickness) and clinical treatment (e.g., the type of embryo transfer) to induce systemic inflammation and affect pregnancy duration (45, 46). A thin endometrium is associated with poor vascularization and reduced blood supply to the placenta, resulting in increased oxidative stress and inflammatory reactions (47). As a result, this condition can potentially compromise the process of placentation and hinder fetal growth, thereby contributing to adverse maternal and perinatal outcomes (48). Our observations indicated that subjects who had an endometrial thickness <11 mm rather than an endometrial thickness ≥ 11 mm had a greater risk of preterm birth when exposed to PM2.5 after biochemical pregnancy. In addition, our findings indicate that the adverse effects of transferring frozen embryos might contribute to an increased susceptibility to preterm birth when exposed to high levels of PM2.5. Retrospective studies suggested that frozen embryo transfer significantly increased the chance of preterm birth and abnormal placentation even after adjusting for BMI, maternal age and other confounders (49, 50). The combination of frozen embryo transfer and exposure to high levels of PM2.5 may have a synergistic effect, further increasing vulnerability to preterm birth. The underlying mechanisms of this interaction are not fully understood but may involve the compromised uterine environment and altered immune responses associated with frozen embryo transfer (51). Therefore, women who bear these risk factors may be a specific target group for future interventions and preventive measures.
Evidence from animal models suggests that mitochondrial dysfunction, oxidative stress, inflammation, and epigenetic alterations could be the underlying mechanisms of the reproductive toxicity of PM2.5 (52–54). High doses of PM2.5 induce the apoptosis of ovarian granulosa cells and oocytes and disrupt embryo development and impair placentation (54). PM2.5 was also associated with more severe lipid peroxidation and inadequate antioxidant capacity in women who suffer a miscarriage than in the normal pregnant population (55). Exposure to PM2.5 might interfere with the transport of oxygen and nutrients and/or accumulation in the placenta to directly cause placental inflammation and senescence, which can lead to preterm birth and miscarriage (56). Further investigation is needed to understand the mechanisms underlying the observed associations between PM2.5 exposure and miscarriage and preterm birth.
There are several strengths in our study. To our knowledge, this is one of the few studies to examine the relationship between PM2.5 exposure and adverse pregnancy outcomes in ART population. Our findings suggest that PM2.5 exposure is associated with a considerable burden of miscarriage and preterm birth among women undergoing infertility treatment. By avoiding outdoor activities in areas with air pollution, pregnant women who undergo IVF treatment may reduce their exposure to PM2.5 particles and mitigate the associated risks to their pregnancy. In addition, previous cohort studies were mainly conducted in women who conceived naturally (2, 7, 8), for whom it is challenging to identify the specific susceptible exposure. Instead, the stage of pregnancy during ART treatment is clearly recorded (e.g., dates of gonadotropin stimulation, embryo transfer, hCG test, and ultrasound examinations). This study used a characterized model with well-defined exposure timelines to study potential susceptible windows, which provides important insights for further study. Moreover, the satellite-based PM2.5 exposure assessment in our study could provide more detailed and additional useful information than the ground-based exposure assessment (57).
There are a few limitations of our study. First, this study analyzed only embryo transfer cycles, excluding cycles that were terminated or without viable embryos, potentially causing selection bias. The explanation for this is that this particular population exhibits the most serious consequences of IVF treatment, making it difficult to determine the impact of PM2.5 pollution on these occurrences. However, this selection bias may cause the adverse effects of PM2.5 to decrease to null values. Second, our data were obtained from medical records as a retrospective study, containing only limited information on individual covariates; thus, residual confounding effects could not be completely ruled out. Third, the absence of details regarding the utilization of fresh air filtration could result in misclassification of the exposure. Hence, it is necessary to conduct multicenter prospective studies to validate the results of this study.
This study demonstrated that women who received ART and who were exposed to PM 2.5 after pregnancy had a greater risk of preterm birth and miscarriage. Women with an endometrial thickness <11 mm or who underwent frozen embryo transfer were more susceptible to increased risks of preterm birth associated with PM2.5. Additionally, an association between PM2.5 exposure and miscarriage after pregnancy was found in all subgroups, encompassing a wide range of endometrial thickness levels and various types of embryo transfer. Nevertheless, the underlying mechanism of the adverse effects of PM2.5 on pregnancy outcomes among women undergoing ART needs further investigation.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
This study received approval from the Research Ethics Committee of Shanghai First Maternity and Infant Hospital (KS22298). Written informed consent was waived due to the nature of retrospective, and anonymous clinical data was used and analyzed.
MC: Conceptualization, Data curation, Funding acquisition, Writing – review & editing. QC: Data curation, Writing – original draft, Writing – review & editing. GL: Conceptualization, Data curation, Formal analysis, Writing – review & editing. CS: Data curation, Writing – review & editing. CL: Methodology, Writing – review & editing. XM: Methodology, Writing – review & editing. WL: Writing – review & editing. AQ: Writing – review & editing. OB: Writing – review & editing. HK: Methodology, Writing – review & editing. FW: Writing – review & editing. LT: Conceptualization, Supervision, Writing – review & editing. XT: Project administration, Resources, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by a grant from the Science and Technology Commission of Shanghai Municipality (23Y11909600), a grant from the Clinical Research Plan of Shanghai Hospital Development Center (SHDC2020CR4080), and two grants from the National Natural Science Foundation of China (81871213, 81671468). The funders had no role in the study design, data collection and analysis, interpretation of the data, or manuscript preparation.
The authors are grateful to our patients and all participants in the data collection. The authors thank Dr Jaber Firas for his valuable comments on the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1460976/full#supplementary-material
PM2.5, aerodynamic diameters ≤ 2.5 μm; ART, assisted reproductive technology; IVF, in vitro fertilization; hCG, human chorionic gonadotropin; SOP, standard operating procedure; COS, controlled ovarian stimulation; ICSI, intracytoplasmic sperm injection; BMI, body mass index; MAIAC, Multi-Angle Implementation of Atmospheric Correction; AOD, aerosol optical depth; MERRA-2, Modern-Era Retrospective Analysis for Research and Applications; AQGs, Air Quality Guidelines; AOR, adjusted odds ratio; 95% CI, 95% confidence interval; SD, standard deviation; IQR, interquartile range.
1. Burnett R, Chen H, Szyszkowicz M, Fann N, Hubbell B, Pope CA 3rd, et al. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc Natl Acad Sci U.S.A. (2018) 115:9592–7. doi: 10.1073/pnas.1803222115
2. Wang L, Luo D, Liu X, Zhu J, Wang F, Li B, et al. Effects of PM(2.5) exposure on reproductive system and its mechanisms. Chemosphere. (2021) 264:128436. doi: 10.1016/j.chemosphere.2020.128436
3. Zhu X, Liu Y, Chen Y, Yao C, Che Z, Cao J. Maternal exposure to fine particulate matter (PM2.5) and pregnancy outcomes: a meta-analysis. Environ Sci Pollut Res Int. (2015) 22:3383–96. doi: 10.1007/s11356-014-3458-7
4. Li Q, Zheng D, Wang Y, Li R, Wu H, Xu S, et al. Association between exposure to airborne particulate matter less than 2.5 μm and human fecundity in China. Environ Int. (2021) 146:106231. doi: 10.1016/j.envint.2020.106231
5. Quraishi SM, Lin PC, Richter KS, Hinckley MD, Yee B, Neal-Perry G, et al. Ambient air pollution exposure and fecundability in women undergoing in vitro fertilization. Environmental epidemiology (Philadelphia, pa). Environ Epidemiol. (2019) 3(1):e036. doi: 10.1097/EE9.0000000000000036
6. Xue T, Zhang Q. Associating ambient exposure to fine particles and human fertility rates in China. Environ pollut. (2018) 235:497–504. doi: 10.1016/j.envpol.2018.01.009
7. Leung M, Weisskopf MG, Laden F, Coull BA, Modest AM, Hacker MR, et al. Exposure to PM2.5 during pregnancy and fetal growth in eastern massachusetts, USA. Environ Health Perspect. (2022) 130:17004. doi: 10.1289/EHP9824
8. Wang Q, Benmarhnia T, Zhang H, Knibbs LD, Sheridan P, Li C, et al. Identifying windows of susceptibility for maternal exposure to ambient air pollution and preterm birth. Environ Int. (2018) 121:317–24. doi: 10.1016/j.envint.2018.09.021
9. Bachwenkizi J, Liu C, Meng X, Zhang L, Wang W, van Donkelaar A, et al. Maternal exposure to fine particulate matter and preterm birth and low birth weight in Africa. Environ Int. (2022) 160:107053. doi: 10.1016/j.envint.2021.107053
10. Tatum M. China’s fertility treatment boom. Lancet. (2020) 396:1622–3. doi: 10.1016/S0140-6736(20)32475-2
11. Niederberger C, Pellicer A, Cohen J, Gardner DK, Palermo GD, O’Neill CL, et al. Forty years of IVF. Fertil Steril. (2018) 110:185–324 e5. doi: 10.1016/j.fertnstert.2018.06.005
12. Cai J, Zhao Y, Kan J, Chen R, Martin R, van Donkelaar A, et al. Prenatal exposure to specific PM(2.5) chemical constituents and preterm birth in China: A nationwide cohort study. Environ Sci Technol. (2020) 54:14494–501. doi: 10.1021/acs.est.0c02373
13. Zeng X, Jin S, Chen XL, Qiu Y. Association between ambient air pollution and pregnancy outcomes in patients undergoing in vitro fertilization in Chengdu, China: A retrospective study. Environ Res. (2020) 184:109304. doi: 10.1016/j.envres.2020.109304
14. Legro RS, Sauer MV, Mottla GL, Richter KS, Li X, Dodson WC, et al. Effect of air quality on assisted human reproduction(dagger). Hum Reprod. (2010) 25:1317–24. doi: 10.1093/humrep/deq021
15. Gaskins AJ, Fong KC, Abu Awad Y, Di Q, Minguez-Alarcon L, Chavarro JE, et al. Time-varying exposure to air pollution and outcomes of in vitro fertilization among couples from a fertility clinic. Environ Health Persp. (2019) 127(7):77002. doi: 10.1289/EHP4601
16. Zhang C, Yao N, Lu Y, Ni J, Liu X, Zhou J, et al. Ambient air pollution on fecundity and live birth in women undergoing assisted reproductive technology in the Yangtze River Delta of China. Environ Int. (2022) 162:107181. doi: 10.1016/j.envint.2022.107181
17. Li L, Zhou L, Feng T, Hao G, Yang S, Wang N, et al. Ambient air pollution exposed during preantral-antral follicle transition stage was sensitive to associate with clinical pregnancy for women receiving IVF. Environ pollut. (2020) 265:114973. doi: 10.1016/j.envpol.2020.114973
18. Wu S, Zhang Y, Wu X, Hao G, Ren H, Qiu J, et al. Association between exposure to ambient air pollutants and the outcomes of in vitro fertilization treatment: A multicenter retrospective study. Environ Int. (2021) 153:106544. doi: 10.1016/j.envint.2021.106544
19. Gaskins AJ, Minguez-Alarcon L, Williams PL, Chavarro JE, Schwartz JD, Kloog I, et al. Ambient air pollution and risk of pregnancy loss among women undergoing assisted reproduction. Environ Res. (2020) 191:110201. doi: 10.1016/j.envres.2020.110201
20. Boulet SL, Zhou Y, Shriber J, Kissin DM, Strosnider H, Shin M. Ambient air pollution and in vitro fertilization treatment outcomes. Hum Reprod. (2019) 34:2036–43. doi: 10.1093/humrep/dez128
21. Shi W, Sun C, Chen Q, Ye M, Niu J, Meng Z, et al. Association between ambient air pollution and pregnancy outcomes in patients undergoing in vitro fertilization in Shanghai, China: A retrospective cohort study. Environ Int. (2021) 148:106377. doi: 10.1016/j.envint.2021.106377
22. Pinborg A, Wennerholm UB, Romundstad LB, Loft A, Aittomaki K, Soderstrom-Anttila V, et al. Why do singletons conceived after assisted reproduction technology have adverse perinatal outcome? Systematic review and meta-analysis. Hum Reprod Update. (2013) 19:87–104. doi: 10.1093/humupd/dms044
23. Shi W, Jiang M, Kan L, Zhang T, Yu Q, Wu Z, et al. Association between ambient air pollutants exposure and preterm birth in women who underwent in vitro fertilization: A retrospective cohort study from Hangzhou, China. Front Med (Lausanne). (2021) 8:785600. doi: 10.3389/fmed.2021.785600
24. WHO. Global air quality guidelines. (2021). Available online at: https://www.who.int/news-room/questions-and-answers/item/who-global-air-quality-guidelines (Accessed December 11, 2021).
25. Wang Y, Qiu Y, Huang B, Du J, Liu L, Jiang T, et al. Association between PM(2.5) exposure and the outcomes of ART treatment: A prospective birth cohort study. Sci Total Environ. (2023) 889:164099. doi: 10.1016/j.scitotenv.2023.164099
26. Guo Y, Liu W, Wang Y, Pan J, Liang S, Ruan J, et al. Polarization microscopy imaging for the identification of unfertilized oocytes after short-term insemination. Fertil Steril. (2017) 108:78–83. doi: 10.1016/j.fertnstert.2017.05.009
27. Chen ZQ, Wang Y, Ng EHY, Zhao M, Pan JP, Wu HX, et al. A randomized triple blind controlled trial comparing the live birth rate of IVF following brief incubation versus standard incubation of gametes. Hum Reprod. (2019) 34:100–8. doi: 10.1093/humrep/dey333
28. Annan JJ, Gudi A, Bhide P, Shah A, Homburg R. Biochemical pregnancy during assisted conception: a little bit pregnant. J Clin Med Res. (2013) 5:269–74. doi: 10.4021/jocmr1008w
29. Sun C, Ye M, Wu Y, Chen Q, Meng Z, Geng L, et al. Clinical outcomes after fresh versus frozen embryo transfer in women with advanced reproductive age undergoing in vitro fertilization: a propensity score-matched cohort study. Hum Fertil (Camb). (2023) 26:1459–68. doi: 10.1080/14647273.2023.2189025
30. Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, et al. The international committee for monitoring assisted reproductive technology (ICMART) and the world health organization (WHO) revised glossary on ART terminology, 2009. Hum Reprod. (2009) 24:2683–7. doi: 10.1093/humrep/dep343
31. Quinn JA, Munoz FM, Gonik B, Frau L, Cutland C, Mallett-Moore T, et al. Preterm birth: Case definition & guidelines for data collection, analysis, and presentation of immunisation safety data. Vaccine. (2016) 34:6047–56. doi: 10.1016/j.vaccine.2016.03.045
32. Sun D, Liu C, Zhu Y, Yu C, Guo Y, Sun D, et al. Long-term exposure to fine particulate matter and incidence of esophageal cancer: A prospective study of 0.5 million Chinese adults. Gastroenterology. (2023) 165:61–70.e5. doi: 10.1053/j.gastro.2023.03.233
33. WHO. Ambient (outdoor) air pollution. (2022). Available online at: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (Accessed May 24, 2023).
34. Zhao S, Lou Y, Chiu APY, He D. Modelling the skip-and-resurgence of Japanese encephalitis epidemics in Hong Kong. J Theor Biol. (2018) 454:1–10. doi: 10.1016/j.jtbi.2018.05.017
35. Mahutte N, Hartman M, Meng L, Lanes A, Luo ZC, Liu KE. Optimal endometrial thickness in fresh and frozen-thaw in vitro fertilization cycles: an analysis of live birth rates from 96,000 autologous embryo transfers. Fertil Steril. (2022) 117:792–800. doi: 10.1016/j.fertnstert.2021.12.025
36. Roque M, Haahr T, Geber S, Esteves SC, Humaidan P. Fresh versus elective frozen embryo transfer in IVF/ICSI cycles: a systematic review and meta-analysis of reproductive outcomes. Hum Reprod Update. (2019) 25:2–14. doi: 10.1093/humupd/dmy033
37. Liu KE, Hartman M, Hartman A, Luo ZC, Mahutte N. The impact of a thin endometrial lining on fresh and frozen-thaw IVF outcomes: an analysis of over 40 000 embryo transfers. Hum Reprod. (2018) 33:1883–8. doi: 10.1093/humrep/dey281
38. Zhang Y, Wang J, Chen L, Yang H, Zhang B, Wang Q, et al. Ambient PM2.5 and clinically recognized early pregnancy loss: A case-control study with spatiotemporal exposure predictions. Environ Int. (2019) 126:422–9. doi: 10.1016/j.envint.2019.02.062
39. Enkhmaa D, Warburton N, Javzandulam B, Uyanga J, Khishigsuren Y, Lodoysamba S, et al. Seasonal ambient air pollution correlates strongly with spontaneous abortion in Mongolia. BMC Pregnancy Childbirth. (2014) 14:146. doi: 10.1186/1471-2393-14-146
40. Malley CS, Kuylenstierna JC, Vallack HW, Henze DK, Blencowe H, Ashmore MR. Preterm birth associated with maternal fine particulate matter exposure: A global, regional and national assessment. Environ Int. (2017) 101:173–82. doi: 10.1016/j.envint.2017.01.023
41. Kim JJ, Axelrad DA, Dockins C. Preterm birth and economic benefits of reduced maternal exposure to fine particulate matter. Environ Res. (2019) 170:178–86. doi: 10.1016/j.envres.2018.12.013
42. Aqicn. Shanghai air pollution: real-time air quality index (AQI). (2022). Available online at: https://aqicn.org/city/shanghai/.
43. Ombelet W, De Sutter P, van der Elst J, Martens G. Multiple gestation and infertility treatment: registration, reflection and reaction–the Belgian project. Hum Reprod Update. (2005) 11:3–14. doi: 10.1093/humupd/dmh048
44. Rodriguez-Wallberg KA, Veleva Z. Higher obstetric and perinatal risks for twins and the need for single embryo transfers in assisted reproduction. Acta Obstet Gynecol Scand. (2023) 102:968–9. doi: 10.1111/aogs.v102.8
45. Liu W, Zhang M, Feng J, Fan A, Zhou Y, Xu Y. The influence of quercetin on maternal immunity, oxidative stress, and inflammation in mice with exposure of fine particulate matter during gestation. Int J Environ Res Public Health. (2017) 14(6):592. doi: 10.3390/ijerph14060592
46. Pope CA 3rd, Bhatnagar A, McCracken JP, Abplanalp W, Conklin DJ, O’Toole T. Exposure to fine particulate air pollution is associated with endothelial injury and systemic inflammation. Circ Res. (2016) 119:1204–14. doi: 10.1161/CIRCRESAHA.116.309279
47. Schoots MH, Gordijn SJ, Scherjon SA, van Goor H, Hillebrands JL. Oxidative stress in placental pathology. Placenta. (2018) 69:153–61. doi: 10.1016/j.placenta.2018.03.003
48. Zheng Y, Chen B, Dai J, Xu B, Ai J, Jin L, et al. Thin endometrium is associated with higher risks of preterm birth and low birth weight after frozen single blastocyst transfer. Front Endocrinol (Lausanne). (2022) 13:1040140. doi: 10.3389/fendo.2022.1040140
49. Bu Z, Zhang J, Hu L, Sun Y. Preterm birth in assisted reproductive technology: an analysis of more than 20,000 singleton newborns. Front Endocrinol (Lausanne). (2020) 11:558819. doi: 10.3389/fendo.2020.558819
50. Sacha CR, Harris AL, James K, Basnet K, Freret TS, Yeh J, et al. Placental pathology in live births conceived with in vitro fertilization after fresh and frozen embryo transfer. Am J obstetrics gynecology. (2020) 222:360 e1–e16. doi: 10.1016/j.ajog.2019.09.047
51. Zhang J. Risk of preeclampsia in artificial cycles of frozen embryo transfer in vitro fertilization pregnancies: a response. Am J Obstet Gynecol. (2021) 225:467–8. doi: 10.1016/j.ajog.2021.06.064
52. Janssen BG, Byun HM, Gyselaers W, Lefebvre W, Baccarelli AA, Nawrot TS. Placental mitochondrial methylation and exposure to airborne particulate matter in the early life environment: An ENVIRONAGE birth cohort study. Epigenetics. (2015) 10:536–44. doi: 10.1080/15592294.2015.1048412
53. Jiang Y, Li J, Ren F, Ji C, Aniagu S, Chen T. PM2.5-induced extensive DNA methylation changes in the heart of zebrafish embryos and the protective effect of folic acid. Environ pollut. (2019) 255:113331. doi: 10.1016/j.envpol.2019.113331
54. Liao BQ, Liu CB, Xie SJ, Liu Y, Deng YB, He SW, et al. Effects of fine particulate matter (PM2.5) on ovarian function and embryo quality in mice. Environ Int. (2020) 135:105338. doi: 10.1016/j.envint.2019.105338
55. Zhang Y, Wang J, Gong X, Chen L, Zhang B, Wang Q, et al. Ambient PM2.5 exposures and systemic biomarkers of lipid peroxidation and total antioxidant capacity in early pregnancy. Environ pollut. (2020) 266:115301. doi: 10.1016/j.envpol.2020.115301
56. Liu Y, Wang L, Wang F, Li C. Effect of fine particulate matter (PM2.5) on rat placenta pathology and perinatal outcomes. Med Sci Monit. (2016) 22:3274–80. doi: 10.12659/MSM.897808
Keywords: PM2.5 exposure, preterm birth, miscarriage, particulate matter, in vitro fertilization
Citation: Chen M, Chen Q, Liao G, Sun C, Liu C, Meng X, Li W, Qiu A, Bukulmez O, Kan H, Wang F, Tse LA and Teng X (2025) Associations of maternal PM2.5 exposure with preterm birth and miscarriage in women undergoing in vitro fertilization: a retrospective cohort study. Front. Endocrinol. 16:1460976. doi: 10.3389/fendo.2025.1460976
Received: 07 July 2024; Accepted: 07 January 2025;
Published: 27 January 2025.
Edited by:
Dolors Manau, Hospital Clinic of Barcelona, SpainCopyright © 2025 Chen, Chen, Liao, Sun, Liu, Meng, Li, Qiu, Bukulmez, Kan, Wang, Tse and Teng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lap Ah Tse, c2hlbGx5QGN1aGsuZWR1Lmhr; Xiaoming Teng, dGVuZ3hpYW9taW5nQGhvdG1haWwuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.