
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol. , 17 February 2025
Sec. Gut Endocrinology
Volume 16 - 2025 | https://doi.org/10.3389/fendo.2025.1409119
This article is part of the Research Topic The Mechanism in Gut Microbiota of Diabetes and Endocrine Complications: Preventive and Therapeutic Target View all 10 articles
 Yan Zeng1,2,3,4†
Yan Zeng1,2,3,4† Qi Wu1,3,4,5†
Qi Wu1,3,4,5† Man Guo2,3,4†
Man Guo2,3,4† Fangyuan Teng3,4,6
Fangyuan Teng3,4,6 Chunxia Jiang1,2,3,4
Chunxia Jiang1,2,3,4 Jiao Chen2,3,4
Jiao Chen2,3,4 Xiaozhen Tan3,4,6
Xiaozhen Tan3,4,6 Chen Zeng2,3,4
Chen Zeng2,3,4 Yang Long3,4,6*
Yang Long3,4,6* Betty Yuen-Kwan Law1*
Betty Yuen-Kwan Law1* Yong Xu1,2,3,4*
Yong Xu1,2,3,4*Despite significant advancements in prevention and treatment, cardiometabolic diseases continue to pose a high burden of incidence and mortality. The chronic progression of these diseases necessitates the identification of early and complementary therapeutic targets to elucidate and mitigate residual risks in patient care. The gut microbiota acts as a sentinel between internal and external environments, transmitting modified risks associated with these factors to the host. Imidazole propionate (ImP), a histidine metabolite originating from the gut microbiota, gained attention after being found to impair glucose tolerance and insulin signaling several years ago. Epidemiological studies over the past five years have demonstrated a robust correlation between ImP and an increased risk of onset of type 2 diabetes (T2D) and obesity, exacerbation of kidney traits in chronic kidney disease (CKD), progression of atherosclerotic plaques, and elevated mortality rates in heart failure (HF). These findings suggest that ImP may serve as a pivotal target for the prevention and treatment of cardiometabolic diseases. Mechanistic insights have uncovered associations between ImP and insulin resistance, impaired glucose metabolism, chronic inflammation, and intestinal barrier damage. This review provides a comprehensive summary of the current evidence regarding the association between ImP and cardiometabolic impairment, highlighting its potential in advancing personalized approaches to disease prevention and management, and exploring the intricate interplay of diet, gut microbiota, and ImP in cardiovascular metabolic impairment. Overall, this review offers valuable insights into the multifaceted roles of ImP in cardiometabolic diseases, identifies current knowledge gaps, and discusses future research directions.
Cardiometabolic diseases impose a significant global health burden, surpassing other disorders in terms of morbidity and mortality, with projections indicating a sharp increase over the next 25 years (1, 2). This category encompasses a range of chronic conditions affecting both cardiovascular and metabolic health, including cardiovascular disease (CVD), insulin resistance, obesity, diabetes, chronic kidney disease (CKD), and nonalcoholic fatty liver disease (NAFLD) (3, 4). Managing these diseases presents challenges for healthcare providers due to their often-asymptomatic nature until advanced stages, highlighting the pressing need for more effective prevention and intervention strategies.
Accumulating evidence implicates imbalances or compositional changes in intestinal microbes in both physiological and pathological alterations in the host (5). The causal contribution of gut microbiota to cardiometabolic diseases is further supported by a plethora of direct experimental evidence (6). A pivotal mechanism involves the production of small molecules by gut microbes, capable of exerting effects at or beyond the host gut barrier. Initially, research primarily focused on bile acids, short-chain fatty acids (SCFAs), branched-chain amino acids, and carnitine-derived metabolites (7–10). Advances in metabolomics have facilitated the identification of increasingly crucial intestinal metabolites, hastening the discovery of potential biomarkers to enhance the diagnosis and prognosis estimation of various diseases.
Recently, imidazole propionate (ImP), a histidine-derived metabolite produced by gut microbes, has garnered increasing attention for its close correlation with metabolic disorder. The investigation into ImP’s role in human disease traces back to 1972 when it was discovered to be excreted by patients with intestinal disorders. Interestingly, it was almost absent in feces and urine from healthy subjects, suggesting ImP’s potential as a microbial metabolite with adverse health effects (11). However, for a considerable period thereafter, ImP seemed to fade into obscurity. It wasn’t until 2018 when researchers from the University of Gothenburg and Sahlgrenska University Hospital (12) discovered its association with impaired insulin signaling in mice and humans that ImP came back into the spotlight. Subsequently, increasing clinical studies reveled close links between circulating ImP levels and metabolic disorder and CVD, including type 2 diabetes (T2D) (13), blood pressure (14), obesity (15), non−alcoholic steatohepatitis (NASH) (16), CKD (17), artery atherosclerosis (18–20), and heart failure (HF) (21). Supplementation with ImP has been demonstrated to exacerbate glucose intolerance (12), impair wound healing (22), and compromise the integrity of the intestinal barrier (23) in mice.
Here, we comprehensively review the available evidence on the biological effects of ImP, emphasizing its potential therapeutic applications as a target for treating cardiometabolic diseases, and discuss future research directions.
ImP, also referred to as dihydrourocanate or deamino-histidine, arises from the metabolic activity of gut microbiota on dietary histidine. Histidine, an essential amino acid obtained from the host diet, serves as a fundamental substrate for protein synthesis and acts as a precursor for the biogenic amine histamine, catalyzed by histidine decarboxylase. Moreover, surplus histidine undergoes metabolic conversion to trans-urocanate via histidine ammonia-lyase (EC:4.3.1.3, encoded by the hutH gene) (24). Subsequently, trans-urocanate is primarily metabolized to cis-urocanate in the skin and to glutamate and NH3 in the liver (24, 25). In the colon, urocanate reductase (EC:1.3.99.33, encoded by the urdA gene), produced by the intestinal microbiota, facilitates the reduction of trans-urocanate into the non-metabolizable product, ImP (26, 27). Ultimately, ImP is excreted either directly through feces or absorbed by the intestines and subsequently excreted through urine (11).
Under physiological conditions, circulating ImP levels exhibit minimal individual variation, ranging from a few to several tens of nanomolars (12–14). However, under pathological conditions such as T2D and CVD, its concentration can escalate by approximately a hundredfold (12, 13).
Despite deriving from histidine metabolism, ImP levels in the body are not determined by histidine intake (13), but are primarily affected by enzyme activity and the composition of intestinal microbiota (12, 13, 26). Large-scale screening for UrdA, which encodes the urocanate reductase responsible for ImP production, has identified bacteria harboring “Y” or “M” UrdA homologs as authentic ImP producers from urocanate (12). Urocanate reductase exhibits optimal activity at neutral pH (12, 26, 28). These UrdA-containing bacteria- encompass species such as Aerococcus urinae, Streptococcus mutans, Anaerococcus prevotii, Adlercreutzia quolifaciens, Eggerthella lenta, Lactobacillus paraplantarum, Brevibacillus laterosporus, and Shewanella oneidensis (12). Over the past five years, an increasing number of intestinal bacteria have been implicated in direct or indirect associations with ImP production, as summarized in Table 1.
Bacterial metabolites originating in the gut traverse to the liver via the portal vein before entering systemic circulation. The crosstalk between the gut and liver ultimately leaded to insulin resistance and even diabetes (29). Koh et al. investigated amino acid-derived microbial metabolites potentially linked to insulin resistance and T2D (12). In their initial study involving 15 obese subjects (body mass index [BMI] > 40), higher concentrations of ImP were observed in both portal and peripheral blood of 5 T2D subjects compared to 10 BMI-matched controls. This finding was corroborated in a larger cohort of 649 middle-aged individuals from the Swedish community, where ImP levels remained significantly elevated in treatment-naive T2D subjects after adjusting for BMI, sex, and age (12). Subsequently, numerous studies successively reported the association between ImP levels and T2D. In a large European multicentric cohort (MetaCardis) comprising 1,958 subjects from France, Germany, and Denmark, progressively elevated ImP levels were observed across patients with normal glucose tolerance, prediabetes, and overt T2D (13). Additionally, circulating ImP levels exhibited positive correlations with HbA1C (13, 30), HOMA-IR (13), insulinemia (13), fasting glucose (13, 15) and postprandial glucose (13, 31). These findings indicate that ImP is not only associated with impaired glucose metabolism but also with diabetic status.
The analysis of gut microbiota in T2D patients effectively addressed the reasons for the changes in circulating ImP levels. In an in vitro gut simulator experiment monitoring ImP production kinetics, the gut microbiota of T2D patients demonstrated ImP production capability, unlike non-T2D patients (12). Further investigation revealed the enrichment of ImP-producing bacteria, characterized by “Y” or “M” UrdA homologs, in the intestines of T2D patients (12), as well as in the intestine and skin of T2D mice (22). These included strains previously associated with an elevated risk of T2D in large population cohorts, such as Streptococcus mutans (32), Eggerthella lenta (33), and Lactobacillus gasseri (33).
However, ImP serves not only as a disease marker but also correlates with an increased risk of prediabetes and T2D, as demonstrated in cohort studies (13, 15), suggesting its biological impact on T2D progression. In animal experiments, ImP injection induced glucose intolerance and decreased hepatic insulin signaling (12). Mechanistically, ImP disrupts insulin signaling by activating p38γ mitogen-activated protein kinase (MAPK), leading to p62 phosphorylation and subsequent activation of mechanistic target of rapamycin complex 1 (mTORC1). This results in the phosphorylation and degradation of insulin receptor substrates 1 and 2 (IRS1 and IRS2). Consistently, phosphorylation of p62 and S6K1 were elevated in the human liver compared to healthy controls (12), highlighting the role of ImP in impairing insulin signaling through the p62/mTORC1 pathway.
In addition to its impact on T2D itself, ImP has also been found to influence the hypoglycemic effects of metformin, the first-line therapy for T2D. Metformin exhibits substantial variability in efficacy among individuals, and genetic variations, particularly in genes encoding transporters such as organic cation transporter 1 (OCT1) (34) and glucose transporter 2 (GLUT2) (35), have been identified as influencing metformin response. In addition to gene polymorphisms, Koh et al. discovered that intestinal ImP levels contribute to this variability (36). T2D patients on metformin with persistently high blood glucose levels showed elevated ImP concentrations.
Further experimental studies demonstrated that ImP diminishes the glucose-lowering effect of metformin and inhibits metformin-induced activation of adenosine 5′-monophosphate-activated protein kinase (AMPK) by impeding AMPK serine phosphorylation through the p38γ/Akt pathway. However, as the authors point out, the study has certain limitations worth noting. Given its cross-sectional design, it remains unclear whether individuals with higher blood glucose values (and consequently higher plasma ImP levels) actually responded poorly to metformin or had more severe diabetes prior to treatment initiation. Hence, a longitudinal cohort study is warranted to ascertain whether ImP directly undermines metformin efficacy and whether metformin contributes to the proliferation of ImP-producing bacteria.
The sub-analysis of the MetaCardis study, including 20% of participants with CVD, revealed a significant increase in circulating ImP concentrations among CVD patients after adjusting for traditional risk factors (age, gender, BMI, ethnicity), kidney function, and presence of T2D (13). Correlation analysis demonstrated a strong positive correlation between ImP levels and serum inflammatory markers, including total leukocyte count, high-sensitivity C-reactive protein (hs-CRP), and interferon gamma-induced protein 10 (IP-10) (13), suggesting a potential association between ImP, inflammation, and CVD progression, warranting further investigation.
Recent large-scale clinical studies have linked ImP to atherosclerosis (18–20), a chronic inflammatory vascular disease and the major cause of CVD (37). The role of gut microbiota in atherosclerosis has been supported by increasing mechanistic evidence (38). In fact, significant changes in gut microbiota have been observed in patients with subclinical coronary atherosclerosis before plaque formation. A study involving 8, 973 participants without overt atherosclerotic disease revealed significant alterations in gut microbiota, including oral microbial species like Streptococcus spp, which correlated significantly with ImP levels and systemic inflammation markers (hs-CRP levels and neutrophil counts) (20). However, due to its cross-sectional design and lack of experimental evidence, the study failed to extrapolate the predictive value of ImP on plaque formation and its potential implications in atherogenesis.
Nevertheless, data from HIV patients have provided indications of a possible association between ImP and the presence of plaques in carotid and coronary arteries (18, 19). HIV infection is linked to chronic inflammation and immune activation, critical factors in atherosclerosis and thrombosis development (39). HIV-induced disruptions in gut microbiota exacerbate chronic inflammation and metabolic irregularities, increasing atherosclerosis risk (40, 41).
In a study of 320 females living with or at risk of HIV infection, with 26% having carotid artery plaque, a distinct shift in gut microbiota composition and increased ImP plasma levels were observed (18). Circulating ImP levels were inversely correlated with potentially beneficial microbial species linked to reduced carotid artery plaque. Additionally, ImP levels positively correlated with serum inflammatory markers, including CX3CL1, TNFSRF9, and LIF-R, associated with immune activation and inflammation pathways related to atherosclerotic plaques (42–44). Notably, after adjusting for plasma ImP levels or inflammatory markers, the association between gut bacterial species and plaque weakened, indicating that circulating ImP levels and related inflammatory markers may partly explain these associations. Further analysis identified 17 ImP-associated species (as illustrated in Table 1), with 8 correlating with the functional enzyme hutH. A gut microbiota score derived from these ImP-associated species showed a positive correlation with plaque formation and several pro-inflammatory markers, even after adjusting for multiple factors. Collectively, gut bacteria may contribute to plaque formation by modulating host immune activation and inflammation through elevated IMP levels.
Recent research suggests that ImP is not only associated with plaque formation but also with plaque obstruction. HIV-infected patients with obstructive coronary artery disease (CAD) exhibited lower gut microbiota diversity and significant compositional changes compared to HIV-infected individuals without CAD or non-obstructive CAD, with increased abundance of known ImP producers such as Rumiococcus gnavus and Veillonella (19). ImP plasma levels were associated with this dysbiosis, significantly elevated in participants with obstructive CAD (19). However, after adjustment for traditional and HIV-related risk factors, gut dysbiosis but not plasma ImP was independently associated with obstructive CAD, indicating that the effects of gut microbiota may extend beyond ImP in plaque obstruction. Longitudinal studies are needed to establish a causal relationship between ImP levels and plaque formation, shedding light on its predictive value for atherosclerosis. Additionally, exploring ImP in the context of HIV-related CVD is crucial, highlighting the importance of understanding the role of ImP and gut dysbiosis in driving CVD risk among HIV patients.
The recent findings offer a strong foundation for future investigations, yet additional experiments are necessary to move beyond associations and establish causal evidence linking ImP to atherosclerosis. Firstly, further research is needed to elucidate the mechanistic connection between ImP and inflammation, addressing the significant correlations observed in multiple studies. Secondly, Atherosclerosis initiates with endothelial injury, leading to the accumulation of macrophage foam cells and infiltration of smooth muscle cells, resulting in fatty streak formation (45). Inflammatory processes play a pivotal role in the development of vulnerable plaques (46). Rupture of the plaque’s cap triggers platelet aggregation, precipitating thrombosis and vascular obstruction (47). Therefore, understanding whether ImP is involved in endothelial injury, foam cell formation, and platelet aggregation should be the central focus of future research into the progression of atherosclerosis.
Individuals with T2D face more than a twofold increased risk of developing HF compared to non-T2D patients, with higher risks of incident cases and mortality among diagnosed patients (48). Profiling the metabolic connections and shared components of T2D and HF could unveil new disease pathways, enhance risk prediction, and enable tailored prevention and management strategies (49). The elevation of intestinal metabolite ImP in the circulation of T2D patients, along with its induction of insulin resistance in animal models (12), offers a novel perspective on unraveling the molecular signatures and metabolic remodeling of HF and its associated factors.
Targeted metabolomic analysis of 260 individuals with diverse glucose metabolism from the Risk Evaluation and Management of Heart Failure (REM-HF) cohort in China identified ImP as a microbial signature contributing to the shared etiologies of T2D, HF, and CKD (21). Data from the Boston Puerto Rican Health Study (BPRHS) cohort (50) and European Prospective Investigation into Cancer (EPIC)-Norfolk study (49) further corroborated this finding. Impressively, serum ImP levels increased by 1.1–1.6 fold with each additional chronic HF comorbidity (21), supporting ImP as a component of the metabolic connections among T2D, HF, and CKD.
Recently, Molinaro et al. from Sweden (51) investigated the association between circulating ImP levels, HF, and incident mortality risk. In the population-based MetaCardis cohort, significantly higher ImP levels were observed in individuals with established CVD or HF compared to those without, with the highest levels detected in HF patients. Individuals in the highest quartile of ImP levels had a threefold increased risk of HF compared to those in the lowest quartile, even after adjusting for multiple traditional cardiovascular risk factors. Moreover, ImP levels were inversely associated with left ventricular ejection fraction (LVEF) and positively correlated with pro-atrial natriuretic peptide (proANP) and N-terminal pro–B-type natriuretic peptide (NT-proBNP) levels. These findings were consistent across the GeneBank cohort from North America, predominantly comprising patients with HF (n = 407), CVD (n = 1,331), and without CVD or HF (n = 417). Additionally, longitudinal follow-up data from the North American cohort revealed that the highest quartile of ImP was independently associated with an increased risk of overall mortality, even after adjusting for traditional risk factors and baseline covariates (adjusted HR = 1.85, 95% CI [1.20, 2.88], P < 0.01). Overall, this study, drawing from two large independent cohorts, offers compelling evidence supporting a substantial correlation between ImP levels and CVD, HF, and HF-associated phenotypes including reduced left ventricular ejection fraction and heightened natriuretic peptide levels. Crucially, this correlation persists regardless of obesity and T2D, known contributors to disease progression. In vitro experiments conducted on H9c2 cardiomyoblast cells pretreated with hypoxia/reoxygenation further supported a causal link between ImP and distinct HF-relevant phenotypes. In this study, the intervention of 0.1 μM ImP for 24 hours significantly elevated the expression of the Natriuretic Peptide B gene (NPPB), which encodes the B-type natriuretic peptide (BNP), and disrupted cardiomyoblast functions, as indicated by significantly reduced mitochondrial membrane potential (21).
The current research on the association between elevated circulating ImP levels and HF presents valuable insights, yet it also reveals several limitations and areas for future investigation. One major concern is the lack of clarity regarding the underlying reasons for the elevation of ImP in HF patients, posing a significant gap in our understanding. Moreover, the reliance on cross-sectional data underscores the need for longitudinal research to establish definitive causal relationships. Furthermore, the predominantly focused research on specific populations calls for more diverse cohorts to ensure the generalizability of findings. Future studies should prioritize exploring the mechanistic understanding of ImP’s role in HF development and conducting interventional trials to assess the therapeutic potential of targeting ImP levels. Consideration of confounding factors such as medication use and lifestyle variables is essential, and efforts to validate ImP as a diagnostic and prognostic biomarker are warranted. Addressing these gaps through comprehensive research endeavors will enhance our understanding of the involvement of ImP in HF and facilitate the development of effective therapeutic strategies.
NAFLD, affecting approximately one quarter of the global population, encompasses a spectrum of conditions ranging from simple hepatic steatosis, often linked to obesity, to NASH, which can progress to fibrosis, cirrhosis, and hepatocellular carcinoma (52). The gut and liver are interconnected through the portal vein, forming the gut-liver axis, which serves as a direct pathway for gut microbiota and their metabolic by-products to reach the liver (53). Bidirectional communication along the gut-liver axis plays a pivotal role in NAFLD pathogenesis (54). In NAFLD, microbial dysbiosis in the gut, particularly a decrease in SCFAs-producing microbiota, has been documented (55). This reduction in SCFAs production leads to an elevation in intestinal pH (56), influencing bacterial metabolite production and subsequent absorption into the host circulation (57).
In Göttingen minipigs fed a choline-deficient amino acid-defined high-fat diet (CDAHFD), serving as a NASH animal model, notable increases in serum ImP concentration and pancreatic glucagon levels were observed. These changes were accompanied by liver activation of mTORC1, as indicated by increased expression of liver RHEB and MTOR genes, along with impaired hepatic insulin signaling, demonstrated by decreased expression of IRS1 and IRS2 (16). Moreover, multiple linear regression analysis identified ImP as a statistically significant predictor for glucagon levels (P = 0.0068). Additionally, 16S rRNA analysis showed significant downregulation of intestinal SCFAs-producing bacteria in CDAHFD-fed minipigs, including butyrate-producing members of Lachnospiraceae and propionate producers from the Muribaculaceae family (16). This resulted in an elevation of colon luminal pH, creating an environment conducive to enzymatic activity of bacterial urocanate reductase, thereby facilitating ImP production from histidine metabolism (12, 16, 26, 28). Following production, ImP is likely transported from the intestines to the liver via the portal vein (58), where it subsequently contributes to impaired hepatic insulin signaling, hyperglucagonemia, decreased expression of the glucagon receptor, and disruption of the liver-α-cell axis (16). This aligns with emerging evidence showing a negative correlation between ImP levels and fibroblast growth factor 21 (FGF-21), an endogenous regulator of lipid and glucose metabolism (30). Recently, ImP were enriched in cirrhotic patients with chronic hepatitis B compared to healthy subjects, signifying its potential role in chronic hepatitis B progression, but its role remains to be further clarified.
Future research should delve into elucidating the exact role of ImP in the pathogenesis of NAFLD and other liver disease. Specifically, efforts should focus on determining the association of ImP with the progression of liver diseases and its potential diagnostic and therapeutic implications. Moreover, researchers could explore strategies to modulate ImP levels by manipulating gut microbiota composition or intervening in gut SCFAs production, thereby developing novel treatment approaches.
Globally, over 10% of the population suffers from CKD, characterized by kidney damage, typically indicated by urinary albumin, or decreased kidney function, measured by glomerular filtration rate (59). Individuals with CKD face a significantly elevated risk of CVD and cardiovascular-related mortality (60), underscoring the importance of early detection for effective management. The gut microbiota and their associated metabolites play a crucial role in the microbiota-gut-kidney axis, offering a promising avenue for early diagnosis and personalized treatment to slow renal progression (61).
Recent studies have identified ImP as another metabolite linked to kidney traits, prospectively associated with CKD incidence over time (17). In a large study involving 2,438 Hispanic/Latino adults (12% with CKD), elevated ImP levels were correlated with worsening kidney traits, including reduced eGFR, increased urinary albumin-to-creatinine (UAC) ratio, and CKD incidence over approximately 6 years (17). Similarly, in another cohort from China with varying glucose tolerances, ImP levels exhibited a strong association with creatinine, Cystatin C, and estimated glomerular filtration rate (eGFR). Furthermore, serum ImP levels increased by 1.5 times with the occurrence of CKD in patients with T2D and chronic HF (21). Notably, ImP was more strongly associated with biomarkers of CKD than with those of HF and T2D, indicating its potential pathogenic role in all three conditions and suggesting shared etiologies mediated by ImP among these diseases.
The understanding of the mechanisms underlying increased ImP levels in CKD is still evolving. Alterations in the gut microbiota may be a contributing factor, as CKD progression leads to factors such as sodium and water retention, increased circulatory system pressure, visceral congestion, intestinal wall edema, and impaired intestinal barrier function, resulting in bacterial translocation and gut dysbiosis (62). Studies like the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) study (17) have shown that higher CKD incidence rates and UAC ratios, along with lower eGFR, are associated with reduced gut microbiota diversity and alterations in overall microbial composition, which may directly contribute to increased ImP production. Future research should further explore the relationship between CKD progression, gut dysbiosis, and ImP production to uncover potential therapeutic strategies for managing CKD-related complications and reducing the risk of ImP-associated health issues.
Another interesting discovery is the observed prospective association of ImP with changes in renal function and impairment in CKD, particularly prominent in patients with diabetes (17). This is consistent with animal studies where serum ImP levels were significantly elevated in diabetic mice models (63). ImP levels positively correlated with renal functional parameters like the UAC ratio. In cellular studies, ImP was found to stimulate inflammation and fibrosis by promoting toll-like receptor 4 (TLR4)-mediated phosphorylation of NF-κB and Stat3 and expression of IL-6, TGF-β1, and MyD88 (63). Moreover, downregulating ImP-producing bacteria, such as certain genera of Bacteroides and Unidentified Ruminococcaceae, was shown to ameliorate diabetic kidney disease by suppressing ImP-induced protein expression of the TLR4 signaling pathway in vivo and in vitro (63). Understanding the mechanistic role of ImP in CKD progression and its associations with other renal conditions, along with validating these mechanisms through extensive cell and animal model studies, could lead to improved clinical applications in the future.
The interconnection among obesity, hypertension, diabetes, and CVD underscores the importance of investigating shared metabolic pathways to improve risk assessment and develop personalized prevention and management strategies. Studies exploring the association between ImP, T2D, and CVD, such as those by Koh and Molinaro, et al. (12, 13) have included patients with overt metabolic diseases or CVD, complicating the differentiation of ImP’s correlation with known risk factors like weight gain, hypertension, and cholesterol. Insights from microbiome community typing analyses within the MetaCardis cohort provided some clues, revealing elevated ImP levels in individuals with the Bacteroides2 (Bact2) enterotype (13), a gut microbiota profile associated with systemic inflammation and obesity (64). Research involving 1,018 females from the UK Adult Twin Registry (TwinsUK) cohort further supports the correlation between ImP and obesity (15). This study found that serum ImP levels were positively correlated with BMI (Pearson correlation coefficient [r] = 0.18, P = 5E-9), visceral fat mass (r = 0.067, P = 0.06), and an increased risk of obesity (r = 0.2, P = 8E-9), indicating circulating ImP levels as a potential marker for obesity.
In another cohort of overweight/obese subjects without T2D and not on any CVD medication, the association between circulating plasma ImP concentrations and CVD risk factors, including blood pressure, HDL cholesterol, and LDL cholesterol, was investigated (14). This study revealed a positive correlation between plasma ImP concentrations and diastolic blood pressure (Spearman rank correlation coefficient [rs] = 0.285, P = 0.004), with borderline significance for systolic blood pressure (rs = 0.187, P = 0.060). Interestingly, no significant association was found between plasma ImP concentrations and peripheral or hepatic insulin resistance, contrary to the findings of Koh et al. (12). This discrepancy could be attributed to differences in the study populations, as Koh et al.’s study included patients with varying BMI and metabolic disease severity. However, the data revealing the association between ImP and blood pressure comes from cohort comprising subjects without T2D, with a very homogeneous range of BMI, and without any medication or overt chronic diseases except for metabolic syndrome (14), suggests that if ImP influences blood pressure, it may do so through mechanisms other than insulin resistance.
While the association between ImP and obesity, as well as blood pressure has been observed, the underlying mechanisms remain unclear. Future research should delve deeper into the molecular pathways linking ImP to obesity and hypertension and explore potential therapeutic targets to mitigate its effects on cardiovascular health. Additionally, the discrepancy in findings regarding the association between ImP and insulin resistance highlights the need for further clarification through well-controlled studies involving diverse populations.
Unhealthy dietary patterns, such as the contemporary Western diet characterized by low fiber content and high levels of animal proteins, saturated fats, sodium, and sugar, have been strongly linked to cardiometabolic disorders (65, 66). Conversely, diets rich in fiber and vegetable proteins, such as the Mediterranean, vegetarian, or plant-based low-protein diets, have shown metabolic benefits (67, 68). Increasing evidence suggests that metabolites produced by gut microbiota play a crucial role in mediating the effects of dietary patterns on host metabolism (69). In the MetaCardis study, circulating ImP levels were positively correlated with saturated fat intake (primarily driven by high cheese consumption) and negatively correlated with fiber and unsaturated fat intake (due to increased consumption of vegetables and nuts) (13). ImP levels were also inversely associated with dietary quality indices like the Alternate Healthy Eating Index, dietary diversity score, and Mediterranean diet scores (13). In a two-week dietary intervention study involving healthy individuals, transitioning from a Western diet to one rich in fiber, fruits, vegetables, and protein led to increased creatinine-normalized urinary ImP levels (70). Similarly, another intervention study focusing on subjects with HbA1c levels exceeding 6% showed that intake of resistant maltodextrin (a type of dietary fiber) reduced fecal ImP levels, particularly in individuals with elevated ImP levels before intervention (71). Overall, dietary fiber intake, unsaturated fat consumption, and adherence to healthy dietary patterns may inversely correlate with glucose metabolism disorders by reducing ImP levels.
Healthy dietary patterns, including high-fiber diets, are associated with greater intestinal microbial diversity and bacterial gene richness (72–74). Decreased intestinal microbial diversity and bacterial gene richness have been linked to metabolic disturbances (75–77). ImP has emerged as a circulating metabolite reflective of gut microbiome α diversity metrics, with a Shannon diversity index of approximately -0.2 (P < 0.01) (15), and is elevated in individuals with low bacterial gene richness (13). Thus, ImP may serve as an important biomarker reflecting the combined influence of diet, gut microbiota, and genetic diversity on cardiometabolic health.
As a natural source of SCFAs, slight differences in dietary fiber structure can lead to distinct effects on gut microbiome composition, resulting in targeted shifts in the production of SCFAs (78). Increased consumption of vegetables and fruits has been associated with higher abundance of SCFA-producing bacteria. For instance, fruit and vegetable intakes were positively associated with Coprococcus species, Faecalibacterium prausnitzii, Roseburia hominis, and Firmicutes bacterium CAG:95 across multiple studies (79–83), which have been reported to have a significant negative correlation with ImP production, as depicted in Table 1. Additionally, studies indicate that decreased SCFA production leads to an increase in intestinal pH (16, 56), providing an optimal environment for urocanate reductase, the bacteria responsible for ImP production, to exert maximal activity. While current evidence is limited, given the observed link between decreased SCFAs and metabolic impairments in numerous studies, this hypothesis seems plausible, suggesting that ImP could serve as a biomarker of dysregulated gut microbiome—due to an unhealthy diet or disease. Therefore, implementing dietary modifications to promote healthier eating habits, along with interventions targeting the regulation of intestinal microbiota composition—such as fecal microbiota transplantation, specific microbiota transplantation or supplementation with probiotics/prebiotics—may offer effective strategies to reduce ImP levels. However, significant gaps remain in understanding the mechanistic relationship between ImP, SCFA production, gut microbiota composition, and intestinal environment. Future research endeavors should aim to elucidate these mechanisms and explore clinical interventions targeting dietary adjustments and microbiota modulation to mitigate ImP-related cardiometabolic risks.
The association between elevated ImP levels and cardiovascular metabolic impairment underscores the importance of exploring the mechanisms underlying ImP’s effects, which may elucidate the potential for targeting ImP to improve cardiovascular metabolism and identify novel drug targets. Insights from the work of Koh and Molinaro et al. (12, 13, 36) have shed light on this field. Their research team elucidated the molecular mechanism by which ImP impairs glucose tolerance and insulin signaling through the activation of the p38γ/p62/mTORC1 signaling pathway. Further studies have revealed that ImP inhibits the hypoglycemic activity of metformin via the p38γ/Akt/AMPK pathway. Structural analysis has unveiled the interaction between ImP and the adenosine triphosphate (ATP) binding pocket of p38γ. In silico analysis suggests that pirfenidone, used to treat idiopathic pulmonary fibrosis, may compete with ImP for binding to this site of p38γ, implying its potential as a candidate for combination therapy in individuals with T2D who are unresponsive to metformin. Future research should focus on elucidating the detailed mechanism of ImP binding to p38γ for structure-based drug design. While pirfenidone shows promise, clinical trials are needed to confirm its efficacy and safety in T2D patients, along with investigations into potential drug interactions.
Besides opposing the interaction involving ImP, strategies aimed at reducing ImP production, like inhibiting enzymes such as urocanate reductase, present promising new therapeutic avenues for T2D. In this regard, Venskutonytė and Koh et al. elucidated the X-ray structures of its ligand-binding domains (26), providing valuable insights for structure-based drug design and aiding in the development of inhibitors for potential treatment of metabolic disorders.
While the existing research provides valuable insights into the role of ImP in cardiometabolic diseases, several limitations and areas for future investigation should be acknowledged. Firstly, much of the current research focuses on observational and cross-sectional studies, limiting our ability to establish causal relationships between ImP and cardiometabolic diseases. Longitudinal follow-up studies are needed to elucidate the temporal relationship between ImP levels and disease onset and progression.
Secondly, there is a noticeable lack of diversity in study populations, with a predominant inclusion of individuals of European descent in many studies. Given that the gut microbiome exhibits significant variation across geographical regions (84, 85), and considering that the production of ImP is highly dependent on bacterial activity, it becomes imperative for future studies to encompass more diverse populations. This approach will not only enhance the generalizability of findings but also allow for a comprehensive understanding of how ethnic and geographic factors may influence ImP production and its implications for cardiometabolic diseases.
Thirdly, while circulating ImP levels have been correlated with systemic inflammation in metabolic disorder (13, 18, 20), the underlying mechanisms remain unclear, highlighting the necessity for further research into ImP’s regulation of inflammatory pathways. Notably, rectal administration of ImP in mice resulted in a significant increase in NF-κB, iNOS, and IL-6 expression, accompanied by a reduction in goblet cell count (23). These findings suggest ImP’s potential to induce intestinal inflammation, disrupt the intestinal barrier, and alter goblet cell proliferation. Given the crucial role of intestinal barrier integrity in cardiometabolic diseases, targeting ImP to modulate intestinal barrier function holds promise for cardiometabolic disease treatment. However, additional evidence is required to substantiate ImP’s regulatory role and elucidate its molecular mechanisms in this context.
Fourthly, optimal enzymatic activity of UrdA occurs under neutral pH conditions (28), while the proximal colon tends to maintain an acidic environment. Reduced production of SCFAs and increased protein fermentation in the gut contribute to the elevation of colonic pH (86), potentially facilitating ImP production. This inference partially elucidates the observed association between decreased fiber intake and increased ImP levels in certain populations, along with the correlation between a Western diet and elevated ImP content (13, 70, 71). Therefore, future research should focus on elucidating the contributions and mechanisms of factors influencing intestinal pH on ImP levels, thereby providing theoretical insights into targeting ImP-mediated pathways of cardiovascular metabolic damage. Additionally, the inhibitory effect of ImP on the hypoglycemic efficacy of metformin (36) underscores the profound impact of gut microbiota and their metabolites on drug therapy. Exploring the effects of drugs related to the treatment of cardiovascular metabolic diseases on ImP-producing bacteria and ImP content presents a novel and intriguing topic, offering potential new insights into the therapeutic mechanisms of drugs.
Furthermore, while diet has been shown to significantly influence ImP production via its effects on gut microbiota composition, it should be noted that other potential factors influencing ImP levels, such as genetic predisposition or medication use, have not yet been thoroughly investigated. Future research will be needed to explore these dimensions, as well as to further elucidate the mechanistic links between ImP and cardiometabolic diseases.
Lastly, as an increasing number of studies shed light on the correlation between gut bacteria and ImP production, as demonstrated in Table 1, it becomes evident that, like many other investigations focused on the gut microbiome, these studies raise more questions than they answer. Unveiling the precise contributions and mechanisms of these bacteria to ImP production is a vast and intricate endeavor, yet it constitutes a crucial aspect of research on ImP and warrants significant attention in future studies.
The interplay among cardiometabolic diseases underscores the importance of exploring shared metabolic pathways to enhance risk prediction and develop tailored prevention and management strategies. Over the past few years, multiple clinical studies have identified the intestinal metabolite ImP as a potential microbial signature linking insulin resistance, T2D, hypertension, obesity, NAFLD, CKD, atherosclerosis, and HF (Table 2, Figure 1). Our study emphasizes the significance of ImP as a potential biomarker and therapeutic target, highlighting the need for longitudinal research and diverse population participation to validate these associations. Future investigations should prioritize elucidating the molecular mechanisms underlying ImP’s role and its contribution to the pathogenesis of cardiometabolic diseases and related comorbidities, thereby advancing treatments for these conditions.
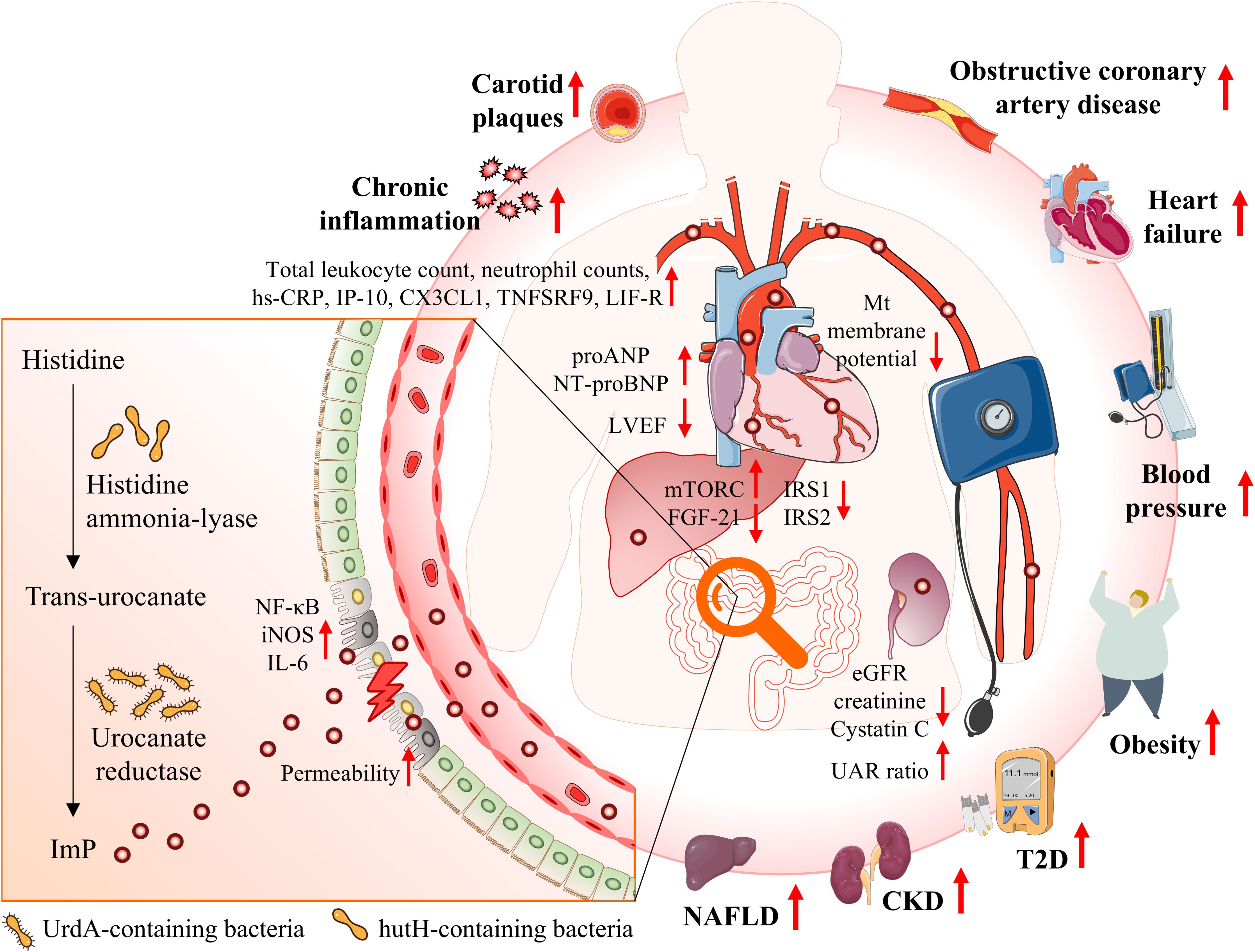
Figure 1. Illustrates the interplay between gut microbiota, ImP production, and cardiometabolic diseases. Epidemiological studies have identified a strong association between elevated ImP levels and an increased risk of onset of T2D and obesity, exacerbation of kidney traits in CKD, progression of atherosclerotic plaques, and elevated mortality rates in HF. ImP is a microbial metabolite derived from histidine via urocanate reductase, an enzyme encoded by the UrdA gene. This pathway is predominantly associated with certain gut bacteria. ImP may contribute to the heightened risk of cardiometabolic diseases through mechanisms such as impaired intestinal barrier function, activation of the p38γ/p62/mTORC1 signaling pathway, promoting systemic inflammation, and impairing cardiac and renal function. CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HF, heart failure; ImP, imidazole propionate; IRS1, insulin receptor substrates 1; IRS2, insulin receptor substrates 2; MT, mitochondria; mTORC1, mechanistic target of rapamycin complex 1; NAFLD, non-alcoholic fatty liver disease; SCFAs, short-chain fatty acids; T2D, type 2 diabetes; UAC, urinary albumin-to-creatinine.
YZ: Data curation, Visualization, Writing – original draft, Writing – review & editing. QW: Writing – original draft. MG: Funding acquisition, Writing – original draft. FT: Visualization, Writing – review & editing. CJ: Visualization, Writing – review & editing. JC: Writing – review & editing, Validation. XT: Writing – review & editing, Validation. CZ: Writing – review & editing, Validation. YL: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. BL: Conceptualization, Supervision, Writing – review & editing. YX: Funding acquisition, Methodology, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study received financial support from the Natural Science Foundation of China (Grant No. 82300911), the Sichuan Science and Technology Program (Grant Nos. 2023ZYD0095, 2023YFS0471, 2022YFS0617, and 2022NSFSC0730), and Scientific Research Funding of Luzhou-Southwest Medical University (Grant No. 2021LZXNYD-J12).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AMPK, adenosine 5′-monophosphate-activated protein kinase; BMI, body mass index; CAD, coronary artery disease; CDAHFD, choline-deficient amino acid-defined high-fat diet; CKD, chronic kidney disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HF, heart failure; ImP, imidazole propionate; IRS1, insulin receptor substrates 1; IRS2, insulin receptor substrates 2; MAPK, mitogen-activated protein kinase; MetaCardis, European multicentric cohort; mTORC1, mechanistic target of rapamycin complex 1; NAFLD, non-alcoholic fatty liver disease; NASH, non−alcoholic steatohepatitis; r, Pearson correlation coefficient; rs, Spearman rank correlation coefficient; SCFAs, short-chain fatty acids; T2D, type 2 diabetes; TLR4, toll-like receptor 4; UAC, urinary albumin-to-creatinine.
1. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. (2020) 76:2982–3021. doi: 10.1016/j.jacc.2020.11.010
2. WHO. Gobal Health Estimates (2020). Available online at: https://www.who.int/data/global-health-estimates (Accessed 03, 2024).
3. Misra S, Aguilar-Salinas CA, Chikowore T, Konradsen F, Ma RCW, Mbau L, et al. The case for precision medicine in the prevention, diagnosis, and treatment of cardiometabolic diseases in low-income and middle-income countries. Lancet Diabetes Endocrinol. (2023) 11:836–47. doi: 10.1016/S2213-8587(23)00164-X
4. Flood D, Guwatudde D, Damasceno A, Manne-Goehler J, Davies JI. Maximising use of population data on cardiometabolic diseases. Lancet Diabetes Endocrinol. (2022) 10:154–7. doi: 10.1016/S2213-8587(21)00328-4
5. Chakaroun RM, Olsson LM, Bäckhed F. The potential of tailoring the gut microbiome to prevent and treat cardiometabolic disease. Nat Rev Cardiol. (2023) 20:217–35. doi: 10.1038/s41569-022-00771-0
6. Witkowski M, Weeks TL, Hazen SL. Gut microbiota and cardiovascular disease. Circ Res. (2020) 127:553–70. doi: 10.1161/CIRCRESAHA.120.316242
7. Yntema T, Koonen DPY, Kuipers F. Emerging roles of gut microbial modulation of bile acid composition in the etiology of cardiovascular diseases. Nutrients. (2023) 15(8):1850. doi: 10.3390/nu15081850
8. Hu T, Wu Q, Yao Q, Jiang K, Yu J, Tang Q. Short-chain fatty acid metabolism and multiple effects on cardiovascular diseases. Ageing Res Rev. (2022) 81:101706. doi: 10.1016/j.arr.2022.101706
9. McGarrah RW, White PJ. Branched-chain amino acids in cardiovascular disease. Nat Rev Cardiol. (2023) 20:77–89. doi: 10.1038/s41569-022-00760-3
10. Dannenberg L, Zikeli D, Benkhoff M, Ahlbrecht S, Kelm M, Levkau B, et al. Targeting the human microbiome and its metabolite TMAO in cardiovascular prevention and therapy. Pharmacol Ther. (2020) 213:107584. doi: 10.1016/j.pharmthera.2020.107584
11. van der Heiden C, Wadman SK, de Bree PK, Wauters EA. Increased urinary imidazolepropionic acid, N-acetylhistamine and other imidazole compounds in patients with intestinal disorders. Clin Chim Acta. (1972) 39:201–14. doi: 10.1016/0009-8981(72)90317-8
12. Koh A, Molinaro A, Ståhlman M, Khan MT, Schmidt C, Mannerås-Holm L, et al. Microbially Produced Imidazole Propionate Impairs Insulin Signaling through mTORC1. Cell. (2018) 175:947–61.e17. doi: 10.1016/j.cell.2018.09.055
13. Molinaro A, Bel Lassen P, Henricsson M, Wu H, Adriouch S, Belda E, et al. Imidazole propionate is increased in diabetes and associated with dietary patterns and altered microbial ecology. Nat Commun. (2020) 11:5881. doi: 10.1038/s41467-020-19589-w
14. van Son J, Serlie MJ, Ståhlman M, Bäckhed F, Nieuwdorp M, Aron-Wisnewsky J. Plasma imidazole propionate is positively correlated with blood pressure in overweight and obese humans. Nutrients. (2021) 13(8):2706. doi: 10.3390/nu13082706
15. Menni C, Zhu J, Le Roy CI, Mompeo O, Young K, Rebholz CM, et al. Serum metabolites reflecting gut microbiome alpha diversity predict type 2 diabetes. Gut Microbes. (2020) 11:1632–42. doi: 10.1080/19490976.2020.1778261
16. Lützhøft DO, Sinioja T, Christoffersen B, Jakobsen RR, Geng D, Ahmad HFB, et al. Marked gut microbiota dysbiosis and increased imidazole propionate are associated with a NASH Göttingen Minipig model. BMC Microbiol. (2022) 22(1):287. doi: 10.1186/s12866-022-02704-w
17. Peters BA, Qi Q, Usyk M, Daviglus ML, Cai J, Franceschini N, et al. Association of the gut microbiome with kidney function and damage in the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Gut Microbes. (2023) 15:2186685. doi: 10.1080/19490976.2023.2186685
18. Wang Z, Peters BA, Bryant M, Hanna DB, Schwartz T, Wang T, et al. Gut microbiota, circulating inflammatory markers and metabolites, and carotid artery atherosclerosis in HIV infection. Microbiome. (2023) 11:119. doi: 10.1186/s40168-023-01566-2
19. Trøseid M, Molinaro A, Gelpi M, Vestad B, Kofoed KF, Fuchs A, et al. Gut microbiota alterations and circulating imidazole propionate levels are associated with obstructive coronary artery disease in people living with HIV. J Infect Dis. (2024) 229(3):898–907. doi: 10.1093/infdis/jiad604
20. Sayols-Baixeras S, Dekkers KF, Baldanzi G, Jönsson D, Hammar U, Lin YT, et al. Streptococcus species abundance in the gut is linked to subclinical coronary atherosclerosis in 8973 participants from the SCAPIS cohort. Circulation. (2023) 148:459–72. doi: 10.1161/CIRCULATIONAHA.123.063914
21. Hua S, Lv B, Qiu Z, Li Z, Wang Z, Chen Y, et al. Microbial metabolites in chronic heart failure and its common comorbidities. EMBO Mol Med. (2023) 15:e16928. doi: 10.15252/emmm.202216928
22. Zheng S, Wang H, Han J, Dai X, Lv Y, Sun T, et al. Microbiota-derived imidazole propionate inhibits type 2 diabetic skin wound healing by targeting SPNS2-mediated S1P transport. iScience. (2023) 26:108092. doi: 10.1016/j.isci.2023.108092
23. Wu J, Wu Y, Feng W, Chen Q, Wang D, Liu M, et al. Role of microbial metabolites of histidine in the development of colitis. Mol Nutr Food Res. (2022) 66:e2101175. doi: 10.1002/mnfr.202101175
24. Acuña I, Ruiz A, Cerdó T, Cantarero S, López-Moreno A, Aguilera M, et al. Rapid and simultaneous determination of histidine metabolism intermediates in human and mouse microbiota and biomatrices. Biofactors. (2022) 48:315–28. doi: 10.1002/biof.v48.2
25. Brosnan ME, Brosnan JT. Histidine metabolism and function. J Nutr. (2020) 150:2570s–5s. doi: 10.1093/jn/nxaa079
26. Venskutonytė R, Koh A, Stenström O, Khan MT, Lundqvist A, Akke M, et al. Structural characterization of the microbial enzyme urocanate reductase mediating imidazole propionate production. Nat Commun. (2021) 12:1347. doi: 10.1038/s41467-021-21548-y
27. Bender RA. Regulation of the histidine utilization (hut) system in bacteria. Microbiol Mol Biol Rev. (2012) 76:565–84. doi: 10.1128/MMBR.00014-12
28. Bogachev AV, Bertsova YV, Bloch DA, Verkhovsky MI. Urocanate reductase: identification of a novel anaerobic respiratory pathway in Shewanella oneidensis MR-1. Mol Microbiol. (2012) 86:1452–63. doi: 10.1111/mmi.2012.86.issue-6
29. Shi C, Han X, Guo W, Wu Q, Yang X, Wang Y, et al. Disturbed Gut-Liver axis indicating oral exposure to polystyrene microplastic potentially increases the risk of insulin resistance. Environ Int. (2022) 164:107273. doi: 10.1016/j.envint.2022.107273
30. Wang Y, Sharma A, Weber KM, Topper E, Appleton AA, Gustafson D, et al. The menopause-related gut microbiome: associations with metabolomics, inflammatory protein markers, and cardiometabolic health in women with HIV. Menopause. (2024) 31:52–64. doi: 10.1097/GME.0000000000002287
31. Wu B, Tan L, Wang W, Feng X, Yan D. Imidazole propionate is increased in diabetes and associated with stool consistency. Diabetes Metab Syndr Obes. (2022) 15:1715–24. doi: 10.2147/DMSO.S362715
32. Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. (2012) 490:55–60. doi: 10.1038/nature11450
33. Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. (2013) 498:99–103. doi: 10.1038/nature12198
34. Shu Y, Sheardown SA, Brown C, Owen RP, Zhang S, Castro RA, et al. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J Clin Invest. (2007) 117(5):1422–31. doi: 10.1172/JCI30558
35. Zhou K, Yee SW, Seiser EL, van Leeuwen N, Tavendale R, Bennett AJ, et al. Variation in the glucose transporter gene SLC2A2 is associated with glycemic response to metformin. Nat Genet. (2016) 48:1055–9. doi: 10.1038/ng.3632
36. Koh A, Mannerås-Holm L, Yunn NO, Nilsson PM, Ryu SH, Molinaro A, et al. Microbial Imidazole Propionate Affects Responses to Metformin through p38γ-Dependent Inhibitory AMPK Phosphorylation. Cell Metab. (2020) 32:643–53.e4. doi: 10.1016/j.cmet.2020.07.012
37. Kong P, Cui ZY, Huang XF, Zhang DD, Guo RJ, Han M. Inflammation and atherosclerosis: signaling pathways and therapeutic intervention. Signal Transduct Target Ther. (2022) 7:131. doi: 10.1038/s41392-022-00955-7
38. Jonsson AL, Bäckhed F. Role of gut microbiota in atherosclerosis. Nat Rev Cardiol. (2017) 14:79–87. doi: 10.1038/nrcardio.2016.183
39. Perkins MV, Joseph SB, Dittmer DP, Mackman N. Cardiovascular disease and thrombosis in HIV infection. Arterioscler Thromb Vasc Biol. (2023) 43:175–91. doi: 10.1161/ATVBAHA.122.318232
40. Shan Z, Clish CB, Hua S, Scott JM, Hanna DB, Burk RD, et al. Gut microbial-related choline metabolite trimethylamine-N-oxide is associated with progression of carotid artery atherosclerosis in HIV infection. J Infect Dis. (2018) 218:1474–9. doi: 10.1093/infdis/jiy356
41. Hsue PY. Mechanisms of cardiovascular disease in the setting of HIV infection. Can J Cardiol. (2019) 35:238–48. doi: 10.1016/j.cjca.2018.12.024
42. Simons KH, de Jong A, Jukema JW, de Vries MR, Arens R, Quax PHA. T cell co-stimulation and co-inhibition in cardiovascular disease: a double-edged sword. Nat Rev Cardiol. (2019) 16:325–43. doi: 10.1038/s41569-019-0164-7
43. Apostolakis S, Spandidos D. Chemokines and atherosclerosis: focus on the CX3CL1/CX3CR1 pathway. Acta Pharmacol Sin. (2013) 34:1251–6. doi: 10.1038/aps.2013.92
44. van Keulen D, van Koeverden ID, Boltjes A, Princen HMG, van Gool AJ, de Borst GJ, et al. Common variants associated with OSMR expression contribute to carotid plaque vulnerability, but not to cardiovascular disease in humans. Front Cardiovasc Med. (2021) 8:658915. doi: 10.3389/fcvm.2021.658915
45. Pickett JR, Wu Y, Zacchi LF, Ta HT. Targeting endothelial vascular cell adhesion molecule-1 in atherosclerosis: drug discovery and development of vascular cell adhesion molecule-1-directed novel therapeutics. Cardiovasc Res. (2023) 119:2278–93. doi: 10.1093/cvr/cvad130
46. Libby P. The changing landscape of atherosclerosis. Nature. (2021) 592:524–33. doi: 10.1038/s41586-021-03392-8
47. Badimon L, Vilahur G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J Intern Med. (2014) 276:618–32. doi: 10.1111/joim.2014.276.issue-6
48. Dunlay SM, Givertz MM, Aguilar D, Allen LA, Chan M, Desai AS, et al. Type 2 Diabetes Mellitus and Heart Failure: A Scientific Statement From the American Heart Association and the Heart Failure Society of America: This statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation. (2019) 140:e294–324. doi: 10.1161/CIR.0000000000000691
49. Pietzner M, Stewart ID, Raffler J, Khaw KT, Michelotti GA, Kastenmüller G, et al. Plasma metabolites to profile pathways in noncommunicable disease multimorbidity. Nat Med. (2021) 27:471–9. doi: 10.1038/s41591-021-01266-0
50. Murthy VL, Yu B, Wang W, Zhang X, Alkis T, Pico AR, et al. Molecular signature of multisystem cardiometabolic stress and its association with prognosis. JAMA Cardiol. (2020) 5:1144–53. doi: 10.1001/jamacardio.2020.2686
51. Molinaro A, Nemet I, Bel Lassen P, Chakaroun R, Nielsen T, Aron-Wisnewsky J, et al. Microbially produced imidazole propionate is associated with heart failure and mortality. JACC Heart Fail. (2023) 11:810–21. doi: 10.1016/j.jchf.2023.03.008
52. Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. (2021) 397:2212–24. doi: 10.1016/S0140-6736(20)32511-3
53. Tilg H, Adolph TE, Trauner M. Gut-liver axis: Pathophysiological concepts and clinical implications. Cell Metab. (2022) 34:1700–18. doi: 10.1016/j.cmet.2022.09.017
54. Yang S, Yu D, Liu J, Qiao Y, Gu S, Yang R, et al. Global publication trends and research hotspots of the gut-liver axis in NAFLD: A bibliometric analysis. Front Endocrinol (Lausanne). (2023) 14:1121540. doi: 10.3389/fendo.2023.1121540
55. Shashni B, Tajika Y, Ikeda Y, Nishikawa Y, Nagasaki Y. Self-assembling polymer-based short chain fatty acid prodrugs ameliorate non-alcoholic steatohepatitis and liver fibrosis. Biomaterials. (2023) 295:122047. doi: 10.1016/j.biomaterials.2023.122047
56. Blaak EE, Canfora EE, Theis S, Frost G, Groen AK, Mithieux G, et al. Short chain fatty acids in human gut and metabolic health. Benef Microbes. (2020) 11:411–55. doi: 10.3920/BM2020.0057
57. Firrman J, Liu L, Mahalak K, Tanes C, Bittinger K, Tu V, et al. The impact of environmental pH on the gut microbiota community structure and short chain fatty acid production. FEMS Microbiol Ecol. (2022) 98(5):fiac038. doi: 10.1093/femsec/fiac038
58. Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol. (2020) 72:558–77. doi: 10.1016/j.jhep.2019.10.003
59. Ruilope LM, Ortiz A, Lucia A, Miranda B, Alvarez-Llamas G, Barderas MG, et al. Prevention of cardiorenal damage: importance of albuminuria. Eur Heart J. (2023) 44:1112–23. doi: 10.1093/eurheartj/ehac683
60. Zoccali C, Mark PB, Sarafidis P, Agarwal R, Adamczak M, Bueno de Oliveira R, et al. Diagnosis of cardiovascular disease in patients with chronic kidney disease. Nat Rev Nephrol. (2023) 19(11):733–46. doi: 10.1038/s41581-023-00747-4
61. Krukowski H, Valkenburg S, Madella AM, Garssen J, van Bergenhenegouwen J, Overbeek SA, et al. Gut microbiome studies in CKD: opportunities, pitfalls and therapeutic potential. Nat Rev Nephrol. (2023) 19(2):87–101. doi: 10.1038/s41581-022-00647-z
62. Jazani NH, Savoj J, Lustgarten M, Lau WL, Vaziri ND. Impact of gut dysbiosis on neurohormonal pathways in chronic kidney disease. Diseases. (2019) 7(1):21. doi: 10.3390/diseases7010021
63. Chen Q, Ren D, Liu L, Xu J, Wu Y, Yu H, et al. Ginsenoside compound K ameliorates development of diabetic kidney disease through inhibiting TLR4 activation induced by microbially produced imidazole propionate. Int J Mol Sci. (2022) 23(21):12863. doi: 10.3390/ijms232112863
64. Vieira-Silva S, Falony G, Belda E, Nielsen T, Aron-Wisnewsky J, Chakaroun R, et al. Statin therapy is associated with lower prevalence of gut microbiota dysbiosis. Nature. (2020) 581:310–5. doi: 10.1038/s41586-020-2269-x
65. Clemente-Suárez VJ, Beltrán-Velasco AI, Redondo-Flórez L, Martín-Rodríguez A, Tornero-Aguilera JF. Global impacts of western diet and its effects on metabolism and health: A narrative review. Nutrients. (2023) 15(12):2749. doi: 10.3390/nu15122749
66. Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: A comprehensive review. Circulation. (2016) 133:187–225. doi: 10.1161/CIRCULATIONAHA.115.018585
67. Threapleton DE, Greenwood DC, Evans CE, Cleghorn CL, Nykjaer C, Woodhead C, et al. Dietary fibre intake and risk of cardiovascular disease: systematic review and meta-analysis. Bmj. (2013) 347:f6879. doi: 10.1136/bmj.f6879
68. Alissa EM, Ferns GA. Dietary fruits and vegetables and cardiovascular diseases risk. Crit Rev Food Sci Nutr. (2017) 57(9):1950–62. doi: 10.1080/10408398.2015.1040487
69. Gold A, Zhu J. Not just a gut feeling: a deep exploration of functional bacterial metabolites that can modulate host health. Gut Microbes. (2022) 14:2125734. doi: 10.1080/19490976.2022.2125734
70. Wellington N, Shanmuganathan M, de Souza RJ, Zulyniak MA, Azab S, Bloomfield J, et al. Metabolic trajectories following contrasting prudent and western diets from food provisions: identifying robust biomarkers of short-term changes in habitual diet. Nutrients. (2019) 11(10):2407. doi: 10.3390/nu11102407
71. Nishimoto Y, Mizuguchi Y, Mori Y, Ito M, Miyazato S, Kishimoto Y, et al. Resistant maltodextrin intake reduces virulent metabolites in the gut environment: A randomized control study in a Japanese cohort. Front Microbiol. (2022) 13:644146. doi: 10.3389/fmicb.2022.644146
72. Simpson HL, Campbell BJ. Review article: dietary fibre-microbiota interactions. Aliment Pharmacol Ther. (2015) 42:158–79. doi: 10.1111/apt.13248
73. Requena T, Martínez-Cuesta MC, Peláez C. Diet and microbiota linked in health and disease. Food Funct. (2018) 9:688–704. doi: 10.1039/C7FO01820G
74. Meslier V, Laiola M, Roager HM, De Filippis F, Roume H, Quinquis B, et al. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut. (2020) 69:1258–68. doi: 10.1136/gutjnl-2019-320438
75. Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. (2013) 500:541–6. doi: 10.1038/nature12506
76. Gomes AC, Hoffmann C, Mota JF. The human gut microbiota: Metabolism and perspective in obesity. Gut Microbes. (2018) 9:308–25. doi: 10.1080/19490976.2018.1465157
77. Chen Z, Radjabzadeh D, Chen L, Kurilshikov A, Kavousi M, Ahmadizar F, et al. Association of insulin resistance and type 2 diabetes with gut microbial diversity: A microbiome-wide analysis from population studies. JAMA Netw Open. (2021) 4:e2118811. doi: 10.1001/jamanetworkopen.2021.18811
78. Deehan EC, Yang C, Perez-Muñoz ME, Nguyen NK, Cheng CC, Triador L, et al. Precision microbiome modulation with discrete dietary fiber structures directs short-chain fatty acid production. Cell Host Microbe. (2020) 27:389–404.e6. doi: 10.1016/j.chom.2020.01.006
79. Gou W, Miao Z, Deng K, Zheng JS. Nutri-microbiome epidemiology, an emerging field to disentangle the interplay between nutrition and microbiome for human health. Protein Cell. (2023) 14:787–806. doi: 10.1093/procel/pwad023
80. Jiang Z, Sun TY, He Y, Gou W, Zuo LS, Fu Y, et al. Dietary fruit and vegetable intake, gut microbiota, and type 2 diabetes: results from two large human cohort studies. BMC Med. (2020) 18:371. doi: 10.1186/s12916-020-01842-0
81. Breuninger TA, Wawro N, Breuninger J, Reitmeier S, Clavel T, Six-Merker J, et al. Associations between habitual diet, metabolic disease, and the gut microbiota using latent Dirichlet allocation. Microbiome. (2021) 9:61. doi: 10.1186/s40168-020-00969-9
82. Asnicar F, Berry SE, Valdes AM, Nguyen LH, Piccinno G, Drew DA, et al. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Nat Med. (2021) 27:321–32. doi: 10.1038/s41591-020-01183-8
83. Liu Y, Ajami NJ, El-Serag HB, Hair C, Graham DY, White DL, et al. Dietary quality and the colonic mucosa-associated gut microbiome in humans. Am J Clin Nutr. (2019) 110:701–12. doi: 10.1093/ajcn/nqz139
84. Deschasaux M, Bouter KE, Prodan A, Levin E, Groen AK, Herrema H, et al. Depicting the composition of gut microbiota in a population with varied ethnic origins but shared geography. Nat Med. (2018) 24:1526–31. doi: 10.1038/s41591-018-0160-1
85. He Y, Wu W, Zheng HM, Li P, McDonald D, Sheng HF, et al. Regional variation limits applications of healthy gut microbiome reference ranges and disease models. Nat Med. (2018) 24:1532–5. doi: 10.1038/s41591-018-0164-x
86. Windey K, De Preter V, Verbeke K. Relevance of protein fermentation to gut health. Mol Nutr Food Res. (2012) 56:184–96. doi: 10.1002/mnfr.201100542
Keywords: imidazole propionate, cardiometabolic disease, diabetes, microbiome, microbial metabolites, biomarker
Citation: Zeng Y, Wu Q, Guo M, Teng F, Jiang C, Chen J, Tan X, Zeng C, Long Y, Law BY-K and Xu Y (2025) Gut microbiota-derived imidazole propionate: an emerging target for the prevention and treatment of cardiometabolic diseases. Front. Endocrinol. 16:1409119. doi: 10.3389/fendo.2025.1409119
Received: 29 March 2024; Accepted: 23 January 2025;
Published: 17 February 2025.
Edited by:
Nigel Irwin, Ulster University, United KingdomReviewed by:
Amélia M Sarmento, Fernando Pessoa University, PortugalCopyright © 2025 Zeng, Wu, Guo, Teng, Jiang, Chen, Tan, Zeng, Long, Law and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Xu, eHl3eWxsQHN3bXUuZWR1LmNu; Betty Yuen-Kwan Law, eWtsYXdAbXVzdC5lZHUubW8=; Yang Long, bG9uZ3lhbmcwMjE3QHN3bXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.