
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 10 January 2025
Sec. Clinical Diabetes
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1527936
This article is part of the Research Topic Digital Technology in the Management and Prevention of Diabetes: Volume II View all 12 articles
Background: The clinical characteristics and risk factors for opportunistic infections in HIV patients with concomitant diabetes mellitus are unclear and worth studying. Explore the risk factors and construct a predictive model for opportunistic infections in HIV-DM patients.
Methods: Clinical data were retrospectively collected from 1,669 HIV-DM admitted to the Public Health Clinical Center of Chengdu from December 2018 to November 2023.Based on the occurrence of opportunistic infections, the patients were divided into two groups. Comparative analyses were conducted to assess nutritional status, plasma glucose levels, comorbidities of chronic noncommunicable diseases, and immune status. We applied univariate logistic regression and LASSO regression to screen independent variables. Multivariable logistic regression analysis was performed to identify risk factors and establish a prediction model for opportunistic infections.
Results: In our study population of 1,669 patients (median age was 59 years, 83.22% male), 868 developed opportunistic infections, while the remaining 801 patients did not develop opportunistic infections. Additionally, 1,598 patients survived, and 71 patients died. The opportunistic infection group had a greater median age, lower median BMI, longer median length of hospital stay and greater number of deaths. Logistic regression analysis identified nutritional risk, duration of HIV infection, HbA1c, albumin, and CD4+ T cell counts as significant risk factors and predictors of opportunistic infections. Nutritional risk (OR=2.888) and HbA1c showed positive associations (P<0.001), while duration of HIV infection, albumin, and CD4+ T cell counts demonstrated negative associations with infection risk (P<0.05). The comprehensive model evaluation, based on receiver operating characteristic (ROC) curve, calibration curve, and decision curve analysis (DCA), validated the acceptable predictive performance of our model.
Conclusion: This study identified nutritional risk, duration of HIV infection, HbA1c and albumin as significant risk factors and predictors of opportunistic infections in HIV-DM patients, further highlighting the importance of nutritional screening and good glucose control.
In recent years, the application of highly active antiretroviral therapy (HAART) has significantly improved the life expectancy of patients who are human immunodeficiency virus (HIV)-positive, bringing their lifespan closer to that of the general population (1). However, this advancement has concurrently led to a rising prevalence of noncommunicable diseases, including chronic metabolic and cardiovascular diseases such as diabetes mellitus. Diabetes mellitus is relatively common among individuals living with human immunodeficiency virus (2). The use of highly active antiretroviral therapy appears to exacerbate metabolic and endocrine dysfunctions, including insulin resistance, dyslipidemia, and lipodystrophy, thus contributing to an increased disease burden (3–5). More significantly, the coexistence of human immunodeficiency virus infection and diabetes mellitus can intensify immune dysfunction and inflammatory responses (6), consequently increasing susceptibility to opportunistic infections.
Opportunistic infections refer to a spectrum of infections caused by organisms (bacterial, viral, fungal, and protozoal pathogens) that are generally harmless in individuals with normal immune function but manifest as significant infections in immunocompromised hosts, most notably in HIV-infected individuals with reduced CD4+ T-lymphocyte counts (7). Despite opportunistic infections remaining the leading cause of mortality and substantial economic burden in HIV patients, the implementation of preventive strategies has successfully improved survival outcomes while reducing healthcare expenditures (8).The risk of opportunistic infections can be mitigated not only through conventional preventive measures such as co-infection screening, antimicrobial prophylaxis, vaccinations, and exposure risk reduction (9), but also through comprehensive clinical management incorporating nutritional risk assessment, immunological monitoring, and systematic disease evaluation.
Individuals with human immunodeficiency virus infection who develop opportunistic infections frequently demonstrate compromised nutritional and immunological status, characterized by lower BMI,CD4+ T-cell counts and reduced baseline hemoglobin levels (10–13).Poor glycemic homeostasis can synergistically amplify immune system dysfunction and inflammatory cascades, consequently heightening the risk of opportunistic infections (6). While chronic comorbidities have been established as risk factors for opportunistic infections (14), the relative contribution of particular comorbid conditions to infection susceptibility remains to be determined.
Although extensive research has been conducted on opportunistic infections in patients with human immunodeficiency virus, these studies have paid limited attention to the concurrent presence of diabetes mellitus. In the context of this dual diagnosis, elucidating the complex interplay between nutritional status, glycemic level, and immune function, additional comorbid conditions, and the development of opportunistic infections remains a significant clinical and research challenge. The objective of this study was to investigate the impact of nutritional status, glycemic level, immune function and multiple comorbidities on the incidence of opportunistic infections in human immunodeficiency virus infection and diabetes mellitus (HIV-DM) patients.
In this retrospective study, we analyzed data from 1,669 patients who were hospitalized at the Public Health Clinical Center of Chengdu between December 2018 and November 2023.The inclusion criteria were as follows: 1. Age ≥ 18 years; 2. Met the diagnostic criteria for HIV and diabetes mellitus. Exclusion criteria: 1.Hospitalization duration ≤ 1 day;2.All baseline data and laboratory diagnostic indicators are missing.
The diagnosis of AIDS and AIDS-related opportunistic infections conformed to the criteria set forth in the “Chinese Guidelines for the Diagnosis and Treatment of HIV/AIDS (2018 Edition) (15). “ The diagnosis and classification criteria for diabetes adhered to those outlined in the “Chinese Guidelines for the Prevention and Treatment of Type 2 Diabetes in China (2020 Edition) (16). “
The 1,669 HIV-infected patients with diabetes were categorized into two sets of groups based on different criteria. Patients were classified into the opportunistic infections group (OIs group, n=868) and the non-opportunistic infections group (non-OIs group, n=801) according to the presence or absence of opportunistic infections. (Tables 1, 2).
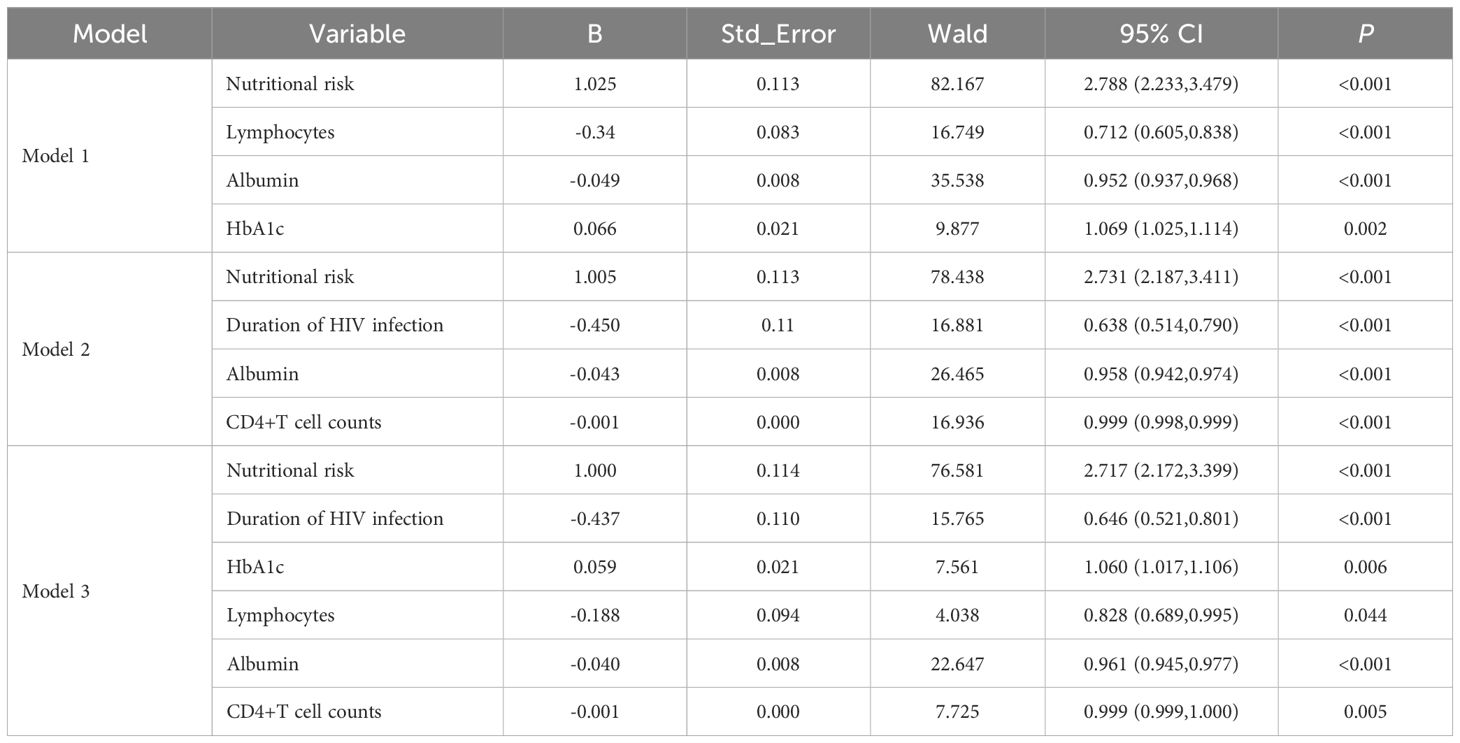
Table 2. Results of multivariate logistic regression of risk factor analysis models for opportunistic infections.
Researchers collected data from 1,669 patients hospitalized at the Public Health Clinical Center of Chengdu between December 2018 and November 2023. The collected data included demographic details, clinical information, and laboratory results. Upon admission, the patients underwent nutritional risk assessment using the Nutritional Risk Screening 2002 (NRS2002) tool. The NRS2002 score ranges from 0 to 7, with scores ≥3 indicating risk of malnutrition and scores <3 indicating no nutritional risk (17). Non-achievement of glycemic targets at discharge was defined as elevated fasting or random plasma glucose levels above normal ranges on either the discharge day or the day prior. A database was established with stringent control measures implemented to ensure the accuracy, completeness, and authenticity of all the data. All personal identifiers have been removed from the dataset to ensure complete anonymization and protect patient privacy in accordance with ethical guidelines and data protection regulations.
Missing covariates were handled using multiple imputation. We conducted a comprehensive assessment of missing data patterns before performing multiple imputation. The proportion of missing values varied across variables (range: 0.72%-37.21%), with HbA1c having a missing rate of 37.21%. Despite this relatively higher missing rate, HbA1c was retained in the analysis given its established clinical significance and the acceptable missing rate (18), while all other variables demonstrated acceptable missing rates below 30%. The normality of continuous variables was assessed using the Shapiro-Wilk test. All continuous variables showed non-normal distribution (P<0.05). Based on these results, we employed non-parametric statistical methods: medians with IQRs for descriptive statistics and Mann-Whitney U tests for group comparisons. For categorical variables, chi-square tests were used for nominal variables, while rank-sum tests were applied for ordinal variables. Variable selection was conducted through univariate logistic regression and least absolute shrinkage and selection operator (LASSO) regression. LASSO regression is a regularized regression method that combines variable selection and regularization to enhance model prediction accuracy and interpretability. By adding a penalty term (L1 regularization) to the regression, LASSO effectively shrinks some coefficients to exactly zero, thereby automatically performing feature selection and producing more parsimonious models. This characteristic makes LASSO particularly valuable in scenarios with multiple potential predictors, as it helps identify the most relevant variables while reducing the risk of overfitting. Subsequently, a multivariate logistic regression model was constructed. Model performance was evaluated using receiver operating characteristic (ROC) curves. For model development, the dataset was randomly partitioned into a training set (70% of the data) and a test set (30%). The prediction model was developed using logistic regression algorithm, based on independent variables from the model with an optimal area under the curve (AUC). Model stability and generalizability were assessed through ten-fold cross-validation prior to construction. Model performance was evaluated on both training and test datasets using receiver operating characteristic curves, calibration plots, and decision curve analysis (DCA). DCA is a method for evaluating prediction models and diagnostic tests that takes into account clinical consequences. The analysis produces a ‘net benefit’ for prediction models by summing the benefits (true positives) and subtracting the harms (false positives). The DCA plots show the net benefit against threshold probability, where the threshold probability represents the probability at which a clinician would choose to treat. The analysis compares the prediction model with two extreme scenarios: treating all patients and treating no patients. A model is considered to have clinical utility if it shows higher net benefit than both the treat-all and treat-none strategies across a reasonable range of threshold probabilities. A nomogram was constructed to provide a visual representation of the prediction model. Statistical analyses were performed using R software (version 4.4.2).
Neither patients nor the public were involved in developing research questions or designing this study. Although all patients received verbal and written information about the study, they were not involved in participant recruitment or study conduct. The burden of participation was assessed by the research team. Patient eligibility assessment and data collection were performed by the investigators. The Ethics Committee of the Public Health Clinical Center of Chengdu waived the requirement for informed consent due to the retrospective nature of this study. All participants were informed that any published data (without personal identifiers) would be freely accessible to the public via the internet. General results will be available to participants upon request.
This study enrolled 1,669 patients with HIV-DM. The study population consisted primarily of middle-aged male patients. The data indicated that 52.01% of patients had opportunistic infections, with 16.02% presenting with multiple types of opportunistic infections. Comorbidities were present in 51.71% of patients, with 50.75% of these cases involving multiple concurrent conditions (Table 1).
Statistical analyses revealed distinct clinical characteristics between opportunistic infections and non-opportunistic infections groups (Table 1). Patients with opportunistic infections exhibited characteristics higher age, lower body mass index, and extended hospital stays compared to those without opportunistic infections(P<0.001). Laboratory analyses demonstrated reduced levels of nutritional indicators in the opportunistic infections group, including albumin (ALB) and total protein, accompanied by an increased prevalence of nutritional risk. Immunological parameters differed significantly between groups. The opportunistic infections group demonstrated reduced lymphocyte counts (LYM) and lower T-cell subset levels, including CD3+T cell counts, CD4+T cell counts and CD8+T cell counts (Figures 1A–D).
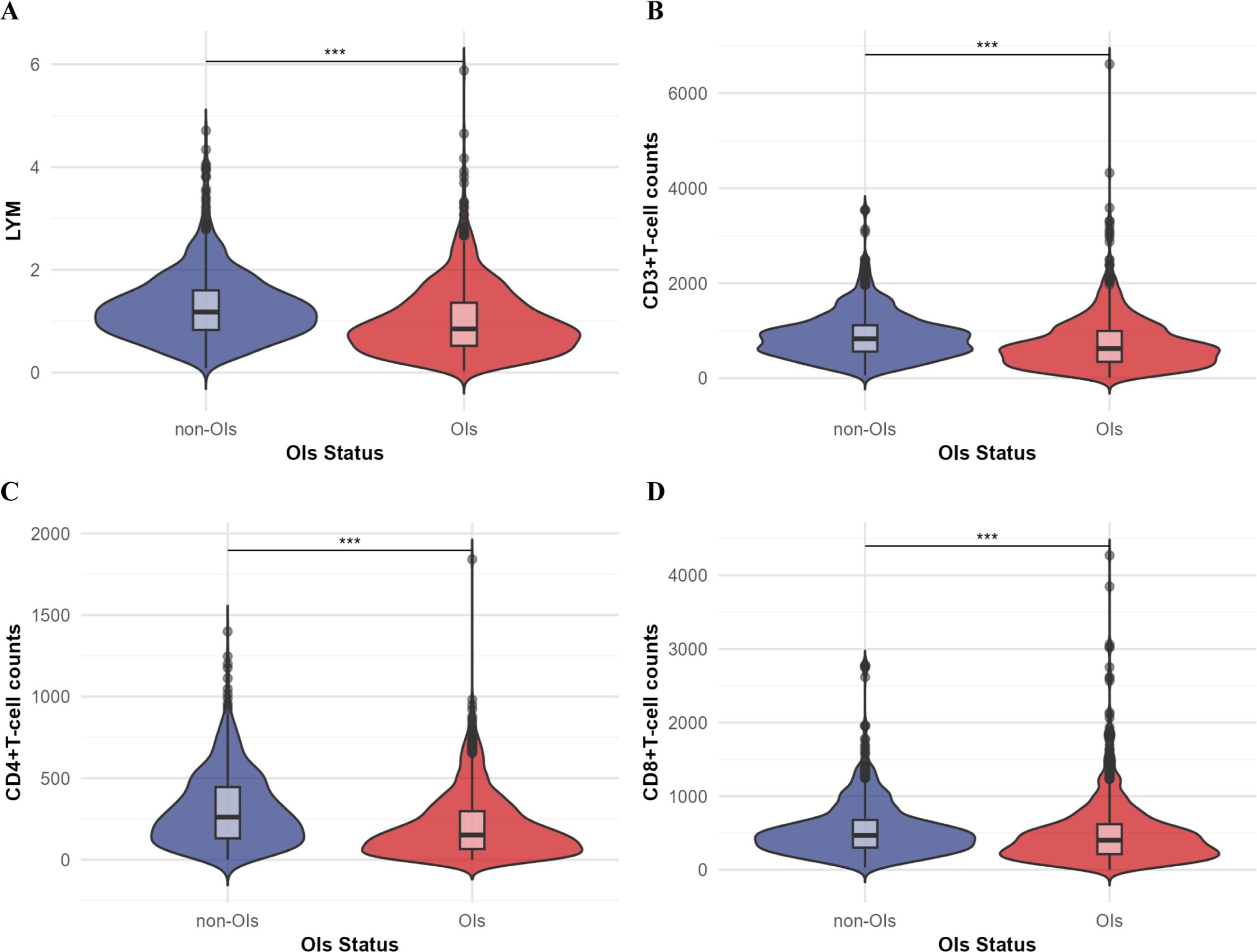
Figure 1. Comparison of T-cell subsets (unit: cells/ul) and lymphocyte counts (LYM) between the OIs group and the non-OIs group. (A) Lymphocyte counts; (B) CD3+ T-cell counts; (C) CD4+ T-cell counts; (D) CD8+ T-cell counts. Comparison between the two groups was performed by Mann-Whitney U test, ***P<0.001.
Patients in the opportunistic infections group demonstrated impaired glycemic control, characterized by elevated glycosylated hemoglobin (HbA1c) levels and lower proportion of patients achieving glycemic targets at hospital discharge. This group also exhibited higher incidence of acute diabetic complications and increased mortality rates.
The correlations among all independent variables and key independent variables are presented in Supplementary Figures 1, 2, respectively.
The potential risk factors for opportunistic infections were initially analyzed using univariate logistic regression and LASSO regression. The univariate analysis included 27 candidate variables (Figure 2). In the LASSO regression, we selected the optimal λ value within one standard deviation of the minimum binomial deviation (Figures 3A, B). This approach identified six significant variables including nutritional risk, duration of HIV infection, high-sensitivity C-reactive protein (h-CRP), lymphocyte counts, albumin, and CD4+ T-cell counts. Multivariate logistic regression analysis was performed on variables selected by both methods, establishing Model 1 with variables identified from univariate logistic regression and Model 2 with variables derived from LASSO regression. Variables that remained significant (P<0.05) in both models were then combined to create Model 3 (Table 2). Based on these analyses, we established three multivariate logistic regression models to identify significant risk factors. In Model 1, the presence of nutritional risk, low lymphocyte counts, low serum albumin levels and elevated HbA1c levels were identified as significant risk factors for opportunistic infections(P<0.05). Model 2 identified nutritional risk, duration of HIV infection, low serum albumin levels, and low CD4+ T-cell counts as significant risk factors (P<0.001). Model 3 integrated the consistently significant risk factors from the previous two models, further validating their associations with opportunistic infections(P<0.05).
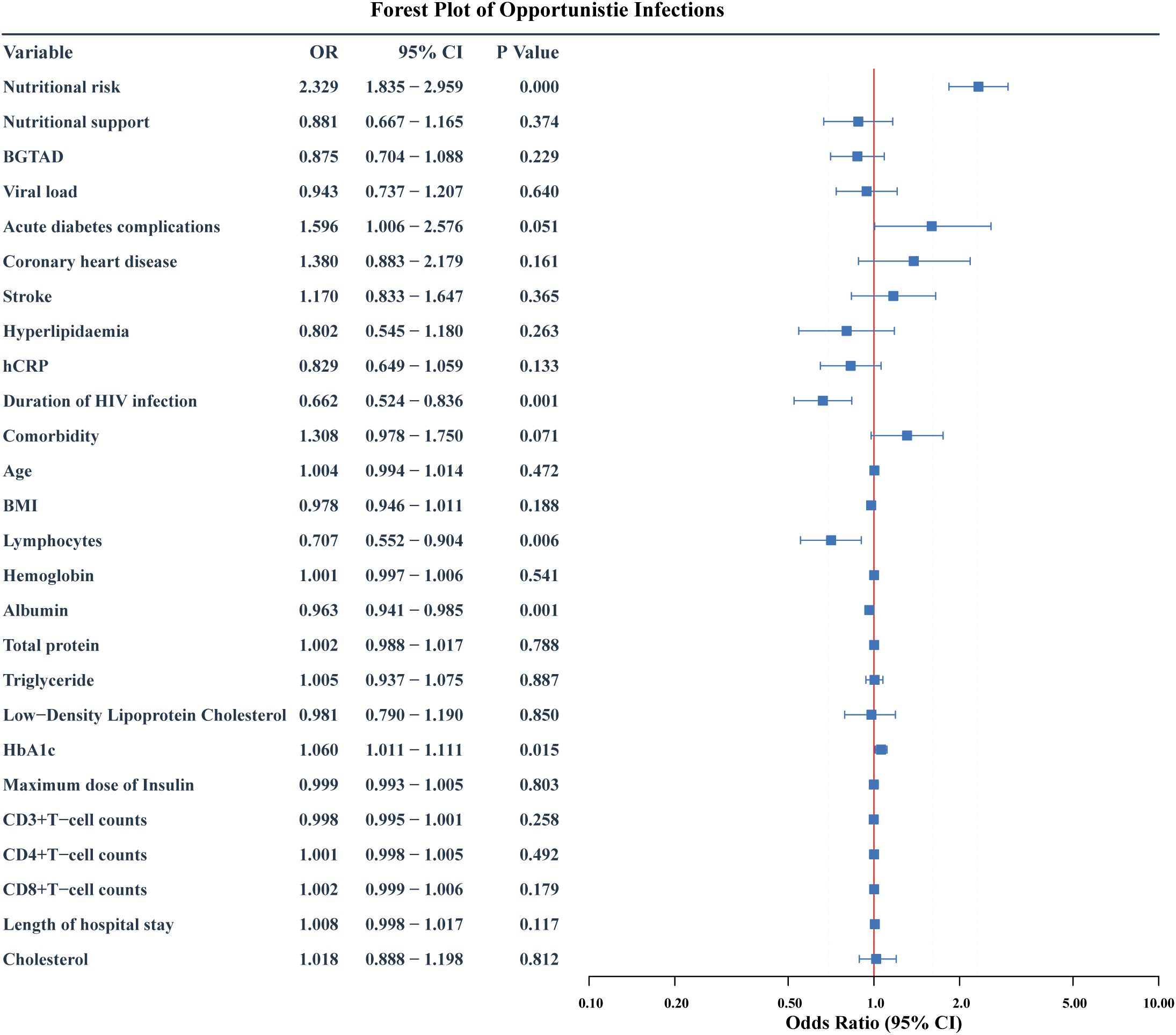
Figure 2. Forest plot of risk factors for opportunistic infections: initial multivariate Model 1. The forest plot shows the odds ratio (OR), 95% confidence interval (95%CI) and p value of HIV-DM patients for developing opportunistic infection after multivariate logistic regression. BGTAD, Blood glucose target achievement at discharge.
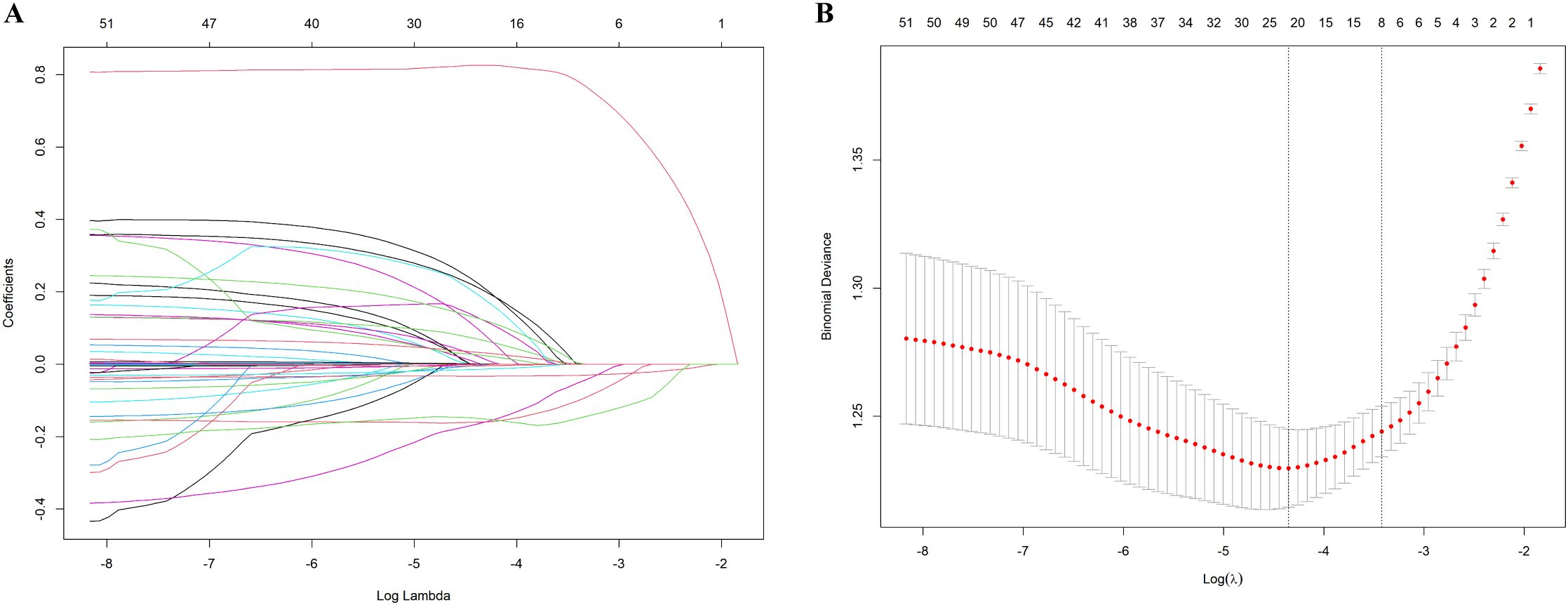
Figure 3. Results of the LASSO regression. (A) The optimal parameter λ in LASSO regression model with logλ as abscissa and regression coefficient as ordinate; (B) 10-fold cross-validation by minimum criteria, adjusting the parameter (λ) selection in the LASSO model, where log(λ) is the lower abscissa, binomial deviation is the ordinate, and the number of variables is the upper abscissa.
The discriminative ability of the three models was assessed using ROC curve analysis, which showed moderate performance with areas under the curve of 0.719(95%CI:0.694-0.743), 0.724(95%CI:0.699-0.748), and 0.730(95%CI:0.705-0.754) for Models 1, 2 and 3, respectively (Figure 4).
A prediction model was developed using the six independent variables selected from Model 3, including nutritional risk, duration of HIV infection, lymphocyte counts, albumin, HbA1c and CD4+ T-cell counts. Multivariate logistic regression analysis (Table 3) revealed that nutritional risk (OR=2.859, 95%CI: 2.184-3.750) and HbA1c (OR=1.054, 95%CI:1.002-1.109) were positively associated with opportunistic infections (P<0.05). Duration of HIV infection (OR=0.576, 95%CI:0.444-0.747), albumin (OR=0.956, 95%CI:0.936, 0.976) and CD4+T cell counts (OR=0.999, 95%CI: 0.998,1.000) showed negative associations with opportunistic infections (P<0.05). No significant association was found between lymphocyte counts and opportunistic infections (P>0.05).
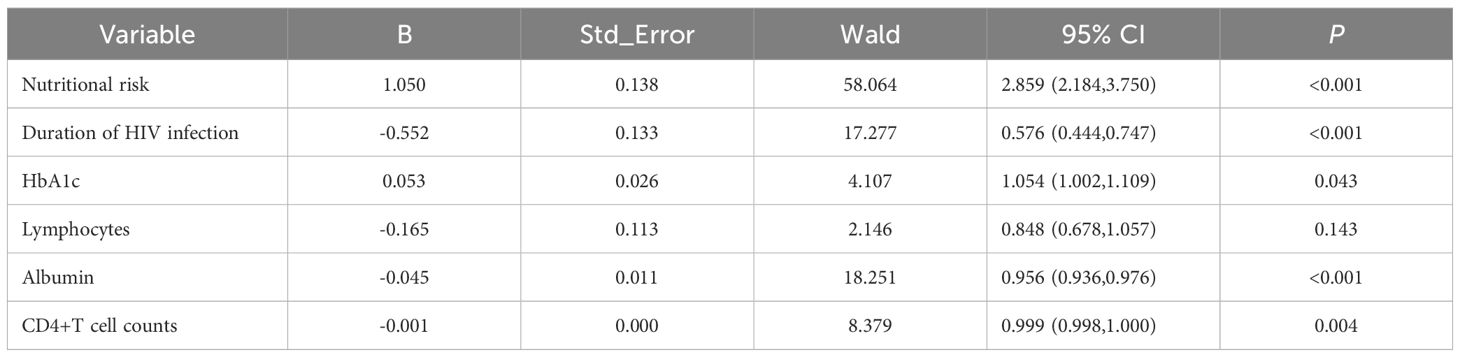
Table 3. Results of multivariate logistic regression of predictive model for opportunistic infections.
In the training dataset, the model demonstrated consistency across different data partitions with an area under the curve of 0.744 ± 0.034 in ten-fold cross-validation.
Evaluation revealed area under the curves of 0.748(95%CI:0.720-0.777) and 0.682(95%CI:0.635-0.728) for the training and test datasets, respectively (Figure 5A). The calibration plot showed good agreement between predicted and observed probabilities across the entire probability range in the training dataset. For the test dataset, while slight overestimation was observed in the low-probability region (0.2-0.4), the model maintained good calibration performance in both middle and high-probability regions (Figures 5B, C).Decision curve analysis demonstrated that the predictive model provided greater net benefits compared to the ‘treat-all’ or ‘treat-none’ strategies, with threshold probabilities ranging from 0.2 to 0.75 in the training dataset and 0.3 to 0.8 in the test dataset (Figure 5D).The nomogram incorporated six variables: HbA1c, CD4+T cell counts, serum albumin, lymphocyte counts, duration of HIV infection and nutritional risk. The prediction probability ranges from 0.06 to 0.90, with total points ranging from approximately 280 to 480. The example patient (red dots) achieved a total score of 461 points, corresponding to a predicted opportunistic infections probability of 0.897 (Figure 6).
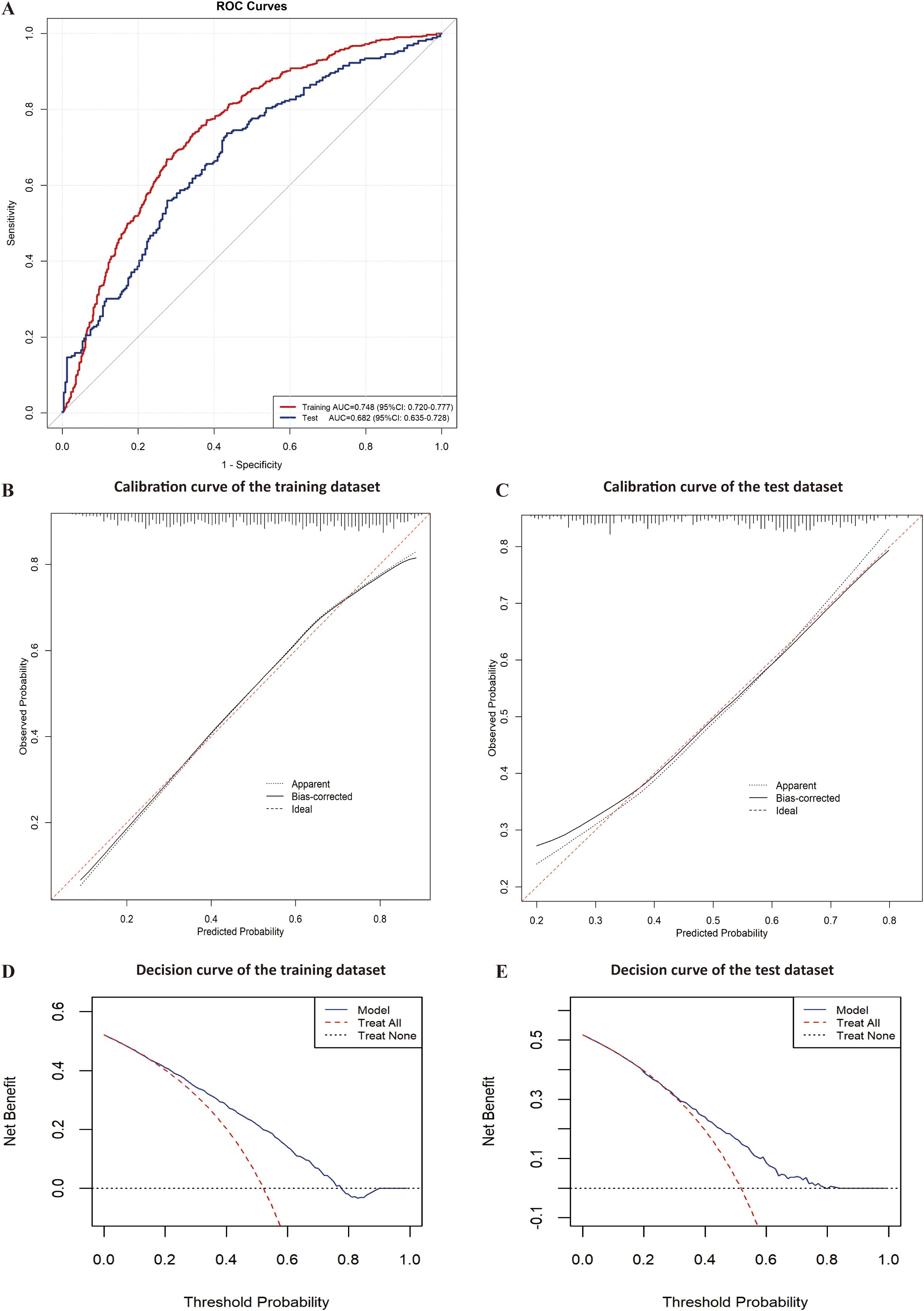
Figure 5. Evaluation of the predictive model for opportunistic infections. (A) ROC curve of the training and test datasets for the predictive model; (B) Calibration curve of the training dataset for the predictive model; (C) Calibration curve of the test dataset for the predictive model; (D) Decision curve of the training dataset for the predictive model; (E) Decision curve of the test dataset for the predictive model.
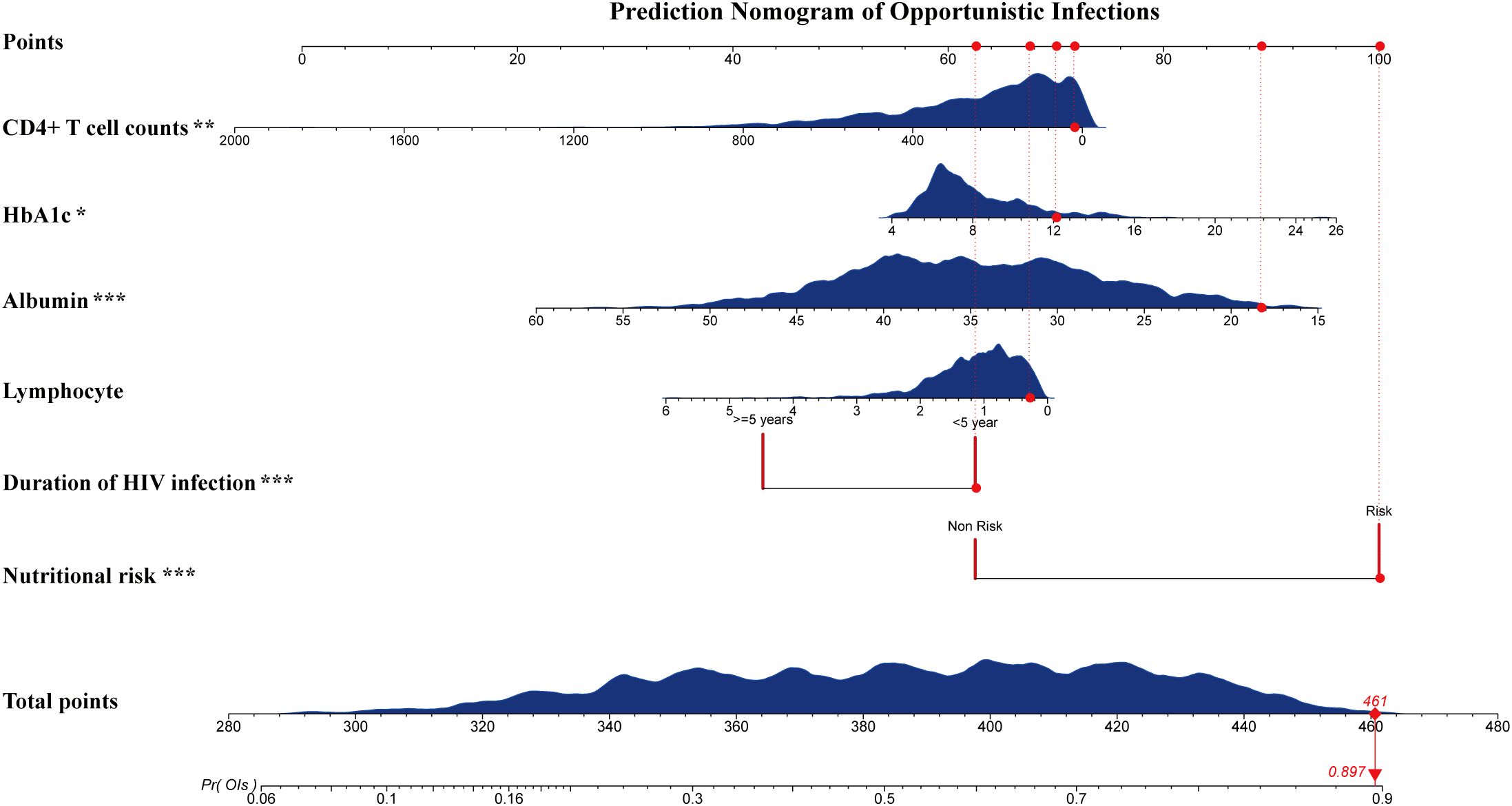
Figure 6. Nomograms of the predictive model for opportunistic infection. Locate the patient’s value for each variable on the corresponding axis, and draw a vertical line to the ‘Points’ axis to determine scores. Sum the scores and find the total on the ‘Total Points’ axis to assess the predicted probability of opportunistic infections. The example patient (red dots, with nutritional risk; duration of HIV infection: <5 years; Lymphocyte: 0.29(10^9/L); Albumin: 18.3 g/L; HbA1c: 12.1%; CD4+ T cell counts: 20 cells/μL) achieved a total score of 461 points, corresponding to a predicted opportunistic infections probability of 0.897. Nutritional risk, identified as ‘Non risk’ (without malnutrition risk) or ‘Risk’ (with malnutrition risk). *P<0.05, **P<0.01, ***P<0.001.
With the advent of HAART and the increase in the prevalence of chronic noncommunicable diseases, the number of patients with HIV infection combined with diabetes is gradually increasing. Opportunistic infections remain a major threat to HIV-DM patients, not only increasing the medication burden (19)but also potentially delaying the initiation of antiretroviral therapy (20), which is extremely detrimental to patient prognosis. In this retrospective study, opportunistic infections in HIV-DM patients accounted for 52.01% of the total HIV-DM patients, with a mortality rate of 4.25%. The age of patients in the opportunistic infections group was greater than that in the non-opportunistic infections group, and they had a lower BMI and longer length of hospital stay consistent with previous studies (21).
In our study, serum albumin level was identified as both a significant predictor and protective factor against opportunistic infections. Previous studies have primarily focused on a low albumin level as a mortality risk factor in HIV-infected patients (22), with both low albumin level and opportunistic infections being independent predictors of poor survival (23, 24). Our findings establish a direct link between albumin level and the occurrence of opportunistic infections, suggesting a potential mechanistic pathway. Low albumin level, indicating poor nutritional status (25) and impaired immune function (26), may increase susceptibility to opportunistic infections, which could subsequently contribute to increased mortality risk. This interconnection highlights the dual clinical significance of serum albumin monitoring and maintenance in HIV-infected patients, serving as both a preventive measure against opportunistic infections and a potential determinant of survival outcomes. This finding not only demonstrates albumin’s predictive value but also emphasizes the importance of maintaining adequate nutritional status in HIV-infected patients. Additionally, our study revealed that 58.84% of hospitalized HIV-DM patients were at nutritional risk at admission (Table 2). Nutritional risk emerged as a crucial determinant in both the occurrence and prediction of opportunistic infections, a finding previously underexplored in the literature. These results underscore the importance of implementing routine nutritional screening during admission. And a comprehensive nutritional assessment should integrate multiple parameters, including BMI, serum albumin, total protein, and standardized nutritional assessment scales (27).
HbA1c levels are widely recognized as predictors of poor health outcomes and comorbidities, including coronary heart disease and stroke (28, 29). However, limited research has examined the relationship between HbA1c and opportunistic infections. In our study, multivariate logistic regression analyses demonstrated that HbA1c served as both a significant risk factor and a valuable predictor for opportunistic infections in HIV-DM patients. In the risk factor analysis, elevated HbA1c levels were significantly associated with increased odds of opportunistic infections (P<0.05). These findings were further validated in the prediction model, where HbA1c demonstrated similar predictive value. The consistency of HbA1c’s significance across both analyses strengthens the evidence for its role in opportunistic infections among HIV-DM patients. While HbA1c showed significant associations in both analyses, admission and discharge plasma glucose levels did not demonstrate such correlations. This discrepancy might be attributed to HbA1c’s ability to reflect long-term glycemic control over the preceding 2-3 months, providing a more comprehensive assessment compared to point-in-time glucose measurements (30).The relationship between elevated HbA1c and increased risk of opportunistic infections could be explained by the detrimental effects of chronic hyperglycemia on immune function and inflammatory impairment (6).Although HbA1c is widely recognized as the gold standard for long-term glycemic control assessment, its diagnostic accuracy may be compromised in the presence of anemia or hemoglobin disorders (16). Previous investigations have demonstrated a positive correlation between hemoglobin levels and HbA1c, suggesting that HbA1c-based diagnostic criteria might underestimate diabetes prevalence in HIV patients with concurrent anemia (31).A limitation of our study is that we did not specifically examine the presence of anemia among participants. Future research directions should incorporate comprehensive hematological parameters and specific diagnoses to elucidate the complex relationships between anemia, hemoglobin abnormalities, and HbA1c, as well as their collective impact on opportunistic infection occurrence. Furthermore, our findings confirmed previous observations (32–34) that acute diabetic complications serve as a prognostic risk factor (Supplementary Table 1). Given that these complications are strongly associated with markedly elevated HbA1c levels (>10%) (32), our results emphasize the clinical imperative for enhanced surveillance and management of patients with high HbA1c levels, both for preventing opportunistic infections and optimizing clinical outcomes.
The multivariate analyses indicated that both CD4+ T-cell counts and lymphocyte counts were significant predictors of opportunistic infections. In the prediction model, CD4+ T-cell counts showed a protective effect, which was consistently observed in the risk factor analysis. Similarly, lymphocyte counts demonstrated a protective association in the risk factor analysis. These findings align with the established understanding (35) that both CD4+ T-cell counts and lymphocyte counts serve as crucial indicators of immune function in HIV-infected patients. Duration of HIV infection was identified as a significant protective predictor against opportunistic infections, demonstrating an inverse association. For each unit increase in duration of HIV infection, the risk of opportunistic infections decreased by approximately 42.4%.This protective effect could be explained by prolonged exposure to standardized antiretroviral therapy (36) and consistent medical management (37).
The moderate association observed in our study merits careful interpretation. The odds ratio approaching 1 for CD4+ T-cell counts in the risk factor analysis, despite statistical significance, suggests limited clinical significance when considered as a continuous variable. These results suggest that small changes in CD4+ T-cell counts may have limited immediate impact. But sustained abnormal levels might significantly affect clinical outcomes. During model development, we attempted to incorporate CD4T cell counts as a categorical variable. However, this categorization diminished the statistical significance of other clinically relevant predictors, notably HbA1c. This finding suggests potential interactions between variables and underscores the importance of maintaining the continuous nature of certain predictors to preserve their predictive value. Additionally, the inconsistent significance of lymphocyte counts between the risk factor analysis (P<0.05) and prediction model (P>0.05) highlights the complexity of using immunological parameters as predictive markers (38).While lymphocyte counts has been investigated as a potential surrogate marker in certain clinical settings (39), its predictive value may vary depending on the specific context and outcome measures being assessed.
In contrast to previous studies (15), our findings revealed that despite a higher prevalence of comorbidities in the opportunistic infection group compared to the non-opportunistic infection group, comorbidities were not identified as a risk factor for opportunistic infections among HIV-DM patients.
Our findings have important implications for clinical practice guidelines and regional healthcare policies regarding opportunistic infection management in HIV-DM patients. First, the prediction model could be integrated into existing clinical guidelines (40) to provide standardized risk assessment protocols for this specific patient population. This would enable healthcare providers to implement more targeted preventive strategies based on individual risk profiles. Second, our risk prediction approach could inform the development of regional screening protocols, helping to optimize resource allocation in different healthcare settings (41). Patients identified as high-risk by the model might benefit from more frequent monitoring and enhanced preventive measures, while those at lower risk could follow standard care protocols. Furthermore, the identified risk factors and their relative importance could guide the development of preventive healthcare policies, particularly in regions with high prevalence of HIV-DM comorbidity. Healthcare systems could use this information to establish more effective surveillance programs and allocate resources more efficiently for opportunistic infection prevention (42).
There are some limitations of this study. First, while multiple imputation was utilized to handle missing data, we acknowledged that potential bias might persist, which could influence the internal validity of our findings despite our best methodological efforts. Second, the predictive performance of our model was moderate, suggesting room for improvement in discriminative ability. Future studies could consider incorporating additional clinical biomarkers and baseline characteristics to enhance the model’s predictive performance. Potential improvements include integrating comprehensive immunological parameters (e.g., CD19, CD56 cell counts, and inflammatory markers), incorporating sensitive diabetes-related indicators (e.g., glycated albumin), and accounting for medication adherence and treatment history. Furthermore, the adoption of more advanced machine learning techniques and larger sample sizes might help improve both model discrimination and calibration. Finally, this study was conducted as a single-center retrospective analysis, which inherently carries methodological limitations such as potential selection bias and incomplete data capture. As a retrospective analysis, causative relationships cannot be firmly established, and there may be residual confounding factors that we have not accounted for, such as unmeasured lifestyle variables or socioeconomic factors. To validate and generalize our findings, future research should employ multi-center prospective cohort designs with larger sample sizes, which would provide more robust evidence and potentially better account for regional variations in patient characteristics and treatment practices.
This study identified significant risk factors for opportunistic infections through multifactorial logistic regression analysis, including nutritional risk, duration of HIV infection, HbA1c levels, lymphocyte counts, albumin level, and CD4+ T cell counts. Based on these clinical indicators, we developed a predictive model for HIV-DM hospitalized patients. Our findings provide a scientific framework for the early identification and prevention of opportunistic infections in this vulnerable population, with the ultimate goal of improving patient outcomes and quality of life.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The requirement of ethical approval was waived by The Ethics Committee of the Public Health Clinical Center of Chengdu for the studies involving humans because this is a retrospective nature of this study and the exclusion of direct subject participation. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board also waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because all patients were provided with comprehensive verbal and written information regarding the study’s purpose and potential implications. The studies were conducted in accordance with the local legislation and institutional requirements.
QZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. FG: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. XR: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. RL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JK: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. DL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. ML: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the Chengdu Municipal Health Commission (2019079), the Chengdu Science and Technology Bureau (2021-YF05-00536-SN), the Chinese Nutrition Society Dietitian Empowerment Program (CNS-DNEP2022-34), and the Sichuan Traditional Chinese Medicine Administration Research Program (2023MS097,2024MS147).
We thank Dr. Lin Cai and Tongtong Yang (Public Health Clinic Center of Chengdu, Infectious Diseases Department).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1527936/full#supplementary-material
1. Organization WH. World health statistics 2024: monitoring health for the SDGs, Sustainable Development Goals. Geneva: World Health Organization (2024). Available online at: https://www.who.int/publications/i/item/9789240094703
2. Sarkar S, Brown TT. Diabetes in people with HIV. Curr Diabetes Rep. (2021) 21:13. doi: 10.1007/s11892-021-01382-8
3. Atun R, Davies JI, Gale E, Barnighausen T, Beran D. Diabetes in sub-Saharan Africa: from clinical care to health policy. Lancet Diabetes Endocrinol. (2017) 5:622–67. doi: 10.1016/S2213-8587(17)30181-X
4. Liu DF, Zhang XY, Zhou RF, Cai L, Yan DM, Lan LJ, et al. Glucose metabolism continuous deteriorating in male patients with human immunodeficiency virus accepted antiretroviral therapy for 156 weeks. World J Diabetes. (2023) 14:299–312. doi: 10.4239/wjd.v14.i3.299
5. Liu D, Zhang X, Kang J, Gao F, He Y, He S. Gradual increasing dyslipidemia in treatment-naive male patients with human immunodeficiency virus and treated with tenofovir plus lamivudine plus efavirenz for 3 years. Diabetol Metab Syndr. (2021) 13:135. doi: 10.1186/s13098-021-00756-y
6. Chukwuanukwu RC, Nwosu NB, Ifeanyichukwu MO, Nsonwu-Anyanwu AC, Manafa PO. Evaluation of some immune and inflammatory responses in diabetes and HIV co-morbidity. Afr Health Sci. (2023) 23:120–8. doi: 10.4314/ahs.v23i1.14
7. Anglaret X, Minga A, Gabillard D, Ouassa T, Messou E, Morris B, et al. AIDS and non-AIDS morbidity and mortality across the spectrum of CD4 cell counts in HIV-infected adults before starting antiretroviral therapy in Cote d’Ivoire. Clin Infect Dis. (2012) 54:714–23. doi: 10.1093/cid/cir898
8. B-Lajoie MR, Drouin O, Bartlett G, Nguyen Q, Low A, Gavriilidis G, et al. Incidence and prevalence of opportunistic and other infections and the impact of antiretroviral therapy among HIV-infected children in low- and middle-income countries: A systematic review and meta-analysis. Clin Infect Dis. (2016) 62:1586–94. doi: 10.1093/cid/ciw139
9. Sadiq U, Shrestha U, Guzman N. Prevention of Opportunistic Infections in HIV/AIDS (2023), Sadiq U SUGN.
10. Gelaw WB, Selomon N, Tiruneh CM, Chekole B, Kassaw A, Wubneh M, et al. Prevalence and determinants of under-nutrition among children on ART in Ethiopia: A systematic review and meta-analysis. PLoS One. (2024) 19:e0303292. doi: 10.1371/journal.pone.0303292
11. Girma D, Dejene H, Adugna GL, Tesema M, Bati F. Time to occurrence, predictors, and patterns of opportunistic infections incidence among HIV-positive patients attending Antiretroviral Therapy Clinic of Salale University Comprehensive Specialized Hospital: A retrospective cohort study. Med (Baltimore). (2022) 101:e29905. doi: 10.1097/MD.0000000000029905
12. Khanal VK, Jha N, Karki P, Paudel IS. Opportunistic infections and clinical profile of HIV/AIDS patients: A study from eastern region of Nepal. Nepal Med Coll J. (2014) 16:194–7.
13. Salomon J, De TP, Melchior JC. Nutrition and HIV infection. Br J Nutr. (2002) 87 Suppl 1:S111–9. doi: 10.1079/bjn2001464
14. Mohamud AK, Ahmed OA, Mohamud AA, Dirie NI. Magnitude of opportunistic infections and associated factors among adult people living with human immune deficient virus on art at selected public hospital, mogadishu Somalia: cross-sectional study. Ann Med Surg (Lond). (2023) 85:3364–71. doi: 10.1097/MS9.0000000000000880
15. Aids And Hepatitis C Professional Group SOID, Prevention CCFD. Chinese guidelines for diagnosis and treatment of HIV/AIDS (2018). Zhonghua Nei Ke Za Zhi. (2018) 57:867–84. doi: 10.3760/cma.j.issn.0578-1426.2018.12.002
16. Zhu D, Society C. Guideline for the prevention and treatment of type 2 diabetes mellitus in China (2020 edition). Chin J Endocrinol Metab. (2021) 37:311–98. doi: 10.3760/cma.j.cn311282-20210304-00142
17. Chinese Society of Critical Care Medicine. Clinical practice guidelines for nutritional assessment and monitoring of adult ICU patients in China. Chin Crit Care Med. (2023) 35:1121–46. doi: 10.3760/cma.j.cn121430-20230829-00710
18. Lee JH, Huber JJ. Evaluation of multiple imputation with large proportions of missing data: how much is too much? Iran J Public Health. (2021) 50:1372–80. doi: 10.18502/ijph.v50i7.6626
19. Staehelin C, Elzi L, Weber R, Furrer H. Treatment and prevention of opportunistic infections. Ther Umsch. (2014) 71:475–82. doi: 10.1024/0040-5930/a000540
20. Bantie B, Kassaw YG, Abate MW, Amare AT, Nigat AB, Tigabu A, et al. Delayed ART initiation in “Test and Treat era” and its associated factors among adults receiving antiretroviral therapy at public health institutions in Northwest Ethiopia: A multicenter cross-sectional study. PLoS One. (2022) 17:e0271127. doi: 10.1371/journal.pone.0271127
21. Dembelu M, Kote M, Gilano G, Mohammed T. Incidence and predictors of reoccurrence of opportunistic infection among adult HIV/AIDS patients attending ART clinic at public health facilities in Arba Minch town, southern Ethiopia: A retrospective cohort study. PLoS One. (2021) 16:e0261454. doi: 10.1371/journal.pone.0261454
22. Bretschera C, Boesiger F, Kaegi-Braun N, Hersberger L, Lobo DN, Evans DC, et al. Admission serum albumin concentrations and response to nutritional therapy in hospitalised patients at malnutrition risk: Secondary analysis of a randomised clinical trial. EClinicalMedicine. (2022) 45:101301. doi: 10.1016/j.eclinm.2022.101301
23. Bisson GP, Ramchandani R, Miyahara S, Mngqibisa R, Matoga M, Ngongondo M, et al. Risk factors for early mortality on antiretroviral therapy in advanced HIV-infected adults. Aids. (2017) 31:2217–25. doi: 10.1097/QAD.0000000000001606
24. Laranjinha I, Joao MP, Dickson J, Estibeiro H, Boquinhas H, Barata JD. Prognostic factors of human immunodeficiency virus-infected patients on chronic hemodialysis. Blood Purif. (2017) 44:244–50. doi: 10.1159/000478966
25. Jain R, Dotan E. Nutrition and aging: a practicing oncologist’s perspective. Curr Oncol Rep. (2017) 19:71. doi: 10.1007/s11912-017-0630-5
26. Wiedermann CJ. Hypoalbuminemia as surrogate and culprit of infections. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22094496
27. Dent E, Hoogendijk EO, Visvanathan R, Wright O. Malnutrition screening and assessment in hospitalised older people: a review. J Nutr Health Aging. (2019) 23:431–41. doi: 10.1007/s12603-019-1176-z
28. Mukherjee S, Ray SK, Jadhav AA, Wakode SL. Multi-level analysis of hbA1c in diagnosis and prognosis of diabetic patients. Curr Diabetes Rev. (2024) 20:e1915119529. doi: 10.2174/0115733998262501231015051317
29. Zhu Z, Mao Y, Chen G. Predictive value of HbA1c for in-hospital adverse prognosis in COVID-19: A systematic review and meta-analysis. Prim Care Diabetes. (2021) 15:910–7. doi: 10.1016/j.pcd.2021.07.013
30. Sherwani SI, Khan HA, Ekhzaimy A, Masood A, Sakharkar MK. Significance of HbA1c test in diagnosis and prognosis of diabetic patients. biomark Insights. (2016) 11:95–104. doi: 10.4137/BMI.S38440
31. Faurholt-Jepsen D, Olsen MF, Andersen AB, Kaestel P, Abdissa A, Amare H, et al. Hyperglycemia and insulin function in antiretroviral treatment-naive HIV patients in Ethiopia: a potential new entity of diabetes in HIV? Aids. (2019) 33:1595–602. doi: 10.1097/QAD.0000000000002249
32. Mccoy RG, Galindo RJ, Swarna KS, Van Houten HK, O’Connor PJ, Umpierrez GE, et al. Sociodemographic, clinical, and treatment-related factors associated with hyperglycemic crises among adults with type 1 or type 2 diabetes in the US from 2014 to 2020. JAMA Netw Open. (2021) 4:e2123471. doi: 10.1001/jamanetworkopen.2021.23471
33. Coregliano-Ring L, Goia-Nishide K, Rangel EB. Hypokalemia in diabetes mellitus setting. Medicina (Kaunas). (2022) 58. doi: 10.3390/medicina58030431
34. Saydah S, Imperatore G, Cheng Y, Geiss LS, Albright A. Disparities in diabetes deaths among children and adolescents - United States, 2000-2014. MMWR Morb Mortal Wkly Rep. (2017) 66:502–5. doi: 10.15585/mmwr.mm6619a4
35. Woldegeorgis BZ, Zekarias Z, Adem BG, Obsa MS, Kerbo AA. Prevalence and determinants of opportunistic infections among HIV-infected adults receiving antiretroviral therapy in Ethiopia: A systematic review and meta-analysis. Front Med (Lausanne). (2023) 10:1087086. doi: 10.3389/fmed.2023.1087086
36. Woldegeorgis BZ, Diro CW, Yohannes B, Kerbo AA, Asgedom YS. Incidence and predictors of opportunistic infections in adolescents and adults after the initiation of antiretroviral therapy: A 10-year retrospective cohort study in Ethiopia. Front Public Health. (2022) 10:1064859. doi: 10.3389/fpubh.2022.1064859
37. Siripurapu R, Ota Y. Human immunodeficiency virus: opportunistic infections and beyond. Neuroimaging Clin N Am. (2023) 33:147–65. doi: 10.1016/j.nic.2022.07.014
38. Berger A, Preiser W, Doerr HW. The role of viral load determination for the management of human immunodeficiency virus, hepatitis B virus and hepatitis C virus infection. J Clin Virol. (2001) 20:23–30. doi: 10.1016/s1386-6532(00)00151-7
39. Schreibman T, Friedland G. Use of total lymphocyte count for monitoring response to antiretroviral therapy. Clin Infect Dis. (2004) 38:257–62. doi: 10.1086/380792
40. Acquired Immunodeficiency Syndrome Professional Group SOID, Prevention CCFD. Chinese guidelines for diagnosis and treatment of human immunodeficiency virus infection/acquired immunodeficiency syndrome (2024 edition). Chin J Infect Dis. (2024) 42:257–84. doi: 10.3760/cma.j.cn311365-20240328-00081
41. Derbie A, Mekonnen D, Woldeamanuel Y, Abebe T. Cryptococcal antigenemia and its predictors among HIV infected patients in resource limited settings: a systematic review. BMC Infect Dis. (2020) 20:407. doi: 10.1186/s12879-020-05129-w
Keywords: HIV, diabetes, nutritional risk, glycosylated hemoglobin, opportunistic Infections
Citation: Zhu Q, Gao F, Ren X, Li R, Kang J, Li M and Liu D (2025) Nutritional risk and HbA1c as critical risk factors and predictors of opportunistic infections in HIV-DM comorbid patients: a retrospective cross-sectional study. Front. Endocrinol. 15:1527936. doi: 10.3389/fendo.2024.1527936
Received: 14 November 2024; Accepted: 17 December 2024;
Published: 10 January 2025.
Edited by:
Xiantong Zou, Peking University People’s Hospital, ChinaReviewed by:
Jiahe Li, University of Pittsburgh, United StatesCopyright © 2025 Zhu, Gao, Ren, Li, Kang, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dafeng Liu, bGRmMzEyQDEyNi5jb20=; Maoquan Li, bGltcXVhbkBjbWMuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.