- 1Department of Endocrinology, The Second Affiliated Hospital of Hainan Medical University, Haikou, China
- 2Key Laboratory of Tropical Translational Medicine of Ministry of Education, School of Basic Medicine and Life Sciences, Hainan Medical University, Haikou, China
- 3Department of Biochemistry and Molecular Biology, Hainan Medical University, Haikou, China
- 4Department of Respiratory and Critical Care Medicine, Haikou Affiliated Hospital of Central South University Xiangya School of Medicine (Haikou People’s Hospital), Haikou, China
Background: Adipokines have been implicated in the pathogenesis of type 2 diabetes mellitus (T2DM) and related complications due to their roles in metabolic regulation and inflammation. However, the relationship between these adipokines and diabetic peripheral neuropathy (DPN) remains unclear.
Methods: A case-control study was performed with 198 patients with DPN and 205 T2DM patients without DPN from the Endocrinology Department at the Second Affiliated Hospital of Hainan Medical University. Circulating adiponectin and leptin levels were quantified via enzyme-linked immunosorbent assays. Logistic regression models, adjusting for age, sex, BMI, smoking status, and diabetes duration, were applied to evaluate the associations between adiponectin and leptin levels and DPN risk.
Results: DPN patients exhibited lower adiponectin (P=0.001) and higher leptin (P=0.007) levels than diabetic controls. Confounders-adjusted analyses revealed that higher adiponectin levels correlated with reduced DPN risk (OR, tertile 3 vs. tertile 1: 0.52; 95% CI: 0.30-0.90), whereas elevated leptin levels were linked to increased DPN risk (OR, tertile 3 vs. tertile 1: 1.91; 95% CI: 1.10-3.32). Stratified analyses confirmed consistent findings across subgroups without statistically significant interactions.
Conclusions: Circulating adiponectin and leptin levels correlate with DPN risk in diabetic patients, suggesting their potential as biomarkers for high-risk DPN identification and guiding targeted prevention and management.
Introduction
Diabetic peripheral neuropathy (DPN), a prevalent diabetic complication affecting half of all diabetic patients, is marked by peripheral nerve damage, primarily in the limbs (1). This condition significantly impacts patient well-being through chronic pain, sensory deficits, and heightened risks of foot complications, imposing substantial healthcare costs and diminished quality of life (2). Given the incomplete understanding of its pathophysiology and the scarcity of efficacious management strategies, the identification of modifiable DPN risk factors is imperative for tailoring preventive and therapeutic interventions aimed at curbing its incidence and severity (3, 4).
The potential role of adipokines, including adiponectin and leptin, in the development of diabetic complications like DPN is gaining recognition due to their involvement in metabolic regulation and inflammatory processes, key factors in diabetes and its sequelae (5). Adiponectin, known for its anti-inflammatory and insulin-sensitizing effects, offers protection against conditions such as atherosclerosis and type 2 diabetes (6, 7). Conversely, leptin, with its pro-inflammatory characteristics, often elevated in obesity and type 2 diabetes, contributes to insulin resistance and metabolic dysfunction (6, 7). Given these roles, adiponectin and leptin may influence DPN development.
Several studies have explored the link between adiponectin and DPN risk among patients with type 2 diabetes, yielding mixed results: some report an inverse association (8, 9), others find no significant correlation (10) or even a positive link (11, 12). Research on leptin and DPN is limited and also inconsistent (11, 13, 14). Prior investigations often suffer from small sample sizes (8, 10, 11, 13, 14), lack of adjustment for confounders (8, 10, 13, 14), or aggregation of various diabetic complications into a single outcome variable (12). Therefore, relationship between adiponectin and leptin levels and DPN risk requires further investigations.
This study aims to investigate the relationship between circulating levels of adiponectin and leptin and the risk of developing DPN in individuals with diabetes. By elucidating these associations, our study might contribute to the growing body of evidence on the role of adipokines in diabetic complications and inform the development of strategies for the prevention and management of DPN.
Methods
Study population
This case-control study was conducted on type 2 diabetes mellitus (T2DM) patients, recruited from the Endocrinology Department at the Second Affiliated Hospital of Hainan Medical University, China, between August 2019 and December 2022. A total of 198 patients diagnosed with diabetic peripheral neuropathy (DPN group) and 205 diabetic patients without neuropathy (diabetic control group) were recruited. The DPN group and the diabetic control group were frequency matched by 5-year age groups and sex. All participants were diagnosed with T2DM according to the American Diabetes Association criteria (15). Participants were excluded if they had significant comorbidities such as major cardiovascular disease, infectious and inflammatory conditions, severe renal or hepatic impairment or cancer. The study protocol was reviewed and approved by the hospital’s ethics committee, and written informed consent was obtained from all participants.
DPN diagnosis
Diagnosis of DPN was determined through a comprehensive approach encompassing clinical symptom evaluation and neurological assessments. Key assessments included the identification of neuropathic symptoms (numbness, tingling, pain), examination of ankle reflexes, and sensory testing using a 128-Hz tuning fork for vibration perception and a 10-g monofilament for light touch sensitivity. A diagnosis of DPN was established if patients without clinical symptoms presented with at least two abnormalities in neurological assessments, or if those with symptoms showed at least one abnormality (16). This diagnostic protocol ensured a thorough and accurate assessment of DPN status among participants.
Data collection
Demographic data, including age, sex, smoking status were obtained via a self-reported questionnaire. Clinical data on height, weight, hypertension and duration of diabetes were extracted from medical records. Body mass index (BMI) was calculated as the weight (kg) divided by the height squared (m2). Comprehensive laboratory assessments conducted at the time of recruitment provided vital biochemical data, including levels of glycated hemoglobin (HbA1c), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C). These laboratory results were accessed through the hospital’s sophisticated laboratory information system, ensuring accuracy and reliability of the biochemical data.
Measurements of adiponectin and leptin
Fasting blood samples were collected from all participants using EDTA tubes and processed within two hours of collection. Plasma samples were separated by centrifugation at 3000 rpm for 15 minutes and stored at -80°C until analysis. The circulating levels of adiponectin and leptin were measured using commercial enzyme-linked immunosorbent assay (ELISA) kits (CUSABIO, China) following the manufacturer’s instructions. The minimum detectable levels of adiponectin and leptin were both 0.156 ng/ml. The intra-assay and inter-assay coefficients of variation for the two assays were below 8% and 10%, respectively.
Statistical analysis
Descriptive statistics were used to summarize the characteristics of the study population, with continuous variables presented as means ± standard deviations or medians with interquartile ranges, and categorical variables expressed as frequencies and percentages. Comparisons between the DPN and diabetic control groups were performed using the chi-square test for categorical variables and the independent t-test or Mann-Whitney U test for continuous variables, depending on the data distribution.
To examine the association between circulating adiponectin and leptin levels and the risk of DPN, logistic regression models were employed. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated, adjusting for potential confounders including age, sex, educational level, BMI, smoking status and duration of diabetes. The levels of adiponectin and leptin were analyzed both as continuous variables and as categorical variables (divided into tertiles based on the distribution in the study population). Stratified analysis for associations of adiponectin and leptin levels with DPN were performed by patients’ characteristics, and interaction analysis were also conducted by including the cross-product term in the logistic regression models.
Statistical significance was defined as a two-tailed p-value of less than 0.05. All analyses were performed using SPSS software, version 22.0 (SPSS Inc., Chicago, IL, USA).
Results
DPN and control groups matched for demographic characteristics
The characteristics of the participants are presented in Table 1. The distributions of age, sex, educational levels, body mass index (BMI), and smoking status were comparable between the DPN group and diabetic control group (P > 0.05 for all comparisons). Glycemic control, as indicated by HbA1c levels, was significantly poorer in the DPN group (8.7 ± 2.0%) compared to the control group (8.3 ± 1.8%, P = 0.030). The levels of TG, but not other lipid indications, were higher in DPN group (1.89 ± 1.18 mmol/L) than those in the control group (1.67 ± 1.05 mmol/L, P=0.049). Additionally, the DPN group had a significantly longer duration of diabetes (median: 11.7 years [IQR: 8.5, 16.8]) compared to the control group (median: 7.3 years [IQR: 5.0, 11.8], P < 0.001).
DPN group had lower adiponectin and higher leptin levels
The box plot presented in Figure 1 illustrates the distribution of adiponectin and leptin levels among two groups. For adiponectin, the levels in DPN patients (median, 8.5 ug/mL; interquartile, 6.2-11.3 ug/mL) were significantly lower compared to those in diabetic controls (median, 9.9 ug/mL; interquartile, 7.0, 13.0 ug/mL; P=0.001). Regarding leptin, the levels are shown to be higher in DPN patients (median, 19.7 ng/mL; interquartile, 12.3-25.8 ng/mL) than in diabetic controls (median, 15.8 ng/mL; interquartile, 11.3-23.5 ng/mL; P=0.007).
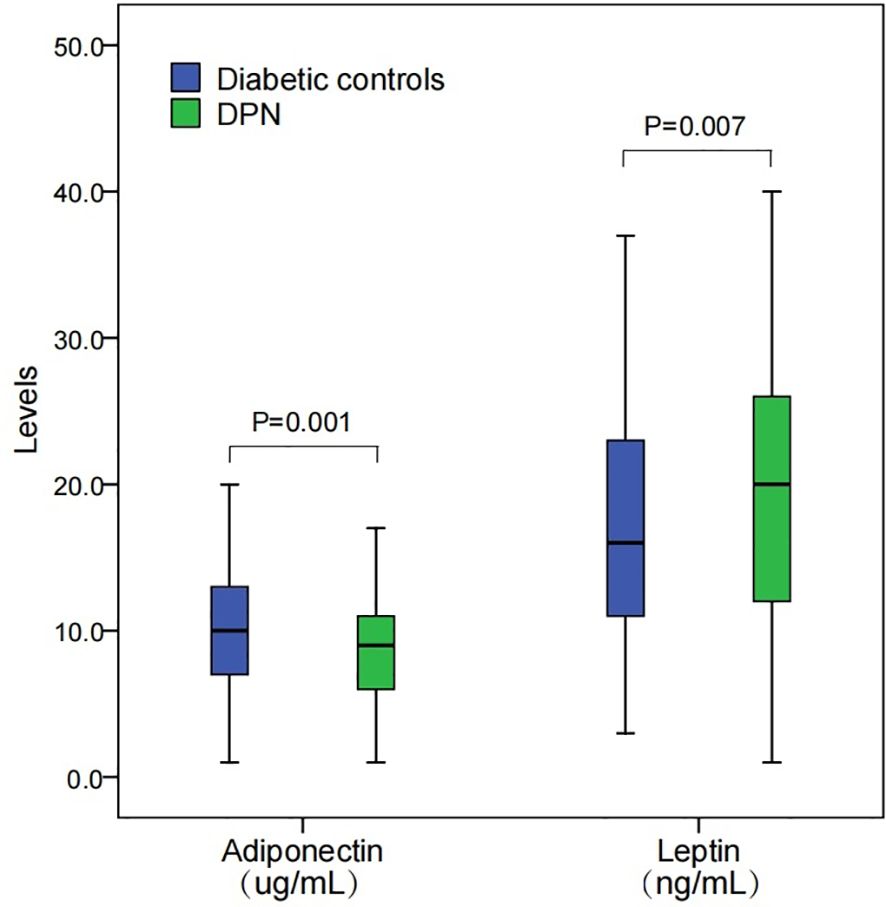
Figure 1. Levels of adiponectin and leptin among DPN patients and diabetic controls. DPN, diabetic peripheral neuropathy. Levels between groups were compared using a Mann-Whitney U test.
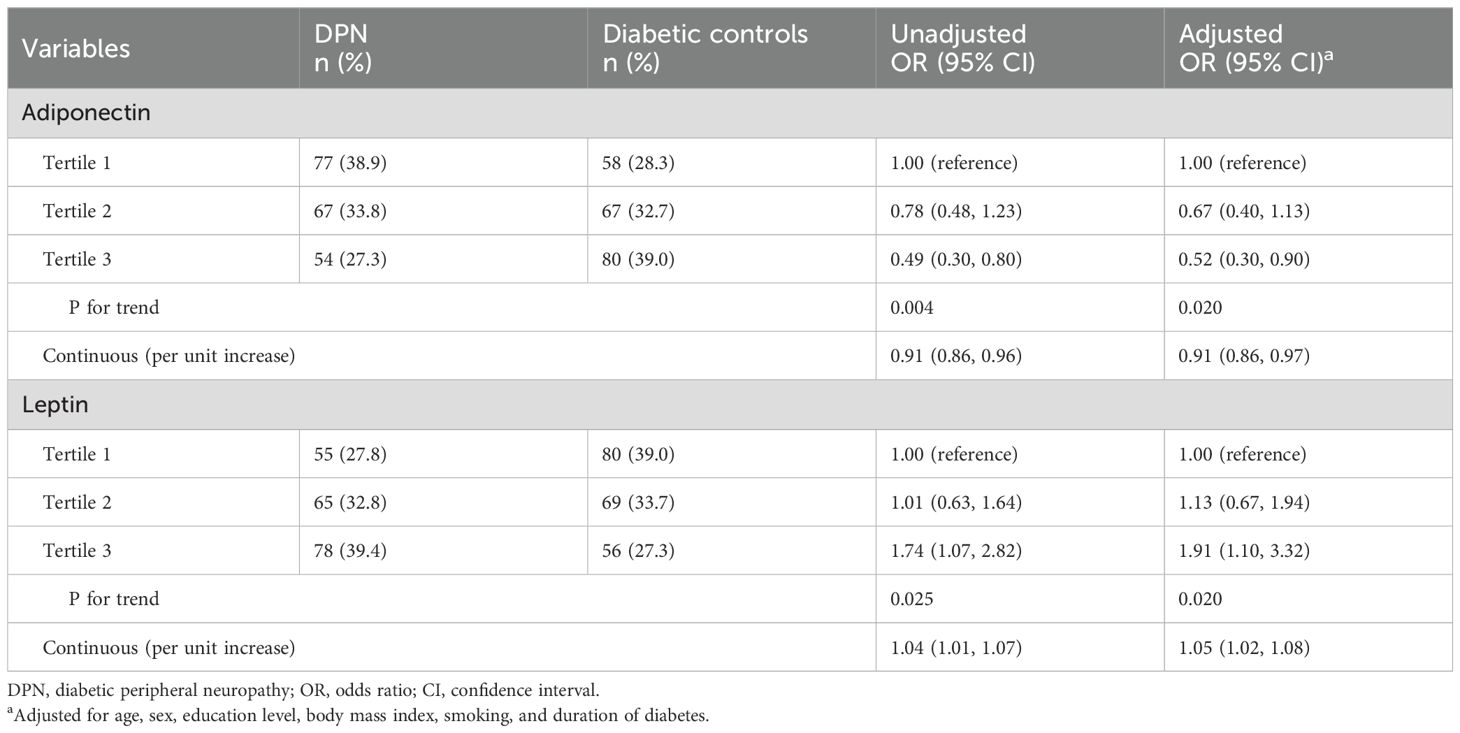
Continuous increase in adiponectin levels decreased DPN risk
The association between circulating adiponectin levels and the risk of DPN is shown in Table 2. In the unadjusted model, participants in the highest tertile of adiponectin had a significantly reduced risk of DPN compared to those in the lowest tertile (crude OR, 0.49; 95% CI, 0.30-0.80). After adjusting for potential confounders, including age, sex, education level, BMI, smoking status, and duration of diabetes, this association remained significant (adjusted OR, 0.52; 95% CI, 0.30-0.90). A trend analysis across the three tertiles also supports this association (P for trend = 0.020). Furthermore, a continuous increase in adiponectin levels was associated with a decreased risk of DPN (adjusted OR, 0.91 per unit increase; 95% CI: 0.86, 0.97).
Higher leptin levels associated with increased DPN risk
Association with DPN risk for leptin levels appears to be opposite to that for adiponectin (Table 2). The unadjusted analysis indicated that participants in the highest tertile of leptin had significantly higher risk of DPN compared to those in the lowest tertile (crude OR, 1.74; 95% CI, 1.07-2.82). This association persisted after adjustment for confounders (adjusted OR, 1.91; 95% CI, 1.10-3.32). Additionally, each unit increase in leptin was associated with a 5% increase in the risk of DPN (adjusted OR, 1.05 per unit increase; 95% CI, 1.02-1.08).
Subgroup-specific associations of adiponectin and leptin with DPN risk
Table 3 presents the results of the stratified analysis by age, sex, education level, BMI, and smoking status to assess whether the association between adiponectin and leptin levels and DPN risk varied across these subgroups. For adiponectin, a significant reduction in DPN risk was found among participants with a primary school education or below who were in the highest adiponectin tertile (OR = 0.22, 95% CI: 0.07, 0.68), while the strength of association among participants with a junior middle school or above appeared weaker. However, the interaction between education level and adiponectin was not statistically significant (P for interaction = 0.649). Similarly, higher adiponectin levels were associated with a lower risk of DPN (Tertile 3: OR = 0.38, 95% CI: 0.19, 0.75) among non-smokers, but no significant association was found in smokers. The interaction between smoking status and adiponectin was not statistically significant (P for interaction = 0.363). For leptin, the association with DPN risk tends to be stronger among participants who were aged 60 years or younger, female or smoke than those in their corresponding comparison subgroups. However, the interaction terms were not statistically significant (P for interaction > 0.05). Overall, no significant interaction was observed between patients’ characteristics and adiponectin and leptin levels on DPN risk.
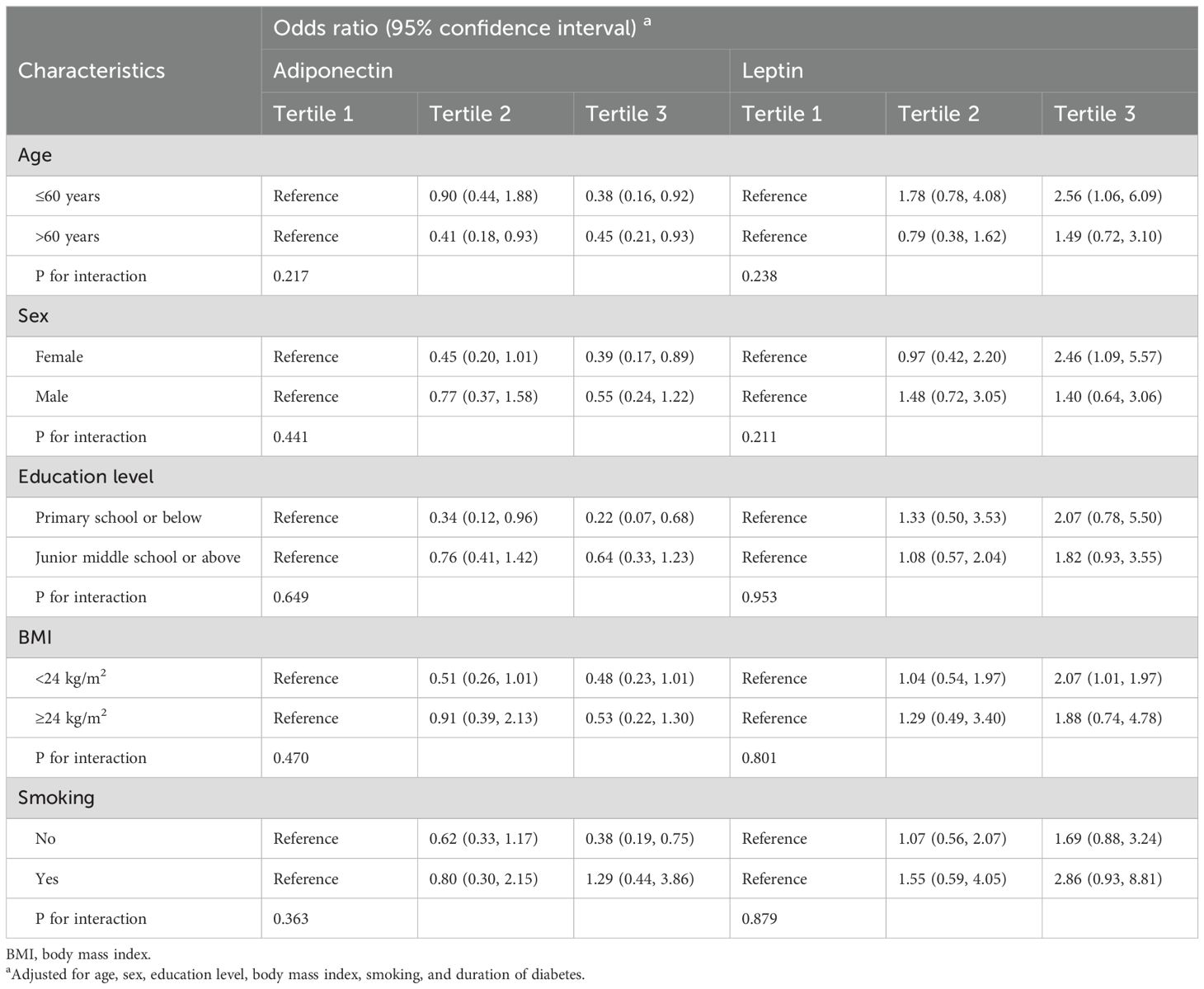
Table 3. Stratified analysis for associations between adipokines and DPN by patients’ characteristics.
Discussion
Summary of findings
In this study, we investigated the association between circulating levels of adiponectin and leptin with the risk of DPN. Our findings demonstrate that higher levels of adiponectin are associated with a lower risk of DPN, while higher levels of leptin are associated with an increased risk of DPN. These associations remained significant even after adjusting for potential confounders, including age, sex, body mass index (BMI), smoking, and duration of diabetes. These results highlight the potential role of adipokines in the pathogenesis of DPN and suggest that adiponectin and leptin may serve as valuable biomarkers for predicting the risk of DPN in diabetic patients.
Comparison with previous studies
Previous studies have reported associations between adiponectin and diabetic complications among type 2 diabetes patients, although the evidence has been limited and inconsistent. In line with our finding, a study in China (mean age, ~55 years; mean BMI, ~24 kg/m2) found that plasma adiponectin levels were lower in DPN patients (n=90) that those non-DPN diabetic patients (n=90) (8). Similarly, a German study (mean age, ~72 years; mean BMI, ~31 kg/m2) of 43 DPN and 168 diabetic controls found that serum adiponectin levels were lower in the case group (9). However, an Indian study (mean age, ~54 years; mean BMI, ~26 kg/m2) of 43 DPN and 43 diabetic controls reported no association between serum adiponectin levels and DPN (10). Another study (mean age, ~60 years; mean BMI, ~26 kg/m2) of Chinese DPN (n=98) and non-DPN patients (n=121) reported that serum adiponectin levels were positively associated with DPN risk after adjusting for potential confounders (11). A study in India (mean age, ~50 years; mean BMI, ~25 kg/m2) also observed that serum adiponectin levels were higher in 138 diabetic neuropathy than those in 349 diabetic controls (12).
The positive association between leptin and DPN observed in our study is also consistent to a previous Chinese study (mean age, ~52 years; mean BMI, ~26 kg/m2) of 82 DPN and 200 diabetic controls, where higher levels of serum leptin were observed in the case group (13). In contrast, a Turkish study (mean age, ~57 years; mean BMI, ~28 kg/m2) found that the plasma leptin levels in diabetic patients with Sensorial neuropathy (n=38) or autonomic neuropathy (n=13) were not statistically different from those without these complications (14). In a Chinese study (mean age, ~60 years; mean BMI, ~26 kg/m2), serum leptin levels were positively associated with DPN risk when adjusting for age, sex, BMI, hypertension, HbA1c level, alcohol intake, smoking status, physical activity, and LDL, but this association was attenuated and became statistically insignificant when the regression model was further adjusted for lipid-lowering medications, eGFR, and disease duration (11).
The reason for the discrepancies in results across studies is unclear. It might be related to differences in population characteristics (e.g. age and BMI), genetic background and study design. For example, most of previous studies had small sample size (<100 cases) and did not consider confounders in the analysis. Further studies with better design are still needed to verify the relationship between adiponectin and leptin levels and DPN risk.
Potential mechanisms underlying the associations
The observed associations between adiponectin, leptin, and the risk of DPN can be explained by several biological mechanisms. Adiponectin is known for its anti-inflammatory and insulin-sensitizing properties (6, 7), which may protect against the development of DPN by reducing systemic inflammation and improving glucose metabolism. Inflammation plays a central role in the pathogenesis of DPN, contributing to nerve damage and dysfunction (3). By attenuating inflammatory pathways, adiponectin may help preserve nerve function and prevent the onset of neuropathy. Additionally, adiponectin has been shown to enhance endothelial function and promote vascular health (17, 18), which could further contribute to its protective effects against DPN by ensuring adequate blood supply to peripheral nerves. Lower levels of adiponectin have been linked to increased oxidative stress (19), which is also contributor to nerve damage in diabetes. Leptin’s role in DPN also appears to be complex. Leptin has also been shown to promote the production of pro-inflammatory cytokines, such as TNF-α and IL-6, which contribute to nerve damage through inflammatory pathways (6, 7). Furthermore, leptin has been shown to impair endothelial function and promote atherogenesis (20, 21), potentially leading to microvascular complications that affect nerve health.
Implications of findings
Our findings might have important implications for the understanding development of DPN. The adiponectin and leptin could be used as biomarkers for DPN risk. Measuring circulating levels of these adipokines could help identify individuals at higher risk for DPN, allowing for earlier intervention and more personalized management of diabetic patients. In addition, the inverse relationship between adiponectin levels and DPN risk suggests that increasing adiponectin levels could be a promising strategy for preventing or mitigating DPN in diabetic patients. Interventions that enhance adiponectin secretion or mimic its effects, such as lifestyle modifications (e.g., weight loss, physical activity) (22) or pharmacological agents (e.g., thiazolidinediones (23)), may hold potential in reducing the burden of DPN. Similarly, the positive association between leptin levels and DPN risk highlights the need for strategies to mitigate leptin resistance and reduce leptin levels in diabetic patients. This could be achieved through weight management, dietary interventions, or targeted therapies aimed at modulating leptin levels (24, 25). Further research is needed to validate these findings and explore the clinical utility of adiponectin and leptin measurements in routine practice.
Limitations and strengths
While this study provides valuable insights into the associations between adiponectin, leptin, and DPN risk, it is important to acknowledge several limitations. First, the cross-sectional design of the study limits the ability to infer causality. Although the associations observed are biologically plausible and supported by previous research, longitudinal studies are needed to establish a temporal relationship between adipokine levels and the development of DPN. Second, the study population consisted of diabetic patients from a specific geographic region, which may limit the generalizability of the findings to other populations. Differences in genetic background, lifestyle factors, and healthcare practices could influence the relationship between adipokines and DPN.
Despite these limitations, the study has several strengths that enhance the validity of the findings. The sample size of our study is largest compared to previous studies. The use of a well-characterized cohort of diabetic patients with detailed clinical and biochemical assessments allowed for a comprehensive analysis of the associations between adipokines and DPN. The adjustment for multiple confounders, including age, sex, BMI, and duration of diabetes, helps to mitigate the potential for confounding.
Conclusions
Our study provides evidence that higher circulating levels of adiponectin are associated with a reduced risk of DPN, while higher levels of leptin are associated with an increased risk. These findings suggest that adipokines play a significant role in the pathogenesis of DPN and may serve as useful biomarkers for identifying individuals at risk for this debilitating complication of diabetes. Future research should focus on longitudinal studies to confirm these associations and explore the underlying mechanisms.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Second Affiliated Hospital of Hainan Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ZC: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. SF: Data curation, Writing – review & editing. SL: Data curation, Writing – review & editing. MF: Conceptualization, Funding acquisition, Project administration, Writing – review & editing. GD: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Hainan Provincial Natural Science Foundation of China (821MS138), Joint Program on Health Science & Technology Innovation of Hainan Province (WSJK2024MS200, WSJK2024QN090), Hainan Province Science and Technology Special Fund (ZDYF2021SHFZ080) and National Natural Science Foundation of China (No. 82460791).
Acknowledgments
We thank the individuals who participated in this project. We also thank the nurse and students who were involved in the recruitment and administration of the questionnaire interview.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Selvarajah D, Kar D, Khunti K, Davies MJ, Scott AR, Walker J, et al. Diabetic peripheral neuropathy: advances in diagnosis and strategies for screening and early intervention. Lancet Diabetes endocrinology. (2019) 7:938–48. doi: 10.1016/S2213-8587(19)30081-6
2. Elafros MA, Andersen H, Bennett DL, Savelieff MG, Viswanathan V, Callaghan BC, et al. Towards prevention of diabetic peripheral neuropathy: clinical presentation, pathogenesis, and new treatments. Lancet Neurology. (2022) 21:922–36. doi: 10.1016/S1474-4422(22)00188-0
3. Galiero R, Caturano A, Vetrano E, Beccia D, Brin C, Alfano M, et al. Peripheral neuropathy in diabetes mellitus: pathogenetic mechanisms and diagnostic options. Int J Mol Sci. (2023) 24:3554. doi: 10.3390/ijms24043554
4. Smith S, Normahani P, Lane T, Hohenschurz-Schmidt D, Oliver N, Davies AH. Prevention and management strategies for diabetic neuropathy. Life (Basel). (2022) 12:1185. doi: 10.3390/life12081185
5. Kwon H, Pessin JE. Adipokines mediate inflammation and insulin resistance. Front endocrinology. (2013) 4:71. doi: 10.3389/fendo.2013.00071
6. Clemente-Suarez VJ, Redondo-Florez L, Beltran-Velasco AI, Martin-Rodriguez A, Martinez-Guardado I, Navarro-Jimenez E, et al. The role of adipokines in health and disease. Biomedicines. (2023) 11:1290. doi: 10.3390/biomedicines11051290
7. Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. (2011) 11:85–97. doi: 10.1038/nri2921
8. Ji ZY, Li HF, Lei Y, Rao YW, Tan ZX, Liu HJ, et al. Association of adiponectin gene polymorphisms with an elevated risk of diabetic peripheral neuropathy in type 2 diabetes patients. J Diabetes its complications. (2015) 29:887–92. doi: 10.1016/j.jdiacomp.2015.06.008
9. Herder C, Bongaerts BW, Ouwens DM, Rathmann W, Heier M, Carstensen-Kirberg M, et al. Low serum omentin levels in the elderly population with Type 2 diabetes and polyneuropathy. Diabetic medicine: J Br Diabetic Assoc. (2015) 32:1479–83. doi: 10.1111/dme.2015.32.issue-11
10. Mohanraj PS, Das A, Sen A, Ranjan A, Rajendran V, Velu A, et al. Evaluating the diagnostic potential of serum vascular endothelial growth factor and adiponectin in diabetic peripheral neuropathy. Cureus. (2024) 16:e53017. doi: 10.7759/cureus.53017
11. Sun Q, Yan B, Yang D, Guo J, Wang C, Zhang Q, et al. Serum adiponectin levels are positively associated with diabetic peripheral neuropathy in Chinese patients with type 2 diabetes. Front endocrinology. (2020) 11:567959. doi: 10.3389/fendo.2020.567959
12. Pradeepa R, Surendar J, Indulekha K, Chella S, Anjana RM, Mohan V. Association of serum adiponectin with diabetic microvascular complications among south Indian type 2 diabetic subjects - (CURES-133). Clin Biochem. (2015) 48:33–8. doi: 10.1016/j.clinbiochem.2014.10.009
13. Nan J, Zhang X, Li D, Jin M, Guo G, Dong J, et al. Effect of leptin levels of type 2 diabetes mellitus and diabetic complications. Clin Invest Med Medecine clinique experimentale. (2022) 45:E32–46. doi: 10.25011/cim.v45i3.38873
14. Sari R, Balci MK, Apaydin C. The relationship between plasma leptin levels and chronic complication in patients with type 2 diabetes mellitus. Metab syndrome related Disord. (2010) 8:499–503. doi: 10.1089/met.2009.0127
15. American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes—2018. Diabetes Care. (2018) 41:S13–27. doi: 10.2337/dc18-S002
16. Jia W, Weng J, Zhu D, Ji L, Lu J, Zhou Z, et al. Standards of medical care for type 2 diabetes in China 2019. Diabetes/metabolism Res Rev. (2019) 35:e3158. doi: 10.1002/dmrr.v35.6
17. Peng J, Chen Q, Wu C. The role of adiponectin in cardiovascular disease. Cardiovasc pathology: Off J Soc Cardiovasc Pathology. (2023) 64:107514. doi: 10.1016/j.carpath.2022.107514
18. Ebrahimi-Mamaeghani M, Mohammadi S, Arefhosseini SR, Fallah P, Bazi Z. Adiponectin as a potential biomarker of vascular disease. Vasc Health Risk management. (2015) 11:55–70. doi: 10.2147/VHRM.S48753
19. Matsuda M, Shimomura I. Roles of adiponectin and oxidative stress in obesity-associated metabolic and cardiovascular diseases. Rev endocrine Metab Disord. (2014) 15:1–10. doi: 10.1007/s11154-013-9271-7
20. Mellott E, Faulkner JL. Mechanisms of leptin-induced endothelial dysfunction. Curr Opin Nephrol hypertension. (2023) 32:118–23. doi: 10.1097/MNH.0000000000000867
21. Goldstein BJ, Scalia R. Adiponectin: A novel adipokine linking adipocytes and vascular function. J Clin Endocrinol Metab. (2004) 89:2563–8. doi: 10.1210/jc.2004-0518
22. Abdalla MMI. Therapeutic potential of adiponectin in prediabetes: strategies, challenges, and future directions. Ther Adv Endocrinol Metab. (2024) 15:20420188231222371. doi: 10.1177/20420188231222371
23. Yu JG, Javorschi S, Hevener AL, Kruszynska YT, Norman RA, Sinha M, et al. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes. (2002) 51:2968–74. doi: 10.2337/diabetes.51.10.2968
24. Rashad NM, Sayed SE, Sherif MH, Sitohy MZ. Effect of a 24-week weight management program on serum leptin level in correlation to anthropometric measures in obese female: A randomized controlled clinical trial. Diabetes Metab syndrome. (2019) 13:2230–5. doi: 10.1016/j.dsx.2019.05.027
Keywords: diabetic peripheral neuropathy, adiponectin, leptin, association, case-control study
Citation: Chen Z, Fu S, Lai S, Fu M and Du G (2024) Association of circulating adiponectin and leptin levels with the risk of diabetic peripheral neuropathy. Front. Endocrinol. 15:1505082. doi: 10.3389/fendo.2024.1505082
Received: 02 October 2024; Accepted: 21 November 2024;
Published: 13 December 2024.
Edited by:
Paramita Basu, University of Pittsburgh, United StatesReviewed by:
Brianne Guilford, Southern Illinois University Edwardsville, United StatesMagdalena Chottova Dvorakova, Charles University, Czechia
Copyright © 2024 Chen, Fu, Lai, Fu and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guankui Du, ZHVndWFua3VpQDE2My5jb20=; Maoxiong Fu, bGp5bHcxMjNAc29odS5jb20=
†These authors share first authorship
 Zongcun Chen
Zongcun Chen Shasha Fu4†
Shasha Fu4† Guankui Du
Guankui Du