
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 10 January 2025
Sec. Cardiovascular Endocrinology
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1499713
This article is part of the Research Topic Molecular Biomarkers of Cardiometabolic Disease - Volume II View all 10 articles
Background: Diabetes has become a global pandemic, posing a sustained threat to human health, primarily due to its associated complications. Left ventricular diastolic dysfunction (LVDD) is a prevalent cardiac complication among patients with diabetes. Since most patients are asymptomatic and lack relevant biomarkers, LVDD has not attracted significant attention from clinicians. The neutrophil-to-lymphocyte ratio (NLR) is a widely studied inflammation biomarker that has been suggested to be linked to various medical conditions, including cardiac diseases. However, its association with LVDD among patients with type 2 diabetes mellitus (T2DM) has not been explored.
Aim: To clarify the relationship between NLR and LVDD among patients with type 2 diabetes.
Methods: We conducted a cross-sectional study using medical records from 855 patients diagnosed with T2DM who were admitted to the Endocrinology department at Wuhan Union Hospital. According to the ASE/EACVI 2016 recommendations, these patients were categorized into two groups based on sonographic parameters: patients with normal left ventricular diastolic function (the non-LVDD group) and patients with LVDD (the LVDD group). NLR values were calculated and divided into three different levels. Statistical analysis was conducted to evaluate the correlation between NLR levels and the prevalence of LVDD.
Results: The prevalence of LVDD among hospitalized patients with T2DM in our study was 47.8% (409/855). The mean NLR value of the LVDD group was significantly higher compared with the non-LVDD group [1.60 (1.24-2.05) vs 1.85 (1.44-2.31), P<0.001]. The prevalence of LVDD in the three different NLR levels was 35.51% (76/214), 49.27% (203/412), and 56.77% (130/229), respectively. Unjustified logistic analysis showed that NLR levels were positively associated with the prevalence of LVDD (P <0.001). Compared to the low level of NLR, the unadjusted odds ratios (OR) of LVDD at the medium and high levels were 1.764 (1.255-2.478, P=0.001) and 2.384 (1.626-3.497, P<0.001), respectively (P for trend <0.001).
Conclusion: Our findings suggest that the NLR is a potential indicator for assisting clinicians in identifying LVDD in patients with T2DM. Patients with elevated NLR levels may be at a greater risk of developing LVDD than those with lower NLR levels, which may require attention and interventions to prevent patients from progressing into heart failure.
According to the latest reports by the World Health Organization (WHO), the global prevalence of diabetes in adults increased from 7% in 1990 to 14% in 2022, with patients exceeding 800 million—representing more than a fourfold increase (1). Heart failure (HF) is a frequent complication of diabetes, affecting up to 22% of individuals with the condition in the American population (2). However, left ventricular diastolic dysfunction (LVDD), diagnosed by echocardiography, is considered a myocardial functional impairment phenotype of diabetic cardiomyopathy and has a prevalence of up to 43% in diabetic patients (3). LVDD is recognized as an early stage of heart failure, also referred to as Stage B heart failure, where patients exhibit structural or functional cardiac abnormalities without overt clinical symptoms or signs (4). However, there is a lack of serum biomarkers for identifying LVDD in high-risk individuals, as conventional heart failure-related biomarkers, such as serum brain natriuretic peptides (BNP), typically remain within the normal range during the early stages of HF. Therefore, it is imperative to explore novel biomarkers capable of identifying LVDD to prevent the onset and progression of overt HF.
Inflammation serves both as a cause and a consequence of HF, playing a pivotal role in its pathogenesis and progression (5, 6). Comorbidities frequently associated with HF, such as diabetes, obesity, and chronic kidney disease, contribute to a state of chronic low-grade inflammation. The neutrophil-to-lymphocyte ratio (NLR), derived from a complete blood count, is a marker of systemic inflammation. It has been confirmed to be linked with multiple inflammatory conditions, such as cardiovascular diseases, malignancies, infections, and hemorrhagic disorders (7). Previous clinical studies have primarily examined the predictive value of NLR for adverse disease outcomes, concluding that elevated NLR levels may signify a more severe disease prognosis in patients (8–11). The relationship between NLR and LVDD in patients with T2DM remains unexplored, with no published studies addressing this issue. Examining these relationships may assist clinicians in identifying patients at elevated risk of LVDD.
Clinical data were collected from 855 patients diagnosed with T2DM who were hospitalized at the Endocrinology Department of Wuhan Union Hospital between January 2019 and January 2021. Detailed clinical information included the patient’s age, gender, BMI, blood pressure, smoking history, family history of diabetes, duration of diabetes, HbA1c, fast blood glucose, liver function, kidney function, lipid profile, uric acid, neutrophil and lymphocyte count. All patients included in the study were of Han ethnicity. The inclusion criteria were as follows: age>18 years old; a diagnosis of type 2 diabetes; access to complete blood count and echocardiography examination; ejection fraction (EF) greater than 50%. T2DM was defined according to the 2023 ADA Standards of Care in Diabetes (12). The following criteria were used for exclusion: patients with previous histories of coronary heart disease, valvular heart diseases, or known clinical heart failure; patients with severe liver or kidney dysfunction; patients with acute infectious diseases; patients diagnosed with hematological diseases; and patients with a history of malignant tumors. Heart failure (HF) was diagnosed according to the 2022 AHA/ACC/HFSA Guidelines (13). Severe hepatic or renal dysfunction was characterized by ALT levels surpassing three times the upper limit of normal or an eGFR below 30 mL/min/1.73 m². Acute infectious and hemorrhagic diseases were identified through pertinent clinical manifestations and hematological indicators.
Echocardiographic tests were conducted using echocardiographic systems (GE Vivid 7; Vingmed; Philips IE33 and Philips EPIQ 7C) with 3-8 MHz transducers. Two experienced ultrasonography specialists identified signs of diastolic dysfunction based on the E/A ratio values of the mitral and septal basal regions. The left ventricular end-diastolic diameter (LVEDD), left atrial diameter (LAD), interventricular septum thickness (IVST), and left ventricular ejection fraction (LVEF) were measured. LVEF was determined using the biplane Simpson’s approach. Peak velocities in the early (E-wave) (MVE) and late (A-wave) (MVA) phases of the mitral inflow pattern were determined using apical four-chamber images. According to the ASE/EACVI 2016 recommendations, patients can be identified as LVDD if the following criteria are met: average E/e ratio > 14 or E/e’ ratio < 14 with an E/A ratio < 0.8 (14).
The NLR values were calculated using the formula NLR = N/L from the absolute peripheral granulocyte (N; 109/Liter) and lymphocyte (L; 109/Liter) blood counts. Our study defined the first 25% of NLR values as the low-level group, the middle 50% as the moderate-level group, and the last 25% as the high-level group.
The analysis was conducted using IBM SPSS Statistics, Version 22 (IBM Corporation, Armonk, NY, USA). A two-sided P value of <0.05 was considered significant. Continuous variables were expressed as mean ± SD or median (interquartile range, IQR), while categorical variables were presented as percentages (%) based on the data distribution and types. Logistic regression analysis assessed the trend of variable changes across different NLR levels, providing ORs and P values for adjusted models. The data were summarized as ORs and regression coefficients (95% CI).
Our data analysis involved 855 hospitalized patients diagnosed with T2DM, as detailed in Table 1. The study population had a mean age of 53.39 ± 11.37 years, with females comprising 30.9% of the total patients. The average HbA1c level was 9.20 ± 3.62%, and the mean BMI was 25.28 ± 3.69 kg/m². Patients were divided into two groups based on the evaluation of left ventricular diastolic function: the normal group (n=466, non-LVDD group) and the diastolic dysfunction group (LVDD group, n=409). In comparison to the non-LVDD group, patients in the LVDD group were older (58.75 ± 8.10 years old vs. 48.64 ± 11.86 years old, P<0.001), had a longer duration of diabetes (8.00 (3.00-15.00) vs. 5.00 (0.94-10.00) years, P<0.001), lower estimated glomerular filtration rate (eGFR) (104.97 (96.31-116.00) vs. 95.43 (77.23-102.87) ml/min/1.73m2, P<0.001), and higher systolic blood pressure (SBP) (133.4 ± 17.66 vs. 129.20 ± 16.71 mmHg, P<0.001). No significant differences were observed in fasting blood glucose (FBG), diastolic blood pressure, and lipid profiles (all P values >0.05). A complete blood cell count was obtained from all patients, and the NLR values were calculated. The results showed significant differences in NLR values between the two groups. The NLR of LVDD group patients was significantly higher compared to the non-LVDD group (1.85 (1.44-2.31) vs 1.60 (1.24-2.05), P<0.001).
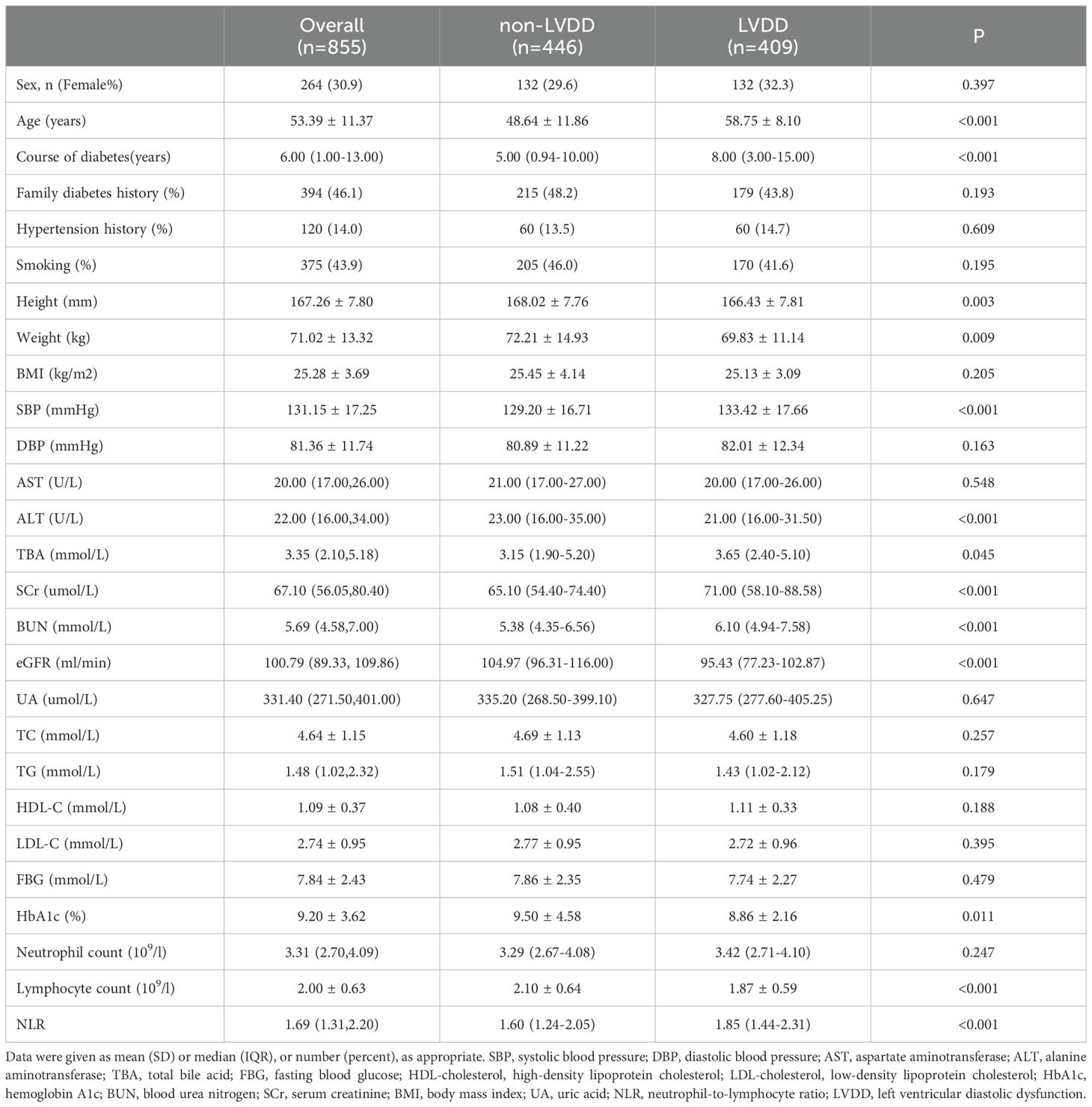
Table 1. Basic characteristics of patients with type 2 diabetes grouped by left ventricular function.
As shown in Table 2, univariate regression analysis showed that the LVDD prevalence was significantly associated with the patient’s age, course of diabetes, HbA1c, SBP, creatinine, eGFR, and NLR (all P values<0.05), while no significant relationships were found regarding FBG, BMI, DBP, uric acid, and TBA (all P values>0.05). Concerning the blood lipid profile, only triglycerides demonstrated a slight but statistically significant association with LVDD prevalence (P=0.047). By performing multivariate regression analysis, the following variables remained significantly associated with LVDD: patient’s age, SBP, and creatinine level, while NLR, course of diabetes, and HbA1c did not show an independent association with LVDD (all P values >0.05).
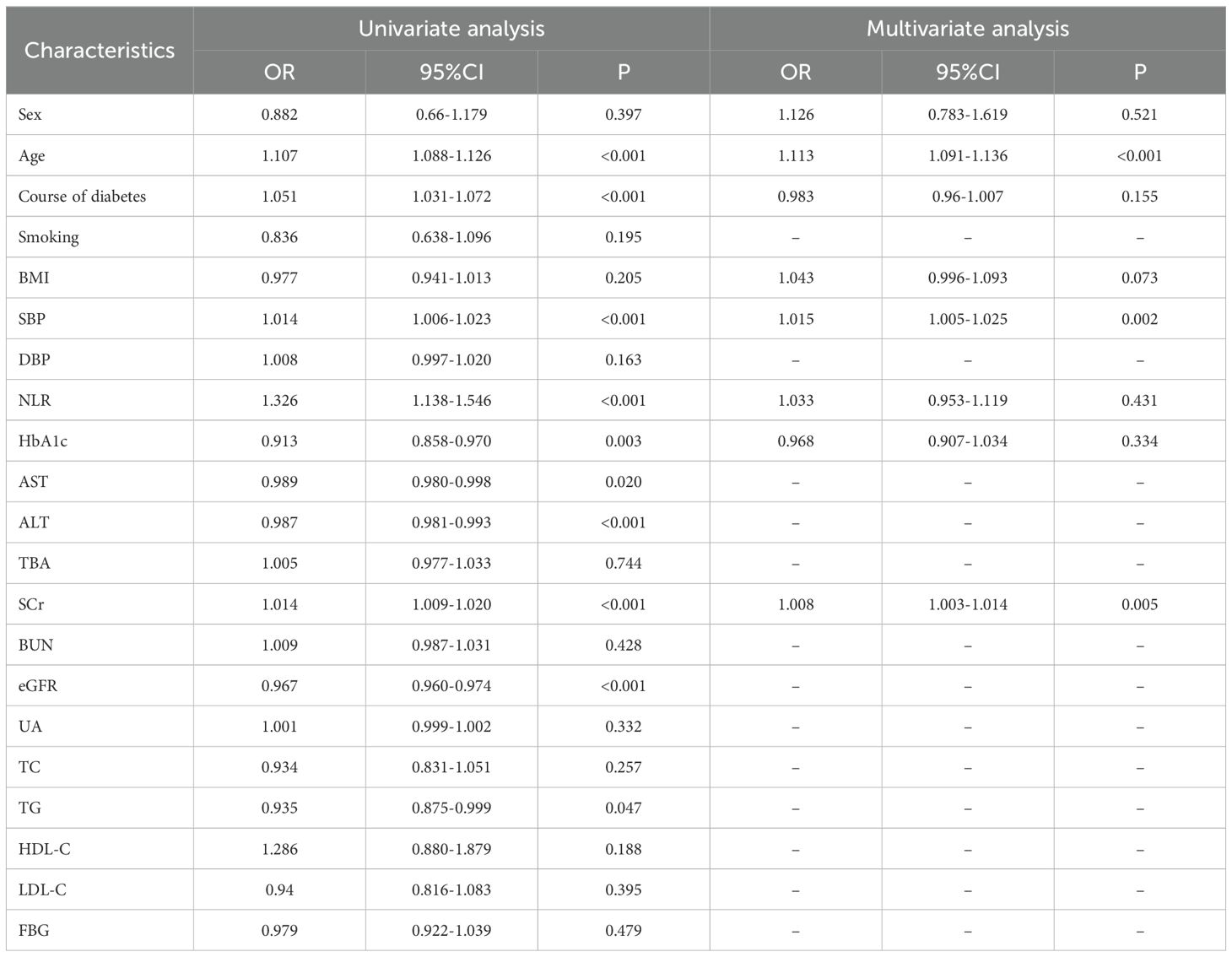
Table 2. Univariate and multivariate logistic regression analyses on the relationship between clinical parameters and prevalence of LVDD.
NLR values were divided into three groups using the following methods: the lowest 25% were considered as the low-level group (0.601,1.316), the middle 50% as the medium-level group (1.316,2.156), and the highest 25% as the high-level group (2.156,15.622). The prevalence of LVDD was 35.51% (76/214) for the low-level NLR group, 49.27% (203/412) for the medium-level group, and 56.77% (130/229) for the high-level group (P < 0.001), respectively. Baseline data showed that the patient’s sex, age, course of diabetes, serum creatinine (SCr), and eGFR were statistically different across the three groups. Echocardiographic parameters related to LVDD were measured, and the results indicated significant changes in left atrial dimension (LAD), interventricular septal thickness (IVST), mitral septal, and lateral velocity among the three groups (P < 0.001) (Table 3).
Table 4 showed the crude and adjusted logistical models evaluating the correlation between different NLR levels and the prevalence of LVDD. Compared with the low-level NLR group, the OR value for the medium-level group was 1.764(1.255-2.478, P<0.001) and 2.384(1.626-3.497, P<0.001) for the high-level group. Additionally, after adjustment for the course of diabetes and HbA1c (defined as model 2), the OR value was 1.764 (1.255-2.478, P<0.001) and 2.384 (1.626-3.497, P<0.001) respectively, and the P-value for the trend was also significant (P<0.001). After further adjustment for age, sex, and serum creatinine (defined as model 3), the OR value for the high-level group, as well as the P-value for the trend, did not achieve statistical significance(P>0.05).
Individuals with T2DM suffer metabolic irregularities, increased production of advanced glycation end products (AGEs), and inflammatory cytokines due to prolonged hyperglycemia. Together with comorbidities such as hypertension, obesity, and renal dysfunction, T2DM can result in varying degrees of damage to cardiac structure and function, hence promoting the development of diabetic cardiomyopathy and HF (5, 6, 15). LVDD, also called preclinical HF, serves as an early stage of the detrimental effects of diabetes mellitus on the heart and exhibits high prevalence among diabetic patients (16). In our study, the prevalence of LVDD among recruited hospitalized patients with T2DM was 47.8% (409/855), consistent with previous reports (17–21). Logistic regression analysis of patients’ clinical parameters revealed that the patient’s age, SBP, and SCr were independently associated with the prevalence of LVDD. In contrast to previous studies, this research did not find a positive correlation between FBG, HBA1c, and the prevalence of LVDD (20, 22, 23). A cross-sectional survey by Rishi T. Guria et al. found that the prevalence of LVDD among T2DM patients was 54%. The research indicated that the average HbA1c level was markedly elevated in the LVDD group relative to the non-LVDD group (11.07 ± 3.66% vs. 9.11 ± 2.95%, P = 0.004) (20). The results may be attributable to the baseline characteristics of our study participants, which predominantly comprised hospitalized T2DM patients with poor glycemic control. Data from outpatient or health screening populations could better elucidate the impact of differential glycemic control on LVDD prevalence. Moreover, the effects of hyperglycemia on the heart depend on elevated blood glucose levels and the duration of sustained hyperglycemia, which may play a more critical role. Consistent with findings from other researchers, our study suggests that diabetic patients with coexisting LVDD tend to have a longer disease course (24–26).
Inflammation plays a pivotal role in the onset and progression of heart failure. Research has shown that patients with LVDD exhibit significantly elevated levels of inflammatory markers, both systemically and in cardiac tissues, including TNFα, IL-6, and IL-1β, compared to individuals with normal cardiac function (27, 28). NLR, an inflammatory biomarker reflecting the body’s inflammatory state, has been associated with various cardiovascular diseases, such as coronary artery disease and heart failure (29). Additionally, increased NLR levels have been linked to diabetes and may serve as a marker of the low-grade chronic inflammation commonly observed in diabetes and its complications (30–33). Studies investigating the relationship between NLR and LVDD are scarce. In our study, the mean NLR was higher in patients with LVDD than those without LVDD. Furthermore, an increasing trend in LVDD prevalence was observed with higher NLR levels. However, after adjusting for confounding factors such as age, gender, eGFR, and BMI, this trend was no longer statistically significant (P for trend = 0.219). When stratifying patients into three NLR groups, significant differences in age and SCr were identified across the groups. This suggests that age and SCr may be confounding factors in the analysis. Nevertheless, this does not negate the potential utility of NLR as an inflammatory biomarker for identifying LVDD risk. Multiple factors, including glycemic control, renal function, and age, influence NLR, which should be considered when interpreting its clinical implications (7, 9, 34).
Our study highlights the critical concern of LVDD in individuals with T2DM, a group at elevated risk for heart failure. We present a reliable and generalizable dataset derived from data analysis from 855 patients. The study identified a significant finding: The prevalence of LVDD increased progressively with rising NLR levels, indicating the potential utility of NLR as a biomarker for identifying LVDD in patients with type 2 diabetes. Echocardiographic screening for LVDD is recommended for patients with elevated NLR levels, especially among the elderly with hypertension and declining renal function. For those diagnosed with LVDD via echocardiography, treatment strategies should prioritize antidiabetic medications with proven benefits in preventing or managing HF, particularly sodium-glucose cotransporter 2 inhibitors (SGLT2i) (35). Further research is needed to confirm whether SGLT2i have a therapeutic or preventive role in halting the progression of advanced HF in patients with diabetes and coexisting LVDD.
The study has some limitations that should be considered. First, a cross-sectional research design cannot establish a causal relationship between NLR values and LVDD. Additionally, the inclusion criteria could not allow the findings to be generalized to the broader population of individuals with type 2 diabetes. Including a more diverse range of individuals from community populations or outpatient settings in the study could improve the generalizability of the results. Lastly, the lack of data on patients’ use of antidiabetic medications and other diabetes complications prevents us from assessing their associations with NLR or LVDD prevalence.
Our research findings indicate a significant positive correlation between NLR values and the prevalence of LVDD in individuals with type 2 diabetes. NLR could potentially be used as a biomarker to allow patients’ risk stratification and detection of LVDD in early asymptomatic phases, significantly reducing the burden of heart failure. Further validation of the predictive value of the NLR on developing LVDD warrants robust prospective studies. Such efforts will not only enhance our understanding of the link between chronic inflammation and LVDD in diabetic individuals but also aid in the clinical management of diabetes-related cardiomyopathy.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
XY: Conceptualization, Data curation, Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing. YS: Conceptualization, Data curation, Investigation, Software, Writing – original draft, Writing – review & editing. HZ: Conceptualization, Data curation, Resources, Writing – original draft. LH: Conceptualization, Data curation, Investigation, Software, Writing – original draft. JZ: Investigation, Methodology, Writing – review & editing. JM: Writing – review & editing. LC: Funding acquisition, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82170822, 82070809, 82300895, and 81900734).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1499713/full#supplementary-material
1. Zhou B, Rayner AW, Gregg EW, Sheffer KE, Carrillo-Larco RM, Bennett JE, et al. Worldwide trends in diabetes prevalence and treatment from 1990 to 2022: a pooled analysis of 1108 population-representative studies with 141 million participants. Lancet. (2024) 404:2077–93. doi: 10.1016/S0140-6736(24)02317-1
2. Dunlay SM, Givertz MM, Aguilar D, Allen LA, Chan M, Desai AS, et al. Type 2 Diabetes Mellitus and Heart Failure: A Scientific Statement from the American Heart Association and the Heart Failure Society of America: This statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation. (2019) 140:e294-e324. doi: 10.1161/CIR.0000000000000691
3. Hoek AG, Dal Canto E, Wenker E, Bindraban N, Handoko ML, Elders PJM, et al. Epidemiology of heart failure in diabetes: a disease in disguise. Diabetologia. (2024) 67:574–601. doi: 10.1007/s00125-023-06068-2
4. Kosmala W, Marwick TH. Asymptomatic left ventricular diastolic dysfunction. JACC: Cardiovasc Imaging. (2020) 13:215–27. doi: 10.1016/j.jcmg.2018.10.039
5. Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res. (2018) 122:624–38. doi: 10.1161/CIRCRESAHA.117.311586
6. Murphy SP, Kakkar R, McCarthy CP, Januzzi JL. Inflammation in heart failure. J Am Coll Cardiol. (2020) 75:1324–40. doi: 10.1016/j.jacc.2020.01.014
7. Buonacera A, Stancanelli B, Colaci M, Malatino L. Neutrophil to lymphocyte ratio: an emerging marker of the relationships between the immune system and diseases. IJMS. (2022) 23:3636. doi: 10.3390/ijms23073636
8. Bagyura Z, Kiss L, Lux Á, Csobay-Novák C, Jermendy ÁL, Polgár L, et al. Neutrophil-to-lymphocyte ratio is an independent risk factor for coronary artery disease in central obesity. IJMS. (2023) 24:7397. doi: 10.3390/ijms24087397
9. Yuan Q, Wang J, Peng Z, Zhou Q, Xiao X, Xie Y, et al. Neutrophil-to-lymphocyte ratio and incident end-stage renal disease in Chinese patients with chronic kidney disease: results from the Chinese Cohort Study of Chronic Kidney Disease (C-STRIDE). J Transl Med. (2019) 17:86. doi: 10.1186/s12967-019-1808-4
10. Zahorec R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. BLL. (2021) 122:474–88. doi: 10.4149/BLL_2021_078
11. Fest J, Ruiter TR, Groot Koerkamp B, Rizopoulos D, Ikram MA, Van Eijck CHJ, et al. The neutrophil-to-lymphocyte ratio is associated with mortality in the general population: The Rotterdam Study. Eur J Epidemiol. (2019) 34:463–70. doi: 10.1007/s10654-018-0472-y
12. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 2. Classification and diagnosis of diabetes: standards of care in diabetes—2023. Diabetes Care. (2023) 46:S19–40. doi: 10.2337/dc23-S002
13. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: A report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. (2022) 145:e895-e1032. doi: 10.1161/CIR.0000000000001063
14. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. (2016) 17:1321–60. doi: 10.1093/ehjci/jew082
15. Pandey A, Khan MS, Patel KV, Bhatt DL, Verma S. Predicting and preventing heart failure in type 2 diabetes. Lancet Diabetes Endocrinol. (2023) 11:607–24. doi: 10.1016/S2213-8587(23)00128-6
16. Bouthoorn S, Valstar GB, Gohar A, Den Ruijter HM, Reitsma HB, Hoes AW, et al. The prevalence of left ventricular diastolic dysfunction and heart failure with preserved ejection fraction in men and women with type 2 diabetes: A systematic review and meta-analysis. Diabetes Vasc Dis Res. (2018) 15:477–93. doi: 10.1177/1479164118787415
17. Chee KH, Tan KL, Luqman I, Saiful SS, Chew YY, Chinna K, et al. Prevalence and predictors of left ventricular diastolic dysfunction in Malaysian patients with type 2 diabetes mellitus without prior known cardiovascular disease. Front Cardiovasc Med. (2021) 8:676862. doi: 10.3389/fcvm.2021.676862
18. Cm W, Pillai G, Divakar A, Bhaskaran R. Left ventricular diastolic dysfunction in type 2 diabetes mellitus: A single-centre observational study from a tertiary care hospital in south India. Cureus. (2023) 5. doi: 10.7759/cureus.34667
19. Fontes-Carvalho R, Ladeiras-Lopes R, Bettencourt P, Leite-Moreira A, Azevedo A. Diastolic dysfunction in the diabetic continuum: association with insulin resistance, metabolic syndrome and type 2 diabetes. Cardiovasc Diabetol. (2015) 14:4. doi: 10.1186/s12933-014-0168-x
20. Guria RT, Prasad MK, Mishra B, Marandi S, Kumar A, Dungdung A. Association of glycosylated haemoglobin (HbA1c) level with left ventricular diastolic dysfunction in patients with type 2 diabetes. Cureus. (2022) 14. doi: 10.7759/cureus.31626
21. Jin J, Wang W, Zhu L, Gu T, Niu Q, Li P, et al. Cardiovascular autonomic neuropathy is an independent risk factor for left ventricular diastolic dysfunction in patients with type 2 diabetes. BioMed Res Int. (2017) 2017:1–6. doi: 10.1155/2017/3270617
22. Maiello M, Zito A, Cecere A, Ciccone MM, Palmiero P. Left ventricular diastolic dysfunction in normotensive postmenopausal women with type 2 diabetes mellitus. Cardiol J. (2017) 24:51–6. doi: 10.5603/CJ.a2016.0064
23. Porel R, Shyama S, Ahmad S, Kumar N, Ahmad S, Biswas R, et al. Can glycated haemoglobin (HbA1c) be used as a predictor of left ventricular diastolic dysfunction in non-hypertensive patients with newly diagnosed type 2 diabetes mellitus: a cross-sectional study at a tertiary care centre in Eastern India. BMJ Open. (2024) 14:e081269. doi: 10.1136/bmjopen-2023-081269
24. Yang H-H, Li F-R, Zhou M-G, Xie L-F, Jin Y-Y, Ligcchen Z-H. Duration of diabetes, glycemic control, and risk of heart failure among adults with diabetes: A cohort study. J Clin Endocrinol. (2022) 108:1166–72. doi: 10.2139/ssrn.4109078
25. Echouffo-Tcheugui JB, Zhang S, Florido R, Hamo C, Pankow JS, Michos ED, et al. Duration of diabetes and incident heart failure. JACC: Heart Failure. (2021) 9:594–603. doi: 10.1016/j.jchf.2021.06.005
26. Zoppini G, Bonapace S, Bergamini C, Rossi A, Trombetta M, Lanzoni L, et al. Evidence of left atrial remodeling and left ventricular diastolic dysfunction in type 2 diabetes mellitus with preserved systolic function. Nutrition Metab Cardiovasc Dis. (2016) 26:1026–32. doi: 10.1016/j.numecd.2016.05.004
27. Dinh W, Füth R, Nickl W, Krahn T, Ellinghaus P, Scheffold T, et al. Elevated plasma levels of TNF-alpha and Interleukin-6 in patients with diastolic dysfunction and glucose metabolism disorders. Cardiovasc Diabetol. (2009) 8:58. doi: 10.1186/1475-2840-8-58
28. Mocan M, Mocan Hognogi LD, Anton FP, Chiorescu RM, Goidescu CM, Stoia MA, et al. Biomarkers of inflammation in left ventricular diastolic dysfunction. Dis Markers. (2019) 2019:1–14. doi: 10.1155/2019/7583690
29. Afari ME, Bhat T. Neutrophil to lymphocyte ratio (NLR) and cardiovascular diseases: an update. Expert Rev Cardiovasc Ther. (2016) 14:573–7. doi: 10.1586/14779072.2016.1154788
30. Adane T, Melku M, Worku YB, Fasil A, Aynalem M, Kelem A, et al. The association between neutrophil-to-lymphocyte ratio and glycemic control in type 2 diabetes mellitus: A systematic review and meta-analysis. J Diabetes Res. (2023) 2023:1–11. doi: 10.1155/2023/3117396
31. Wan H, Wang Y, Fang S, Chen Y, Zhang W, Xia F, et al. Associations between the neutrophil-to-lymphocyte ratio and diabetic complications in adults with diabetes: A cross-sectional study. J Diabetes Res. (2020) 2020:1–9. doi: 10.1155/2020/6219545
32. Wang J-R, Chen Z, Yang K, Yang H-J, Tao W-Y, Li Y-P, et al. Association between neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and diabetic retinopathy among diabetic patients without a related family history. Diabetol Metab Syndr. (2020) 12:55. doi: 10.1186/s13098-020-00562-y
33. Ning P, Yang F, Kang J, Yang J, Zhang J, Tang Y, et al. Predictive value of novel inflammatory markers platelet-to-lymphocyte ratio, neutrophil-to-lymphocyte ratio, and monocyte-to-lymphocyte ratio in arterial stiffness in patients with diabetes: A propensity score–matched analysis. Front Endocrinol. (2022) 13:1039700. doi: 10.3389/fendo.2022.1039700
34. Verdoia M, Schaffer A, Barbieri L, Aimaretti G, Marino P, Sinigaglia F, et al. Impact of diabetes on neutrophil-to-lymphocyte ratio and its relationship to coronary artery disease. Diabetes Metab. (2015) 41:304–11. doi: 10.1016/j.diabet.2015.01.001
Keywords: neutrophil-to-lymphocyte ratio, type 2 diabetes mellitus, left ventricular diastolic dysfunction, heart failure, inflammatory biomarkers
Citation: Yang X, Shi Y, Zhang H, Huang L, Zhang J, Min J and Chen L (2025) Association between neutrophil-to-lymphocyte ratio and left ventricular diastolic dysfunction in patients with type 2 diabetes mellitus. Front. Endocrinol. 15:1499713. doi: 10.3389/fendo.2024.1499713
Received: 21 September 2024; Accepted: 23 December 2024;
Published: 10 January 2025.
Edited by:
Aleksandra Klisic, Primary Health Care Center Podgorica, MontenegroReviewed by:
Emmanouil Korakas, University General Hospital Attikon, GreeceCopyright © 2025 Yang, Shi, Zhang, Huang, Zhang, Min and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lulu Chen, Y2hlcmlhX2NoZW5AMTI2LmNvbQ==; Jie Min, bWluamllNzdAb3V0bG9vay5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.