- 1Medizinische Klinik und Poliklinik IV, Klinikum der Universität München, Ludwig-Maximilians-Universität (LMU) München, Munich, Germany
- 2Klinik für Endokrinologie, Diabetologie und Klinische Ernährung, Universitätsspital Zürich (USZ) und Universität Zürich (UZH), Zurich, Switzerland
- 3The LOOP Zurich - Medical Research Center, Zurich, Switzerland
- 4Department of Internal Medicine I, Division of Endocrinology and Diabetes, University Hospital of Würzburg, Würzburg, Germany
Context: Primary aldosteronism (PA) is the most common form of endocrine hypertension. According to the Endocrine Society Practice Guidelines, the diagnosis of PA requires a pathological screening test result and non-suppressible aldosterone levels during confirmatory testing. Sequential testing with more than one confirmatory test may result in discordant test results.
Objective and patients: We investigated the association of discordant results of captopril challenge test (CCT) and saline infusion test (SIT) on patient subtype classification by adrenal vein sampling (AVS) and outcome in 111 consecutive patients from the German Conn’s Registry. Concordance was defined as non-suppressible aldosterone levels upon both tests, while discordance was defined as conflicting test results. Patients with unilateral disease were offered adrenalectomy (ADX). Biochemical and clinical outcomes were assessed using the PASO criteria.
Results: 85 of 111 (77%) patients had concordant results of CCT and SIT. Although baseline characteristics were comparable between patients with concordant and discordant tests, the latter had significantly lower aldosterone levels after testing (CCT: 170 vs. 114pg/ml; SIT: 139 vs. 101pg/ml; p=0.004). In 35% of patients with discordant (n=9) and 46% of concordant test results (n=39), AVS suggested lateralized PA. In 36 of 48 cases ADX was performed. 86% of patients with discordant and 72% with concordant results had complete biochemical success.
Conclusion: The use of two confirmatory tests in patients with PA results in discordant results in approximately 23% of cases. Patients having discordant confirmatory test results had a comparable rate of lateralized PA and underwent adrenalectomy with similar long-term outcome.
Introduction
Primary aldosteronism (PA) is the most frequent cause of endocrine arterial hypertension and affects about 5-15% of hypertensive patients (1, 2). Patients with PA have a higher risk for cardiovascular complications such as stroke and myocardial infarction as well as metabolic diseases like diabetes mellitus type 2, compared to matched patients with essential hypertension (3–7). The diagnosis is often protracted due to challenges including adjustment of antihypertensive medication before screening and confirmation testing (8, 9). The fact that only 1% of patients with treatment-resistant hypertension undergo guideline-recommended screening for PA underlines the importance of increasing the awareness for PA, as the early diagnosis is essential to reduce cardiovascular risks (10, 11).
The Clinical Practice Guideline for PA recommends screening by measuring the plasma aldosterone to renin ratio (ARR) with adjusted medication to identify potential cases of PA. Whereas patients with a strong biochemical phenotype can directly bypass to adrenal vein sampling (AVS) according to Clinical Practice Guidelines, patients with a pathological ARR usually undergo at least one confirmatory test to ensure or reject the diagnosis (9, 12, 13). In clinical routine either the saline infusion test (SIT) or the captopril challenge test (CCT) is performed. Because of the high variability of aldosterone and confirmatory tests, there might be discordant results in SIT and CCT (14, 15). Considering limited sensitivity and specificity, the Japanese Endocrine Society Guideline recommends performing at least two confirmatory tests, regardless of the result of the first one to make a definitive diagnosis (16).
In this context, in 2021 Fukumoto et al. reported that most of the patients with a pathological result in only one confirmatory test (discordant) had milder forms of PA indicated by 96% of patients showing a non-lateralized disease (17). Populations from Asian countries exhibit differences in the relative proportions of each PA subtype and of mutation status in aldosterone-producing adenomas, which predominantly cause unilateral PA (16, 18). These factors can influence the results of confirmatory tests. We investigated patients with diagnosed PA from the Munich Center of the German Conn’s Registry who underwent two confirmatory tests (CCT and SIT) on subtyping and outcome of PA.
Subjects and methods
Patients
Between 2017 and 2020, 257 consecutive patients were diagnosed with PA in the Munich center of the German Conn’s Registry. All patients were supposed to undergo SIT as well as CCT. Due to Covid-19 pandemic and long traveling distances, only 200 patients underwent both confirmatory tests and had a pathological result in at least one of them. Of the latter, 177 underwent AVS with 131 patients showing successful AVS and receiving appropriate medication with limited impact on the ARR according to the Endocrine Society Guidelines (9). Thereof, 111 patients had at least one follow-up after six to 12 months, which represents the cohort included in the study (Figure 1).
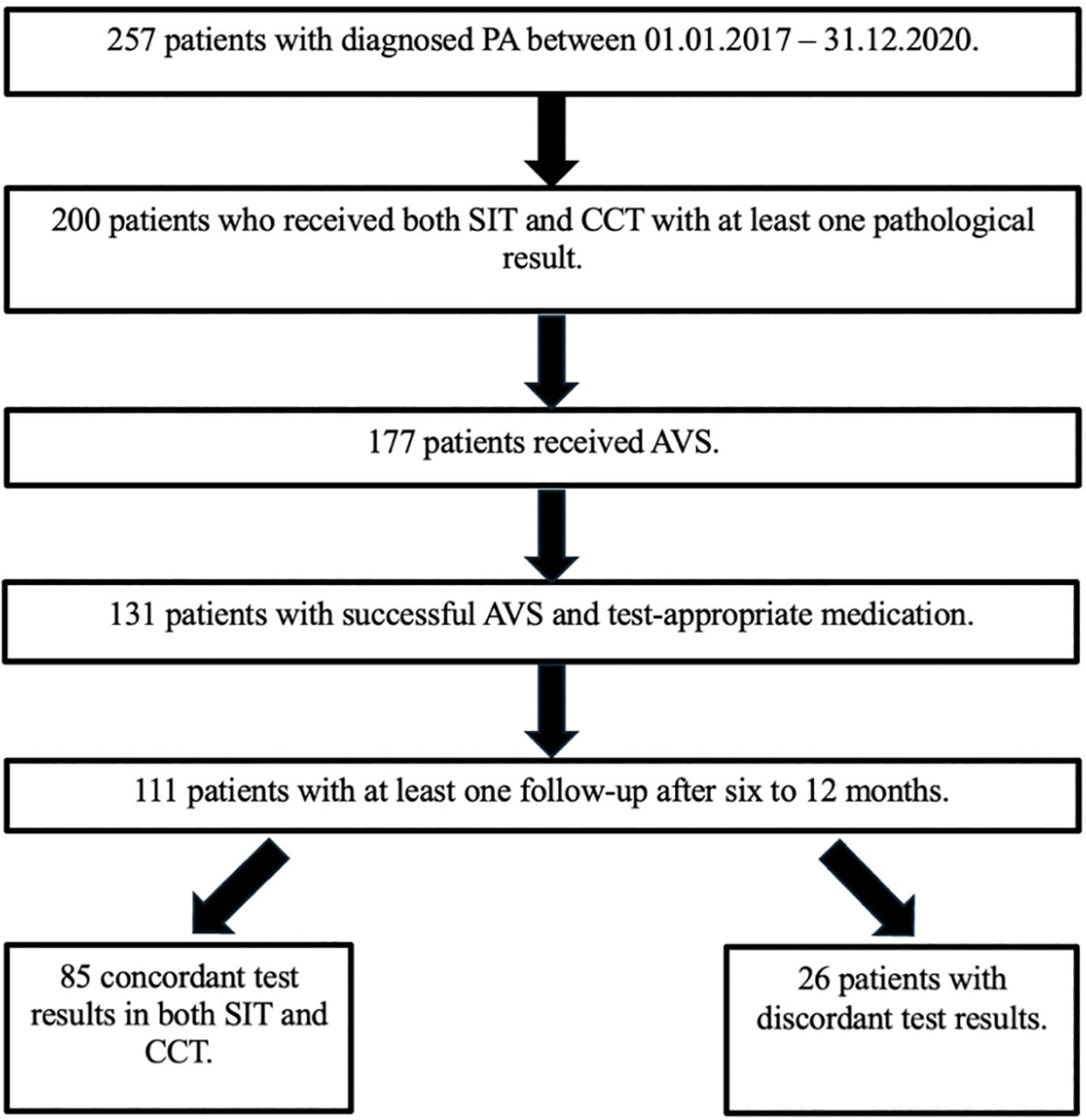
Figure 1. Patient flowchart. AVS, adrenal vein sampling; CCT, captopril challenge test; PA, primary aldosteronism; SIT, saline infusion test.
Study protocol
Screening and confirmation tests
All patients were screened according to the Endocrine Society Guideline (9) using the ARR (cut-off 12.0 pg/ml/μU/ml, sitting position),with a cut-off of at least 60 pg/ml for PAC (19). Before screening or confirmatory testing, antihypertensive medication was withdrawn for at least one or four weeks (contraceptives, mineralocorticoid receptor antagonists). If not possible, patients received medication with limited impact on the ARR, such as the alpha 1-adrenergic receptor antagonist doxazosin or the calcium-channel antagonist verapamil (9). Patients with pathological ARR ≥ 12 pg/ml/μU/ml underwent CCT and SIT independently of the results of the first test. Both tests were performed in a seated position as this measurement shows superiority to the recumbent position (20).
For the SIT, 2000 ml sodium chloride solution (0.9%) was applied by infusion in a seated position over a duration of 4 hours in the morning (8-12 am). Blood samples were drawn before (0h) and after infusion (4h). During the whole test the patients were monitored. SIT was considered pathological if aldosterone value after testing was ≥ 60 pg/ml and baseline ARR was ≥ 12 pg/ml/μU/ml (9).
For the CCT, blood samples were drawn after the patient was resting for 30 min followed by the oral administration of 50 mg captopril. After 60 min and 120 min blood was collected again. If the aldosterone was suppressed less than 30% of baseline value with a baseline ARR ≥ 12 pg/ml/μU/ml, PA was diagnosed (9).
For assessing cortisol co-secretion, a low-dose dexamethasone suppression test was performed by oral administration of 1 mg dexamethasone at 11 pm. Blood was collected at 8 am the next day. Cortisol co-secretion was diagnosed if the cortisol concentration was equal to or above 1.8µg/dl.
Classification of patients
Patients were classified as concordant if both confirmatory tests (SIT and CCT) showed a pathological result with non-suppressible aldosterone. If only one confirmatory test showed a pathological result, they were classified as discordant.
Subtyping and outcome assessment
After diagnosis of PA all patients underwent further subtyping with AVS as previously reported (21). Successful cannulation of the adrenal vein was assumed when the selectivity index (SI), which is defined as the measured plasma cortisol concentration in the respective adrenal vein to the peripheral vein, was ≥ 2.0. For patients with a lateralization index ≥ 4 unilateral disease was assumed and unilateral ADX was offered. Patients without lateralization, those who refused surgery or had contraindications for surgery received medical treatment with mineralocorticoid receptor antagonists (MRA).
All patients were re-evaluated approximately six to 12 months after initiation of specific treatment by ADX or MRA. The clinical and biochemical outcome was assessed according to the PASO criteria six to 12 months after surgery (22).
Laboratory analysis
Blood collection was performed standardized between 8 am and 10 am in a fasting state after a rest of 15 minutes in a seated position at baseline and at follow-up. Samples were either analyzed directly or processed and stored at -80°C until analysis.
Aldosterone, renin and cortisol were measured using the Diasorin Liaison CLIA. All other analyses were performed in our central laboratory using standard methods.
Statistical analysis
Data between groups was compared using Mann-Whitney U test or chi-square test for numerical or categorical variable, respectively. In this study, continuous variables are reported as mean values with ± standard deviation (SD), while categorical variables are presented as frequencies and percentage. Two-tailed probability values of <5% were considered to be statistically significant. Statistical analysis was performed using standard statistical software (IBM SPSS Statistics for Windows, Version 25. Armonk, NY: IBM Corp.).
Results
Clinical and biochemical characteristics of the total cohort
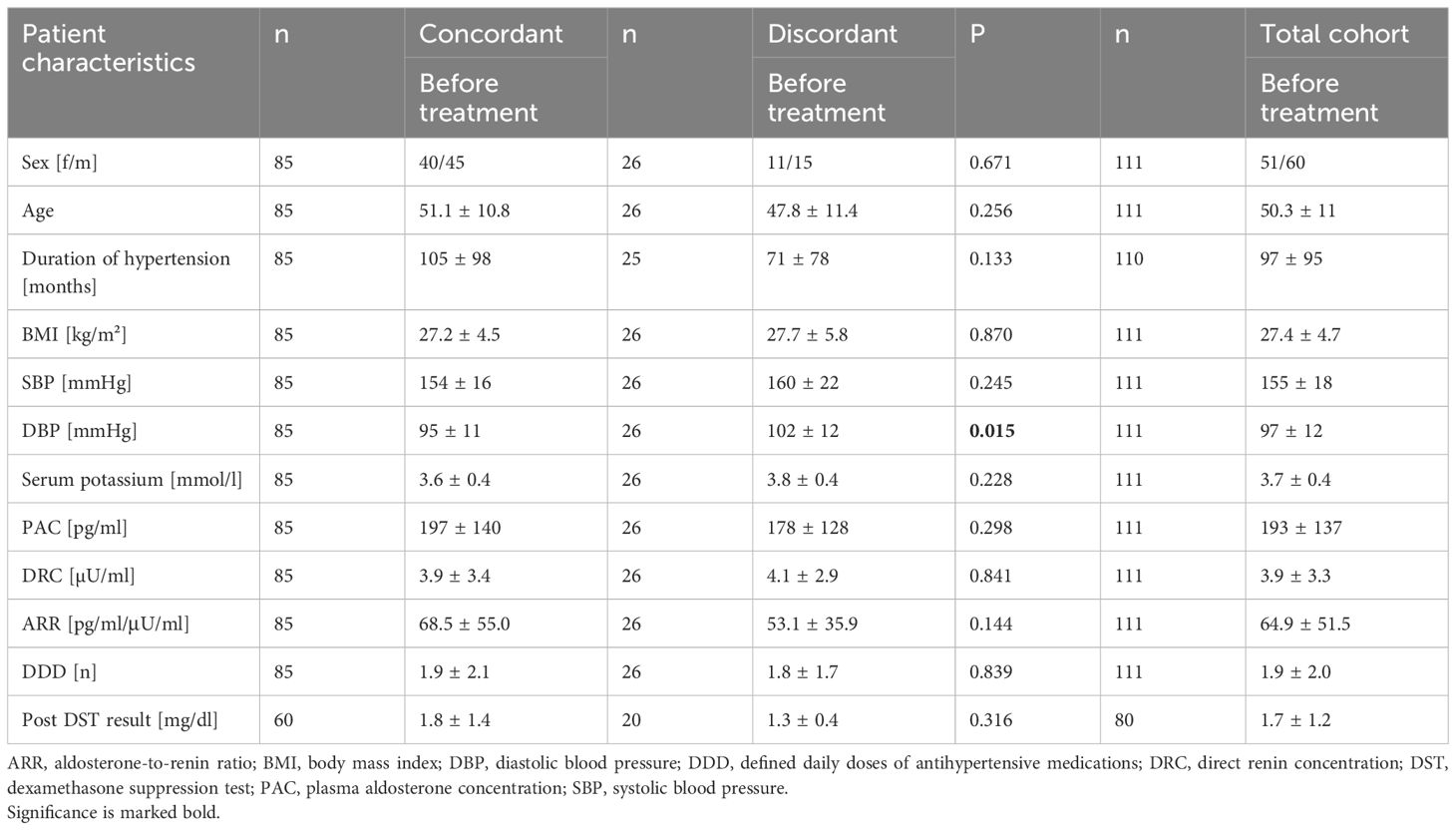
Out of 200 patients with PA who underwent both confirmatory tests and had a pathological result in at least one of them, 111 were included in the study. The mean age was 50 ± 11 years and the mean BMI was 27.4 ± 4.7 kg/m². On average, PA was diagnosed 8 years after the known onset of hypertension. In total, a higher proportion of men were diagnosed with PA than women (male: n=60; 54.1%; female: n=51; 45.9%). Besides plasma aldosterone concentration (193 pg/ml), mean systolic (SBP) and diastolic (DBP) blood pressure levels were still elevated despite a defined daily dose of antihypertensives (DDD) of 1.9 (SBP 155 mmHg; DBP 97 mmHg).
Characteristics of patients with concordant and discordant confirmatory tests
Out of the total cohort 77% (n=85) showed concordant and 23% (n=26) discordant results in SIT and CCT. 15 of these patients showed a positive test result in SIT and 11 in CCT (Table 1). Patients with concordant and discordant tests were comparable for baseline characteristics such as gender, age, duration of hypertension, BMI, plasma aldosterone and renin concentration. Although systolic blood pressure was comparable, diastolic blood pressure was significantly higher in patients with discordant test results (95 vs. 102 mmHg; p=0.015).
However, aldosterone was less suppressible in both tests in the patient group with concordant test results in comparison to patients with discordant test results (SIT: 139 vs. 101 pg/ml; p= 0.014; CCT: 170 vs. 114 pg/ml; p=0.004) and renin levels tended to be lower at baseline (2.7 vs. 4.0 µU/ml; p= 0.018). After CCT, the discordant patient group featured a more pronounced raise in renin (3.3 vs. 4.9 µU/ml; p=0.010).
There was no difference concerning cortisol co-secretion between patients with concordant and discordant test results (1.8 vs. 1.3 mg/dl; p=0.316).
47% (n=40) of patients with concordant and 46% (n=12) with discordant test results revealed nodules of the adrenal gland in imaging (p=0.936). Patients with concordant test results tended to have larger nodules (17 vs. 13mm; p=0.061) (Table 2).
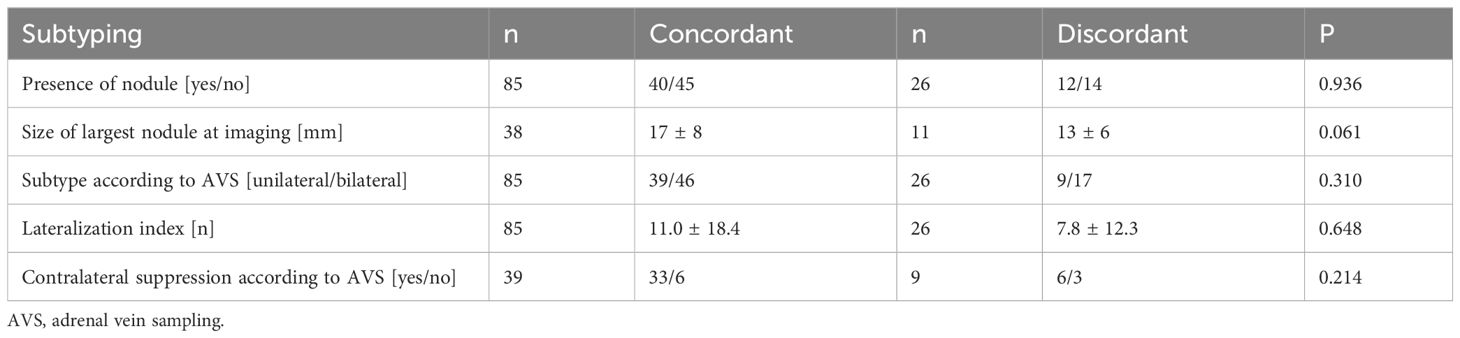
Table 2. Characteristics of patients with concordant and discordant confirmatory tests according to subtype diagnosis.
According to AVS subtyping, 54% (n=46) of patients with concordant and 65% (n=17) with discordant test results showed a non-lateralized disease. 46% of patients in the group with concordant (n=39) and 35% with discordant results (n=9) were lateralized (p=0.310).
Characteristics of patients with concordant and discordant confirmatory tests according to subtype diagnosis
Considering patients with non-lateralized disease, the ARR was significantly higher in patients with concordant confirmatory test results at baseline (60.9 vs. 42.5 pg/ml/μU/ml; p=0.037), whereas other parameters were comparable in both groups (Table 3). In confirmatory testing, renin was significantly lower in the group with concordant results before (4.3 vs. 2.5 µU/ml; p=0.013) and after CCT (4.6 vs. 3.0 µU/ml; p=0.041). The drop of aldosterone in CCT was also significantly smaller in patients with concordant testing (5 vs. 24%; p=0.003). Patients with discordant and concordant confirmatory tests with unilateral disease did not differ significantly in baseline characteristics, but patients with discordant test results had stronger suppression during confirmatory test by SIT and CCT compared to those with concordant confirmatory tests (SIT: 22 vs. 36%; p=0.023; CCT: 3 vs. 29%; p=0.008).
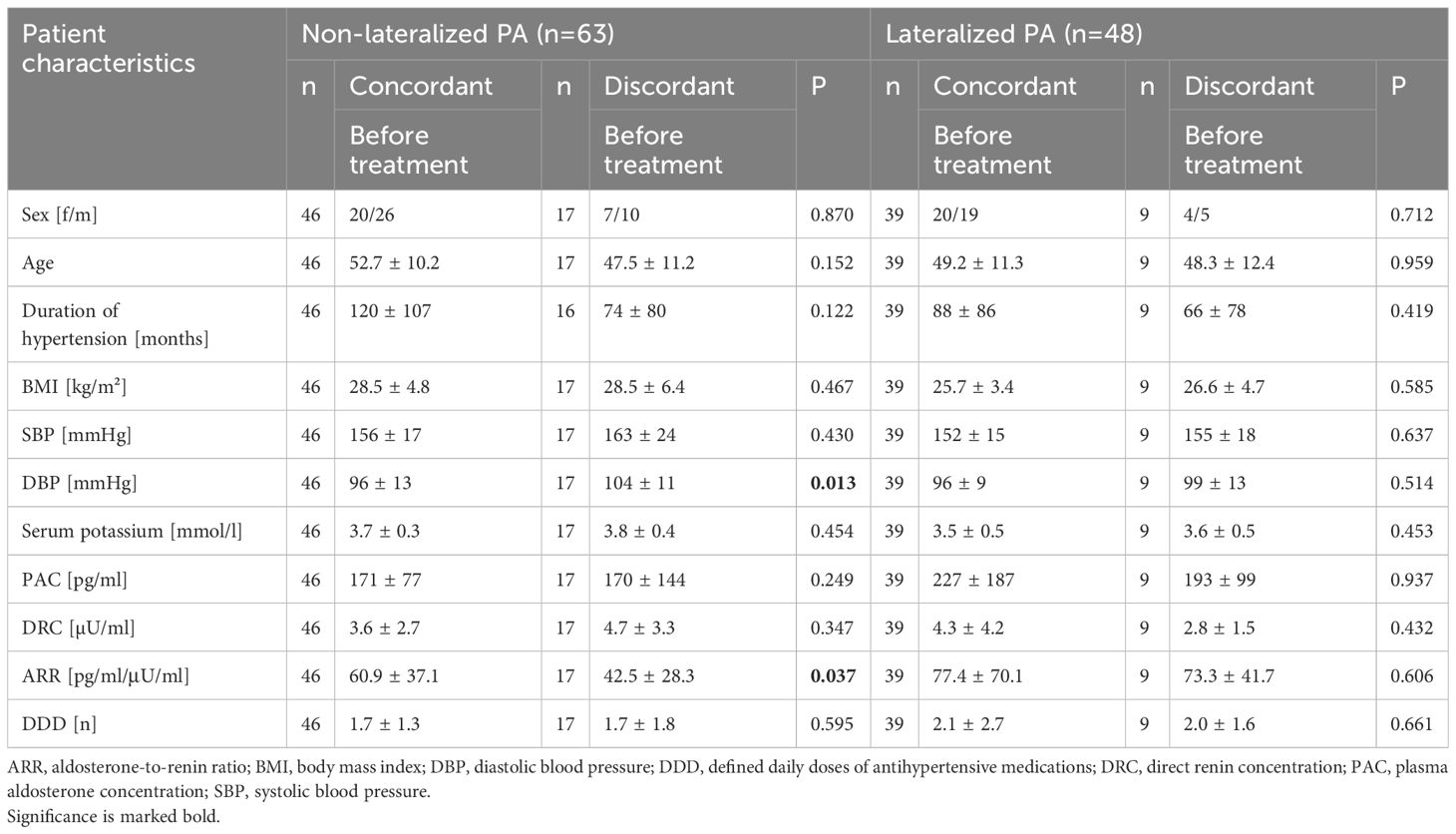
Table 3. Characteristics of bilateral and unilateral patients with concordant and discordant confirmatory tests.
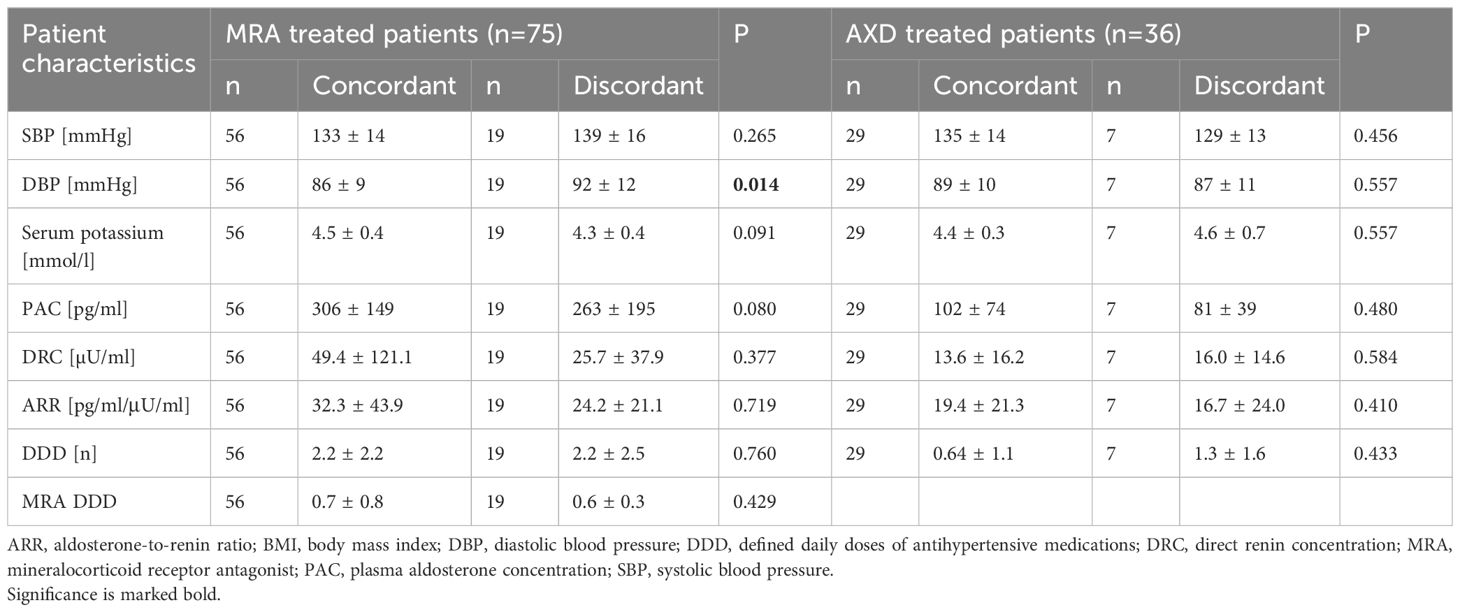
Clinical and biochemical outcome of patients treated by MRA or ADX
Neither patients treated with MRA (n=75) nor patients after ADX showed differences in baseline plasma aldosterone, renin and potassium when comparing the cohort with concordant and discordant test results in follow-up visit (Table 4).
Of 48 patients with unilateral disease, 36 underwent ADX. Tumor tissue was evaluated for PA-driver mutations in 21 patients. Almost 50% of adenomas (n=10) carried a KCNJ5 mutation, of which 90% (n=9) were female. 90% (n=9) of all patients with KCNJ5 mutation had concordant results in confirmatory testing.
We evaluated the biochemical and clinical outcome of patients with concordant (n=29) and discordant (n=7) test results after ADX according to the PASO criteria (19). Overall, 72% (n=21) of patients with concordant and 86% (n=6) with discordant confirmatory tests had a complete biochemical remission (p=0.375). 14% (n=14) of the group with concordant results revealed a partial biochemical remission, whereas none of those with discordant tests showed this result (p=0.297). 14% (n=4) of patients with concordant and 14% (n=1) with discordant confirmatory tests had an absent biochemical remission (p=0.851) (Figure 2).
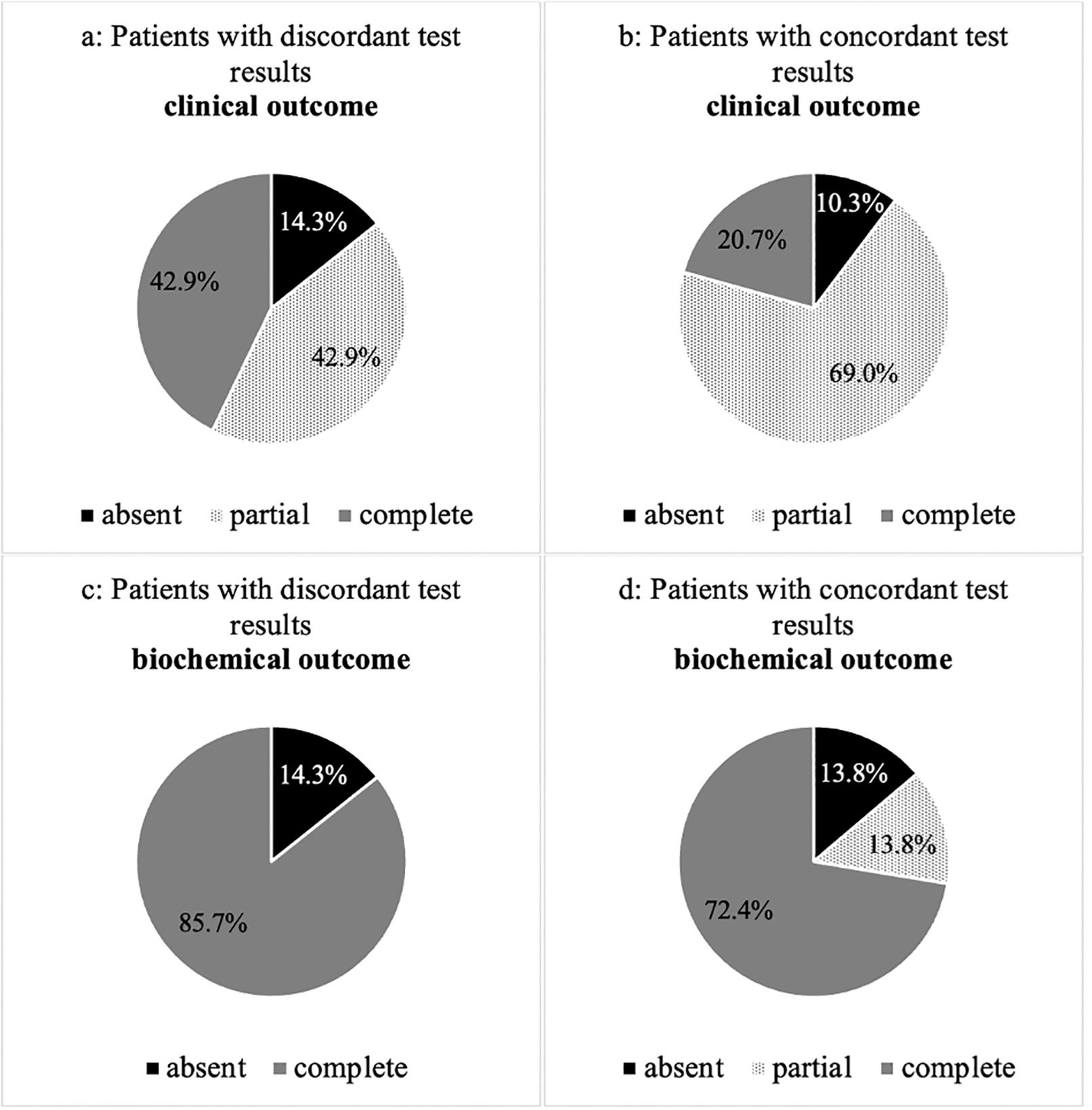
Figure 2. (A–D) Clinical and biochemical outcome of patients with concordant and discordant test results after adrenalectomy according to PASO criteria.
Considering the clinical outcome, 69% (n=20) of the group with concordant and 43% (n=3) with discordant results had a complete clinical remission (p=0.224). 21% (n=6) with concordant and 43% (n=3) with discordant testing revealed a partial clinical remission (p=0.270). 10% (n=3) of patients with concordant and 14% (n=1) with discordant results had an absent clinical remission (p=0.973).
Characteristics of unilateral patients with discordant confirmatory tests and pathology in either SIT or CCT
Despite significantly higher plasma aldosterone concentration (PAC) values in patients with pathological SIT results (90 vs. 240 pg/ml; p=0.024), there were no significant differences in baseline characteristics when splitting patients with unilateral disease and discordant confirmatory tests into subgroups of patients with pathological SIT or CCT.
Considering subtyping, 6 out of 11 patients with pathology in CCT and 6 out of 15 patients with pathology in SIT revealed nodules in imaging (p=0.462). Patients with pathological results in CCT tended to show larger nodules than patients with pathological SIT, even if the difference did not reach statistical significance (12.0 vs. 7.0 mm; p=0.333).
In AVS, there was no difference between patients with pathology in CCT (8 out of 11 were non-lateralized; 72%) and patients with pathology in SIT (9 out of 15; 60%) (p=0.500).
Discussion
In clinical routine PA is highly underdiagnosed due to the complex diagnostic work up (10, 23). The present study adds further evidence that confirmatory tests lack sensitivity, even if performed properly (14, 24, 25).
In this study almost one fourth (23%) of patients with PA would have been missed, if only one confirmatory test had been performed. In this context, it is of particular importance that this would also negatively impact patients with non-lateralized PA with a strong clinical and biochemical phenotype, as these patients can often undergo surgery to achieve biochemical cure.
To improve the diagnostic procedure and to reduce the burden of utilized healthcare resources, machine learning-based models and steroid metabolomics-driven diagnostic strategies are currently developed (26, 27). Whereas for patients with strong biochemical and clinical phenotype Clinical Practice Guidelines offer the possibility to bypass the confirmatory tests, this pathway is not recommended for milder cases of PA, although this topic is also controversially discussed (12, 28).
In the present study patients with concordant and discordant test results were comparable for gender, age, biochemical and clinical phenotype and could hardly be divided from each other at first presentation. In addition, the AVS lateralization index was not different between patients with concordant and discordant test results (11.0 vs. 7.8; p= 0.648), as well as between patients with or without cortisol co-secretion (p=0.886), whose impact on AVS parameters is still under debate (29, 30).
Interestingly, one third of discordant patients showed a unilateral subtype, and outcome after surgery in patients diagnosed with unilateral disease was not different between patients with discordant and concordant test results. Moreover, there was no evidence in favor of either confirmatory test for the diagnosis of PA or in predicting either lateralization or outcome of the disease (14).
The results of our present study are in contrast to the findings of Fukumoto et al. who reported that 96% of all discordant and 57% of all patients with concordant confirmatory tests were diagnosed with non-lateralized PA, resulting in the conclusion that discordant patients could consequently be treated with MRA without further testing (17).
In our current cohort, lateralized disease was driven by a KCNJ5-mutated APA in 50% of patients, which is comparable with other studies. Interestingly, most patients with APA-KCNJ5 mutations had concordant test results, which could explain the differences compared to the Fukumoto group as there is a much higher frequency of KCNJ5 mutation (over 80%) in patients with lateralized disease in Asian countries (17).
Based on these findings, further testing can be reasonable in Western countries if patients with highly suspected PA cannot bypass confirmatory testing. However, our findings do not support the routine implementation of two confirmatory tests due to the logistical challenges and the substantial burden of utilized healthcare resources.
As evidence suggests an ongoing increased cardiovascular risk despite MRA treatment - at least if renin levels are suppressed (31–33) - discordant test results should not be used to withhold AVS testing. This is further underlined by the fact that the biochemical and clinical outcome were comparable between patients with concordant and discordant confirmatory test results.
We acknowledge the limitation that the measurements in this study were performed with immunoassay. Compared to the commercial immunoassay, the LC-MS/MS seems to show more accurate results and is considered to be more reliable (34, 35). Although LC-MS/MS performs superior in terms of sensitivity and specificity, it must be pointed out that it tends to show lower values compared to immunoassay, which calls for specifically established reference intervals for this particular method. Moreover, a relevant number of patients were excluded because of our strict inclusion criteria. Due to the small sample size of the cohort included, no definitive conclusion can be drawn. Therefore, more investigation is necessary to address this issue. The strengths of our study include a structured and detailed patient phenotyping of the homogeneously characterized study population including data on AVS and mutation status.
In conclusion, our data show that the combined use of SIT and CCT in patients with PA results in discordant results in one fourth of cases. Patients with discordant confirmatory test results had a comparable rate of lateralized PA and underwent adrenalectomy with similar long-term outcome. Thus, further improvements in the diagnostic workup are necessary.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by ethics committee at the LMU Munich. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HD: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft. DB: Data curation, Writing – review & editing. IS: Writing – review & editing. DH: Writing – review & editing. MB: Methodology, Writing – review & editing. FB: Writing – review & editing. LK: Writing – review & editing. TW: Data curation, Writing – review & editing. MR: Funding acquisition, Resources, Supervision, Writing – review & editing. HS: Writing – review & editing. CA: Conceptualization, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Else Kröner-Fresenius Stiftung in support of the German Conn’s Registry-Else-Kröner Hyperaldosteronism Registry (2013_A182, 2015_A171 and 2019_A104 to MR), the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement No 694913 to MR), by the Deutsche Forschungsgemeinschaft (DFG) (within the CRC/Transregio 205/1 “The Adrenal: Central Relay in Health and Disease” to CA, DH, MB, HS, and MR and within the Clinician Scientist PRogram In Vascular MEdicine (PRIME) MA 2186/14-1 to HS). This work was further supported by the Clinical Research Priority Program of the University of Zurich for the CRPP HYRENE (to FB).
Acknowledgments
The study was only feasible due to the support of our clinical PA team, the Endocrine laboratory team in Munich.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1495959/full#supplementary-material
References
1. Monticone S, Burrello J, Tizzani D, Bertello C, Viola A, Buffolo F, et al. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J Am Coll Cardiol. (2017) 69:1811–20. doi: 10.1016/j.jacc.2017.01.052
2. Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. (2006) 48:2293–300. doi: 10.1016/j.jacc.2006.07.059
3. Monticone S, D’Ascenzo F, Moretti C, Williams TA, Veglio F, Gaita F, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. (2018) 6:41–50. doi: 10.1016/S2213-8587(17)30319-4
4. Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. (2005) 45:1243–8. doi: 10.1016/j.jacc.2005.01.015
5. Rocha R, Rudolph AE, Frierdich GE, Nachowiak DA, Kekec BK, Blomme EA, et al. Aldosterone induces a vascular inflammatory phenotype in the rat heart. Am J Physiol Heart Circ Physiol. (2002) 283:H1802–10. doi: 10.1152/ajpheart.01096.2001
6. Brilla CG, Weber KT. Mineralocorticoid excess, dietary sodium, and myocardial fibrosis. J Lab Clin Med. (1992) 120:893–901.
7. Young M, Fullerton M, Dilley R, Funder J. Mineralocorticoids, hypertension, and cardiac fibrosis. J Clin Invest. (1994) 93:2578–83. doi: 10.1172/JCI117269
8. Fischer E, Beuschlein F, Bidlingmaier M, Reincke M. Commentary on the Endocrine Society Practice Guidelines: Consequences of adjustment of antihypertensive medication in screening of primary aldosteronism. Rev Endocr Metab Disord. (2011) 12:43–8. doi: 10.1007/s11154-011-9163-7
9. Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2016) 101:1889–916. doi: 10.1210/jc.2015-4061
10. Brown JM, Siddiqui M, Calhoun DA, Carey RM, Hopkins PN, Williams GH, et al. The unrecognized prevalence of primary aldosteronism: A cross-sectional study. Ann Intern Med. (2020) 173:10–20. doi: 10.7326/M20-0065
11. Cohen JB, Cohen DL, Herman DS, Leppert JT, Byrd JB, Bhalla V. Testing for primary aldosteronism and mineralocorticoid receptor antagonist use among U.S. Veterans: A retrospective cohort study. Ann Intern Med. (2021) 174:289–97. doi: 10.7326/M20-4873
12. Maiolino G, Rossitto G, Bisogni V, Cesari M, Seccia TM, Plebani M, et al. Quantitative value of aldosterone-renin ratio for detection of aldosterone-producing adenoma: the aldosterone-renin ratio for primary aldosteronism (AQUARR) study. J Am Heart Assoc. (2017) 6. doi: 10.1161/JAHA.117.005574
13. Reincke M, Bancos I, Mulatero P, Scholl UI, Stowasser M, Williams TA. Diagnosis and treatment of primary aldosteronism. Lancet Diabetes Endocrinol. (2021) 9:876–92. doi: 10.1016/S2213-8587(21)00210-2
14. Song Y, Yang S, He W, Hu J, Cheng Q, Wang Y, et al. Confirmatory tests for the diagnosis of primary aldosteronism: A prospective diagnostic accuracy study. Hypertension. (2018) 71:118–24. doi: 10.1161/HYPERTENSIONAHA.117.10197
15. Yozamp N, Hundemer GL, Moussa M, Underhill J, Fudim T, Sacks B, et al. Intraindividual variability of aldosterone concentrations in primary aldosteronism: implications for case detection. Hypertension. (2021) 77:891–9. doi: 10.1161/HYPERTENSIONAHA.120.16429
16. Naruse M, Katabami T, Shibata H, Sone M, Takahashi K, Tanabe A, et al. Japan Endocrine Society clinical practice guideline for the diagnosis and management of primary aldosteronism 2021. Endocr J. (2022) 69:327–59. doi: 10.1507/endocrj.EJ21-0508
17. Fukumoto T, Umakoshi H, Ogata M, Yokomoto-Umakoshi M, Matsuda Y, Motoya M, et al. Significance of discordant results between confirmatory tests in diagnosis of primary aldosteronism. J Clin Endocrinol Metab. (2021) 106:e866–e74. doi: 10.1210/clinem/dgaa812
18. Nanba K, Rainey WE. GENETICS IN ENDOCRINOLOGY: Impact of race and sex on genetic causes of aldosterone-producing adenomas. Eur J Endocrinol. (2021) 185:R1–R11. doi: 10.1530/EJE-21-0031
19. Manolopoulou J, Fischer E, Dietz A, Diederich S, Holmes D, Junnila R, et al. Clinical validation for the aldosterone-to-renin ratio and aldosterone suppression testing using simultaneous fully automated chemiluminescence immunoassays. J Hypertens. (2015) 33:2500–11. doi: 10.1097/HJH.0000000000000727
20. Stowasser M, Ahmed AH, Cowley D, Wolley M, Guo Z, McWhinney BC, et al. Comparison of seated with recumbent saline suppression testing for the diagnosis of primary aldosteronism. J Clin Endocrinol Metab. (2018) 103:4113–24. doi: 10.1210/jc.2018-01394
21. Betz MJ, Degenhart C, Fischer E, Pallauf A, Brand V, Linsenmaier U, et al. Adrenal vein sampling using rapid cortisol assays in primary aldosteronism is useful in centers with low success rates. Eur J Endocrinol. (2011) 165:301–6. doi: 10.1530/EJE-11-0287
22. Williams TA, Lenders JWM, Mulatero P, Burrello J, Rottenkolber M, Adolf C, et al. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. (2017) 5:689–99. doi: 10.1016/S2213-8587(17)30135-3
23. Hundemer GL, Kline GA, Leung AA. How common is primary aldosteronism? Curr Opin Nephrol Hypertens. (2021) 30:353–60. doi: 10.1097/MNH.0000000000000702
24. Wu VC, Chang HW, Liu KL, Lin YH, Chueh SC, Lin WC, et al. Primary aldosteronism: diagnostic accuracy of the losartan and captopril tests. Am J Hypertens. (2009) 22:821–7. doi: 10.1038/ajh.2009.89
25. Westerdahl C, Bergenfelz A, Isaksson A, Valdemarsson S. Captopril suppression: limitations for confirmation of primary aldosteronism. J Renin Angiotensin Aldosterone Syst. (2011) 12:326–32. doi: 10.1177/1470320310390405
26. Prete A, Lang K, Pavlov D, Rhayem Y, Sitch AJ, Franke AS, et al. Urine steroid metabolomics as a diagnostic tool in primary aldosteronism. J Steroid Biochem Mol Biol. (2024) 237:106445. doi: 10.1016/j.jsbmb.2023.106445
27. Buffolo F, Burrello J, Burrello A, Heinrich D, Adolf C, Muller LM, et al. Clinical score and machine learning-based model to predict diagnosis of primary aldosteronism in arterial hypertension. Hypertension. (2021) 78:1595–604. doi: 10.1161/HYPERTENSIONAHA.121.17444
28. Mysliwiec J, Zukowski L, Grodzka A, Pilaszewicz A, Dragowski S, Gorska M. Diagnostics of primary aldosteronism: is obligatory use of confirmatory tests justified? J Renin Angiotensin Aldosterone Syst. (2012) 13:367–71. doi: 10.1177/1470320312438791
29. O’Toole SM, Sze WC, Chung TT, Akker SA, Druce MR, Waterhouse M, et al. Low-grade cortisol cosecretion has limited impact on ACTH-stimulated AVS parameters in primary aldosteronism. J Clin Endocrinol Metab. (2020) 105. doi: 10.1210/clinem/dgaa519
30. Heinrich DA, Quinkler M, Adolf C, Handgriff L, Muller L, Schneider H, et al. Influence of cortisol cosecretion on non-ACTH-stimulated adrenal venous sampling in primary aldosteronism: a retrospective cohort study. Eur J Endocrinol. (2022) 187:637–50. doi: 10.1530/EJE-21-0541
31. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. (2018) 6:51–9. doi: 10.1016/S2213-8587(17)30367-4
32. Chang YH, Chung SD, Wu CH, Chueh JS, Chen L, Lin PC, et al. Surgery decreases the long-term incident stroke risk in patients with primary aldosteronism. Surgery. (2020) 167:367–77. doi: 10.1016/j.surg.2019.08.017
33. Ahmed S, Hundemer GL. Benefits of surgical over medical treatment for unilateral primary aldosteronism. Front Endocrinol (Lausanne). (2022) 13:861581. doi: 10.3389/fendo.2022.861581
34. Ray JA, Kushnir MM, Palmer J, Sadjadi S, Rockwood AL, Meikle AW. Enhancement of specificity of aldosterone measurement in human serum and plasma using 2D-LC-MS/MS and comparison with commercial immunoassays. J Chromatogr B Analyt Technol BioMed Life Sci. (2014) 970:102–7. doi: 10.1016/j.jchromb.2014.08.042
Keywords: aldosterone, confirmatory test, saline infusion test, captopril challenge test, PASO criteria
Citation: Daneshpour H, Brüdgam D, Stüfchen I, Heinrich DA, Bidlingmaier M, Beuschlein F, Kürzinger L, Williams TA, Reincke M, Schneider H and Adolf C (2024) Impact of confirmatory test results on subtype classification and biochemical outcome following unilateral adrenalectomy in patients with primary aldosteronism. Front. Endocrinol. 15:1495959. doi: 10.3389/fendo.2024.1495959
Received: 13 September 2024; Accepted: 05 November 2024;
Published: 29 November 2024.
Edited by:
Mirko Parasiliti-Caprino, University of Turin, ItalyReviewed by:
Marilda Mormando, Regina Elena National Cancer Institute (IRCCS), ItalyMartina Bollati, Università degli Studi di Torino, Italy
Copyright © 2024 Daneshpour, Brüdgam, Stüfchen, Heinrich, Bidlingmaier, Beuschlein, Kürzinger, Williams, Reincke, Schneider and Adolf. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christian Adolf, Y2hyaXN0aWFuLmFkb2xmQG1lZC51bmktbXVlbmNoZW4uZGU=
†ORCID: Christian Adolf, orcid.org/0000-0002-9206-0953
 Hediyeh Daneshpour
Hediyeh Daneshpour Denise Brüdgam1
Denise Brüdgam1 Martin Bidlingmaier
Martin Bidlingmaier Felix Beuschlein
Felix Beuschlein Lydia Kürzinger
Lydia Kürzinger Holger Schneider
Holger Schneider Christian Adolf
Christian Adolf