- Department of Reproductive Medicine, Women’s Hospital of Nanjing Medical University, Nanjing Women and Children’s Healthcare Hospital, Nanjing, China
Objectives: The increasing prevalence of obesity underscores the need to explore its impact on assisted reproductive technology (ART) outcomes. This study aims to evaluate the association between visceral fat area (VFA), measured by bioelectrical impedance analysis (BIA), and pregnancy outcomes following frozen embryo transfer (FET).
Methods: In this retrospective clinical study, the data of 1,510 patients who underwent FET between April 2022 and April 2023 were analyzed. The VFA was measured by BIA, and patients were categorized into low and high VFA groups based on a threshold of 65 cm². Pregnancy outcomes were compared between the two groups. Univariable and multivariate logistic regression analyses, along with restricted cubic spline (RCS) modeling, were used to adjust for age, body mass index (BMI), and basal estradiol (E2) levels to determine the relationship between VFA and FET outcomes.
Results: There were significant differences in baseline characteristics and outcomes between the two groups. The high VFA group was characterized by older age and a lower basal estradiol (E2) level. The biochemical pregnancy rate, implantation rate, clinical pregnancy rate (CPR), and live birth rate (LBR) were significantly lower in the high VFA group. Logistic regression revealed a significant negative correlation between the high VFA group and both CPR and LBR. The RCS model demonstrated that the VFA was nonlinearly correlated with CPR and LBR. Subgroup analysis showed that among individuals under 35 years of age or with a BMI < 24, high VFA was significantly associated with poorer CPR and LBR.
Conclusions: High VFA is associated with poorer pregnancy outcomes after FET in female patients with infertility, with both CPR and LBR decreasing as VFA increases. Clinicians should consider VFA as an important reference for targeted fat management interventions to optimize reproductive success, especially when VFA exceeds 65 cm².
1 Introduction
The increasing prevalence of obesity is a significant public health challenge that extends beyond metabolic disorders to encompass reproductive health (1, 2). Evidence suggests that obesity adversely affects both male and female reproductive health, leading to complications ranging from hormonal imbalances to reduced efficacy of fertility treatments (3). In patients undergoing assisted reproductive technology (ART), obesity is associated with diminished ovarian response to stimulation, which negatively impacts oocyte quality and endometrial function. This, in turn, increases miscarriage rates and reduces both the clinical pregnancy rate (CPR) (odds ratio [OR] 0.50, 95% confidence interval [CI] 0.31–0.82) and the live birth rate (LBR) (OR 0.51, 95% CI 0.29–0.87) (4). Therefore, identifying an indicator that accurately reflects the impact of obesity on ART outcomes and using this indicator as a basis to provide targeted adiposity interventions is critical to improving the success of ART.
While body mass index (BMI) has traditionally been used as the standard measure of obesity, it fails to capture the complexities of body composition, particularly the impact of fat distribution on fertility and pregnancy outcomes (5–7). This limitation can obscure how fat, particularly visceral adiposity, influences the endocrine environment and reproductive function. In light of these shortcomings, the present study shifts focus from BMI to a more precise measure of adiposity that directly affects reproductive outcomes: visceral fat area (VFA). Visceral fat, which is stored within the abdominal cavity and around internal organs, has been associated with insulin resistance, inflammation, and altered sex hormone metabolism—factors known to negatively impact fertility and pregnancy outcomes (8–10). Among the methods available for measuring visceral fat, such as computed tomography, magnetic resonance imaging, ultrasound, and bioelectrical impedance analysis (BIA) (11), BIA was selected for the present study owing to its non-invasive nature, cost-effectiveness, convenience, and non-exposure to radiation, offering a practical approach for large-scale studies and clinical applications (12–14).
By using BIA to measure VFA, this study aims to evaluate the relationship between visceral fat and female reproductive outcomes. Isolating the effects of visceral fat will enable a more accurate assessment of its role in fertility and ART success, specifically frozen embryo transfer (FET). This nuanced understanding is essential for developing targeted interventions that could improve reproductive outcomes in women with elevated adiposity. By using VFA as a more precise measure of relevant fat content, we aim to elucidate the pathways through which adiposity affects fertility and pregnancy success, beyond the generalized and somewhat crude metric of BMI.
2 Materials and methods
2.1 Study population
This retrospective cohort study included female patients who underwent FET at the Reproductive Center of Women’s Hospital of Nanjing Medical University between April 2022 and April 2023. The exclusion criteria were as follows: severe chronic diseases (e.g., heart disease, diabetes mellitus, kidney disease), chromosomal abnormalities or genetic diseases in either partner, uterine malformations, endometrial thickness < 6 mm on the embryo transfer (ET) day, and incomplete cycle data.
2.2 Study groups and propensity score matching
Patients who met the inclusion criteria were stratified using a median VFA of 65 cm² as the cutoff point, dividing the patients into two groups: those with a VFA less than 65 cm² were defined as the low VFA group, and those with a VFA of 65 cm² or greater were categorized into the high VFA group. Propensity score matching (PSM) was employed to reduce potential bias between the groups. Patients were matched in a 1:1 ratio based on age, endometrial preparation protocols, and the type of embryos transferred (Supplementary Table S1).
2.3 Ethics approval and consent to participate
This retrospective cohort study was conducted according to the Declaration of Helsinki and was approved by the Ethics Committee of Women’s Hospital of Nanjing Medical University (approval number 2022KY-046). Given the retrospective and anonymized nature of the data analysis, the ethics committee waived the requirement for written informed consent.
2.4 Treatment protocol
2.4.1 Body composition data collection before FET
On the initial day of progesterone (P) exposure, each patient’s body composition was measured by trained professionals using InBody 720, which utilizes BIA to rapidly evaluate body composition without the use of radiation (15). The patients were required to empty their bladder; remove their coats, shoes, socks, accessories, and any metallic items; and stand bare foot on the device, holding onto the measurement handles with the arms extended so that the arms and legs were in direct contact with the electrodes. Data were collected via a computer connected to the device.
2.4.2 FET protocol and luteal phase support
In this study, the endometrial preparation protocols for patients undergoing FET included natural cycle (NC), ovulation induction (OI), and hormone replacement therapy (HRT). The choice of protocol was made by clinicians based on each patient’s medical history and professional judgment.
For evaluation of Day 3 (D3) cleavage-stage embryos, we applied the scoring system established by Scott et al. (16), defining embryos with a score of ≥3 as high-quality. For Day 5 (D5) or Day 6 (D6) blastocysts, grading was performed according to the criteria proposed by Gardner et al. (17), with blastocysts graded ≥3BB considered high-quality. All embryos underwent vitrification followed by thawing, and no embryos were subjected to preimplantation genetic testing (PGT).
Embryo transfer was performed under transabdominal ultrasound guidance. D3 embryos were transferred on Day 3 after progesterone administration (P+3), while D5 or D6 blastocysts were transferred on Day 5 after progesterone administration (P+5). The luteal phase was supported until the 10th week of pregnancy.
2.4.3 Pregnancy outcome
Serum human chorionic gonadotropin (hCG) concentration was measured 14 days after FET to diagnose biochemical pregnancy. An ultrasound examination was conducted 28 days after FET to confirm clinical pregnancy based on the identification of a gestational sac. Miscarriage was defined as the natural termination of a clinical pregnancy before 28 weeks of gestation, with early miscarriage specifically referring to fetal loss before the 12th week of pregnancy. Live birth was defined as the delivery of a living infant at or beyond 28 weeks of gestation. The implantation rate was calculated as the ratio of gestational sacs to the number of embryos transferred. The primary outcome of interest was the CPR, while the secondary outcomes included the implantation rate, biochemical pregnancy rate, miscarriage rate and LBR.
2.5 Statistical analysis
All continuous variables are presented as the mean ± standard deviation, unless otherwise indicated. The comparison of descriptive statistics between the two groups was conducted using the independent-samples t-test. Categorical data were analyzed using Pearson’s chi-square (χ2) test or Fisher’s exact test, and the data are described as frequency (percentage). The Mann–Whitney U test was used to compare non-parametric variables. The univariable and multivariate logistic regression analysis was used to assess the correlation between the VFA and FET outcomes, with VFA categorized into high and low VFA groups based on a threshold of 65 cm². Restricted cubic splines (RCS) were applied to characterize the relationship between the VFA as a continuous variable and FET outcomes. Both the logistic regression analysis and RCS were adjusted for confounding variables, including age, BMI and basal E2 to minimize residual confounding. The statistical analyses were performed using SPSS software (v26.0, IBM Corp., Armonk, NY, US) and R statistical software (v4.2.0, R Foundation, Vienna, Austria). Statistical significance was defined as a two-sided P-value of <0.05.
3 Results
3.1 Baseline characteristics of study participants
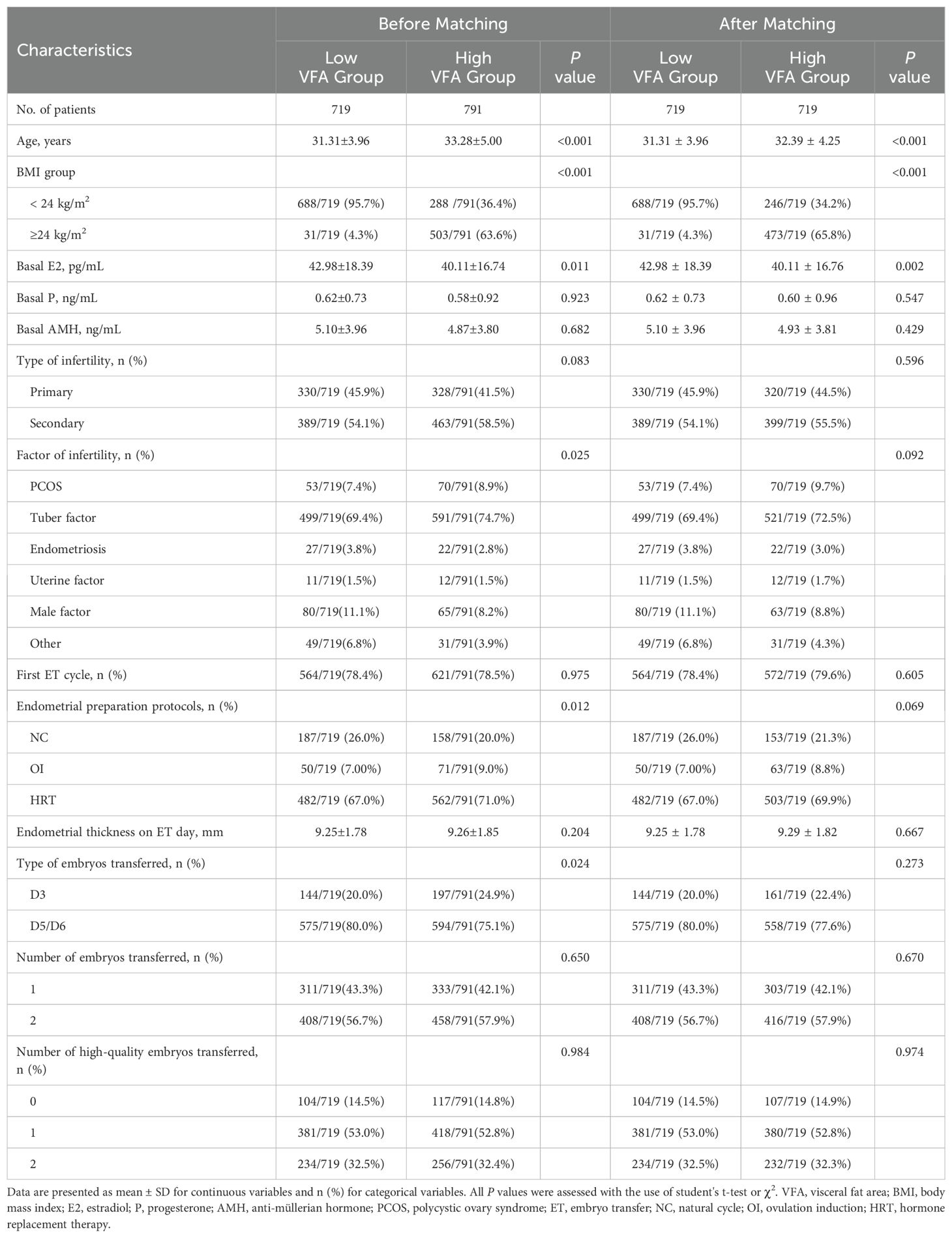
The study included 1,510 patients who underwent in vitro fertilization and embryo transfer cycles. The patients were categorized into two groups based on their VFA based on a cutoff threshold of 65 cm2: the low VFA group (VFA < 65 cm2, n = 719) and the high VFA group (VFA ≥ 65 cm2, n = 791). After PSM, 719 paired patients were included for analysis (Figure 1). The baseline characteristics are presented in Table 1. In this study, the low VFA group included 31 patients (4.3%) with a BMI that reached or exceeded 24 kg/m²; conversely, the high VFA group comprised 246 patients (34.2%) with a BMI that was below 24 kg/m². This suggests that even when the BMI is within the normal range or is low, individuals may still have a high accumulation of visceral fat, which could have adverse effects on reproductive health. Compared with the low VFA group, the high VFA group was significantly older (32.39 ± 4.25 vs. 31.31 ± 3.96 years, P < 0.001), had a significantly lower basal E2 levels (40.11 ± 16.76 vs. 42.98 ± 18.39 pg/mL, P = 0.002). The basal P, basal AMH level, type of infertility, factor of infertility, whether it was the first ET cycle, endometrial preparation protocols, endometrial thickness on ET day, type of embryos transferred, number of embryos transferred, number of high-quality embryos transferred were not significantly different between the two groups (all P > 0.05).
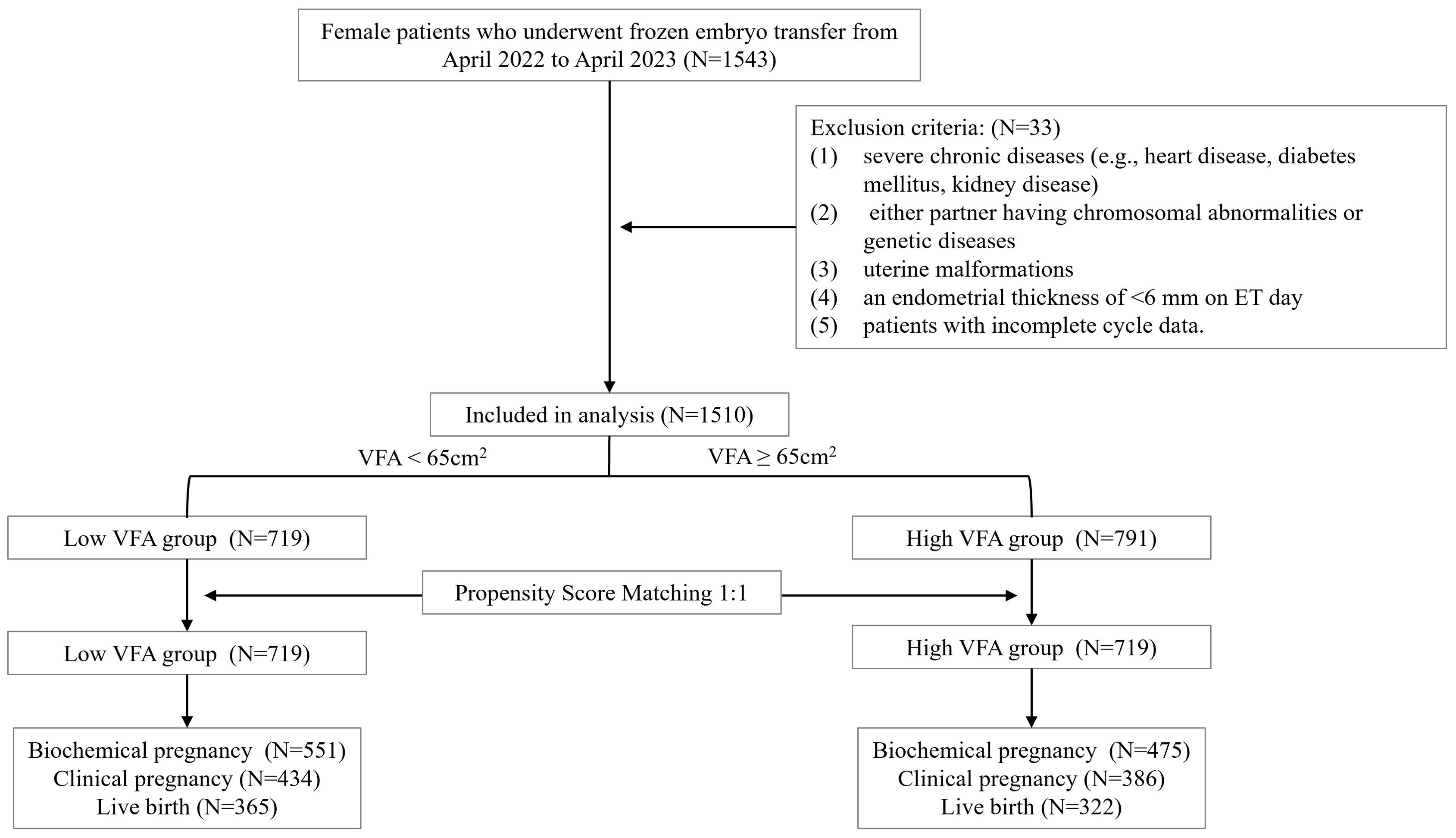
Figure 1. Flow chart of the participants included in this study. VFA, visceral fat area; ET, embryo transfer.
3.2 Clinical outcomes between two groups
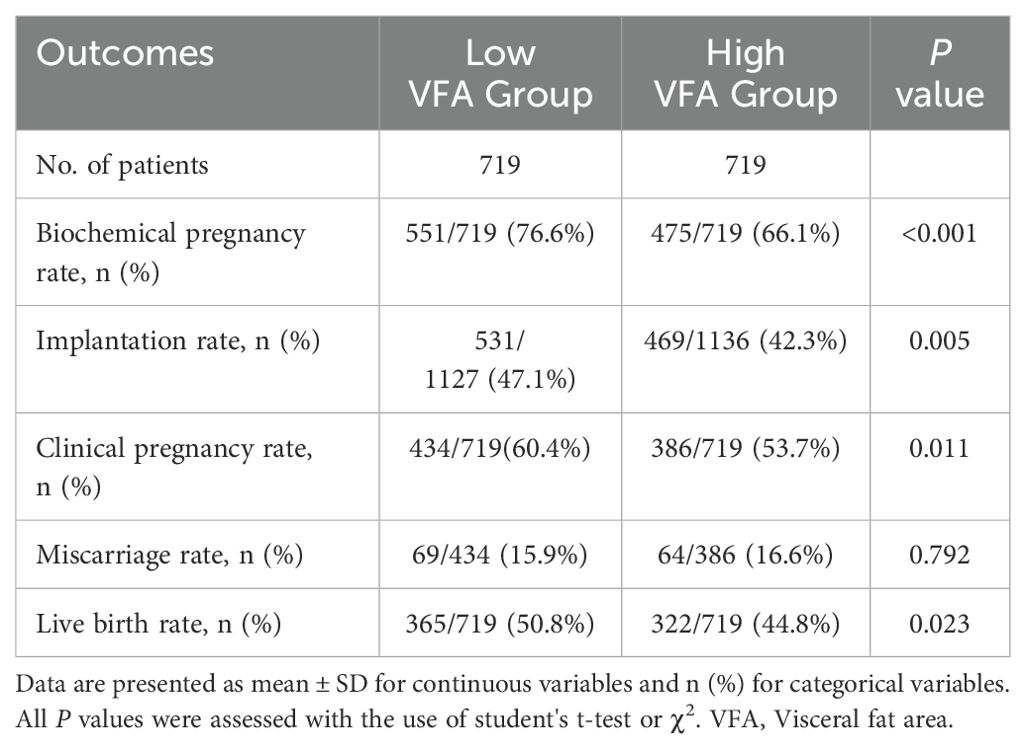
We compared the clinical outcomes between the two groups (Table 2). Compared with the low VFA group, the high VFA group demonstrated a significantly lower biochemical pregnancy rate (66.1% vs. 76.6%, P < 0.001), implantation rate (42.3% vs. 47.1%, P = 0.005), CPR (53.7% vs. 60.4%, P = 0.011), and LBR (44.8% vs. 50.8%, P = 0.023). There was no significant difference in the miscarriage rate was observed between the two groups of patients (16.6% vs. 15.9%, P = 0.792).
3.3 Relationship between VFA and clinical outcomes
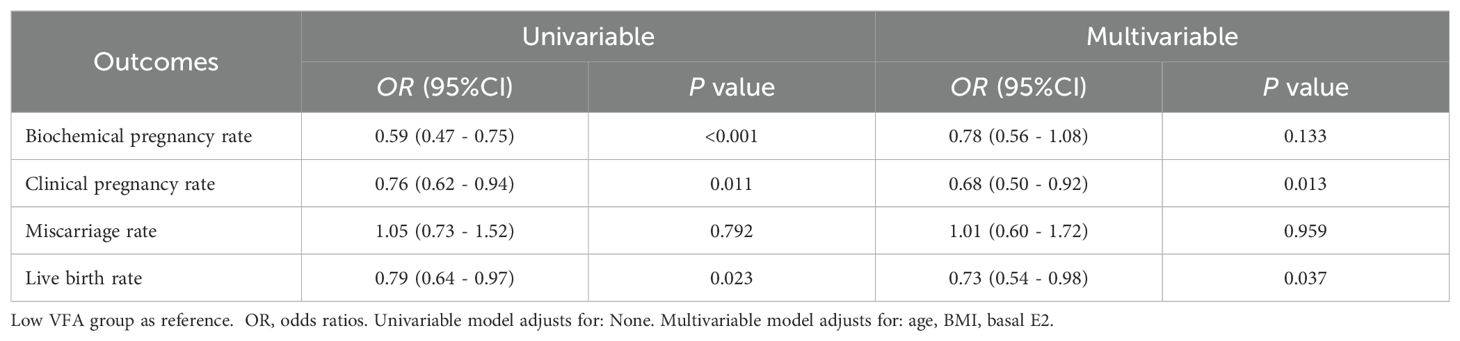
Table 3 displays the univariate and multiple logistic regression analysis that was utilized to explore the relationship between the VFA and clinical outcomes. In the univariable model, compared to the low VFA group, the biochemical pregnancy rate (OR 0.59, 95% CI 0.47 - 0.75, P < 0.001), CPR (OR 0.76, 95% CI 0.62 - 0.94, P = 0.011), and LBR (OR 0.79, 95% CI 0.64 - 0.97, P = 0.023) were significantly reduced in the high VFA group, while the miscarriage rate did not show a significant difference. In the multivariate model, after adjusting for age, BMI, and basal E2 levels, the CPR (OR 0.68, 95% CI 0.50 - 0.92, P = 0.013) and LBR (OR 0.73, 95% CI 0.54 - 0.98, P = 0.037) were significantly reduced in the high VFA group compared to the low VFA group, while the biochemical pregnancy rate (OR 0.78, 95% CI 0.56 - 1.08, P = 0.133) and miscarriage rate (OR 1.01, 95% CI 0.60 - 1.72, P = 0.959) showed no significant differences.
Figure 2 visualizes the association between the continuous variable of VFA and clinical outcomes after controlling for potential confounders such as age, BMI, and basal E2 levels using RCS models. We found that as VFA increased, the CPR (P-nonlinear = 0.072, P = 0.029) and LBR (P-nonlinear = 0.088, P = 0.040) significantly decreased, while the biochemical pregnancy rate (P-nonlinear = 0.364, P = 0.413) and miscarriage rate (P-nonlinear = 0.904, P = 0.884) were not significantly associated with VFA.
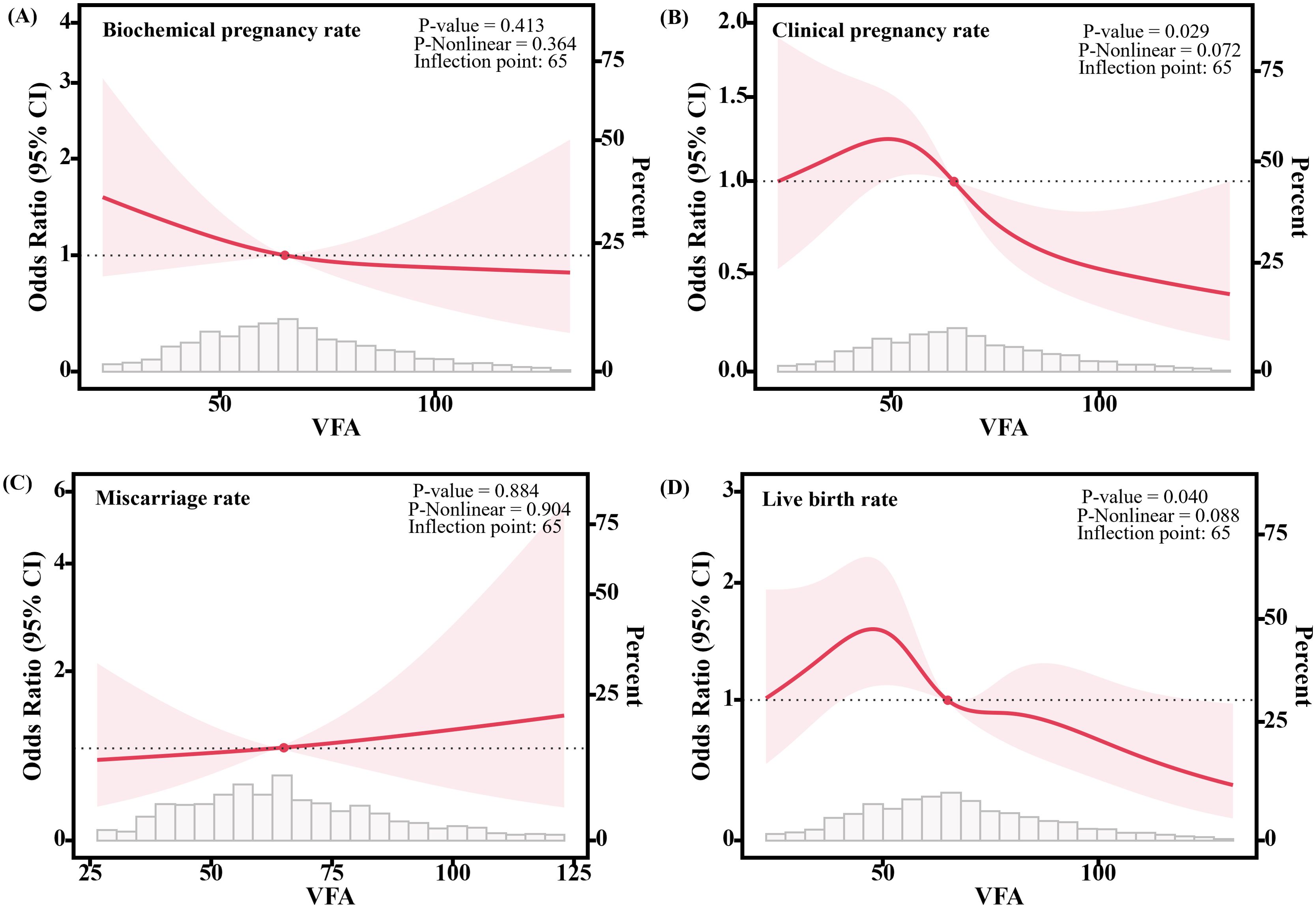
Figure 2. Association between VFA and clinical outcomes of frozen embryo transfer patients. (A) Relationship with the biochemical pregnancy rate. (B) Relationship with the clinical pregnancy rate. (C) Relationship with the miscarriage rate. (D) Relationship with the live birth rate. Solid lines show the estimation of the difference in clinical outcomes when using VFA=65 as the odds ratios. 95% confidence intervals (CIs) are indicated by shaded areas. The models were adjusted for age, BMI, basal E2. VFA, Visceral fat area.
3.4 Subgroup analysis
As shown in Table 4, we performed subgroup analysis to stratify the association between high VFA group and clinical outcomes. In patients younger than 35 years, after adjusting for confounding factors, a high VFA was associated with a significant reduction in the CPR (OR 0.65, 95% CI 0.46 - 0.93, P = 0.018) and LBR (OR 0.70, 95% CI 0.49 - 0.98, P = 0.038); however, in patients aged 35 or older, regardless of whether confounding factors were adjusted for, there was no significant association between high VFA and CPR or LBR (all P > 0.05). Among patients with a BMI less than 24 kg/m², a high VFA significantly reduced CPR (univariable model: OR 0.72, 95% CI 0.54 - 0.96, P = 0.027; multivariable model: OR 0.66, 95% CI 0.46 - 0.94, P = 0.020) and LBR (univariable model: OR 0.73, 95% CI 0.54 - 0.98, P = 0.034; multivariable model: OR 0.65, 95% CI 0.45 - 0.92, P = 0.015), regardless of confounding factors; in contrast, in patients with a BMI of 24 kg/m² or greater, there was no significant correlation between high VFA and CPR or LBR (P > 0.05).
4 Discussion
This study, which utilized BIA to measure VFA, provides an in-depth retrospective analysis of data from patients who underwent FET, with the goal of elucidating the complex relationship between visceral fat and pregnancy outcomes. The results revealed a significant correlation between VFA ≥ 65 cm² and diminished ART success, as evidenced by lower CPR and LBR.
Previous research on the impact of excess fat on the pregnancy outcomes of ART has yielded inconsistent results. While some studies have reported no significant correlation between obesity and ART outcomes (18–20), a larger body of clinical research suggests a more nuanced relationship (5, 21–23) which is consistent with our findings. In this study, 4.3% of patients were classified as overweight or obese based on BMI criteria but had a lower VFA, whereas 34.2% were considered normal weight or underweight based on BMI but had a higher VFA. These findings align with Jia et al.’s perspective that BMI alone may no longer be sufficient to accurately assess obesity, and that VFA offers a more precise indication (24, 25). Specifically, in the context of reproductive medicine, measuring VFA provides a more accurate evaluation of a patient’s fertility potential and can help guide more effective and individualized treatment strategies.
In this study, a VFA of ≥65 cm² was associated with poorer pregnancy outcomes following FET. Using this VFA threshold, we observed that patients in the high VFA group were older and had lower basal E2 levels. Previous studies have suggested that advanced age and lower basal E2 levels are linked to adverse reproductive outcomes (26, 27). However, even after adjusting for various confounding factors that could influence pregnancy outcomes, our results still demonstrated a significant negative correlation between VFA and both CPR and LBR, highlighting the potential adverse effects of visceral fat on FET success rates. Excessive fat accumulation may impair endometrial receptivity, with growing evidence indicating that obesity in females can disrupt the conditions necessary for successful implantation. A 2013 study using oocyte donation cycles from healthy-weight donors to isolate the impact of obesity on oocyte and embryo quality found that recipients who were overweight or obese exhibited lower implantation rates, as well as lower CPR and LBR, compared to those with a healthy weight (28). In China, where legislation and regulation regarding oocyte donation remain stringent, many studies have controlled for embryo quality through high-quality autologous embryo transfers, adjusting for potential confounders, and still found that obesity was associated with lower implantation rates, CPR, and LBR (21, 29). These clinical findings are consistent with our conclusions and further refine the understanding of how visceral fat accumulation affects ART pregnancy outcomes. High levels of visceral fat may interfere with healthy embryo implantation and endometrial preparation through several mechanisms, including pro-inflammatory responses (30), hormone regulation (31), and cellular signal disruption (32). A transcriptomic study demonstrated that women with obesity—83.3% of whom had central obesity—showed distinct endometrial gene expression profiles during the embryo implantation window compared to controls (33). This analysis underscores the critical role of visceral fat in reproductive health, suggesting that its impact extends beyond general obesity as indicated by BMI.
The direct association between visceral adiposity and impaired reproductive outcomes highlights the need for targeted interventions to manage and reduce visceral fat in order to improve ART success rates. In our stratified analysis, we found that an increase in VFA levels was significantly correlated with poorer CPR and LBR in women under the age of 35, while no significant correlation was observed in women aged 35 or older. This suggests that the adverse effects of visceral fat accumulation on reproductive health are more pronounced in younger women, whereas in older women, VFA may not be the primary factor influencing reproductive outcomes. Several studies have reported similar findings (5, 6). Among women with a normal or underweight BMI, 43.2% had a high VFA, which was significantly associated with reduced CPR and LBR. This suggests that the specificity of fat distribution, particularly the accumulation of visceral fat, should be an important consideration when assessing fertility in this population. In contrast, no significant correlation was found between VFA and CPR or LBR in the overweight or obese group, likely due to the generally high VFA levels in this cohort—only 4.3% of patients in this group had low VFA, rendering the impact of VFA as a single variable relatively weak in the overall analysis. Therefore, for younger women, even those with a normal or underweight BMI, managing body fat distribution, particularly reducing visceral fat accumulation, should be considered an important strategy for maintaining reproductive health and improving fertility potential. For older women, however, a comprehensive approach that considers multiple factors is necessary to avoid delaying treatment.
In summary, our study utilized BIA to accurately measure VFA, offering a novel approach to examining the effects of visceral fat on ART outcomes. This method provides a more detailed understanding of how specific body composition factors, beyond BMI, influence FET success. Moreover, the adjustment for various confounding factors enhances the robustness and credibility of the findings, facilitating a reliable exploration of the relationship between visceral fat and pregnancy outcomes. However, as this was a single-center retrospective study with a relatively small sample size, the generalizability of the findings to diverse populations with different ethnic backgrounds or health profiles may be limited. Therefore, future prospective studies with larger sample sizes are needed to further investigate the relationship between visceral fat and FET pregnancy outcomes.
5 Conclusions
This study identified a significant association between VFA and pregnancy outcomes following FET through an in-depth retrospective analysis. After adjusting for age, BMI, and basal E2 levels, a high VFA was significantly correlated with lower CPR and LBR, with a VFA threshold of 65 cm². This VFA threshold provides a novel benchmark for clinical guidance on adiposity management in ART, potentially enabling more effective and personalized interventions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This retrospective cohort study was conducted according to the Declaration of Helsinki and was approved by the Ethics Committee of Women’s Hospital of Nanjing Medical University (approval number 2022KY-046). Given the retrospective and anonymized nature of the data analysis, the ethics committee waived the requirement for written informed consent.
Author contributions
DN: Formal analysis, Writing – original draft, Writing – review & editing. YW: Methodology, Software, Writing – review & editing. QX: Formal analysis, Writing – review & editing. XW: Investigation, Writing – review & editing. KY: Investigation, Writing – review & editing. WJ: Data curation, Writing – review & editing. YY: Funding acquisition, Writing – review & editing. XL: Funding acquisition, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by the National Key Research and Development Program of China (2022YFC2702201, 2021YFC2700601-1), the National Natural Science Foundation of China (81871210, 82171645, 82371670), and Jiangsu Province Capability Improvement Project through Science, Technology and Education Jiangsu Provincial Medical Key Discipline (ZDXK202211).
Acknowledgments
The Center for Reproductive Medicine, Women’s Hospital of Nanjing Medical University ‘s physicians and scientific team are acknowledged by the authors.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1474201/full#supplementary-material
References
1. Mu L, Liu J, Zhou G, Wu C, Chen B, Lu Y, et al. Obesity prevalence and risks among Chinese adults: findings from the China PEACE million persons project, 2014-2018. Circ Cardiovasc Qual Outcomes. (2021) 14:e007292. doi: 10.1161/circoutcomes.120.007292
2. Broughton DE, Moley KH. Obesity and female infertility: potential mediators of obesity’s impact. Fertil Steril. (2017) 107:840–7. doi: 10.1016/j.fertnstert.2017.01.017
3. Mintziori G, Nigdelis MP, Mathew H, Mousiolis A, Goulis DG, Mantzoros CS. The effect of excess body fat on female and male reproduction. Metabolism. (2020) 107:154193. doi: 10.1016/j.metabol.2020.154193
4. Shah DK, Missmer SA, Berry KF, Racowsky C, Ginsburg ES. Effect of obesity on oocyte and embryo quality in women undergoing in vitro fertilization. Obstet Gynecol. (2011) 118:63–70. doi: 10.1097/AOG.0b013e31821fd360
5. Zheng D, Wang Y, Chen L, Zeng L, Li R. Association between body mass index and in vitro fertilization/intra-cytoplasmic sperm injection outcomes: An analysis of 15,124 normal ovarian responders in China. Chin Med J (Engl). (2024) 137:837–45. doi: 10.1097/cm9.0000000000002992
6. Rafael F, Rodrigues MD, Bellver J, Canelas-Pais M, Garrido N, Garcia-Velasco JA, et al. The combined effect of BMI and age on ART outcomes. Hum Reprod. (2023) 38:886–94. doi: 10.1093/humrep/dead042
7. Borges MC, Clayton GL, Freathy RM, Felix JF, Fernández-Sanlés A, Soares AG, et al. Integrating multiple lines of evidence to assess the effects of maternal BMI on pregnancy and perinatal outcomes. BMC Med. (2024) 22:32. doi: 10.1186/s12916-023-03167-0
8. Tchernof A, Després JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. (2013) 93:359–404. doi: 10.1152/physrev.00033.2011
9. Bensussen A, Torres-Magallanes JA, Roces De Álvarez-Buylla E. Molecular tracking of insulin resistance and inflammation development on visceral adipose tissue. Front Immunol. (2023) 14:1014778. doi: 10.3389/fimmu.2023.1014778
10. Brenes-Martín F, Melero-Jiménez V, López-Guerrero M, Calero-Ruiz MM, Vázquez-Fonseca L, Ábalos-Martínez J, et al. First trimester evaluation of maternal visceral fat and its relationship with adverse pregnancy outcomes. Biol (Basel). (2023) 12:144. doi: 10.3390/biology12020144
11. Naboush A, Hamdy O. Measuring visceral and hepatic fat in clinical practice and clinical research. Endocr Pract. (2013) 19:587–9. doi: 10.4158/ep12331.Or
12. Browning LM, Mugridge O, Chatfield MD, Dixon AK, Aitken SW, Joubert I, et al. Validity of a new abdominal bioelectrical impedance device to measure abdominal and visceral fat: comparison with MRI. Obes (Silver Spring). (2010) 18:2385–91. doi: 10.1038/oby.2010.71
13. Unno M, Furusyo N, Mukae H, Koga T, Eiraku K, Hayashi J. The utility of visceral fat level by bioelectrical impedance analysis in the screening of metabolic syndrome - the results of the Kyushu and Okinawa Population Study (KOPS). J Atheroscler Thromb. (2012) 19:462–70. doi: 10.5551/jat.11528
14. Sukkriang N, Chanprasertpinyo W, Wattanapisit A, Punsawad C, Thamrongrat N, Sangpoom S. Correlation of body visceral fat rating with serum lipid profile and fasting blood sugar in obese adults using a noninvasive machine. Heliyon. (2021) 7:e06264. doi: 10.1016/j.heliyon.2021.e06264
15. Zając-Gawlak I, Kłapcińska B, Kroemeke A, Pośpiech D, Pelclová J, Přidalová M. Associations of visceral fat area and physical activity levels with the risk of metabolic syndrome in postmenopausal women. Biogerontology. (2017) 18:357–66. doi: 10.1007/s10522-017-9693-9
16. Scott L, Finn A, O’leary T, Mclellan S, Hill J. Morphologic parameters of early cleavage-stage embryos that correlate with fetal development and delivery: prospective and applied data for increased pregnancy rates. Hum Reprod. (2007) 22:230–40. doi: 10.1093/humrep/del358
17. Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. (2000) 73:1155–8. doi: 10.1016/s0015-0282(00)00518-5
18. Dechaud H, Anahory T, Reyftmann L, Loup V, Hamamah S, Hedon B. Obesity does not adversely affect results in patients who are undergoing in vitro fertilization and embryo transfer. Eur J Obstet Gynecol Reprod Biol. (2006) 127:88–93. doi: 10.1016/j.ejogrb.2005.12.009
19. Matalliotakis I, Cakmak H, Sakkas D, Mahutte N, Koumantakis G, Arici A. Impact of body mass index on IVF and ICSI outcome: a retrospective study. Reprod BioMed Online. (2008) 16:778–83. doi: 10.1016/s1472-6483(10)60142-3
20. Prost E, Reignier A, Leperlier F, Caillet P, Barrière P, Fréour T, et al. Female obesity does not impact live birth rate after frozen-thawed blastocyst transfer. Hum Reprod. (2020) 35:859–65. doi: 10.1093/humrep/deaa010
21. Zhang J, Liu H, Mao X, Chen Q, Fan Y, Xiao Y, et al. Effect of body mass index on pregnancy outcomes in a freeze-all policy: an analysis of 22,043 first autologous frozen-thawed embryo transfer cycles in China. BMC Med. (2019) 17:114. doi: 10.1186/s12916-019-1354-1
22. Sermondade N, Huberlant S, Bourhis-Lefebvre V, Arbo E, Gallot V, Colombani M, et al. Female obesity is negatively associated with live birth rate following IVF: a systematic review and meta-analysis. Hum Reprod Update. (2019) 25:439–51. doi: 10.1093/humupd/dmz011
23. Bakkensen JB, Strom D, Boots CE. Frozen embryo transfer outcomes decline with increasing female body mass index in female but not male factor infertility: analysis of 56,564 euploid blastocyst transfers. Fertil Steril. (2024) 121:271–80. doi: 10.1016/j.fertnstert.2023.07.027
24. Zheng J, Hu Y, Xu H, Lei Y, Zhang J, Zheng Q, et al. Normal-weight visceral obesity promotes a higher 10-year atherosclerotic cardiovascular disease risk in patients with type 2 diabetes mellitus-a multicenter study in China. Cardiovasc Diabetol. (2023) 22:137. doi: 10.1186/s12933-023-01876-7
25. Huang H, Zheng X, Wen X, Zhong J, Zhou Y, Xu L. Visceral fat correlates with insulin secretion and sensitivity independent of BMI and subcutaneous fat in Chinese with type 2 diabetes. Front Endocrinol (Lausanne). (2023) 14:1144834. doi: 10.3389/fendo.2023.1144834
26. Vitagliano A, Paffoni A, Viganò P. Does maternal age affect assisted reproduction technology success rates after euploid embryo transfer? A systematic review and meta-analysis. Fertil Steril. (2023) 120:251–65. doi: 10.1016/j.fertnstert.2023.02.036
27. He Y, Lu Y, Zhu Q, Wang Y, Lindheim SR, Qi J, et al. Influence of metabolic syndrome on female fertility and in vitro fertilization outcomes in PCOS women. Am J Obstet Gynecol. (2019) 221:138.e131–138.e112. doi: 10.1016/j.ajog.2019.03.011
28. Bellver J, Pellicer A, García-Velasco JA, Ballesteros A, Remohí J, Meseguer M. Obesity reduces uterine receptivity: clinical experience from 9,587 first cycles of ovum donation with normal weight donors. Fertil Steril. (2013) 100:1050–8. doi: 10.1016/j.fertnstert.2013.06.001
29. Bellver J, Ayllón Y, Ferrando M, Melo M, Goyri E, Pellicer A, et al. Female obesity impairs in vitro fertilization outcome without affecting embryo quality. Fertil Steril. (2010) 93:447–54. doi: 10.1016/j.fertnstert.2008.12.032
30. Kolb H. Obese visceral fat tissue inflammation: from protective to detrimental? BMC Med. (2022) 20:494. doi: 10.1186/s12916-022-02672-y
31. Frank AP, De Souza Santos R, Palmer BF, Clegg DJ. Determinants of body fat distribution in humans may provide insight about obesity-related health risks. J Lipid Res. (2019) 60:1710–9. doi: 10.1194/jlr.R086975
32. Serfaty L, Capeau J. Hepatitis C, insulin resistance and diabetes: clinical and pathogenic data. Liver Int. (2009) 29 Suppl 2:13–25. doi: 10.1111/j.1478-3231.2008.01952.x
33. Bellver J, Martínez-Conejero JA, Labarta E, Alamá P, Melo MA, Remohí J, et al. Endometrial gene expression in the window of implantation is altered in obese women especially in association with polycystic ovary syndrome. Fertil Steril. (2011) 95:2335–2341, 2341.e2331-2338. doi: 10.1016/j.fertnstert.2011.03.021
Keywords: visceral fat area (VFA), bioelectrical impedance analysis (BIA), frozen embryo transfer (FET), clinical pregnancy rate (CPR), live birth rate (LBR)
Citation: Ni D, Wei Y, Xie Q, Wang X, Yu K, Jiang W, Yang Y and Ling X (2025) Evaluating the impact of visceral fat on the outcomes of frozen embryo transfer via bioelectrical impedance analysis. Front. Endocrinol. 15:1474201. doi: 10.3389/fendo.2024.1474201
Received: 01 August 2024; Accepted: 18 December 2024;
Published: 10 January 2025.
Edited by:
Richard Ivell, University of Nottingham, United KingdomReviewed by:
Xiaowei Nie, Jiangsu Provincial Hospital of Traditional Chinese Medicine, ChinaLing Zhang, Hangzhou Medical College, China
Copyright © 2025 Ni, Wei, Xie, Wang, Yu, Jiang, Yang and Ling. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiufeng Ling, bGluZ3hpdWZlbmdfbmpmeUAxNjMuY29t; Ye Yang, eWV5YW5nODlAaG90bWFpbC5jb20=
 Danyu Ni
Danyu Ni Qijun Xie
Qijun Xie Xiufeng Ling
Xiufeng Ling