- 1Division of Medicine, KK Women's and Children's Hospital, Singapore, Singapore
- 2Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, Singapore
- 3Pediatric Academic Clinical Programme, Duke-NUS Medical School, Singapore, Singapore
- 4Pediatric Academic Clinical Programme, Yong Loo Lin School of Medicine, Singapore, Singapore
Diazoxide (DZX) remains the first-line medication for the treatment of prolonged and persistent forms of hyperinsulinemic hypoglycemia (HH). In nearly 40%–50% of cases of HH, the genetic mechanism is unidentified. Almost half of the infants with permanent or genetic causes are DZX sensitive, but hypersensitivity to DZX is extremely rare, and the mechanism is poorly understood. Here, we report for the first time a case of DZX hypersensitivity in a neonate with HH who inherited a novel HNF1A variant from the mother. A term, male large-for-gestational-age infant of a diabetic mother presented with early onset of severe, recurrent hypoglycemia. Critical blood samples when hypoglycemic confirmed HH. Diazoxide was initiated at conventional doses of 5 mg/kg/day, which resulted in hyperglycemia (blood glucose, 16.6 mmol/L) within 48 h. Glucose infusion was rapidly weaned off. DZX was withheld and eventually stopped. Following 3 days of milk feeds alone with a normal glucose profile, suspecting a resolution of HH, he underwent a 6-h fasting study and passed. While on glucose monitoring in the hospital, he again developed hypoglycemic episodes, and the critical blood samples confirmed HH. DZX was restarted at a lower dose of 3 mg/kg/day, which required further down-titration to 0.7 mg/kg/day before steady euglycemia was obtained. No more episodes of hypo- or hyperglycemia occurred, and he passed a safety fasting study before discharge. Molecular genetic testing identified a novel HNF1A mutation in the mother–child dyad, whereas the father tested negative. We concluded that the HH phenotype due to this novel HNF1A mutation can be mutation specific and require a very low dose of DZX. Clinicians should observe closely for the risk of diabetic ketoacidosis and hyperglycemic hyperosmolar state while initiating DZX therapy.
Introduction
Transient or prolonged hypoglycemia may occur in high-risk newborn infants due to impaired metabolic transition from fetal to neonatal life. Navigating the glucose nadir successfully in the first 2–3 h of life fundamentally depends on the availability of substrate and intact neuroendocrine systems to generate glucose (1). Additionally, adequate hepatic glycogen stores with unimpaired enzyme systems for glycogenolysis, gluconeogenesis, and appropriate β-cell and counter-regulatory hormone response are essential for maintaining euglycemia (2). Infants of diabetic mothers (IDMs), infants of obese mothers (IOMs), small-for-gestational-age (SGA), large-for-gestational-age (LGA), and preterm infants are prone to hypoglycemia unless they can have access to early milk feeds and, where necessary, buccal glucose gel with supplementary feeds to correct the borderline low glucose levels (3). Some infants may also require short-term parenteral glucose. Inappropriate insulin response leads to hyperinsulinemic hypoglycemia (HH), characterized by detectable insulin levels with hypoglycemia, hypoketonemia, and hypofattyacidemia. HH more often develops in IDM, IOM, SGA, LGA, and infants who had perinatal asphyxia, and the resultant neuroglycopenia enhances cerebral injury risk (4, 5).
Molecular defects in ABCC8, KCNJ11, GLUD1, GCK, HADH1, SLC16A1, UCP2, HNF4A, HNF1A, HK1, PGM1, FOX A2, CACNA1D, EIF2S3, and PMM2 genes have been reported in patients with congenital HH. In particular, mutations in ABCC8 and KCNJ11 genes encoding the pancreatic KATP channel proteins, sulfonylurea receptor (SUR1), and potassium inward rectifier (Kir6.2), respectively, cause almost 50% of the genetic forms of HH. As a KATP channel agonist, diazoxide (DZX) is a first-line drug approved by the FDA (USA) for the medical treatment of HH. Consequently, genetic evaluation is warranted in HH patients who are DZX unresponsive, as mutations in KATP channel-regulating genes are more common (6–8).
In contrast to HH, maturity-onset diabetes of the young (MODY) is an autosomal dominant disease characterized by the onset of hyperglycemia before 25 years of age. There are at least 14 known MODY mutations, five of which involve the HH genes HNF1A, HNF4A, GCK, ABCC8, and KCNJ11. Of these, HNF1A and HNF4A encode hepatocyte nuclear factor 1α (HNF1α) and 4α (HNF4α), respectively, both of which are transcription factors for nuclear hormone receptors that are expressed in the β cells of the pancreas, thereby influencing the regulation of glucose-mediated insulin secretion (9). Therefore, mutations in these transcription factors regulating β-cell differentiation and function can cause both HH and MODY diabetes (9, 10). Heterozygous inactivating mutations in HNF1A and HNF4A genes can have a bi-phasic presentation, with transient or prolonged HH in infancy and diabetes in adolescence or young adulthood (10, 11). Additionally, we have shown that a single HNF4A mutation can cause variable phenotypes among members of the same family (12). HH due to HNF1A, and HNF4A gene mutations respond well to DZX. Interestingly, Arya et al. reported exceptional diazoxide sensitivity to a conventional dose of DZX in an HH infant with a novel HNF4A mutation requiring lower doses of DZX for glucose control (13). Here, we describe an HH infant with a novel maternally inherited HNF1A mutation presenting with an ultra-responsiveness to a traditional dose of DZX.
Case presentation
A term, male infant was born at 38 + 3 weeks via cesarean section for cephalopelvic disproportion to a non-consanguineous Chinese couple. The mother had gestational diabetes on diet control (HbA1c 8.4%, 32 weeks gestation). The baby was non-dysmorphic, LGA (4,280 g, 98th percentile, +2.16 SDS), had an occipitofrontal circumference of 34 cm (45th percentile, −0.13 SDS), and had length of 56 cm (99th percentile, +2.71 SDS). There was a family history of type 2 diabetes and obesity in the maternal grandfather and type 2 diabetes in the grandmother.
At the hospital of birth, the infant had symptomatic hypoglycemia at 2 h of life, with jitteriness, and the capillary blood glucose (CBG) was 0.7 mmol/L. Hypoglycemia was refractory to a mini-bolus of 10% dextrose, requiring a progressive escalation of glucose infusion rate (GIR) to a maximum of 22.4 mg/kg/min to achieve euglycemia. Hence, he was transferred to our tertiary neonatal center on day 7 of life and underwent a controlled GIR reduction test to obtain the critical diagnostic blood, which confirmed HH (plasma glucose, 2.1 mmol/L; insulin, 42.3 mIU/L; ketone, 0.1 mmol/L; and free fatty acids, 0.12 mmol/L). Growth hormone and cortisol response to hypoglycemia were appropriate. Blood gas, renal and liver function tests, an inborn error of metabolism screen, acylcarnitine, and infective markers were unremarkable. An echocardiogram prior to initiation of DZX revealed asymmetric septal hypertrophy.
For the symptomatic hypoglycemia and high GIR requirements, he was initiated on a standard oral dose of DZX, 5 mg/kg/day in three divided doses on day 8 of life, and hydrochlorothiazide was added. Over 48 h, he responded to DZX with a glucose profile in the 3.5–8 mmol/L range. On day 10, CBG peaked at 10 mmol/L, and on day 11, while weaning of GIR was in progress, his CBG levels reached 16.6 mmol/L, indicating a possible spontaneous resolution of HH. DZX was withheld, and glucose infusion was progressively weaned and ceased. He was kept on close CBG monitoring while continuing on milk feeds, and the glucose profile remained stable for the following 72 h. To confirm a resolution of HH, a 6-h fasting study was conducted on day 15 of life, which he passed.
On day 16 of life, the infant again developed hypoglycemia while on enteral nutrition, which spontaneously resolved with feeds. Recurrent hypoglycemic episodes were noted again on days 17–18 of life, even to unrecordable glucose levels. Glucose infusion was restarted, and the maximal GIR required was 18 mg/kg/min. Critical blood samples (plasma glucose, 1.5 mmol/L; insulin, 26.2 mIU/L; ketone, 0.0 mmol/L; and free fatty acids, 0.18 mmol/L) again confirmed HH. Thus, a lower dose of DZX, 3 mg/kg/day, was initiated on day 18 of life. Again, a rise in CBG levels up to 14 mmol/L necessitated further down-titration of DZX, and the CBG levels became stable on a dose of 0.7 mg/kg/day of DZX (Figure 1). On this low dose of DZX, he passed a 6-h safety fasting study before discharge to ensure the maintenance of CBG levels during an inadvertent fast at home. Parents underwent counseling and caregiver training with an action plan for hyperglycemia and hypoglycemia. Genetic testing was done using the Invitae (San Francisco, CA, USA) hypoglycemia panel (120 genes). A novel heterozygous mutation in exon 5,c.1064G>T(p.Gly355Val) was identified, replacing glycine with valine at codon 355 of the HNF1A protein. Further family genetic testing revealed the same genetic mutation in the mother, and the father tested negative (Figure 2).

Figure 1. Timeline of events from day 7 to day 28 of life. Glucose profile and glucose infusion rates are depicted. Diazoxide dose given is shown in the box below. (A) Controlled GIR reduction test to confirm HH; (B) confirmation of HH; (C) initiation of DZX at 5 mg/kg/day; (D) DZX discontinuation; (E) passed fasting study; (F) hypoglycemia, reinstating glucose infusion followed by DZX at 3mg/kg/day; (G–I) DZX dose down-titrated to 1.5 mg/kg/day, 1.2 mg/kg/day, and 1 mg/kg/day; (J) omitted a dose of DZX; (K) DZX dose reduced to 0.7 mg/kg/day; (L) passed safety fasting study; (M) discharged home. DZX, diazoxide; GIR, glucose infusion rate; HH, hyperinsulinemic hypoglycemia.
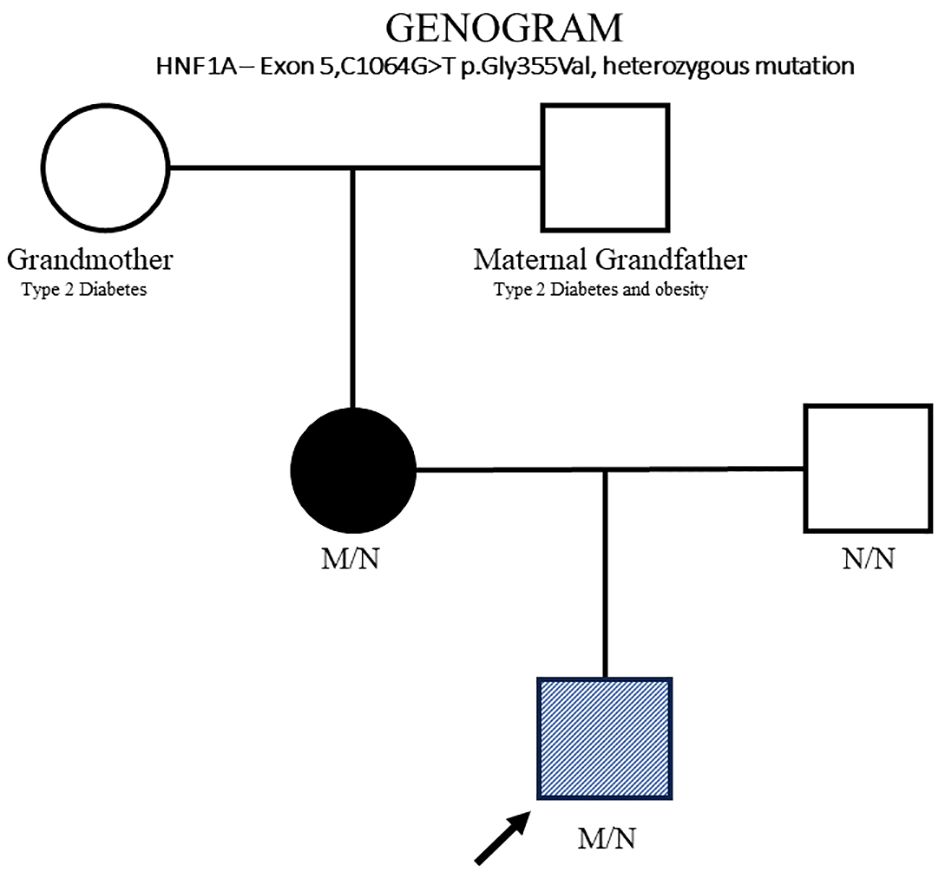
Figure 2. HNF1A family pedigree. Circles (female) and squares (male). Diagonal hatching denotes HH (proband). Filled symbols indicates MODY (mother). Unfilled symbol indicates normal genotype (father) and maternal grandfather and grandmother. M/N, heterozygous HNF1A genotype; N/N, normal genotype.
Outcome and follow-up
He remained on home glucose monitoring, and the CBG chart was reviewed online by the hypoglycemia nurse on a day-to-day basis and weekly by a team consultant. Till 5 months of age, his CBG remained stable. Then, occasional nocturnal glucose levels became borderline low, and the dose of DZX was raised from 0.7 mg/kg/day to 1 mg/kg/day, implying the persistence of HH. He required a further stepwise increase in the DZX dose over the next 5 months to 2.5 mg/kg/day, as borderline low glucose levels were recorded at night while on formula milk, corn starch, and weaning foods under the dietitian’s advice. Neurodevelopmental assessment and growth parameters, namely, weight of 11.5 kg (95th percentile, +1.62 SDS), occipitofrontal circumference of 48 cm (93rd percentile, +1.51 SDS), and length of 81 cm (99th percentile, +2.21 SDS) were all above the 90th percentile at 12 months of age.
Discussion
We report a term LGA infant of a diabetic mother with congenital hyperinsulinism who demonstrated exquisite sensitivity to 3–5mg/kg/day of DZX, with CBG reaching the diabetic range. Hyperinsulinism was not transient but recurred, and the baby required a very low dose of DZX <1 mg/kg/day to maintain euglycemia. The very low dose requirement was transient such that by 5 months old, a higher but lower than standard dosing of DZX was needed to maintain his normal glucose profile. The baby had a unique HNF1A variant, and we postulate that this may be responsible for the changing requirements in the first year of life.
Arya et al. reported a neonate with exceptional DZX sensitivity with a dose of 5 mg/kg/day in the background of a novel HNF4A mutation in the mother–child dyad. Later, a reduced dose of DZX, 1.5mg/kg/day, stabilized the CBG levels (13). To the best of our knowledge, there is no report of DZX hypersensitivity needing an extremely low dose of DZX (0.7 mg/kg/day) to maintain euglycemia in a confirmed case of HH having an HNF1A mutation. HNF1A mutation is novel in our case, and the predisposition to DZX hypersensitivity could be mutation specific.
MODY accounts for at least 1% of all cases of diabetes mellitus (14). In the UK, 80% of MODY variants were misdiagnosed as T1/T2 diabetes (15). With increasing awareness of MODY, more novel mutations are identified using genetic testing and reported with diabetes in pregnancy and adulthood and HH in infants. Pearson et al. and Fajan et al. identified HNF4A mutations causing a biphasic phenotype, HH, and MODY1 (16, 17). Earlier reports by Pearson et al. did not find a higher prevalence of HH in HNF1A variants. But later, Tung et al. identified 12 cases of diazoxide-responsive HH in the USA, of which 58% had HNF1A mutation, and Rozenkova et al. reported that HNF1A mutations represented the second most frequent cause of HH in the Czech Republic (18, 19).
Our described neonate was LGA and had symptomatic hypoglycemia within hours of birth. Heterozygous mutations in HNF4A were known to cause macrosomia but not with HNF1A (16). However, two recent reports point to a fetal overgrowth in utero and neonatal HH with HNF1A mutation (20, 21), as noted in our case. Most cases of HH in IDM are transient and resolve within several days to a week (22). The hypoglycemia in our infant was due to hyperinsulinism. Even though HH was hypersensitive to DZX initially, the response to treatment was appropriate for HH while on a very low dose. The course of HH continues beyond infancy, and the requirement of DZX dose rose with age, supporting the nature of some of the MODY gene mutations (12, 13).
Determining whether the given novel HNF1A variant of uncertain significance (VUS) is responsible for HH is challenging. Still, GDM, fetal overgrowth, and persistent neonatal HH with the same novel mutation in the mother–child dyad suggest a MODY phenotypic presentation. Cromer et al. and Yau et al. reported a similar scenario to our case, where a single genetic VUS in HNF1A caused gestational diabetes, fetal overgrowth, and neonatal HH (11, 20). The mutation identified in our case is currently a VUS, as this variant is not present in population databases (Exome Aggregation Consortium). The identified mutation was in exon 5,c.1064G>T (p.Gly355Val), replacing glycine with valine at codon 355 of the HNF1A protein. The glycine residue is moderately conserved, and there is a moderate physicochemical difference between glycine and valine. Further advanced modeling of protein sequence and biophysical properties performed at Invitae laboratory (structural, functional, and spatial information, amino acid conservation, physicochemical variation, residue mobility, and thermodynamic stability) indicates that this missense variant is expected to disrupt HNF1A protein function. This observation further confirms that the location or characteristics of the specific genetic variant can derange the protein function or degree of penetrance and can present with different phenotypes. Given the absence of population data and lack of functional studies on protein function, available evidence is currently insufficient to determine the role of this variant in disease.
Successful use of DZX to treat hypoglycemia began in 1960 (23). The recommended dose of DZX to treat HH is 5–20 mg/kg/day. A history of non-ketotic hyperosmolar coma with DZX therapy was first reported in a 13-month-old boy with HH by Balsam et al. The infant was on 7.5 mg/kg/day of DZX and developed listlessness and seizures within a week of initiation of DZX. The plasma glucose was noted to be 2,000 mg/dL (111.1 mmol/L). On recovery from hyperglycemia, he was restarted on a lower dose of DZX at 4 mg/kg/day and maintained optimal glucose control. No genetic testing was done in this case (24). In 2018, Mangala et al. reported a 16-month-old with hyperglycemic (>22 mmol/L) hyperosmolar coma and ketoacidosis while on 15 mg/kg/day of DZX for HH, diagnosed at 4 months of age. No pathogenic variant was detected in genetic testing (25). In both cases, hyperglycemia occurred during intercurrent illnesses, and the dose of DZX used was much higher than in our case. Diazoxide is metabolized in the liver and excreted through the kidneys. While on treatment with DZX, intercurrent illnesses could impair hepatic and renal functions, and the DZX levels could rise exponentially to toxic levels manifesting as hyperglycemia and hyperosmolar coma. There was no record of hepatic function in the former, but in the latter, they reported marked hepatic and renal dysfunction, which could account for the DZX toxicity (24, 25). In our reported case, he developed hyperglycemia initially while on a recommended lower end of conventional DZX dose. He was screened negative for infections. Hyperosmolar ketosis was prevented in the index case because of close monitoring and timely intervention. Recently, there were reports of increasing adverse reactions to DZX in newborn infants (26). Two recent publications have shown the safety and efficacy of low-dose DZX in treating HH in SGA, non-SGA, preterm, and term infants (27, 28).
The mechanisms underlying the hyperresponsiveness to DZX are unknown. Higher blood glucose levels while on DZX treatment could involve a mechanism similar to the dual phenotypic presentation in the HNF1A/HNF4A mutations, switching from hypoglycemia to hyperglycemia. Stanescu et al. suggested a possible relationship between HNF1A, HNF4A, GLUT2, and the KATP channel. She described two HNF1A children with congenital hyperinsulinism, who, unlike ours, had variants that were paternally inherited, and neither child demonstrated diazoxide hypersensitivity. A potential mechanism for diazoxide hypersensitivity involves interaction between the transcription factors HNF1A and HNF4A, and the KATP channel (10). It is presently unclear whether the inheritance pattern may play a role.
This case study identified a strong family history of diabetes in the grandparents of the index case, but due to old-age illnesses, no genetic testing was done. MODY is an autosomal dominantly inherited disease, with gestational diabetes in the mother who has the same novel mutation as the proband. It is plausible that the maternal grandparents, too, have the same mutation. Long-term management of infants with an HNF1A variant includes understanding its triphasic nature and the transitions from hypoglycemia to normoglycemia to diabetes. Genetic counseling for future pregnancies will need to include highlighting the autosomal dominant nature of this variant, and the risks of diabetes in pregnancy, neonatal hypoglycemia, and early-onset hyperglycemia in individuals who have the variant.
Strengths and limitations
As with all case reports, the key limitation lies in its generalizability, except that this is the second report of a hepatocyte nuclear factor variant linked with exquisite DZX sensitivity.
Conclusion
Like hypoglycemia, DZX itself is not benign. This case study implies that a very low dose of DZX may be sufficient in infants with this reported novel HNF1A variant. We postulate that abnormalities in genes encoding transcription factors may alter the affected infant’s sensitivity to DZX. It is justifiable to do a genetic study in prolonged and persistent HH in IDM, especially in the background of a strong family history of early onset diabetes. Parents should be educated on the possibility of hyperglycemia and hypoglycemia while on DZX treatment to prevent hyperglycemia and hyperosmolar coma to avoid neuronal injury.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
SC: Conceptualization, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. DV: Investigation, Resources, Writing – original draft. VR: Investigation, Resources, Supervision, Writing – review & editing. FY: Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank our hypoglycemia team, including doctors and nurses from the Departments of Neonatology and Pediatric Endocrinology of KK Women’s and Children’s Hospital, Singapore, for contributing to the management of this baby. We also thank SSN Mohanambal Arumugham and Ningjuan Zhang, Department of Neonatology, and SNC Lim Soo Ting Joyce for providing home care services and conducting the fasting studies.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Srinivasan G, Pildes RS, Cattamanchi G, Voora S, Lilien LD. Plasma glucose values in normal neonates: a new look. J Pediatr. (1986) 109:114–17. doi: 10.1016/S0022-3476(86)80588-1
2. Chong JH, Chandran S, Agarwal P, Rajadurai VS. Delayed presentation of prolonged hyperinsulinaemic hypoglycaemia in a preterm small-for-gestational age neonate. BMJ Case Rep. (2013) 2013:bcr2013200920. doi: 10.1136/bcr-2013-200920
3. Chandran S, Siew JX, Rajadurai VS, Lim RWS, Chau MC, Yap F. A feed-centric hypoglycaemia pathway ensures appropriate care escalation in at-risk infants. BMJ Open Qual. (2021) 10:e001296. doi: 10.1136/bmj0q-2020-001296
4. Kureshi A, Khalak R, Gifford J, Munshi U. Maternal obesity-associated neonatal morbidities in early newborn period. Front Pediatr. (2022) 10:867171. doi: 10.3389/fped.2022.867171
5. Chandran S, Teoh KW, Janardhan K, Yap F. Case report: neurodevelopment outcome in a small-for-gestational-age infant with symptomatic hyperinsulinemic hypoglycemia, gaze preference, and infantile spasms. Front Endocrinol (Lausanne). (2022) 13:818252. doi: 10.3389/fendo.2022.818252
6. Guemes M, Hussain K. Hyperinsulinemic hypoglycemia. Pediatr Clin. (2015) 62:1017–36. doi: 10.1016/j.pcl.2015.04.010
7. Hu S, Xu Z, Yan J, Liu M, Sun B, Li W, et al. The treatment effect of diazoxide on 44 patients with congenital hyperinsulinism. J Pediatr Endocrinol Metab. (2012) 25:1119–22. doi: 10.1515/jpem-2012-0224
8. Guemes M, Rahman SA, Kapoor RR, Flanagan S, Houghton JAL, Misra S, et al. Hyperinsulinemic Hypoglycemia in Children and Adolescents: Recent advances in understanding of pathophysiology and management. Rev Endocr Metab Disord. (2020) 21:577–97. doi: 10.1007/s11154-020-09548-7
9. Younis H, Ha SE, Jorgensen BG, Verma A, Ro S. Maturity-onset diabetes of the young: mutations, physiological consequences, and treatment options. J Pers Med. (2022) 12:1762. doi: 10.3390/jpm12111762
10. Stanescu DE, Hughes N, Kaplan B, Stanley CA, De Leon DD. Novel Presentations of Congenital Hyperinsulinism due to Mutations in the MODY genes: HNF1A and HNF4A. J Clin Endocrinol Metab. (2012) 97:E2026–30. doi: 10.1210/jc.2012-1356
11. Yau D, Colclough K, Natarajan A, Parikh R, Canham N, Didi M, et al. Congenital hyperinsulinism due to mutations in HNF1A. Eur J Med Genet. (2020) 63:103928. doi: 10.1016/j.ejmg.2020.103928
12. Chandran S, Rajadurai VS, Hoi WH, Flanagan SE, Hussain K, Yap F. A novel HNF4A mutation causing three phenotypic forms of glucose dysregulation in a family. Front Pediatr. (2020) 8:320. doi: 10.3389/fped.2020.00320
13. Arya B, Kalitsi J, Hickey A, Flanagan SE, Kapoor RR. Exceptional Diazoxide Sensitivity in Hyperisnulinemic Hypoglycemia due to a Novel HNF4A mutation. Endocrinol Diabetes Metab Case Rep. (2019) 2019:19–0013. doi: 10.1530/EDM-19-0013
14. Kleinberger JW, Pollin TI. Undiagnosed MODY: time for action. Curr Diabetes Rep. (2015) 15:110. doi: 10.1007/s11892-015-0681-7
15. Shields BM, Hicks S, Shepherd MH, Colclough K, Hattersley AT, Ellard S. Maturity onset diabetes of the young (MODY): How many cases are we missing? Diabetologia. (2010) 53:2504–8. doi: 10.1007/s00125-010-1799-4
16. Pearson ER, Boj SF, Steele AM, Barrett T, Stals K, Shield JP, et al. Macrosomia and hyperinsulinaemic hypoglycaemia in patients with heterozygous mutations in the HNF4A gene. PloS Med. (2007) 4:e118. doi: 10.1371/journal.pmed.0040118
17. Fajans SS, Bell GI. Macrosomia and neonatal hypoglycaemia in RW pedigree subjects with a mutation (Q268X) in the gene encoding hepatocyte nuclear factor 4alpha (HNF4A). Diabetologia. (2007) 50:2600–1. doi: 10.1007/s00125-007-0833-7
18. Tung JY, Boodhansingh K, Stanley CA, De León DD. Clinical heterogeneity of hyperinsulinism due to HNF1A and HNF4A mutations. Pediatr Diabetes. (2018) 19:910–16. doi: 10.1111/pedi.12655
19. Rozenkova K, Malikova J, Nessa A, Dusatkova L, Bjørkhaug L, Obermannova B, et al. High incidence of heterozygous ABCC8 and HNF1A mutations in czech patients with congenital hyperinsulinism. J Clin Endocrinol Metab. (2015) 100:E1540–9. doi: 10.1210/jc.2015-2763
20. Cromer SJ, Sella AC, Rosenberg E, Scully K, McDonnell M, Abreu AP, et al. Report of prolonged neonatal hypoglycemia in three infants of mothers with variants in HNF1A. AACE Clin Case Rep. (2022) 8:224–30. doi: 10.1016/j.aace.2022.07.004
21. Hermann FM, Kjærgaard MF, Tian C, Tiemann U, Jackson A, Olsen LR, et al. An insulin hypersecretion phenotype precedes pancreatic β cell failure in MODY3 patient-specific cells. Cell Stem Cell. (2023) 30:38–51.e8. doi: 10.1016/j.stem.2022.12.001
22. Senniappan S, Shanti B, James C, Hussain K. Hyperinsulinaemic hypoglycaemia: genetic mechanisms, diagnosis and management. J Inherit Metab Dis. (2012) 35:589–601. doi: 10.1007/s10545-011-9441-2
23. Drash A, Wolff F. Drug therapy in Leucine-Sensitive hypoglycemia. Metabolism. (1964) 13:487–92. doi: 10.1016/0026-0495(64)90133-7
24. Balsam MJ, Baker L, Kaye R. Hyperosmolar nonketotic coma associated with diazoxide therapy for hypoglycemia. J Pediatr. (1971) 78:523–5. doi: 10.1016/s0022-3476(71)80241-x
25. Mangla P, Hussain K, Ellard S, Flanagan SE, Bhatia V. Diazoxide toxicity in a child with persistent hyperinsulinemic hypoglycemia of infancy: mixed hyperglycemic hyperosmolar coma and ketoacidosis. J Pediatr Endocrinol Metab. (2018) 31:943–45. doi: 10.1515/jpem-2018-0112
26. Desai J, Key L, Swindall A, Gaston K, Talati AJ. The danger of diazoxide in the neonatal intensive care unit. Ther Adv Drug Saf. (2021) 12:20420986211011338. doi: 10.1177/20420986211011338
27. Ng AY, Agrawal P, Vijayan R, Arya VB, Kapoor RR, Shah P. Low-dose diazoxide therapy in hyperinsulinaemic hypoglycaemia. Clin Endocrinol (Oxf). (2024) 100:140–42. doi: 10.1111/cen.14991
Keywords: gestational diabetes, infant of diabetic mother, HNF1A variant, hypoglycemia, congenital hyperinsulinism, diazoxide hypersensitivity
Citation: Chandran S, Verma D, Rajadurai VS and Yap F (2024) Case report: A novel HNF1A variant linked to gestational diabetes, congenital hyperinsulinism, and diazoxide hypersensitivity. Front. Endocrinol. 15:1471596. doi: 10.3389/fendo.2024.1471596
Received: 27 July 2024; Accepted: 04 September 2024;
Published: 03 October 2024.
Edited by:
Natalia da Silva Lima, University of Santiago de Compostela, SpainReviewed by:
Simona Zampetti, Sapienza University of Rome, ItalyVioleta Heras Dominguez, University of Malaga, Spain
Copyright © 2024 Chandran, Verma, Rajadurai and Yap. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suresh Chandran, c3VyZXNoLmNoYW5kcmFuQHNpbmdoZWFsdGguY29tLnNn; Fabian Yap, ZmFiaWFuLnlhcC5rLnBAc2luZ2hlYWx0aC5jb20uc2c=
 Suresh Chandran
Suresh Chandran Deepti Verma1
Deepti Verma1 Victor Samuel Rajadurai
Victor Samuel Rajadurai Fabian Yap
Fabian Yap