- Department of Gynecological Oncology, Sichuan Provincial Women’s and Children’s Hospital/The Affiliated Women's and Children's Hospital of Chengdu Medical College, Chengdu, China
Objectives: To investigate various supplements that improve insulin resistance, hormonal status, and oxidative stress in overweight or obese women with polycystic ovarian syndrome (PCOS).
Methods: A literature search was conducted on four different databases, which led to the discovery of twenty - five randomized controlled trials (RCTs). These RCTs evaluated the efficacy of various supplements in improving insulin resistance (IR), hormonal status, and oxidative stress among overweight or obese women diagnosed with PCOS. Subsequently, data extraction and analysis were carried out to determine the quality of the study’s methodological design and the potential for bias. Moreover, a meta-analysis was performed using the data from the RCTs.
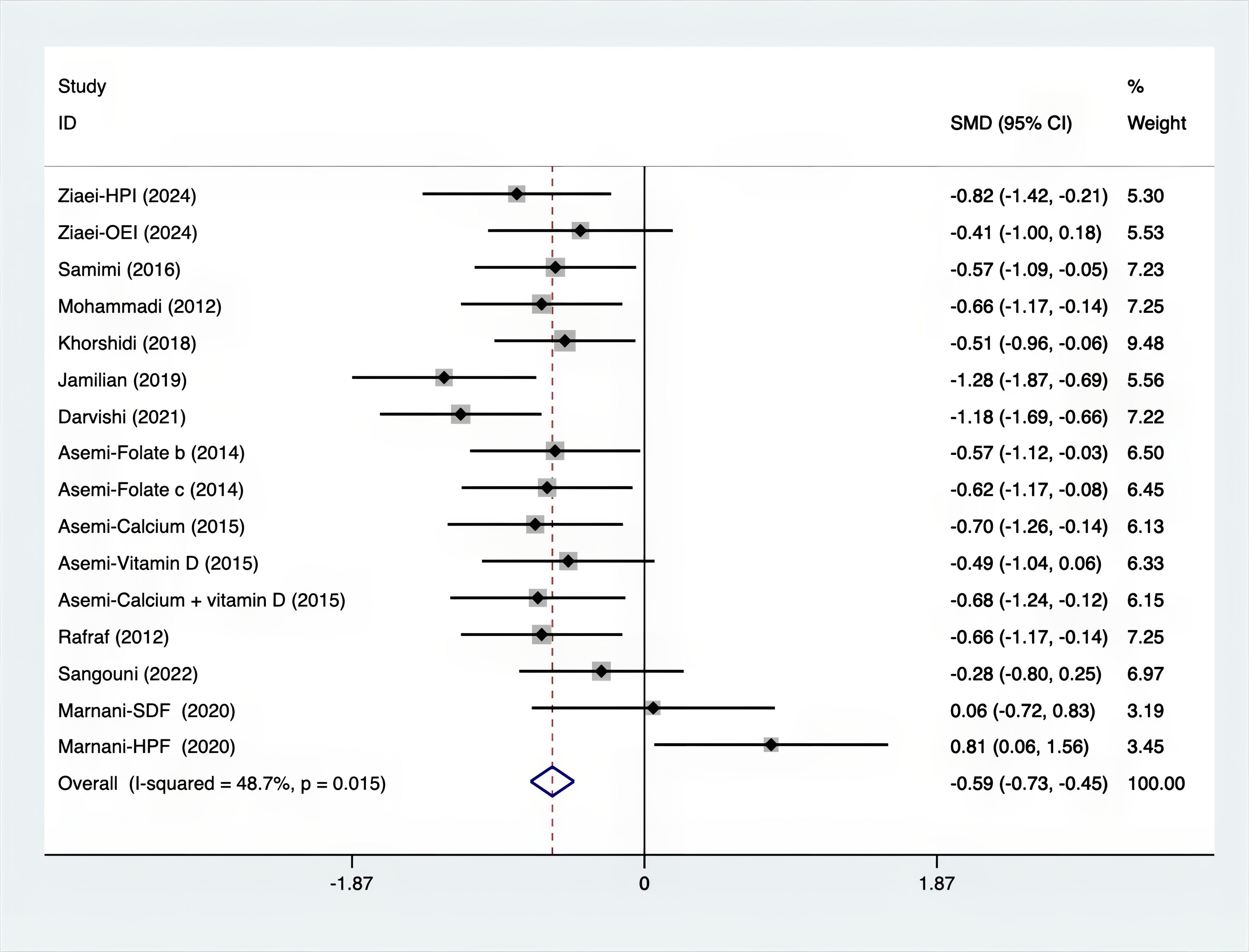
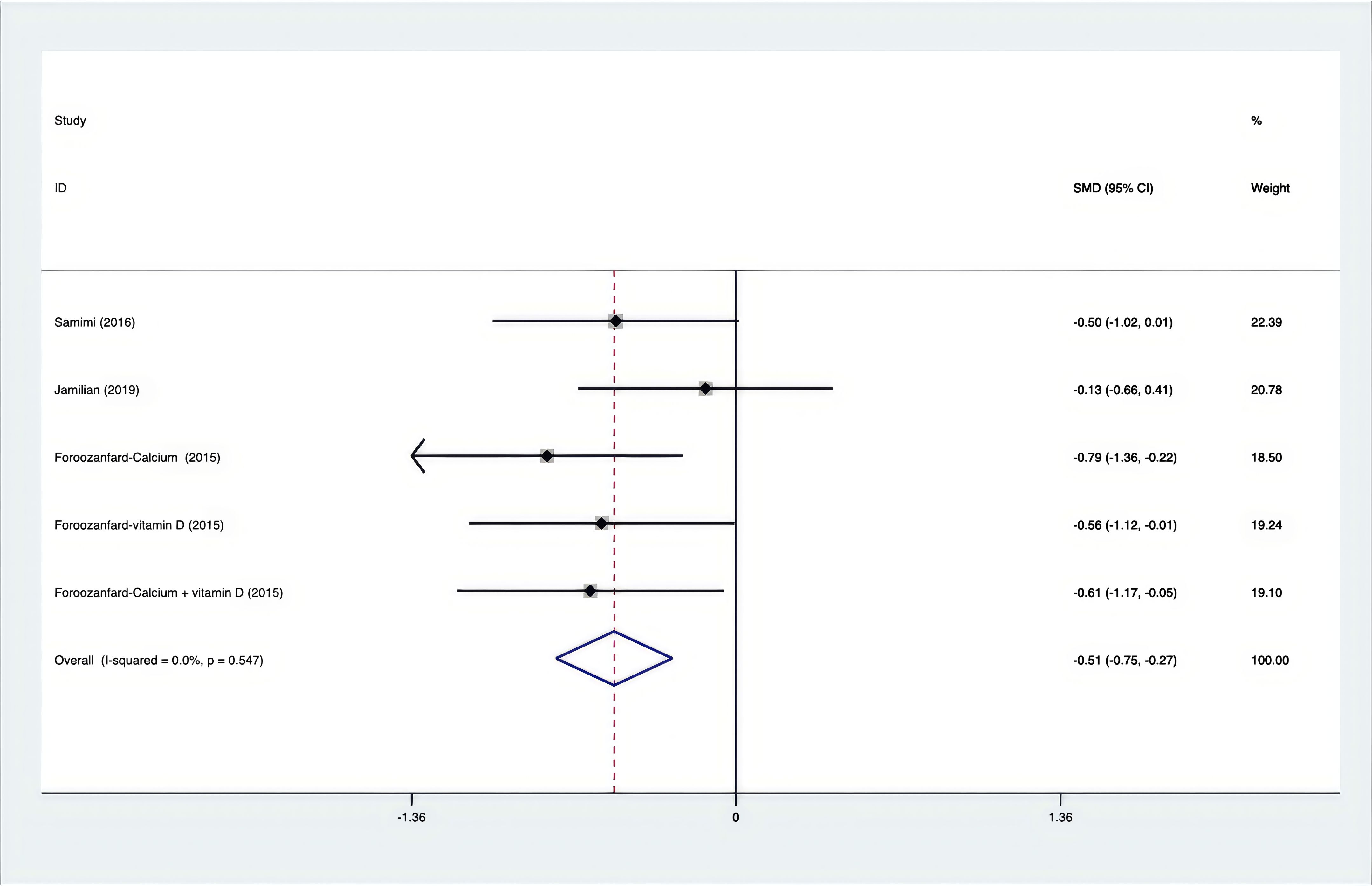
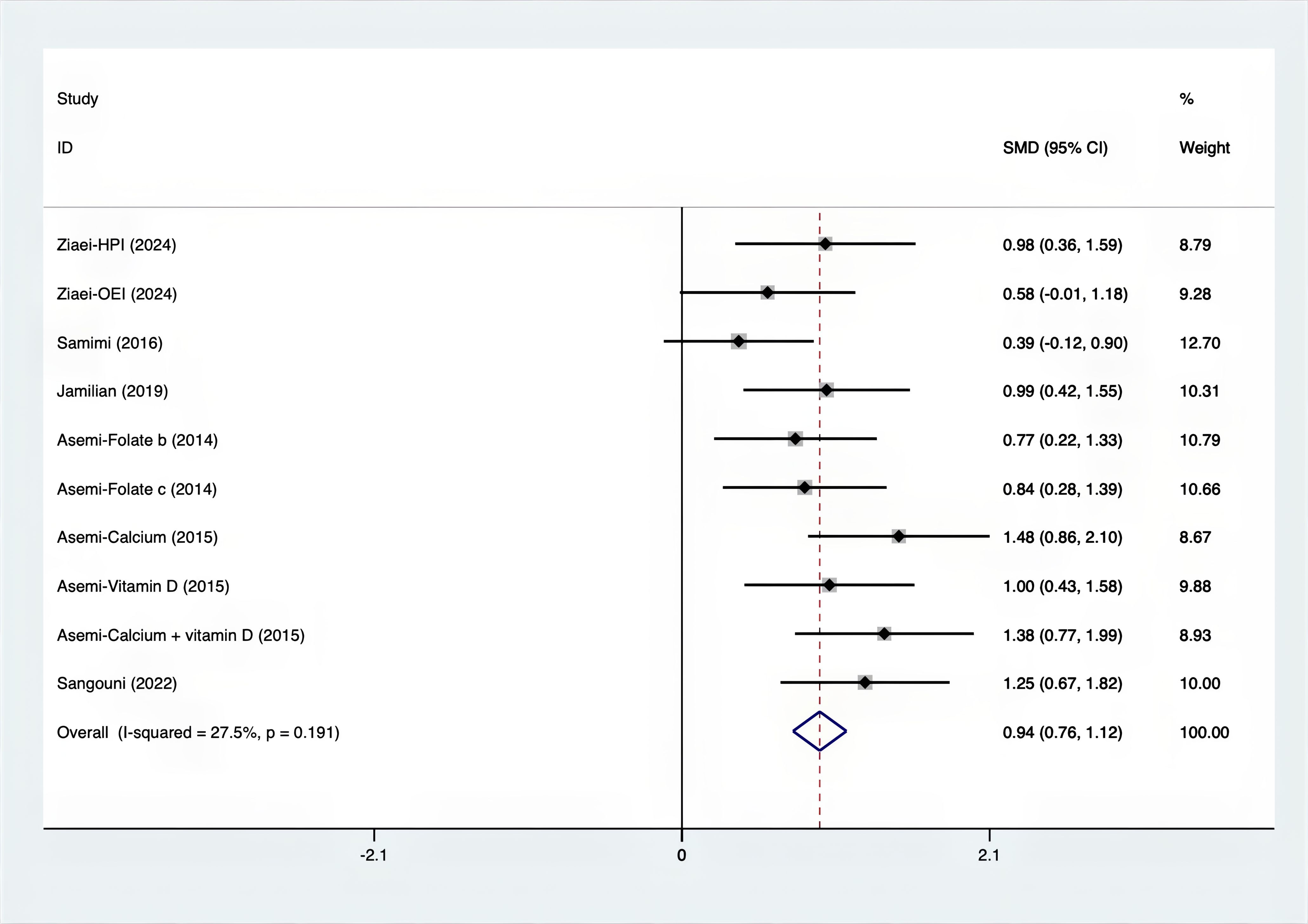
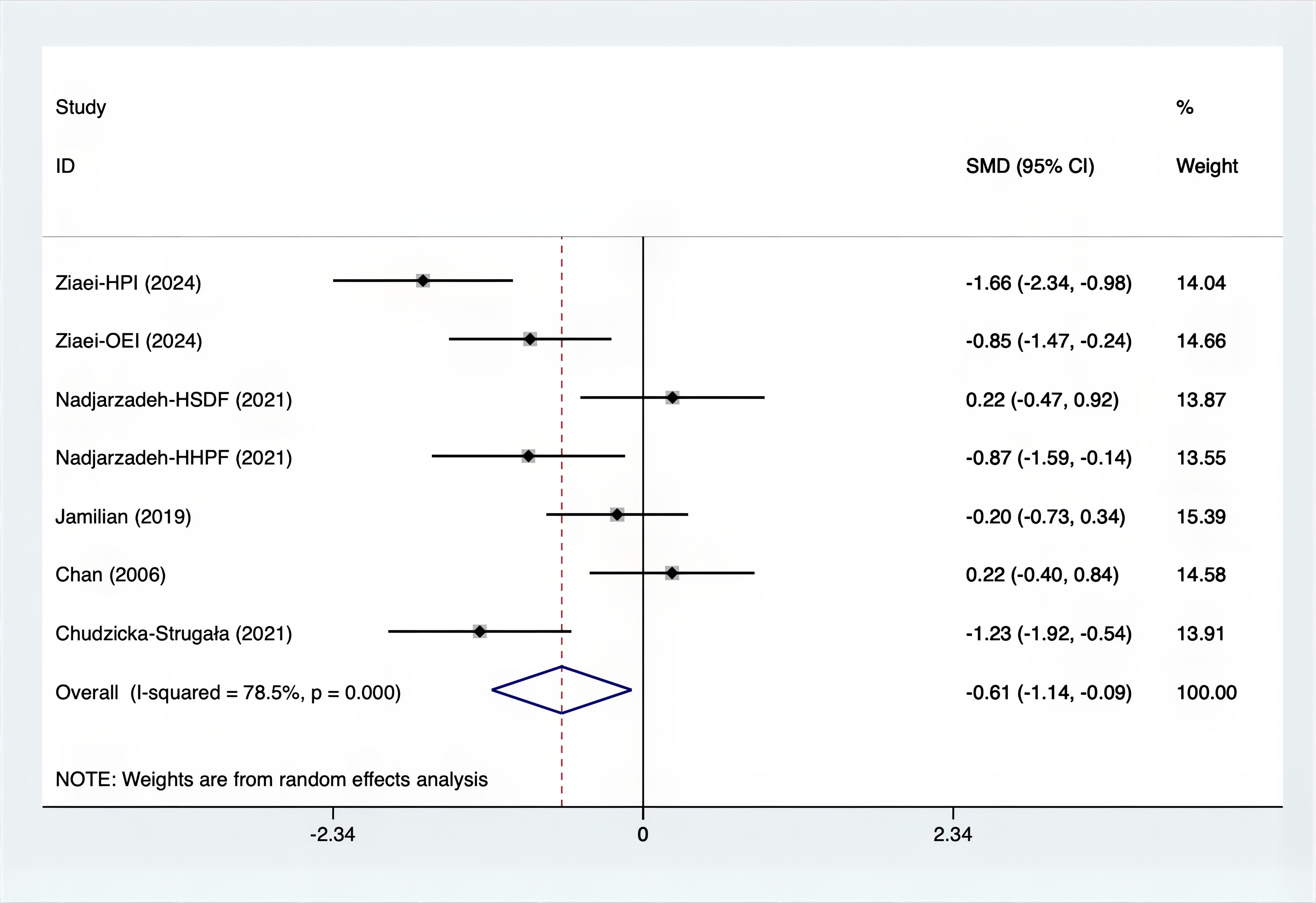
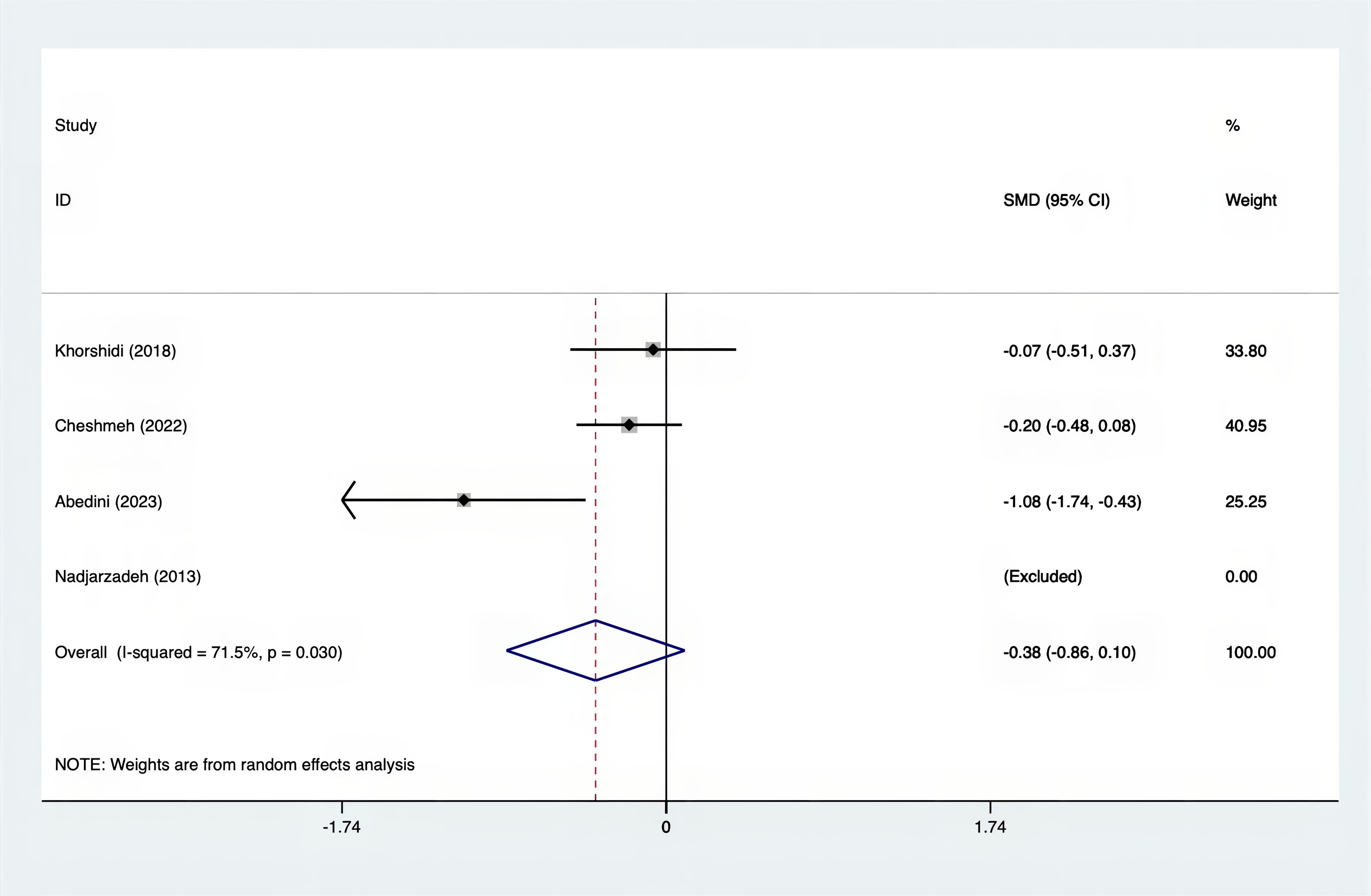
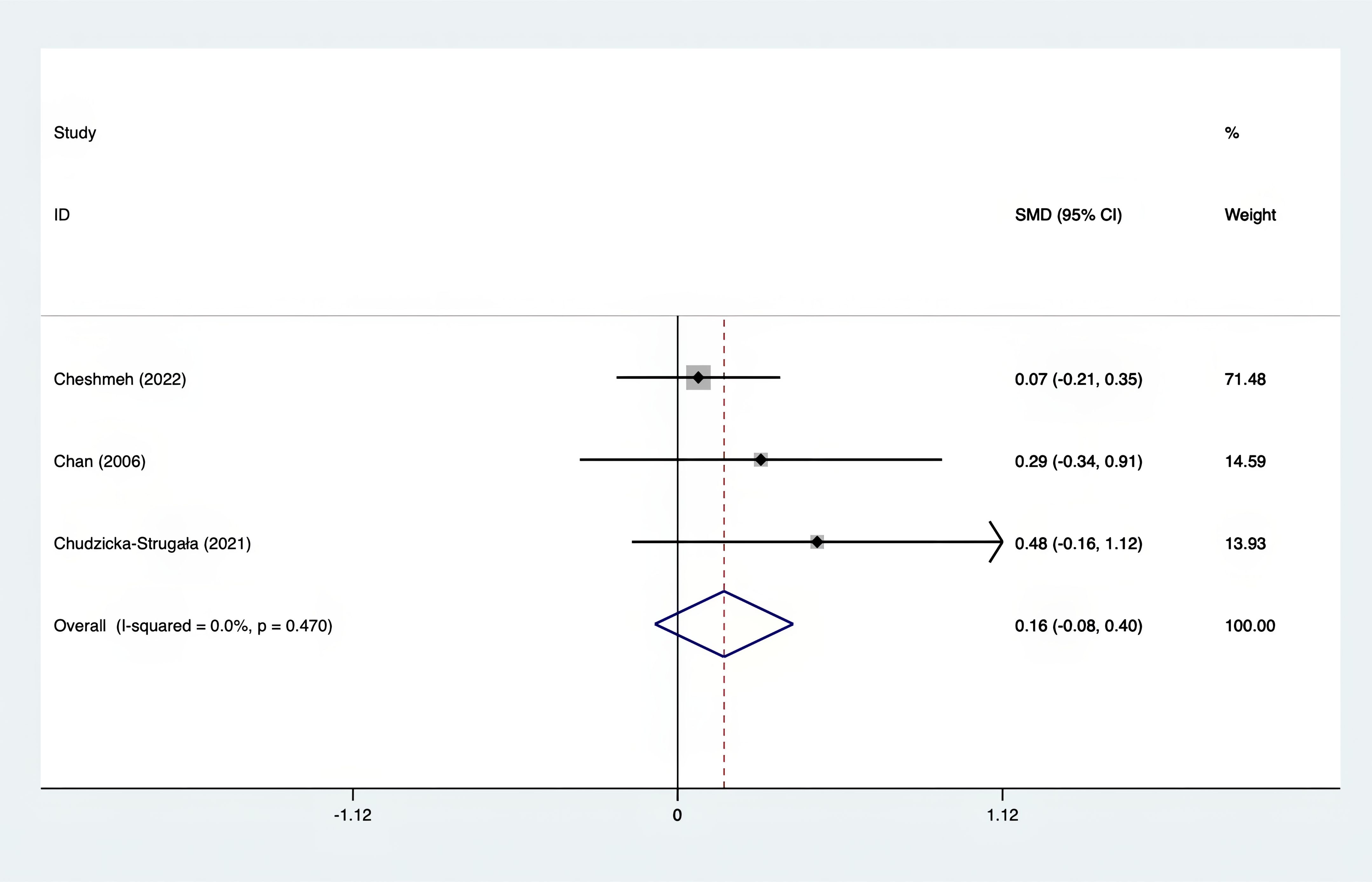
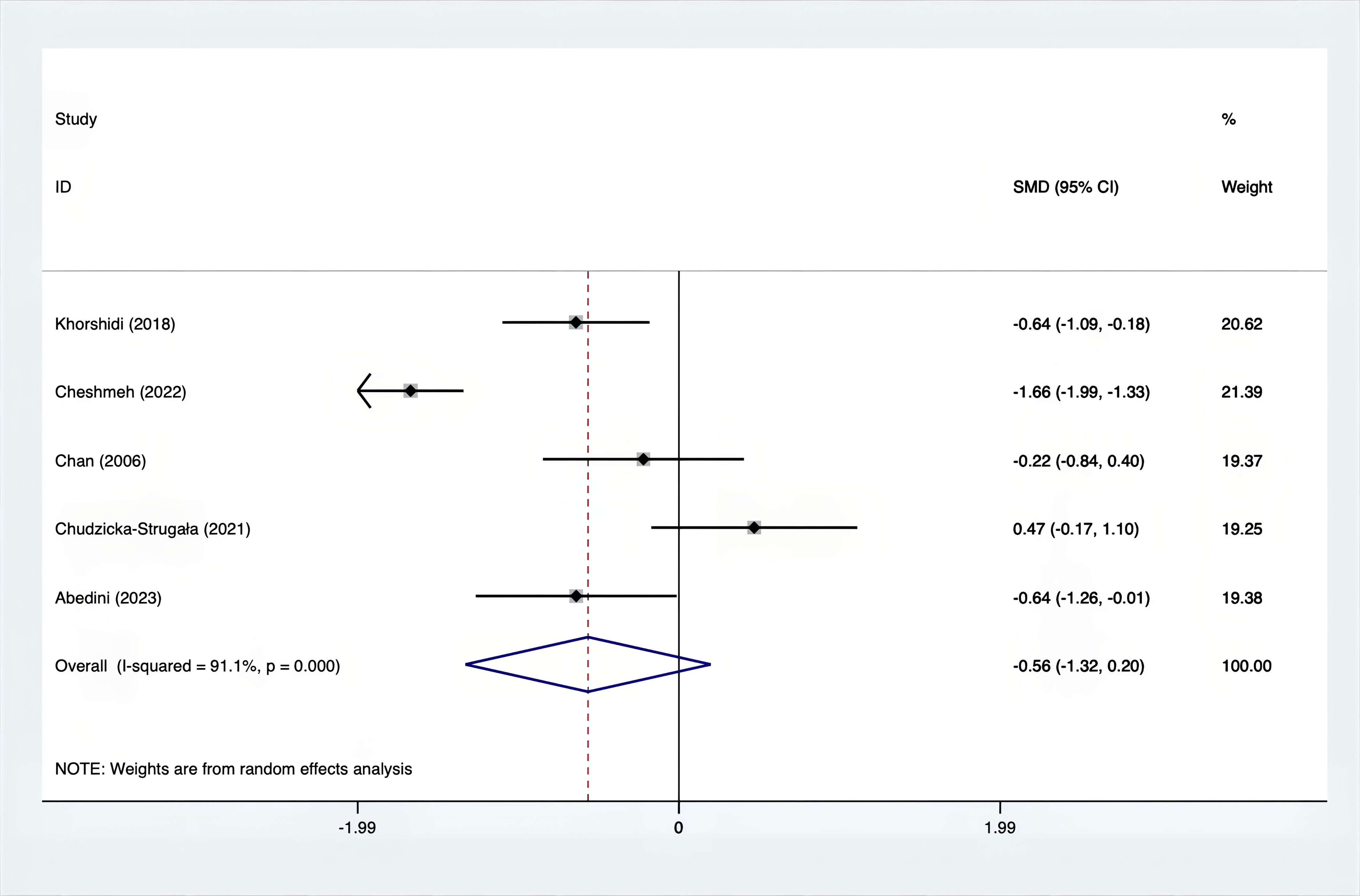
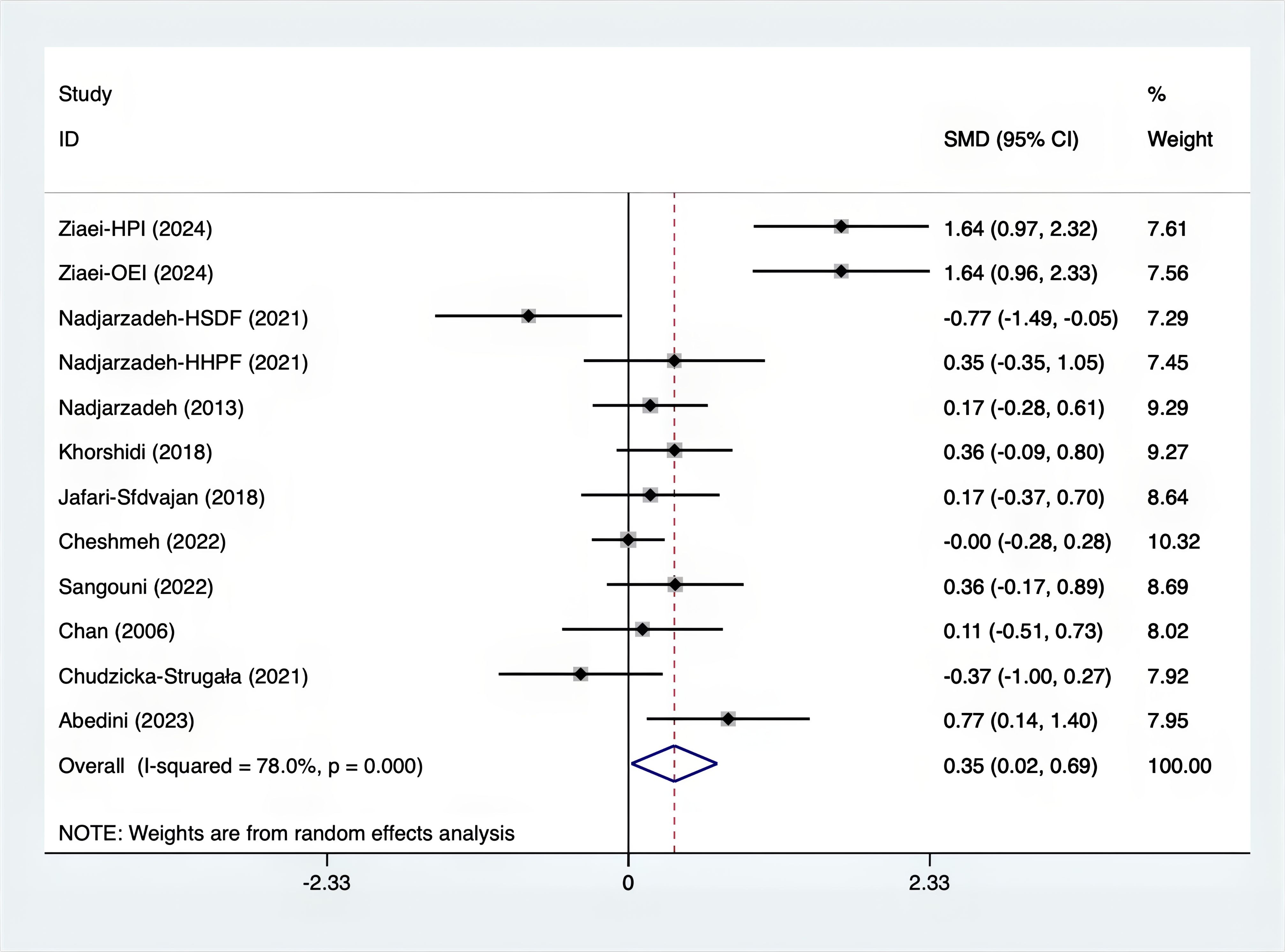
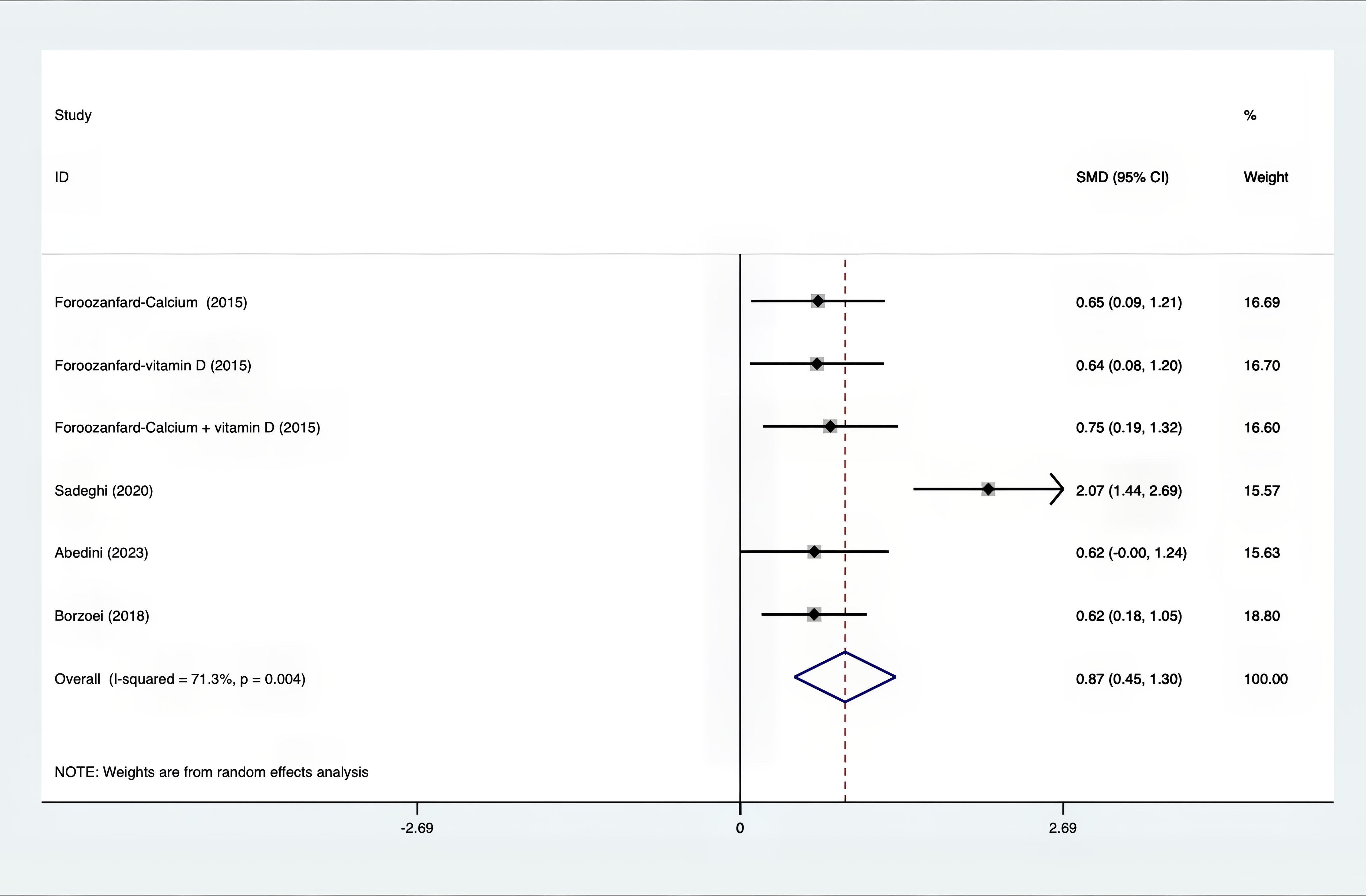
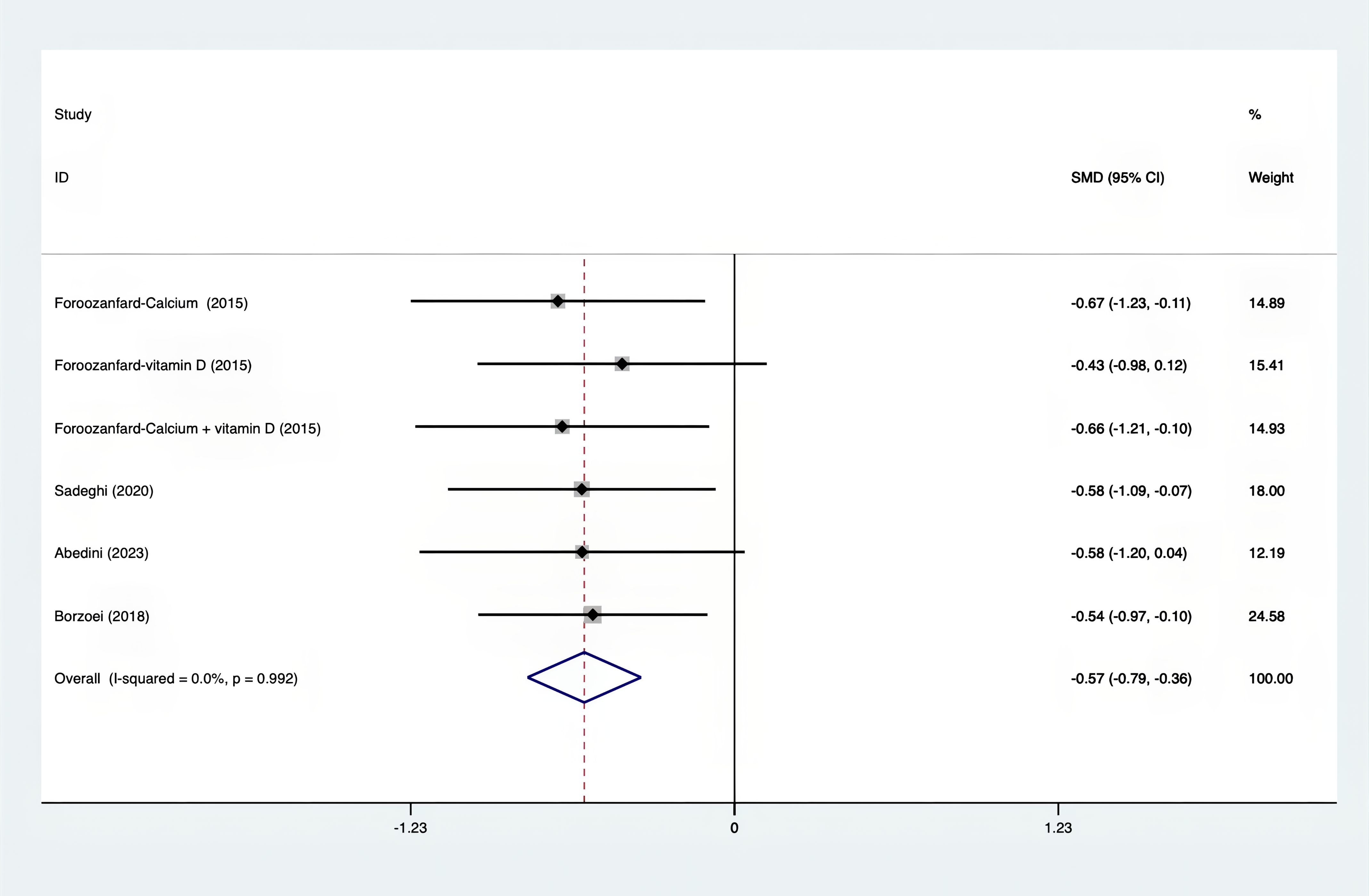
Results: A total of 25 RCTs were carried out, and 1636 women were enrolled. All participants were overweight or obese. The standardized mean differences (SMD) were as follows: For fasting plasma glucose (FPG), it was -0.34 (95% confidence interval [CI], -0.49 to -0.19, p = 0.123, I2 = 30.8%); for insulin, it was -0.67 (95% CI, -0.83 to -0.52, p = 0.208, I2 = 24%); for fasting insulin (FI), it was -0.26 (95% CI, -0.52 to -0.00, p = 0.269, I2 = 21.9%); for homeostatic model assessment-insulin resistance index (HOMA-IR), it was -0.59 (95% CI, -0.73 to -0.45, p = 0.015, I2 = 48.7%); for homoeostatic model assessment beta - cell function (HOMA-B), it was -0.51 (95% CI, -0.75 to -0.27, p = 0.547, I2 = 0%); for quantitative insulin sensitivity check index (QUICKI), it was 0.94 (95% CI, 0.76 to -1.12, p = 0.191, I2 = 27.5%); for total testosterone, it was -0.61 (95% CI, -1.14 to -0.09, p = 0.00, I2 = 78.5%); for testosterone, it was -0.38 (95% CI, -0.86 to 0.10, p = 0.03, I2 = 71.5%); for follicle - stimulating hormone (FSH), it was 0.16 (95% CI, -0.08 to 0.40, p = 0.470, I2 = 0%); for luteinizing hormone (LH), it was -0.56 (95% CI, -1.32 to 0.20, p = 0.000, I2 = 91.1%); for sex hormone - binding globulin (SHBG), it was 0.35 (95% CI, 0.02 to 0.69, p = 0.000, I2 = 78%); for dehydroepiandrosterone (DHEAS), it was -0.27 (95% CI, -0.76 to 0.21, p = 0.001, I2 = 78.7%); for plasma total antioxidant capacity (TAC), it was 0.87 (95% CI, 0.45 to 1.30, p = 0.004, I2 = 71.3%); for plasma malondialdehyde (MDA), it was -0.57 (95% CI, -0.79 to -0.36, p = 0.992, I2 = 0.0%).
Conclusion: This study’s findings indicate that, in comparison with a placebo, supplements have a favorable effect on IR, hormonal functions, and oxidative stress in PCOS. Nevertheless, it is crucial to note that the above-drawn conclusions need to be verified by more high-quality studies, given the limitations regarding the number and quality of the included studies.
1 Introduction
PCOS, a complex reproductive endocrine and metabolic disorder, is more likely to affect women of childbearing age. Globally, its incidence ranges from 3–10% (1) A survey shows that 28 percent of women with PCOS in Spain are overweight (2), and most overweight patients suffer from insulin resistance (IR). In the long term, it not only affects the operation of the reproductive endocrine system but also increases the probability of developing problems such as metabolic syndrome, glycosuria (3), endometrial cancer, cardiovascular disease, and hypertension (4, 5). These health problems pose a significant risk to women’s overall well - being.
The pathogenesis of PCOS remains unknown. However, variables such as IR (insulin resistance), hyperandrogenemia (HA), genetics, endocrine disorder, obesity, inflammation, and immune system malfunction are considered to contribute to or aggravate PCOS (4, 6). IR leads to reduced responsiveness and sensitivity to insulin stimulation (7), which is a major factor in the development of the disease. In the insulin signaling pathway (8–10), the inflammatory response, oxidative stress, and gut flora are all related to the formation of IR. It has been found that IR is significantly associated with abnormalities in glucose and lipid metabolism, amino acid metabolism (11), HA, ovulation problems, and changes in the physiological function of the endometrium. In overweight or obese women with PCOS, IR is a factor contributing to excessive fat production and worsening obesity. Moreover, HA can exacerbate IR, which may lead to a vicious cycle of more severe symptoms (12).
PCOS is an endocrine and metabolic disorder. Thus, the key to its long-term management is to address the underlying endocrine and metabolic imbalances and implement appropriate treatment strategies. Therapeutic diets and nutritional supplements have been shown to have positive effects on reducing appetite, increasing satiety, promoting weight loss, and reducing fat mass. Currently, a wide variety of supplements are used in the treatment of obese patients with PCOS, such as inulin, CoQ10, thylakoid, carnitine, omega-3, vitamin D, fennel, quercetin, myo-inositol, synbiotic, green cardamom, folate, calcium, probiotic, green tea, and concentrated pomegranate juice (13), etc. A great deal of clinical research has demonstrated the potential of these supplements in treating insulin resistance, hypertension, oxidative stress, inflammation, and metabolic disorders associated with PCOS.
Regarding the technique and data consolidation, there have been no comprehensive and systematic studies comparing supplements with placebo alone. To determine whether supplements, as opposed to placebo alone, can potentially improve IR, hormonal status, and oxidative stress successfully, the main objective of this study was to conduct a comprehensive review and meta-analysis. In this way, overweight individuals with PCOS may have access to a more effective treatment alternative.
2 Methods
Throughout the process of conducting this systematic review, the Preferred Reporting of Items for Systematic Reviews and Meta-analyses (PRISMA) (14) checklist was used. This review has been registered with PROSPERO (CRD 42024544518). There is no review mechanism established for this specific review.
2.1 Requirements for participation in this study
2.1.1 Categories of individuals involved
This review included PCOS persons who met the diagnostic criteria for PCOS and had a Body Mass Index (BMI) >25 kg/m2. The participants were aged between 18 and 45. The 2003 Rotterdam criteria (15) were used for diagnosing PCOS, which involved one of the following signs and symptoms: (a) absent or infrequent menstrual cycles (at least eight cycles in the past year), (b) symptoms of excessive male hormone levels, either detected by laboratory tests or shown as clinical symptoms, and (c) the presence of polycystic ovaries as confirmed by ultrasound, characterized by more than twelve follicles with a diameter between two and nine millimeters and/or an ovarian volume exceeding ten milliliters. The study excluded those who met the following criteria: Participants were excluded if they were under 18 or over 45 years old, had a BMI of 25 kg/m² or less, had used certain medications or interventions within the past three months (such as oral contraceptives, hormone therapy, weight - loss interventions, probiotics, prebiotics, antacids, or antibiotics), had certain medical conditions (such as autoimmune diseases, cancer, cardiovascular disorders, abnormal liver function, kidney diseases, or other endocrine disorders), were allergic to placebo, adhered to specific diet or exercise programs, were current smokers, or were pregnant.
2.1.2 Types of interventions
The treatments included a variety of supplements, such as inulin, CoQ10, thylakoid, carnitine, omega-3, vitamin D, fennel, quercetin, myo-inositol, synbiotic, green cardamom, folate, calcium, probiotics, green tea and concentrated pomegranate juice.
2.1.3 Types of comparators
Comparators encompassed placebo.
2.1.4 Types of outcomes
The primary variables of interest were changes in indices related to IR (FPG, insulin, FI, HOMA-IR, HOMA-B, QUICKI), hormonal profile (total testosterone, testosterone, FSH, LH, SHBG, DHEAS), and oxidative stress (TAC, MDA). The secondary outcomes involved the assessment of anthropometric variables like weight, BMI, Waist-to-Hip Ratio (WHR), and waist circumference (WC). Moreover, the lipid profile markers measured were plasma triglycerides (TG), total cholesterol (TC), and high - density lipoprotein (HDL) cholesterol.
2.1.5 Types of study
This systematic review merely included RCTs.
2.2 Search strategy and study selection
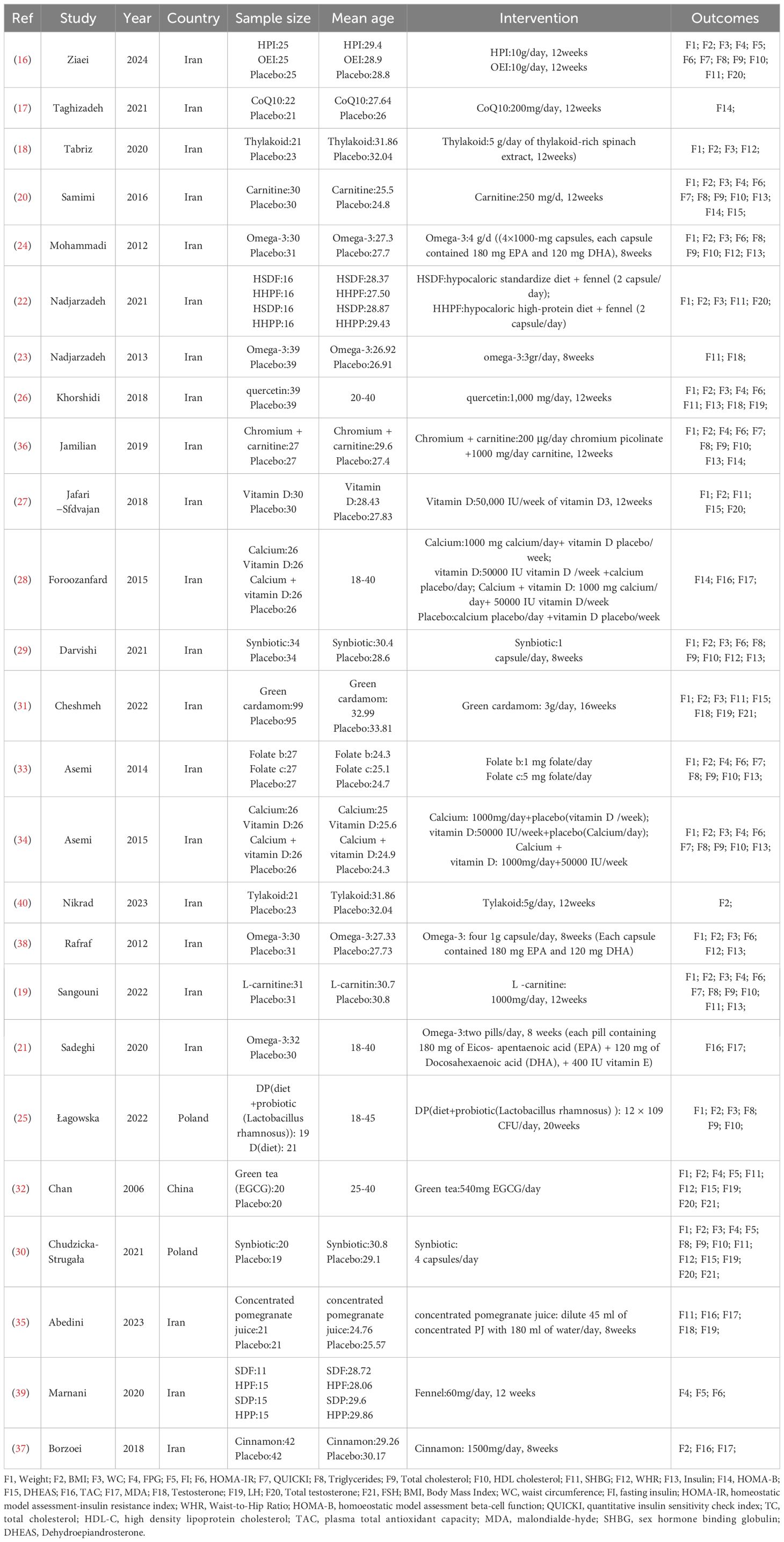
Several English-language databases, including PubMed, EMBASE, Cochrane, and Web of Science, were searched for relevant information. Additionally, information was gathered from ClinicalTrials.gov and relevant papers in reference lists. For this search in PubMed, medical subject headings (MeSH) and keywords such as “Polycystic Ovary Syndrome,” “Overweight,” and “Dietary Supplements” were used. The search covered the period from the inception of each database until March 2024. A comprehensive list of search strategies is provided in the Appendix. Initially, all retrieved titles and abstracts were imported into the EndNote X9 library, and duplicates were removed. Subsequently, the titles and abstracts of the papers were assessed, and then the full texts of the articles were examined to determine whether they met the inclusion criteria. Finally, the reference lists of the included papers and relevant reviews were scoured for new potentially useful research. The search and screening methods were independently carried out by two researchers, and any discrepancies were resolved through consultation with a third researcher. After that, a fourth researcher extracted key information from the studies included in the analysis. This information comprised the first author, the publication year, the sample size, the age of the participants, the treatments, the outcome measures, and other relevant elements (Table 1).
2.3 Assessment of methodological quality
The Cochrane Handbook V.5.1.0 was used to assess the risk of bias in RCTs. In case of any disagreements, two researchers (WL and FQL) carried out independent evaluations. If a consensus was needed, a third researcher (JXW) would participate. The evaluation covered seven aspects, namely: random sequence generation (related to selection bias), allocation concealment (also related to selection bias), blinding of implementers and participants (related to implementation bias), blinding of outcome assessors (related to observation bias), completeness of outcome data (related to follow-up bias), selective reporting of study results (related to reporting bias), and other biases. According to these criteria, each study was comprehensively evaluated. Studies that met all the criteria were classified as having a ‘low risk’ of bias, meaning they were of good quality with low overall bias. Those that only partially met the criteria were categorized as ‘unclear risk’, indicating a moderate chance of bias. Studies that did not meet any of the criteria were classified as ‘high risk’, suggesting poor quality and a high risk of bias.
2.4 Data extraction
During the data extraction process, two researchers independently utilized standardized forms. Should any discrepancies arise, a third researcher was tasked with resolving them. The standardized forms included the following information: authors, publication year, country, research design, subjects, diagnostic methods, sample size, interventions for experimental and control groups, duration, and results. Systematic evaluation encompassed intervention data across a range of parameters. The factors taken into account in this study consisted of various IR indicators (such as FPG, insulin, FI, HOMA-IR, HOMA-B, QUICKI, and DHEAS), hormonal status (such as Total Testosterone, Testosterone, FSH, LH, and SHBG), oxidative stress (such as TAC and MDA), anthropometric outcomes (such as weight, BMI, WHR, and WC), and lipid profiles (such as plasma TG, TC, and HDL). Subsequently, these factors were analyzed in the meta-analysis.
2.5 Statistical analysis
To conduct statistical analysis, Stata 15 (Stata Corporation, College Station, Texas, USA) was used. Firstly, clinical heterogeneity was evaluated. If present, the meta-analysis was terminated, and the results were reported descriptively through text and comprehensive tables. After eliminating clinical heterogeneity, the chi-square test was used to further assess the statistical heterogeneity of the subjects. Mean differences (MD) or SMD for continuous variables were calculated with 95% confidence intervals (CI) on each side. A significance threshold of p < 0.05 was used to declare statistical significance. Based on the goals of the investigation, an evaluation was made to determine the degree of similarity in clinical outcome indicators between the intervention group and the control group. The I2 test was applied to check for statistical heterogeneity. I2 values of 0%, 25%, 50%, and 75% represented no heterogeneity, low heterogeneity, medium heterogeneity, and high heterogeneity respectively. If the heterogeneity was less than 50%, the fixed-effects model was used. Additionally, the Egger test and the random-effects model were used to evaluate the publication bias involved.
3 Results
3.1 Study selection and description of study characteristics
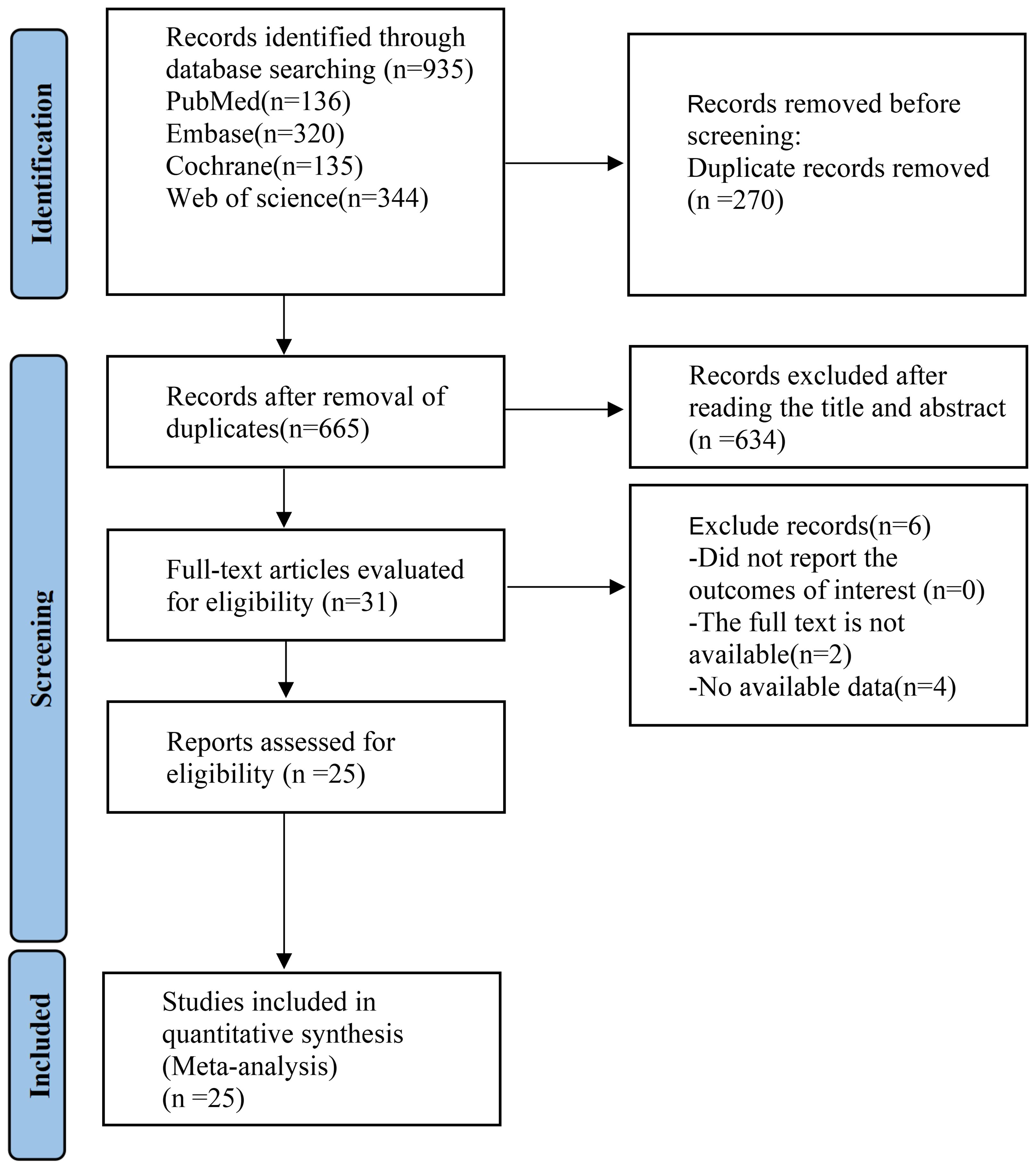
The PRISMA flow diagram provided an illustration of the selection procedure for the systematic review and meta-analysis. Initially, a total of 935 relevant research studies were evaluated. After multiple rounds of screening, 25 (16–40)studies were eligible for inclusion. The study included a cohort of 925 adults diagnosed with obesity and PCOS. These participants were given supplements as part of 25 RCTs (Figure 1). A summary of the included RCTs is shown in Table 1.
3.2 The quality of the methodology
The risk-bias assessment information for each RCT is presented in Figure 2. Since other bias is not mentioned in the article, it was regarded as unclear. 6 RCTs (16, 18, 21, 23, 39, 40) were assigned a relatively low risk of bias because information for each item (except other bias) was comprehensively reported. 19 RCTs (17, 19, 20, 22, 24–38) were given an uncertain risk of bias due to insufficient reporting. Among the included studies, 6 RCTs (25, 29, 32–35) lacked sufficient information regarding the random sequence generation method, which might have introduced selection bias. The allocation concealment information of 9 RCTs (24, 25, 29, 32–35, 37, 38) was incomplete, and the design and blinding implementation of 4 RCTs (22, 24, 35, 37) were not clear. Also, the blinding of 19 RCT (17, 19, 20, 22, 24–38) outcome assessors was unclear.
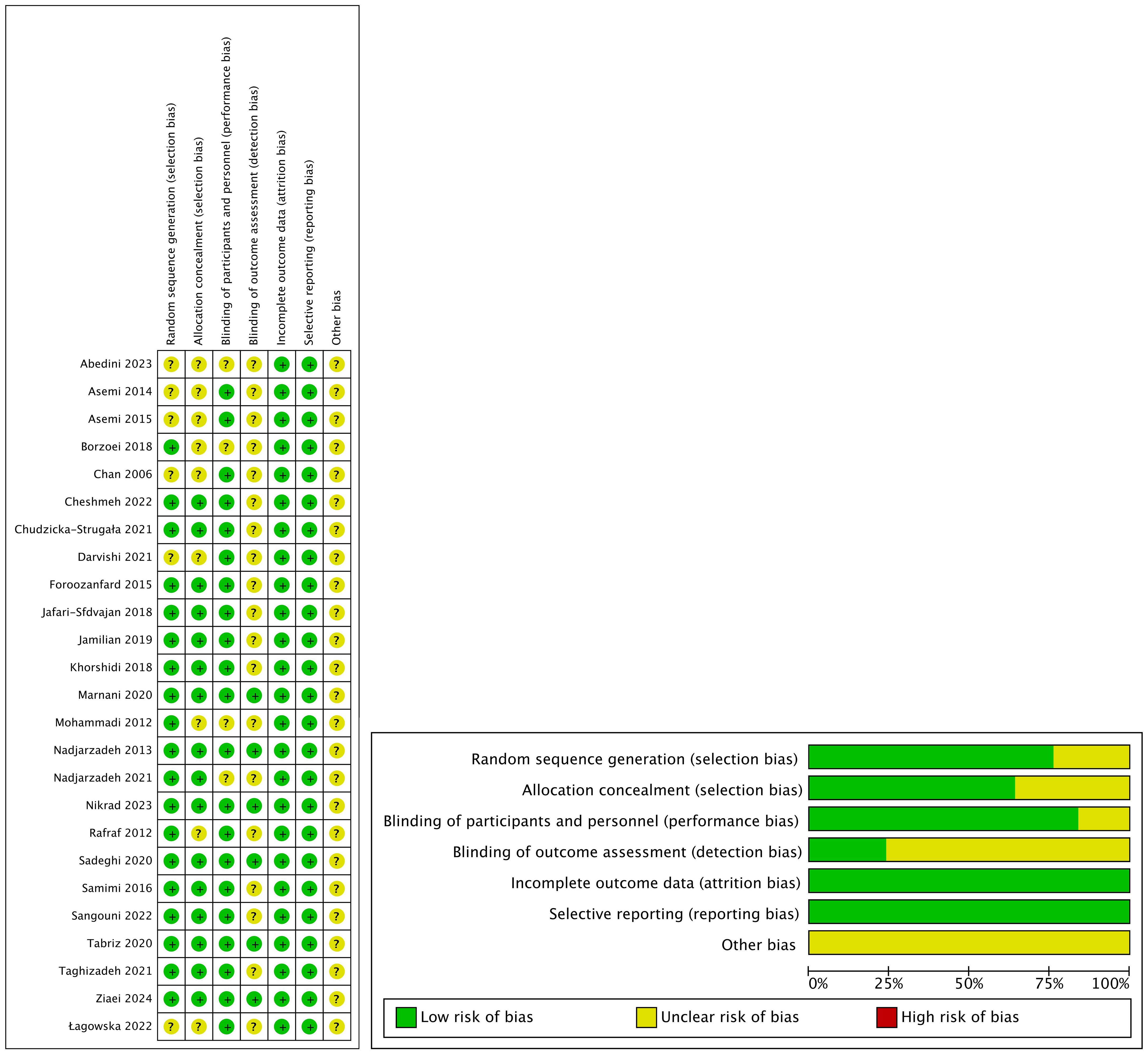
Figure 2. Risk of bias assessed in the included randomized controlled trials. Plus-sign represents ‘low risk’, Question mark represents ‘unclear risk’.
3.3 Impacts of interventions
3.3.1 FPG and insulin resistance related index
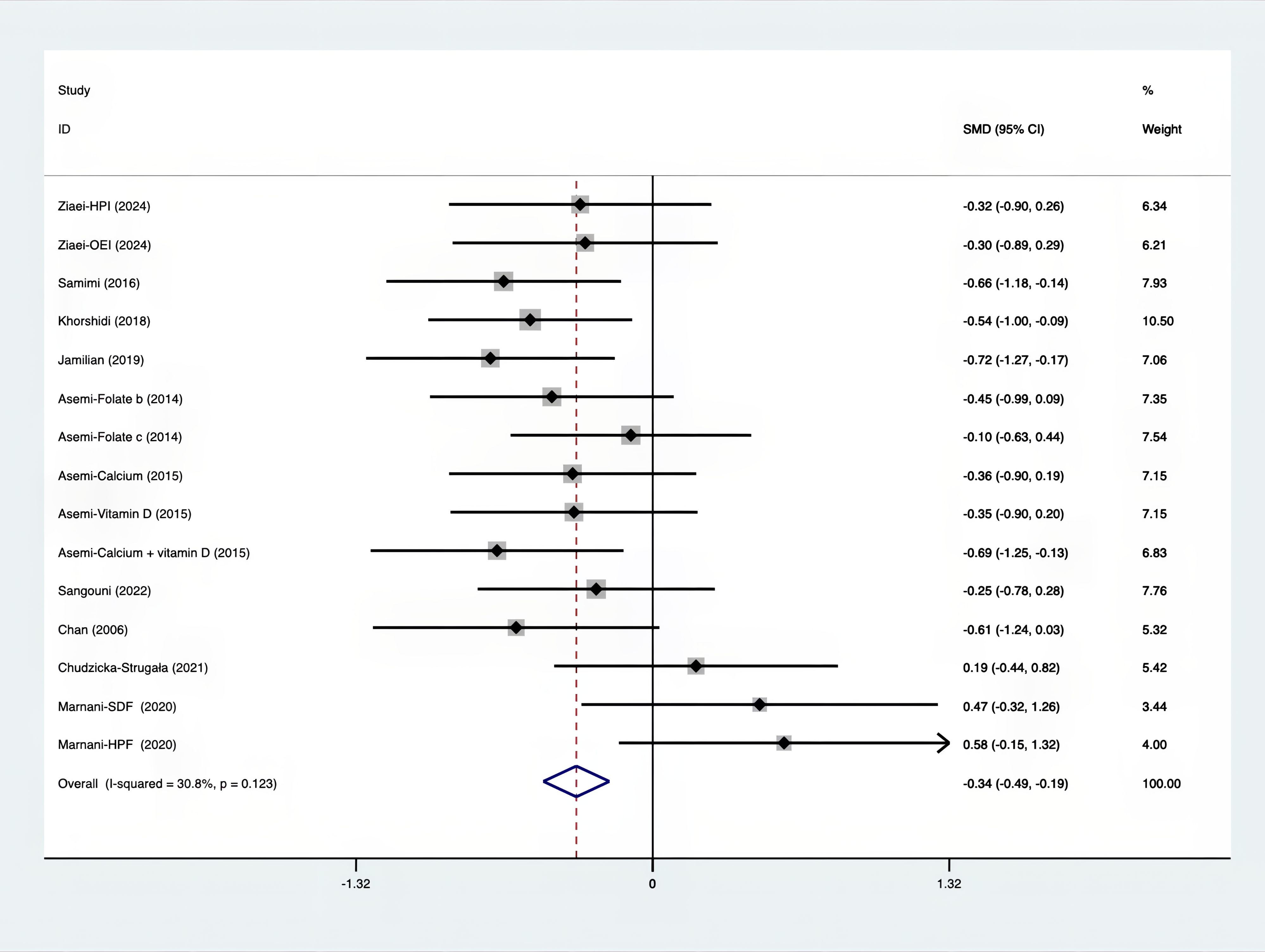
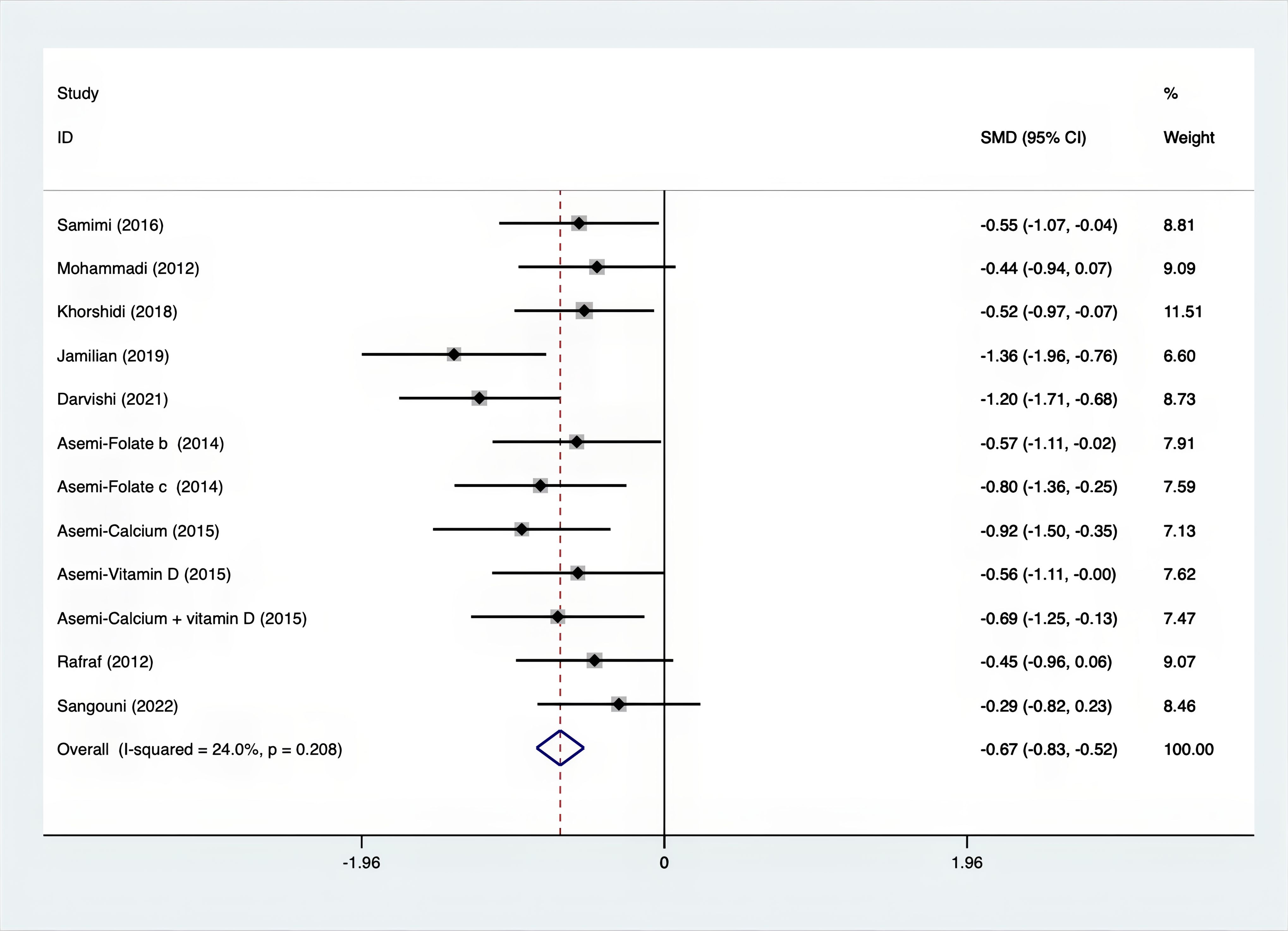
As shown in Figures 3–8, both the FPG, insulin, FI, HOMA-IR, HOMA-B, and QUICKI measurements were affected by the difference between the supplements and the placebo. In terms of FPG, insulin, FI, HOMA-IR, HOMA-B, and QUICKI, there was a statistically significant difference between the groups receiving the supplement and the group receiving the placebo. According to the SMD, for FPG it was -0.34 (95% CI, -0.49 to -0.19, p=0.123, I2 = 30.8%); for insulin -0.67 (95% CI, -0.83 to -0.52, p=0.208, I2 = 24%); for FI -0.26 (95% CI, -0.52 to -0.00, p=0.269, I2 = 21.9%); for HOMA-IR -0.59 (95% CI, -0.73 to -0.45, p=0.015, I2 = 48.7%); for HOMA-B -0.51 (95% CI, -0.75 to -0.27, p=0.547, I2 = 0%); and for QUICKI 0.94 (95% CI, 0.76 to -1.12, p=0.191, I2 = 27.5%). Based on these findings, compared with the use of a placebo, the use of supplements was associated with greater improvement in the markers of FPG, insulin, FI, HOMA-IR, and HOMA-B.
3.3.2 Hormonal functions related index
The SMD values for Total Testosterone, Testosterone, FSH, LH, SHBG, and DHEAS were provided in Figures 9–14. The effect sizes and statistical significance for the various hormones were as follows: Total Testosterone had an effect size of -0.61 (95% CI, -1.14 to -0.09, p=0.00, I2 = 78.5%); Testosterone had an effect size of -0.38 (95% CI, -0.86 to 0.10, p=0.03, I2 = 71.5%); FSH had an effect size of 0.16 (95% CI, -0.08 to 0.40, p=0.470, I2 = 0%); LH had an effect size of -0.56 (95% CI, -1.32 to 0.20, p=0.000, I2 = 91.1%); SHBG had an effect size of 0.35 (95% CI, 0.02 to 0.69, p=0.000, I2 = 78%); and DHEAS had an effect size of -0.27 (95% CI, -0.76 to 0.21, p=0.001, I2 = 78.7%). Supplements were more effective than placebo in reducing Total Testosterone levels and increasing SHBG levels. A sensitivity analysis was carried out by systematically removing each study one by one (see Supplementary Material S1). The analytical results showed the robustness of the findings, remaining consistent throughout the entire process.
3.3.3 Oxidative stress related index
The following was a list of the SMD of TAC and MDA, which were presented in Figures 15, 16: MDA was found to have a value of -0.57 (95% CI, -0.79 to -0.36, p=0.992, I2 = 0.0%), whereas TAC had a value of 0.87 (95% CI, 0.45 to 1.30, p=0.004, I2 = 71.3%). It was proven that supplements were superior to placebo in terms of MDA. A sensitivity analysis was conducted by systematically removing each study one by one in a step-by-step manner (Supplementary Material S2). The results of the study were confirmed to be reliable, as they remained consistently unchanged throughout the entire process.
3.3.4 anthropometric outcomes
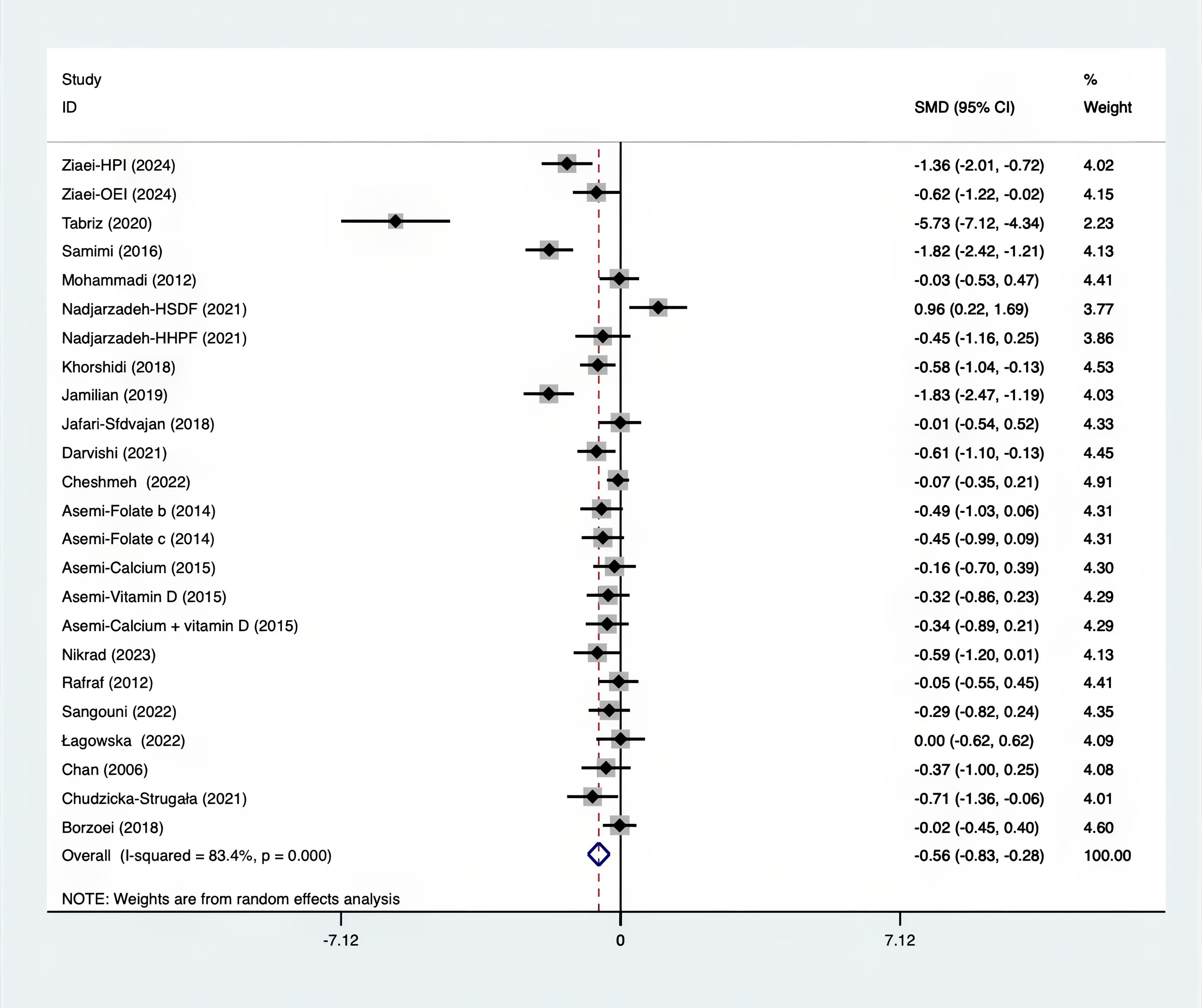
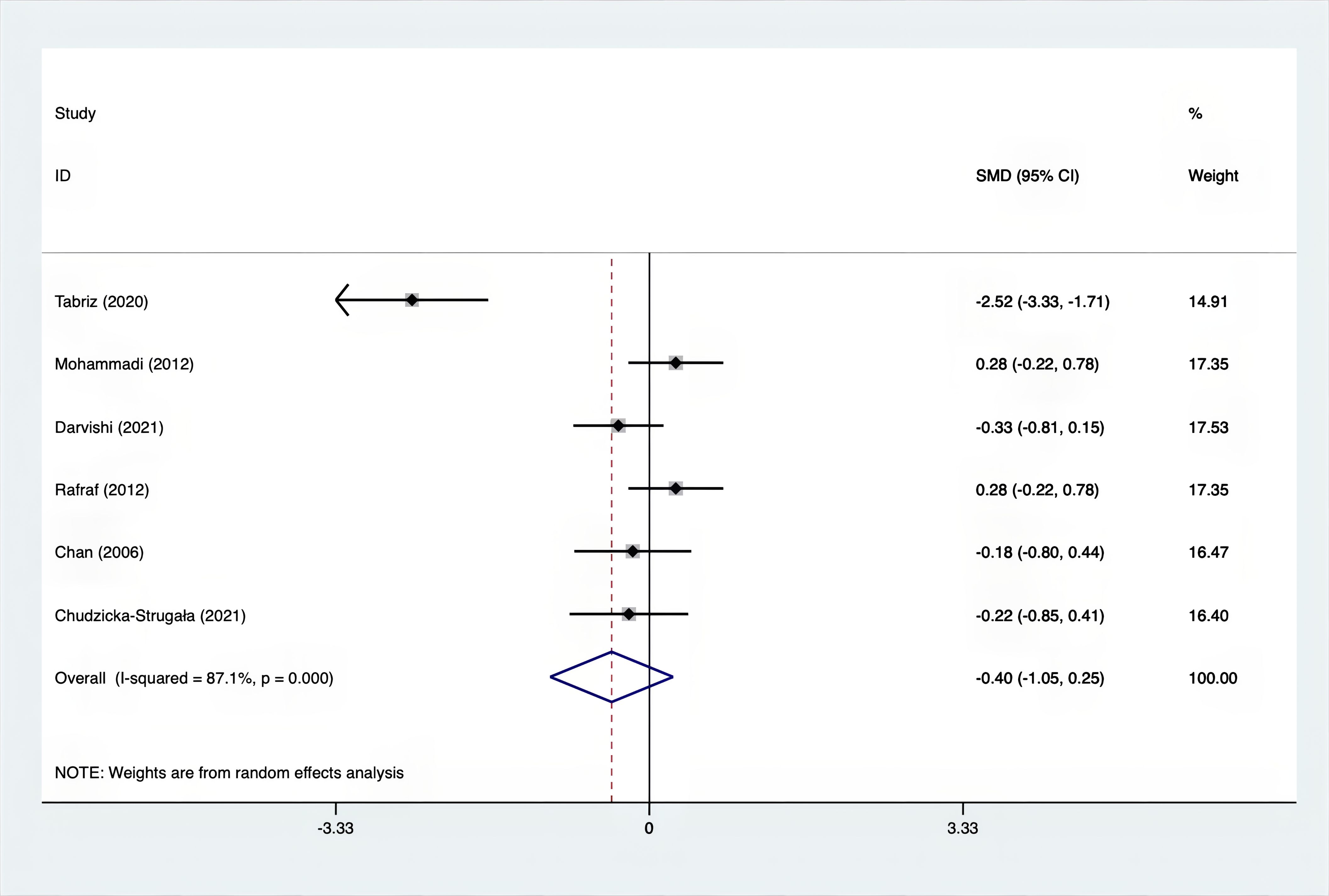
The SMD for weight, BMI, WHR and WC were as shown in Figures 17–20. The weight had a negative effect size of -0.59 (95% CI: -0.88 to -0.30, p=0.000, I2 = 83.5%). The BMI also had a negative effect size of -0.56 (95% CI: -0.83 to -0.28, p=0.000, I2 = 83.4%). The WHR had a relatively smaller negative effect size of -0.40 (95% CI: -1.05 to 0.25, p=0.000, I2 = 87.1%). Lastly, the WC had a negative effect size of -0.50 (95% CI: -0.74 to -0.27, p=0.000, I2 = 69.4%). Supplements showed outperformed placebo in relation to weight, BMI, WC, and weight. A sensitivity analysis was carried out by systematically excluding each study (Supplementary Material S3). The study results were proven to be robust because they remained consistent throughout the entire process.
3.3.5 Ester blood test indicators
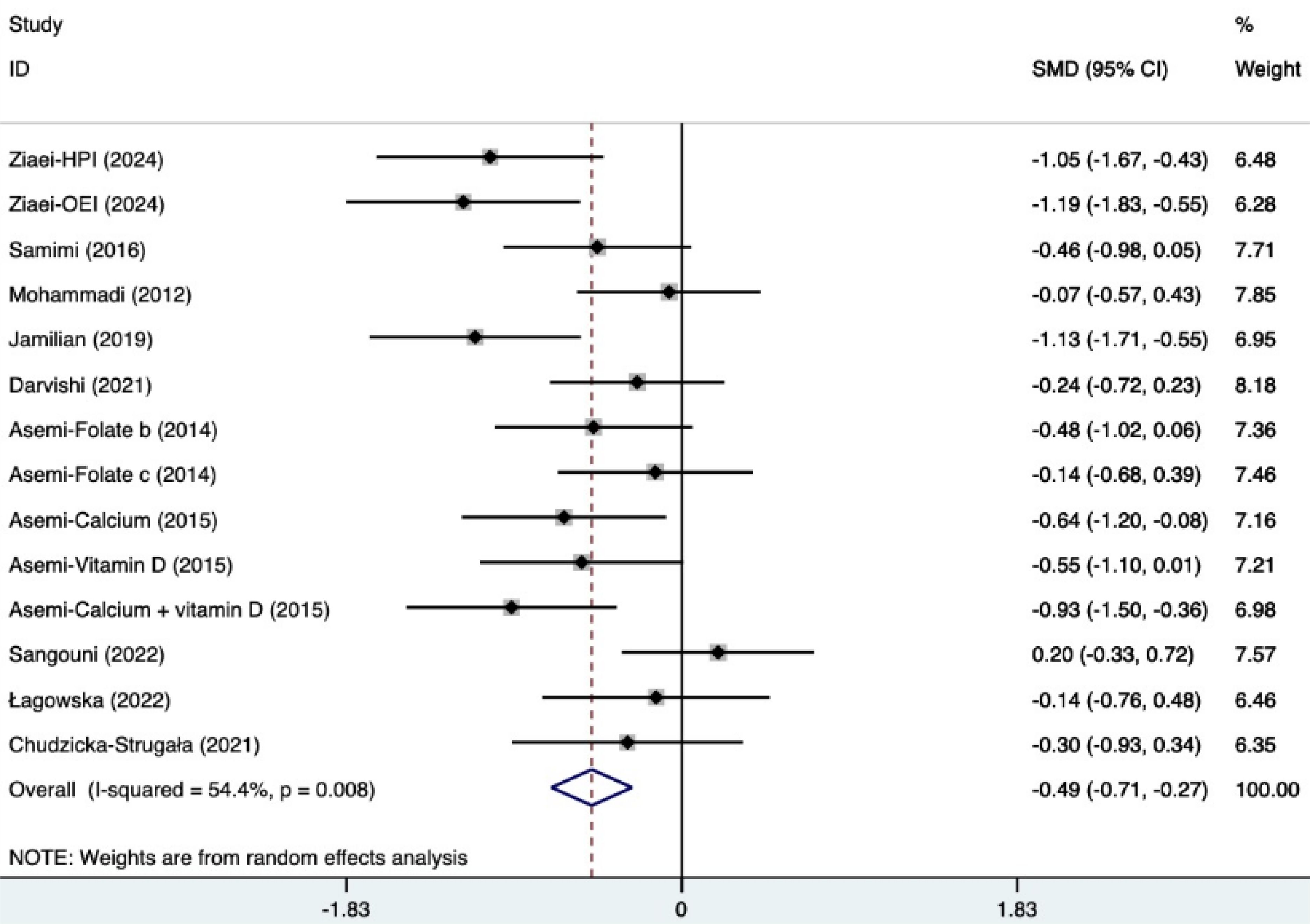
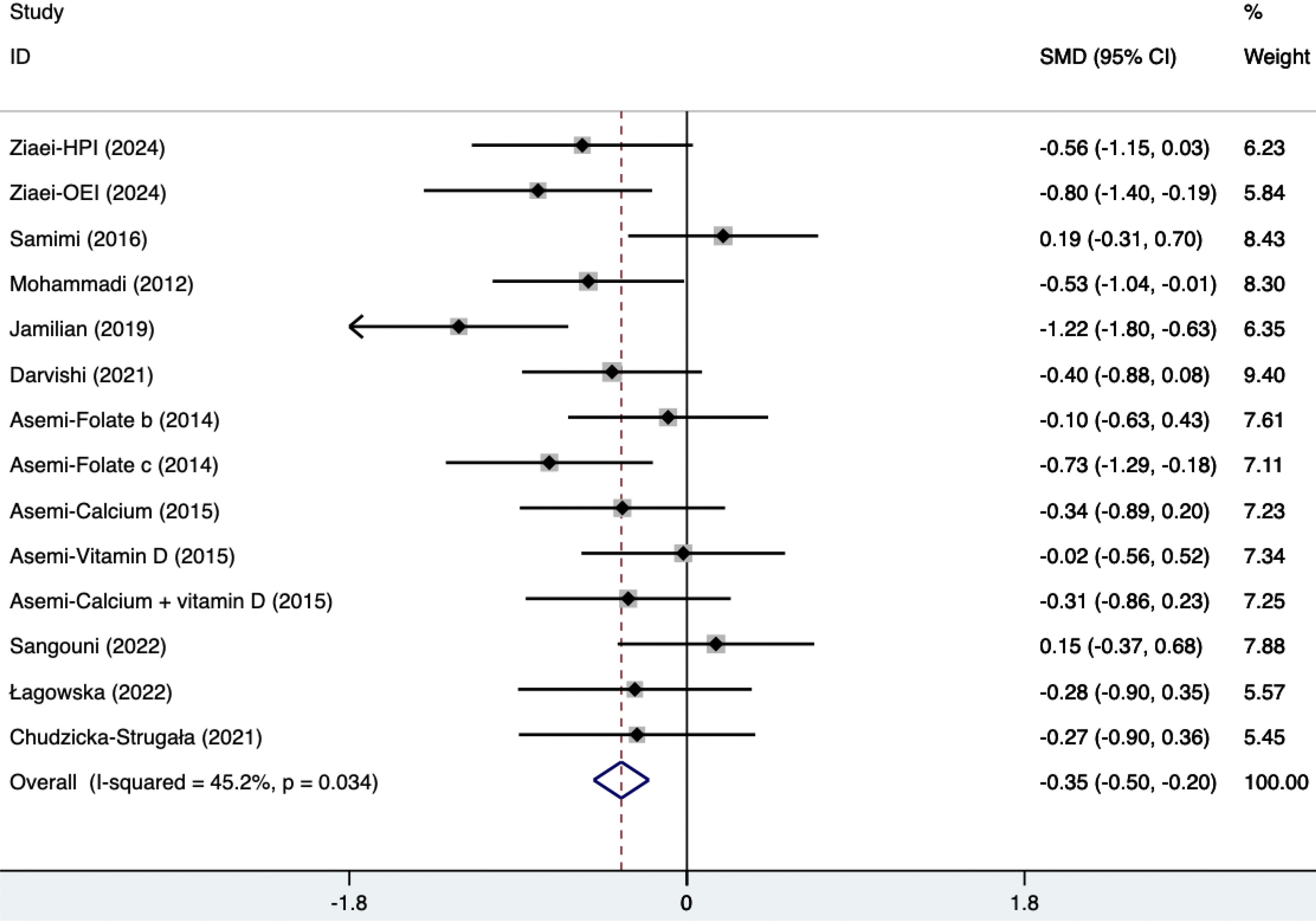
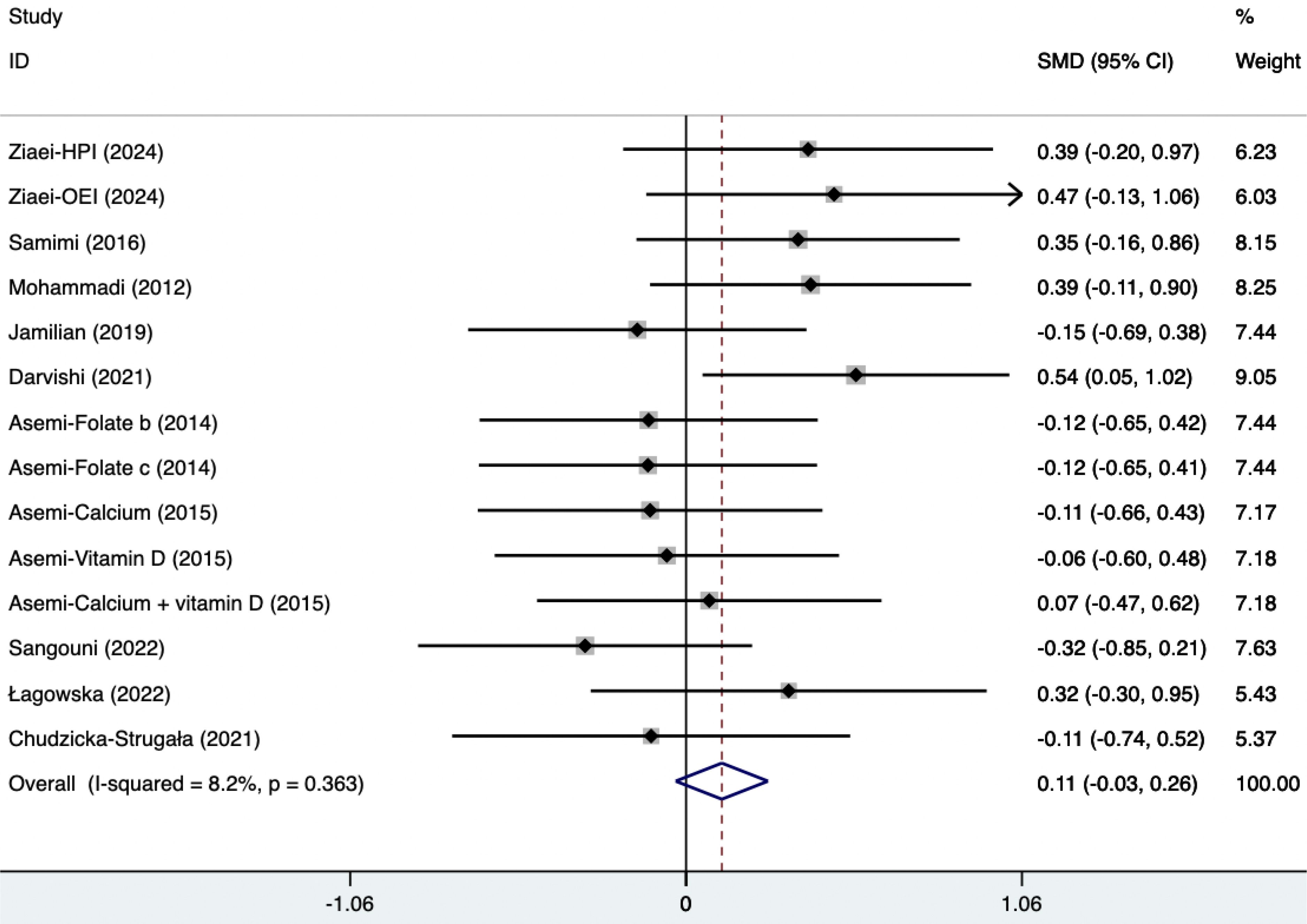
The SMD of plasma TG, TC, and HDL were as follows, which were shown in Figures 21–23: -0.49 (95% CI, -0.71 to -0.27, p=0.008, I2 = 54.4%) for plasma TG, -0.35 (95% CI, -0.50 to -0.20, p=0.034, I2 = 45.2%) for plasma TC, 0.11 (95% CI, -0.03 to 0.26, p=0.363, I2 = 8.2%) for plasma HDL. Supplements were better than placebo in plasma TG, TC, and HDL. A sensitivity analysis was performed by systematically omitting each study one by one (Supplementary Material S4). The analysis results showed the durability of the findings, as they remained stable throughout the entire process.
3.4 Publication bias
The Egger test was used to assess the presence of publication bias across a range of variables, such as FPG, insulin, FI, HOMA-IR, HOMA-B, QUICKI, DHEAS, Total Testosterone, Testosterone, FSH, LH, SHBG, TAC, MDA, Weight, BMI, WHR, WC, TG, TC, and HDL. The variables Weight (P=0.007), BMI (P=0.006), FPG (P=0.004), QUICKI (P=0.016), TG (P=0.042), DHEAS (P=0.018), and LH (P=0.029) were shown to be more susceptible to publication bias, as indicated by the results presented in Supplementary Figures 5A–G. On the other hand, the following outcomes: WC (P=0.062), FI (P=0.574), HOMA-IR (P=0.054), TC (P=0.187), HDL (P=0.746), SHBG (P=0.267), WHR (P=0.056), insulin (P=0.089), HOMA-B (P=0.320), TAC (P=0.338), MDA (P=0.598), Testosterone (P=0.485), Total Testosterone (P=0.402), and FSH (P=0.209) were found to have a lower likelihood of exhibiting publication bias (Supplementary Figures 6A–N).
4 Discussion
Groundbreaking research has been carried out to investigate the impact of supplements on overweight or obese individuals with PCOS. The inclusion of a greater number of research studies increases confidence in the conclusions, thereby contributing to a more comprehensive data set. Moreover, all the studies included in this paper use the Rotterdam criteria (2003) as the diagnostic criteria for PCOS. This helps to minimize variation in diagnostic criteria and reduce heterogeneity. IR is a crucial factor in the progression and manifestation of PCOS. Severe IR can significantly influence hyperandrogenemia, glucose-metabolism disorders, and ovulation disorders, etc (3). Additionally, when using contraceptives as part of medical treatments for PCOS in obese patients (4), caution should be taken, as hypoglycemic drugs are strongly associated with the occurrence of adverse side effects. Consequently, alternative treatments and adjuvant therapies for PCOS have been explored. In recent years, supplements have shown the potential to improve insulin resistance and alleviate long-term and short-term complications (41).
Compared with placebo, the results of this systematic review and meta-analysis suggest that supplements have a beneficial effect on IR, hyperandrogenism, oxidative stress, BMI, and lipid profiles (13) in overweight or obese individuals with PCOS. These findings are consistent with previous meta-analyses and some RCTs (42–44).
When analyzing supplement types, current evidence suggests that Carnitine, quercetin, Synbiotic, Folate, and Calcium may significantly reduce insulin levels compared to the baseline and placebo. It is also possible that Carnitine, Omega-3, quercetin, Synbiotic, Folate, and Calcium can reduce HOMA-IR relative to the baseline. Calcium might lower HOMA-B relative to the baseline, and Calcium, Omega-3, and Cinnamon could lower MDA relative to the baseline. We speculate that the improvement of insulin resistance by these supplements may be achieved through enhancing glucose and lipid metabolism and reducing oxidative stress (45). For instance, Omega-3 can increase insulin sensitivity by producing and secreting anti-inflammatory adipokines and decreasing inflammation and pro-inflammatory cytokines (46). There was high heterogeneity in Total Testosterone, Testosterone, LH, SHBG, and DHEAS. Further subgroup analysis (Supplementary Figures 7A–E) showed that synbiotic and inulin might significantly reduce Total Testosterone relative to the baseline, and concentrated pomegranate juice and inulin could significantly increase SHBG relative to the baseline. Although the forest maps indicated that supplements had no significant effect on Testosterone, LH, and DHEAS, a subgroup analysis (Supplementary Figures 7B, C, E) of different supplement types found that concentrated pomegranate juice may significantly reduce Testosterone relative to the baseline, and quercetin, green cardamom, and concentrated pomegranate juice may significantly reduce DHEAS relative to the baseline. We hypothesize that the mechanism by which the above supplements balance sex hormones is complex and may involve the androgen metabolic pathway, oxidative stress, and the balance of intestinal flora. For instance, Anthocyanins and ellagic acid derivatives in concentrated pomegranate juice function as antioxidants (47). Meanwhile, an animal study has shown that its antiandrogenic phenolic compounds may lower testosterone levels by inhibiting DHT receptor complexes and enhancing aromatase activity (48). In addition, prebiotics and probiotics are capable of regulating gut flora, suppressing inflammation, and ameliorating obesity or metabolic disorders, particularly in the bile acid metabolic pathway (49). It should be noted that although these findings appear to offer hope, they need to be interpreted with caution. Mainly because the risk of detection bias is not clear, the sample size is small, and most subgroups only included one study. Consequently, a large number of future studies are required to investigate the effects of supplements on obese PCOS.
The primary goal for managing PCOS is weight loss (44). As per the findings of Balen (50), a 5-10 percent weight loss can effectively restore ovulation and normal menstrual cycles in overweight or obese individuals. This is achieved by reducing blood insulin levels, increasing the levels of SHBG and insulin-like growth factor binding protein, and decreasing blood androgen levels. This article shows evidence that supplements like quercetin, synbiotic, and inulin have been proven effective in promoting weight reduction. However, omega-3, vitamin D, green cardamom, folate, calcium, probiotics, green tea, and thylakoid do not show significant differences from placebo, perhaps because of inconsistent baseline doses. Additionally, given the limited number of participants in these investigations, it may be essential to conduct trials with a larger sample size to get more conclusive results.
PCOS can manifest in a variety of clinical ways, such as infertility, metabolic abnormalities, and psychosocial problems. However, this paper mainly concentrates on endocrine function and metabolic disorders rather than infertility, which is a shortcoming of this study. Moreover, all the participants in this study were overweight or obese, and there were no participants of normal or low weight. Consequently, the results are more relevant to overweight and obese individuals with PCOS. It is crucial to note that endocrine and metabolic dysfunctions also exist in lean or normal-weight individuals with PCOS (5). Thus, it is suggested that future studies should include both obese and lean or normal-weight individuals with PCOS.
4.1 Limitations
This evaluation has certain limitations. First of all, the interventions varied greatly in terms of the type, form, dosage, and duration of the supplement, leading to a high degree of variability among the studies. Moreover, there were differences in the initial regulation of nutritional intake, including carbohydrates, proteins, and other components. The observed differences can be attributed to changes in the inclusion criteria, the types of lifestyle interventions used, and the length of these treatments. In view of this, it is important to recognize the potential presence of selection bias, performance bias, and follow-up bias, all of which pose further limitations.
4.2 Implications for practice
Recently, there has been an increasing amount of evidence indicating that supplements can be beneficial in enhancing insulin resistance and facilitating weight loss. However, because there are many different types of supplements to choose from and the number of participants in each supplement category is limited, further RCTs are necessary in the future to thoroughly investigate various metabolic markers, such as IR, hyperandrogenemia, and oxidative stress.
5 Conclusion
This study represents the first attempt to carry out a systematic review and meta - analysis in order to investigate the effects of supplement therapy on PCOS in overweight or obese individuals. Supplements are more effective than placebo in dealing with insulin resistance, androgenemia, oxidative stress, and lipid status in overweight or obese individuals with PCOS. However, our findings are specifically related to adults and are not applicable to children or the elderly.
Author contributions
XR: Writing – review & editing, Writing – original draft, Methodology. WW: Writing – review & editing, Supervision, Conceptualization. QL: Writing – original draft, Formal analysis, Data curation. WL: Writing – original draft, Methodology, Data curation. XW: Writing – original draft, Software, Data curation. GW: Writing – review & editing, Funding acquisition, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the application of retroperitoneal lymphatic tracing and prediction of lymph node metastasis in early cervical cancer (2022YFS0252).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1464959/full#supplementary-material
References
1. Wolf WM, Wattick RA, Kinkade ON, Olfert MD. Geographical prevalence of polycystic ovary syndrome as determined by region and race/ethnicity. Int J Environ Res Public Health. (2018) 15. doi: 10.3390/ijerph15112589
2. Lim SS, Davies MJ, Norman RJ, Moran LJ. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. (2012) 18:618–37. doi: 10.1093/humupd/dms030
3. Christ JP, Gunning MN, Meun C, Eijkemans MJC, van Rijn BB, Bonsel GJ, et al. Pre-conception characteristics predict obstetrical and neonatal outcomes in women with polycystic ovary syndrome. J Clin Endocrinol Metab. (2019) 104:809–18. doi: 10.1210/jc.2018-01787
4. Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. (2018) 14:270–84. doi: 10.1038/nrendo.2018.24
5. Gilbert EW, Tay CT, Hiam DS, Teede HJ, Moran LJ. Comorbidities and complications of polycystic ovary syndrome: An overview of systematic reviews. Clin Endocrinol (Oxf). (2018) 89:683–99. doi: 10.1111/cen.2018.89.issue-6
6. Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol. (2013) 6:1–13. doi: 10.2147/CLEP.S37559
7. Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. (2012) 33:981–1030. doi: 10.1210/er.2011-1034
8. Gutiérrez-Rodelo C, Roura-Guiberna A, Olivares-Reyes JA. Molecular mechanisms of insulin resistance: an update. Gac Med Mex. (2017) 153:214–28.
9. Li Y, Zheng Q, Sun D, Cui X, Chen S, Bulbul A, et al. Dehydroepiandrosterone stimulates inflammation and impairs ovarian functions of polycystic ovary syndrome. J Cell Physiol. (2019) 234:7435–47. doi: 10.1002/jcp.v234.5
10. Wu C, Jiang F, Wei K, Jiang Z. Exercise activates the PI3K-AKT signal pathway by decreasing the expression of 5α-reductase type 1 in PCOS rats. Sci Rep. (2018) 8:7982. doi: 10.1038/s41598-018-26210-0
11. Guo S. Insulin signaling, resistance, and the metabolic syndrome: insights from mouse models into disease mechanisms. J Endocrinol. (2014) 220:T1–t23. doi: 10.1530/JOE-13-0327
12. Sanchez-Garrido MA, Tena-Sempere M. Metabolic dysfunction in polycystic ovary syndrome: Pathogenic role of androgen excess and potential therapeutic strategies. Mol Metab. (2020) 35:100937. doi: 10.1016/j.molmet.2020.01.001
13. Günalan E, Yaba A, Yılmaz B. The effect of nutrient supplementation in the management of polycystic ovary syndrome-associated metabolic dysfunctions: A critical review. J Turk Ger Gynecol Assoc. (2018) 19:220–32.
14. Li Y, He J, Hu Y. Comparison of the efficiency and safety of total ankle replacement and ankle arthrodesis in the treatment of osteoarthritis: an updated systematic review and meta-analysis. Orthop Surg. (2020) 12:372–7. doi: 10.1111/os.12635
15. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. (2004) 19:41–7. doi: 10.1093/humrep/deh098
16. Ziaei R, Shahshahan Z, Ghasemi-Tehrani H, Heidari Z, Nehls MS, Ghiasvand R. Inulin-type fructans with different degrees of polymerization improve insulin resistance, metabolic parameters, and hormonal status in overweight and obese women with polycystic ovary syndrome: a randomized double-blind, placebo-controlled clinical trial. Food Sci Nutr. (2024) 12:2016–28. doi: 10.1002/fsn3.v12.3
17. Taghizadeh S, Izadi A, Shirazi S, Parizad M, Pourghassem Gargari B. The effect of coenzyme Q10 supplementation on inflammatory and endothelial dysfunction markers in overweight/obese polycystic ovary syndrome patients. Gynecological Endocrinol. (2021) 37:26–30. doi: 10.1080/09513590.2020.1779689
18. Tabrizi FPF, Farhangi MA, Vaezi M, Hemmati S. Changes of body composition and circulating neopterin, omentin-1, and chemerin in response to thylakoid-rich spinach extract with a hypocaloric diet in obese women with polycystic ovary syndrome: a randomized controlled trial. Phytotherapy Res. (2021) 2020:1–13.
19. Sangouni AA, Pakravanfar F, Ghadiri-Anari A, Nadjarzadeh A, Fallahzadeh H, Hosseinzadeh M. The effect of L-carnitine supplementation on insulin resistance, sex hormone-binding globulin and lipid profile in overweight/obese women with polycystic ovary syndrome: a randomized clinical trial. Eur J Nutr. (2022) 61:1199–207. doi: 10.1007/s00394-021-02659-0
20. Samimi M, Jamilian M, Ebrahimi FA, Rahimi M, Tajbakhsh B, Asemi Z. Oral carnitine supplementation reduces body weight and insulin resistance in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Clin Endocrinol. (2016) 84:851–7. doi: 10.1111/cen.2016.84.issue-6
21. Sadeghi F, Alavi-Naeini A, Mardanian F, Ghazvini MR, Mahaki B. Omega-3 and vitamin E co-supplementation can improve antioxidant markers in obese/overweight women with polycystic ovary syndrome. Int J vitamin Nutr Res Internationale Z fur Vitamin- und Ernahrungsforschung J Int vitaminologie Nutr. (2020) 90:477–83. doi: 10.1024/0300-9831/a000588
22. Nadjarzadeh A, Ghadiri-Anari A, Ramezani-Jolfaie N, Mohammadi M, Salehi-Abargouei A, Namayande SM, et al. Effect of hypocaloric high-protein, low-carbohydrate diet supplemented with fennel on androgenic and anthropometric indices in overweight and obese women with polycystic ovary syndrome: a randomized placebo-controlled trial. Complementary therapies Med. (2021) 56:102633. doi: 10.1016/j.ctim.2020.102633
23. Nadjarzadeh A, Dehghani Firouzabadi R, Vaziri N, Daneshbodi H, Lotfi MH, Mozaffari-Khosravi H. The effect of omega-3 supplementation on androgen profile and menstrual status in women with polycystic ovary syndrome: A randomized clinical trial. Iranian J Reprod Med. (2013) 11:665–72.
24. Mohammadi E, Rafraf M, Farzadi L, Asghari-Jafarabadi M, Sabour S. Effects of omega-3 fatty acids supplementation on serum adiponectin levels and some metabolic risk factors in women with polycystic ovary syndrome. Asia Pacific J Clin Nutr. (2012) 21:511–8.
25. Łagowska K. Drzymała-Czyż S: A low glycemic index, energy-restricted diet but not Lactobacillus rhamnosus supplementation changes fecal short-chain fatty acid and serum lipid concentrations in women with overweight or obesity and polycystic ovary syndrome. Eur Rev Med Pharmacol Sci. (2022) 26:917–26.
26. Khorshidi M, Moini A, Alipoor E, Rezvan N, Gorgani-Firuzjaee S, Yaseri M, et al. The effects of quercetin supplementation on metabolic and hormonal parameters as well as plasma concentration and gene expression of resistin in overweight or obese women with polycystic ovary syndrome. Phytotherapy research: PTR. (2018) 32:2282–9. doi: 10.1002/ptr.v32.11
27. Jafari-Sfidvajani S, Ahangari R, Hozoori M, Mozaffari-Khosravi H, Fallahzadeh H, Nadjarzadeh A. The effect of vitamin D supplementation in combination with low-calorie diet on anthropometric indices and androgen hormones in women with polycystic ovary syndrome: a double-blind, randomized, placebo-controlled trial. J endocrinological Invest. (2018) 41:597–607. doi: 10.1007/s40618-017-0785-9
28. Foroozanfard F, Jamilian M, Bahmani F, Talaee R, Talaee N, Hashemi T, et al. Calcium plus vitamin D supplementation influences biomarkers of inflammation and oxidative stress in overweight and vitamin D-deficient women with polycystic ovary syndrome: a randomized double-blind placebo-controlled clinical trial. Clin Endocrinol. (2015) 83:888–94. doi: 10.1111/cen.2015.83.issue-6
29. Darvishi S, Rafraf M, Asghari-Jafarabadi M, Farzadi L. Synbiotic supplementation improves metabolic factors and obesity values in women with polycystic ovary syndrome independent of affecting apelin levels: a randomized double-blind placebo - controlled clinical trial. Int J fertility sterility. (2021) 15:51–9.
30. Chudzicka-Strugała I, Kubiak A, Banaszewska B, Zwozdziak B, Siakowska M, Pawelczyk L, et al. Effects of synbiotic supplementation and lifestyle modifications on women with polycystic ovary syndrome. J Clin Endocrinol Metab. (2021) 106:2566–73. doi: 10.1210/clinem/dgab369
31. Cheshmeh S, Ghayyem M, Khamooshi F, Heidarzadeh-Esfahani N, Rahmani N, Hojati N, et al. Green cardamom plus low-calorie diet can decrease the expression of inflammatory genes among obese women with polycystic ovary syndrome: a double-blind randomized clinical trial. Eating weight Disord. (2022) 27:821–30. doi: 10.1007/s40519-021-01223-3
32. Chan CC, Koo MW, Ng EH, Tang OS, Yeung WS, Ho PC. Effects of Chinese green tea on weight, and hormonal and biochemical profiles in obese patients with polycystic ovary syndrome–a randomized placebo-controlled trial. J Soc Gynecologic Invest. (2006) 13:63–8.
33. Asemi Z, Karamali M, Esmaillzadeh A. Metabolic response to folate supplementation in overweight women with polycystic ovary syndrome: a randomized double-blind placebo-controlled clinical trial. Mol Nutr Food Res. (2014) 58:1465–73. doi: 10.1002/mnfr.201400033
34. Asemi Z, Foroozanfard F, Hashemi T, Bahmani F, Jamilian M, Esmaillzadeh A. Calcium plus vitamin D supplementation affects glucose metabolism and lipid concentrations in overweight and obese vitamin D deficient women with polycystic ovary syndrome. Clin Nutr (Edinburgh Scotland). (2015) 34:586–92. doi: 10.1016/j.clnu.2014.09.015
35. Abedini M, Ramezani-Jolfaie N, Ghasemi-Tehrani H, Tarrahi MJ, Amani R. The effect of concentrated pomegranate juice on biomarkers of inflammation, oxidative stress, and sex hormones in overweight and obese women with polycystic ovary syndrome: a randomized controlled trial. Phytotherapy research: PTR. (2023) 37:2255–61. doi: 10.1002/ptr.v37.6
36. Jamilian M, Foroozanfard F, Kavossian E, Kia M, Aghadavod E, Amirani E, et al. Effects of chromium and carnitine co-supplementation on body weight and metabolic profiles in overweight and obese women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial (Publication with expression of concern). Biol Trace Element Res. (2020) 193:334–41. doi: 10.1007/s12011-019-01720-8
37. Borzoei A, Rafraf M, Niromanesh S, Farzadi L, Narimani F, Doostan F. Effects of cinnamon supplementation on antioxidant status and serum lipids in women with polycystic ovary syndrome. J Tradit Complement Med. (2018) 8:128–33. doi: 10.1016/j.jtcme.2017.04.008
38. Rafraf M, Mohammadi E, Asghari-Jafarabadi M, Farzadi L. Omega-3 fatty acids improve glucose metabolism without effects on obesity values and serum visfatin levels in women with polycystic ovary syndrome. J Am Coll Nutr. (2012) 31:361–8. doi: 10.1080/07315724.2012.10720443
39. Marnani EH, Ghadiri-Anari A, Ramezani-Jolfaie N, Mohammadi M, Abdollahi N, Namayandeh SM, et al. Effect of fennel supplementation along with high-protein, low-carbohydrate weight-loss diet on insulin resistance and percentage of fat and muscle mass in overweight/obese women with polycystic ovary syndrome. J Funct Foods. (2020) 67.
40. Nikrad N, Ardekani AM, Farhangi MA, Tousi AZ, Tabrizi F, Vaezi M, et al. The effect of thylakoid membranes of spinach extract supplementation on atherogenic, glycemic, and anthropometric indices and renal function in obese PCOS women under a hypo-caloric diet: A randomized, double-blind, controlled trial. J Food Biochem. (2023) 2023. doi: 10.1155/2023/9408072
41. Alesi S, Ee C, Moran LJ, Rao V, Mousa A. Nutritional supplements and complementary therapies in polycystic ovary syndrome. Adv Nutr. (2022) 13:1243–66. doi: 10.1093/advances/nmab141
42. He J, Deng R, Wei Y, Zhang S, Su M, Tang M, et al. Efficacy of antioxidant supplementation in improving endocrine, hormonal, inflammatory, and metabolic statuses of PCOS: a meta-analysis and systematic review. Food Funct. (2024) 15:1779–802. doi: 10.1039/D3FO02824K
43. Mohd Shukri MF, Norhayati MN, Badrin S, Abdul Kadir A. Effects of L-carnitine supplementation for women with polycystic ovary syndrome: a systematic review and meta-analysis. PeerJ. (2022) 10:e13992.
44. Tefagh G, Payab M, Qorbani M, Sharifi F, Sharifi Y, Ebrahimnegad Shirvani MS, et al. Rezaei N et al: Effect of vitamin E supplementation on cardiometabolic risk factors, inflammatory and oxidative markers and hormonal functions in PCOS (polycystic ovary syndrome): a systematic review and meta-analysis. Sci Rep. (2022) 12:5770. doi: 10.1038/s41598-022-09082-3
45. Liao D, Liu X, Yuan X, Feng P, Ouyang Z, Liu Y, et al. Clinical evidence of the effects of carnitine supplementation on body weight, glycemic control and serum lipids in women with polycystic ovary syndrome: a systematic review and meta-analysis. Gynecol Endocrinol. (2022) 38:110–5. doi: 10.1080/09513590.2021.1988559
46. Jamilian M, Shojaei A, Samimi M, Afshar Ebrahimi F, Aghadavod E, Karamali M, et al. The effects of omega-3 and vitamin E co-supplementation on parameters of mental health and gene expression related to insulin and inflammation in subjects with polycystic ovary syndrome. J Affect Disord. (2018) 229:41–7. doi: 10.1016/j.jad.2017.12.049
47. Esmaeilinezhad Z, Babajafari S, Sohrabi Z, Eskandari MH, Amooee S, Barati-Boldaji R. Effect of synbiotic pomegranate juice on glycemic, sex hormone profile and anthropometric indices in PCOS: A randomized, triple blind, controlled trial. Nutr Metab Cardiovasc Dis. (2019) 29:201–8. doi: 10.1016/j.numecd.2018.07.002
48. Ibrahim MAA, Sadek MT, Sharaf Eldin HEM. Role of pomegranate extract in restoring endometrial androgen receptor expression, proliferation, and pinopodes in a rat model of polycystic ovary syndrome. Morphologie. (2022) 106:145–54. doi: 10.1016/j.morpho.2021.04.004
49. Li T, Zhang Y, Song J, Chen L, Du M, Mao X. Yogurt enriched with inulin ameliorated reproductive functions and regulated gut microbiota in dehydroepiandrosterone-induced polycystic ovary syndrome mice. Nutrients. (2022) 14. doi: 10.3390/nu14020279
Keywords: polycystic ovary syndrome, overweight, supplements, insulin resistance, hyperandrogenemian
Citation: Ren X, Wu W, Li Q, Li W, Wang X and Wang G (2024) Different supplements improve insulin resistance, hormonal functions, and oxidative stress on overweight and obese women with polycystic ovary syndrome: a systematic review and meta-analysis. Front. Endocrinol. 15:1464959. doi: 10.3389/fendo.2024.1464959
Received: 15 July 2024; Accepted: 16 October 2024;
Published: 11 December 2024.
Edited by:
Dimitrios Patoulias, Aristotle University of Thessaloniki, GreeceReviewed by:
Ashot Avagimyan, Yerevan State Medical University, ArmeniaPaschalis Karakasis, Aristotle University of Thessaloniki, Greece
Copyright © 2024 Ren, Wu, Li, Li, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Wang, d2FsbGFjZTE5NzFAMTYzLmNvbQ==
 Xiaoyan Ren
Xiaoyan Ren Wenjuan Wu
Wenjuan Wu