- 1The First Clinical Medical College of Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 2School of Basic Medical Sciences, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 3The Second Clinical Medical College of Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 4Department of Clinical Evaluation Center, The First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 5Department of Infectious Diseases, The First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
Objective: Current research suggests that irisin is closely linked to the pathogenesis and progression of metabolic dysfunction-associated fatty liver disease (MAFLD). This systematic review and meta-analysis updates our previous meta-analysis and further explores the relevance between circulating irisin levels and MAFLD.
Methods: Nine databases (PubMed, EMBASE, Cochrane Library, CNKI, Wanfang, Weipu, CBM, Clinicaltrials.gov and gray literature) were retrieved as of 1st August, 2024. The standardized mean difference (SMD) and 95% confidence interval (CI) represent pooled effect size. We used the Newcastle–Ottawa Scale to evaluate the quality of articles and the certainty of evidence assessed by GRADE system. All statistical analyses were performed using RevMan 5.3 and Stata 12(Stata Corporation, yi TX).
Results: Fifteen case-control studies were included. Circulating irisin levels in the MAFLD group were markedly lower than those in the healthy group (SMD=-1.04 [-1.93, -0.14]). Subgroup analyses by race, age, severity and T2DM revealed that circulating irisin levels were lower in the MAFLD group compared to those in the healthy controls in the Asian population (SMD=-1.38 [-2.44, -0.31], P<0.05) and in those above 50 years old (SMD=-2.23 [-3.64, -0.81], P<0.05) and higher in the mild MAFLD groups than those in moderate to severe MAFLD groups (SMD = 11.68 [9.05, 14.31], P<0.05). And the circulating irisin levels in MAFLD patients with T2DM were significantly lower than those in healthy group (SMD = -2.90 [-4.49, -1.30]). ELISA kits from different companies also presented different relationships.
Conclusions: There were significantly lower circulating irisin levels in the MAFLD group than in the healthy control group. Although these results differed from our previous results, there is no denying that circulating irisin levels are closely associated with the advancement of MAFLD.
Introduction
Recently, non-alcoholic fatty liver disease (NAFLD) has emerged as the most prevalent chronic liver disease, affecting nearly a quarter of the worldwide population (1). NAFLD is intensely affiliated with metabolic syndromes and was renamed metabolic dysfunction-associated fatty liver disease (MAFLD) in 2020 (2). This innovative designation focuses on the presence of metabolic comorbidities and steatosis (3). MAFLD can progress from relatively benign isolated hepatic steatosis (HS) to more severe non-alcoholic steatohepatitis (NASH), liver fibrosis, cirrhosis, and hepatocellular carcinoma (HCC). With changes in people’s lifestyle and dietary habits, the incidence of MAFLD has increased rapidly year after year (4). The average age of onset is also decreasing, and the prevalence of MAFLD in people under 60 years of age has gradually increased (by 17.8% from 2007 to 2010 and by 46.5% from 2015 to 2018) more than it has in people over 60 years of age (by 23.9% from 2007 to 2010 and by 30.9% from 2015 to 2018) (5). Currently, liver biopsy is still the gold standard for the diagnosis of NAFLD, but due to its invasiveness, sampling variability, high cost, and other limitations, its acceptance is low (6). The accuracy of imaging tests needs to be improved, and because the diagnostic ability of many molecular markers is also limited, more accurate biomarkers are needed to effectively predict MAFLD disease and its progression.
Irisin is a hormone-like myokine that is released by cleavage of the ectodomain of the transmembrane receptor fibronectin type III domain-containing 5 (FNDC5), which is conveyed in skeletal muscle and other tissues (7). Since the publication of Bostrom et al. (8) on irisin in 2012, it has rapidly become a research hotspot and has been related to a series of metabolic disorders and various liver diseases. Irisin can increase energy consumption by stimulating “browning” of white adipose tissue, participates in energy metabolism, and improves glucose homeostasis by lowering insulin resistance (9). In addition, irisin can also serve as an adipokine that regulates the metabolic rate of human adipose tissue (10). Recently, Armandi et al. (11) found that circulating irisin levels may serve as a potential biomarker for predicting NAFLD severity. In addition, other scientific studies have shown that circulating irisin levels may be negatively correlated with obesity, may also be associated with fat degeneration, and may decrease with liver damage (12). Furthermore, Zhu W. et al. (13) explained the important regulatory role of irisin in NAFLD at the mechanistic level using a high-fat diet (HFD)-induced NAFLD mouse model. Irisin is inextricably linked to the development of MAFLD, and its expression level may reflect the degree of disease.
Numerous scholars have explored the circulating irisin levels in MAFLD patients, but the results have been conflicting. We also performed a meta-analysis in 2020. However, because of the limited number of incorporated studies, the inadequate sample size of some studies, and the bias of selection of the study population, we updated the previous studies, increased the number of studies, and further detailed the inclusion criteria, hoping to obtain more comprehensive and accurate conclusions to facilitate clinical and scientific research, better assess the relationship between MAFLD and circulating irisin levels, and explore potential biomarkers and novel treatment targets for MAFLD.
2 Methods
2.1 Data sources and search strategy
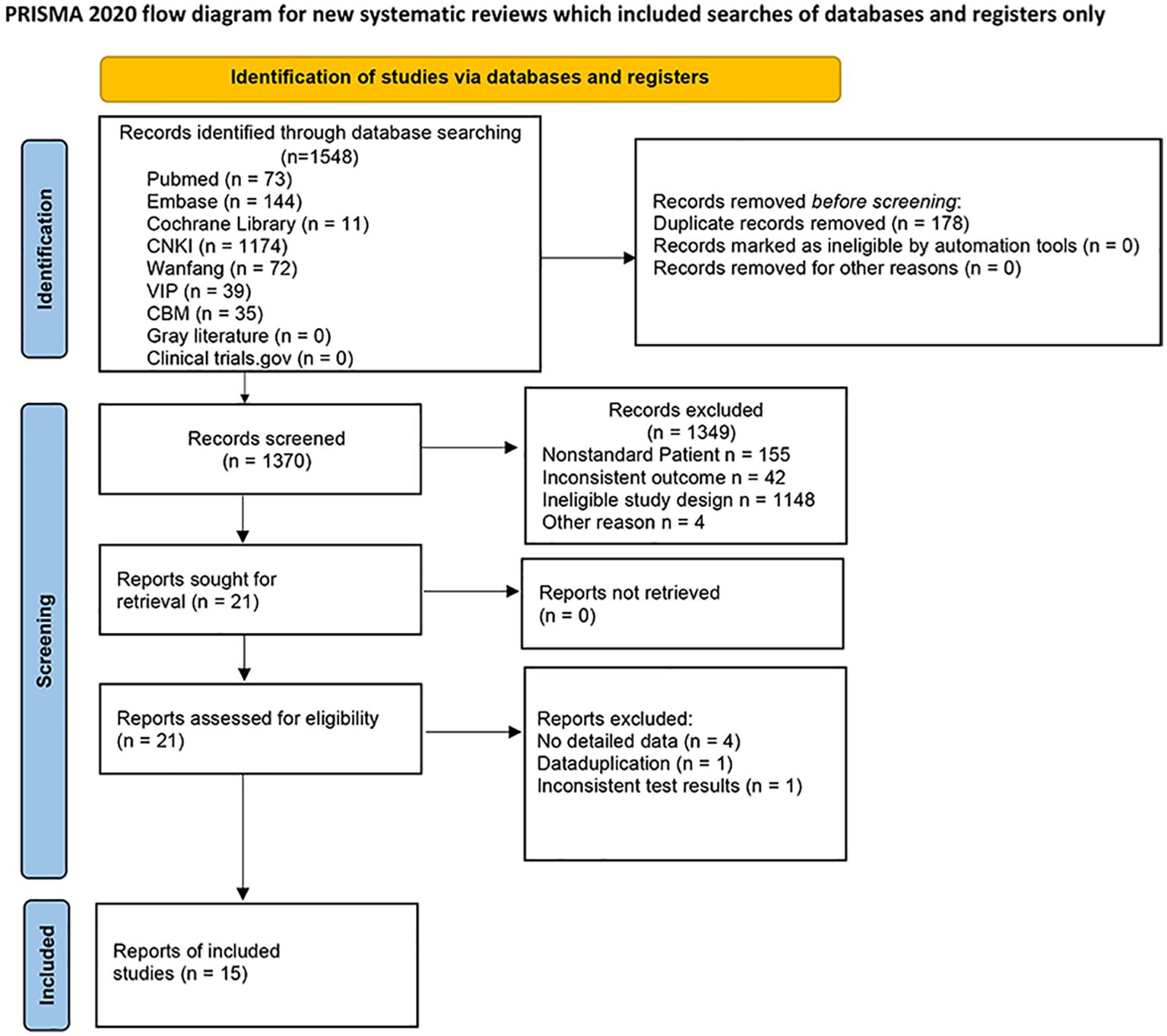
The study was proceeded in accordance with Cochrane Collaboration guidelines and reported based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria (Figure 1). The research protocol was registered in PROSPERO (number CRD42019130962) (Supplementary Material 1).
All articles originated from nine databases (PubMed, EMBASE, Cochrane Library, CNKI, Wanfang, Weipu, CBM, Clinicaltrials.gov, grey literature) until August 01, 2024. The following free and MeSH search terms were used: (“Non-alcoholic Fatty Liver Disease”, “NASH”, “Nonalcoholic fatty liver disease”, “Non alcoholic fatty liver disease”, “Non alcoholic Fatty Liver Disease”, “NAFLD”, “Nonalcoholic Fatty Liver Disease”, “Nonalcoholic Fatty Liver”, “Nonalcoholic Steatohepatitis”, “Nonalcoholic Steatohepatitides”, “fatty liver”, “Liver, Nonalcoholic Fatty”, “Steatohepatitides, Nonalcoholic”, “Steatohepatitis, Nonalcoholic”, “Metabolic Associated Fatty Liver Disease”, “MAFLD”, “Metabolic dysfunction-associated fatty liver disease”, “Metabolic associated steatohepatitis”, “MASH”, “Metabolically Associated Liver Steatosis”) and (“irisin”, “fibronectin type III domain containing protein 5”, “Fndc5 protein”, “FNDC5”, “FRCP2 protein”), The complete search strategy is fully elaborated in Supplementary Material 2. In addition, to ensure no omissions, relevant citations in the literature were searched. For documents with incomplete content, the authors were contacted via e-mail for more detailed data. No language restrictions were applied.
2.2 Inclusion and exclusion criteria
Each piece of literature was reviewed by two researchers individually, and the controversial parts were handed over to a third member for arbitration based on our protocol.
The inclusion criteria of studies were (1) patients aged 18 years or older with MAFLD, NAFLD, simple fatty liver (SFL), nonalcoholic fatty liver (NAFL) or NASH; (2) MAFLD diagnosed in accordance with significant hepatocellular steatosis on liver biopsy histology or diffuse fatty liver disease on imaging; (3) control group comprising of healthy individuals who don’t have any metabolic diseases; (4) circulating irisin levels from serum or plasma and (5) a case-control or cohort study design. The case group included patients with NAFLD or MAFLD, and the control group consisted of healthy individuals without NAFLD in case-control studies. In cohort studies, the groups were divided into high irisin level and low irisin level groups based on circulating irisin levels.
The exclusion criteria were detailed below: (1) studies including secondary liver fat accumulation caused by heavy alcohol drinking and other well-defined liver-damaging factors (such as hereditary factors, viruses, and drugs); (2) no comparison with healthy individuals; (3) full text that does not focus on circulating irisin levels (serum or plasma); (4) clinical randomized controlled trials, case reports, animal experimental studies, or literature review; (5) repetitive publications; (6) articles with insufficient data and obstruction in data retrieval due to corresponding authors who are unresponsive.
2.3 Data extraction
Two investigators separately fetched information which included the first author’s name, publication year, country, Newcastle–Ottawa Scale (NOS) score, diagnosis method of the disease, the number and basic information of the case and control groups (including age and body mass index [BMI]), diagnosis standards of MAFLD, circulating levels of irisin in the case and control groups, and Homeostasis model assessment of insulin resistance (HOMA-IR) levels of the case and control groups.
2.4 Quality evaluation and risk of bias
The included studies were evaluated employing the NOS (14), which consists of selection, comparability, and exposure. The Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) approach provides guidance for assessing the quality of the study (https://gdt.gradepro.org). For publication bias, the Egger’s test (15) was used. Quality appraisal was carried out autonomously by two researchers, and the results would be validated by a third researcher if there was any divergence.
2.5 Statistical analysis
Revman 5.3 and Stata 12 (Stata Corporation, College Station, TX, USA) were utilized for statistics analyses. Normally distributed data were extracted as mean ± Standard Deviation (SD), and those with a non-normal distribution were transformed to the mean ± SD (https://www.math.hkbu.edu.hk/~tongt/papers/median2mean.html) (16–18).
We utilized the standardized mean difference (SMD) to extract all circulating levels of irisin as continuous variables. The heterogeneity level was estimated on basis of the P-value and I2 test. When there was observed significant statistical heterogeneity (I2 ≥ 50%), the random effects model (shown as “D+L”) was recommended (19, 20). In the absence of significant statistical heterogeneity (I2 < 50%), the fixed effects model (shown as “I-V”) was adopted. Subgroup analysis and meta-regression were applied to identify the fundamental causes of high heterogeneity. Subgroup analysis were executed from several perspectives, including race, BMI, age, HOMA-IR, NOS score, severity, ELISA kits and presence or absence of T2DM. As BMI ≥25 kg/m2 was considered overweight, patients were classified into two subgroups in accordance with BMI≥25 and BMI <25 kg/m2 (21). For univariate meta-regression results showing P < 0.1 (22), it is meaningful to explain the partial heterogeneity of our study. Sensitivity analysis was conducted by omitting each study at a time.
3 Results
3.1 Study selection
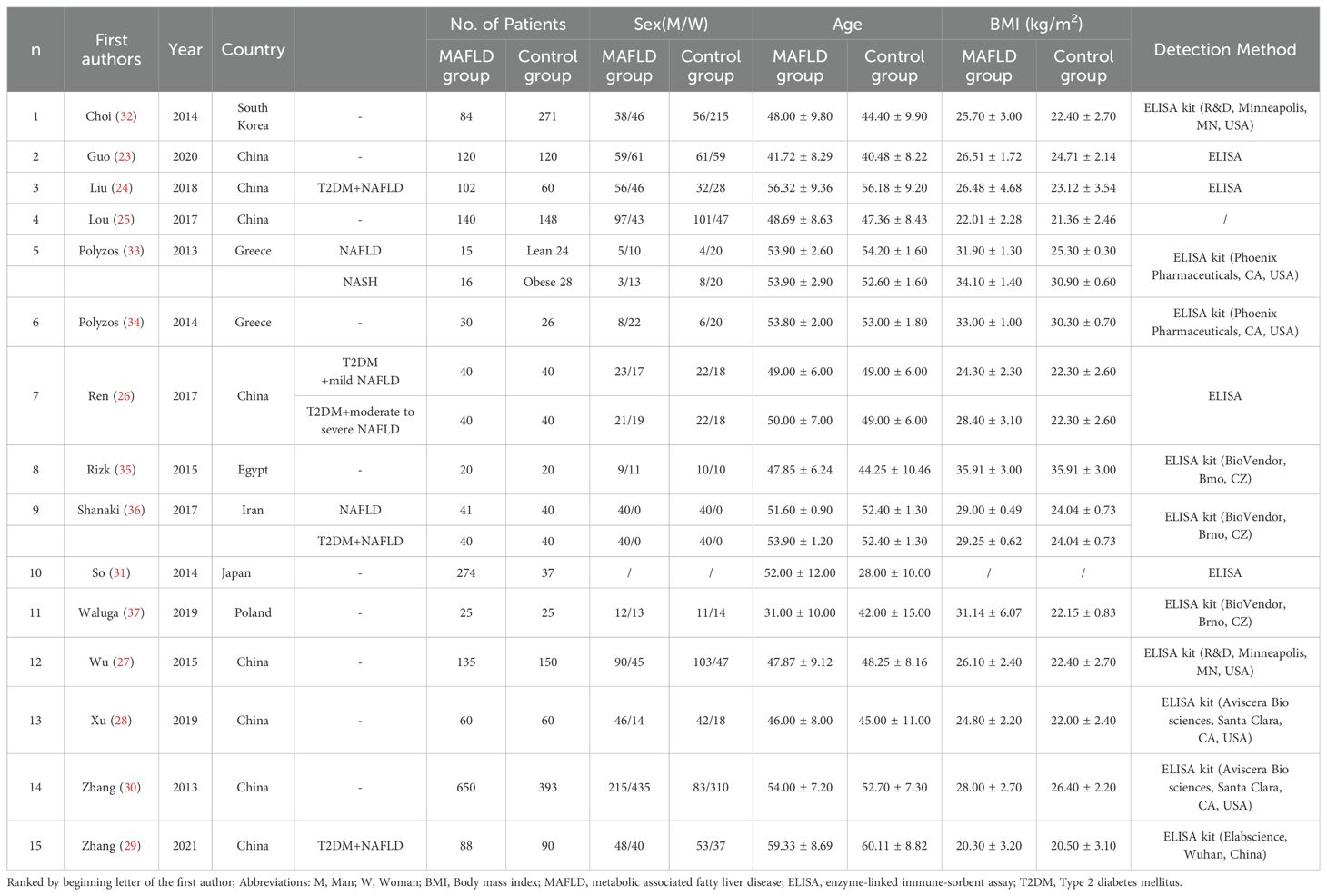
The initial search yielded 1548 articles. After removing duplicates and screening titles, abstracts, and entire texts according to the inclusion and exclusion criteria, 15 studies were included in the data analysis (23–37). The detailed literature screening flow is illustrated in Figure 1. Fifteen studies were included in the meta-analysis. Eight studies were conducted in China (23–30), two in Greece (33, 34), one in South Korea (31), one in Iran (36), one in Poland (37), one in Japan (32), and one in Egypt (35). All the studies followed a case-control study design. The patient and control groups were not restricted by sex, and the average age ranged from 33 to 68 years. The BMI of patients with MAFLD ranged from 20.3 to 35.91 kg/m2, whereas that of healthy controls had a range of 20.5 to 30.9 kg/m2 (details are shown in Table 1).
3.2 Quality assessment
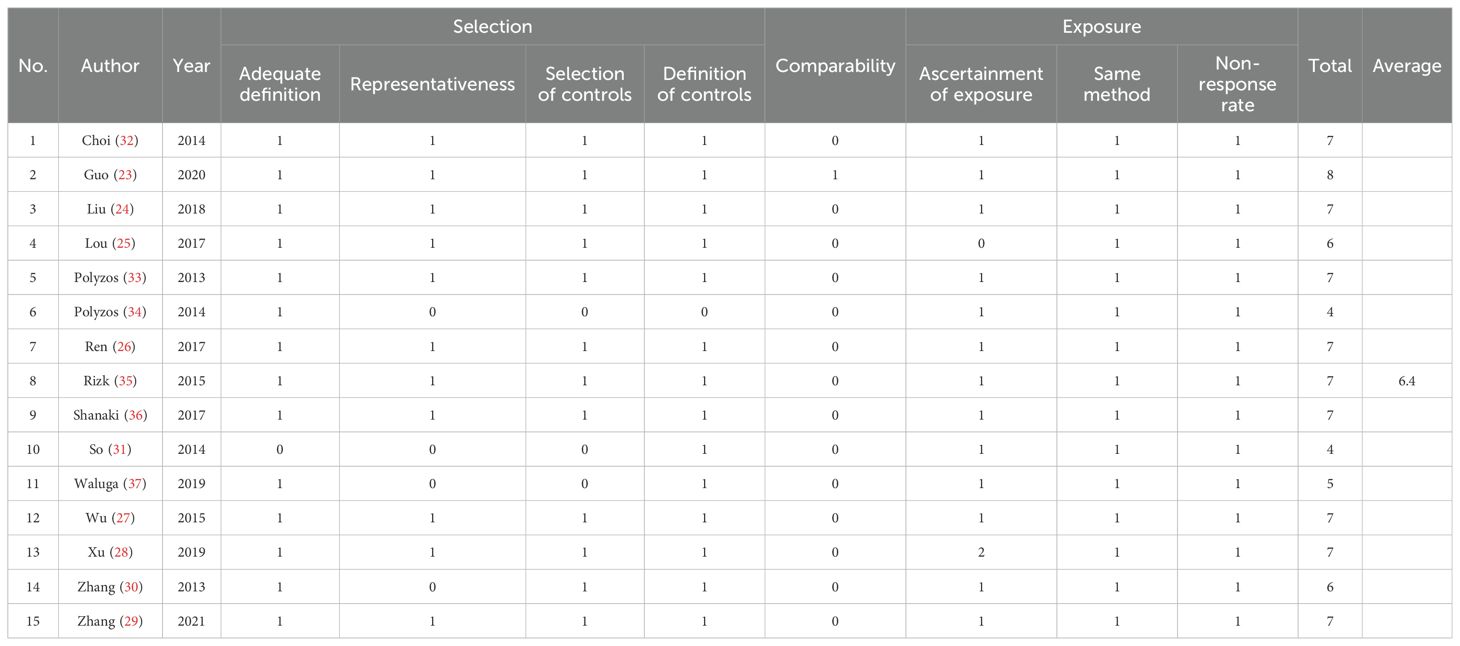
The average NOS score for all included studies was 6.4, indicating that most of the studies were of high quality. Except for the studies by So et al. (32), Lou et al. (25), Zhang et al. (30), Waluga et al. (37), and Polyzos et al. (2014) (33), the other 10 studies all scored 7 or higher. The representativeness of cases and the selection or definition of controls were not adequately described, which is a problem for the three articles with scores below 7 (Table 2). The GRADE approach results indicated that the general quality of evidence was exceedingly low (shown in Supplementary Material 3). Observational studies were scored low. Significant heterogeneity among the included studies led to serious inconsistencies (I2 > 90%). In addition, the basic information of the case and control groups in some articles was not strictly matched, which also has an impact on the determination of the evidence.
3.3 Association between circulating irisin levels and MAFLD
As the results of the pooled meta-analysis (Figure 2) showed significant heterogeneity (I2 = 99%, P < 0.001), a random-effects model was used. The results demonstrated that in the MAFLD group had significantly lower circulating irisin level than the healthy group, with an overall SMD (95% confidence interval [CI]) of -1.04 (-1.93, -0.14) (Figure 2). Simultaneously, we performed heterogeneity analyses using the Galbraith test, which suggested that fewer than 50% of the studies were within an acceptable limit (Figure 3). Consequently, it was necessary to further explore potential reasons of heterogeneity through subgroup analysis and meta-regression.
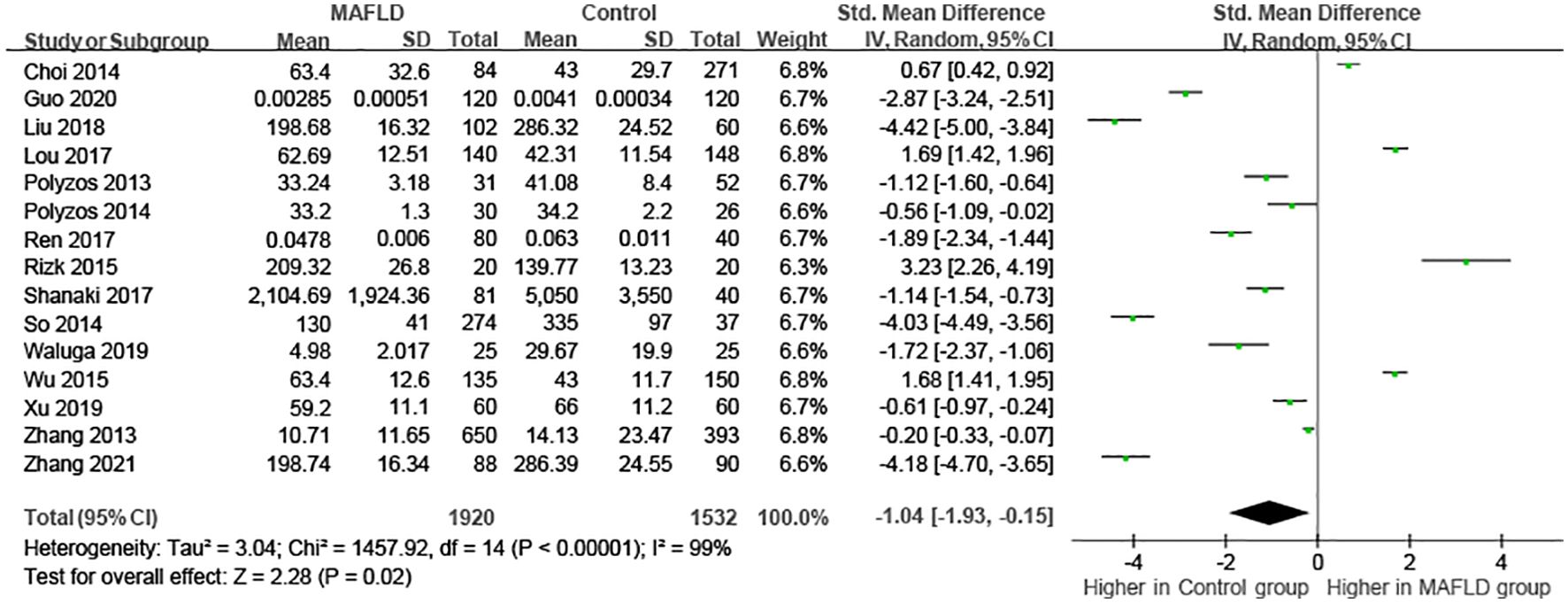
Figure 2. Forest plot of circulating irisin levels between MAFLD and the healthy control group (Random-Effects Model, SMD).
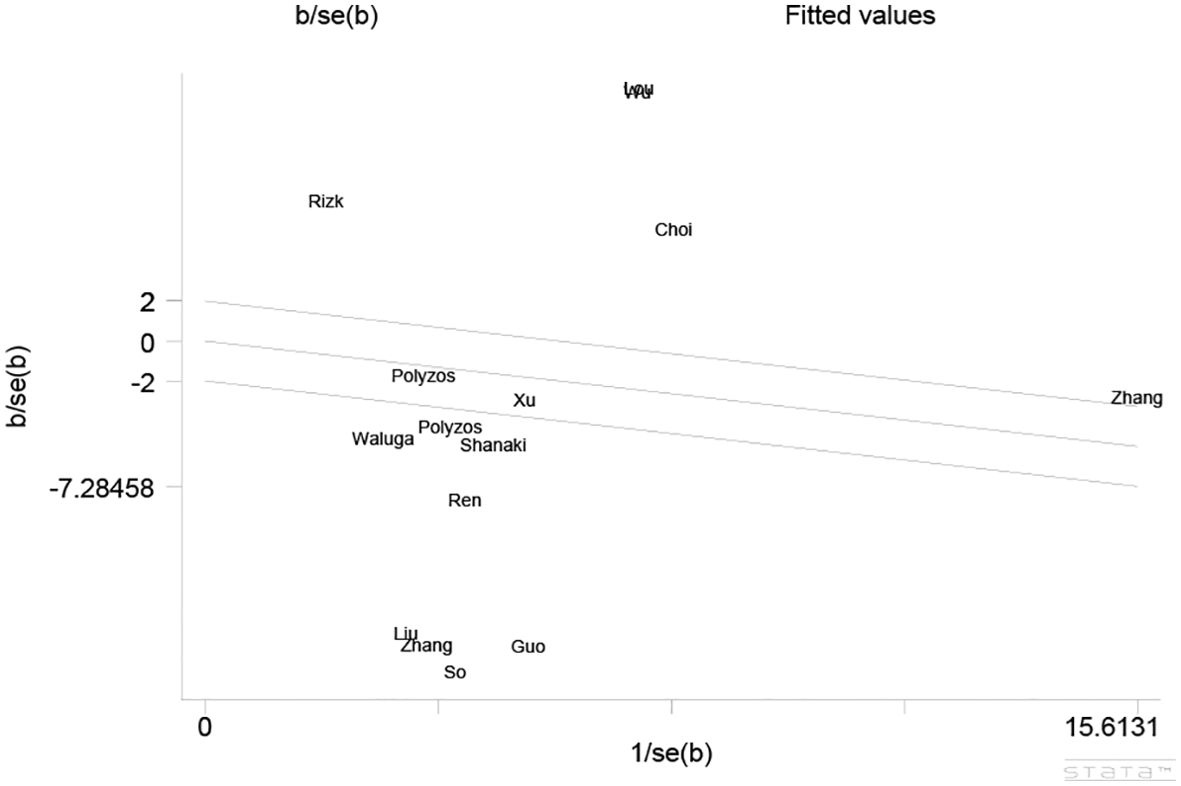
Figure 3. Galbraith Test result of circulating irisin levels between MAFLD and the healthy control group.
3.4 Subgroup analysis
The results of the subgroup analysis are shown in Table 3, with high heterogeneity accounting for the use of the random-effects model. Subgroup analyses were performed in light of race, BMI, age, HOMA-IR, NOS, disease severity, different ELISA kits and presence or absence of T2DM (forest plot of subgroups is shown in Supplementary Material 4). Most subgroup results showed no prominent variation in circulating irisin levels comparing the MAFLD group to healthy controls. However, it is important to note that the findings from subgroup analysis stratified by race indicated that in Asian populations, circulating irisin levels were lower in the MAFLD group than in healthy controls, with an overall SMD (95% CI) of -1.38 (-2.44, -0.31). Subgroup analysis results by age showed that in patients aged >50 years, circulating irisin levels in the MAFLD group were lower than those in the healthy group (SMD = -2.23 [-3.64, -0.81]), and subgroup analyses classified by severity revealed that higher circulating irisin levels were found in both mild and moderate to severe MAFLD groups than in the healthy group (mild: SMD =1.69 [0.80, 2.57]; moderate to severe: SMD = 1.03 [0.77, 1.29]). In addition, we also compared mild MAFLD with moderate to severe MAFLD and showed that the circulating irisin levels in the mild MAFLD group were observed higher as compared to those in the moderate to severe MAFLD group (SMD = 11.68 [9.05, 14.31]). Besides, we also performed a subgroup analysis based on inclusion or exclusion of T2DM, and the results showed that the circulating irisin levels in MAFLD patients with T2DM were significantly lower than those in healthy group (SMD = -2.90 [-4.49, -1.30]). Moreover, ELISA kits had significant influence on the results. Irisin detected with ELISA kits (Aviscera Bio sciences, Santa Clara, CA, USA; BioVendor, Bmo, CZ; Unspecified) were irrelevant (SMD = -0.37 [-0.76, 0.03]; SMD = 0.08 [-2.21, 2.38]; SMD = -2.30 [-4.82, 0.22]), ELISA kits produced by R&D, Minneapolis, MN, USA was positively correlated (SMD = 1.17 [0.18, 2.16]), whereas the ELISA kits (Phoenix Pharmaceuticals, CA, USA; Elabscience, Wuhan, China) are lower than in healthy people (SMD = -0.85 [-1.40, -0.30]; SMD = -4.18 [-4.70, -3.65]). However, no clear source of heterogeneity was identified in subgroup analyses.
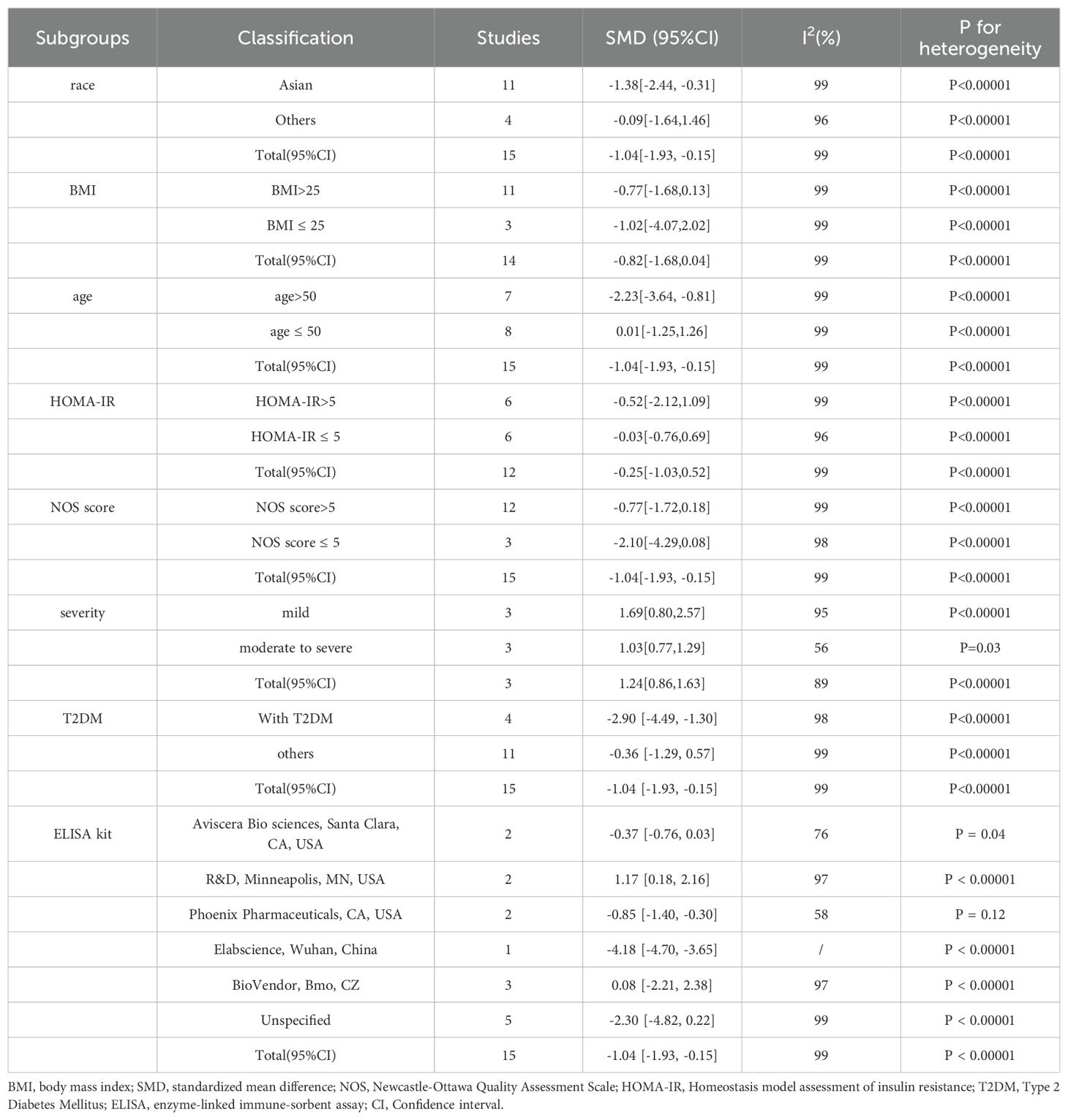
Table 3. Subgroup analysis of the circulating irisin levels in MAFLD patients compared with controls.
3.5 Meta-regression
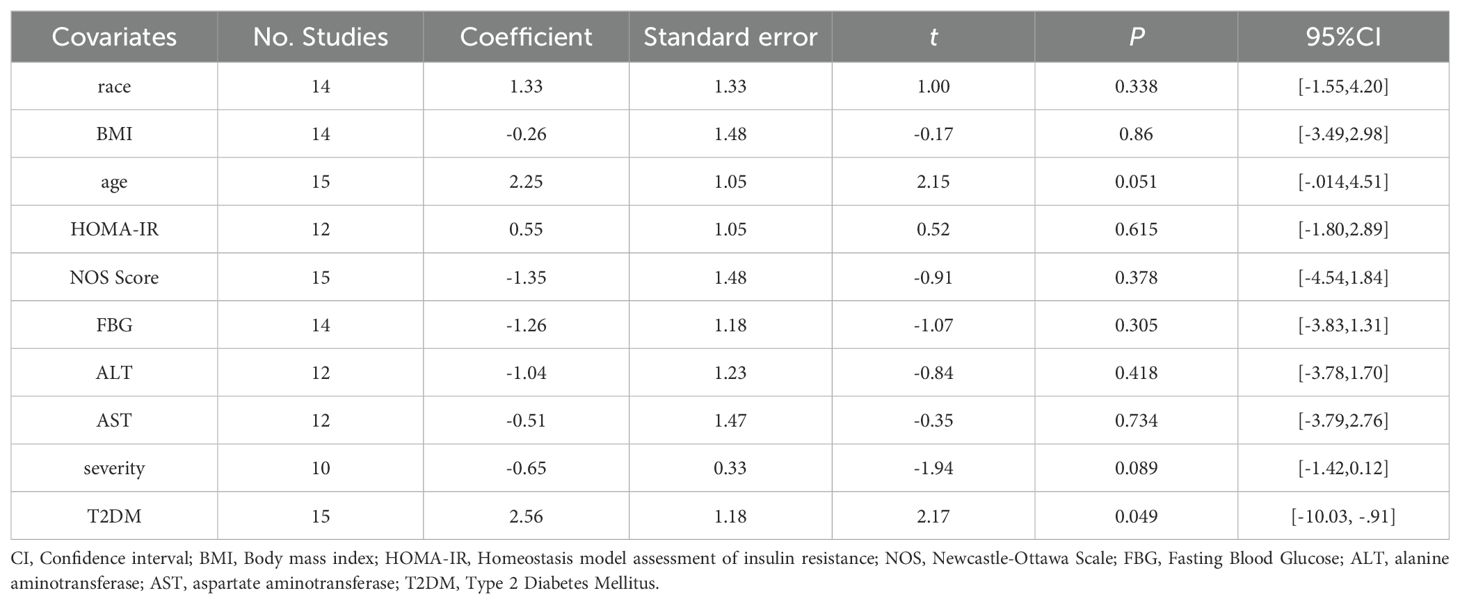
The sources of heterogeneity were further explored using a meta-regression. Univariate regression analysis showed that the group classified based on the presence of T2DM has a P-value <0.05, which can be interpreted as a source of heterogeneity. Age and severity P-values were <0.1. Race, BMI, HOMA-IR, NOS score, Fasting Blood Glucose (FBG), and alanine transaminase (ALT) and aspartate transaminase (AST) levels may not account for the origins of heterogeneity (race, P = 0.338; BMI: P = 0.865; HOMA-IR: P = 0.865; FBG: P = 0.305; ALT: P = 0.418; AST: P = 0.734). Multivariate meta-regression was not performed because of the insufficient number of studies available for analysis and too few studies in which the two factors overlapped. Due to the paucity of studies, univariate regression of the ELISA kits was not feasible. The results of the meta-regression analysis are presented in Table 4 (see Supplementary Material 5 for details).
3.6 Sensitivity analysis
One-by-one removal of the included studies was used for sensitivity analysis to observe changes in the pooled effects. As shown in Figure 4, the result was not influenced by any of the studies, confirming the stability of this study.
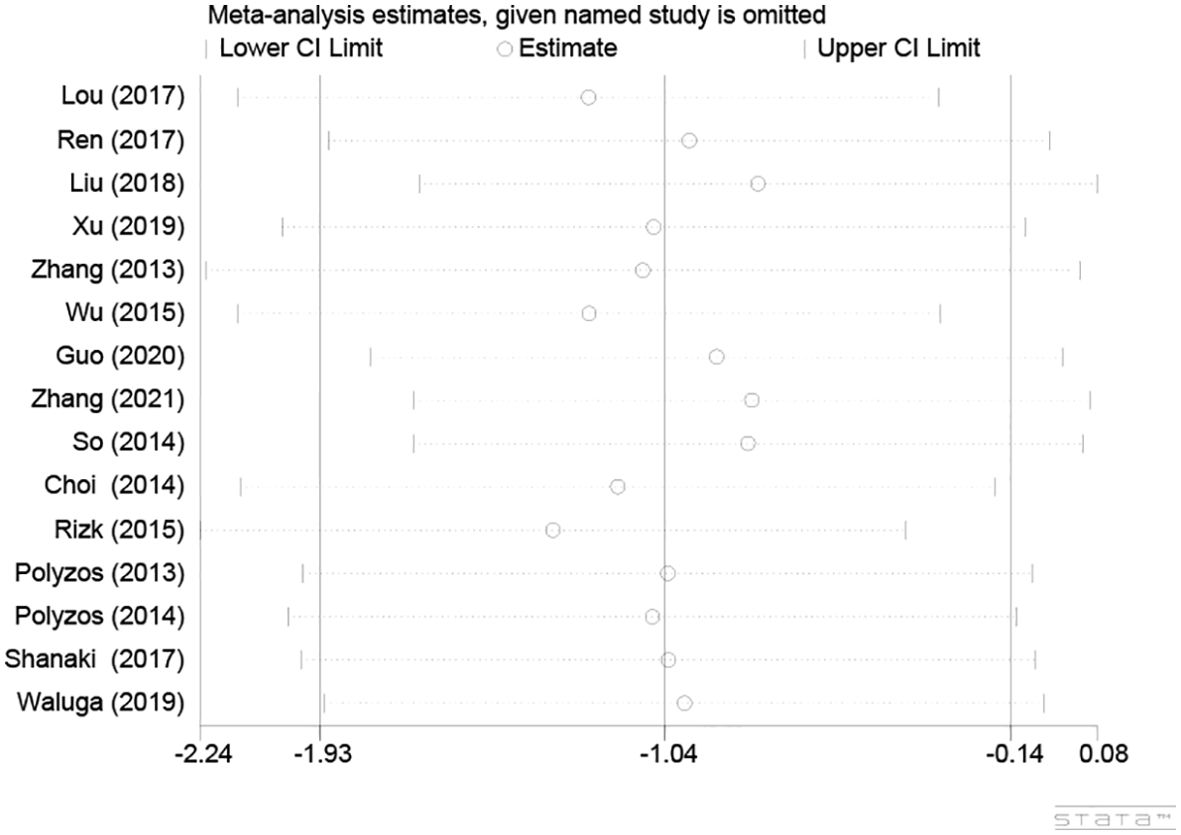
Figure 4. Sensitivity analysis plot of circulating irisin levels between MAFLD and the healthy control group.
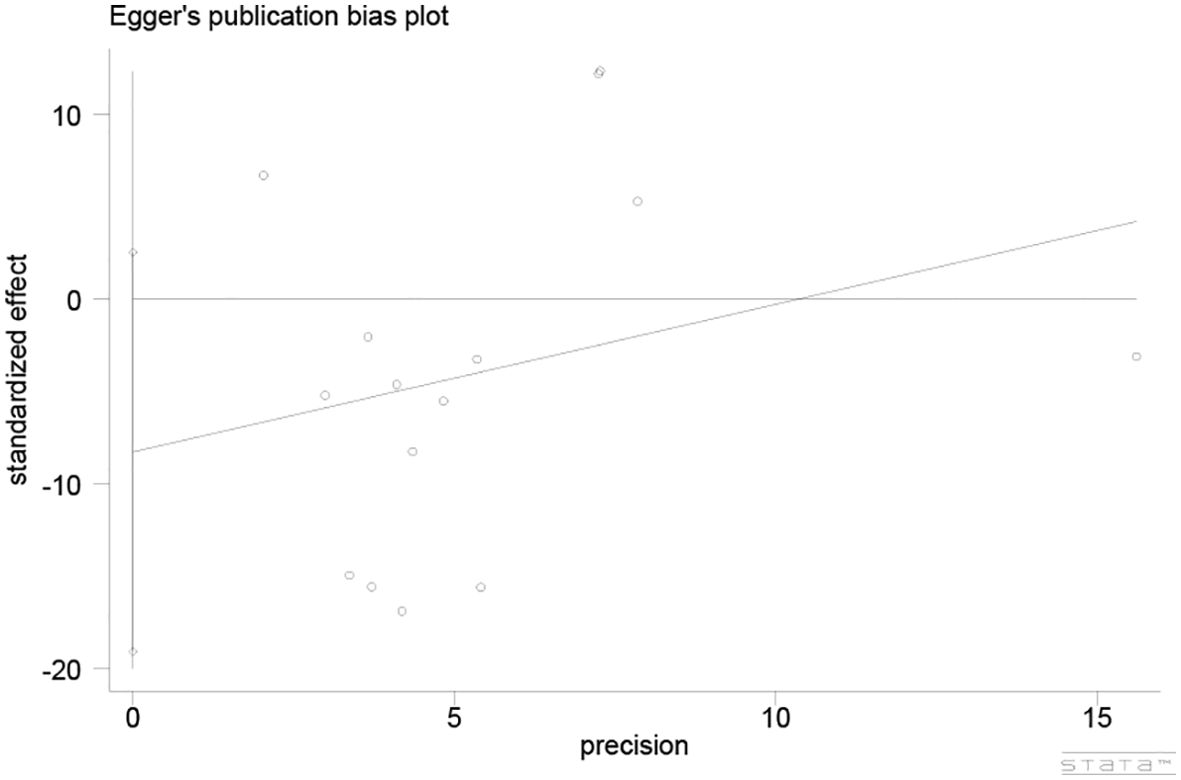
3.7 Publication bias
The Egger’s test was utilized to assess publication bias (Figure 5), which demonstrated a low potential for publication bias (P = 0.122, P > 0.05).
4 Discussion
This current meta-analysis aimed to explore the connection between circulating irisin level and MAFLD. Currently, MAFLD is considered the most common chronic liver disease. MAFLD is considered a more appropriate general term than NAFLD (38). Many studies have suggested that overweight or obesity (39), type 2 diabetes mellitus (T2DM), and metabolic dysfunction (40, 41) are closely related to the complicated mechanism underlying the prevalence and development of MAFLD (2).
Irisin, a well-defined factor secreted by muscles, regulates metabolism and prevents obesity, which is associated with the occurrence and progression of MAFLD. Irisin can activate liver autophagy, reduce liver inflammation, and promote the browning of white fat cells (42, 43) through sports activities (44, 45), thus improving MAFLD. Polyzos et al. (33) showed that serum irisin levels were lower in obese controls and in patients with NAFLD and NASH than those in lean controls. Zhang et al. (46) also indicated that serum levels of irisin were reduced in NAFLD- afflicted obese adults. Ulualan et al. (12) found that serum irisin levels were lower in obese patients with and without NAFLD than in a control group. Gonzales-Gil et al. (47) reported that patients with obesity and metabolic syndrome (MS) had significantly lower plasma irisin levels compared to normal-weight controls. In addition, Choi et al. (31) reported that serum irisin levels were lower in patients with severe steatosis than in those with mild steatosis. Related research on irisin and MAFLD is constantly updated. Therefore, the latest systematic review and meta-analysis of the relationship between irisin and MAFLD was implemented for early clinical diagnosis and further scientific exploration.
In this study, 1548 articles were retrieved from both English and Chinese databases (PubMed, EMBASE, Cochrane Library, CNKI, Wanfang, CBM, VIP, Clinicaltrials.gov and gray literature), and 15 studies were ultimately enrolled, containing eight studies from China, two from Greece, one from South Korea, one from Iran, one from Poland, one from Japan, and one from Egypt. The circulating irisin level in patients with MAFLD was significantly lower than that in healthy controls. Patients with MAFLD have worse liver impairment than healthy people, and previous studies have found that circulating irisin levels decrease with liver damage (31, 36, 46). Meanwhile, irisin is produced during physical exercise (8, 48, 49) and decreases after long periods of inactivity. However, many diseases such as obesity, T2DM, and metabolic dysfunction are caused by a sedentary lifestyle, which may not be conducive to circulating irisin (50). High heterogeneity cannot be ignored; therefore, subgroup analysis and meta-regression were crucial to find the potential root causes of heterogeneity. From the perspective of race, the circulating irisin level was lower in the MAFLD group than that in healthy controls in Asian populations, but there was no significant difference in other subgroups. Subgroup analysis based on severity observed that both mild and moderate to severe MAFLD groups had higher circulating irisin levels than healthy controls, likewise mild MAFLD groups had higher irisin levels than moderate to severe MAFLD groups. It was indicated that the higher severity of MAFLD was connected with the lower irisin levels, which was not in conflict with our general results and conclusions. In terms of combined with T2DM, obvious lower circulating irisin levels were shown in MAFLD patients with T2DM compared to those in healthy controls. However, when BMI, age, HOMA-IR, and NOS score were used for subgroup analysis, it could not perceive significant difference between the MAFLD and healthy groups. Based on univariate meta-regression results, age and severity may be the sources of heterogeneity. However, as there was no common study comparing age and severity, multivariate meta-regression could not be conducted. Sensitivity and publication bias analyses were conducted. Excluding any study did not significantly influence the results. In addition, the Egger’s test demonstrated a low possibility of publication bias. These results indicate that our study is persuasive and representative to some extent.
Fifteen articles were included in this study, consisting of 1920 patients and 1532 healthy controls, which improved the validity of publication bias over previously published studies. Several studies, including one by as Shanaki et al. (36), on MAFLD patients with overweight, obesity, T2DM, and metabolic dysfunction were also included based on the updated definition of MAFLD. However, many studies were excluded for various reasons. For example, Zhang et al. published two studies (30, 46), and since the data were the same, we included a more detailed one (30). A study by Metwally et al. (51) had no controls, which did not conform to our study design criteria. The control group in Canivet et al. (52) included lean subjects and non-healthy controls, which was inconsistent with the study design criteria and was therefore also excluded. Clinicaltrials.gov and the gray literature were also scrutinizeed to resolve publication bias, which was shown to be low according to the Egger’s test (P = 0.122, P > 0.05). Regarding the use of different ELISA kits to measure irisin levels, the SMD of irisin was chosen as the primary result rather than the weighted mean difference (WMD), so variations in the different reference ranges of different ELISA kits were adjusted based on their associated SDs. Different ELISA kits significantly affect the results. Circlulating irisin levels detected with ELISA kits [Aviscera Bio sciences, Santa Clara, CA, USA (28, 30); BioVendor, Bmo, CZ (35–37); Unspecified (23–26, 31)] were irrelevant. There were higher circlulating irisin levels obtained by R&D, Minneapolis, MN, USA (27, 32) in MAFLD individuals compared to healthy group. Serum irisin levels in the MAFLD group were less than those in the healthy control group when ELISA kits produced by Pharmaceuticals, CA, USA (33, 34) and Elabscience, Wuhan, China (29). (See Table 1 for details.)
Recently, Qiu et al. (53) updated a meta-analysis of NAFLD versus circulating irisin levels and found no difference exciting in the level of circulating irisin between NAFLD and non-NAFLD patients. First, in terms of the literature search, Qiu et al. (53) mainly searched English databases, and our team added a search of Chinese databases to make the included studies more comprehensive. Second, there were also differences in literature inclusion, and the control population included by Qiu et al. (53) was more extensive than the non-NAFLD control group or patients in different disease stages of NAFLD. We developed screening criteria in greater detail based on the latest MAFLD guidelines, with a greater focus on rigorous screening of the included population. Among the studies included by Qiu et al. (53), five studies were not included by us, namely Canivet et al. (52), Metwally et al. (51), Monserrat-Mesquida et al. (54), Moreno-Perez et al. (55), and Petta et al. (56) The reasons for the exclusion of Canivet et al. (52) and Metwally et al. (51) are mentioned above. The study by Monserrat-Mesquida et al. (54) did not explicitly mention that the included population was NAFLD patients, and Moreno-Perez et al. (55) included HIV patients, which did not meet our inclusion criteria. Petta et al. (56) included patients with compensated cirrhosis caused by NASH, whereas our criteria excluded patients with NAFLD-associated cirrhosis. More importantly, the results of Qiu et al. (53) were inconsistent with those of our group, as we found that the circulating irisin level in patients with MAFLD was lower than that in healthy controls.
Similarly, our updated meta-analysis also differed from previous conclusions, and differences in the included articles may be the main reason. In addition, contrary to our previous study, this meta-analysis suggests that the MAFLD group might exhibit significantly reduced circulating irisin levels than the healthy group. However, previous study indicated that the circulating irisin levels were not significantly different between the NAFLD group and healthy controls. First of all, with regard to literature retrieval, the updated study retrieved more than 1,000 additional literatures. Secondly, there were differences in literature inclusion, the updated paper incorporated more extensive articles than previous one. Studies on NAFLD combined with obesity or T2DM were not excluded in this updated article due to the MAFLD standard guidelines and the inclusion criteria adjustments. Meanwhile, the updated research demonstrated that the circulating irisin levels in MAFLD patients with T2DM were significantly lower than those in healthy group (24, 26, 29, 36). So it can be deduced that the insufficient number of included articles and analysis samples in our original study might affect the accuracy of previous research results.
Besides, most observational studies have shown that MAFLD patients present lower circulating irisin levels than healthy individuals. Aydin et al. (57) found through animal experiments that the circulating irisin level in rats with liver damage was reduced, and if MAFLD patients allow the disease to progress, it may lead to liver cell damage, which also provides a basis for our research results. Du et al. (58) suggested that circulating irisin levels were decreased in patients with T2DM, and relevant studies shown that the prevalence of MAFLD is higher in people with diabetes than in people without diabetes (59, 60).
Therefore, the updated discovery could not only help to diagnose the disease earlier but also develop the diets of patients. But there is relatively few food related literature about improving the circulating irisin levels. Fortunately, because irisin is produced primarily by exercise-induced muscle secretion and is associated with vitamin D, several studies have provided dietary information indirectly. For example, high-protein foods (8, 61, 62) and high-vitamin foods (63–66) like milk, fish, meat, vegetables, fruits, can help increase the circulating irisin levels, which can provide more precious and beneficial dietary guidance for MAFLD patients. More and higher-quality researches are needed to determine and foster daily dietary guidelines for MAFLD individuals.
4.1 Study strengths and limitations
This meta-analysis was primarily focused on the association between circulating irisin levels and MAFLD. To avoid local literature bias, studies in both English and Chinese languages were included, and Clinicaltrials.gov and gray literature were also searched to reduce publication bias. Subgroup univariate regression analysis was used to study the data, and the results were interpreted and discussed. In addition, the stability of the merged results was affirmed by the Egger’s test and sensitivity analysis. However, this meta-analysis had several limitations that cannot be ignored. First of all, only 15 articles were included in this updated study, the number of articles in this study was insufficient and not entirely representative. In addition, more than 70% of the research population in the articles was from Asia. Second, as no study was concerned with both age and severity, multivariate meta-regression could not be performed. If more comprehensive and systematic studies are available, more convincing results can be obtained. Finally, circulating irisin levels in some studies [e.g., Liu et al. (24), Guo et al. (23), and Shanaki et al. (36)] still differed greatly from those in others after unit homogenization, and different ELISA kits were utilized to measure circulating irisin levels, and the reliability of commercially available irisin ELISA kits is still debatable (67–70). Therefore, SMD was adopted instead of WMD to eliminate the impact of different ELISA kits resulting in significant differences in serum irisin levels. It is essential to conduct more serviceable analytical studies so as to explore detection methods of ELISA kits (69), patients physical activities (68), and sampling time. Moreover, several studies were not included owing to the lack of data, and no relevant information was available despite contacting the authors by email. Furthermore, different experimental personnel, operations, and equipment may also affect the reliability of the outcomes. All of these conditions influenced the reliability of the final results. Finally, only three studies with small sample sizes from Asia performed subgroup analysis of disease severity classification. In the future, it is necessary to strictly control inclusion and exclusion criteria, retrieve a larger sample size, develop a more rigorous design, and conduct high-quality randomized controlled trials to explore the accurate effect of irisin signaling in MAFLD pathogenesis, which will provide a new method for the intervention and projection of blood irisin levels related to MAFLD.
5 Conclusions
Overall, this meta-analysis suggested that circulating irisin levels in patients with MAFLD were lower than in healthy controls. In the Asian population subgroup and in the subgroup of patients aged >50 years, the circulating irisin levels of patients with MAFLD were lower than those of the healthy control group. Within the severity subgroup, patients with mild, moderate, and severe MAFLD had higher circulating irisin levels compared with the healthy controls and the irisin levels in mild MAFLD patients were higher than those in moderate to severe MAFLD patients. The patients of MAFLD combined with T2DM presented apparently lower circulating irisin levels compared with the healthy groups. Subgroup analysis revealed no sources of heterogeneity. Univariate regression analysis revealed that age and severity P-values were less than 0.1, T2DM P-value was lower than 0.05.
Therefore, the relationship between irisin and MAFLD can be summarized as follows: irisin may serve as a key indicator in MAFLD diagnosis, which is universally challenging to diagnose and treat during initial stages. Furthermore, we discovered that irisin could be an indicator of the progression of MAFLD to a certain extent, which has significant clinical impacts for the detection and treatment of disease progression. However, multi-regional, multi-faceted, multi-center research evidence is required to confirm and consolidate the current conclusions.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
CS: Writing – original draft. KW: Writing – original draft. YK: Writing – original draft. QZ: Writing – original draft. SC: Writing – original draft. QL: Writing – original draft. YR: Writing – original draft. XY: Writing – original draft. SL: Writing – review & editing. JH: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Medical science and Technology Project of Zhejiang Province (2023KY140), Medical science and Technology Project of Zhejiang Province (2022KY921), Major Medical science and Technology Project of Zhejiang Province (WKJ-ZJ-2537).
Acknowledgments
It’s our great honor to give sincere thanks to all the participants in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1464951/full#supplementary-material
Supplementary Material 1 | The published protocol in PROSPERO.
Supplementary Material 2 | Databases retrieval strategy.
Supplementary Material 3 | The results of GRADE system.
Supplementary Material 4 | Forest plot of circulating irisin levels between MAFLD and the healthy control group by different subgroups (Random-Effects Model, SMD). (A) Forest plot of circulating irisin levels between MAFLD and the healthy control group by race (Random-Effects Model, SMD). (B) Forest plot of circulating irisin levels between MAFLD and the healthy control group by BMI (Random-Effects Model, SMD). (C) Forest plot of circulating irisin levels between MAFLD and the healthy control group by age (Random-Effects Model, SMD). (D) Forest plot of circulating irisin levels between MAFLD and the healthy control group by HOMA-IR (Random-Effects Model, SMD). (E) Forest plot of circulating irisin levels between MAFLD and the healthy control group by NOS score (Random-Effects Model, SMD). (F) Forest plot of circulating irisin levels between MAFLD and the healthy control group by severity (Random-Effects Model, SMD). (G) Forest plot of circulating irisin levels between mild MAFLD and moderate to severe MAFLD (Random-Effects Model, SMD). (H) Forest plot of circulating irisin levels between MAFLD and the healthy control group by whether to merge T2DM or not (Random-Effects Model, SMD). (I) Forest plot of circulating irisin levels between MAFLD and the healthy control group by ELISA kits (Random-Effects Model, SMD).
Supplementary Material 5 | Figures of meta-regression. (A) Result of meta-regression by race. (B) Result of meta-regression by BMI. (C) Result of meta-regression by Age. (D) Result of meta-regression by HOMA-IR. (E) Result of meta-regression by NOS score. (F) Result of meta-regression by severity. (G) Result of meta-regression by FBG. (H) Result of meta-regression by ALT. (I) Result of meta-regression by AST. (J) Result of meta-regression by T2DM.
Abbreviations
MAFLD, metabolic dysfunction-associated fatty liver disease; BMI, body mass index; CI, confidence interval; SD, Standard Deviation; SMD, standardized mean difference; NOS, Newcastle-Ottawa Quality Assessment Scale; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; NAFLD, Non-alcoholic fatty liver disease; HS, hepatic steatosis; NASH, nonalcoholic steatohepatitis; HCC, hepatocellular carcinoma; FNDC5, fibronectin type III domain–containing 5; HFD, high-fat diet; SFL, simple fatty liver; HOMA-IR, Homeostasis model assessment of insulin resistance; T2DM, type 2 diabetes mellitus; FBG, Fasting Blood Glucose; ALT, alanine transaminase; AST, aspartate transaminase; MS, obesity and metabolic syndrome; WMD, weighted mean difference.
References
1. Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J Hepatol. (2017) 67:862–73. doi: 10.1016/j.jhep.2017.06.003
2. Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. (2020) 73:202–9. doi: 10.1016/j.jhep.2020.03.039
3. Ayada I, van Kleef LA, Alferink LJM, Li P, de Knegt RJ, Pan Q. Systematically comparing epidemiological and clinical features of MAFLD and NAFLD by meta-analysis: Focusing on the non-overlap groups. Liver Int. (2022) 42:277–87. doi: 10.1111/liv.15139
4. Li J, Zou B, Yeo YH, Feng Y, Xie X, Lee DH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. (2019) 4:389–98. doi: 10.1016/s2468-1253(19)30039-1
5. Zhou J, Zhou F, Wang W, Zhang XJ, Ji YX, Zhang P, et al. Epidemiological features of NAFLD from 1999 to 2018 in China. Hepatology. (2020) 71:1851–64. doi: 10.1002/hep.31150
6. Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. (2019) 156:1264–1281.e1264. doi: 10.1053/j.gastro.2018.12.036
7. Colaianni G, Cinti S, Colucci S, Grano M. Irisin and musculoskeletal health. Ann N Y Acad Sci. (2017) 1402:5–9. doi: 10.1111/nyas.13345
8. Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. (2012) 481:463–8. doi: 10.1038/nature10777
9. Perakakis N, Triantafyllou GA, Fernández-Real JM, Huh JY, Park KH, Seufert J, et al. Physiology and role of irisin in glucose homeostasis. Nat Rev Endocrinol. (2017) 13:324–37. doi: 10.1038/nrendo.2016.221
10. Wang X, Mao L, Li C, Hui Y, Yu Z, Sun M, et al. The potential role of FNDC5/irisin in various liver diseases: awakening the sleeping beauties. Expert Rev Mol Med. (2022) 24:e23. doi: 10.1017/erm.2022.19
11. Armandi A, Rosso C, Nicolosi A, Caviglia GP, Abate ML, Olivero A, et al. Crosstalk between irisin levels, liver fibrogenesis and liver damage in non-obese, non-diabetic individuals with non-alcoholic fatty liver disease. J Clin Med. (2022) 11:635. doi: 10.3390/jcm11030635
12. Ulualan G, Kiraz ZK, Kırel B. Relation of serum irisin levels to obesity and non-alcoholic fatty liver disease. Turk J Pediatr. (2022) 64:246–54. doi: 10.24953/turkjped.2020.3003
13. Zhu W, Sahar NE, Javaid HMA, Pak ES, Liang G, Wang Y, et al. Exercise-induced irisin decreases inflammation and improves NAFLD by competitive binding with MD2. Cells. (2021) 10:3306. doi: 10.3390/cells10123306
14. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
15. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
16. Shi JD, Luo DH, Weng H, Zeng XT, Lin L, Chu HT, et al. Optimally estimating the sample standard deviation from the five-number summary. Res Synth Methods. (2020) 11:641–54. doi: 10.1002/jrsm.1429
17. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. (2018) 27:1785–805. doi: 10.1177/0962280216669183
18. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
19. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
20. Schmidt FL, Oh IS, Hayes TL. Fixed- versus random-effects models in meta-analysis: model properties and an empirical comparison of differences in results. Br J Math Stat Psychol. (2009) 62:97–128. doi: 10.1348/000711007x255327
21. World Health Organization, Regional Office for the Western Pacific (WPRO), International Association for the Study of Obesity International Obesity Task Force. The Asia-Pacific Perspective: Redefining obesity and its treatment. Sydney, Australia: Health Communications Australia Pty Ltd (2000).
23. Guo YD, Deng YJ, Sun HL, Liu JG. To investigate the levels of adipsin, visfatin and irisin in patients with nonalcoholic fatty liver disease (NAFLD) and their correlation. J Pract Med. (2020) 36:2376–80. doi: 10.3969/j.issn.1006-5725.2020.17.012
24. Liu N, Du AS, Jia J, Zhao YX, Wang YL. Study on correlation between serum irisin level and type 2 diabetes mellitus combined with nonalcoholic fatty liver disease. Med Pharm J Chin PLA. (2018) 30:41–4. doi: 10.3969/j.issn.2095-140X.2018.09.012
25. Lou HF, Shi XY, Fang X. A study on the correlation between serum Irisin level and non-alcoholic fatty liver disease. Chin J Integr Tradit West Med Dig. (2017) 25:607–11. doi: 10.3969/j.issn.1671-038X.2017.08.12
26. Ren R, Guo ZX. Relationship between serum irisin silencing regulatory protein 1 and type 2 diabetes mellitus with nonalcoholic fatty liver disease. Chin Rem Clin. (2017) 17:1042–4. doi: 10.11655/zgywylc2017.07.043
27. Wu HJ, Ouyang XH, Xia YJ. Changes of serum irisin level in patients with non-alcoholic fatty liver disease. Anhui Med Pharm J. (2015) 19:1095–8. doi: 10.3969/j.issn.1009-6469.2015.06.022
28. Xu F, Liu J, Wei YC, Ye Y, Chen HX, Gao XS, et al. Association between serum Irisin and fatty liver of Kazakh in Xinjiang. Xinjiang Med J. (2019) 49:9–12.
29. Zhang GD. Analysis of serum hs-CRP, TNF-α, IL-6, IL-10 and irigenin levels in patients with non-alcoholic fatty liver disease complicated with type 2 diabetes mellitus. Clin Res Pract. (2021) 6:24–6. doi: 10.19347/j.cnki.2096-1413.202132008
30. Zhang HJ. The efficacy and safety of intensive-induced conventional exercise and conventional exercise for nonalcoholic fatty liver disease in obese humans. Shanghai, China: Shanghai Jiaotong University (2013).
31. So R, Oh S, Shida T, Tanaka K, Shoda J. Irisin is associated with physical activity level and hepatic steatosis grade in non-alcoholic fatty liver disease subjects: 841. Hepatology. (2014) 60:606A.
32. Choi ES, Kim MK, Song MK, Kim JM, Kim ES, Chung WJ, et al. Association between serum irisin levels and non-alcoholic fatty liver disease in health screen examinees. PloS One. (2014) 9:e110680. doi: 10.1371/journal.pone.0110680
33. Polyzos SA, Kountouras J, Anastasilakis AD, Geladari EV, Mantzoros CS. Irisin in patients with nonalcoholic fatty liver disease. Metabolism. (2014) 63:207–17. doi: 10.1016/j.metabol.2013.09.013
34. Polyzos SA, Kountouras J, Anastasilakis AD, Margouta A, Mantzoros CS. Association between circulating irisin and homocysteine in patients with nonalcoholic fatty liver disease. Endocrine. (2015) 49:560–2. doi: 10.1007/s12020-014-0473-x
35. Rizk FH, Elshweikh SA, Abd El-Naby AY. Irisin levels in relation to metabolic and liver functions in Egyptian patients with metabolic syndrome. Can J Physiol Pharmacol. (2016) 94:359–62. doi: 10.1139/cjpp-2015-0371
36. Shanaki M, Moradi N, Emamgholipour S, Fadaei R, Poustchi H. Lower circulating irisin is associated with nonalcoholic fatty liver disease and type 2 diabetes. Diabetes Metab Syndr. (2017) 11 Suppl 1:S467–72. doi: 10.1016/j.dsx.2017.03.037
37. Waluga M, Kukla M, Kotulski R, Zorniak M, Boryczka G, Kajor M, et al. Omentin, vaspin and irisin in chronic liver diseases. J Physiol Pharmacol. (2019) 70:277–85. doi: 10.26402/jpp.2019.2.11
38. Eslam M, Sanyal AJ, George J. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. (2020) 158:1999–2014.e1991. doi: 10.1053/j.gastro.2019.11.312
39. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. (2018) 15:11–20. doi: 10.1038/nrgastro.2017.109
40. Bril F, Barb D, Portillo-Sanchez P, Biernacki D, LoMonaco R, Suman A, et al. Metabolic and histological implications of intrahepatic triglyceride content in nonalcoholic fatty liver disease. Hepatology. (2017) 65:1132–44. doi: 10.1002/hep.28985
41. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. (2016) 64:73–84. doi: 10.1002/hep.28431
42. Arhire LI, Mihalache L, Covasa M. Irisin: A hope in understanding and managing obesity and metabolic syndrome. Front Endocrinol (Lausanne). (2019) 10:524. doi: 10.3389/fendo.2019.00524
43. Elizondo-Montemayor L, Mendoza-Lara G, Gutierrez-DelBosque G, Peschard-Franco M, Nieblas B, Garcia-Rivas G. Relationship of circulating irisin with body composition, physical activity, and cardiovascular and metabolic disorders in the pediatric population. Int J Mol Sci. (2018) 19:3727. doi: 10.3390/ijms19123727
44. Tsuchiya Y, Ando D, Goto K, Kiuchi M, Yamakita M, Koyama K. High-intensity exercise causes greater irisin response compared with low-intensity exercise under similar energy consumption. Tohoku J Exp Med. (2014) 233:135–40. doi: 10.1620/tjem.233.135
45. Tsuchiya Y, Ando D, Takamatsu K, Goto K. Resistance exercise induces a greater irisin response than endurance exercise. Metabolism. (2015) 64:1042–50. doi: 10.1016/j.metabol.2015.05.010
46. Zhang HJ, Zhang XF, Ma ZM, Pan LL, Chen Z, Han HW, et al. Irisin is inversely associated with intrahepatic triglyceride contents in obese adults. J Hepatol. (2013) 59:557–62. doi: 10.1016/j.jhep.2013.04.030
47. Gonzalez-Gil AM, Peschard-Franco M, Castillo EC, Gutierrez-DelBosque G, Treviño V, Silva-Platas C, et al. Myokine-adipokine cross-talk: potential mechanisms for the association between plasma irisin and adipokines and cardiometabolic risk factors in Mexican children with obesity and the metabolic syndrome. Diabetol Metab Syndr. (2019) 11:63. doi: 10.1186/s13098-019-0458-2
48. Huh JY, Siopi A, Mougios V, Park KH, Mantzoros CS. Irisin in response to exercise in humans with and without metabolic syndrome. J Clin Endocrinol Metab. (2015) 100:E453–457. doi: 10.1210/jc.2014-2416
49. Moreno M, Moreno-Navarrete JM, Serrano M, Ortega F, Delgado E, Sanchez-Ragnarsson C, et al. Circulating irisin levels are positively associated with metabolic risk factors in sedentary subjects. PloS One. (2015) 10:e0124100. doi: 10.1371/journal.pone.0124100
50. Korta P, Pocheć E, Mazur-Biały A. Irisin as a multifunctional protein: implications for health and certain diseases. Medicina (Kaunas). (2019) 55:485. doi: 10.3390/medicina55080485
51. Metwally M, Bayoumi A, Romero-Gomez M, Thabet K, John M, Adams LA, et al. A polymorphism in the Irisin-encoding gene (FNDC5) associates with hepatic steatosis by differential miRNA binding to the 3’UTR. J Hepatol. (2019) 70:494–500. doi: 10.1016/j.jhep.2018.10.021
52. Canivet CM, Bonnafous S, Rousseau D, Leclere PS, Lacas-Gervais S, Patouraux S, et al. Hepatic FNDC5 is a potential local protective factor against Non-Alcoholic Fatty Liver. Biochim Biophys Acta Mol Basis Dis. (2020) 1866:165705. doi: 10.1016/j.bbadis.2020.165705
53. Qiu S, Wang Q, Cai X, Sun Z, Wu T. Circulating irisin in nonalcoholic fatty liver disease: an updated meta-analysis. Endokrynol Pol. (2022) 74:47–54. doi: 10.5603/EP.a2022.0067
54. Monserrat-Mesquida M, Quetglas-Llabrés M, Abbate M, Montemayor S, Mascaró CM, Casares M, et al. Oxidative stress and pro-inflammatory status in patients with non-alcoholic fatty liver disease. Antioxidants (Basel). (2020) 9:759. doi: 10.3390/antiox9080759
55. Moreno-Perez O, Reyes-Garcia R, Muñoz-Torres M, Merino E, Boix V, Reus S, et al. High Irisin levels in nondiabetic HIV-infected males are associated with insulin resistance, nonalcoholic fatty liver disease, and subclinical atherosclerosis. Clin Endocrinol (Oxf). (2018) 89:414–23. doi: 10.1111/cen.13800
56. Petta S, Valenti L, Svegliati-Baroni G, Ruscica M, Pipitone RM, Dongiovanni P, et al. Fibronectin type III domain-containing protein 5 rs3480 A>G polymorphism, irisin, and liver fibrosis in patients with nonalcoholic fatty liver disease. J Clin Endocrinol Metab. (2017) 102:2660–9. doi: 10.1210/jc.2017-00056
57. Aydin S, Ogeturk M, Kuloglu T, Kavakli A, Aydin S. Effect of carnosine supplementation on apoptosis and irisin, total oxidant and antioxidants levels in the serum, liver and lung tissues in rats exposed to formaldehyde inhalation. Peptides. (2015) 64:14–23. doi: 10.1016/j.peptides.2014.11.008
58. Du XL, Jiang WX, Lv ZT. Lower circulating irisin level in patients with diabetes mellitus: A systematic review and meta-analysis. Horm Metab Res. (2016) 48:644–52. doi: 10.1055/s-0042-108730
59. Boccatonda A, Andreetto L, D’Ardes D, Cocco G, Rossi I, Vicari S, et al. From NAFLD to MAFLD: definition, pathophysiological basis and cardiovascular implications. Biomedicines. (2023) 11:883. doi: 10.3390/biomedicines11030883
60. Al Hashmi K, Giglio RV, Pantea Stoian A, Patti AM, Al Waili K, Al Rasadi K, et al. Metabolic dysfunction-associated fatty liver disease: current therapeutic strategies. Front Nutr. (2024) 11:1355732. doi: 10.3389/fnut.2024.1355732
61. Zhang J, Liu YL. MicroRNA in skeletal muscle: its crucial roles in signal proteins, mus cle fiber type, and muscle protein synthesis. Curr Protein Pept Sci. (2017) 18:579–88. doi: 10.2174/1389203717666160621122405
62. Yang XY, Tse MCL, Hu X, Jia WH, Du GH, Chan CB. Interaction of CREB and PGC-1α Induces fibronectin type III domain-containing protein 5 expression in C2C12 myotubes. Cell Physiol Biochem. (2018) 50:1574–84. doi: 10.1159/000494655
63. Safarpour P, Daneshi-Maskooni M, Vafa M, Nourbakhsh M, Janani L, Maddah M, et al. Vitamin D supplementation improves SIRT1, Irisin, and glucose indices in overweight or obese type 2 diabetic patients: a double-blind randomized placebo-controlled clinical trial. BMC Fam Pract. (2020) 21:26. doi: 10.1186/s12875-020-1096-3
64. Benedik E. Sources of vitamin D for humans. Int J Vitam Nutr Res. (2022) 92:118–25. doi: 10.1024/0300-9831/a000733
65. Yildiz Kopuz TN, Dagdeviren M, Fisunoglu M. Serum irisin levels in newly diagnosed type-II diabetic patients: No association with the overall diet quality but strong association with fruit intake. Clin Nutr ESPEN. (2022) 49:357–64. doi: 10.1016/j.clnesp.2022.03.022
66. Sanesi L, Dicarlo M, Pignataro P, Zerlotin R, Pugliese F, Columbu C, et al. Vitamin D increases irisin serum levels and the expression of its precursor in skeletal muscle. Int J Mol Sci. (2023) 24:4129. doi: 10.3390/ijms24044129
67. Polyzos SA, Mantzoros CS. An update on the validity of irisin assays and the link between irisin and hepatic metabolism. Metabolism. (2015) 64:937–42. doi: 10.1016/j.metabol.2015.06.005
68. Jedrychowski MP, Wrann CD, Paulo JA, Gerber KK, Szpyt J, Robinson MM, et al. Detection and quantitation of circulating human irisin by tandem mass spectrometry. Cell Metab. (2015) 22:734–40. doi: 10.1016/j.cmet.2015.08.001
69. Bubak MP, Heesch MWS, Shute RJ, Dinan NE, Laursen TL, LA Salle DT, et al. Irisin and fibronectin type III domain-containing 5 responses to exercise in different environmental conditions. Int J Exerc Sci. (2017) 10:666–80. doi: 10.70252/XKMW8211
Keywords: non-alcoholic fatty liver disease, metabolic dysfunction-associated fatty liver disease, irisin, systematic review, meta-analysis
Citation: Shen C, Wu K, Ke Y, Zhang Q, Chen S, Li Q, Ruan Y, Yang X, Liu S and Hu J (2024) Circulating irisin levels in patients with MAFLD: an updated systematic review and meta-analysis. Front. Endocrinol. 15:1464951. doi: 10.3389/fendo.2024.1464951
Received: 15 July 2024; Accepted: 18 November 2024;
Published: 17 December 2024.
Edited by:
Prabu Paramasivam, of New Mexico Health Sciences Center, United StatesReviewed by:
Jing-Fu Bao, Southern Medical University, ChinaZiwei Guo, China Academy of Chinese Medical Sciences, China
Copyright © 2024 Shen, Wu, Ke, Zhang, Chen, Li, Ruan, Yang, Liu and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shan Liu, Z3JheXN0YXI5MkAxNjMuY29t; Jie Hu, bXVodWRpZTExMDZAMTYzLmNvbQ==
†These authors share first authorship
‡ORCID: Shan Liu, orcid.org/0000-0001-9139-3257
Jie Hu, orcid.org/0000-0001-9142-0446
 Chenglu Shen
Chenglu Shen Kaihan Wu
Kaihan Wu Yani Ke
Yani Ke Qin Zhang
Qin Zhang Shuaihang Chen
Shuaihang Chen Qicong Li
Qicong Li Yuting Ruan
Yuting Ruan Xudan Yang
Xudan Yang Shan Liu
Shan Liu Jie Hu
Jie Hu