- Department of Neurosurgery, The Second Affiliated Hospital of Harbin Medical University, Harbin, China
Background: Pituitary tumors (PTs) are common benign intracranial tumors. Investigating the metabolites in serum and cerebrospinal fluid in PTs is essential to understanding the underlying biological mechanisms and identifying new biomarkers and therapeutic strategies.
Methods: We used the GWAS dataset of PTs from the FinnGen database, a dataset of 486 plasma metabolites from the GWAS catalog database, and a dataset of 338 cerebrospinal fluid (CSF) metabolites from the WADRC and WRAP study collections. An inverse variance weighting (IVW) approach was utilized as the mainly method to investigate causality between metabolites and PTs, supplemented by four complementary methods to strengthen our findings. Additionally, we utilized several sensitivity methods to guarantee the robustness of our findings.
Results: The study identified 17 plasma metabolites and 10 CSF metabolites related to PTs. Among these, 11 metabolites indicated a significant positive causality with PTs, while 16 displayed a remarkable negative causality. Particularly, plasma levels of 3-dehydrocarnitine (OR = 2.73, 95% CI = 1.55–4.83, P = 0.001) and acetylcarnitine (OR = 0.35, 95% CI = 0.19–0.63, P = 0.001) were found to be significant exposure factors for PTs. Multiple sensitivity analyses confirm the robustness of the results. The study found no evidence of a reverse causality between PTs and the plasma levels of 3-dehydrocarnitine and acetylcarnitine.
Conclusions: The present study identified 27 metabolites associated with the incidence of PTs, among which 3-dehydrocarnitine and acetylcarnitine are the most noteworthy.
1 Introduction
Pituitary tumors (PTs) are the second most common primary brain neoplasm, accounting for about 10-15% of all brain tumors, and are the most common benign tumor in the saddle region (1). Larger PTs often cause patients to experience visual field defects, headaches or hypopituitarism (2). Additionally, functional PTs can produce excessive amounts of hormones, resulting in various endocrine symptoms (3). The first-line treatment option for PTs is typically endoscopic transsphenoidal pituitary surgery, except for prolactinomas, for which the first-line treatment option is medication with bromocriptine or carbamazepine (4).
In recent years, the rapid development of metabolomics has provided new research methods for studying the pathogenesis of diseases, including PTs, and for searching for biomarkers (5). Previous studies have shown that choline is negatively correlated with growth inhibitory receptor type 2 expression in growth hormone-secreting pituitary adenomas, and positively related to magnetic resonance imaging T2 signals and Ki-67 indices (6). In addition, growth inhibitor stimulates BKCa channels in rat PT cells via lipoxygenase metabolites of arachidonic acid (7). Various studies suggest that metabolites are closely related to the development of PTs (8, 9). Nevertheless, it remains unknown which metabolites are causally associated with PTs.
Mendelian randomization (MR) is an epidemiological technique that uses genome-wide association study (GWAS) data to investigate causality between exposures and outcomes (10). It reduces the effects of reverse causality and confounding factors. Therefore, we intended to investigate the effects of serum and cerebrospinal fluid (CSF) metabolites on PTs employing MR.
2 Methods
2.1 Study design
This paper is based on the STROBE-MR report and strictly follows the three main assumptions of MR analysis (11): 1) the designated instrumental variables(IVs) are strongly related to serum and CSF metabolites; 2) the IVs are not confounded by other factors; and 3) the IVs influence the occurrence of PTs only through serum and CSF metabolites, not through other pathways (Figure 1).
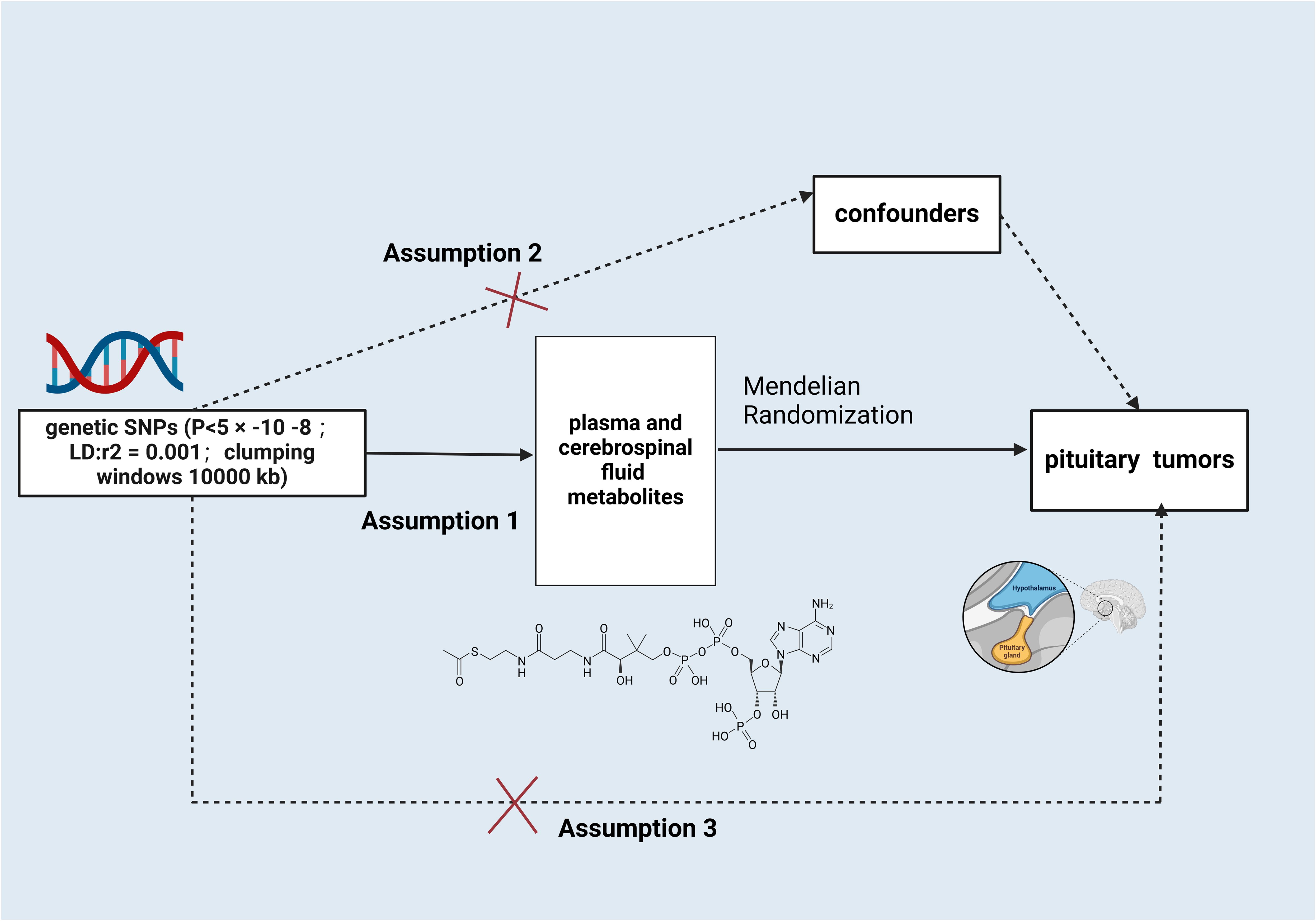
Figure 1. Schematic illustration of the study design. snps, single nucleotide polymorphisms. Created with BioRender.com.
2.2 GWAS data source
The GWAS dataset of PTs as defined by the International Classification of Diseases, 10th Revision (ICD-10) is from round 9 of the FinnGen database. It includes 1,402 European patients with PTs and 375,875 control European patients (12). The serum metabolite GWAS dataset consists primarily of 486 metabolites from the German KORA F4 study (n = 1,768 individuals) and the UK TwinsUK study (n = 6,056 individuals) (13). The 338 CSF Metabolites GWAS dataset was obtained from CSF specimens from 689 participants in the Wisconsin Alzheimer’s Disease Research Centre (532 participants) and the Wisconsin Alzheimer’s Disease Prevention Registry (168 participants). The study received approval from the Institutional Ethics Review Board of the University of Wisconsin Health Sciences (14).
2.3 IVs selection
We took the following steps in screening the IVs:1) We used a threshold of p<5×10−8 to screen SNPs; 2) We used the criteria of r2<0.001 and LD = 10,000 kb to remove linkage disequilibrium; 3) We calculated the F-statistic for each SNP, excluding those with F-statistics smaller than 10 and retaining those larger than 10; 4) We excluded SNPs in palindromic sequences; 5) Finally, we used PhenoScanner to remove SNPs related to potential confounding factors (15).
2.4 Statistical analysis
Our MR analyses were all performed using the TwoSampleMR package in R Studio (16). First, serum and CSF metabolites were selected as IVs, and PTs were considered endpoints. The inverse variance weighting (IVW) technique was used as the mainly analysis method. Furthermore, weighted modal, weighted median, MR-Egger, and simple modal methods were used as supplementary analyses. Given the exploratory nature of this study, we did not apply the Bonferroni correction method; instead, results with a P-value of less than 0.05 were considered significant.
We performed sensitivity analyses of our MR results using a variety of methods.MR-Egger regression intercepts and Mendelian randomized polytomous residuals and outliers (MR-PRESSO) were used to detect potential horizontal pleiotropy. Cochrane’s Q-statistics and their corresponding p-values were computed in the IVW test and MR-Egger regression to evaluate potential heterogeneity among IVs. If significant heterogeneity was found (p<0.05), we used the random effects model of IVW to obtain more unbiased and robust estimates. The robustness of our MR analysis results was verified through scatterplots and funnel plots. Additionally, leave-one-out analyses were conducted to evaluate the potential impact of individual SNPs on the observed causal relationships.
2.5 Metabolic pathway analysis
We performed metabolic pathway analyses of metabolites causally associated with PTs using Metaconflict 5.0. (https://www.metaboanalyst.ca/) (17). In summary, 11 metabolic pathways were identified from the metabolite database, with 9 pathways sourced from both the Small Molecule Pathway (SMP) database and the KEGG database, and 2 pathways derived exclusively from KEGG.
3 Results
3.1 MR analysis of 486 plasma metabolites’ impact on PTs
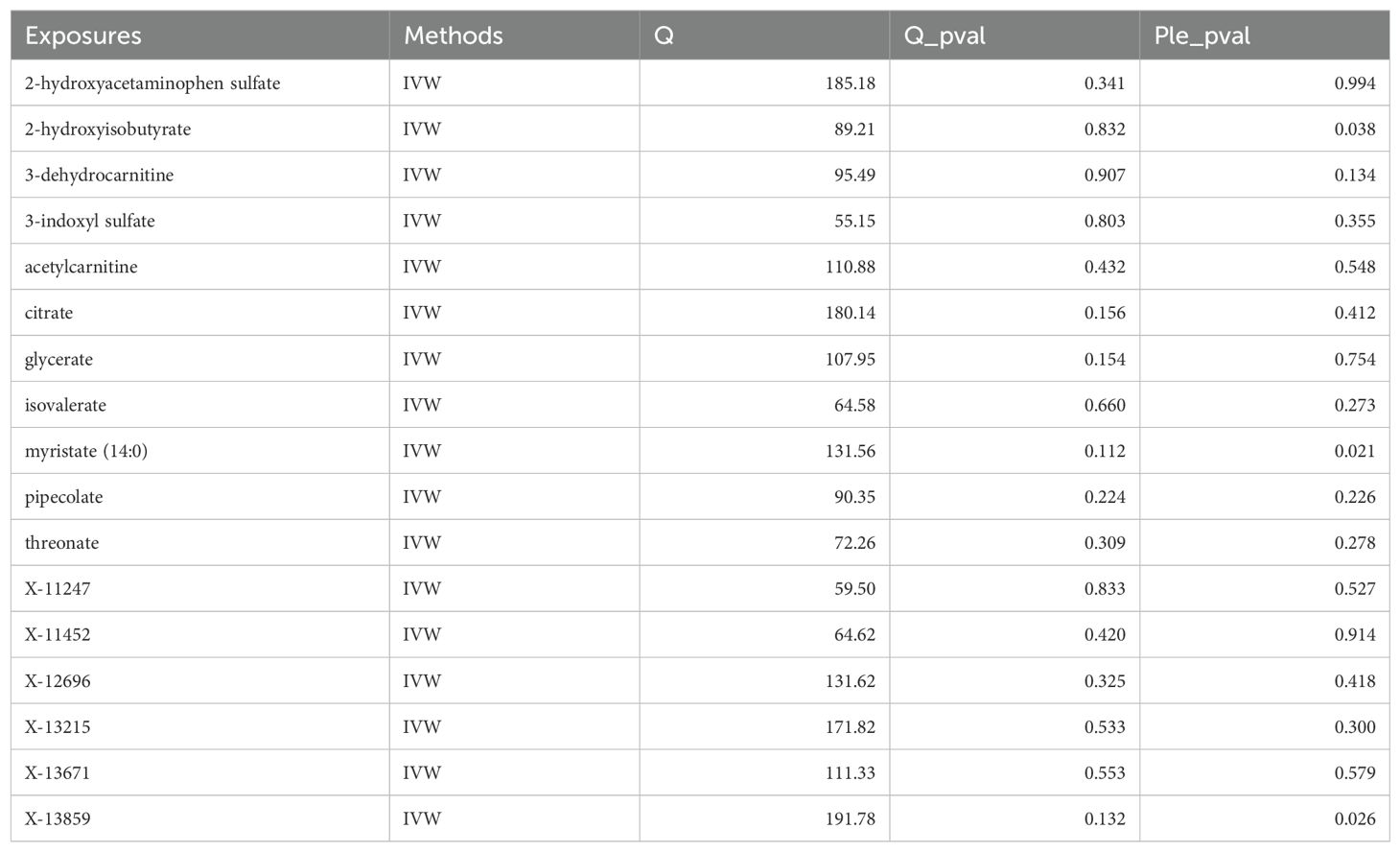
The MR analysis identified significant associations between plasma metabolites and the risk of PTs. Specifically, 2−hydroxyacetaminophen sulfate (OR=0.97, 95%CI: 0.95-1.00, P=0.024), acetylcarnitine (OR=0.35, 95%CI: 0.19-0.63, P=0.001), isovalerate (OR=0.45, 95%CI: 0.24-0.84, P=0.012), myristate (OR=0.48, 95%CI: 0.27-0.86, P=0.014), pipecolate (OR=0.63, 95%CI: 0.41-0.96, P=0.034), threonate (OR=0.65, 95%CI: 0.43-0.97, P=0.035) showed inverse correlations with PTs’ risk. Conversely, 2−hydroxyisobutyrate (OR=2.27, 95%CI: 1.34-3.84, P=0.002), 3−dehydrocarnitine (OR=2.73, 95%CI: 1.55-4.83, P=0.001), 3−indoxyl sulfate (OR=1.62, 95%CI: 1.03-2.53, P=0.036), citrate (OR=2.17, 95%CI: 1.03-4.55, P=0.040), and glycerate (OR=2.01, 95%CI: 1.01-4.02, P=0.048) were positively related to the risk of PTs. Additionally, six unknown metabolites were discovered to be causally linked to the occurrence of PTs. (Figures 2, 3) Scatter plots illustrating these causal relationships are provided in Figure 4. However, reverse MR analysis revealed no causal association between PTs and these plasma metabolites(P<0.05).
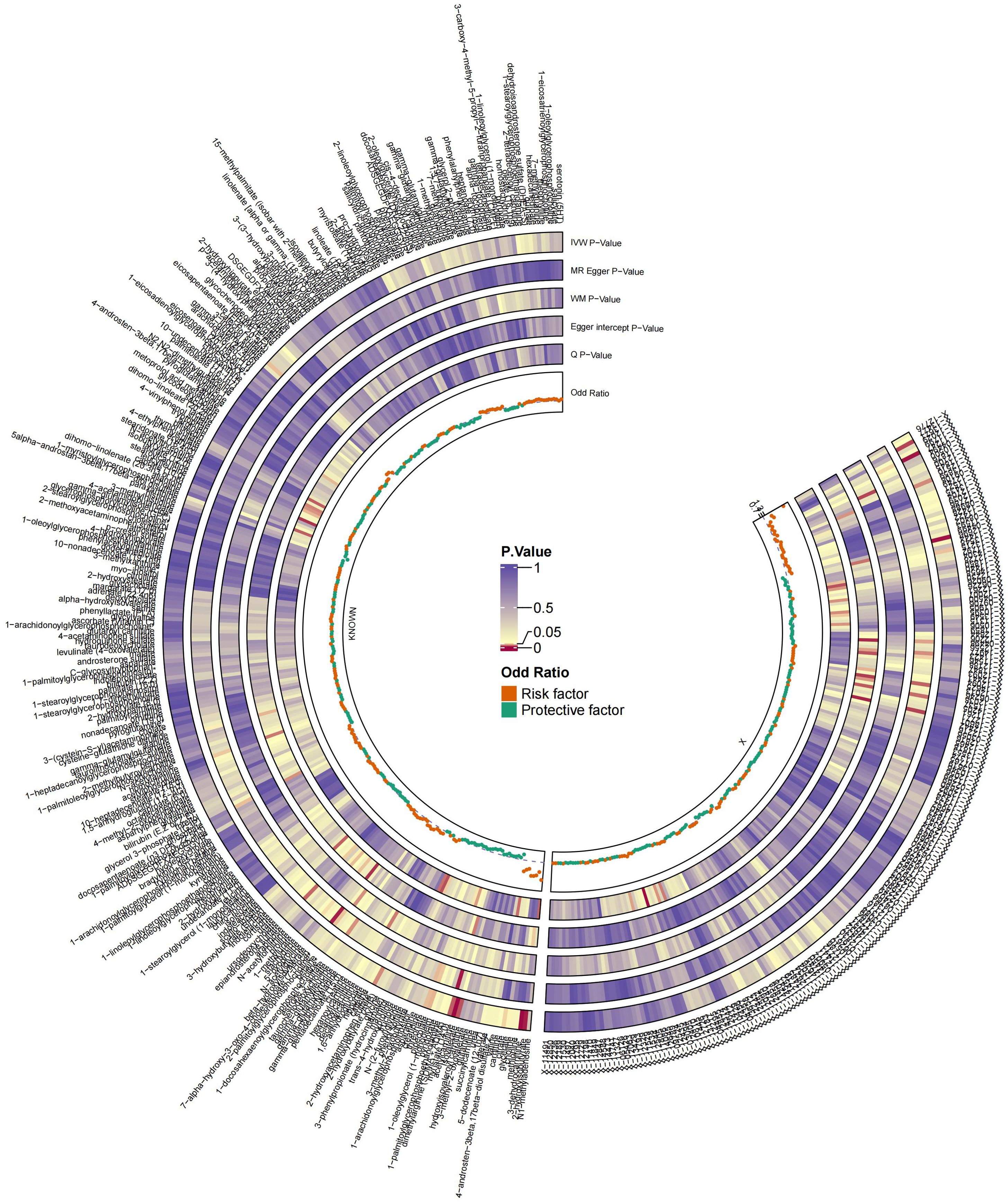
Figure 2. Summary of causal estimates regarding the impact of all plasma metabolites on PTs in MR analysis. From outside to inside, the corresponding P-values of IVW, MR-Egger, WM, Egger intercept, MRPRESSO Global Test, Q, odds ratio are represented, respectively. MR, Mendelian randomization; IVW, inverse-variance weighted; WM, weighted median.
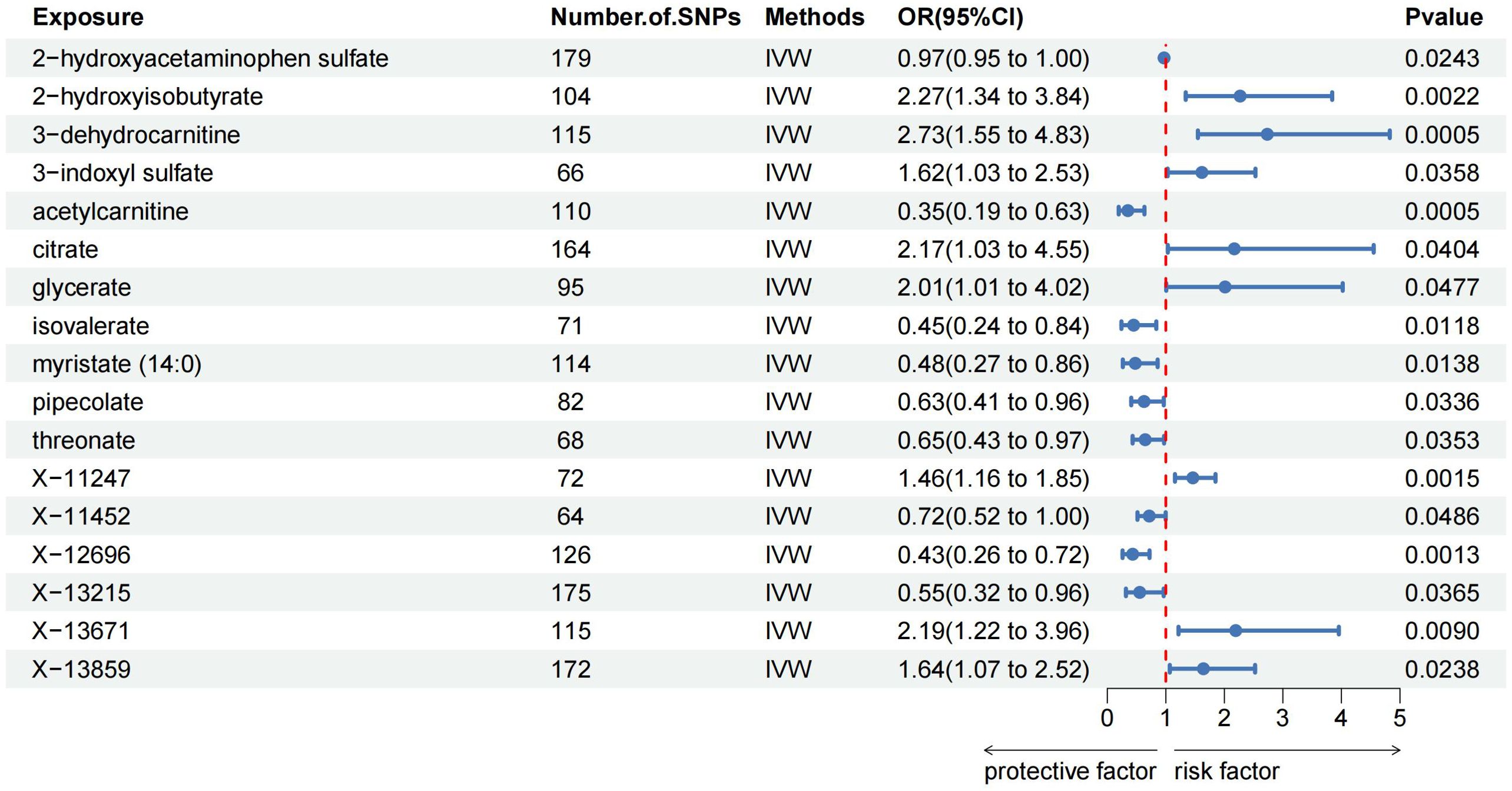
Figure 3. Forest plots illustrating the significant causal estimates of plasma metabolites on PTs are presented. SNPs, single nucleotide polymorphisms; OR, odds ratio; IVW, inverse-variance weighted.
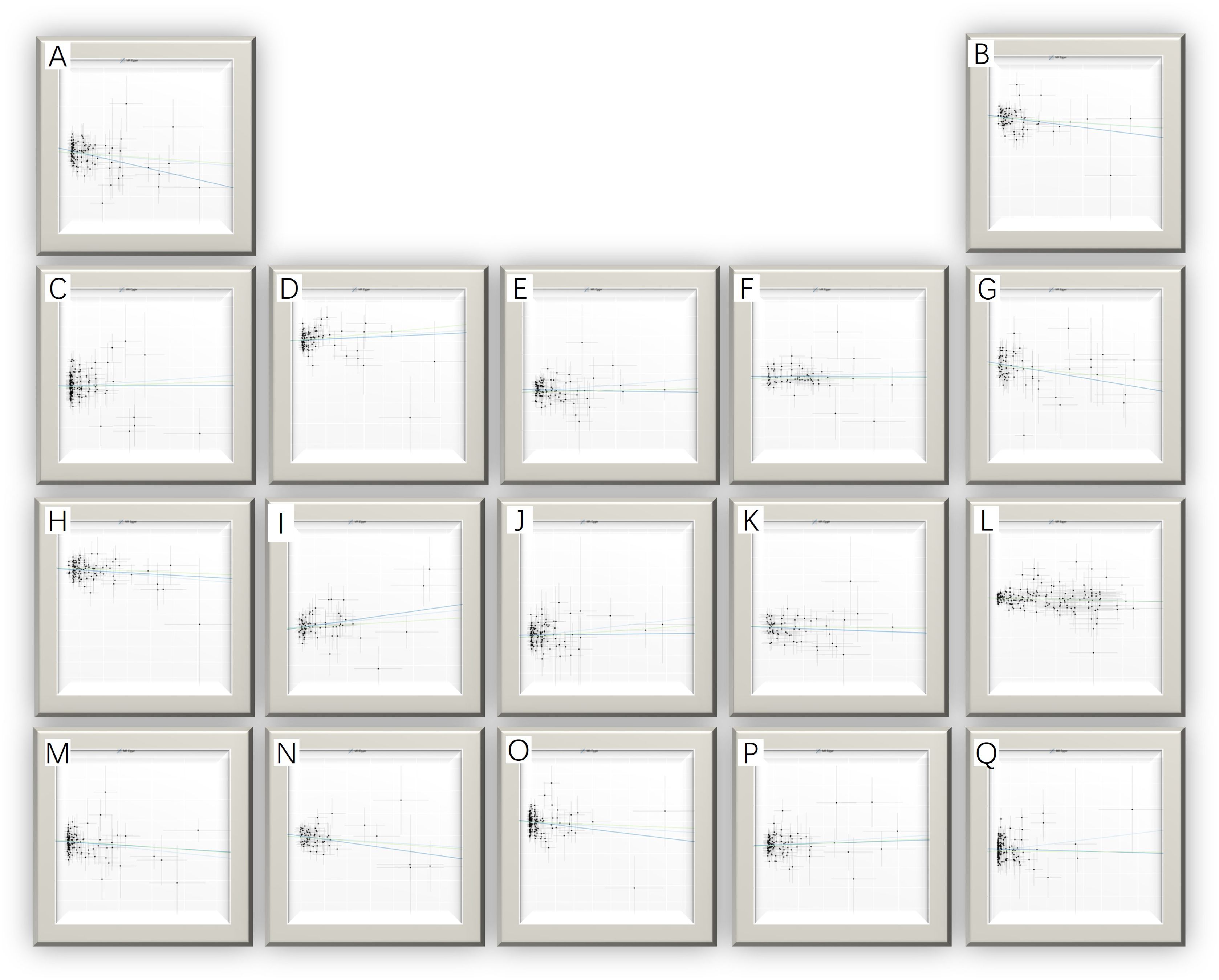
Figure 4. Scatter plots for the causal association between plasma metabolites and PTs. (A) myristate (14:0) (B) pipecolate (C) citrate (D) glycerate (E) 2-hydroxyisobutyrate (F) 3-indoxyl sulfate (G) threonate (H) acetylcarnitine (I) X-11247 (J) 3-dehydrocarnitine (K) X-11452 (L) 2-hydroxyacetaminophen sulfate (M) X-12696 (N) isovalerate (O) X-13215 (P) X-13671 (Q) X-13859.
3.2 MR analysis of 338 CSF metabolites’ impact on PTs
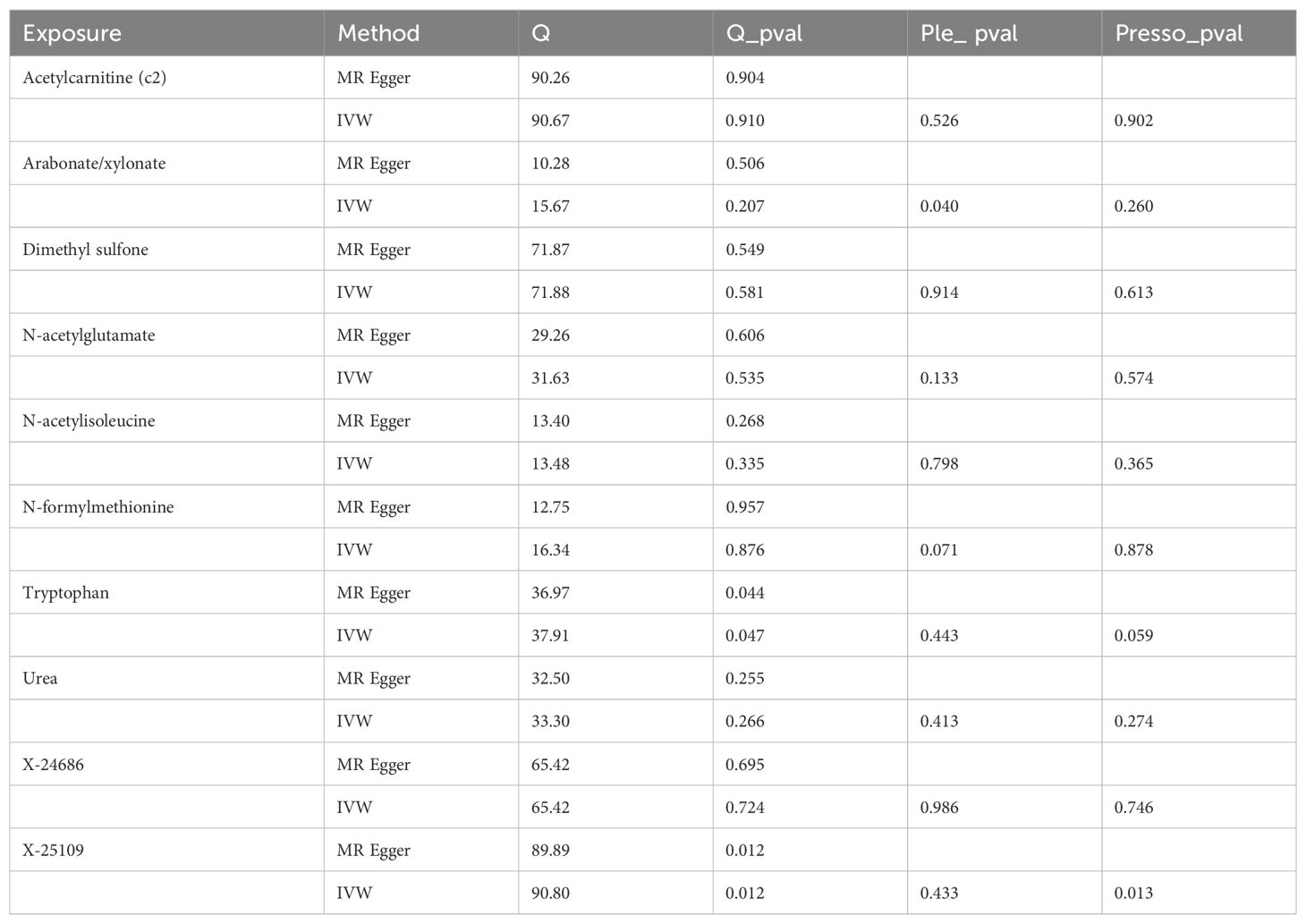
The MR analysis displayed significant associations between CSF metabolites and the risk of PTs. Specifically, Acetylcarnitine (c2) (OR=0.90, 95%CI: 0.83-0.97, P=0.005), Arabonate/xylonate (OR=0.65, 95%CI: 0.44-0.98, P=0.037), Dimethyl sulfone (OR=0.95, 95%CI: 0.90-1.00, P=0.034), N-formylmethionine (OR=0.78, 95%CI: 0.63-0.95, P=0.016), Tryptophan (OR=0.57, 95%CI: 0.33-0.96, P=0.036), Urea (OR=0.71, 95%CI: 0.52-0.97, P=0.0353) showed inverse correlations with PTs’ risk. Conversely, N-acetylglutamate (OR=1.21, 95%CI: 1.02-1.44, P=0.027) and N-acetylisoleucine (OR=1.54, 95%CI: 1.04-2.28, P=0.032) were positively associated with the risk of PTs. Additionally, two unknown CSF metabolites were discovered to be causally linked to the occurrence of PTs. (Table 1) Scatter plots illustrating these causal relationships are provided in Figure 5. However, reverse MR analysis revealed no causal association between PTs and these CSF metabolites (P<0.05).
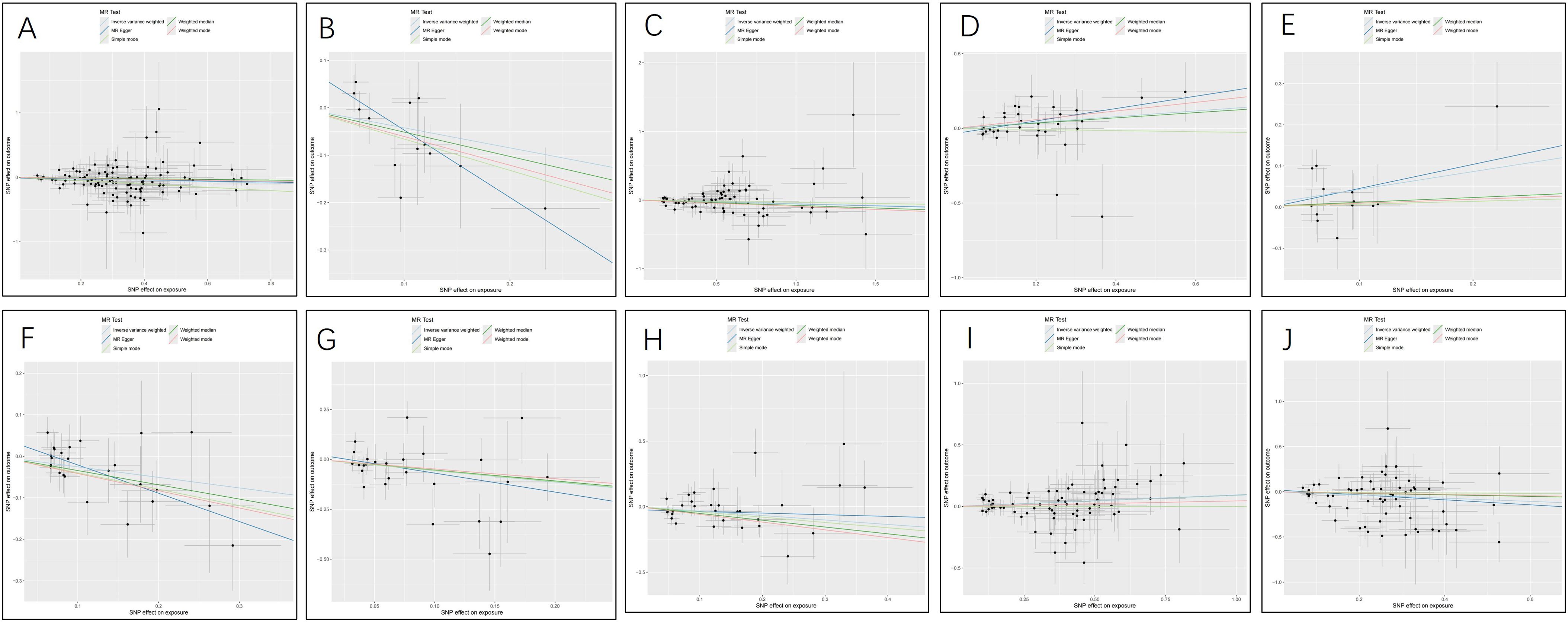
Figure 5. Scatter plots for the causal association between cerebrospinal fluid metabolites and PTs. (A) Acetylcarnitine(c2) (B) Arabonate/xylonate (C) Dimethyl sulfone (D) N-acetylglutamate (E) N-acetylisoleucine (F) N-formylmethionine (G) Tryptophan (H) Urea (I) X-24686 (J) X-25109.
3.3 Sensitivity analyses
To ensure the robustness of our findings, sensitivity analyses were conducted, confirming the integrity of IVs and hypothesis of the MR pattern through MR-Egger tests and Cochran’s Q. Regarding plasma metabolites and PTs, no evidence of heterogeneity was observed across the seventeen types of plasma metabolites. Except for 2-hydroxyisobutyrate, myristate (14:0) and X-13859, there was no pleiotropy in the other fourteen plasma metabolites. Concerning CSF metabolites and PTs, except for tryptophan and X-25109, there was no heterogeneity in the other eleven CSF metabolites. Except for arabonate/xylonate, there was no pleiotropy in the other eleven CSF metabolites. Furthermore, both leave-one-out and MR-PRESSO analyses detected no underlying instrumental outliers, providing further support for the robustness of our results. Detailed summaries of sensitivity analyses are available in Tables 2, 3, while illustrations of leave-one-out analysis and funnel plots can be discovered in Supplementary Figures 1, 2.
3.4 Metabolic pathway analyses
In the metabolic pathway analysis, two significant metabolic pathways primarily involved in PTs were identified. The results showed that the “Arginine biosynthesis” pathway (P = 0.002) and the “Glyoxylate and dicarboxylate metabolism” pathway (P = 0.008) may be involved in the development and occurrence of PTs.
4 Discussion
We used serum metabolites, CSF metabolites, and PTs for MR analysis to investigate the causality between metabolites and PTs. In our research, we identified 17 plasma metabolites and 10 CSF metabolites associated with PTs. Among them, 11 metabolites showed significant positive causality with PTs, while 16 metabolites showed significant negative causality with PTs. Our study identified a variety of serum metabolites that may function as biomarkers for PTs. Among them, serum 3-dehydrocarnitine and acetylcarnitine are the most noteworthy.
MR is analogous to randomized controlled trials in nature and uses genetic variations as IVs to make causal inferences. It is able to avoid to some extent the problems of confounders (e.g., overall patient frailty) (18) and reverse causation in traditional observational studies because genes are determined at birth and are not affected by acquired environment or disease. With the increasing application of metabolomics in various diseases within neuroendocrinology and neurosurgery, it has provided new insights and ideas for exploring therapeutic targets and biomarkers for various brain tumors. While metabolomics research on PTs remains in its infancy, numerous researches have consistently demonstrated that metabolomics plays a crucial role and holds promising prospects for studying the mechanisms of PTs (5, 19).
Metabolites play a crucial role in living organisms, participating in processes such as energy metabolism, cell signaling, and maintaining intracellular stability. Abnormal changes in metabolites can reflect the development of specific diseases. Metabolomics has shown that different subtypes of pituitary adenomas exhibit distinct metabolic profiles. Previous studies have identified differences in the levels of metabolites such as deoxycholic acid, pyridoxine, and methyl 3-adipate in ACTH adenomas (20). In prolactinomas, downregulation of N-acetylaspartate, phosphoethanolamine, and inositol expression, along with upregulation of glutamine, aspartate, and glutamate expression, has been observed (21). In invasive non-functioning pituitary adenomas, the levels of creatinine, desipramine, taurine, hypotaurine, lactate, and succinic acid were upregulated, whereas the levels of cis-11-eicosatetraenoic acid, glyceric acid, docosahexaenoic acid, arachidonic acid, hypoxanthine, lysine, linoleic acid, xanthine, valine, uracil, and oleic acid were downregulated (8). In contrast, differences in amino acids, lipids and glucose metabolites were also present in acromegaly (19). Our discovery that acetylcarnitine is inversely associated with PTs suggests that it could represent a significant therapeutic target for PTs. Acetylcarnitine, a derivative of carnitine, plays a crucial role in multiple neural pathways and has demonstrated utility in treating various neurological disorders, including dementia, cerebral ischemia, and neuroblastoma (22–25). Acetyl L-carnitine has been proposed to modulate the tumor microenvironment through its impact on the target glandular axis and subsequent neuroendocrine secretion (26). 3-Dehydrocarnitine, belonging to the carnitine family, functions as an intermediate in the degradation of carnitine. Carnitine has traditionally been linked with fatty acid metabolism. Metabolites of fatty acids might influence PTs growth by modulating the release of inflammatory factors and neurotransmitters from pertinent signaling pathways (6, 27).
Lipid metabolism is pivotal in the initiation and advancement of PTs (28). Lipid metabolism represents a significant component of the metabolic reprogramming observed in tumor cells (29). Large quantities of fatty acids can either be utilized for constructing cell membranes or oxidized to generate energy (30, 31). In addition, lipids serve not only as structural components and sources of energy for tumor cells but also regulate cell growth, differentiation, and proliferation through signaling pathways (32). Our study indicates a causal association between acetylcarnitine, myristate, isovalerate, 2-hydroxyisobutyric acid, and 3-dehydrocarnitine—lipids and their related compounds—and the development of pituitary tumors. Earlier research has also linked taurine and glyceric acid to IGF-1 levels, suggesting their potential as metabolomic biomarkers for active acromegaly (33). Additionally, integrated protein-metabolite pathway analyses demonstrated significant enrichment of multiple metabolites within the fatty acid metabolic pathway in ACTH-secreting pituitary adenomas (34). Thus, these results collectively indicate that lipid metabolism could indeed play a significant role in the development of pituitary tumors, potentially influencing metabolic reprogramming and signaling pathways like the phosphatidylinositol-3-kinase (PI3K)/Akt pathway (35–38). Furthermore, fluctuations in the levels of these lipid metabolites in pituitary tumors can serve as biomarkers for tumor diagnosis and treatment.
The relationship between amino acid metabolism and PTs is complex (39). Our study suggests that some amino acid metabolites such as Arabonate, N-formylmethionine, Tryptophan, N-acetylglutamate and N-acetylisoleucine are causally associated with the development of PTs. Notably, N-acetylglutamate is related to the glutamine metabolic pathway. One of the most important mechanisms linking amino acid metabolism and PTs is glutamine metabolism, which is altered in pituitary tumorigenesis and may vary across different clinical types of PTs (40). Glutamine provides ATP to tumor cells, supplies critical precursors for nucleotides, and helps tumor cells survive in hostile environments through the synthesis of glutathione (41). Additionally, glutamine metabolism is intricately linked to the mTOR signaling pathway, which is crucial for tumor cell proliferation and growth (42, 43). Furthermore, certain amino acids, such as tryptophan, may influence the pituitary gland via the neuroendocrine pathway, indirectly affecting pituitary cell proliferation and tumor formation (44).
Further transcriptome analysis suggests that ligands associated with 3-dehydrocarnitine, including PPARα, AMPK, SREBP, ChREBP, PGC-1α, and SIRT, may be involved in the signaling pathways of gonadotroph tumors, while PPARα, AMPK, SREBP, and ChREBP ligands may play a role in the signaling pathways of prolactinoma. Similarly, ligands associated with acetylcarnitine, such as PPARα, PGC-1α, SIRT1, PPARδ, SREBP-1c, LXRα/β, and PPARγ, are likely involved in the signaling of gonadotroph tumors. Additionally, PPARα, PGC-1α, and FOXO1 ligands linked to acetylcarnitine may participate in the signaling pathways of prolactinoma, while SREBP-1c, LXRα/β, PPARγ, HIF-1α, PPARα, PGC-1α, and FOXO1 are likely involved in the signaling of nonfunctional pituitary tumors (45). These transcriptome ligands jointly regulate the expression of related metabolites and genes, modulating signaling pathways that ultimately impact tumor development and progression (Supplementary Table 1).
The arginine biosynthetic pathway consists of a series of chemical reactions that convert amino acids into arginine. Arginine is an essential amino acid with several cellular functions, including protein synthesis and cell proliferation (46). Tumor cells may upregulate the arginine synthesis pathway to meet the demands of rapid proliferation, particularly in pituitary tumors (47). Furthermore, arginine metabolism is closely related to immune regulation (48). Pituitary tumor cells can inhibit T-cell activity by depleting arginine, thereby evading the immune system’s attack (49). Key enzymes in the arginine synthesis pathway, such as arginase and nitric oxide synthase, are upregulated in pituitary tumors and may serve as diagnostic markers or therapeutic targets. Glyoxylate is metabolized via the glyoxylate cycle or glycine pathway to produce the intermediate product glyoxylate, which can be further metabolized to oxalate or converted into other intermediates that enter the tricarboxylic acid cycle. The accumulation of glyoxylate and its metabolites may influence the oxidative stress levels and metabolic state of pituitary tumor cells, thereby promoting tumor growth and survival (50). Dicarboxylic acids, including oxalic acid, fumaric acid, and succinic acid, are crucial intermediates of the TCA cycle, involved in energy production and cellular metabolism (51). Pituitary tumor cells may undergo metabolic reprogramming in the dicarboxylate metabolic pathway, promoting cell proliferation and anti-apoptosis by affecting ATP production, redox state, and the accumulation of metabolic by-products (38). Investigating abnormalities in the arginine metabolic pathway, as well as the glyoxylate and dicarboxylate metabolic pathways, may help identify pituitary tumor-specific biomarkers for diagnosis and monitoring disease progression. Drugs targeting these metabolic pathways could be utilized in the treatment of pituitary tumors. For example, inhibiting key enzymes in these pathways may reduce the energy supply and growth advantage of tumor cells. Additionally, by modulating oxidative stress levels, the proliferation of tumor cells may be inhibited, and apoptosis may be promoted.
Our study examined the causality of serum and CSF metabolites with PTs, identifying numerous metabolites that could serve as potential biomarkers for PTs. Notably, serum 3-dehydrocarnitine and acetylcarnitine emerged as the most promising biomarkers. However, several limitations must be acknowledged. Firstly, our MR analyses were based on metabolite GWAS data with a limited sample size, necessitating the collection of more samples and metabolite species across diverse populations to accurately elucidate the relationship between metabolites and PTs. Secondly, our study represents a preliminary exploration; thus, further experimental researches are desired to validate these results and investigate underlying mechanisms in greater detail. Thirdly, our findings may be specific to European populations and might not be generalizable to other ethnic groups. Lastly, due to the exploratory nature of our study, multiple testing adjustments were not applied; however, we ensured the robustness of our results by employing multiple MR algorithms.
5 Conclusions
In conclusion, our comprehensive analysis of 486 plasma metabolites and 338 CSF metabolites successfully identified 27 metabolites associated with PTs. These findings underscore the potential of plasma and CSF metabolites, particularly serum 3-dehydrocarnitine and acetylcarnitine, as biomarkers for PTs. This research opens promising avenues for early detection and risk assessment of PTs.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval and consent are not required for this study in accordance with local or national guidelines.
Author contributions
WW: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. ML: Conceptualization, Data curation, Formal Analysis, Writing – review & editing. ZW: Conceptualization, Project administration, Visualization, Writing – review & editing. WY: Conceptualization, Writing – review & editing. XL: Conceptualization, Funding acquisition, Project administration, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported in part by grants from Neurosurgery First-class Discipline Funding Project of the Second Affiliated Hospital of Harbin Medical University.
Acknowledgments
We thank the investigators and participants of the original GWAS. We are grateful for all GWAS sharing summary data used in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1460278/full#supplementary-material
Abbreviations
PTs, Pituitary tumors; MR, Mendelian randomization; GWAS, genome-wide association study; CSF, cerebrospinal fluid; IVs, instrumental variables; ICD-10, International Classification of Diseases, 10th Revision; IVW, inverse variance weighting; MR-PRESSO, Mendelian randomized polytomous residuals and outliers; SMP, Small Molecule Pathway.
References
1. Laws ER Jr., Penn DL, Repetti CS. Advances and controversies in the classification and grading of pituitary tumors. J Endocrinol Invest. (2019) 42:129–35. doi: 10.1007/s40618-018-0901-5
2. Araujo-Castro M, Berrocal VR, Pascual-Corrales E. Pituitary tumors: epidemiology and clinical presentation spectrum. Hormones (Athens). (2020) 19:145–55. doi: 10.1007/s42000-019-00168-8
4. Tritos NA, Miller KK. Diagnosis and management of pituitary adenomas: A review. Jama. (2023) 329:1386–98. doi: 10.1001/jama.2023.5444
5. Pînzariu O, Georgescu B, Georgescu CE. Metabolomics-A promising approach to pituitary adenomas. Front Endocrinol (Lausanne). (2018) 9:814. doi: 10.3389/fendo.2018.00814
6. Hu J, Yan J, Zheng X, Zhang Y, Ran Q, Tang X, et al. Magnetic resonance spectroscopy may serve as a presurgical predictor of somatostatin analog therapy response in patients with growth hormone-secreting pituitary macroadenomas. J Endocrinol Invest. (2019) 42:443–51. doi: 10.1007/s40618-018-0939-4
7. Duerson K, White RE, Jiang F, Schonbrunn A, Armstrong DL. Somatostatin stimulates BKCa channels in rat pituitary tumor cells through lipoxygenase metabolites of arachidonic acid. Neuropharmacology. (1996) 35:949–61. doi: 10.1016/0028-3908(96)00131-1
8. Lin K, Zhang J, Lin Y, Pei Z, Wang S. Metabolic characteristics and M2 macrophage infiltrates in invasive nonfunctioning pituitary adenomas. Front Endocrinol (Lausanne). (2022) 13:901884. doi: 10.3389/fendo.2022.901884
9. Tang J, Mou M, Zheng X, Yan J, Pan Z, Zhang J, et al. Strategy for identifying a robust metabolomic signature reveals the altered lipid metabolism in pituitary adenoma. Anal Chem. (2024) 96:4745–55. doi: 10.1021/acs.analchem.3c03796
10. Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. Jama. (2017) 318:1925–6. doi: 10.1001/jama.2017.17219
11. Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomization: the STROBE-MR statement. Jama. (2021) 326:1614–21. doi: 10.1001/jama.2021.18236
12. Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner KM, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. (2023) 613:508–18. doi: 10.1038/s41586-022-05473-8
13. Shin SY, Fauman EB, Petersen AK, Krumsiek J, Santos R, Huang J, et al. An atlas of genetic influences on human blood metabolites. Nat Genet. (2014) 46:543–50. doi: 10.1038/ng.2982
14. Panyard DJ, Kim KM, Darst BF, Deming YK, Zhong X, Wu Y, et al. Cerebrospinal fluid metabolomics identifies 19 brain-related phenotype associations. Commun Biol. (2021) 4:63. doi: 10.1038/s42003-020-01583-z
15. Kamat MA, Blackshaw JA, Young R, Surendran P, Burgess S, Danesh J, et al. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics. (2019) 35:4851–3. doi: 10.1093/bioinformatics/btz469
16. Yang M, Wan X, Zheng H, Xu K, Xie J, Yu H, et al. No evidence of a genetic causal relationship between ankylosing spondylitis and gut microbiota: A two-sample mendelian randomization study. Nutrients. (2023) 15:1057. doi: 10.3390/nu15041057
17. Gu Y, Jin Q, Hu J, Wang X, Yu W, Wang Z, et al. Causality of genetically determined metabolites and metabolic pathways on osteoarthritis: a two-sample mendelian randomization study. J Transl Med. (2023) 21:357. doi: 10.1186/s12967-023-04165-9
18. Findlay MC, Rennert RC, Lucke-Wold B, Couldwell WT, Evans JJ, Collopy S, et al. Impact of frailty on surgical outcomes of patients with cushing disease using the multicenter registry of adenomas of the pituitary and related disorders registry. Neurosurgery. (2024), 10.1227. doi: 10.1227/neu.0000000000003090
19. Pînzariu O, Georgescu CE. Metabolomics in acromegaly: a systematic review. J Investig Med. (2023) 71:634–45. doi: 10.1177/10815589231169452
20. Oklu R, Deipolyi AR, Wicky S, Ergul E, Deik AA, Chen JW, et al. Identification of small compound biomarkers of pituitary adenoma: a bilateral inferior petrosal sinus sampling study. J neurointerv Surg. (2014) 6:541–6. doi: 10.1136/neurintsurg-2013-010821
21. Ijare OB, Baskin DS, Pichumani K. Ex Vivo (1)H NMR study of pituitary adenomas to differentiate various immunohistochemical subtypes. Sci Rep. (2019) 9:3007. doi: 10.1038/s41598-019-38542-6
22. Dhitavat S, Ortiz D, Rogers E, Rivera E, Shea TB. Folate, vitamin E, and acetyl-L-carnitine provide synergistic protection against oxidative stress resulting from exposure of human neuroblastoma cells to amyloid-beta. Brain Res. (2005) 1061:114–7. doi: 10.1016/j.brainres.2005.05.074
23. Hudson S, Tabet N. Acetyl-L-carnitine for dementia. Cochrane Database Syst Rev. (2003) 2003:Cd003158. doi: 10.1002/14651858.CD003158
24. Maldonado C, Vázquez M, Fagiolino P. Potential therapeutic role of carnitine and acetylcarnitine in neurological disorders. Curr Pharm Des. (2020) 26:1277–85. doi: 10.2174/1381612826666200212114038
25. Calvani M, Arrigoni-Martelli E. Attenuation by acetyl-L-carnitine of neurological damage and biochemical derangement following brain ischemia and reperfusion. Int J Tissue React. (1999) 21:1–6.
26. Krsmanović LZ, Virmani MA, Stojilković SS, Catt KJ. Actions of acetyl-L-carnitine on the hypothalamo-pituitary-gonadal system in female rats. J Steroid Biochem Mol Biol. (1992) 43:351–8. doi: 10.1016/0960-0760(92)90170-N
27. Bach E, Møller AB, Jørgensen JO, Vendelbo MH, Jessen N, Olesen JF, et al. Intact pituitary function is decisive for the catabolic response to TNF-α: studies of protein, glucose and fatty acid metabolism in hypopituitary and healthy subjects. J Clin Endocrinol Metab. (2015) 100:578–86. doi: 10.1210/jc.2014-2489
28. Hu C, Zhou Y, Feng J, Zhou S, Li C, Zhao S, et al. Untargeted lipidomics reveals specific lipid abnormalities in nonfunctioning human pituitary adenomas. J Proteome Res. (2020) 19:455–63. doi: 10.1021/acs.jproteome.9b00637
29. Onizuka H, Masui K, Amano K, Kawamata T, Yamamoto T, Nagashima Y, et al. Metabolic reprogramming drives pituitary tumor growth through epigenetic regulation of TERT. Acta Histochem Cytochem. (2021) 54:87–96. doi: 10.1267/ahc.21-00007
30. de Carvalho C, Caramujo MJ. The various roles of fatty acids. Molecules. (2018) 23:2583. doi: 10.3390/molecules23102583
31. Currie E, Schulze A, Zechner R, Walther TC, Farese RV Jr. Cellular fatty acid metabolism and cancer. Cell Metab. (2013) 18:153–61. doi: 10.1016/j.cmet.2013.05.017
32. Koundouros N, Poulogiannis G. Reprogramming of fatty acid metabolism in cancer. Br J Cancer. (2020) 122:4–22. doi: 10.1038/s41416-019-0650-z
33. Yu H, Zhao Y, Zhang Y, Zhong L. Metabolic profiling of acromegaly using a GC-MS-based nontargeted metabolomic approach. Endocrine. (2020) 67:433–41. doi: 10.1007/s12020-019-02143-0
34. Feng J, Zhang Q, Zhou Y, Yu S, Hong L, Zhao S, et al. Integration of proteomics and metabolomics revealed metabolite-protein networks in ACTH-secreting pituitary adenoma. Front Endocrinol (Lausanne). (2018) 9:678. doi: 10.3389/fendo.2018.00678
35. Monsalves E, Juraschka K, Tateno T, Agnihotri S, Asa SL, Ezzat S, et al. The PI3K/AKT/mTOR pathway in the pathophysiology and treatment of pituitary adenomas. Endocr Relat Cancer. (2014) 21:R331–44. doi: 10.1530/ERC-14-0188
36. Rubinfeld H, Shimon I. PI3K/Akt/mTOR and Raf/MEK/ERK signaling pathways perturbations in non-functioning pituitary adenomas. Endocrine. (2012) 42:285–91. doi: 10.1007/s12020-012-9682-3
37. Hoxhaj G, Manning BD. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat Rev Cancer. (2020) 20:74–88. doi: 10.1038/s41568-019-0216-7
38. Sabatino ME, Grondona E, De Paul AL. Architects of pituitary tumour growth. Front Endocrinol (Lausanne). (2022) 13:924942. doi: 10.3389/fendo.2022.924942
39. Ijare OB, Holan C, Hebert J, Sharpe MA, Baskin DS, Pichumani K. Elevated levels of circulating betahydroxybutyrate in pituitary tumor patients may differentiate prolactinomas from other immunohistochemical subtypes. Sci Rep. (2020) 10:1334. doi: 10.1038/s41598-020-58244-8
40. Hu J, Chen Q, Ding X, Zheng X, Tang X, Li S, et al. Glutamine metabolism in the proliferation of GS-expression pituitary tumor cells. Endocr Connect. (2020) 9:223–33. doi: 10.1530/EC-19-0515
41. Li X, Peng X, Li Y, Wei S, He G, Liu J, et al. Glutamine addiction in tumor cell: oncogene regulation and clinical treatment. Cell Commun Signal. (2024) 22:12. doi: 10.1186/s12964-023-01449-x
42. Foster R, Griffin S, Grooby S, Feltell R, Christopherson C, Chang M, et al. Multiple metabolic alterations exist in mutant PI3K cancers, but only glucose is essential as a nutrient source. PloS One. (2012) 7:e45061. doi: 10.1371/journal.pone.0045061
43. Chen R, Duan J, Li L, Ma Q, Sun Q, Ma J, et al. mTOR promotes pituitary tumor development through activation of PTTG1. Oncogene. (2017) 36:979–88. doi: 10.1038/onc.2016.264
44. Torres-Aleman I, Redding TW, Schally AV. Inhibition of growth of a prolactin and growth hormone-secreting pituitary tumor in rats by D-tryptophan-6 analog of luteinizing hormone-releasing hormone. Proc Natl Acad Sci U S A. (1985) 82:1252–6. doi: 10.1073/pnas.82.4.1252
45. Batchu S, Diaz MJ, Patel A, Reddy A, Lucke-Wold B. Transcriptome-derived ligand-receptor interactome of major pitNET subgroups. J Neurol Surg B Skull Base. (2024) 85:340–6. doi: 10.1055/a-2088-6594
46. Tong BC, Barbul A. Cellular and physiological effects of arginine. Mini Rev Med Chem. (2004) 4:823–32. doi: 10.2174/1389557043403305
47. Poillet-Perez L, Xie X, Zhan L, Yang Y, Sharp DW, Hu ZS, et al. Autophagy maintains tumour growth through circulating arginine. Nature. (2018) 563:569–73. doi: 10.1038/s41586-018-0697-7
48. Popovic PJ, Zeh HJ 3rd, Ochoa JB. Arginine and immunity. J Nutr. (2007) 137:1681s–6s. doi: 10.1093/jn/137.6.1681S
49. Zhao G, Chen W, He J, Cui C, Zhao L, Zhao Y, et al. Analysis of cyclooxygenase 2, programmed cell death ligand 1, and arginase 1 expression in human pituitary adenoma. World Neurosurg. (2020) 144:e660–e73. doi: 10.1016/j.wneu.2020.09.031
50. Altinoz MA, Ozpinar A. Oxamate targeting aggressive cancers with special emphasis to brain tumors. BioMed Pharmacother. (2022) 147:112686. doi: 10.1016/j.biopha.2022.112686
Keywords: Mendelian randomization, 3-dehydrocarnitine, acetylcarnitine, pituitary tumors, metabolites, pathogenesis
Citation: Wang W, Liu M, Wang Z, Ye W and Li X (2024) Elucidating the causal links between plasma and cerebrospinal fluid metabolites and pituitary tumors: a Mendelian randomization analysis. Front. Endocrinol. 15:1460278. doi: 10.3389/fendo.2024.1460278
Received: 05 July 2024; Accepted: 12 November 2024;
Published: 28 November 2024.
Edited by:
Krzysztof Cezary Lewandowski, Medical University of Lodz, PolandReviewed by:
Nidan Qiao, Fudan University, ChinaBrandon Peter Lucke-Wold, University of Florida, United States
Copyright © 2024 Wang, Liu, Wang, Ye and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianfeng Li, bGl4aWFuZmVuZzIwMDBAMTYzLmNvbQ==
†ORCID: Wencai Wang, orcid.org/0009-0004-5673-1643
Xianfeng Li, orcid.org/0000-0001-8549-537X
 Wencai Wang
Wencai Wang Menghao Liu
Menghao Liu Xianfeng Li
Xianfeng Li