
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 03 September 2024
Sec. Pediatric Endocrinology
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1456541
This article is part of the Research Topic Insights in Pediatric Endocrinology: 2024 View all 18 articles
 Maura Marin1†‡
Maura Marin1†‡ Flora Maria Murru2†‡
Flora Maria Murru2†‡ Francesco Baldo2‡
Francesco Baldo2‡ Gianluca Tamaro2‡
Gianluca Tamaro2‡ Elena Faleschini2‡
Elena Faleschini2‡ Egidio Barbi1,2‡
Egidio Barbi1,2‡ Gianluca Tornese1,2*‡
Gianluca Tornese1,2*‡Background: Brain magnetic resonance imaging (MRI) is mandatory or highly recommended in many pediatric endocrinological conditions to detect causative anatomic anomalies and rule out neoplastic lesions. However, MRI can also show findings associated with the underlying clinical condition, as well as unrelated “incidentalomas”. These latter findings are often abnormalities with a high incidence in the general population for which there is no clear literature regarding their management, especially in pediatric patients. The present study aimed to evaluate the number of unnecessary performed MRIs in pediatric endocrinology.
Methods: Retrospective analysis on 584 MRI scans performed in 414 patients (254 growth hormone deficiency, 41 other causes of short stature, 116 central precocious puberty).
Results: The MRI scans were completely normal in 67% of the individuals, and the prevalence of individuals who underwent more than one MRI was 18%, with no significant differences among the groups. The overall prevalence of incidentalomas was 17%. Among 170 repeated MRI scans, 147 (86%) were not required according to a dedicated protocol. Only five patients (four GHD, one Noonan) correctly repeated the MRI. All the repeated MRI scans did not reveal any progression in the findings. If we include the MRIs performed in cases of OCSS other than Noonan syndrome (n=32) and girls with CPP older than 6 years (n=89), an additional 121 MRIs could have been avoided, leading to a total number of unnecessary MRIs to 268 (46%).
Conclusions: Only a few specific neuroimaging findings in endocrinologic pediatric patients warrant further investigation, while too often repeated imaging is carried out unnecessarily. We advocate the importance of guidelines to reduce costs for both the healthcare system and patients’ families, as well as to alleviate physical and psychological distress for patients and caregivers.
Brain magnetic resonance imaging (MRI) is mandatory or highly recommended to detect causative anatomic anomalies and rule out neoplastic lesions in many pediatric endocrinological conditions, such as central precocious puberty (CPP) (1) and growth hormone deficiency (GHD) (2) or other causes of short stature (OCSS) in which recombinant human growth hormone (rhGH) is prescribed such as Noonan syndrome (3). Often, previously unsuspected anomalies and malformations are found at brain MRI and sometimes these findings are completely unrelated to the clinical aspect for which the radiological examination was requested (4, 5). In the latter case, such a finding is referred to as “incidentaloma”. Due to the lack of clear pediatric guidelines for the management and follow-up of these radiological findings, sometimes the use of radiological exams might become excessive and even disproportionate to the patient’s medical needs. In a previous study, we reported the suggested management of the most frequent brain findings found in pediatric patients affected by GHD and CPP (6), which would result in a reduction of not only economic but also physical and psychological implications. The present study aimed to evaluate the number of unnecessary performed MRIs in pediatric endocrinology. Our goal was to highlight that, based on the current literature, most of the follow-up brain MRIs are probably not required and that only a few neuroimaging findings are worth subsequent investigations.
This is a retrospective cohort study on children who had a brain MRI with pituitary protocol performed at the Institute for Maternal and Child Health IRCSS “Burlo Garofolo” in Trieste, Italy, from 01/07/2007 to 31/12/2020, because of a diagnosis of CPP, GHD, or OCSS conditions in which rhGH is prescribed (i.e., Turner syndrome, chronic renal insufficiency, Prader–Willi syndrome, SHOX deficiency, children born small for gestational age [SGA] without catch-up growth, Noonan syndrome, and idiopathic short stature [ISS] cleared by the regional GH commission) (7). GHD was diagnosed in children with growth defects (height ≤−3 SDS; or height ≤−2 SDS and growth velocity ≤−1 SDS; or growth velocity ≤−2 SDS or ≤−1.5 SDS after 2 consecutive years, even without short stature) based on positive arginine and insulin stimulation tests; a GH peak below 8 μg/L in both tests was considered as pathological (8); sex steroid priming was not used since it is not recommended in our national guidelines; and patients with already known multiple pituitary deficits were excluded from the study. PPC was diagnosed in patients with secondary sexual characteristics before 8 years of age in girls and 9 years in boys with a GnRH test showing an LH peak >5 mU/mL and/or an LH/FSH peak ratio >1. In accordance with our internal protocol, all OCSS patients received an MRI scan prior to commencing rhGH therapy to rule out any preexisting brain tumors.
Patients with previously known abnormal brain MRI scans requiring follow-up (n=8) were excluded from the study. The “G2 clinico” platform (management system specialist activities) was employed to access all patients’ data. Information retrieved included date of birth, sex, indication for brain MRI (GHD, OCSS, CPP), date of brain MRI, and found abnormalities (if any). If multiple MRIs were performed, the number of MRIs, any changes in the findings, and the date of the last MRI were recorded.
Brain MRI with gadolinium-based contrast agents and with specific sequences for the hypothalamic–pituitary region was performed with a 1.5 Tesla Ingenia MR scanner (Philips Healthcare, Best, The Netherlands). Imaging in static mode with axial T2-, T1- and FLAIR (fluid-attenuated inversion recovery)-weighted sequences of the whole brain and subsequent thin-layer evaluation targeted to the pituitary gland in the sagittal and coronal planes, with T2- and T1-weighted sequences, were performed before and after administration of a full-dose contrast agent. Coronal scans allowed visualization of the pituitary gland, pedicle, chiasm, and parasellar regions whereas sagittal images were more suitable for evaluation of the midline plane. The acquisition procedure lasted an average of 20 min–30 min, and sedation was considered as a protocol in children <8 years of age (between 7 and 8 years was evaluated on a case-by-case basis). All the MRI scans were reviewed by an expert pediatric radiologist (FMM) with more than 15 years of experience.
The guidelines for the management and follow-up of MRI brain findings were first introduced in January 2021 (6). The following findings were considered as “alterations with definite or possible clinical/anatomical significance” (ADPCAS): adenohypophysis hypoplasia, pituitary stalk interruption syndrome (PSIS), ectopic neurohypophysis, complete or partial empty sella; Rathke cleft cyst (RCC), pituitary adenoma, craniopharyngiomas (or other tumors in the hypothalamus–pituitary region), and Arnold-Chiari type I. The following findings were included within the group “alterations without clinical significance/incidentalomas”: arachnoid cyst, pineal cyst, choroid plexus cysts, vascular abnormalities, and increased pituitary volume. Other findings with low frequency (n ≤ 4) and not related to GHD and CPP were grouped as “other”. The following findings were considered as “alterations worthy of radiological follow-up”: (1) craniopharyngioma or other tumors (follow-up after surgery ± radio/chemotherapy according to the oncological guidelines); (2) pituitary adenoma: if symptomatic, surgical or medical therapy + MRI follow-up according to the oncological guidelines; if asymptomatic and ≥10 mm, MRI once per year for 3 years and then every 1–2 years, if asymptomatic and ≥5 mm (<10 mm), a single MRI after 1 year; (3) RCC: if symptomatic, radiologic follow-up for at least 5 years postsurgery; if asymptomatic and >5 mm, MRI at 1, 3, and 5 years (regardless of characteristics); (4) arachnoid cyst, if large and in high-risk regions; (5) pineal cysts, if >14 mm and/or with an abnormal radiological pattern or clinical symptoms.
Ethical Committee approval was not requested since General Authorization to Process Personal Data for Scientific Research Purposes (Authorization no. 9/2014) declared that retrospective archive studies that use ID codes, preventing the data from being traced back directly to the data subject, do not need ethics approval (9). Informed consent was signed by parents at the first visit, in which they agreed that “clinical data may be used for clinical research purposes, epidemiology, the study of pathologies and training, to improve knowledge, care and prevention”. All data were collected in an anonymous database.
All statistical analyses were conducted with JMP™ (version 16.1.0, SAS Institute Inc., Cary, NC, United States). Descriptive statistics was used to describe data. Continuous variables were expressed as median with interquartile range, minimum, and maximum. Categorical data were expressed as percentages (%). For the comparison of continuous variables between more than two groups, the Kruskal–Wallis test was used. The chi-square test was used to compare categorical variables between the groups. Statistical significance was considered for p-values <0.05.
The study has been reported in line with the STROBE statement for observational studies (10).
During the study period, a total of 584 MRI scans were performed on 414 patients: 257 GHD, 41 OCSS, 116 CPP. In the OCSS group, 16 were SGA (of which 3 Silver–Russell syndrome), 9 were Noonan syndrome, 7 were ISS, 3 were Turner syndrome, 3 were SHOX deficiency, 2 were IRC, and 1 was Prader–Willi syndrome.
Clinical and imaging features of the entire cohort and the three groups are presented in Table 1. Age at presentation was lower in CPP and OCSS (median 8.7 and 9.1 years, respectively) compared with GHD (median 11.5 years) (p<0.01), whereas CPP had a higher prevalence of women (83%) compared with GHD (37%) and OCSS (46%) (p<0.01). In the CPP group, 7 out of 96 women (7%) were diagnosed below the age of 6 years.
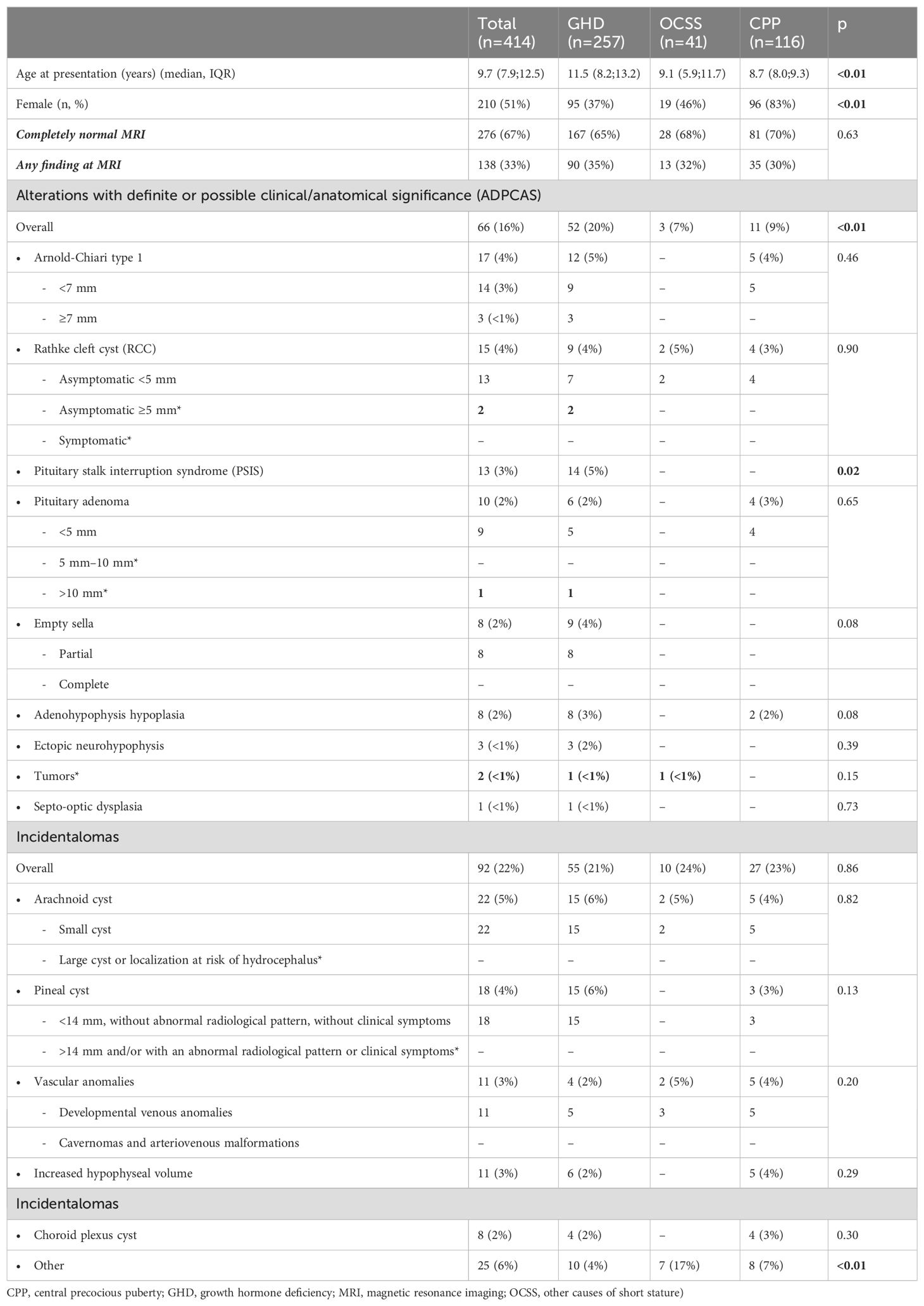
Table 1. Clinical and imaging features of the entire cohort and the three groups (* and in bold: alterations worthy of radiological follow-up.
MRI scan was completely normal in 67% of the individuals (n=276) (Figure 1), with no significant differences among groups (GHD 65%, OCSS 68%, CPP 70%, p=0.63), whereas in 33% of the cases, a finding was reported (Table 1). ADPCAS were found in 16% (n=66) of the entire cohort (Figure 1): the GHD group had a higher prevalence (21%), compared with OCSS (7%) and CPP (9%) (p<0.01); in particular, PSIS (n=13), empty sella (n=8), adenohypophysis hypoplasia (n=8), and ectopic neurohypophysis (n=3) were only found in the GHD group (Table 1). Arnold–Chiari type 1 malformation, pituitary adenoma, and adenohypophysis hypoplasia were also detected in CPP, whereas RCC were found in both CPP and OCSS (1 Noonan, 1 ISS) patients, with no significant differences among groups (Table 1). No craniopharyngiomas were detected during the study period, but a dysgerminoma was found in a patient with GHD and a dysembryoplastic neuroepithelial tumor (DNET) in a patient with Noonan syndrome.
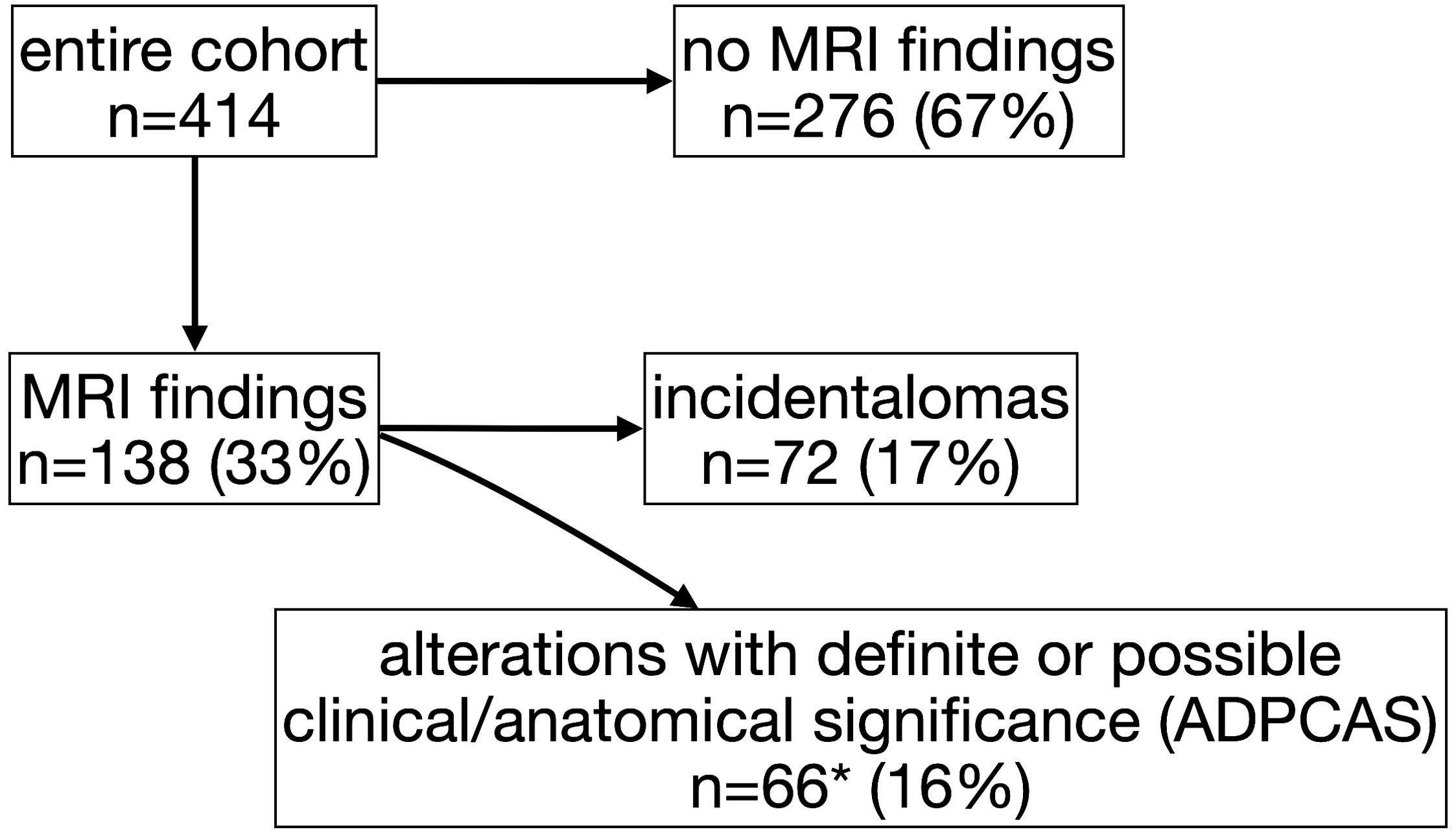
Figure 1. Flow diagram of study participants and magnetic resonance imaging (MRI) findings (*includes 20 patients whit also “incidentalomas”).
There was no significant difference in the median age between GHD patients with ADPCAS (11.3 years [6.7; 13.7]) and those without ADPCAS (11.6 years [8.57; 13.16]) (p=0.82). Similarly, the median age of CPP patients with APCAS (8.2 years [7.9; 9.2]) was not significantly different from those without APCAS (8.7 years [8.0; 9.3]) (p=0.39).
The overall prevalence of incidentalomas was 17% (Figure 1)—22% if we consider also those who had both incidentalomas and ADPCAS (n=92)—with no significant differences among groups (p=0.79). OCSS had a higher prevalence (21%) of “other” findings compared with CPP (7%) and GHD (6%): most of them were related to the underlying syndrome (Table 1).
The prevalence of individuals who performed more than one MRI was 18% (n=73), with no significant differences among diagnostic groups (GHD 19%, OCSS 10%, CPP 18%, p=0.37) (Table 2); the prevalence of patients with ADPCAS that performed more than one MRI (57%) was significantly higher than those without ADPCAS (10%, p<0.01). The median number of MRIs was 3, with a maximum of 11 in GHD, 7 in OCSS, and 5 in CPP, and the median distance from the first to the last MRI was 1.6 years (Table 2). The number of MRIs in patients with ADPCAS (median 4 [IQR 2;5], max 11) was significantly higher than in patients without ADPCAS (median 2 [IQR 2;3], max 6) (p<0.01).
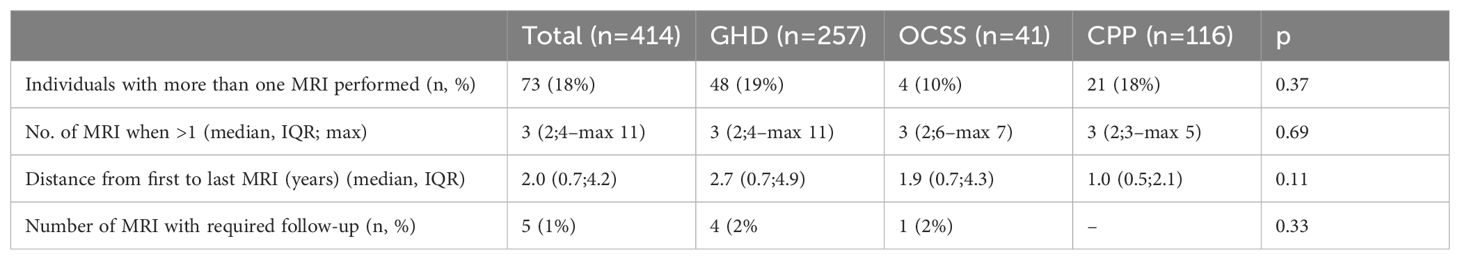
Table 2. Data regarding repeated imaging (CPP, central precocious puberty; GHD, growth hormone deficiency; MRI, magnetic resonance imaging; OCSS, other causes of short stature) .
According to our guidelines (6), only in five patients (7% of those with more than one performed MRI), four with GHD, and one with Noonan syndrome, a follow-up was required: two Rathke cleft cysts >5 mm, one non-functioning pituitary adenoma >10 mm, one dysembryoplastic neuroepithelial tumor, and one dysgerminoma. No patients with CPP or OCSS would have required a follow-up MRI. Details are reported in Table 3.
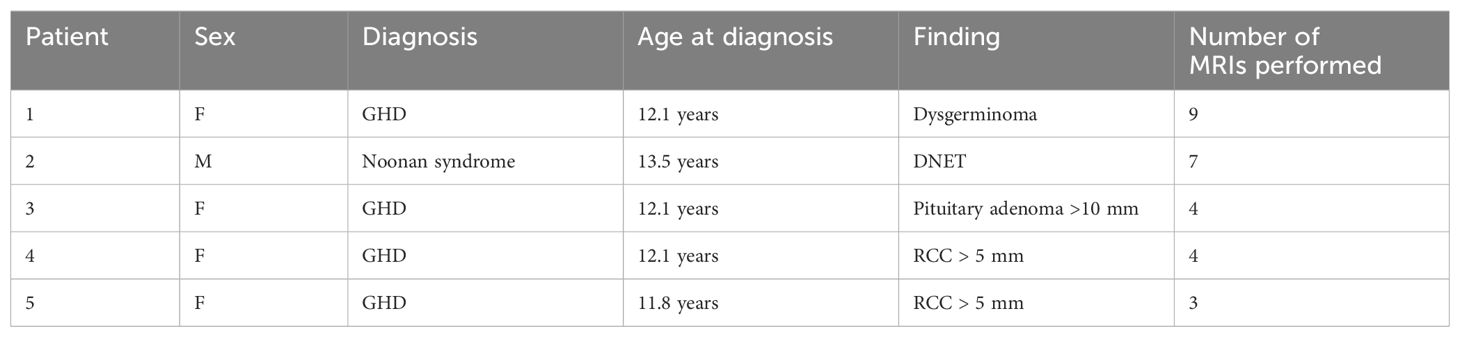
Table 3. Clinical and imaging features of lesions with indicated radiological follow-up (DNET, dysembryoplastic neuroepithelial tumor; GHD, growth hormone deficiency; RCC, Rathke’s cleft cyst) .
Overall, out of 170 repeated MRI scans, 147 (86%) were unnecessary, as none of these scans showed any progression of findings. Considering an estimated cost of 450 euros for each MRI (of which 46.15 euros are paid by the patient’s families), 66,150 euros could have been saved for unnecessary tests during the study period (6,785.05 by the families and 59,365.95 by the National Health System).
If we include the MRIs performed in cases of OCSS other than Noonan syndrome (n=32) and girls with CPP older than 6 years (n=89), an additional 121 MRIs could have been avoided. This brings the total number of unnecessary MRIs to 268 (46%) and the potential savings to 130,950 euros 12,368.20 euros by families and 108,231.80 euros by the National Health System). These figures do not include the costs associated with procedural sedations in younger children.
Brain MRI is essential in many pediatric endocrinological conditions, such as GHD or CPP. However, it is not uncommon (13%–18%) (11, 12) to identify “incidentalomas”, incidental findings serendipitously diagnosed in a patient undergoing imaging for an unrelated reason (13). Even the majority of alterations with definite or possible clinical or anatomical relevance (ADPCAS) do not progress over time and thus would not need radiological follow-up (6). In our previous paper, we emphasized that there was a lack of guidelines regarding the management of these findings in the pediatric population, often leading to repeated MRI scans, excessive and disproportionate to the patient’s needs (6), potentially leading to significant resource expenditure and patient anxiety (14). To mitigate this risk, we have summarized the optimal management strategies for the most frequently identified alterations in patients with GHD and CPP. These strategies could not only reduce costs but also alleviate physical and psychological implications (6). Moreover, performing a brain MRI without a proper indication also raises ethical issues; written informed consent should be obtained after a detailed explanation of the reasons and the risks and benefits (15).
In this retrospective study, we evaluated the number of repeated MRIs performed and estimated the number of avoidable MRIs before the introduction of guidelines for the management and follow-up of brain MRI findings in January 2021 (6). During the study period, we identified a total of 584 MRI scans that were performed on 414 patients with GHD, CPP, or OCSS. The MRI scan was completely normal in two-thirds of the patients, and we did not observe significant differences among the three diagnostic groups (GHD, CPP, OCSS). These results confirm those obtained in many other studies on the topic, where normal MRI represents the most common outcome in children diagnosed with GHD and CPP (16–19).
Nevertheless, one-third of young individuals undergoing a brain MRI will have findings in the report that need explanation and may require follow-up, potentially causing additional anxiety for the family (14). Half of these individuals (17% of the entire cohort) had an “incidentaloma,” with no significant differences in prevalence among groups. Although the number of patients with incidentalomas who underwent follow-up MRIs was significantly lower than those with ADPCAS (10% vs. 57%), and patients with incidentalomas underwent fewer follow-up MRIs compared with those with ADPCAS (median 2 vs. 4), they all performed multiple MRI without a clear indication.
The prevalence of ADPCAS was significantly higher in children with GHD (20%) compared with those with CPP and OCSS (9% and 7%, respectively). PSIS, empty sella, ectopic neurohypophysis, and septo-optic dysplasia were observed exclusively in cases of GHD (2, 20). On the other hand, Arnold-Chiari type 1 malformation (21), pituitary adenoma (22), and adenohypophysis hypoplasia (18) were also detected in CPP patients, whereas RCCs were also found in both CPP and OCSS patients (one Noonan, one ISS), as previously reported (23, 24). No craniopharyngiomas were detected during the study period, despite GHD or CPP often being its first sign of presentation, and MRI is strongly recommended by guidelines to rule out neoplastic lesions (18–20, 25). However, we did identify a dysgerminoma in a patient with GHD and a DNET in a patient with Noonan syndrome. This latter finding represents a rare intracranial tumor, already described in patients with Noonan syndrome (26). Although the median age at GHD diagnosis might seem higher than expected (27, 28), it is consistent with other Italian reports (29, 30), suggesting a possible delay in referrals and diagnosis in Italy. Furthermore, the median age of GHD patients with ADPCAS (indicating definite GHD) was similar to that of patients without ADPCAS (who might be classified as having short stature unresponsive to stimulation tests) (31), corroborating the fact that there is no an error in diagnosis.
Overall, 147 out of 170 repeated MRI scans (86%) were unnecessary, since none of these findings would have needed a follow-up according to current literature, and none showed any progression of findings. An appropriate evidence-based follow-up was carried out in only five cases (32–37). Therefore, adherence to the criteria outlined in our study for follow-up could have reduced costs (66,150 euros) and psychological stress for the patients that imaging studies inevitably entail. According to the guidelines we proposed in our previous study (6), brain MRI findings that did not require radiological follow-up could be adequately managed with clinical and laboratory monitoring alone. Among ADPCAS, the management of an empty sella warrants further discussion, as there is a significant gap in evidence-based guidelines. Some authors advocate for radiological follow-up due to the theoretical risk of progression (38, 39). However, given the very low incidence of neuroradiological progression, which correlates with hormonal deterioration, clinical and laboratory monitoring alone may be sufficient for these patients (39). If new signs, symptoms, or hormonal changes suggestive of progression occur, an MRI evaluation becomes necessary. Our data have revealed that all findings that underwent more than one MRI remained stable over time, suggesting, therefore, that extensive follow-up is not necessary when they do not initially present insidious characteristics.
In addition to reconsidering repeated MRIs, it is important to question the necessity of performing an MRI initially. While the indication for neuroimaging in GHD is clear—to identify anatomical anomalies that may explain the etiology and to exclude neoplastic lesions that could contraindicate rhGH therapy (2)—there are still controversies about the necessity of performing MRI scans on all children with CPP. The likelihood of finding significant intracranial abnormalities in girls over 6 years old is low, although not zero (1, 40). In our cohort, we did not find any neoplastic lesions in CPP patients. By excluding girls over the age of six from MRI screenings, we could have avoided 89 tests.
It is not uncommon to perform an MRI before starting treatment with rhGH in non-GHD patients to rule out neoplastic lesions (3, 41, 42), even though no published guidelines support the routine use of brain MRI in these cases. It should be noted that in conditions where rhGH is indicated, such as Turner syndrome, Noonan syndrome, or SHOX deficiency, GHD is typically not excluded, although coincidences have been reported (43–45). Furthermore, the rate of abnormal MRI findings is similar in short children with normal GH responses and normal IGF-1 levels compared with children with GHD (46). Currently, evidence does not support the use of MRI of the pituitary region in short children born SGA without GHD (47), whereas MRI is recommended in children with Noonan syndrome (3), due to their higher risk of developing tumors, including brain tumors, compared with the general population (48, 49). In our cohort, a DNET was found in a patient with Noonan syndrome. Therefore, we recommend a precautionary brain MRI before initiating rhGH therapy in NS patients to exclude any preexisting brain tumors. In other patients with OCSS, an MRI might not be necessary, unless they present neurological symptoms or signs of hypopituitarism. With this approach, we could have spared an additional 32 MRIs.
Overall, we could have spared 268 MRIs (46% of the total number), resulting in potential savings of more than 130,000 euros, not including the costs for procedural sedations. By fine-tuning our internal protocol, we aim to further reduce the number of MRIs, including repeat scans, in the coming years.
While our study offers valuable insights, it is not without limitations. Primarily, it relies on retrospective data. However, we followed meticulous steps to mitigate potential biases. All available data were gathered to minimize selection bias, and to ensure objectivity, every MRI was scrutinized by an expert pediatric radiologist, mitigating reporting bias. Additionally, being a single-center study, there is a possibility that our findings may not fully represent broader populations. Nevertheless, the consistency of our results with prior literature suggests potential generalizability. Lastly, a notable constraint arises from the absence of a “healthy” control group among the pediatric population undergoing neuroimaging solely for pituitary disorders. This limitation is inherent in many similar studies in the literature exploring brain alterations in children with pituitary-related diseases (4). On the other hand, to our knowledge, this is the first study evaluating the follow-up of MRI findings in children with endocrine disorders. There is a lack of guidelines on incidentalomas and MRI brain abnormalities in the pediatric population. Therefore, our study can support many pediatricians who daily find themselves having to manage such clinical conditions.
With this study, we confirm that only a few neuroimaging findings in pediatric patients warrant further investigation, and we highlight that too often investigations are carried out unnecessarily. We advocate the importance of being familiar with guidelines for prescribing these exams and managing these findings to reduce costs for both the healthcare system and patients’ families, as well as to alleviate physical and psychological distress for patients and caregivers.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical Committee approval was not requested since General Authorization to Process Personal Data for Scientific Research Purposes (Authorization no. 9/2014) declared that retrospective archive studies that use ID codes, preventing the data from being traced back directly to the data subject, do not need ethics approval. All data were collected in an anonymous database. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements because informed consent was signed by parents at the first visit, in which they agreed that “clinical data may be used for clinical research purposes, epidemiology, the study of pathologies and training, to improve knowledge, care and prevention”.
MM: Data curation, Investigation, Writing – original draft, Writing – review & editing. FM: Data curation, Investigation, Writing – original draft, Writing – review & editing. FB: Conceptualization, Validation, Writing – original draft, Writing – review & editing. GTa: Validation, Writing – original draft, Writing – review & editing. EF: Validation, Writing – original draft, Writing – review & editing. EB: Resources, Validation, Writing – original draft, Writing – review & editing. GTo: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Italian Ministry of Health, through the contribution given to the Institute for Maternal and Child Health IRCCS Burlo Garofolo, Trieste, Italy (RC 16/24).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Carel JC, Eugster EA, Rogol A, Ghizzoni L, Palmert MR, ESPE-LWPES GnRH Analogs Consensus Conference Group, et al. Consensus statement on the use of gonadotropin-releasing hormone analogs in children. Pediatrics. (2009) 123:e752–62. doi: 10.1542/peds.2008-1783
2. Growth Hormone Research Society. Consensus guidelines for the diagnosis and treatment of growth hormone (GH) deficiency in childhood and adolescence: summary statement of the GH research society. GH research society. J Clin Endocrinol Metab. (2000) 85:3990–3. doi: 10.1210/jcem.85.11.6984
3. Bangalore Krishna K, Pagan P, Escobar O, Popovic J. Occurrence of cranial neoplasms in pediatric patients with noonan syndrome receiving growth hormone: is screening with brain MRI prior to initiation of growth hormone indicated? Horm Res Paediatr. (2017) 88:423–6. doi: 10.1159/000479107
4. Aguirre RS, Eugster EA. Central precocious puberty: from genetics to treatment. Best Pract Res Clin Endocrinol Metab. (2018) 32:343–54. doi: 10.1016/j.beem.2018.05.008
5. Maghnie M, Lindberg A, Koltowska-Haüggstroüm M, Ranke MB. Magnetic resonance imaging of CNS in 15,043 children with GH deficiency in KIGS (Pfizer international growth database). Eur J Endocrinol. (2013) 168:211–7. doi: 10.1530/EJE-12-0801
6. Baldo F, Marin M, Murru FM, Barbi E, Tornese G. Dealing with brain MRI findings in pediatric patients with endocrinological conditions: less is more? Front Endocrinol (Lausanne). (2022) 12:780763. doi: 10.3389/fendo.2021.780763
7. (2014). Italia. Determinazione dell’Agenzia Italiana del Farmaco 19 giugno 2014. Modifica alla Nota AIFA 39. Gazzetta Ufficiale. Serie Generale n. 154 del 5 luglio.
8. Penco A, Bossini B, Giangreco M, Vidonis V, Vittori G, Grassi N, et al. Should pediatric endocrinologists consider more carefully when to perform a stimulation test? Front Endocrinol (Lausanne). (2021) 12:660692. doi: 10.3389/fendo.2021.660692
9. The Italian Data Protection Authority. Authorisation no. 9/2014 – General Authorisation to Process Personal Data for Scientific Research Purposes. Available online at: https://www.garanteprivacy.it/web/guest/home/docweb/-/docwebdisplay/docweb/3786078. (accessed June 6, 2024).
10. Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. (2014) 12:1495–9. doi: 10.1016/j.ijsu.2014.07.013
11. Oh YR, Kim YJ, Oh KE, Park GH, Kang E, Nam HK, et al. Brain magnetic resonance imaging (MRI) findings in central precocious puberty patients: is routine MRI necessary for newly diagnosed patients? Ann Pediatr Endocrinol Metab. (2023) 28:200–5. doi: 10.6065/apem.2244192.096
12. Schmitt J, Thornton P, Shah AN, Rahman AKMF, Kubota E, Rizzuto P, et al. Brain MRIs may be of low value in most children diagnosed with isolated growth hormone deficiency. J Pediatr Endocrinol Metab. (2021) 34:333–40. doi: 10.1515/jpem-2020-0579
13. O'Sullivan JW, Muntinga T, Grigg S, Ioannidis JPA. Prevalence and outcomes of incidental imaging findings: umbrella review. BMJ. (2018) 361:k2387. doi: 10.1136/bmj.k2387
14. Powell DK. Patient explanation guidelines for incidentalomas: helping patients not to fear the delayed surveillance. AJR Am J Roentgenol. (2014) 202:W602. doi: 10.2214/AJR.13.12337
15. Graham M, Hallowell N, Savulescu J. A just standard: the ethical management of incidental findings in brain imaging research. J Law Med Ethics. (2021) 49:269–81. doi: 10.1017/jme.2021.38
16. Alba P, Tsai S, Mitre N. The severity of growth hormone deficiency does not predict the presence or absence of brain magnetic resonance imaging abnormalities – A retrospective review. Eur Endocrinol. (2020) 16:60–4. doi: 10.17925/EE.2020.16.1.60
17. Cantas-Orsdemir S, Garb JL, Allen HF. Prevalence of cranial MRI findings in girls with central precocious puberty: a systematic review and meta-analysis. J Pediatr Endocrinol Metab. (2018) 31:701–10. doi: 10.1515/jpem-2018-0052
18. Cassio A, Marescotti G, Aversa T, Salerno M, Tornese G, Stancampiano M, et al. Central precocious puberty in italian boys: data from a large nationwide cohort. J Clin Endocrinol Metab. (2024) 109(8):2061–70. doi: 10.1210/clinem/dgae035
19. Alyahyawi NY. Auxological, clinical, and MRI abnormalities in pediatric patients with isolated growth hormone deficiency. Cureus. (2024) 16:e54904. doi: 10.7759/cureus.54904
20. Arslanoğlu I, Kutlu H, Işgüven P, Tokuş F, Işik K. Diagnostic value of pituitary MRI in differentiation of children with normal growth hormone secretion, isolated growth hormone deficiency and multiple pituitary hormone deficiency. J Pediatr Endocrinol Metab. (2001) 14(5):517–23. doi: 10.1515/JPEM.2001.14.5.517
21. Kim MS, Hwang PH, Lee DY. A case of a girl with arnold-chiari type 1 malformation with precocious puberty. Korean J Fam Med. (2018) 39:54–6. doi: 10.4082/kjfm.2018.39.1.54
22. Uhing A, Ahmed A, Salamat S, Chen M. A rare case of precocious puberty secondary to an LH-secreting pituitary adenoma. JCEM Case Rep. (2023) 1:luad055. doi: 10.1210/jcemcr/luad055
23. Acharya SV, Gopal RA, Menon PS, Bandgar TR, Shah NS. Precocious puberty due to rathke cleft cyst in a child. Endocr Pract. (2009) 15:134–7. doi: 10.4158/EP.15.2.134
24. Oh YJ, Park HK, Yang S, Song JH, Hwang IT. Clinical and radiological findings of incidental Rathke's cleft cysts in children and adolescents. Ann Pediatr Endocrinol Metab. (2014) 19:20–6. doi: 10.6065/apem.2014.19.1.20
25. Soriano-Guilleín L, Argente J. Central precocious puberty, functional and tumor- related. Best Pract Res Clin Endocrinol Metab. (2019) 33:101262. doi: 10.1016/j.beem.2019.01.003
26. Pellegrin MC, Tornese G, Cattaruzzi E, Blank E, Kieslich M. Ventura A. A rare brain tumor in noonan syndrome: report of two cases. Horm Res Paediatr. (2014) 82(Suppl. 1):274. doi: 10.1159/000365775
27. Harju S, Saari A, Sund R, Sankilampi U. Epidemiology of disorders associated with short stature in childhood: A 20-year birth cohort study in Finland. Clin Epidemiol. (2022) 14:1205–14. doi: 10.2147/CLEP.S372870
28. Stochholm K, Gravholt CH, Laursen T, Jørgensen JO, Laurberg P, Andersen M, et al. Incidence of GH deficiency - a nationwide study. Eur J Endocrinol. (2006) 155:61–71. doi: 10.1530/eje.1.02191
29. Lonero A, Giotta M, Guerrini G, Calcaterra V, Galazzi E, Iughetti L, et al. Isolated childhood growth hormone deficiency: a 30-year experience on final height and a new prediction model. J Endocrinol Invest. (2022) 45:1709–17. doi: 10.1007/s40618-022-01808-4
30. Rodari G, Profka E, Giacchetti F, Cavenaghi I, Arosio M, Giavoli C. Influence of biochemical diagnosis of growth hormone deficiency on replacement therapy response and retesting results at adult height. Sci Rep. (2021) 11:14553. doi: 10.1038/s41598-021-93963-6
31. Lanzetta MA, Dalla Bona E, Tamaro G, Vidonis V, Vittori G, Faleschini E, et al. Clinical and laboratory characteristics but not response to treatment can distinguish children with definite growth hormone deficiency from short stature unresponsive to stimulation tests. Front Endocrinol (Lausanne). (2024) 15:1288497. doi: 10.3389/fendo.2024.1288497
32. Steele CA, MacFarlane IA, Blair J, Cuthbertson DJ, Didi M, Mallucci C, et al. Pituitary adenomas in childhood, adolescence and young adulthood: presentation, management, endocrine and metabolic outcomes. Eur J Endocrinol. (2010) 163:515–22. doi: 10.1530/EJE-10-0519
33. Mahdi ES, Webb RL, Whitehead MT. Prevalence of pituitary cysts in children using modern magnetic resonance imaging techniques. Pediatr Radiol. (2019) 49:1781–7. doi: 10.1007/s00247-019-04479-1
34. Trifanescu R, Ansorge O, Wass JA, Grossman AB, Karavitaki N. Rathke's cleft cysts. Clin Endocrinol (Oxf). (2012) 76:151–60. doi: 10.1111/j.1365-2265.2011.04235.x
35. Culver SA, Grober Y, Ornan DA, Patrie JT, Oldfield EH, Jane JA Jr, et al. A case for conservative management: characterizing the natural history of radiographically diagnosed rathke cleft cysts. J Clin Endocrinol Metab. (2015) 100:3943–8. doi: 10.1210/jc.2015-2604
36. Thaker VV, Lage AE, Kumari G, Silvera VM, Cohen LE. Clinical course of nonfunctional pituitary microadenoma in children: A single-center experience. J Clin Endocrinol Metab. (2019) 104:5906–12. doi: 10.1210/jc.2019-01252
37. Derrick KM, Gomes WA, Gensure RC. Incidence and outcomes of pituitary microadenomas in children with short stature/growth hormone deficiency. Horm Res Paediatr. (2018) 90:151–60. doi: 10.1159/000489456
38. Carosi G, Brunetti A, Mangone A, Baldelli R, Tresoldi A, Del Sindaco G, et al. A multicenter cohort study in patients with primary empty sella: hormonal and neuroradiological features over a long follow-up. Front Endocrinol (Lausanne). (2022) 13:925378. doi: 10.3389/fendo.2022.925378
39. De Marinis L, Bonadonna S, Bianchi A, Maira G, Giustina A. Primary empty sella. J Clin Endocrinol Metab. (2005) 90:5471–7. doi: 10.1210/jc.2005-0288
40. Kaplowitz PB. Do 6-8 year old girls with central precocious puberty need routine brain imaging? Int J Pediatr Endocrinol. (2016) 2016:9. doi: 10.1186/s13633-016-0027-5
41. Nagel BH, Palmbach M, Petersen D, Ranke MB. Magnetic resonance images of 91 children with different causes of short stature: pituitary size reflects growth hormone secretion. Eur J Pediatr. (1997) 156:758–63. doi: 10.1007/s004310050707
42. Mayer TE, Halbsguth A, Kollmann F, Lorey C. MRI and CT of sella and brain in turner’s syndrome. In: Nadjmi M, editor. Imaging of Brain Metabolism Spine and Cord Interventional Neuroradiology Free Communications. Springer, Berlin, Heidelberg (1989). doi: 10.1007/978-3-642-74337-5_135
43. Cavallo L, Gurrado R. Endogenous growth hormone secretion does not correlate with growth in patients with Turner's syndrome. Italian Study Group for Turner Syndrome. J Pediatr Endocrinol Metab. (1999) 12:623–7. doi: 10.1515/jpem.1999.12.5.623
44. Tamburrino F, Gibertoni D, Rossi C, Scarano E, Perri A, Montanari F, et al. Response to long-term growth hormone therapy in patients affected by RASopathies and growth hormone deficiency: Patterns of growth, puberty and final height data. Am J Med Genet A. (2015) 167A:2786–94. doi: 10.1002/ajmg.a.37260
45. Iughetti L, Vannelli S, Street ME, Pirazzoli P, Bertelloni S, Radetti G, et al. Impaired GH secretion in patients with SHOX deficiency and efficacy of recombinant human GH therapy. Horm Res Paediatr. (2012) 78:279–87. doi: 10.1159/000345354
46. Kemp SF, Alter CA, Dana K, Baptista J, Blethen SL. Use of magnetic resonance imaging in short stature: data from National Cooperative Growth Study (NCGS) Substudy 8. J Pediatr Endocrinol Metab. (2002) 15 Suppl 2:675–9. doi: 10.1515/jpem.2002.15.s2.675
47. Arends NJ, d Lip W V, Robben SG, Hokken-Koelega AC. MRI findings of the pituitary gland in short children born small for gestational age (SGA) in comparison with growth hormone-deficient (GHD) children and children with normal stature. Clin Endocrinol (Oxf). (2002) 57:719–24. doi: 10.1046/j.1365-2265.2002.01605.x
48. Sodero G, Cipolla C, Pane LC, Sessa L, Malavolta E, Arzilli F, et al. Efficacy and safety of growth hormone therapy in children with Noonan syndrome. Growth Horm IGF Res. (2023) 69-70:101532. doi: 10.1016/j.ghir.2023.101532
Keywords: brain magnetic resonance imaging, incidentaloma, growth hormone deficiency, central precocious puberty, follow-up
Citation: Marin M, Murru FM, Baldo F, Tamaro G, Faleschini E, Barbi E and Tornese G (2024) Minimizing unnecessary brain magnetic resonance imaging in pediatric endocrinology: a retrospective cohort analysis. Front. Endocrinol. 15:1456541. doi: 10.3389/fendo.2024.1456541
Received: 28 June 2024; Accepted: 15 August 2024;
Published: 03 September 2024.
Edited by:
Sally Radovick, The State University of New Jersey, United StatesReviewed by:
Gerdi Tuli, Regina Margherita Hospital, ItalyCopyright © 2024 Marin, Murru, Baldo, Tamaro, Faleschini, Barbi and Tornese. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gianluca Tornese, Z2lhbmx1Y2EudG9ybmVzZUBidXJsby50cmllc3RlLml0
†These authors have contributed equally to this work
‡ORCID: Maura Marin, orcid.org/0009-0003-9557-4989
Flora Maria Murru, orcid.org/0000-0001-8954-8889
Francesco Baldo, orcid.org/0000-0002-7523-3154
Gianluca Tamaro, orcid.org/0000-0003-1635-9141
Elena Faleschini, orcid.org/0000-0002-7045-5524
Egidio Barbi, orcid.org/0000-0002-6343-846X
Gianluca Tornese, orcid.org/0000-0002-4395-3915
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.