- 1School of Basic Medical Sciences, Zhejiang Chinese Medical University, Hangzhou, China
- 2Second Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 3Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 4Department of Molecular Imaging and Nuclear Medicine, Tianjin Medical University Cancer Institute and Hospital, Tianjin, China
- 5National Clinical Research Center for Cancer, Tianjin Key Laboratory of Cancer Prevention and Therapy, Tianjin’s Clinical Research Center for China, Tianjin, China
Background: The triglyceride-glucose (TyG) index is a surrogate indicator of insulin resistance. Therefore, we aimed to determine the association between TyG index and heart failure (HF) with preserved ejection fraction (HFpEF) in patients with coronary heart disease (CHD) and to explore whether such associations would be modified by different metabolic states.
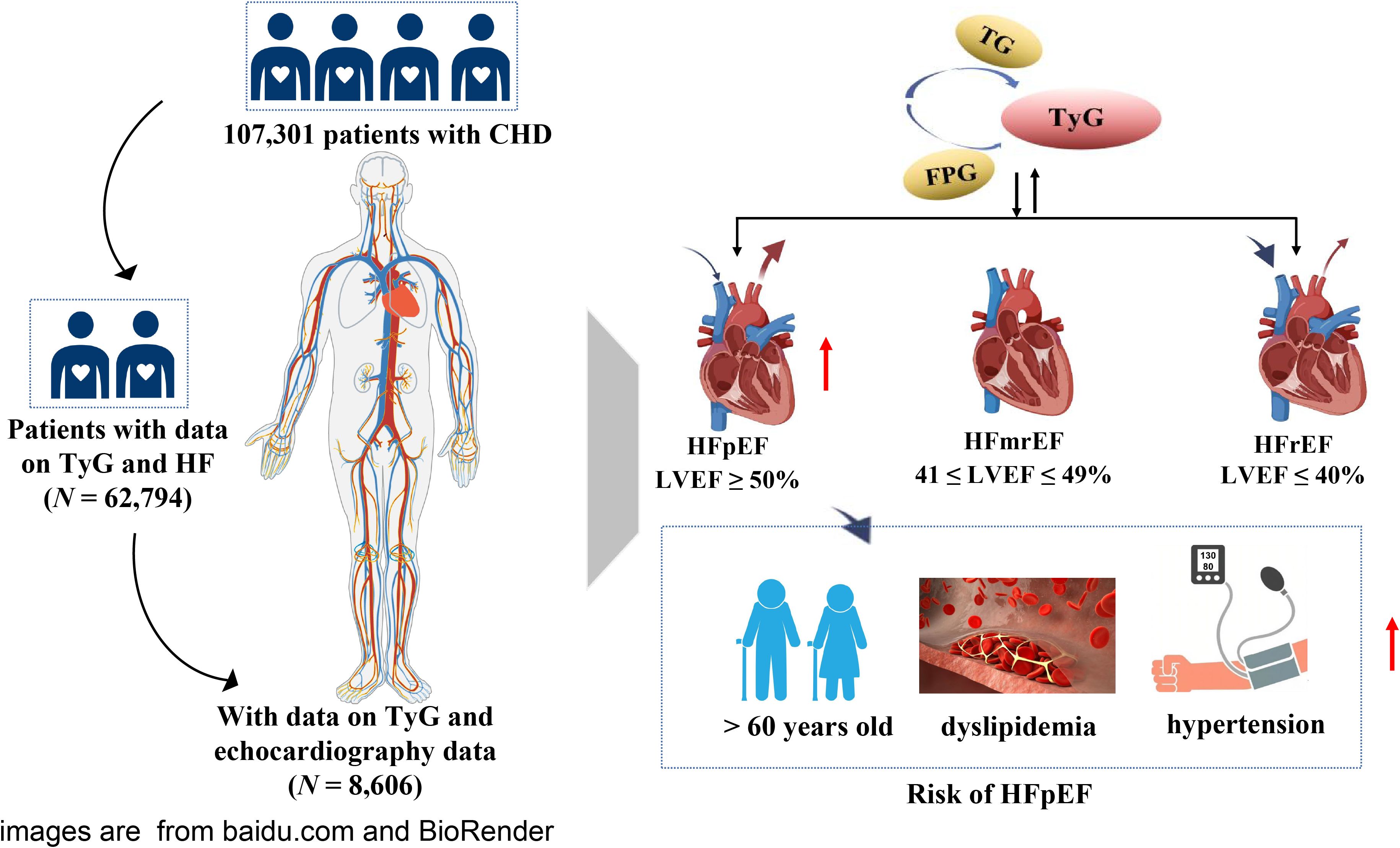
Methods: Among 107,301 CHD patients, 62,794 were included to analyze the relationship between the TyG index and HF. Among them, 8,606 patients who had undergone echocardiography were included to identify different types of HF, including HF with reduced ejection fraction (HFrEF), HF with intermediate-range ejection fraction (HFmrEF), and HFpEF. Among them, 1896 patients were diagnosed with HFpEF. Logistic regression was used to analyze the relationship between the TyG index and HFpEF in CHD patients. In addition, the association between TyG index and HFpEF according to sex, age, blood lipids, and blood pressure was assessed.
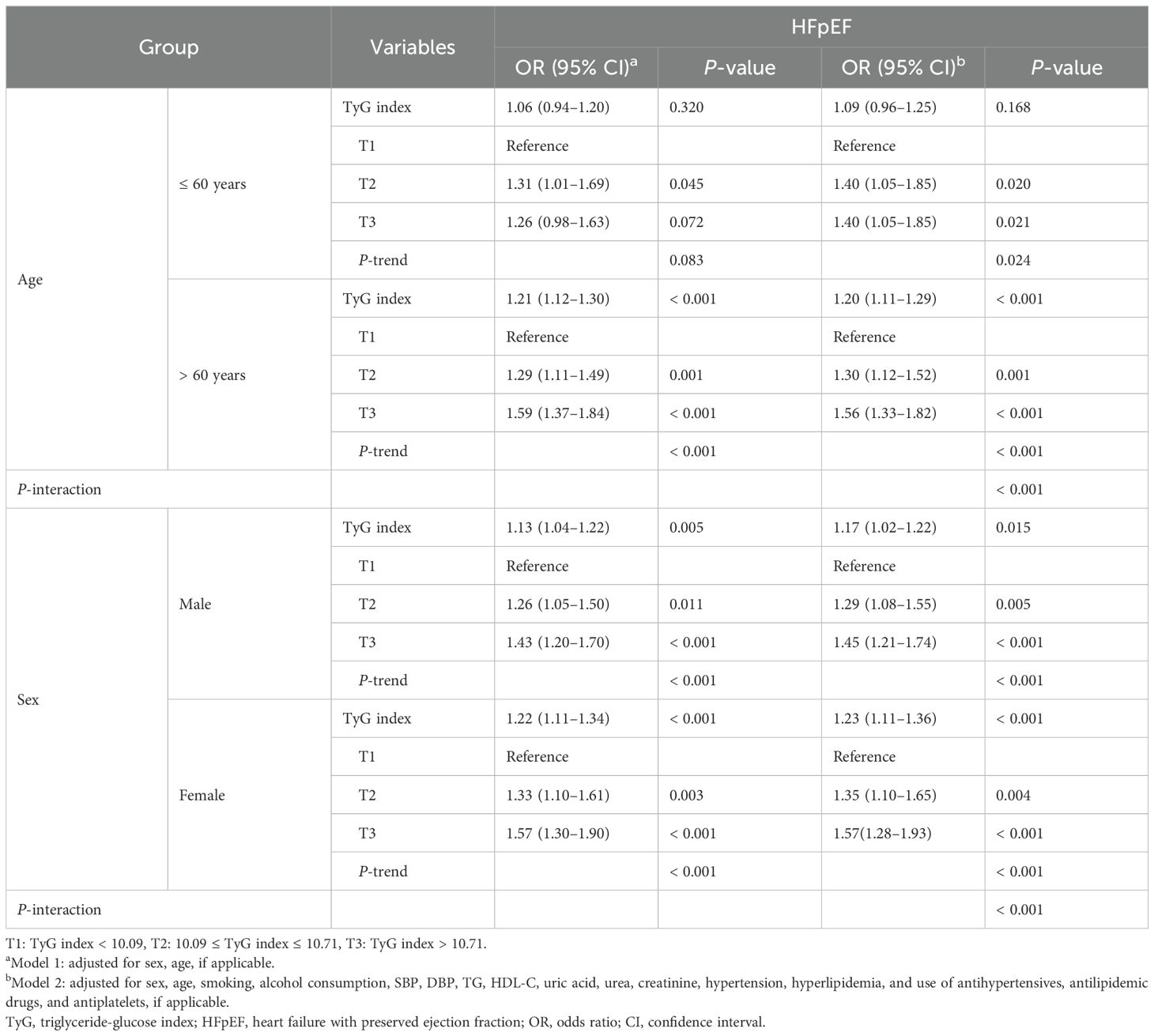
Results: A baseline analysis of CHD patients divided into four groups according to the tertile level of the TyG index showed significant differences in the related parameters between the groups. In the multi-adjusted models, the TyG index was significantly associated with the risk of HFpEF (odds ratio [OR]: 1.17; 95% confidence interval [CI]: 1.09–1.25). After adjustment for multivariates, TyG index levels for T2 (OR: 1.33; 95% CI: 1.16–1.52) and T3 (OR: 1.52; 95% CI: 1.32–1.74) were associated with increased OR in HFpEF. In addition, the TyG index of CHD patients was significantly associated with HFpEF in older adults aged > 60 years (OR: 1.20; 95% CI: 1.11–1.29), hypertension (OR: 1.27; 95% CI: 1.17–1.37), and dyslipidemia (OR: 1.15; 95% CI: 1.08–1.24). Moreover, the OR (OR: 1.23; 95% CI: 1.11–1.36) in women is higher than in men (OR: 1.17; 95% CI: 1.02–1.22, indicating a stronger association between TyG index and HFpEF in women.
Conclusions: Our findings demonstrated a significant association between TyG index and HFpEF in CHD patients. Furthermore, TyG index was independently associated with HFpEF in hypertension, dyslipidemia, and older patients (aged > 60 years). In addition, the association between the TyG index and HFpEF in CHD patients differed according to sex.
1 Introduction
Coronary heart disease (CHD) is an atherosclerotic disease. Its pathological process leads to coronary artery stenosis, which in turn leads to myocardial ischemia, myocardial necrosis, myocardial systolic dysfunction, and heart failure (HF) due to decreased ejection capacity (1, 2). HF with preserved ejection fraction (HFpEF) is the most common type of HF, diagnosed in approximately 50% of HF patients (3). The American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America (4) reported an annual increase in the percentage of hospitalizations due to HFpEF in patients with HF. By 2020, left ventricle ejection fraction (LVEF) exceeding 40% is anticipated in 65% of hospitalized patients with HF. HFpEF is associated with high morbidity, readmission rates, and readmission mortality, therefore, its prevention and treatment require further investigation.
The triglyceride-glucose (TyG) index is used as a marker of insulin resistance (IR), which is implicated in the development of non-communicable diseases (5, 6). The TyG index is associated with a high prevalence of coronary artery disease and an increased risk of major adverse cardiovascular and cerebrovascular events (7–11). TyG index and carotid plaque demonstrated a significant association in CHD patients (12). A Mendelian randomization study showed that the TyG index can be used as a more sensitive pre-diagnostic indicator of cardiovascular disease, which could provide a quantitative risk for cardiometabolic outcomes, including HF (13). The TyG index is a novel biomarker of myocardial fibrosis in HF patients and can be considered a useful risk stratification metric for the management of HF (14). However, no relevant studies have investigated the relationship between the TyG index and HF or the types of HF in CHD patients, especially HFpEF.
Therefore, this study aimed to clarify the association between the TyG index and HF in CHD patients and investigate the association between the TyG index and different types of HF, especially HFpEF. Identifying simpler biochemical indicators to prevent the risk of HF may aid in the clinical management of CHD.
2 Methods
2.1 Patients
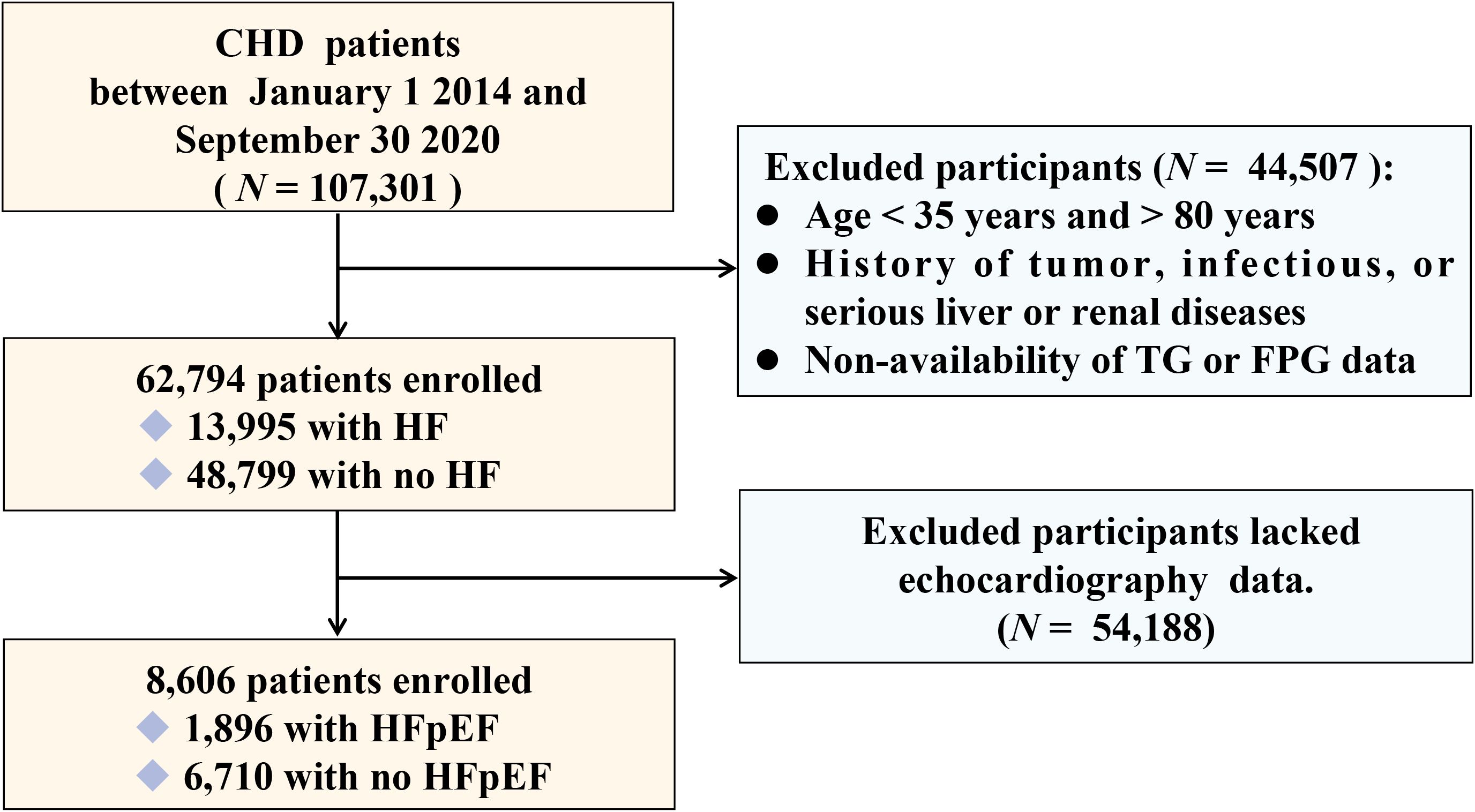
This was a large-scale, multicenter, retrospective, cross-sectional study. Between January 1, 2014, and September 30, 2020, 107,301 CHD patients admitted to six hospitals in Tianjin were included. Following the exclusion of patients aged < 35 years or > 80 years, those with tumor, infectious, or severe liver or kidney diseases, and patients lacking data on triglycerides (TGs) and fasting plasma glucose (FPG),62,794 participants were included in the study. Among them, 8,606 patients who had undergone echocardiography were included to identify different types of HF, including HF with reduced ejection fraction (HFrEF), HF with intermediate-range ejection fraction (HFmrEF), and HFpEF. A flowchart of patient selection is shown in Figure 1. This study was approved by the ethics committee of the Tianjin University of Traditional Chinese Medicine (TJUTCM-EC20190008) and registered with the Chinese Clinical Trial Registry (ChiCTR-1900024535) and ClinicalTrials.gov (NCT04026724).
2.2 Data collection
Age, sex, smoking, alcohol consumption, and medication history were obtained through standard structured questionnaires (15, 16). Fasting venous blood samples were collected from all the participants early in the morning. FPG, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), TG, low-density lipoprotein cholesterol (LDL-C), glycated hemoglobin (HbA1c), uric acid, urea, and creatinine levels were measured using an automatic hematology analyzer. Standard laboratory procedures for quality control were strictly followed. The TyG index was calculated as follows: Ln[TG (mg/dL) × FPG (mg/dL)/2] (17). The hyperlipidemia status of the participants was evaluated based on the National Cholesterol Education Program. Hyperlipidemia was defined as having a TG ≥ 150 mg/dL (1.7 mmol/L), TC ≥ 200 mg/dL (5.18 mmol/L), LDL-C ≥ 130 mg/dL (3.37 mmol/L), or HDL-C ≤ 50 mg/dL (1.30 mmol/L) in women and ≤ 40 mg/dL (1.04 mmol/L) in men. Moreover, participants who reported using lipid-lowering medications were considered to have hyperlipidemia. Participants meeting any one of the aforementioned criteria were diagnosed to have hyperlipidemia (18, 19). Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured by experienced technicians at heart level using automatic blood pressure monitors. Hypertension was defined as having a SBP ≥ 130 mmHg or a DBP ≥ 80 mmHg (20).
CHD was defined according to the International Classification of Diseases 10th revision, primary care health records, and the American College of Cardiology Foundation/American Heart Association criteria for HF. HF is usually diagnosed based on clinical symptoms, physical examination findings, laboratory tests, and imaging studies. Detailed records are available in the RCSCD-TCM database. HF included congestive HF, left ventricular failure, New York Heart Association (NYHA) Heart Function class II–IV (21), and unspecified HF. Different types of HF use the left ventricular ejection fraction (LVEF) measured using echocardiography as a cut-off for the inclusion/exclusion criteria. The European Society of Cardiology Guidelines (22) classified HF patients into the following three groups/categories based on LVEF: HFpEF, patients with LVEF ≥ 50%; HFmrEF, patients with 41 ≤ LVEF ≤ 49%; and HFrEF, patients with LVEF ≤ 40%.
2.3 Statistical analyses
The characteristics of participants in the different groups were compared using χ2 tests for categorical variables, and Mann–Whitney U test and Kruskal–Wallis H test for continuous variables. Odds ratios (ORs) and 95% confidence intervals (CIs) of HFpEF were estimated for the TyG index using logistic regression. To further explore the potential nonlinear association between the TyG index and HFpEF, restricted cubic spline (RCS) regression model with four knots was used. The collinearity of the different models was tested before logistic regression. Sex, age, smoking, alcohol consumption, SBP, DBP, TG, HDL-C, uric acid, urea, creatinine, hypertension, hyperlipidemia, and use of antihypertensives, antilipidemic drugs, and antiplatelets were considered as potential confounders in this association. Missing values were imputed using the multiple imputation method. All statistical analyses were performed using SPSS 24.0 (IBM Corp., Armonk, NY, USA).
3 Results
3.1 Baseline characteristics
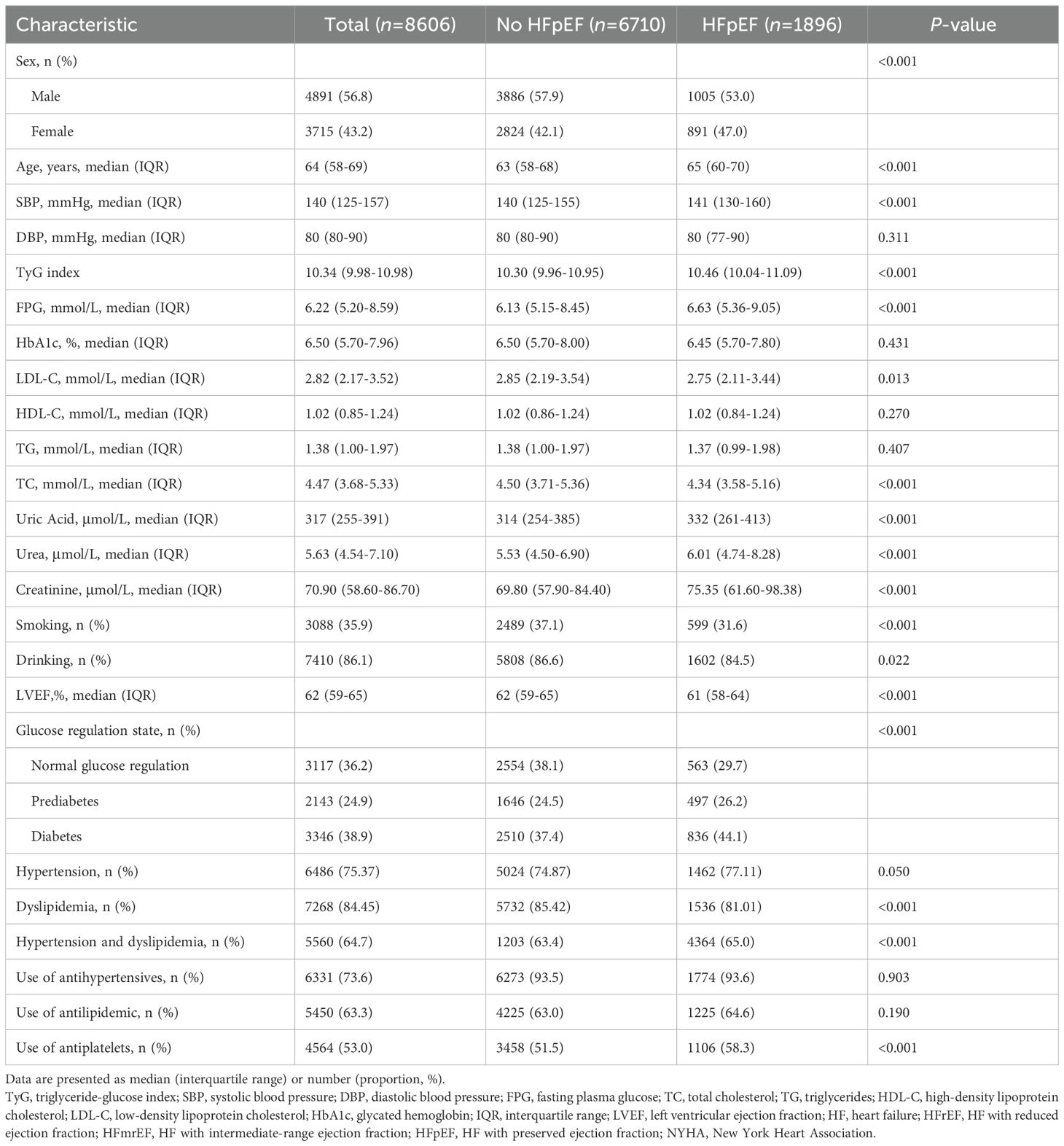
Baseline analysis revealed significant differences in related parameters among 62,794 CHD patients with and without HF (P < 0.001) (Supplementary Table S1). Among them, 1896 out of 8,606 patients who had undergone echocardiography were diagnosed with HFpEF. The average age of the participants was 65 years, and the proportion of men (56.8%) was higher (Table 1). The patients were divided into three groups according to the tertile level of the TyG index (T1: TyG index < 10.09, T2: 10.09 ≤ TyG index ≤ 10.71, T3: TyG index > 10.71). In general, FPG, TC, TG, LDL-C, HbA1c, urea, the proportion of HFrEF, HFmrEF, HFpEF, hyperlipidemia, and use of antihypertensives, antilipidemic drugs, and antiplatelets were positively associated with the tertile level of the TyG index, whereas HDL-C and smoking and alcohol consumption were negatively associated with the tertile level of the TyG index (Supplementary Table S2).
3.2 Association between TyG index and the risk of HFpEF
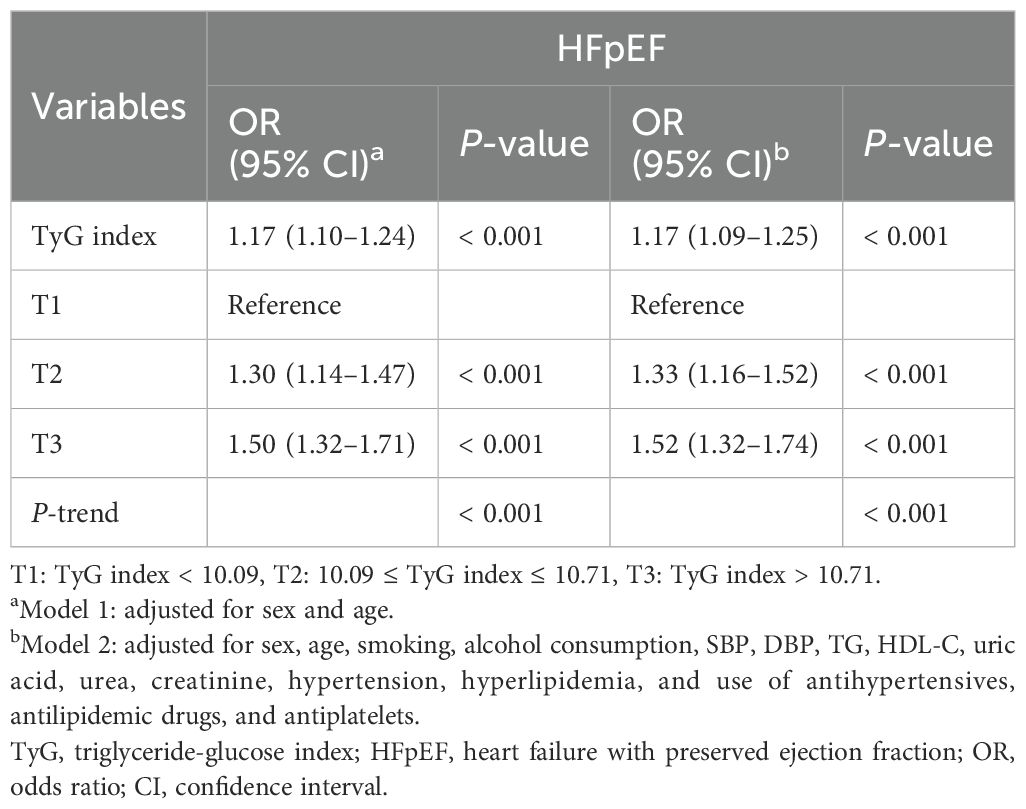
Multivariate logistic regression analysis revealed that TG, FPG, and TyG index were significantly associated with the risk of HF, of which TyG index had the highest OR value (OR: 1.14; 95% CI: 1.06–1.22) (Supplementary Table S3). Logistic regression models were constructed to show that the TyG index was significantly associated with HFpEF before and after multivariate adjustment (P < 0.001) (Table 2). When the TyG index was analyzed as a continuous variable, it was significantly associated with HFpEF (OR: 1.17; 95% CI: 1.09–1.25). When the TyG index was considered a classified variable, the risk of HFpEF for patients in T2 and T3 was 1.33 and 1.52 times higher, respectively, than the risk for patients in T1. The associations between the univariate analysis and risk of HFpEF are analyzed in detail in Supplementary Table S4. The association between TyG index and the risk of different types of HF, including HFrEF, HFmrEF, and HFpEF, was further evaluated. The results indicated that the associations remained significantly different (Supplementary Table S5). The restricted cubic spline models showed that the risk of HFpEF initially remained stable, followed by a rapid increase, and a rapid decrease (Figure 2).
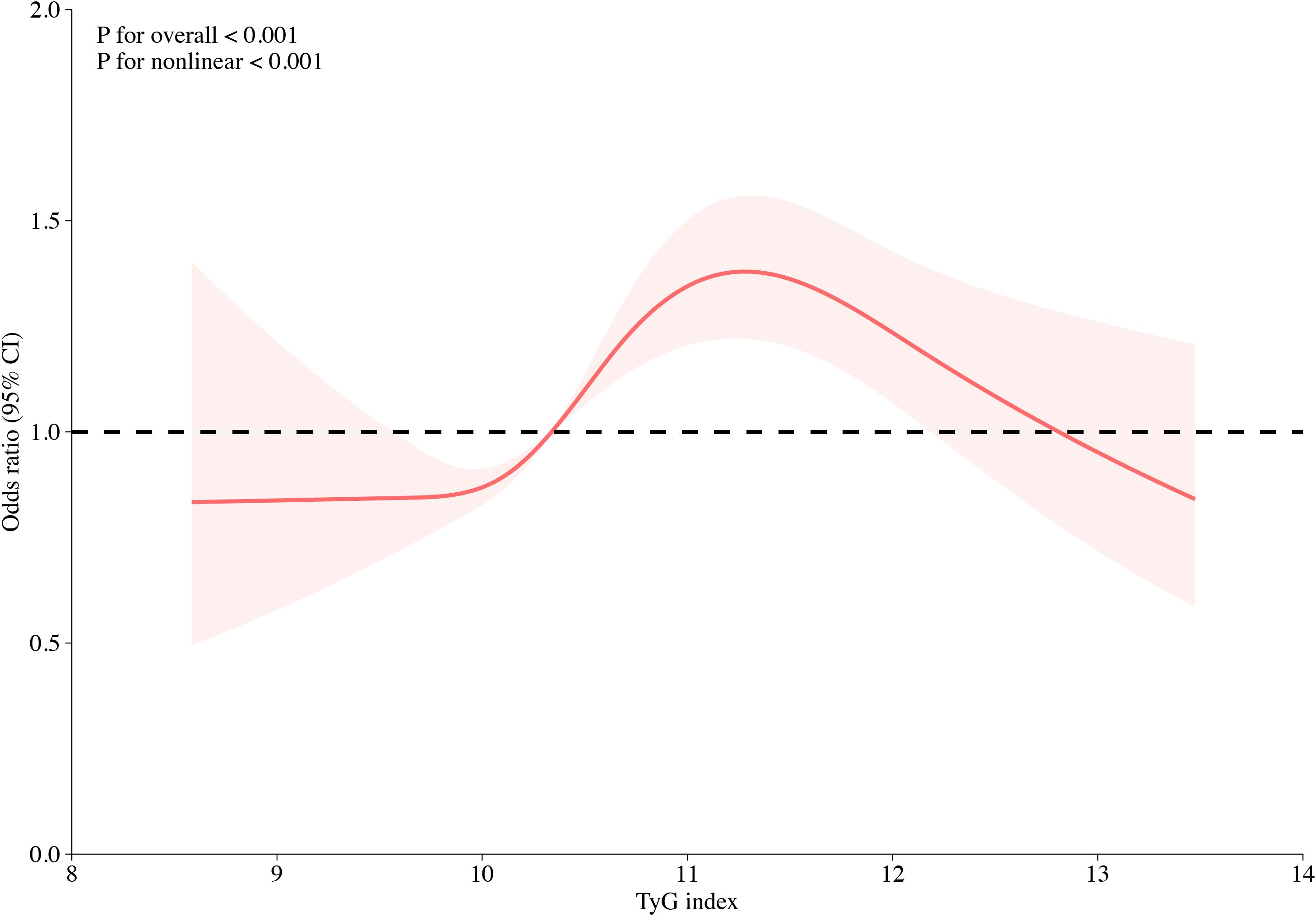
Figure 2. Multivariable-adjusted odds ratios for HFpEF in CHD patients based on restricted cubic spines for the TyG index. Red lines indicate references for hazard ratios, and red areas represent 95% confidence intervals. The model was adjusted for sex, age, smoking, alcohol consumption, SBP, DBP, TG, HDL-C, uric acid, urea, creatinine, hypertension, hyperlipidemia, and use of antihypertensives, antilipidemic agents, and antiplatelets.
3.3 Association between TyG index and the risk of HFpEF according to sex and age
Association between the TyG index and the risk of HFpEF according to age and sex are summarized in Table 3. After multivariate adjustment, the TyG index of CHD patients was significantly associated with HFpEF in older patients aged > 60 years (OR: 1.20; 95% CI: 1.11–1.29). Multivariate logistic regression analysis showed that the TyG index levels for T2 and T3 were associated with an increased OR for HFpEF when T1 was used as a reference, with the highest association observed for T3 in older adults (OR: 1.56; 95% CI: 1.33–1.82). However, no independent association was observed in middle-aged patients aged ≤ 60 years (P > 0.05).
Regardless of sex, this association remained statistically significant before and after multivariate adjustment. After multivariate adjustment, the OR value of TyG index and HFpEF was higher in women (OR: 1.23; 95% CI: 1.11–1.36) than in men (OR: 1.17; 95% CI: 1.02–1.22). Multivariate logistic regression analysis showed that the TyG index levels for T2 and T3 were associated with an increased OR for HFpEF when T1 was used as a reference, with the highest association observed for T3 in both sexes (OR: 1.57; 95% CI: 1.28–1.93 for women and OR: 1.45; 95% CI: 1.21–1.74 for men).
3.4 Association between TyG index and the risk of HFpEF according to different metabolic status
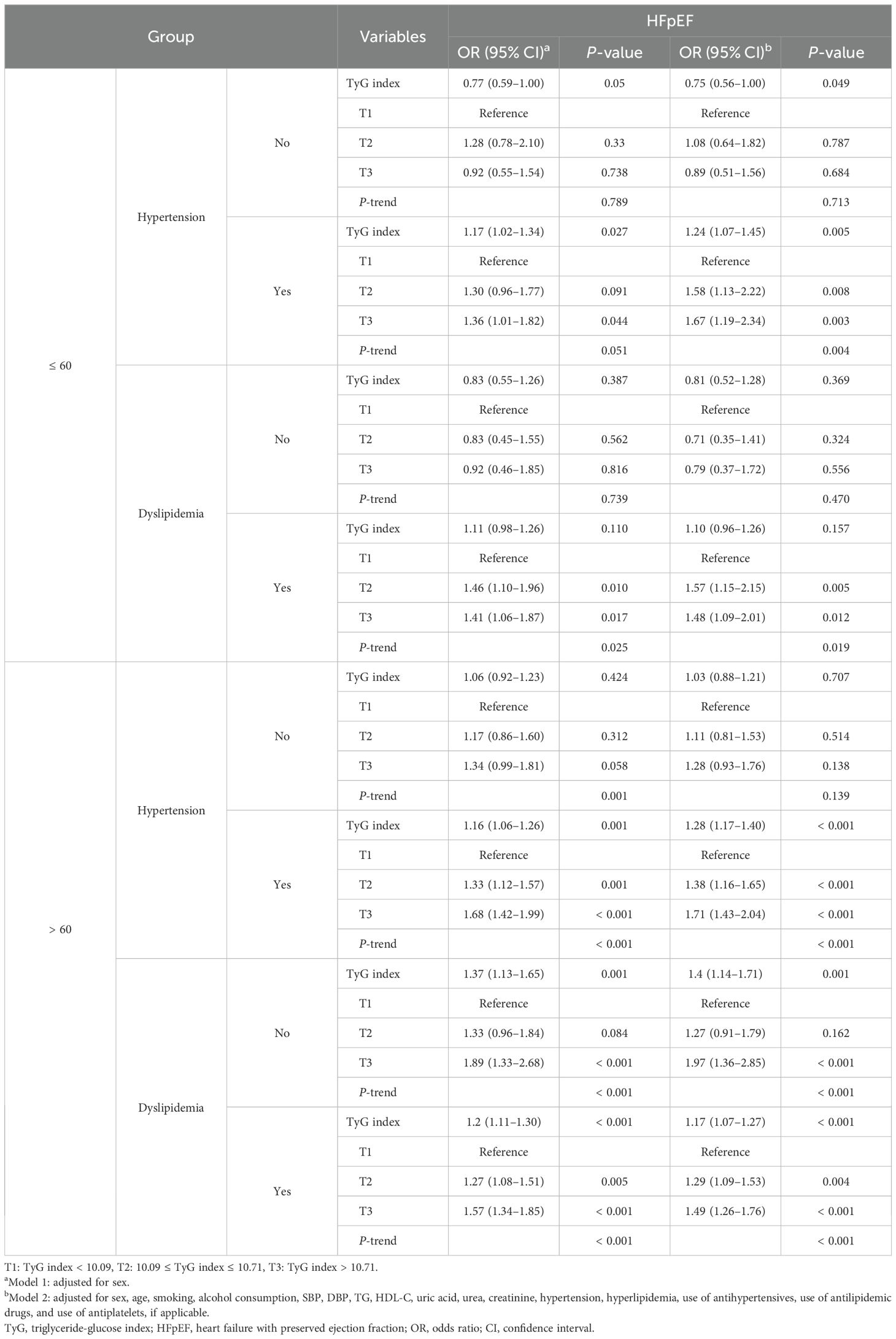
The association between the TyG index and HFpEF varied with blood pressure and lipid status (Table 4). After multivariate adjustment, the TyG index of CHD patients was significantly associated with HFpEF for hypertension (OR: 1.27; 95% CI: 1.17–1.37) and dyslipidemia (OR: 1.15; 95% CI: 1.08–1.24). For both hypertension and dyslipidemia, using T1 as the reference, T2 and T3 were significantly related to the increased risks of HFpEF. Notably, T3 exhibited the strongest association in both hypertension (OR: 1.69; 95% CI: 1.44–1.97) and dyslipidemia (OR: 1.49; 95% CI: 1.29–1.73). This relationship remained significant even after multivariate adjustment.

Table 4. Association between TyG index and the risk of HFpEF according to hypertension and dyslipidemia.
3.5 Association between TyG index and the risk of HFpEF according to age and metabolic status
The TyG index and HFpEF demonstrated a significant association with hypertension (OR: 1.24; 95% CI: 1.07–1.45) among CHD patients aged ≤ 60 years (Table 5). Using T1 as the reference, T2 and T3 were significantly related to the increased risk of HFpEF, and T3 exhibited the strongest association (OR: 1.67; 95% CI: 1.19–2.34). After multivariate adjustment, the TyG index demonstrated a similar association with HFpEF among individuals aged > 60 years, for both hypertension (OR: 1.28; 95% CI: 1.17–1.40) and dyslipidemia (OR: 1.17; 95% CI: 1.07–1.27). For both hypertension and dyslipidemia in patients aged > 60 years, using T1 as the reference, T2 and T3 were significantly related to the increased risk of HFpEF, and T3 exhibited the strongest association for both hypertension (OR: 1.71; 95% CI: 1.43–2.04) and dyslipidemia (OR: 1.49; 95% CI: 1.26–1.76). This relationship remained significant even after multivariate adjustment.
3.6 Association between TyG index and the risk of HFpEF according to sex and metabolic status
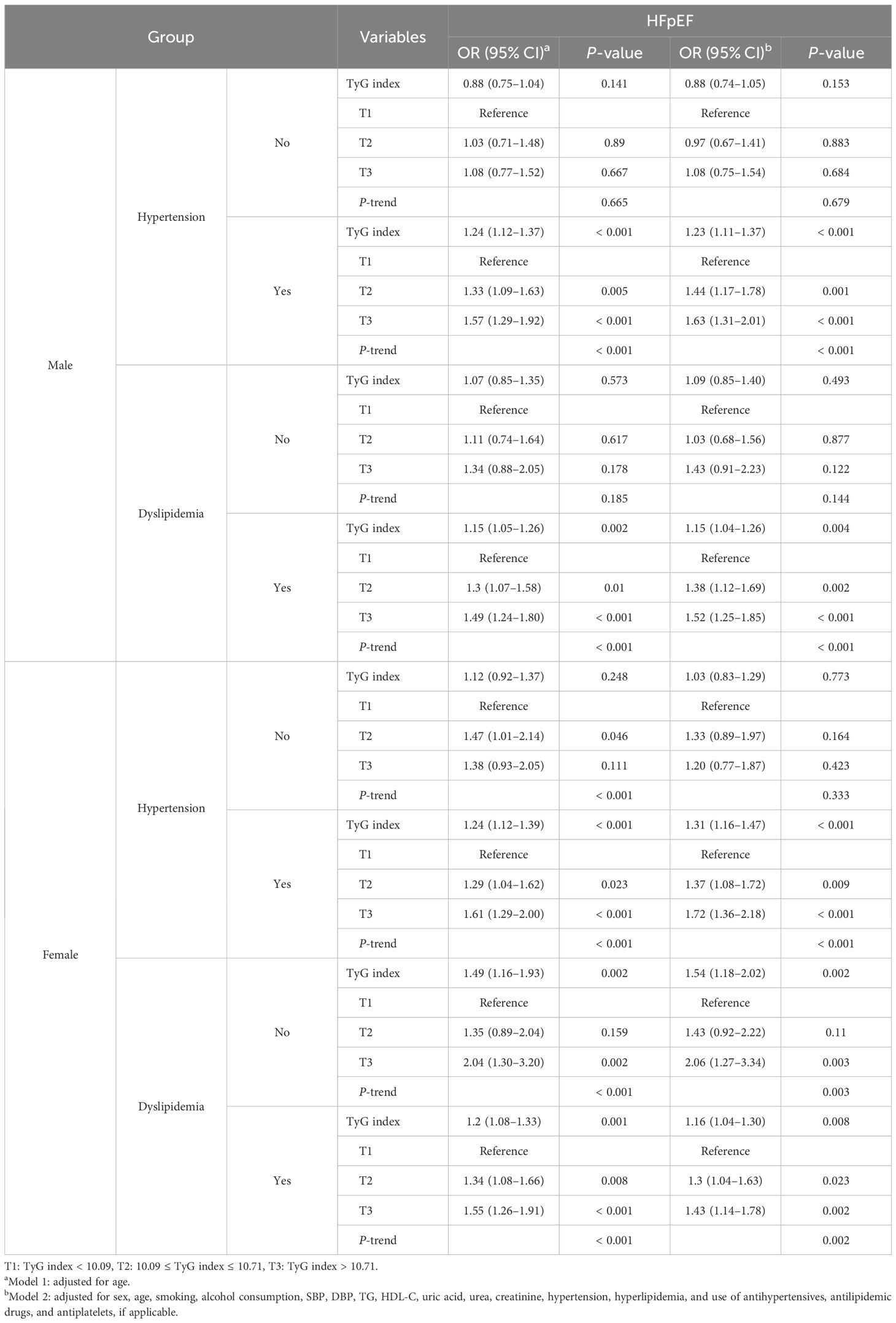
Regardless of sex and different blood pressure and lipid statuses among CHD patients, the association between TyG index and HFpEF was consistent (Table 6). After multivariate adjustment, the TyG index of CHD patients was significantly associated with HFpEF for hypertension (OR: 1.23; 95% CI: 1.11–1.37) and dyslipidemia (OR: 1.15; 95% CI: 1.04–1.26) among men. Similarly, the TyG index of CHD patients was significantly associated with HFpEF among women for both hypertension (OR: 1.31; 95% CI: 1.16–1.47) and dyslipidemia (OR: 1.16; 95% CI: 1.04–1.30). After multivariate adjustment using T1 as the reference, T2 and T3 were significantly related to an increased risk of HFpEF, and T3 showed the strongest association. The association between the TyG index and HFpEF in hypertension was stronger than that in hyperlipidemia in both sexes.
4 Discussion
This is the first large-scale study to demonstrate the relationship between the TyG index and HF and HFpEF in CHD patients and assess this relationship according to sex, age, and metabolic state (blood pressure and blood lipids). Overall, our data suggest that TyG index variability can increased HFpEF risk among Chinese adults.
HF is a global epidemic with an increasing prevalence, and the prognosis of patients with HF remains poor. HF is the leading cause of hospitalization in adults, with a 1-year mortality rate of 10–35%, and is considerably higher in patients with advanced HF (23). This underscores the importance of primary and secondary prevention of underlying HF conditions, including ischemic HF, management of HFpEF, and HF in older adults (24). Moreover, the number of HFpEF patients has been consistently increasing. Inadequate popularization of primary prevention has led to an increase in the number of individuals at high risk for developing HFpEF; however, continuous improvement in HFrEF treatment methods has substantially improved the LVEF of patients by more than 50%. Epidemiological data show that patients with HFpEF have all-cause and HF hospitalization rates similar to those with HFrEF. HFpEF is more common in older adults, women, and patients with hypertension (25–27). Our findings elucidated that HFpEF constituted the predominant subset among HF patients and that HFpEF patients were older, had higher systolic blood pressure, and were predominantly women, which is consistent with those of previous findings.
The TyG index and homeostasis model assessment of insulin resistance (HOMA-IR) demonstrated a close association. Moreover, the predictive value of the TyG index for IR was better than that for HOMA-IR (28). The TyG index is positively correlated with the prognosis of HF (14). The TyG index is a new biomarker of myocardial fibrosis in patients with HF and can be regarded as a useful risk stratification indicator for HF management (29). Multiple studies have indicated that a higher TyG index is associated with an increased risk of cardiovascular events, with varying degrees of risk across different populations (30–34). Therefore, further research is needed to explore the relationship with diabetic patients and HF. In study of NHANES 2007-2018, the TyG index was positively associated with the risk of HF, suggesting that the TyG index could serve as an important therapeutic target and prognostic indicator for HF (35). A high TyG index has been linked to poor prognosis in patients with HFpEF (36, 37). Additionally, the TyG index has demonstrated significant independent prognostic value regarding inpatient mortality and one-year all-cause mortality in patients with HF and chronic kidney disease (38). These studies suggest that the TyG index may play a crucial role in developing new therapeutic strategies aimed at improving the prognosis of high-risk populations with cardiovascular metabolic diseases. Notably, the TyG index is closely associated with the development of cognitive and physical impairments in individuals with insulin resistance and prediabetes (39). Furthermore, a high TyG-BMI is significantly associated with the risk of HF among participants with diabetes or prediabetes (40). These studies validate our findings that TyG has an independent association with HF in CHD patients and that there is a certain association with different types of HFpEF. Therefore, we propose that the TyG index could be considered a more convenient marker of IR and a valuable predictor of HFpEF. The discrepancies between our findings and these studies may relate to the association of the TyG index with HFpEF, as the previous cohorts were derived from the general population, whereas our study includes patients with CHD, specifically those with diabetes and prediabetes.
Established risk factors for atherosclerotic cardiovascular disease (ASCVD) include age, male sex, family history of ASCVD, obesity, hypertension, hypercholesterolemia, and diabetes mellitus (41). Therefore, the association between TyG index and HFpEF under different risk factor stratifications requires further exploration. The results of this study showed that the TyG index was independently associated with HFpEF in hypertension, dyslipidemia, and older patients (aged > 60 years). This relationship was observed in both sexes. A Shanghai-based community-based study on the relationship between macrovascular and microvascular injuries and the TyG index in older adults showed that an elevated TyG index was significantly associated with higher arterial stiffness and risk of renal microvascular injury (9). In middle-aged and older populations, an increase in the TyG index was significantly associated with hypertension and isolated systolic hypertension (42). The TyG index may represent a cost-effective and informative screening tool for metabolically obese individuals of normal weight (elevated blood pressure, hypertriglyceridemia, hyperglycemia, and low HDL cholesterol levels) (43). A high TyG index was independently associated with subclinical atherosclerosis (SA) in non-diabetic women but not in non-diabetic men. The TyG index was not associated with the presence of SA in patients with diabetes (44). Although the prevalence of coronary microvascular dysfunction among men and women with HFpEF is similar, the drivers of microvascular dysfunction may differ according to sex (45). These studies provide evidence for the difference in OR values for the relationship between the TyG index and HFpEF in this study.
In summary, the effect of TyG index in patients with cardiovascular diseases has been extensively investigated, emphasizing its potential clinical significance. Evaluation of the TyG index may have important clinical implications for risk stratification and individualized treatment of CHD patients.
5 Strengths and limitations
This study had certain limitations. First, because this was a multi-center study, there is a possibility of bias in the measurement methods at different research centers. However, the practitioners conducted external quality assessments between clinical laboratories in each center. Second, the retrospective design of the current study might have contributed to recall bias, and residual confounders could not be completely avoided. Therefore, any changes in the TyG index that may occur after HF treatment are unknown and require further investigation. Furthermore, the exact mechanism underlying the relationship between TyG index and HFpEF remains unclear, warranting further prospective large-scale studies.
6 Conclusion
This study demonstrated a significant association between the TyG index and HFpEF in CHD patients. Moreover, the association between TyG index and HFpEF in CHD patients was significantly more pronounced in patients with hypertension, dyslipidemia, and older patients aged > 60 years. In addition, the association between the TyG index and HFpEF in CHD patients showed that the OR value was higher in women than in men. The results of this study emphasize the need for a risk management strategy based on sex, age, and metabolic status to prevent the occurrence of HFpEF in CHD patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was approved by the ethics committee of Tianjin University of Traditional Chinese Medicine (TJUTCM-EC20190008) and registered in the Chinese Clinical Trial Registry (ChiCTR-1900024535) and Clinical Trials.gov (NCT04026724). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
CY: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. ZL: Data curation, Funding acquisition, Methodology, Software, Supervision, Writing – original draft, Writing – review & editing. XF: Conceptualization, Investigation, Methodology, Writing – review & editing. YL: Data curation, Investigation, Software, Formal analysis, Writing – review & editing. LY: Data curation, Software, Supervision, Writing – review & editing. YH: Data curation, Formal analysis, Writing – review & editing. LL: Data curation, Formal analysis, Methodology, Writing – review & editing. SG: Formal analysis, Funding acquisition, Supervision, Writing – review & editing. WC: Data curation, Formal analysis, Methodology, Software, Supervision, Writing – review & editing. RY: Investigation, Software, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the University level Scientific Research Project (Talent Special Project), Zhejiang Chinese Medical University (2023RCZXZK01) and National Natural Science Foundation of China (82405046).
Acknowledgments
We thank all the participants in the study and the members of the survey teams, as well as the financial support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1447072/full#supplementary-material
Abbreviations
TyG, Triglyceride-glucose; HF, Heart failure; HFrEF, Heart failure with reduced ejection fraction; HFmrEF, Heart failure with mid-range ejection fraction; HFpEF, Heart failure with preserved ejection fraction; LVEF, Left ventricle ejection fraction; CHD, Coronary heart disease; ASCVD, Atherosclerotic cardiovascular disease; SBP, Systolic blood pressure; DBP, Diastolic blood pressure; FPG, Fasting plasma glucose; TC, Total cholesterol; HDL-C, High-density lipoprotein cholesterol; TG, Triglycerides; LDL-C, Low-density lipoprotein cholesterol; CRP, C-reactive protein; HbA1c, Glycated hemoglobin; OR, Odds ratio; CIs, Confidence intervals; IR, Insulin resistance; HOMA-IR, Homeostasis model assessment of insulin resistance; SA, Subclinical atherosclerosis.
References
1. Trøseid M, Andersen GØ, Broch K, Hov JR. The gut microbiome in coronary artery disease and heart failure: Current knowledge and future directions. EBio Med. (2020) 52:102649. doi: 10.1016/j.ebiom.2020.102649
2. Schwinger RHG. Pathophysiology of heart failure. Cardiovasc Diagn Ther. (2021) 11:263–76. doi: 10.21037/cdt-20-302
3. Yijia L, Zhu L, Xu W, Tongyao N, Mei M, Yuanyuan H, et al. Effects of adjuvant Chinese patent medicine therapy on major adverse cardiovascular eventsin patients with coronary heart disease anginapectoris: a population-based retrospective cohort study. Acupuncture Herbal Med. (2022) 2:109–17. doi: 10.1097/hm9.0000000000000028
4. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the american college of cardiology/american heart association task force on clinical practice guidelines and the heart failure society of america. Circulation. (2017) 136:e137–61. doi: 10.1161/CIR.0000000000000509
5. Chamroonkiadtikun P, Ananchaisarp T, Wanichanon W. The triglyceride-glucose index, a predictor of type 2 diabetes development: A retrospective cohort study. Prim Care Diabetes. (2020) 14:161–7. doi: 10.1016/j.pcd.2019.08.004
6. Alizargar J, Bai CH, Hsieh NC, Wu SV. Use of the triglyceride-glucose index (TyG) in cardiovascular disease patients. Cardiovasc Diabetol. (2020) 19:8. doi: 10.1186/s12933-019-0982-2
7. da Silva A, Caldas APS, Hermsdorff HHM, Bersch-Ferreira ÂC, Torreglosa CR, Weber B, et al. Triglyceride-glucose index is associated with symptomatic coronary artery disease in patients in secondary care. Cardiovasc Diabetol. (2019) 18:89. doi: 10.1186/s12933-019-0893-2
8. Luo E, Wang D, Yan G, Qiao Y, Liu B, Hou J, et al. High triglyceride-glucose index is associated with poor prognosis in patients with acute ST-elevation myocardial infarction after percutaneous coronary intervention. Cardiovasc Diabetol. (2019) 18:150. doi: 10.1186/s12933-019-0957-3
9. Zhao S, Yu S, Chi C, Fan X, Tang J, Ji H, et al. Association between macro- and microvascular damage and the triglyceride glucose index in community-dwelling elderly individuals: the Northern Shanghai Study. Cardiovasc Diabetol. (2019) 18:95. doi: 10.1186/s12933-019-0898-x
10. Huang R, Lin Y, Ye X, Zhong X, Xie P, Li M, et al. Triglyceride-glucose index in the development of heart failure and left ventricular dysfunction: analysis of the ARIC study. Eur J Prev Cardiol. (2022) 29:1531–41. doi: 10.1093/eurjpc/zwac058
11. Kim MK, Ahn CW, Kang S, Nam JS, Kim KR, Park JS. Relationship between the triglyceride glucose index and coronary artery calcification in Korean adults. Cardiovasc Diabetol. (2017) 16:108. doi: 10.1186/s12933-017-0589-4
12. Li Z, He Y, Wang S, Li L, Yang R, Liu Y, et al. Association between triglyceride glucose index and carotid artery plaque in different glucose metabolic states in patients with coronary heart disease: a RCSCD-TCM study in China. Cardiovasc Diabetol. (2022) 21:38. doi: 10.1186/s12933-022-01470-3
13. Si S, Li J, Li Y, Li W, Chen X, Yuan T, et al. Causal effect of the triglyceride-glucose index and the joint exposure of higher glucose and triglyceride with extensive cardio-cerebrovascular metabolic outcomes in the UK biobank: A mendelian randomization study. Front Cardiovasc Med. (2021) 7:583473. doi: 10.3389/fcvm.2020.583473
14. Yang S, Du Y, Liu Z, Zhang R, Lin X, Ouyang Y, et al. Triglyceride-glucose index and extracellular volume fraction in patients with heart failure. Front Cardiovasc Med. (2021) 8:704462. doi: 10.3389/fcvm.2021.704462
15. Barua RS, Rigotti NA, Benowitz NL, Cummings KM, Jazayeri MA, Morris PB, et al. 2018 ACC expert consensus decision pathway on tobacco cessation treatment: a report of the american college of cardiology task force on clinical expert consensus documents. J Am Coll Cardiol. (2018) 72:3332–65. doi: 10.1016/j.jacc.2018.10.027
16. Fan AZ, Ruan WJ, Chou SP. Re-examining the relationship between alcohol consumption and coronary heart disease with a new lens. Prev Med. (2019) 118:336–43. doi: 10.1016/j.ypmed.2018.11.022
17. Li Z, Cheng Q, Liu Y, Cheng X, Wang S, He Y, et al. Low-/high-density lipoprotein cholesterol ratio and carotid plaques in patients with coronary heart disease: a Chinese cohort study. Lipids Health Dis. (2021) 20:144. doi: 10.1186/s12944-021-01575-w
18. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. (2002) 106:3143–421. doi: 10.1161/circ.106.25.3143
19. Han Y, Jiang X, Qin Y, Zhao Y, Zhang G, Liu C. A cross-sectional study exploring the relationship between the dietary inflammatory index and hyperlipidemia based on the National Health and Nutrition Examination Survey (2005-2018). Lipids Health Dis. (2023) 22:140. doi: 10.1186/s12944-023-01908-x
20. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure. J Am Coll Cardiol. (2022) 79:e263–421. doi: 10.1016/j.jacc.2021.12.012
21. New York Heart Association (NYHA) Heart Function Classification Standard Group. Internal medicine. Beijing: People’s Medical Publishing House (2010) p. 195–6.
22. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. (2016) 37:2129–200. doi: 10.1093/eurheartj/ehw128
23. Savarese G, Stolfo D, Sinagra G, Lund LH. Heart failure with mid-range or mildly reduced ejection fraction. Nat Rev Cardiol. (2022) 19:100–16. doi: 10.1038/s41569-021-00605-5
24. Shimokawa H, Miura M, Nochioka K, Sakata Y. Heart failure as a general pandemic in Asia. Eur J Heart Fail. (2015) 17:884–92. doi: 10.1002/ejhf.319
25. Shiga T, Suzuki A, Haruta S, Mori F, Ota Y, Yagi M, et al. Clinical characteristics of hospitalized heart failure patients with preserved, mid-range, and reduced ejection fractions in Japan. ESC Heart Fail. (2019) 6:475–86. doi: 10.1002/ehf2.12418
26. Savarese G, Vedin O, D’Amario D, Uijl A, Dahlström U, Rosano G, et al. Prevalence and prognostic implications of longitudinal ejection fraction change in heart failure. JACC Heart Fail. (2019) 7:306–17. doi: 10.1016/j.jchf.2018.11.019
27. Liang M, Bian B, Yang Q. Characteristics and long-term prognosis of patients with reduced, mid-range, and preserved ejection fraction: A systemic review and meta-analysis. Clin Cardiol. (2022) 45:5–17. doi: 10.1002/clc.23754
28. Kang SW, Kim SK, Kim YS, Park MS. Risk prediction of the metabolic syndrome using TyG Index and SNPs: a 10-year longitudinal prospective cohort study. Mol Cell Biochem. (2023) 478:39–45. doi: 10.1007/s11010-022-04494-1
29. Nishida K, Otsu K. Inflammation and metabolic cardiomyopathy. Cardiovasc Res. (2017) 113:389–98. doi: 10.1093/cvr/cvx012
30. Haring B, Schumacher H, Mancia G, Teo KK, Lonn EM, Ahfoud F, et al. Triglyceride-glucose index, low-density lipoprotein levels, and cardiovascular outcomes in chronic stable cardiovascular disease: results from the ONTARGET and TRANSCEND trials. Eur J Prev Cardiol. (2024) 31:311–9. doi: 10.1093/eurjpc/zwad340
31. Dang K, Wang X, Hu J, Zhang Y, Cheng L, Qi X, et al. The association between triglyceride-glucose index and its combination with obesity indicators and cardiovascular disease: NHANES 2003-2018. Cardiovasc Diabetol. (2024) 23:8. doi: 10.1186/s12933-023-02115-9
32. Dong W, Gong Y, Zhao J, Wang Y, Li B, Yang Y. A combined analysis of TyG index, SII index, and SIRI index: positive association with CHD risk and coronary atherosclerosis severity in patients with NAFLD. Front Endocrinol (Lausanne). (2024) 14:1281839. doi: 10.3389/fendo.2023.1281839
33. Sun C, Hu L, Li X, Zhang X, Chen J, Li D, et al. Triglyceride-glucose index’s link to cardiovascular outcomes post-percutaneous coronary intervention in China: a meta-analysis. ESC Heart Fail. (2024) 11:1317–28. doi: 10.1002/ehf2.14679
34. Dong J, Yang H, Zhang Y, Chen L, Hu Q. A high triglyceride glucose index is associated with early renal impairment in the hypertensive patients. Front Endocrinol (Lausanne). (2022) 13:1038758. doi: 10.3389/fendo.2022.1038758
35. Zhang F, Hou X. Association between the triglyceride glucose index and heart failure: NHANES 2007-2018. Front Endocrinol (Lausanne). (2024) 14:1322445. doi: 10.3389/fendo.2023.1322445
36. Wang T, Xu J, Zhang H, Tao L, Huang X. Triglyceride-glucose index for the detection of subclinical heart failure with preserved ejection fraction in patients with type 2 diabetes. Front Cardiovasc Med. (2023) 10:1086978. doi: 10.3389/fcvm.2023.1086978
37. Zhou Q, Yang J, Tang H, Guo Z, Dong W, Wang Y, et al. High triglyceride-glucose (TyG) index is associated with poor prognosis of heart failure with preserved ejection fraction. Cardiovasc Diabetol. (2023) 22:263. doi: 10.1186/s12933-023-02001-4
38. Chen Y, Li S, Yang K, Wu B, Xie D, Peng C, et al. Triglyceride-glucose index and prognosis in individuals afflicted with heart failure and chronic kidney disease. ESC Heart Fail. (2024) 11:3120–32. doi: 10.1002/ehf2.14898
39. Santulli G, Visco V, Varzideh F, Guerra G, Kansakar U, Gasperi M, et al. Prediabetes increases the risk of frailty in prefrail older adults with hypertension: beneficial effects of metformin. Hypertension. (2024) 81:1637–43. doi: 10.1161/HYPERTENSIONAHA.124.23087
40. Yang S, Shi X, Liu W, Wang Z, Li R, Xu X, et al. Association between triglyceride glucose-body mass index and heart failure in subjects with diabetes mellitus or prediabetes mellitus: a cross-sectional study. Front Endocrinol (Lausanne). (2023) 14:1294909. doi: 10.3389/fendo.2023.1294909
41. Choi S. The potential role of biomarkers associated with ASCVD risk: risk-enhancing biomarkers. J Lipid Atheroscler. (2019) 8:173–82. doi: 10.12997/jla.2019.8.2.173
42. Zheng R, Mao Y. Triglyceride and glucose (TyG) index as a predictor of incident hypertension: a 9-year longitudinal population-based study. Lipids Health Dis. (2017) 16:175. doi: 10.1186/s12944-017-0562-y
43. Xu X, Bhagavathula AS, Zhang Y, Ryan PM, Rahmani J, Qi X. Sex differences in the tyG index and cardiovascular risk factors in metabolically obese normal weight phenotype. Int J Endocrinol. (2022) 2022:1139045. doi: 10.1155/2022/1139045
44. Lu YW, Chang CC, Chou RH, Tsai YL, Liu LK, Chen LK, et al. Gender difference in the association between TyG index and subclinical atherosclerosis: results from the I-Lan Longitudinal Aging Study. Cardiovasc Diabetol. (2021) 20:206. doi: 10.1186/s12933-021-01391-7
Keywords: triglyceride-glucose index, coronary heart disease, heart failure, heart failure with preserved ejection fraction, metabolic states
Citation: Li Z, Fan X, Liu Y, Yu L, He Y, Li L, Gao S, Chen W, Yang R and Yu C (2024) Triglyceride-glucose index is associated with heart failure with preserved ejection fraction in different metabolic states in patients with coronary heart disease. Front. Endocrinol. 15:1447072. doi: 10.3389/fendo.2024.1447072
Received: 11 June 2024; Accepted: 17 October 2024;
Published: 04 November 2024.
Edited by:
Gaetano Santulli, Albert Einstein College of Medicine, United StatesReviewed by:
Lorenzo Da Dalt, University of Milan, ItalyAndrew Carley, The Ohio State University, United States
Copyright © 2024 Li, Fan, Liu, Yu, He, Li, Gao, Chen, Yang and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunquan Yu, eWNxdGp1dGNtQGZveG1haWwuY29t; Rongrong Yang, cm9uZ3JvbmcwNDIzQGhvdG1haWwuY29t; Wei Chen, d2VpY2hlbkB0bXUuZWR1LmNu
†These authors share first authorship
 Zhu Li
Zhu Li Xiang Fan
Xiang Fan Yijia Liu
Yijia Liu Lu Yu3
Lu Yu3 Rongrong Yang
Rongrong Yang Chunquan Yu
Chunquan Yu