- Department of Endocrinology and Metabolism, The Institute of Endocrinology, National Health Commision of the People's Repiublic of China (NHC) Key Laboratory of Diagnosis and Treatment of Thyroid Diseases, The First Affiliated Hospital of China Medical University, Shenyang, China
Clinicians often consider the use of dietary supplements to assist in lowering thyroid autoantibody titres in patients with Hashimoto’s thyroiditis (HT). Currently, different supplements differ in their ability to reduce autoantibody levels. The purpose of this article is to compare the ability of different supplements to lower autoantibody titres and restore TSH levels through a systematic literature review. We obtained information from the PubMed, Web of Science, Embase, and Cochrane databases, as well as the China National Knowledge Infrastructure (CNKI). Selected studies included those using selenium, Vitamin D, Myo-inositol, and Myo-inositol in combination with selenium for the treatment of HT patients with euthyroidism. These data were combined using standardised mean differences (SMDs) and assessed using a random effects model. A total of 10 quantitative meta-analyses of case-control studies were selected for this meta-analysis. Compared to the placebo group, the use of selenium supplements was able to significantly reduce the levels of thyroid peroxidase autoantibodies (TPOAb) (SMD: -2.44, 95% CI: -4.19, -0.69) and thyroglobulin autoantibodies (TgAb) (SMD: -2.76, 95% CI: -4.50, -1.02). During a 6-month treatment, the use of Myo-inositol, Vitamin D alone, and the combination of selenium, and Myo-inositol did not effectively reduce TPOAb (Myo-inositol: SMD:-1.94, 95% CI: -6.75, 2.87; Vitamin D: SMD: -2.54, 95% CI: -6.51,1.42; Se+Myo-inositol: SMD: -3.01, 95% CI: -8.96,2.93) or TgAb (Myo-inositol: SMD:-2.02, 95% CI: -6.52, 2.48; Vitamin D: SMD: -2.73, 95% CI: -6.44,0.98; Se+Myo-inositol: SMD: -3.64, 95% CI: -9.20,1.92) levels. Therefore, we recommend that patients with HT(Hashimoto’s Thyroiditis) be given an appropriate amount of selenium as an auxiliary treatment during standard-of-care treatment.
1 Introduction
Hashimoto’s thyroiditis(HT) is a common autoimmune disease with a prevalence of 14.19% in China and is 2-3 times more common in women than in men (1).HT is typically characterised by euthyroidism (2). The development of HT is associated with genetic susceptibility (3), iodine intake (4), and other factors. Its pathology is characterised by thyroid cell destruction and lymphocytic infiltration (5). On examination, patients usually present with a significant increase in thyroid peroxidase antibodies (TPOAb) or thyroglobulin antibodies (TgAb), with normal or elevated levels of TSH(Thyroid stimulating hormone) (6). Clinically, levothyroxine is used to manage the symptoms of hypothyroidism (7). However, studies have demonstrated that TPOAb are linked to unfavourable pregnancy outcomes (8) and lower IQ(intelligence quotient) in children (9). Consequently, the use of supplements to reduce TPOAb or TgAb levels is also being considered by clinicians for treatment of HT (10–12).
The highest concentration of selenium across tissues is found in the thyroid (13). Selenium plays a variety of roles in the thyroid, the most important of which is its involvement in the formation of type I deiodinase, which catalyses the deiodination of tetraiodothyronine (T4) to triiodothyronine (T3) (14). A deficiency of delenium has demonstrated to reduce the activity of deiodinase, which consequently alters thyroid hormone metabolism, as evidenced by the blockage of T4 to T3 conversion (15). In an epidemiological study of selenium and goitre in China, the prevalence of pathological thyroid disease was significantly higher in selenium-deficient areas than in selenium-sufficient areas (16), indicating that adequate selenium supplementation may be beneficial in the treatment of HT, in addition to its known anti-inflammatory effects (17–20).
Vitamin D plays a significant role in maintaining calcium and phosphorus balance and bone health (21, 22). There is a bidirectional relationship between human vitamin D levels and the thyroid function (23, 24). Furthermore, numerous studies have documented a negative correlation between antithyroid antibodies and 25(OH)D levels (25–27). Conversely, HT has been demonstrated to impact bone metabolism (28). Many meta-analyses and clinical trials have shown that supplementation with moderate amounts of vitamin D is beneficial in reducing autoantibody levels in patients with HT (29). However, some studies have not yielded comparable outcomes (30).
In addition to selenium and Vitamin D supplementation in HT patients, Krysiak et al. recently used selenium and Vitamin D in combination with inositol (35). This combination was effective in lowering autoantibody levels, but due to the reduced sample size of the study, these findings warrant validation across larger patient cohorts.
In this study, the effects of supplementation with selenium, Vitamin D, and inositol, or a combination of these, in patients with HT is summarised. A systematic review and network meta-analysis was performed to assess the effects of these supplements on TPOAb, TgAb, and TSH, based on recently published randomised controlled trials.
2 Materials and methods
2.1 Search strategy
A systematic literature search was performed using the PubMed, Web of Science, Embase, and Cochrane databases, as well as the China National Knowledge Infrastructure (CNKI) in December 2023 using the following search terms: (Hashimoto Disease[MeSH Terms]) OR (Disease, Hashimoto[Other Term]) OR (Chronic Lymphocytic Thyroiditis[Other Term]) OR (Chronic Lymphocytic Thyroiditides[Other Term]) OR (Lymphocytic Thyroiditides, Chronic[Other Term]) OR (Lymphocytic Thyroiditis, Chronic[Other Term]) OR (Thyroiditides, Chronic Lymphocytic[Other Term]) OR (Thyroiditis, Chronic Lymphocytic[Other Term]) OR (Hashimoto Struma[Other Term]) OR (Hashimoto’s Struma[Other Term]) OR (Hashimoto’s Syndrome[Other Term]) OR (Hashimoto Syndrome[Other Term]) OR (Hashimoto’s Syndromes[Other Term]) OR (Hashimotos Syndrome[Other Term]) OR (Syndrome, Hashimoto’s[Other Term]) OR (Syndromes, Hashimoto’s[Other Term]) OR (Hashimoto’s Disease[Other Term]) OR (Disease, Hashimoto’s[Other Term]) OR (Hashimotos Disease[Other Term]) OR (Hashimoto Thyroiditis[Other Term]) OR (Hashimoto Thyroiditides[Other Term]) OR (Thyroiditides, Hashimoto[Other Term]) OR (Thyroiditis, Hashimoto[Other Term]) AND (selenium[MeSH Terms]) OR (Vitamin d[MeSH Terms]) OR (Myo-inositol[MeSH Terms]).
2.2 Study selection
Relevant literature was selected in PubMed, Cochrane Central Register of trials, and Web of Science. The search terms were in English, and the article language was restricted to English. The words or technical terms used for the search were related to “Autoimmune thyroiditis”, “Hashimoto’s thyroiditis”, “Selenium”, “Vitamin D”, and “Myo-inositol”.
Studies included in this meta-analysis had to meet the following criteria: (I)Participants were diagnosed with HT; (II) Patients enrolled in the study did not use levothyroxine sodium tablets throughout the study; (III) Patients did not suffer from other autoimmune or metabolic disease; (IV) Patients in experimental and control groups did not take any supplements within six months (particularly inositol, Vitamin D, and selenium); (V) Patients with increased TPOAb or TgAb titres; (VII) Patients did not suffer from congestive heart failure, diabetes, renal or hepatic impairment, and were not pregnant or lactating.
Wang Ping and Gu Qing Ling reviewed and quality-assessed each article’s title, abstract, and/or full text retrieved from the literature search to determine eligibility for the meta-analysis.
2.3 Data extraction and quality assessment
Data extraction was performed using an extraction table that focussed on study characteristics (first author, year of publication, and study design), participant characteristics (number, subgroup, age), outcomes (levels of TPOAb, TgAb, and TSH), and adjusted matching factors.
2.4 Quality assessment
Two reviewers completed quality assessment of the selected studies using the Jadad scale. This included assessment of whether the studies were blinded, and randomised, and whether subjects withdrew from the study or were lost to follow-up. The total score ranged from 3 to 5, with higher scores representing a higher assessment quality. All discrepancies or conflicting assessments were resolved via a consensus discussion with a third reviewer.
2.5 Statistical analysis
Mean differences with a 95% CI(Confidential intervals) were used to evaluate the impact of each supplement on HT-related and representative metrics. To account for the heterogeneity among studies, the data were pooled using a random-effects method. Heterogeneity was assessed using the I² statistic, and I² > 50% was considered a level of high heterogeneity. Subgroup analysis was performed according to the time and dose of the supplements used. All analyses were performed using STATA (version 17.0, StataCorp), and a P<0.05 was considered statistically significant.
3 Results
3.1 Characteristics of the included articles
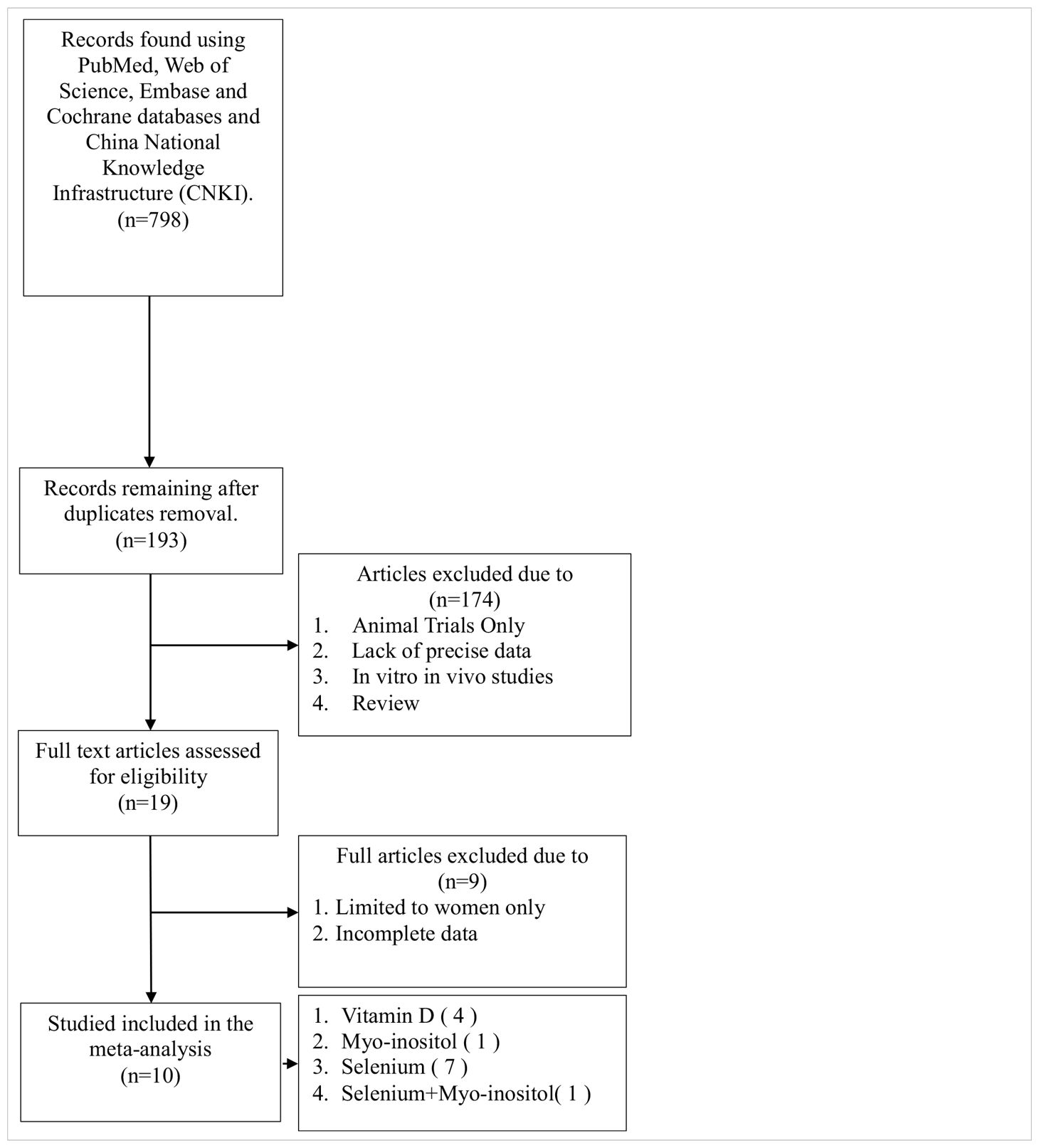
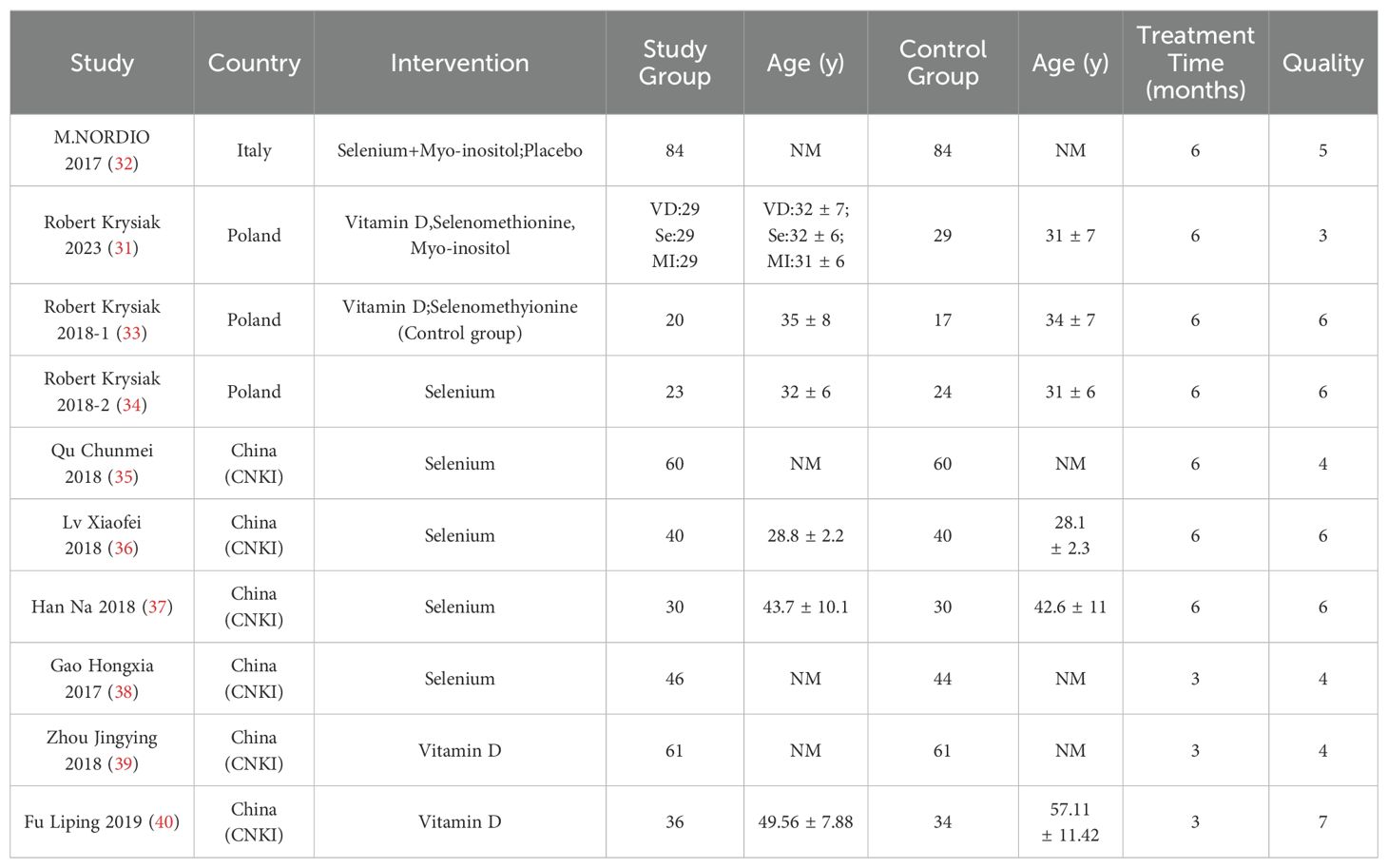
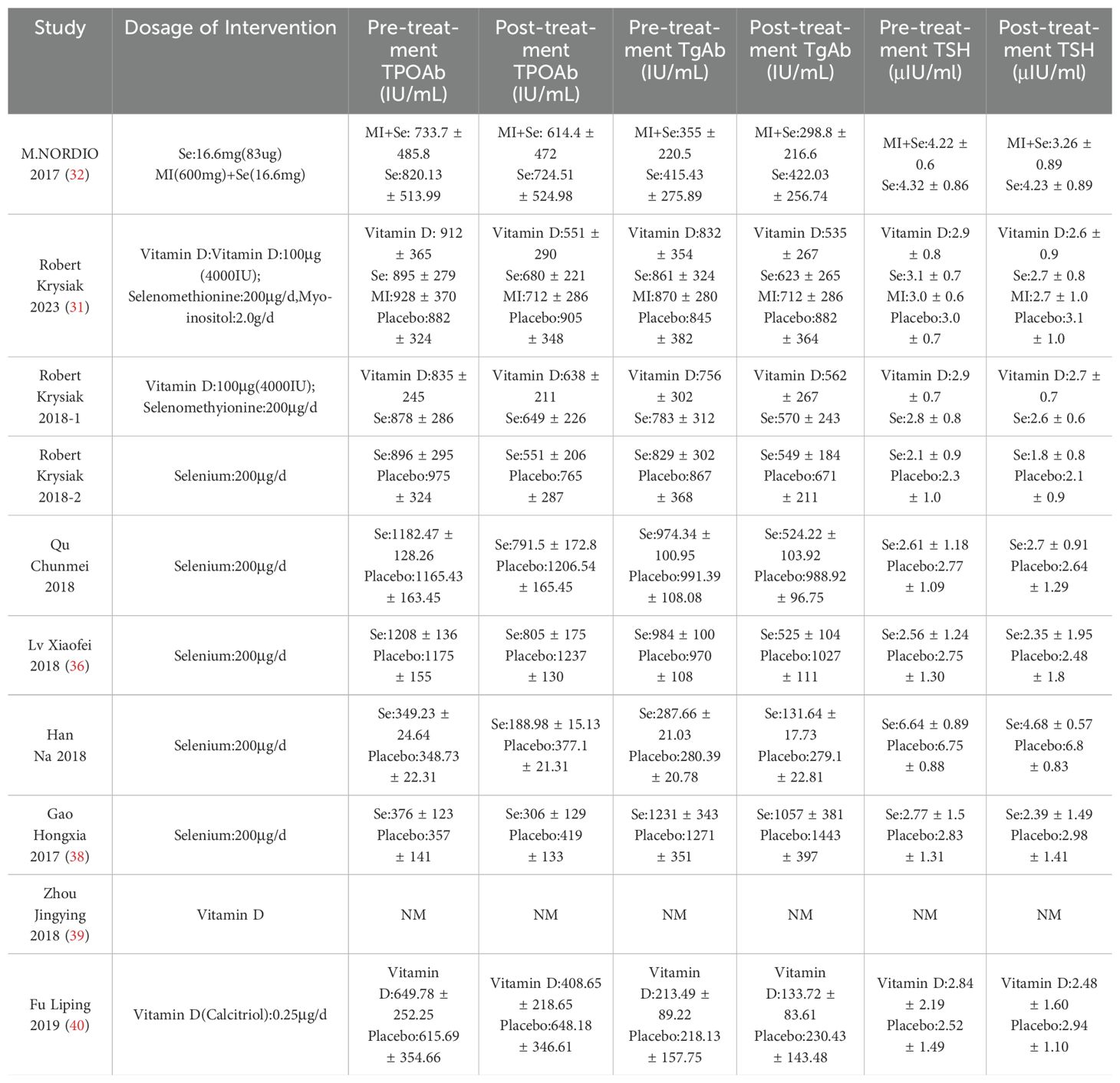
As illustrated in Table 1, the literature search yielded a total of 798 relevant articles. Following a comprehensive examination of the article’s abstracts, 193 studies with full-text publications were subjected to a detailed evaluation. Of these, 184 studies were excluded due to the presence of irrelevant or single-arm results, leaving a total of 10 articles for inclusion in the meta-analysis. Table 2 presents the abstract items for these 10 articles, including the first author, publication year, study design, sample size, results, adjusted matching factors, and quality assessment scores. Table 3 shows the supplements' dosage and changes in TPOAb, TgAb and TSH before and after supplementation.
In 7 of these articles, interventions lasted 6 months, while in 3 articles, interventions lasted 3 months. Of these 10 studies, 1 was an open trial, which was considered of moderate quality, and the remaining studies were randomised controlled trials, all of high quality (Table 2).
3.2 Network meta-analysis plot
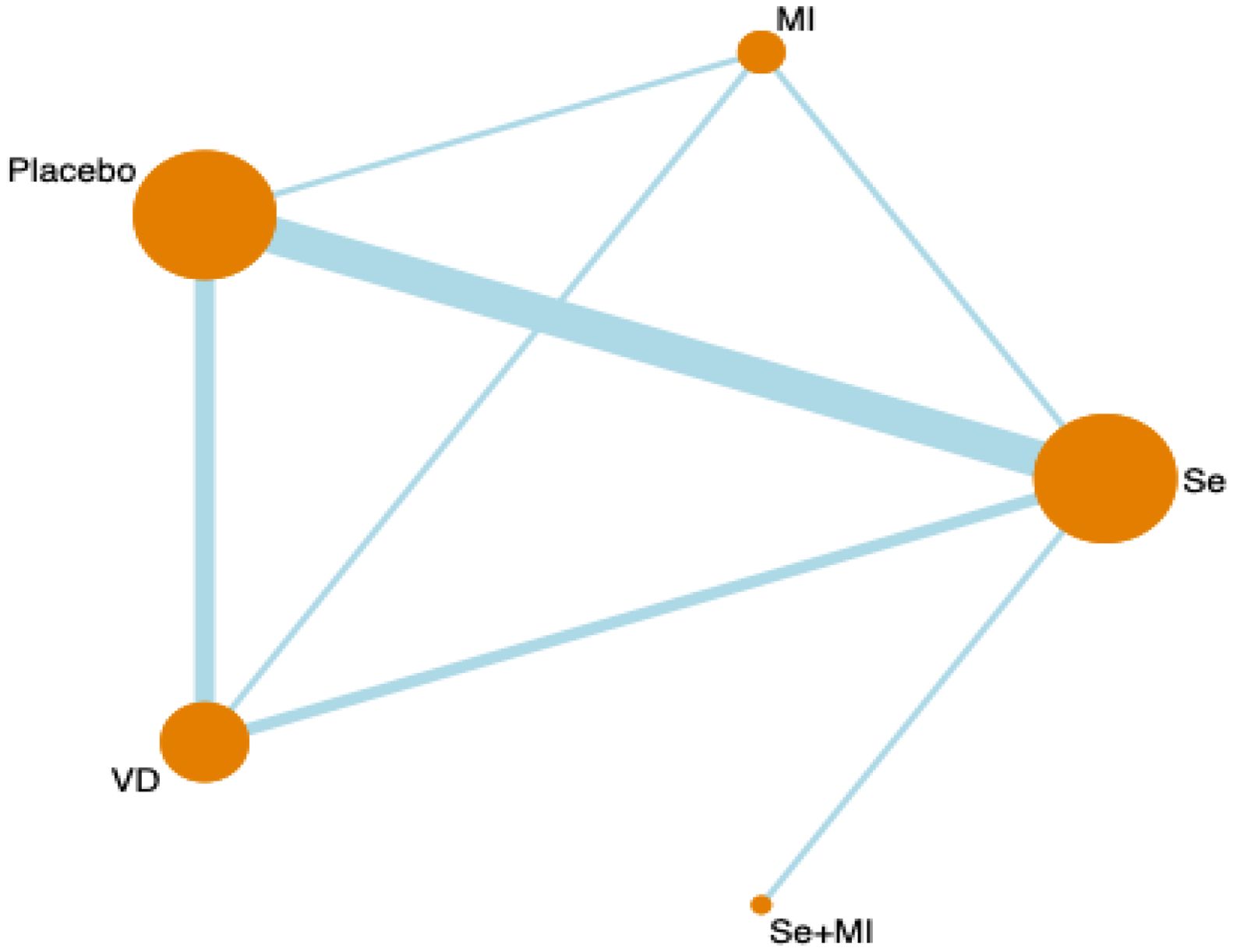
Figure 1 shows a network diagram of trials that tested the effectiveness of different interventions (placebo, Vitamin D, selenium supplements, inositol, Vitamin D + selenium, Vitamin D + inositol) and direct comparisons of these interventions. Node size is the sample size for each type of intervention, and line thickness is proportional to the number of trials for that comparison.
3.3 Forest plots
The use of selenium supplementation was generally able to significantly reduce TPOAb (SMD(Standard mean difference): -2.44, 95% CI(Confidential intervals):-4.19, -0.69) (Figure 2A) and TgAb(SMD: -2.76, 95% CI: -4.50, -1.02) (Figure 2B) levels when compared to the placebo group. The combination of selenium and Myo-inositol supplementation was able to reduce individual autoantibody levels when compared to selenium alone. However these results were not statistically significant in the meta-analysis, which is likely attributable to the limited sample size. In clinical trials, vitamin D may reduce antibody titres in HT patients, but this was not reflected in this study (Figures 2A, B). In clinical trials, the majority of HT patients who received vitamin D supplementation exhibited significant hypothyroidism. However, the sample size was limited, which raises concerns about the reliability of the findings. While inositol alone may not significantly reduce antibody titres, its combination with selenium or vitamin D may enhance the function of these supplements. Nevertheless, further research is needed to clarify its role in clinical practice.
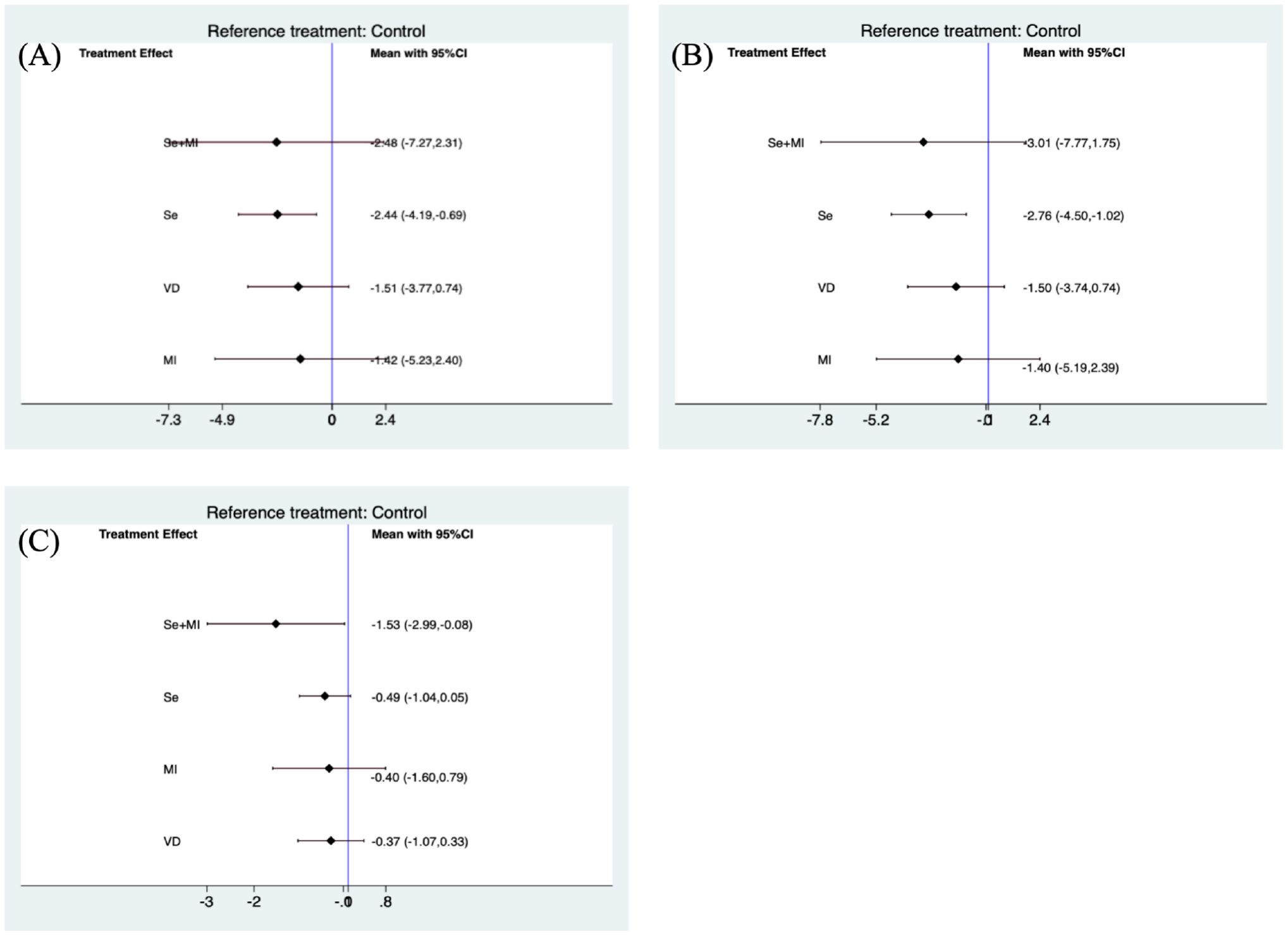
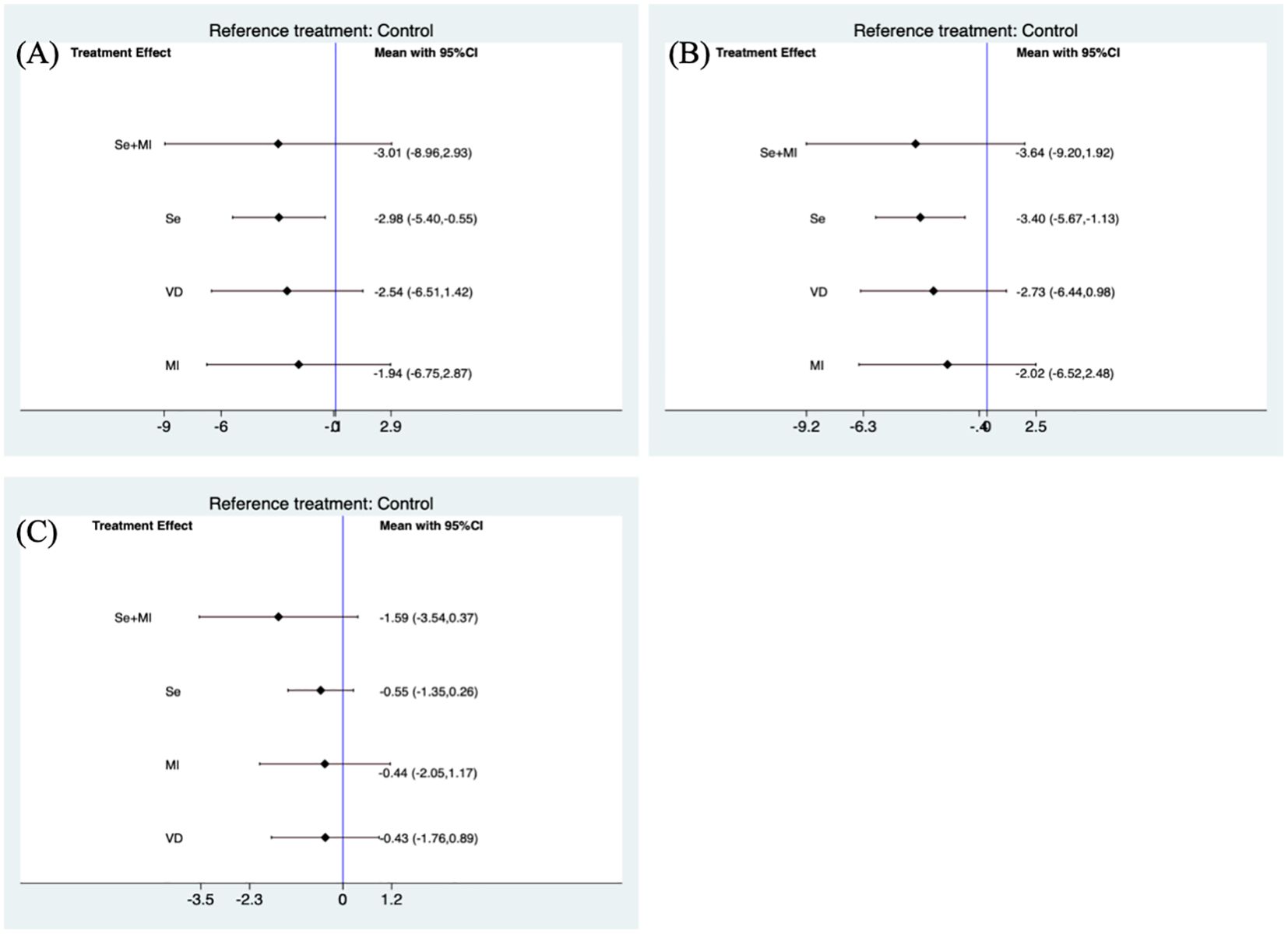
Figure 2. Forest plots of TPOAb and TgAb levels during supplement use. (A) Forest plot of TPOAb levels when using Se, VD, Se+MI and MI supplements vs. placebo. (B) Forest plot of TgAb levels when using Se, VD, Se+MI and MI supplements vs. placebo. (C) Forest plot of TSH levels when using Se, VD, Se+MI and Myo-inositol supplements vs. placebo. Se, Selenium; MI, Myo-inositol; VD, Vitamin D.
Due to inconsistencies in the treatment duration of the included trials, a separate subgroup analysis was conducted for trials that included treatment supplementation for a period of 6 months. The use of selenium supplements was still found to be effective in reducing TPOAb(SMD: -2.98, 95 %CI: -5.40, -0.55) and TgAb levels (SMD: -3.40, 95%CI: -5.67, -1.13).
Furthermore, Myo-inositol used in combination with selenium was able to reduce TSH levels (SMD: -1.53, 95%CI: -2.99, -0.08) (Figure 2C). However these results were not significant when subgroup analyses were performed for studies using treatment supplements for 6 months (Figure 3C).
3.4 Publication bias
Because many of the articles on selenium supplementation came from CNKI, we conducted a publication bias test on the included articles. The results of Egger’s test indicated the presence of publication bias, and the results did not reverse after using the trim and fill method, indicating that the results are stable and publication bias does not affect this result.
3.5 Sensitivity analysis
To assess the stability of the results, we performed a sensitivity analysis. Each article was successively excluded and a meta-analysis was performed on the remaining literature, and we found that the results did not change significantly, which means that the results are stable and reasonable.
4 Discussion
In this network meta-analysis, selenium supplementation during the treatment of HT patients effectively reduced TPOAb (SMD:-2.44, 95% CI:-4.19, -0.69) and TgAb (SMD: -2.76, 95% CI:-4.50, -1.02) levels. If patients have vitamin D deficiency or insufficiency(<20 ng/mL) or low selenium levels(20-30 ng/mL) (41) and still have high autoantibodies after long-term regular treatment, we can consider adding selenium or vitamin D or a combination of the two, or inositol or a combination of the two. If a single supplement does not significantly reduce antibody levels, a combination of supplements is more likely to be recommended. Furthermore, it is recommended that patient levels of selenium and vitamin D be monitored throughout the course of treatment, with adjustments to supplement dosages made as necessary.
The association between autoimmune thyroid disease and low levels of vitamin D has been confirmed by many epidemiological studies (42, 43), with several investigations showing a negative correlation between vitamin D levels and thyroid-associated antibodies (44, 45). Consequently, researchers have investigated the potential of vitamin D supplementation to improve autoimmune thyroiditis and reduce the associated autoantibody levels. While some studies have found that vitamin D supplementation in HT patients treated with levothyroxine can reduce autoantibody levels, it seems to be more effective for patients with low vitamin D levels (34, 46, 47). It is undeniable that vitamin D may be involved in the pathogenesis of HT. Recent studies have also found that vitamin D supplementation under normal conditions is an effective method in reducing the prevalence of autoimmune diseases (48).
There is a growing body of evidence to identify that selenium plays a role in thyroid function (49), and recent studies have shown that selenium levels are negatively correlated with autoantibody titres in patients with HT (50). Therefore, clinicians tend to favour the use of selenium supplements as an adjunctive treatment HT (51). Furthermore, oxidative stress has been suggested to be involved in the pathogenesis of HT (52). Glutathione peroxidase is a crucial antioxidant enzyme in humans, and selenium acts as its catalytic centre during reduction reactions (53). Moreover, selenium has been demonstrated to inhibit inflammatory responses by modulating the ratio of Treg/Th17 cells(Regulatory T-cells/T-helper cell 17) (54). Our results are in agreement with studies reporting that selenium supplementation significantly reduces thyroid-associated autoantibody levels. However, that excessive selenium intake can result in toxicity (55). Therefore, supplementation should be moderate and tailored to the individual.
Myo-inositol is a vital nutrient for human health and an essential component of cellular structural lipids (56). Derivatives of Myo-inositol perform a number of important cellular and metabolic functions. including morphogenesis (57), cytoskeletal rearrangement (58), cell proliferation (59), and the regulation of glucose metabolism (60). In the thyroid gland, an imbalance in Myo-inositol metabolism impairs the hormone biosynthesis, storage, and secretion (61). In recent years, Myo-inositol has been effectively used in combination with selenium or vitamin D as a dietary supplement for the treatment of HT. This combination can adequately reduce autoantibody levels and restore TSH levels, while also restoring TSH signalling (31, 62, 63). Nevertheless, further clinical trials and mechanistic studies are required to provide additional support for these findings.
It is important to note that while the use of Myo-inositol in clinical practice is increasing, Myo-inositol alone is not as effective as when combined with selenium or vitamin D (31). A study by Payer found that combining inositol and selenium in women with subclinical hypothyroidism resulted in a reduction in autoantibody titres and a decline in serum cholesterol levels in patients (62). Krysiak et al. observed that the impact of vitamin D on thyroid autoimmunity and hypothalamic-pituitary-thyroid axis activity was more pronounced in patients with HT who received Myo-inositol (64). Furthermore, Benvenga et al. administered selenium and Myo-inositol to mice with cadmium-induced thyroid damage and observed that mice treated with both inositol and selenium exhibited the same indices as those not treated with cadmium. These findings indicate that the combination of Myo-inositol and selenium protects the thyroid in cadmium-exposed subjects (65). Moreover, the effect of selenium combined with vitamin D on the reduction thyroid-specific autoantibodies should not be overlooked. Krysiak et al. administered vitamin D (4000 IU/day) to 23 women who had been treated with selenomethionine for a minimum of 12 months. The combination of vitamin D and selenothionine was found to be effective in lowering TPOAb and TgAb titres and increasing SPINA (The secretory capacity of the thyroid gland), when compared to women treated with vitamin D alone (24 women). This indicates that selenium intake may enhance the effect of vitamin D in thyroid autoimmunity (33). The combination of multiple supplements is likely to become a future trend in clinical practive for the reduction of HT-specific autoantibody titres.
To our knowledge, this is the meta-analysis to compare the efficacy of divers dietary supplements as an adjunctive therapy for the management of HT. Furthermore, patients treated with levothyroxine were excluded from this analysis, avoiding drug influences on the assessed measures. However, this resulted in some limitations, including a stendency towards a higher number of included selenium-related articles. Conversely, Myo-inositol, which has been proposed as a dietary supplement for HT patients in recent years, has fewer reported clinical studies. In addition, discrepancies in treatment duration, variations in drug dosage, and differences in drugs administration may also influence the reported experimental outcomes. Nevertheless, this meta-analysis revealed a statistically significant reduction in autoantitody titres when selenium was administered. Further research is required to investigate the efficacy of other supplements in this clinical setting.
5 Conclusion
In conclusion, the results of this study demonstrated that selenium supplementation has a significant role in lowering thyroid autoantibody titres in patients with HT. Moreover, large multicentre randomised controlled studies are required to ascertain whether other supplement-assisted treatments for HT can prove beneficial for these patients.
Author contributions
BP: Writing – original draft, Writing – review & editing. WW: Writing – original draft, Writing – review & editing. QG: Data curation, Formal analysis, Methodology, Writing – review & editing. PW: Data curation, Formal analysis, Methodology, Writing – review & editing. ZS: Project administration, Supervision, Writing – review & editing, Funding acquisition. WT: Supervision, Writing – review & editing, Project administration.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (Grant Number: 82270836).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Li Y, Teng D, Ba J, Chen B, Du J, He L, et al. Efficacy and safety of long-term universal salt iodization on thyroid disorders: epidemiological evidence from 31 provinces of Mainland China. Thyroid. (2020) 30:568–79. doi: 10.1089/thy.2019.0067
2. Caturegli P, De Remigis A, Rose NR. Hashimoto thyroiditis: clinical and diagnostic criteria. Autoimmun Rev. (2014) 13:391–7. doi: 10.1016/j.autrev.2014.01.007
3. Skov J, Calissendorff J, Eriksson D, Magnusson P, Kampe O, Bensing S, et al. Limited genetic overlap between overt Hashimoto's thyroiditis and Graves' Disease in twins: A population-based study. J Clin Endocrinol Metab. (2021) 106:1101–10. doi: 10.1210/clinem/dgaa956
4. Shan Z, Li Y, Wang H, Teng Di, Teng X, Chong W, et al. Changing iodine status and the incidence of thyroid disease in mainland China: A prospective 20-year follow-up study. THyroid. (2023) 33:858–66. doi: 10.1089/thy.2022.0505
5. Weetman AP. An update on the pathogenesis of Hashimoto's thyroiditis. J Endocrinol Invest. (2021) 44:883–90. doi: 10.1007/s40618-020-01477-1
6. Ralli M, Angeletti D, Fiore M, D'Aguanno V, Lambiase A, Artico M, et al. Hashimoto's thyroiditis: An update on pathogenic mechanisms, diagnostic protocols, therapeutic strategies, and potential Malignant transformation. Autoimmun Rev. (2020) 19:102649. doi: 10.1016/j.autrev.2020.102649
7. Klubo-Gwiezdzinska J, Wartofsky L. Hashimoto thyroiditis: an evidence-based guide to etiology, diagnosis and treatment. Pol Arch Intern Med. (2022) 132:16222. doi: 10.20452/pamw.16222
8. Dhillon-Smith RK, Coomarasamy A. TPO antibody positivity and adverse pregnancy outcomes. Best Pract Res Clin Endocrinol Metab. (2020) 34:101433. doi: 10.1016/j.beem.2020.101433
9. Zhu L, Teng Y, Guo Y, Ru X, Wu X, Hao J, et al. Thyroid function test abnormalities-isolated TPOAb+, SCH and hypothyroxinemia and preschool children's neurodevelopment. Clin Endocrinol (Oxf). (2023) 99:492–501. doi: 10.1111/cen.14963
10. Winther KH, Papini E, Attanasio R, Negro R, Hegedus L. A 2018 European thyroid association survey on the use of selenium supplementation in Hashimoto's thyroiditis. Eur Thyroid J. (2020) 9:99–105. doi: 10.1159/000504781
11. Mikulska AA, Karazniewicz-Lada M, Filipowicz D, Ruchala M, Glowka FK. Metabolic characteristics of Hashimoto's thyroiditis patients and the role of microelements and diet in the disease management-an overview. Int J Mol Sci. (2022) 23:6580. doi: 10.3390/ijms23126580
12. Kubiak K, Szmidt MK, Kaluza J, Zylka A, Sicinska E. Do dietary supplements affect inflammation, oxidative stress, and antioxidant status in adults with hypothyroidism or Hashimoto's disease?-A systematic review of controlled trials. Antioxidants (Basel). (2023) 12:1798. doi: 10.3390/antiox12101798
13. Gorini F, Sabatino L, Pingitore A, Vassalle C. Selenium: an element of life essential for thyroid function. Molecules. (2021) 26:7084. doi: 10.3390/molecules26237084
14. Schomburg L. Selenium, selenoproteins and the thyroid gland: interactions in health and disease. Nat Rev Endocrinol. (2011) 8:160–71. doi: 10.1038/nrendo.2011.174
15. Sun Q, Oltra E, Dijck-Brouwer DAJ, Chillon TS, Seemann P, Asaad S, et al. Autoantibodies to selenoprotein P in chronic fatigue syndrome suggest selenium transport impairment and acquired resistance to thyroid hormone. Redox Biol. (2023) 65:102796. doi: 10.1016/j.redox.2023.102796
16. Liu. Y, Huang. H, Zeng. J, Sun C. Thyroid volume, goiter prevalence, and selenium levels in an iodine-sufficient area: a cross-sectional study. BMC Public Health BMC Public Health. (2013) 13:1153. doi: 10.1186/1471-2458-13-1153
17. Hu Y, Feng W, Chen H, Shi H, Jiang L, Zheng X, et al. Effect of selenium on thyroid autoimmunity and regulatory T cells in patients with Hashimoto's thyroiditis: A prospective randomized-controlled trial. Clin Transl Sci. (2021) 14:1390–402. doi: 10.1111/cts.12993
18. Huwiler VV, Maissen-Abgottspon S, Stanga Z, Muhlebach S, Trepp R, Bally L, et al. Selenium supplementation in patients with Hashimoto thyroiditis: A systematic review and meta-analysis of randomized clinical trials. Thyroid. (2024) 34:295–313. doi: 10.1089/thy.2023.0556
19. Toulis KA, Anastasilakis AD, Tzellos TG, Goulis DG, Kouvelas D. Selenium supplementation in the treatment of Hashimoto's thyroiditis: a systematic review and a meta-analysis. Thyroid. (2010) 20:1163–73. doi: 10.1089/thy.2009.0351
20. Minnetti M, Sada V, Feola T, Giannetta E, Pozza C, Gianfrilli D, et al. Selenium supplementation in pregnant women with autoimmune thyroiditis: A practical approach. Nutrients. (2022) 14:2234. doi: 10.3390/nu14112234
21. Agostini D, Donati Zeppa S. Vitamin D, diet and musculoskeletal health. Nutrients. (2023) 15:2902. doi: 10.3390/nu15132902
22. Delrue C, Speeckaert MM. Vitamin D and vitamin D-binding protein in health and disease. Int J Mol Sci. (2023) 24:4642. doi: 10.3390/ijms24054642
23. Gallo D, Baci D, Kustrimovic N, Lanzo N, Patera B, Tanda ML, et al. How does vitamin D affect immune cells crosstalk in autoimmune diseases? Int J Mol Sci. (2023) 24:4689. doi: 10.3390/ijms24054689
24. Chen S, Yang W, Guo Z, Lv X, Zou Y. Association between serum vitamin D levels and sensitivity to thyroid hormone indices: a cross-sectional observational study in NHANES 2007-2012. Front Endocrinol (Lausanne). (2023) 14:1243999. doi: 10.3389/fendo.2023.1243999
25. Wang J, Lv S, Chen G, Gao C, He J, Zhong H, et al. Meta-analysis of the association between vitamin D and autoimmune thyroid disease. Nutrients. (2015) 7:2485–98. doi: 10.3390/nu7042485
26. Planck T, Shahida B, Malm J, Manjer J. Vitamin D in Graves disease: levels, correlation with laboratory and clinical parameters, and genetics. Eur Thyroid J. (2018) 7:27–33. doi: 10.1159/000484521
27. Chao G, Zhu Y, Fang L. Correlation between Hashimoto's thyroiditis-related thyroid hormone levels and 25-hydroxyvitamin D. Front Endocrinol (Lausanne). (2020) 11:4. doi: 10.3389/fendo.2020.00004
28. Wu J, Huang H, Yu X. How does Hashimoto's thyroiditis affect bone metabolism? Rev Endocr Metab Disord. (2023) 24:191–205. doi: 10.1007/s11154-022-09778-x
29. Tang J, Shan S, Li F, Yun P. Effects of vitamin D supplementation on autoantibodies and thyroid function in patients with Hashimoto's thyroiditis: A systematic review and meta-analysis. Med (Baltimore). (2023) 102:e36759. doi: 10.1097/MD.0000000000036759
30. Vahabi Anaraki P, Aminorroaya A, Amini M, Momeni F, Feizi A, Iraj B, et al. Effect of Vitamin D deficiency treatment on thyroid function and autoimmunity markers in Hashimoto's thyroiditis: A double-blind randomized placebo-controlled clinical trial. J Res Med Sci. (2017) 22:103. doi: 10.4103/jrms.JRMS_1048_16
31. Krysiak R, Kowalcze K, Szkrobka W, Okopien B. Sexual function and depressive symptoms in young women with Euthyroid Hashimoto's thyroiditis receiving vitamin D, selenomethionine and myo-inositol: A pilot study. Nutrients. (2023) 15:2815. doi: 10.3390/nu15122815
32. Nordio M, Basciani S. Myo-inositol plus selenium supplementation restores euthyroid state in Hashimoto's patients with subclinical hypothyroidism. Eur Rev Med Pharmacol Sci. (2017) 21:51–9.
33. Krysiak R, Szkrobka W, Okopien B. The effect of vitamin D and selenomethionine on thyroid antibody titers, hypothalamic-pituitary-thyroid axis activity and thyroid function tests in men with Hashimoto's thyroiditis: A pilot study. Pharmacol Rep. (2019) 71:243–7. doi: 10.1016/j.pharep.2018.10.012
34. Krysiak R, Kowalcze K, Okopien B. Selenomethionine potentiates the impact of vitamin D on thyroid autoimmunity in euthyroid women with Hashimoto's thyroiditis and low vitamin D status. Pharmacol Rep. (2019) 71:367–73. doi: 10.1016/j.pharep.2018.12.006
35. Qu C, Li Y, Chen S. Effects of selenium supplementation on thyroid function, immune response and oxidative stress in HT patients. J Hainan Med Univ. (2019) 25:367–70. doi: 10.13210/j.cnki.jhmu.20190124.001
36. Lv X, Ye W, Zhang Y, Lai J, Fan L. Analysis of the effect of sleneium on Hashimoto's thyroiditis with normal thyroid function. World Latest Med Inf. (2018) 18, 95–100. doi: 10.19613/j.cnki.1671-3141.2018.61.065
37. Han N. Observation of the clinical efficacy of selenium in patients with autoimmune thyroiditis accompanied by subclinical hypothyroidism. Cardiovasc Dis J integrated traditional Chin Western Med. (2017) 5:183–4. doi: 10.16282/j.cnki.cn11-9336/r.2017.33.147
38. Gao H, Liu J, Feng R. The effect of selenium preparations on autoantibodies in chronic lymphocytic thyroiditis. Health Vocational Educ. (2017) 35:120–1.
39. Zhou J, Long C, Tang N. Effects of vitamin D treatment on thyroid autoantibodies in patients with hashimoto thyroiditis. J Mod Med Health. (2018) 34:1656–8. doi: 10.3969/j.issn.1009-C5519.2018.11.017
40. Liping Fu JJ, Wang B, Xu X. Impact of calcitriol on the thyroid peroxidase antibody, thyroglobulin antibody levels and Th17/Treg related cytokines in serum of patients with Hashimoto's thyroiditis. Zhejiang Med. (2019) 41:2398–401. doi: 10.12056/j.issn.1006-2785.2019.41.22.2019-2290
41. Pludowski P, Kos-Kudla B, Walczak M, Fal A, Zozulinska-Ziolkiewicz D, Sieroszewski P, et al. Guidelines for preventing and treating vitamin D deficiency: A 2023 update in Poland. Nutrients. (2023) 15:695. doi: 10.3390/nu15030695
42. Metwalley KA, Farghaly HS, Sherief T, Hussein A. Vitamin D status in children and adolescents with autoimmune thyroiditis. J Endocrinol Invest. (2016) 39:793–7. doi: 10.1007/s40618-016-0432-x
43. Appunni S, Rubens M, Ramamoorthy V, Saxena A, Tonse R, Veledar E, et al. Association between vitamin D deficiency and hypothyroidism: results from the National Health and Nutrition Examination Survey (NHANES) 2007-2012. BMC Endocr Disord. (2021) 21:224. doi: 10.1186/s12902-021-00897-1
44. Chen Y, Han B, Zhu C, Li Q, Chen C, Zhai H, et al. Bidirectional Mendelian randomization analysis for vitamin D and thyroid peroxidase antibody. Int J Endocrinol. (2022) 2022:2260388. doi: 10.1155/2022/2260388
45. Aktas HS. Vitamin B12 and vitamin D levels in patients with autoimmune hypothyroidism and their correlation with anti-thyroid peroxidase antibodies. Med Princ Pract. (2020) 29:364–70. doi: 10.1159/000505094
46. Simsek Y, Cakir I, Yetmis M, Dizdar OS, Baspinar O, Gokay F. Effects of Vitamin D treatment on thyroid autoimmunity. J Res Med Sci. (2016) 21:85. doi: 10.4103/1735-1995.192501
47. Krysiak R, Szkrobka W, Okopien B. The effect of vitamin D on thyroid autoimmunity in levothyroxine-treated women with Hashimoto's thyroiditis and normal vitamin D status. Exp Clin Endocrinol Diabetes. (2017) 125:229–33. doi: 10.1055/s-0042-123038
48. Hahn J, Cook NR, Alexander EK, Friedman S, Walter J, Bubes V, et al. Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. BMJ. (2022) 376:e066452. doi: 10.1136/bmj-2021-066452
49. Winther KH, Rayman MP, Bonnema SJ, Hegedus L. Selenium in thyroid disorders - essential knowledge for clinicians. Nat Rev Endocrinol. (2020) 16:165–76. doi: 10.1038/s41574-019-0311-6
50. Rostami R, Nourooz-Zadeh S, Mohammadi A, Khalkhali HR, Ferns G, Nourooz-Zadeh J. Serum selenium status and its interrelationship with serum biomarkers of thyroid function and antioxidant defense in Hashimoto's thyroiditis. Antioxidants (Basel). (2020) 9:1070. doi: 10.3390/antiox9111070
51. Ates I, Arikan MF, Altay M, Yilmaz FM, Yilmaz N, Berker D, et al. The effect of oxidative stress on the progression of Hashimoto's thyroiditis. Arch Physiol Biochem. (2018) 124:351–6. doi: 10.1080/13813455.2017.1408660
52. Xu B, Huang S, Peng W, Cai G, Zhou H, Guo Y, et al. Comprehensive analysis of hub biomarkers associated with immune and oxidative stress in Hashimoto's thyroiditis. Arch Biochem biophysics. (2023) 745:10973. doi: 10.1016/j.abb.2023.109713
53. P.A. Rotruck JT, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. (1973) 179:588–90. doi: 10.1126/science.179.4073.588
54. Wang W, Jiang QL, Xu Q, Zeng Y, Jiang R, Jiang J. Selenium regulates T cell differentiation in experimental autoimmune thyroiditis in mice. Int Immunopharmacol. (2023) 124:110993. doi: 10.1016/j.intimp.2023.110993
55. Lv Q, Liang X, Nong K, Gong Z, Qin T, Qin X, et al. Advances in research on the toxicological effects of selenium. Bull Environ Contamination Toxicol. (2021) 106:715–26. doi: 10.1007/s00128-020-03094-3
56. van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. (2008) 9:112–24. doi: 10.1038/nrm2330
57. Kessels MM, Schwintzer L, Schlobinski D, Qualmann B. Controlling actin cytoskeletal organization and dynamics during neuronal morphogenesis. Eur J Cell Biol. (2011) 90:926–33. doi: 10.1016/j.ejcb.2010.08.011
58. Vasilev F, Ezhova Y, Chun JT. Signaling enzymes and ion channels being modulated by the actin cytoskeleton at the plasma membrane. Int J Mol Sci. (2021) 22:10366. doi: 10.3390/ijms221910366
59. Huang F, Zhang F, Huang L, Zhu X, Huang C, Li N, et al. Inositol 1,4,5-trisphosphate receptors regulate vascular smooth muscle cell proliferation and neointima formation in mice. J Am Heart Assoc. (2024) 13:e034203. doi: 10.1161/JAHA.124.034203
60. Fan C, Liang W, Wei M, Gou X, Han S, Bai J. Effects of D-chiro-inositol on glucose metabolism in db/db mice and the associated underlying mechanisms. Front Pharmacol. (2020) 11. doi: 10.3389/fphar.2020.00354
61. Flohe L, Toppo S, Orian L. The glutathione peroxidase family: Discoveries and mechanism. Free Radic Biol Med. (2022) 187:113–22. doi: 10.1016/j.freeradbiomed.2022.05.003
62. Payer J, Jackuliak P, Kuzma M, Dzupon M, Vanuga P. Supplementation with myo-inositol and Selenium improves the clinical conditions and biochemical features of women with or at risk for subclinical hypothyroidism. Front Endocrinol (Lausanne). (2022) 13:1067029. doi: 10.3389/fendo.2022.1067029
63. Nordio M, Basciani S. Treatment with myo-inositol and selenium ensures euthyroidism in patients with autoimmune thyroiditis. Int J Endocrinol. (2017) 2017:2549491. doi: 10.1155/2017/2549491
64. Krysiak R, Kowalcze K, Okopien B. The impact of vitamin D on thyroid autoimmunity and hypothalamic-pituitary-thyroid axis activity in myo-inositol-treated and myo-inositol-naive women with autoimmune thyroiditis: A pilot study. J Clin Pharm Ther. (2022) 47:1759–67. doi: 10.1111/jcpt.v47.11
Keywords: vitamin D, selenium, myo-inositol, Hashimoto’s thyroiditis, meta-analysis
Citation: Peng B, Wang W, Gu Q, Wang P, Teng W and Shan Z (2024) Effects of different supplements on Hashimoto’s thyroiditis: a systematic review and network meta-analysis. Front. Endocrinol. 15:1445878. doi: 10.3389/fendo.2024.1445878
Received: 08 June 2024; Accepted: 19 November 2024;
Published: 04 December 2024.
Edited by:
Roberto Fernandes da Costa, Autonomous University of Chile, ChileReviewed by:
Ying Lu, Shenzhen Second People’s Hospital, ChinaXin Sun, The First Affiliated Hospital of Soochow University, China
Copyright © 2024 Peng, Wang, Gu, Wang, Teng and Shan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongyan Shan, Y211c2hhbnpob25neWFuQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Bingcong Peng
Bingcong Peng Weiwei Wang
Weiwei Wang Qingling Gu
Qingling Gu Weiping Teng
Weiping Teng Zhongyan Shan
Zhongyan Shan