- 1Department of Nephrology, Rheumatology, Endocrinology and Metabolism, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Japan
- 2Department of Diabetology and Metabolism, NHO Okayama Medical Center, Okayama, Japan
- 3Department of Urology, Fukuyama City Hospital, Hiroshima, Japan
- 4Department of Internal Medicine, Tsuyama Chuo Hospital, Okayama, Japan
- 5Department of Gastroenterological Surgery, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Japan
- 6Clinical Cancer Center, Okayama University Hospital, Okayama, Japan
Background: Although immune checkpoint inhibitors (ICIs) are effective cancer drugs, ICI-induced diabetes is a rare but a life-threatening adverse event for patients. The deleterious action of ICI on pancreatic beta-cell function is a concern. However, the influence of ICI on insulin synthesis and secretion in patients with cancer without diabetes remains unknown.
Methods: This study included 87 patients diagnosed with advanced cancer. Glucose metabolism markers (HbA1c, HOMA-IR) and indicators of insulin secretory capacity (HOMA-beta, C-peptide) were prospectively evaluated in patients with ICI-treated cancers to determine their association with cancer prognosis.
Results: Patients with overall survival (OS) ≥ 7 months had substantially higher HOMA-beta levels at baseline (p=0.008) and 1 month after ICI administration (p=0.006) compared to those with OS <7 months. The median OS was significantly longer in patients with HOMA-beta ≥ 64.24 (13 months, 95%CI: 5.849–20.151, 37 events) than in those with HOMA-beta < 64.24 (5 months, 95%CI: 3.280–6.720, 50 events) (p=0.013). Further, the median progression-free survival (PFS) was significantly longer in patients with HOMA-beta ≥ 66.43 (4 months, 95%CI: 3.073–4.927, 33 events) than in those with HOMA-beta < 66.43 (2 months, 95%CI: 1.410–2.590, 54 events) (p=0.025). Additionally, multivariable logistic regression analysis revealed that a HOMA-beta value ≥ 64.24 independently predicted longer OS in ICI-treated patients.
Conclusions: Pre-ICI HOMA-beta level is linked to longer OS in ICI-treated patients. This connection is significant and shows that insulin secretory capacity may predict ICI efficacy.
Introduction
The use of immune checkpoint inhibitors (ICIs) has significantly expanded as a highly efficacious anticancer approach for several types of malignancies (1). Timely identification and effective handling of immune-related adverse events (irAEs) are crucial for mitigating the substantial morbidity arising from the growing utilization of immunotherapeutic interventions (2). The incidence of immune checkpoint inhibitor-induced diabetes mellitus (CPI-DM) among irAEs is rare, less than 1%; however, it is a life-threatening adverse effect that poses a significant risk to a patient’s life, with no means of early detection (3, 4). Previous studies have reported several features of CPI-DM: rapid onset of severe hyperglycemia, the presence of diabetic ketoacidosis or an extreme reduction in C-peptide levels, and continuous insulin dependence for glycemic control following the development of diabetes (5). Moreover, Hisanaga et al. observed that pre-existing diabetes is associated with higher mortality in advanced lung cancer during ICI treatment (6). Matsumura et al. documented that nivolumab administration resulted in the exacerbation of diabetes and partial insulin secretion deficiency in patients diagnosed with lung cancer (7). Although several studies have reported the development of insulin-deficient diabetes mellitus after ICI administration, it is not necessarily a type of insulin-depleted diabetes (3, 4). The potential impact of ICI administration on pancreatic beta-cell activity has been a subject of investigation. However, limited information is available on the effects of ICI treatment on insulin secretion in individuals without diabetes. Gauci et al. retrospectively investigated the impact of ICI administration on blood glucose levels in a cohort of 116 patients, excluding those with CPI-DM. Their findings revealed no statistically significant alterations in blood glucose levels following ICI administration. Furthermore, there is a lack of research investigating the impact of ICI administration on C-peptide, HOMA-beta, and HOMA-IR (8). This prospective observational study was conducted to analyze the impact of ICI administration on indicators of glucose metabolism in cancer patients without diabetes. Additionally, this study aimed to explore the potential association between glucose metabolism markers and the efficacy of ICI.
Materials and methods
Study participants
The present study included a cohort of 87 individuals diagnosed with advanced cancer who were recruited between June 2017 and August 2019. Advanced cancer was defined as a case in which the cancer was histologically stage IV and had progressed or recurred during or after one or more systemic treatments for advanced or metastatic disease. The study included patients who were diagnosed with stage IV cancer, as confirmed via histological examination, and were recruited from Okayama University Hospital. The exclusion criteria were as follows: (1) a documented medical history of diabetes; (2) fasting blood glucose levels ≥ 126 mg/dL (7.0 mmol/L) or glycated hemoglobin (HbA1c) values ≥ 6.5%; and (3) treatment with anti-diabetes medication. All participants were administered a minimum of one dose of intravenous anti-PD1 drug as initial immunotherapy. Participants in this study did not receive concomitant steroid therapy or chemotherapy during the observation period. Prognosis was monitored until September 2022. Written informed consent was obtained from all patients. The study protocol was approved by the Ethics Committee of Okayama University (1704-009) and was conducted in accordance with the Declaration of Helsinki.
Data collection
Blood samples were collected after a 12-h fast. HbA1c, fasting plasma glucose (FPG), C-reactive protein, and creatinine levels were measured within 1 h of blood collection using standard methods and an automated clinical chemistry analyzer (JCA-BM8040G, JEOL Ltd, Tokyo, Japan). Fasting serum C-peptide (Lumipulse System, Fujirebio, Tokyo, Japan) and immunoreactive insulin (IRI) (Chemilumi Insulin, Siemens Healthineers, Tokyo, Japan) levels were measured.
The body mass index (BMI) was calculated using the following formula: body weight (kg)/height2 (m2). The homeostasis model of assessment-insulin resistance (HOMA-IR) was calculated using the following formula: fasting insulin (μU/mL) × fasting glucose (mg/dL)/405. The homeostasis model of assessment-beta cell (HOMA-beta) was calculated as follows: 360 × fasting insulin (μU/mL)/(fasting glucose (mg/dL) − 63). eGFR was calculated using the following formula modified for Japanese subjects: eGFR (mL/min/1.73 m2) = 194 × s-Cr (mg/dL)-1.094 × age (years) -0.287 (× 0.739, for females).
Medical history and current status of primary tumor progression, Eastern Cooperative Oncology Group performance status (ECOG-PS; 0–1 vs. ≥2), and number of metastatic sites were collected from the medical charts of each patient.
Definition
The response to ICI therapy was assessed by evaluating the overall survival (OS) and progression-free survival (PFS) using the Response Evaluation Criteria in Solid Tumors (version 1.1) (9).
OS was defined as the time from the initiation of the first ICI administration to death. PFS was defined as the time from initiation of the first ICI administration to disease progression or death. PFS was determined for patients who did not exhibit disease progression as of September 2022 and was defined as the date of the most recent imaging examination. The cohort of patients who remained alive as of September 2022 was determined based on the time from therapy initiation to the date of their most recent visit.
Statistical analysis
The paired Wilcoxon signed-rank and Friedman tests were performed to evaluate the changes in glucose tolerance before and after ICI administration. The Mann–Whitney U test was used to compare the two groups based on the median overall survival. Receiver operating characteristic (ROC) curves were calculated for glucose metabolism markers (BMI and HOMA-beta) to define the cut-off value by the method with the smallest distance from the point in the upper left corner in two groups with median OS. PFS and OS from the date of the first treatment and survival curves were generated using the Kaplan–Meier method and compared using the log-rank test. Univariate and multivariate Cox proportional hazards models were used to calculate the independent significance of prognostic variables. All statistical analyses were performed using SPSS version 26 (IBM Corp., Armonk, NY, USA). Statistical significance was set at p<0.05.
Results
Baseline characteristics of the study participants
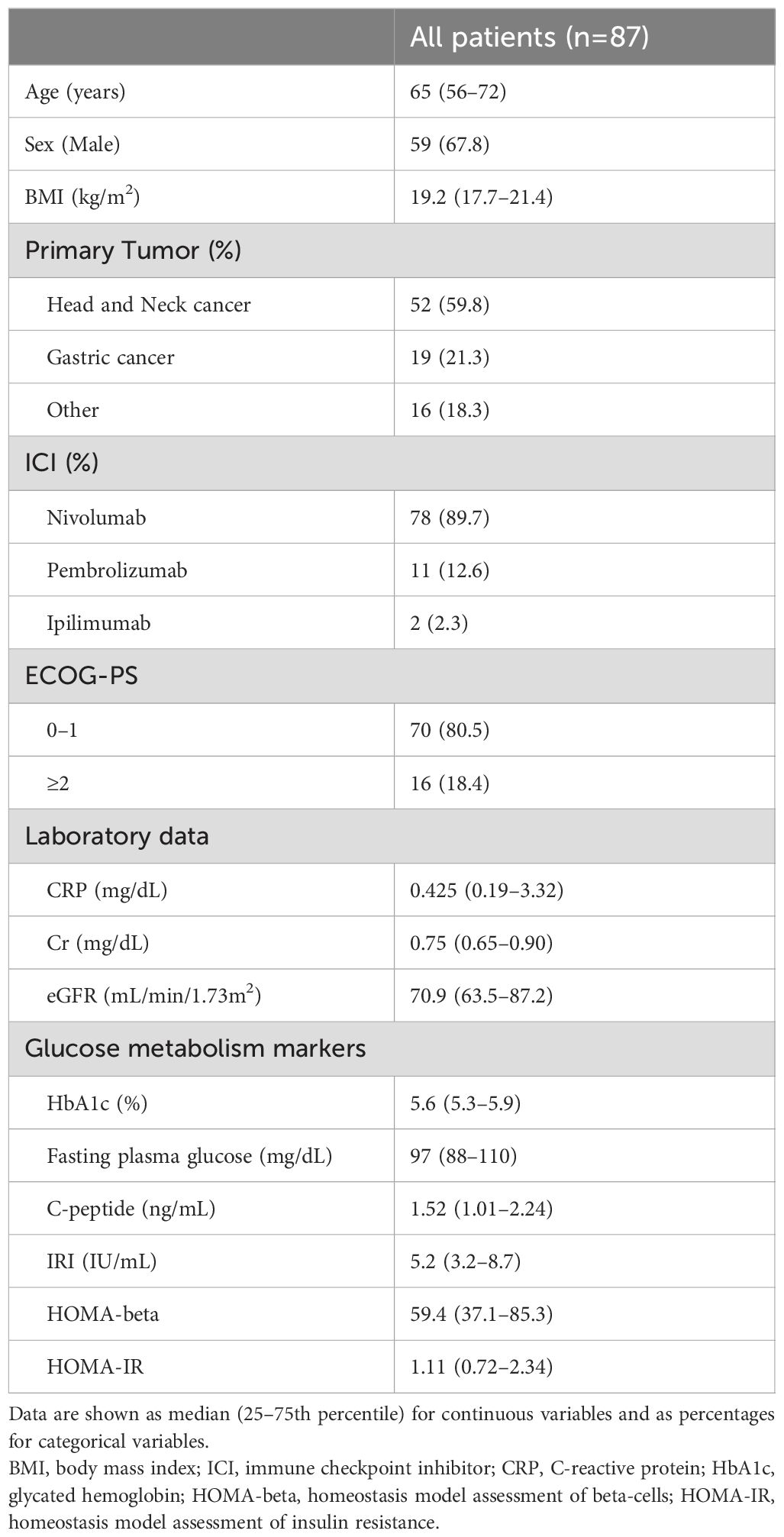
This study included 87 participants (59 male and 28 female). The baseline characteristics of the study participants are presented in Table 1. The median age of the participants was 65 years, with a median BMI of 19.2 kg/m2. Of the participants, 78 individuals used nivolumab, 10 individuals used pembrolizmab, and 1 individual used ipilimumab. Regarding the combination therapy, a single patient received a treatment regimen consisting of nivolumab and pembrolizmab, while another patient received nivolumab in conjunction of ipilimumab. Additionally, the Eastern Cooperative Oncology Group Performance Status (ECOG-PS) was classified as 0-1 in 80.5% of the cases. We observed a total of 52 cases of head and neck cancer, 19 cases of gastric cancer, and 16 cases of various other types of cancer. Specifically, these included six cases of renal cell carcinoma, six cases of urothelial cancer, two cases of colon cancer, one case of cervical esophageal cancer, and one case of malignant melanoma. The laboratory results showed no indications of renal dysfunction or overt diabetes among the individuals.
Glucose metabolism markers after ICI administration
We evaluated the time course of the glucose metabolism markers before and after ICI administration. HbA1c levels before and 1 month after ICI administration were compared in 76 individuals. The median HbA1c level before ICI administration was 5.6% (interquartile range [IQR], 0.58), whereas the median HbA1c level 1 month after ICI administration was 5.4% (IQR, 0.65). This difference was statistically significant (p=0.018). Similarly, C-peptide levels at baseline (1.51 ng/mL [IQR 1.06]) significantly increased 1 month after ICI administration (1.63 ng/mL [IQR 1.17]) (p=0.022, n=55). However, no statistically significant changes were observed in FPG, HOMA-beta, or HOMA-IR (Figure 1). The number of evaluable patients gradually decreased over the course of 6 months as ICI were deemed ineffective, and ICI therapy was discontinued. There were no statistically significant differences in glucose metabolism markers between the 14 patients who were evaluated at 6 months and the 5 patients who were evaluated at 12 months after ICI administration. Since the analysis was performed in participants without diabetes, the ICI-induced changes in HbA1c and C-peptide were not clinically meaningful, but these data suggested that ICI administration affected glucose metabolism markers even in non-diabetic conditions.
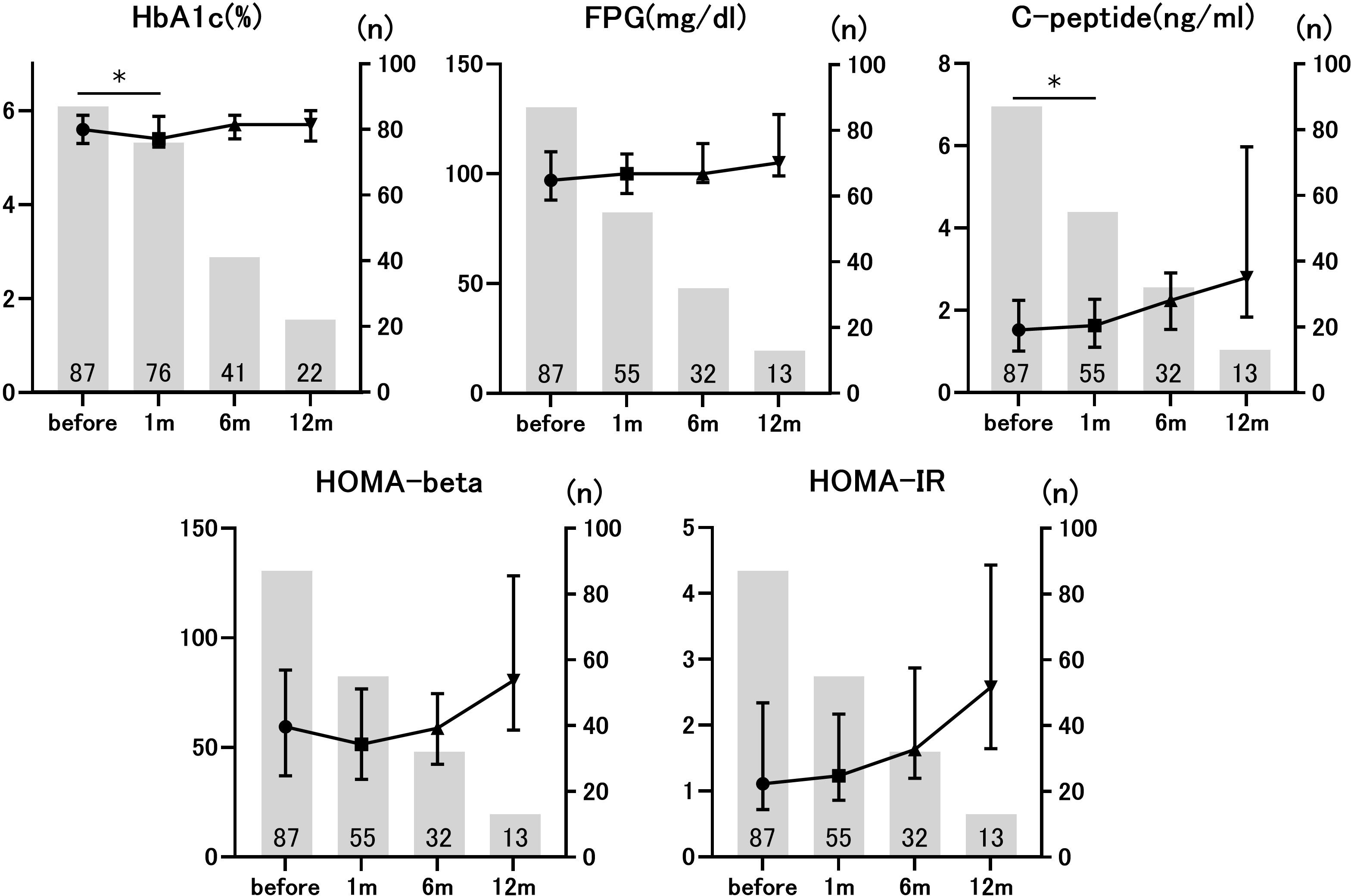
Figure 1. Longitudinal changes in glucose metabolism markers before and after immune checkpoint inhibitors (ICIs) administration. The number of patients applicable to each time point is given as a numerical value. *p<0.05 vs. pre-ICI treatment. Data are presented as mean ± SD.
Glucose metabolism markers and overall survival
Next, we compared glucose metabolism markers based on the median OS. The median OS was 7 months (95%CI: 4.892–9.108). We observed a substantial decrease in HbA1c levels 1 month after ICI treatment in patients with an OS of at least 7 months compared to their pre-treatment levels. Serum C-peptide levels significantly decreased after 1 month of therapy in patients with an OS of less than 7 months (Table 2). Notably, patients with OS greater than 7 months exhibited increased pre-treatment HOMA-beta and HOMA-beta after 1 month of treatment, in comparison to patients with OS less than 7 months (Figure 2). This difference was observed both before (p=0.008) and 1 month after (p=0.006) ICI administration. However, there were no significant differences in HbA1c, FPG, C-peptide, IRI, or HOMA-IR. These data suggest an association between the pre-ICI HOMA-beta and OS.
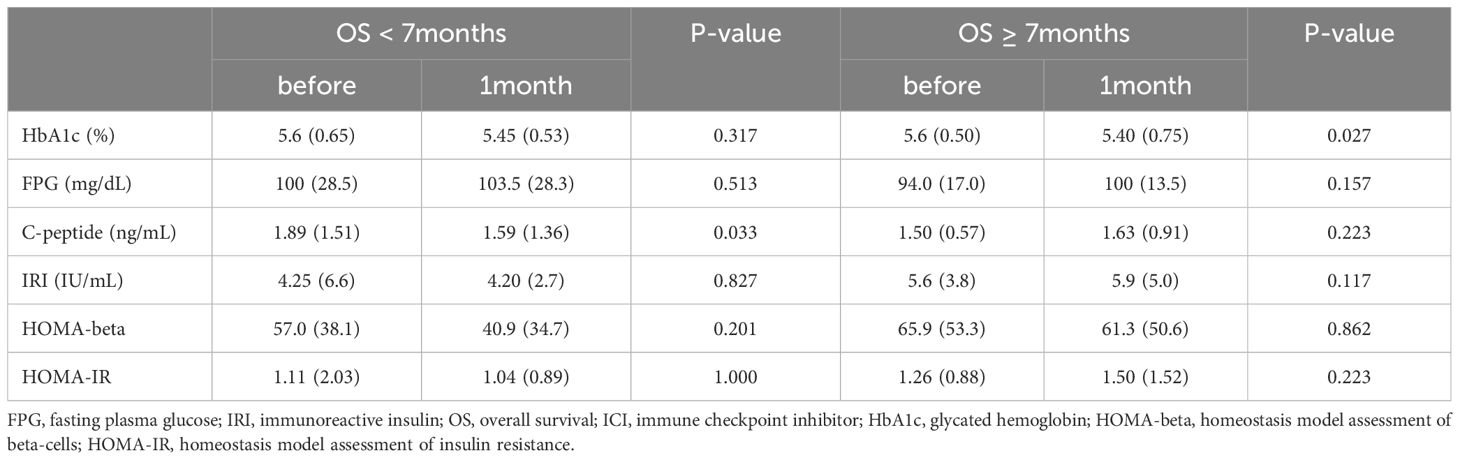
Table 2. Comparison of glucose metabolism markers before and 1 month after ICI administration in the two groups based on median overall survival (OS).
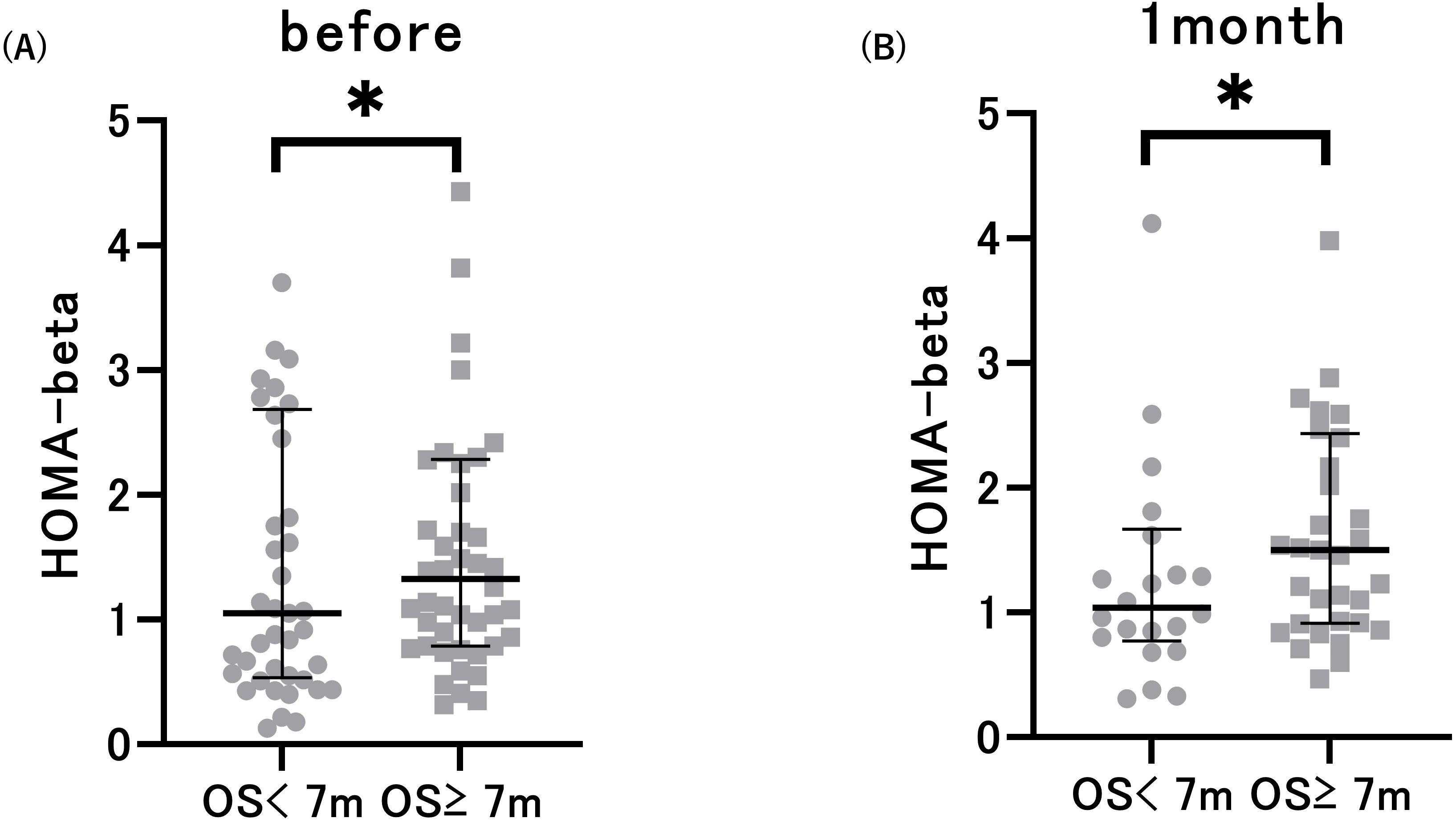
Figure 2. Pre-treatment HOMA-beta (A) and HOMA-beta after 1 month of treatment (B) in patients with overall survival (OS) < 7 months and OS ≥ 7 months. *p<0.05 vs. patients with OS < 7 months. Data are presented as mean ± SD.
Association of glucose metabolism markers with cancer prognosis
At the end of the observation period, disease progression was observed in 75 individuals, accounting for 86.2% of the total participants. Additionally, the mortality rate was 94.3%, with 82 patients succumbing to the condition. The median OS was 7 months (95% CI: 4.892–9.108 months), and the median PFS was 3 months (95% CI: 2.247–3.753 months). We assessed the ability of the area under the ROC curve based on BMI and HOMA-beta to predict the median OS (OS ≥ 7 months) in patients with ICI treatment. The optimal predictive BMI cut-off value was found to be 18.58 kg/m2 with a sensitivity of 0.717, specificity of 0.561, and AUC of 0.665 (p=0.008, Supplementary Figure S1A). HOMA-beta cut-off value was 64.24, with a sensitivity of 0.565, specificity of 0.732, and AUC of 0.665 (p=0.008, Figure 3A). Moreover, we evaluated the ability of the area under the ROC curve based on BMI and HOMA-beta to predict the median PFS (PFS ≥ 3 months) in patients undergoing ICI treatment. The optimal predictive BMI cut-off value was 18.44 kg/m2, with a sensitivity of 0.727, specificity of 0.512, and AUC of 0.631 (p=0.035, Supplementary Figure S1B). The HOMA-beta cut-off value was 66.43, with a sensitivity of 0.500, specificity of 0.744, and AUC of 0.582 (p=0.185, Figure 3B). The median OS was significantly longer in patients with BMI ≥ 18.58 kg/m2 (12 months, 95%CI: 7.633–16.367, 51 events) than in those with BMI < 18.58 kg/m2 (5 months, 95% CI: 3.343–6.657, 36 events) (p=0.001; Supplementary Figure S2A). Additionally, the median OS was significantly longer in patients with HOMA-beta ≥ 64.24 (13 months, 95%CI: 5.849–20.151, 37 events) than in those with HOMA-beta < 64.24 (5 months, 95%CI: 3.280–6.720, 50 events) (p=0.013; Figure 4A). Further, the median PFS was significantly longer in patients with HOMA-beta ≥ 66.43 (4 months, 95%CI: 3.073–4.927, 33 events) than in those with HOMA-beta < 66.43 (2 months, 95%CI: 1.410–2.590, 54 events) (p=0.025; Figure 4B). The median PFS tended to be longer in patients with BMI ≥ 18.44 kg/m2 (4 months, 95%CI: 2.866-5.134, 53 events) than in those with BMI < 18.44 kg/m2 (2 months, 95% CI: 1.454-2.546, 34 events), but the difference was not statistically significant (p=0.085; Supplementary Figure S2B).
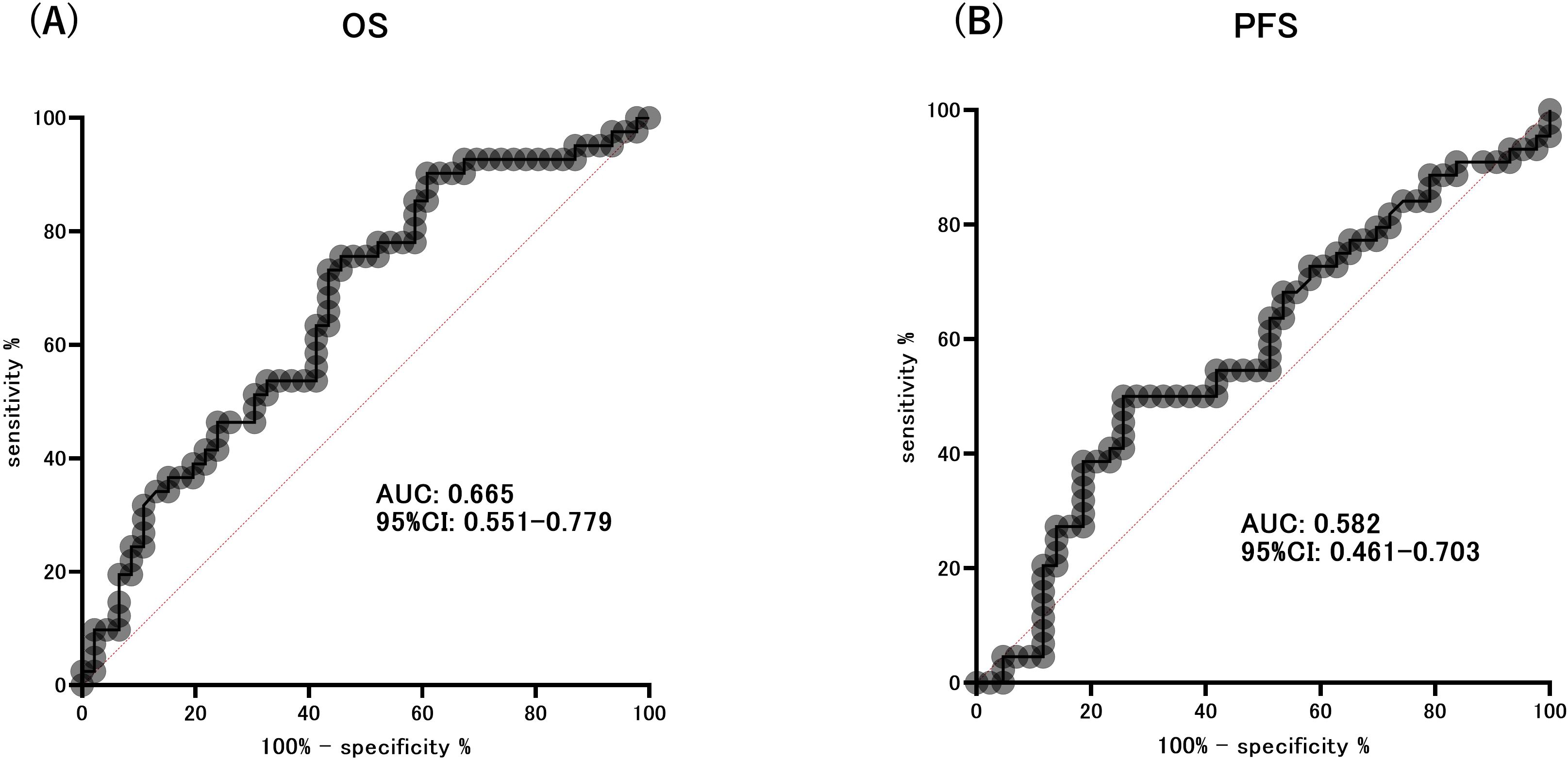
Figure 3. ROC curve analysis of the ability of HOMA-beta to predict the extended overall survival (OS) (A) and progression-free survival (PFS) (B).
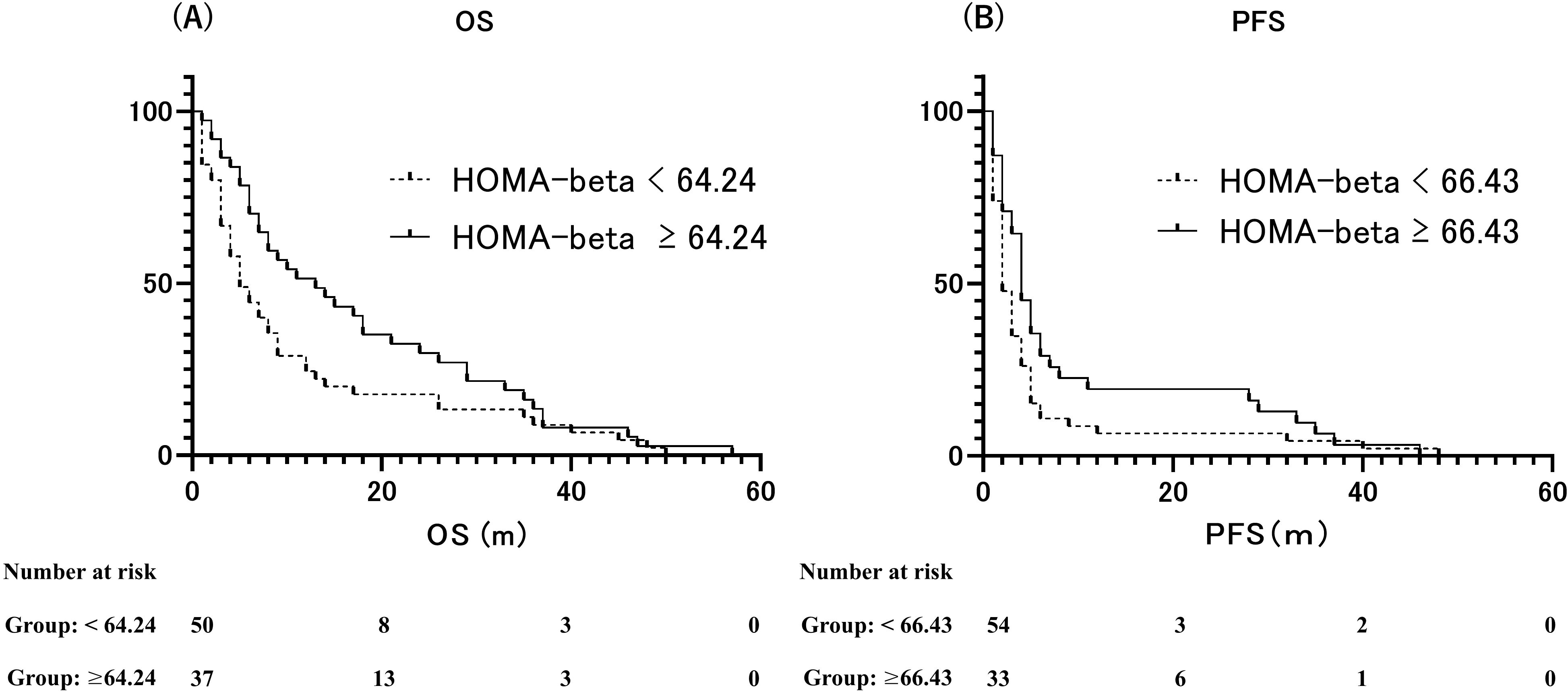
Figure 4. (A) Kaplan–Meier curves of overall survival (OS) for patients with HOMA-beta ≥ 64.24 and HOMA-beta < 64.24. (B) Kaplan–Meier curves of progression-free survival (PFS) for patients with HOMA-beta ≥ 66.43 and HOMA-beta < 66.43.
Finally, we examined the association between glucose metabolism markers and OS or PFS using a multivariate logistic regression analysis to evaluate each variable. A HOMA-beta ≥ 64.24 was an independent factor associated with prolonged OS in patients receiving ICI treatment after adjusting for age, sex, BMI, and eGFR (Table 3). Furthermore, HOMA-beta ≥ 66.43 was identified as an independent factor contributing to prolonged PFS in patients receiving ICI treatment (Table 4).
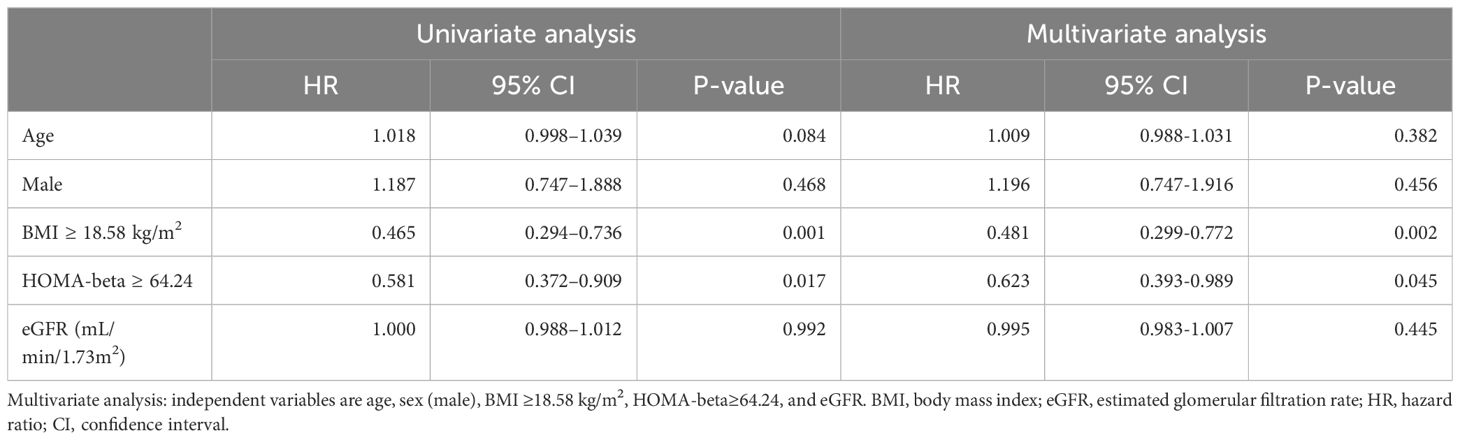
Table 3. Cox proportional-hazards regression: univariate and multivariate analyses of overall survival (OS).
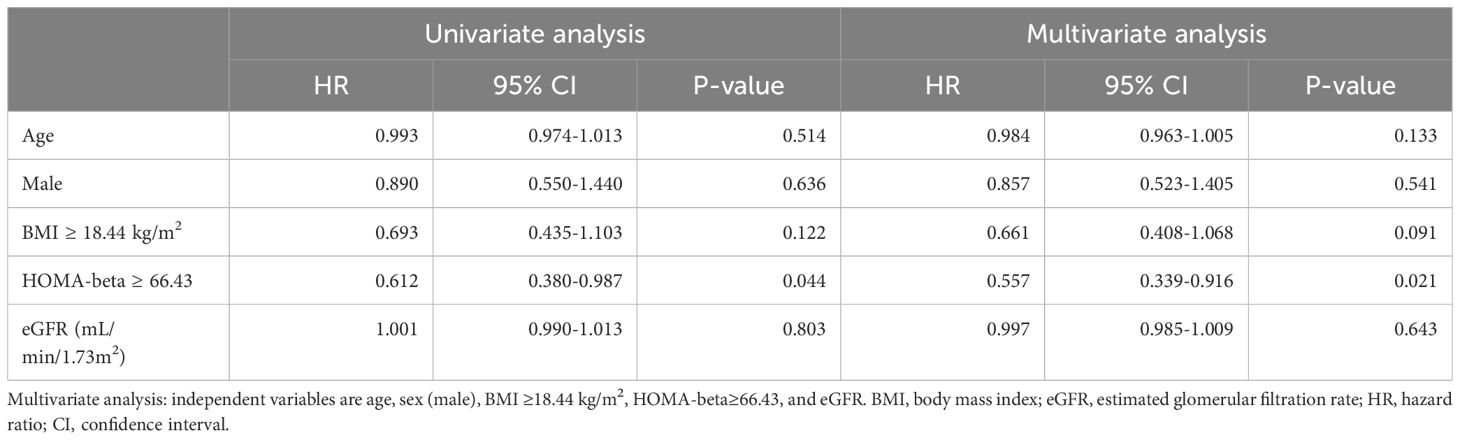
Table 4. Cox proportional-hazards regression: univariate and multivariate analyses of progression-free survival (PFS).
Discussion
In this study, we demonstrated that ICI administration induces fluctuations in glucose metabolism markers even in non-diabetic conditions. Notably, HOMA-beta was significantly higher in patients with OS ≥7 months than in those with OS <7 months, both before and 1 month after ICI administration. This work represents a novel contribution as it is the first to establish an association between HOMA-beta and prolonged OS in the context of ICI therapy, irrespective of the occurrence of diabetes.
The programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) pathway plays a significant role in maintaining immunological homeostasis in the pancreas (10, 11). Typically, the expression of PD-L1 in pancreatic beta-cells is minimal. However, in the presence of inflammation, the upregulation of PD-L1 is induced by cytokines such as interferon. The findings of previous in vitro investigations conducted on non-diabetic human islets demonstrated that interferon-gamma (IFN-γ) plays a role in promoting PD-L1 in pancreatic beta-cells (12, 13). The precise mechanism by which ICIs cause harm to pancreatic beta-cell remains unclear. However, it has been hypothesized to impede inhibitory signals and stimulate T lymphocytes, leading to an increase in the number of CD8+ T cells. This increase in CD8+ T cells may result in the impairment of pancreatic cells and a subsequent decline in pancreatic endocrine function (14–16). However, considering the substantial contribution of local and systemic inflammation to the advancement of malignant tumors and its role in cancer progression and the survival of patients with cancer, it is plausible to suggest that the inflammation-induced PD-1/PD-L1 pathway might play a role in the development of pancreatic endocrine dysfunction (17, 18).
Numerous studies have documented a correlation between the effectiveness of ICI administration and the occurrence of irAEs (19). While the number of patients with CPI-DM is insufficient to make definitive conclusions, it is noteworthy that a significant number of these individuals have exhibited either partial or total antitumor response (20, 21). However, additional research is required to determine whether the occurrence of CPI-DM is linked to improved clinical outcomes. C-peptide serves as a marker of endogenous insulin in individuals who exhibit normal glucose tolerance, while HOMA-beta is utilized as an indication of beta-cell activity (22). In this study, we showed an association between an effectiveness of ICI and insulin secretion capacity. Notably, patients with HOMA-beta value ≥ 65 exhibited a statistically significant increase in OS after ICI administration. In general, hyperinsulinemia is known to influence the development of certain types of cancer, but no reports have been found to support the idea that cancer progression other than pancreatic cancer affects beta-cell function (23). On the other hand, cancer cachexia, which is observed in advanced cancer, causes weight loss with changes in body composition due to reduced caloric intake, chronic inflammation, and abnormal endocrine function. In glucose metabolism, decreased insulin secretion associated with decreased caloric intake and increased hepatic glucose production and hepatic glycogenesis lead to increased insulin resistance (24). However, the effect of advanced cancer on beta-cell function remains unclear. It has been postulated that the impact on the capacity for insulin secretion might indicate the effectiveness of ICI in the treatment of patients with cancer.
The use of ICI has emerged as a promising and efficacious approach for combating cancer across a diverse range of malignancies (1). Several factors influence the efficacy of ICI therapy (25). Hence, it is crucial to ascertain the determinants affecting the effectiveness of ICI therapy. PD-L1 is the initial predictive biomarker approved by the Food and Drug Administration (FDA) for ICI administration in individuals diagnosed with non-small cell lung cancer. Microsatellite instability, or deficient mismatch repair, is the second biomarker that has received FDA approval for the therapeutic management of solid tumors that are either unresectable or metastasized. Additionally, the utilization of tumor mutation burden data has received approval from the FDA for the therapeutic management of solid tumors that are either unresectable or metastasized (26). Furthermore, various patient characteristics, including sex, age, smoking history, and BMI, have been identified in the literature as potential predictors of treatment response to ICI (27–30). Several predictive variables have been documented in the context of ICI therapy. However, a clear predictive factor has not yet been established. Cortellini et al. reported that BMI serves as a predictive indicator for ICI treatment (31). In this study, we found that both HOMA-beta and BMI were significant prognostic indicators. The results regarding the association between BMI and OS were consistent with previous literature. For PFS, however, only HOMA-beta was a predictive factor associated with prolonged PFS. A significant association between BMI and PFS may be found by increasing the number of cases.
Despite these novel findings, this study has certain limitations. First, it had a single-center, prospective, observational design, and limited sample size. Conducting multicenter studies with larger sample sizes is necessary to validate our findings. Second, it is important to ascertain the generalizability of our findings to other forms of cancer, given the inherent limitations associated with focusing only on the specific malignancy examined in this study. Third, HOMA-beta is a rough estimate of endogenous insulin secretory capacity, but it is extremely stable in fasting and non-diabetic studies and is a frequently used test to evaluate beta-cell function (32). Additionally, owing to the inherent characteristics of the condition, the lack of a comprehensive evaluation of beta-cell activity prior to ICI administration makes it impossible to accurately determine the effects of ICI on beta-cells.
In summary, our study findings demonstrate an association between HOMA-beta and extended OS in the context of ICI therapy. Additionally, our work posits that the beta-cell activity prior to ICI administration may potentially serve as an indicator of the effectiveness of ICI therapy in individuals diagnosed with cancer. Future investigations should aim to provide a more comprehensive understanding of the biological mechanisms underlying these findings.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Okayama University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MW: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal Analysis, Data curation, Conceptualization. JE: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal Analysis, Data curation, Conceptualization. AT: Writing – review & editing, Resources, Data curation, Conceptualization. HK: Writing – review & editing, Resources, Data curation, Conceptualization. YN: Writing – review & editing, Resources, Data curation, Conceptualization. SK: Writing – review & editing, Resources, Data curation, Conceptualization. JW: Writing – review & editing, Validation, Supervision, Resources, Investigation, Funding acquisition, Formal Analysis, Data curation, Conceptualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by a Grant-in-Aid for Scientific Research (C) (20K08908) (to JE) and the Japan Agency for Medical Research and Development (AMED, grant no: 17ek0210095h0001, 20ek0109445h0001).
Conflict of interest
JW received speaker honoraria from Astra Zeneca, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Kyowa Kirin, Novo Nordisk, and Mitsubishi Tanabe, and received grant support from Bayer, Chugai, Kyowa Kirin, Otsuka, Shionogi, Sumitomo, and Mitsubishi Tanabe.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1439705/full#supplementary-material
Supplementary Figure 1 | ROC curve analysis of the ability of BMI to predict the extended overall survival (OS) (A) and progression-free survival (PFS) (B).
Supplementary Figure 2 | (A) Kaplan–Meier curves of overall survival (OS) for patients with BMI ≥ 18.58 kg/m2 and < 18.58 kg/m2. (B) Kaplan–Meier curves of progression-free survival (PFS) for patients with BMI ≥ 18.44 kg/m2 and < 18.44 kg/m2.
References
1. Zhang C, Zhang C, Wang H. Immune-checkpoint inhibitor resistance in cancer treatment: Current progress and future directions. Cancer Lett. (2023) 562:216182. doi: 10.1016/j.canlet.2023.216182
2. Darnell EP, Mooradian MJ, Baruch EN, Yilmaz M, Reynolds KL. Immune-related adverse events (irAEs): Diagnosis, management, and clinical pearls. Curr Oncol Rep. (2020) 22:39. doi: 10.1007/s11912-020-0897-9
3. Lo Preiato V, Salvagni S, Ricci C, Ardizzoni A, Pagotto U, Pelusi C. Diabetes mellitus induced by immune checkpoint inhibitors: type 1 diabetes variant or new clinical entity? Review of the literature. Rev Endocr Metab Disord. (2021) 22:337–49. doi: 10.1007/s11154-020-09618-w
4. Tittel SR, Laubner K, Schmid SM, Kress S, Merger S, Karges W, et al. Immune-checkpoint inhibitor-associated diabetes compared to other diabetes types - A prospective, matched control study. J Diabetes. (2021) 13:1007–14. doi: 10.1111/1753-0407.13215
5. Quandt Z, Young A, Anderson M. Immune checkpoint inhibitor diabetes mellitus: a novel form of autoimmune diabetes. Clin Exp Immunol. (2020) 200:131–40. doi: 10.1111/cei.13424
6. Hisanaga K, Uchino H, Kakisu N, Miyagi M, Yoshikawa F, Sato G, et al. Pre-existing diabetes limits survival rate after immune checkpoint inhibitor treatment for advanced lung cancer: A retrospective study in Japan. Diabetes Metab Syndr Obes. (2021) 14:773–81. doi: 10.2147/DMSO.S289446
7. Matsumura K, Nagasawa K, Oshima Y, Kikuno S, Hayashi K, Nishimura A, et al. Aggravation of diabetes, and incompletely deficient insulin secretion in a case with type 1 diabetes-resistant human leukocyte antigen DRB1*15:02 treated with nivolumab. J Diabetes Investig. (2018) 9:438–41. doi: 10.1111/jdi.12679
8. Gauci ML, Boudou P, Baroudjian B, Vidal-Trecan T, Da Meda L, Madelaine-Chambrin I, et al. Occurrence of type 1 and type 2 diabetes in patients treated with immunotherapy (anti-PD-1 and/or anti-CTLA-4) for metastatic melanoma: a retrospective study. Cancer Immunol Immunother. (2018) 67:1197–208. doi: 10.1007/s00262-018-2178-0
9. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
10. Pauken KE, Jenkins MK, Azuma M, Fife BT. PD-1, but not PD-L1, expressed by islet-reactive CD4+ T cells suppresses infiltration of the pancreas during type 1 diabetes. Diabetes. (2013) 62:2859–69. doi: 10.2337/db12-1475
11. Falcone M, Fousteri G. Role of the PD-1/PD-L1 Dyad in the maintenance of pancreatic immune tolerance for prevention of type 1 diabetes. Front Endocrinol (Lausanne). (2020) 11:569. doi: 10.3389/fendo.2020.00569
12. De Burghgrave M, Lourenço C, Berthault C, Aiello V, Villalba A, Fouque A, et al. Pancreatic islet cells response to IFNγ relies on their spatial location within an islet. Cells. (2022) 12:113. doi: 10.3390/cells12010113
13. Osum KC, Burrack AL, Martinov T, Sahli NL, Mitchell JS, Tucker CG, et al. Interferon-gamma drives programmed death-ligand 1 expression on islet beta-cells to limit T cell function during autoimmune diabetes. Sci Rep. (2018) 8:8295. doi: 10.1038/s41598-018-26471-9
14. Marchand L, Thivolet A, Dalle S, Chikh K, Reffet S, Vouillarmet J, et al. Diabetes mellitus induced by PD-1 and PD-L1 inhibitors: description of pancreatic endocrine and exocrine phenotype. Acta Diabetol. (2019) 56:441–8. doi: 10.1007/s00592-018-1234-8
15. Shirwaikar Thomas A, Chari ST. Immune checkpoint inhibitor-induced (Type 3) autoimmune pancreatitis. Curr Gastroenterol Rep. (2023) 25:255–9. doi: 10.1007/s11894-023-00885-6
16. Liu Y, Zhang H, Zhou L, Li W, Yang L, Li W, et al. Immunotherapy-associated pancreatic adverse events: current understanding of their mechanism, diagnosis, and management. Front Oncol. (2021) 11:627612. doi: 10.3389/fonc.2021.627612
17. Mukherji R, Debnath D, Hartley ML, Noel MS. The role of immunotherapy in pancreatic cancer. Curr Oncol. (2022) 29:6864–92. doi: 10.3390/curroncol29100541
18. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. (2014) 15:e493–503. doi: 10.1016/S1470-2045(14)70263-3
19. Hussaini S, Chehade R, Boldt RG, Raphael J, Blanchette P, Maleki Vareki S, et al. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors - A systematic review and meta-analysis. Cancer Treat Rev. (2021) 92:102134. doi: 10.1016/j.ctrv.2020.102134
20. Stamatouli AM, Quandt Z, Perdigoto AL, Clark PL, Kluger H, Weiss SA, et al. Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes. (2018) 67:1471–80. doi: 10.2337/dbi18-0002
21. Tsang V, McGrath RT, Clifton-Bligh RJ, Scolyer RA, Jakrot V, Guminski AD, et al. Checkpoint inhibitor-associated autoimmune diabetes is distinct from type 1 diabetes. J Clin Endocrinol Metab. (2019) 104:5499–506. doi: 10.1210/jc.2019-00423
22. Park SY, Gautier JF, Chon S. Assessment of insulin secretion and insulin resistance in human. Diabetes Metab J. (2021) 45:641–54. doi: 10.4093/dmj.2021.0220
23. Szablewski L. Insulin resistance: the increased risk of cancers. Curr Oncol. (2024) 31:998–1027. doi: 10.3390/curroncol31020075
24. Dev R, Bruera E, Dalal S. Insulin resistance and body composition in cancer patients. Ann Oncol. (2018) 29:ii18–26. doi: 10.1093/annoc/mdx815
25. Fan J, Shen X, Wang Y, Zhou HL, Liu G, Li YL, et al. Biomarkers for immune checkpoint therapy targeting programmed death 1 and programmed death ligand 1. BioMed Pharmacother. (2020) 130:110621. doi: 10.1016/j.biopha.2020.110621
26. Wang Y, Tong Z, Zhang W, Zhang W, Buzdin A, Mu X, et al. FDA-approved and emerging next generation predictive biomarkers for immune checkpoint inhibitors in cancer patients. Front Oncol. (2021) 11:683419. doi: 10.3389/fonc.2021.683419
27. Conforti F, Pala L, Bagnardi V, De Pas T, Martinetti M, Viale G, et al. Cancer immunotherapy efficacy and patients’ sex: a systematic review and meta-analysis. Lancet Oncol. (2018) 19:737–46. doi: 10.1016/S1470-2045(18)30261-4
28. Takamori S, Shimokawa M, Komiya T. Prognostic impact of chronological age on efficacy of immune checkpoint inhibitors in non-small-cell lung cancer: Real-world data from 86 173 patients. Thorac Cancer. (2021) 12:2943–8. doi: 10.1111/1759-7714.14178
29. Gainor JF, Rizvi H, Jimenez Aguilar E, Skoulidis F, Yeap BY, Naidoo J, et al. Clinical activity of programmed cell death 1 (PD-1) blockade in never, light, and heavy smokers with non-small-cell lung cancer and PD-L1 expression ≥50. Ann Oncol. (2020) 31:404–11. doi: 10.1016/j.annpnc.2019.11.015
30. Brueckl WM, Ficker JH, Zeitler G. Clinically relevant prognostic and predictive markers for immune-checkpoint-inhibitor (ICI) therapy in non-small cell lung cancer (NSCLC). BMC Cancer. (2020) 20:1185. doi: 10.1186/s12885-020-07690-8
31. Cortellini A, Bersanelli M, Buti S, Cannita K, Santini D, Perrone F, et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. J Immunother Cancer. (2019) 7:57. doi: 10.1186/s40425-019-0527-y
Keywords: anti-PD1 immune checkpoint inhibitors, insulin secretory capacity, cancer prognosis, insulin secretion, glucose metabolism markers
Citation: Watanabe M, Eguchi J, Takamoto A, Kanzaki H, Noda Y, Kagawa S and Wada J (2024) HOMA-beta independently predicts survival in patients with advanced cancer on treatment with immune checkpoint inhibitors. Front. Endocrinol. 15:1439705. doi: 10.3389/fendo.2024.1439705
Received: 28 May 2024; Accepted: 25 November 2024;
Published: 11 December 2024.
Edited by:
Michaela Luconi, University of Florence, ItalyReviewed by:
Joe Varghese, Christian Medical College and Hospital, IndiaSrinadh Choppara, University of Michigan, United States
Copyright © 2024 Watanabe, Eguchi, Takamoto, Kanzaki, Noda, Kagawa and Wada. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mayu Watanabe, d2F0YW5hYmUubWF5dS5ycEBtYWlsLmhvc3AuZ28uanA=; Jun Eguchi, amVndWNoaUBjYy5va2F5YW1hLXUuYWMuanA=
 Mayu Watanabe
Mayu Watanabe Jun Eguchi
Jun Eguchi Atsushi Takamoto3
Atsushi Takamoto3 Jun Wada
Jun Wada