- 1Department of Neurology, The Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou Municipal Hospital, Suzhou, Jiangsu, China
- 2Biology and Biotechnology Program, School of Science and Technology, Endicott College, Beverly, MA, United States
- 3Department of Neurology, The Affiliated Changshu Hospital of Nantong University, Changshu, Jiangsu, China
- 4Emergency Medicine Department, The Quzhou Affiliated Hospital of Wenzhou Medical University, Quzhou People’s Hospital, Quzhou, Zhejiang, China
- 5Department of Neurology, The Quzhou Affiliated Hospital of Wenzhou Medical University, Quzhou People’s Hospital, Quzhou, Zhejiang, China
Objective: Ischemic stroke-associated pneumonia (iSAP) affects about 10% of acute ischemic stroke patients during hospitalization. Current prediction scales for iSAP are insufficient. Identifying early biomarkers for stroke-associated pneumonia is crucial for improving patient outcomes. This study aimed to investigate the predictive value of euthyroid sick syndrome (ESS) for iSAP in acute-stage of ischemic stroke patients.
Methods: We studied 1767 acute ischemic stroke patients within one week of symptom onset, categorizing them into an infection group (iSAP, n=376) and control group (control, n=1391). COX regression analysis was used to identify the potential risk and protected factors. Kaplan-Meier time-event curves and Log-Rank tests were performed to differentiate infection time in patients with ESS or normal T3 group.
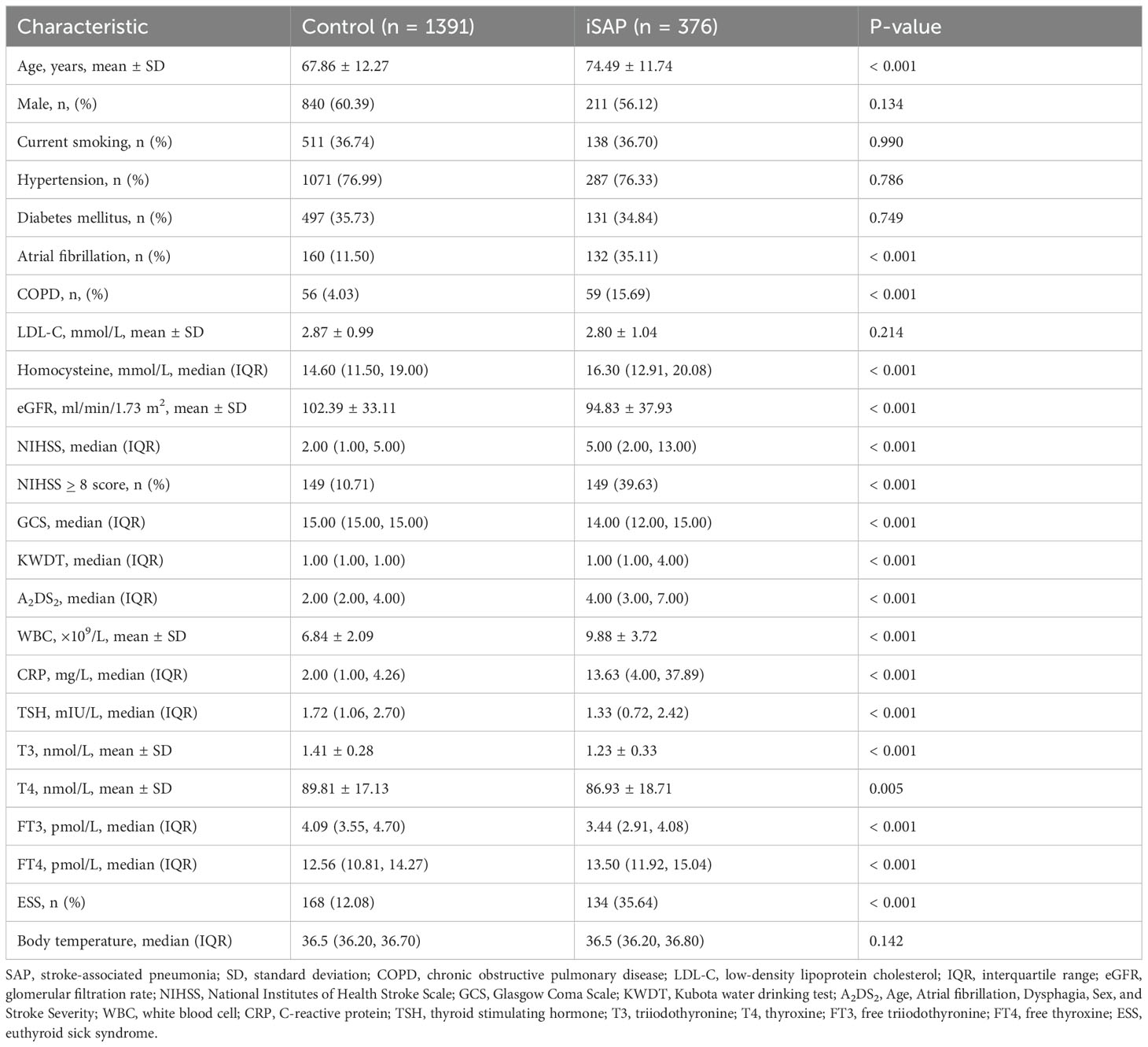
Results: The iSAP group had higher rates of risk factors like older age, atrial fibrillation, COPD, and ESS, along with elevated levels of WBC, CRP,and FT4 levels (all P < 0.001). Conversely, iSAP patients had lower GCS scores, eGFR, TSH, T3, FT3 (all P < 0.001) and T4 levels (P = 0.005) upon admission. No significant differences were observed in sex, smoking history, hypertension, diabetes, or LDL-C levels (P > 0.05). COX regression analysis identified age, KWST scores, leukocyte count, CRP, and ESS (all P < 0.001) as significantly correlated with iSAP. ROC analysis revealed ESS as a predictor with sensitivity of 35.64% and specificity of 87.92% for SAP prediction, like atrial fibrillation and higher than COPD and eGFR.
Conclusion: ESS at admission predicts a higher risk of stroke-associated pneumonia in acute-stage of ischemic stroke.
1 Introduction
Stroke is the second leading cause of death globally and is the third leading cause of disability (1–3). Approximately 60-80% of deaths due to stroke are attributed to ischemic stroke, rather than hemorrhagic or transient stroke (4, 5). Stroke-associated pneumonia (SAP) is a significant early complication following a stroke. During the acute stage of hospitalization for ischemic stroke, about 10% of patients will develop ischemic stroke-related pneumonia (iSAP) (6). SAP is linked to unfavorable functional outcomes and high mortality rates (7, 8). Therefore, early prediction and intervention for stroke-related pneumonia are of paramount importance for improving the prognosis and survival rates of stroke patients. The sensitivity and specificity of commonly used scales for predicting iSAP, such as the A2DS2, AIS-APS, and ISAN, need enhancement through the incorporation of serum biomarkers (9–11). Therefore, identifying biomarkers for early iSAP diagnosis and subsequent treatment initiation is critical for the improvement of ischemic stroke patient prognosis.
Euthyroid sick syndrome (ESS) is one of the prevalent neuroendocrine changes observed in severe illnesses. ESS refers to alterations in thyroid function associated with severe diseases, surgery, and other stressful conditions, primarily characterized by decreased levels of triiodothyronine (T3) and free triiodothyronine (fT3), often without significant changes in thyroid-stimulating hormone (TSH) levels (12). ESS can serve as a predictor of poor outcomes in severe diseases, such as stroke (13–17). However, the relationship between stroke complications, like iSAP, and ESS has not been explored.
Poststroke immunosuppressive syndrome is a contributing factor of poststroke infection (18). Additionally, thyroid hormones influence immune system regulation. Therefore, the predictive value of thyroid hormone level change in poststroke infection is promising. The example of hypothyroidism causing Hashimoto encephalopathy bolsters the hypothesis that thyroid dysregulation could have neurological prognostic value (19–22). Furthermore, the screening of thyroid function upon admission has become a routine diagnostic and treatment measure in most medical institutions in China, setting the stage for prospective studies on the predictive value of thyroid hormones in post-stroke infection (23, 24). This current study aims to analyze the relationship between triiodothyronine level at admission and the incidence of SAP in the acute stages of ischemic stroke. Additionally, it aims to provide insights for accurately predicting iSAP occurrence based on thyroid hormone level changes.
2 Methods
2.1 Subjects
The subject data for this study were obtained from our previously established observational cohort study on cerebral infarction prognosis. The data were collected from patients in our hospitals (Suzhou Municipal Hospital, Quzhou People’s Hospital and Affiliated Changshu Hospital of Nantong University) who met the inclusion criteria between September 2016 and September 2022. The internally collected data is not yet publicly available. Inclusion criteria required that patients had an acute stroke within one week of enrollment into the study and data collection, were at least 18 years old, had thyroid function tests completed at point of admission, and no exclusion criteria were met. Exclusion criteria included patients with a history of chronic thyroid disease, a history of taking medications that affect thyroxine level or function, non-acute stages of stroke, presence or history of brain tumors, encephalitis, brain trauma, and severe multiple organ dysfunction syndrome. The patient data were categorized into an iSAP group (376 patients), a well-matched control group without iSAP (1391 patients), an ESS group (302 patients), and a well-matched control group with normal T3 levels (1465 patients). This study received approval from the Ethics Committee of all participated Hospital. Data collection did not require the access or use of patient private information. Furthermore, all patients provided informed consent for medical services and clinical study enrollment upon admission.
2.2 Research ethics and patient consent
The studies involving human participants were reviewed and approved by ethics committee of the Affiliated Suzhou Hospital of Nanjing Medical University (Suzhou Municipal Hospital), Quzhou Affiliated Hospital of Wenzhou Medical University and Affiliated Changshu Hospital of Nantong University. The patients provided their written informed consent to participate in this study.
2.3 Data collection
Demographic and clinical data at admission were collected, including age, sex, smoking history, history of hypertension, type 2 diabetes, atrial fibrillation, and chronic obstructive pulmonary disease (COPD). Baseline clinical characteristics, including stroke severity according to the National Institutes of Health Stroke Scale (NIHSS), dysphagia according to the Kubota water drinking test (KWDT), and disturbance of consciousness according to the Glasgow Coma Scale (GCS). The anatomical structures involved in the stroke and the extent they were involved were determined by brain magnetic resonance imaging (MRI) or cerebral artery computerized tomography (CT) angiography, cervical cerebrovascular color ultrasound, electrocardiogram, and other patient-specific required examination results within 72 hours of admission (25). A NIHSS score of 8 or above is the criteria for severe stroke (26). In addition, we also calculated the Age, Atrial Fibrillation, Dysphagia, Sex, and Stroke Severity (A2DS2) score, which ranges from 0 to 10 points and is validated for predicting SAP risk (27).
2.4 Laboratory tests
Fasting venous blood was evaluated within 24 hours of admission for laboratory tests, including white blood cells, creatinine, low-density lipoprotein cholesterol (LDL-C), homocysteine, CRP and thyroid hormones. The levels of total triiodothyronine (T3), free triiodothyronine (fT3), total thyroxine (T4), free thyroxine (fT4) and thyroid stimulating hormone (TSH) were determined on an ARCHITECT i2000 (Abbott Diagnostics, US) by chemiluminescent microparticle immunoassay methodology (28). ESS was diagnosed with T3 < 1.21 nmol/L or fT3 < 2.63 pmol/L accompanied by normal TSH level (29).
2.5 Diagnostic criteria for stroke associated pneumonia
Patients suspected of comorbidity with SAP were diagnosed by two attending neurologists independently according to the Pneumonia In Stroke Consensus group recommendations. The attending respiratory physician was consulted to further establish the diagnosis of SAP, if needed. The diagnosis was made during the first 7 days after the onset of stroke, according to the modified Centers for Disease Control and Prevention (CDC) criteria in patients not receiving mechanical ventilation.
The diagnostic criteria included the presence of at least one of the following indicators: 1. Fever (>38°C) without any other known cause; 2. Abnormal white blood cell count, either leukopenia (<4×109 WBC/L) or leukocytosis (>12×109 WBC/L); 3. Altered mental status with no other identified cause, for adults aged ≥70. Additionally, at least two of the following should be observed: 1. Onset of purulent sputum or a change in sputum character over 24 hours, or an increase in respiratory secretions, or elevated suctioning requirements; 2. Emergence of a new or aggravated cough, dyspnea, or tachypnea (respiratory rate >25/min); 3. Detection of rales, crackles, or bronchial breath sounds; 4. Deterioration in gas exchange, such as O2 desaturation (e.g., PaO2/FiO2 ≤240) or elevated oxygen needs. Furthermore, two sequential chest radiographs should reveal at least one of the following: new or progressive, persistent infiltrate, consolidation, or cavitation. In patients without pre-existing pulmonary or cardiac issues, a single definitive chest radiograph suffices. These criteria were chosen for their relevance to the study’s target population and their established effectiveness in diagnosing pneumonia in stroke patients (30). While these criteria apply to all instances of stroke-associated pneumonia, in our research on ischemic stroke-associated pneumonia, we utilized chest CT, which offers superior clarity compared to the recommended chest X-ray.
2.6 Statistical analyses
In our analysis, we employed a range of statistical tests and software tools. Data following a normal distribution were subjected to a t-test, and results are presented as mean ± standard deviation (x-bar ± SD). For data not conforming to a normal distribution, we employed the Mann-Whitney U test, and results are expressed as the median with the lower and upper quartiles. Categorical data were subjected to univariate analysis using the Chi-square test, and the results are reported as the number of cases (percentage). To identify independent risk factors for iSAP, both univariate and multivariate COX regression analyses were conducted. All statistical analyses were carried out using SPSS version 25.0. Additionally, Kaplan-Meier survival curves were generated using GraphPad Prism 9, and group differences in infection-free time were assessed using the Log Rank test. Receiver Operating Characteristic (ROC) curves were created using Medcalc software to evaluate various factors’ predictive performance, including the Area Under the Curve (AUC), sensitivity, and specificity. Statistical significance was defined as a P-value less than 0.05 (α = 0.05).
3 Results
In this study, a total of 1,767 patients diagnosed with ischemic stroke were included, with an average age of 69.27 ± 12.46 years. Among the participants, 612 individuals (34.63%) were under 65 years of age, 766 individuals (43.35%) fell into the 65 to 79 age range, while 389 individuals (22.02%) were aged 80 or older. Of the total cohort, 716 were female (40.52%). Regarding thyroid hormone levels, 234 patients (13.24%) exhibited T3 levels below 1.21 nmol/l, while 68 patients (3.85%) had fT3 levels below 2.63 pmol/l. Both T3 and fT3 values fell below the normal range in 57 patients (3.28%). The severity of stroke, as assessed by the NIHSS score, revealed 298 patients (16.86%) with scores ‗ 8 points upon admission. Among them, 149 cases (39.63%) were in the iSAP group, while 149 cases (10.71%) were categorized under the Control group. This difference in the proportion of severe cases between the two groups was statistically significant (χ² = 176.518, P < 0.001). There were 60 cases (19.86%) in the ESS group and 238 cases (16.24%) in the Normal group. There was no significant difference in the proportion of severe cases between these two groups (χ2 = 2.343, P = 0.126). Additionally, atrial fibrillation was detected in 292 patients, with 132 cases (35.11%) in the iSAP group and 160 cases (11.50%) in the Control group. The prevalence of atrial fibrillation in the iSAP group was notably higher than that in the Control group (χ² = 119.548, P < 0.001). Similarly, 85 cases of atrial fibrillation (28.15%) were observed in the ESS group, and 207 cases (14.13%) were observed in the Normal group, exhibiting a significantly higher prevalence in the ESS group (χ² = 35.658, P < 0.001).
3.1 Baseline characteristics and outcomes of patients with and without iSAP
In our study, we observed notable differences between the iSAP group and the Control group. For example, the proportions of patients in the iSAP group with advanced age, atrial fibrillation, COPD, hyperhomocysteinemia, ESS, high NIHSS score, high KWDT score, high A2DS2 score, elevated white blood cell count, increased CRP levels, and higher fT4 levels were all significantly higher compared to the Control group (all P < 0.001). Conversely, several parameters showed significant differences in the opposite direction. Specifically, the GCS score, eGFR, TSH, T3, fT3 levels (all P < 0.001), and T4 level (P = 0.005) were significantly lower in the iSAP group when compared to the Control group. Notably, there were no significant differences between the two groups in terms of gender, current smoking status, hypertension, diabetes, and LDL-C levels (all P > 0.05). Furthermore, our analysis revealed that 134 cases (35.60%) in the iSAP group exhibited ESS, while only 168 cases (12.10%) in the Control group had ESS. The incongruity in the incidence of ESS between the two groups was highly significant (χ² = 115.953, P < 0.001). Moreover, when examining thyroid hormone levels, we found that T3, T4, fT3, and TSH levels in the iSAP group were significantly lower compared to the Control group (P ≤ 0.005), while the level of fT4 was notably higher in the iSAP group (P < 0.001). These results are presented in Table 1.
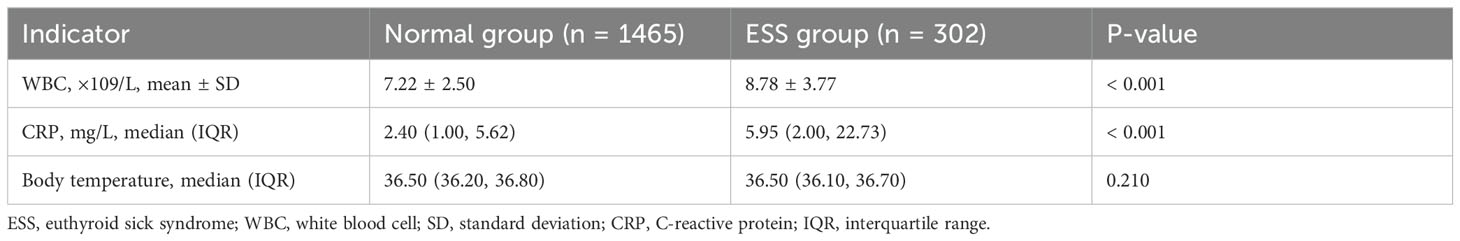
3.2 Comparison of infection biomarkers for patients between the ESS and Normal groups
In the ESS group, 134 patients (44.37%) were diagnosed with iSAP, while in the Normal group, 242 patients (16.5%) had iSAP. This difference in iSAP incidence between the two groups was statistically significant (χ² = 115.953, P < 0.001). Additionally, we observed distinct differences in key health indicators. The white blood cell counts and CRP levels were notably higher in the ESS group compared to the Normal group (all P < 0.001). Furthermore, a significantly greater proportion of patients in the ESS group exhibited elevated body temperature upon admission compared to the Normal group (P = 0.004). However, there were no significant differences in overall body temperature between the two groups (P > 0.05). For a comprehensive overview of infection indicators between the ESS and Normal groups, please refer to Table 2.
3.3 Analysis of independent risk factors for ischemic stroke-associated pneumonia
We conducted a rigorous statistical analysis to explore the factors associated with concurrent iSAP using univariate Cox regression analysis. In this analysis, concurrent iSAP served as the dependent variable, while patients’ demographic characteristics, disease-related scores, and hematological indicators were considered as independent variables. The results illuminated several significant relationships. Notably, advanced age, a history of atrial fibrillation or COPD, higher scores on the NIHSS, A2DS2, or KWDT scales, the presence of concurrent ESS, lower eGFR levels, elevated WBC counts, increased CRP levels, higher homocysteine levels, and elevated body temperature were identified as positively associated factors with concurrent iSAP. Conversely, a higher GCS score emerged as a negative associated factor with concurrent iSAP (Figure 1A).
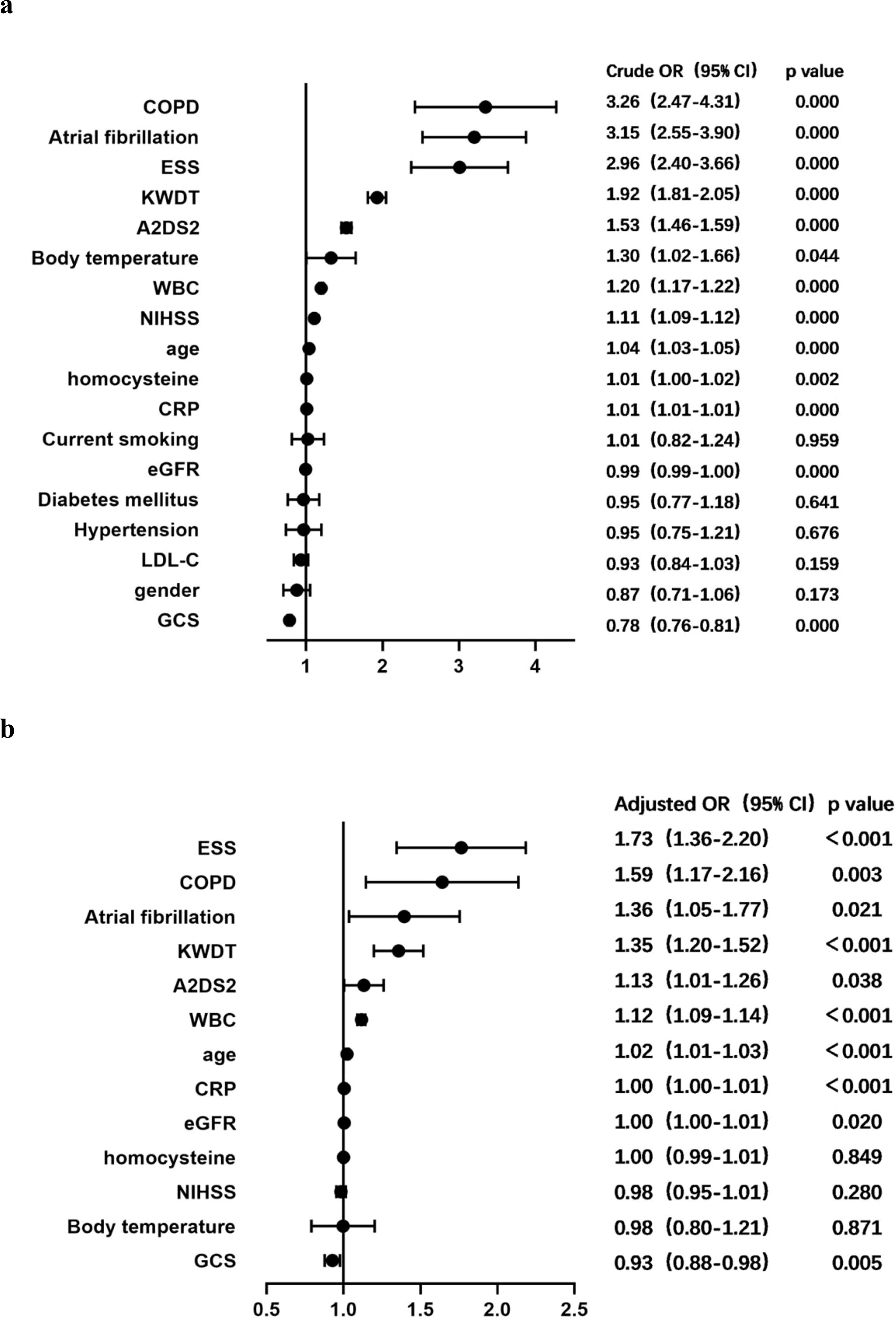
Figure 1. Forest plot of odds ratios for iSAP. (A) Crude ORs for potential factors for predicting iSAP. (B) Adjusted ORs for potential factors for predicting iSAP. ESS, euthyroid sick syndrome; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; KWDT, Kubota water drinking test; eGFR, glomerular filtration rate; NIHSS, National Institutes of Health Stroke Scale; GCS, Glasgow Coma Scale; 95% CI, 95% confidence interval; OR, odds ratio; SAP, stroke-associated pneumonia.
Further analysis was conducted by employing concurrent iSAP as the dependent variable, and variables with univariate analysis results showing significance at P < 0.05 were integrated into a multivariate COX proportional risk model. The related factors were thoroughly examined using the enter method at the significance level of α = 0.05. This analysis confirmed that older age, a history of atrial fibrillation or COPD, high scores on the A2DS2 or KWDT scales, the presence of concurrent ESS, lower eGFR levels, and higher levels of WBC counts or CRP levels all independently emerged as positive factors contributing to the occurrence of iSAP. Conversely, a higher GCS score was identified as an independent negative factor for iSAP (Figure 1B). These insights into the multifaceted factors associated with concurrent iSAP contributes to our understanding of this complex medical condition.
3.4 Kaplan-Meier time-event curves of T3 prediction for infection time in patients with ESS or Normal group
One month after the admission of ischemic stroke, Kaplan-Meier time event curves of ischemic stroke-associated pneumonia in ESS group and normal group were significantly separated (Figure 2). Log-Rank tests for infection time in patients with ESS or Normal group showed the median time of infection was 8.0 days (95%CI 3.0-11.0) in ESS group and 9.0 days (95%CI 6.0-11.0) in Normal group (Log Rank P < 0.001) (Figure 2).
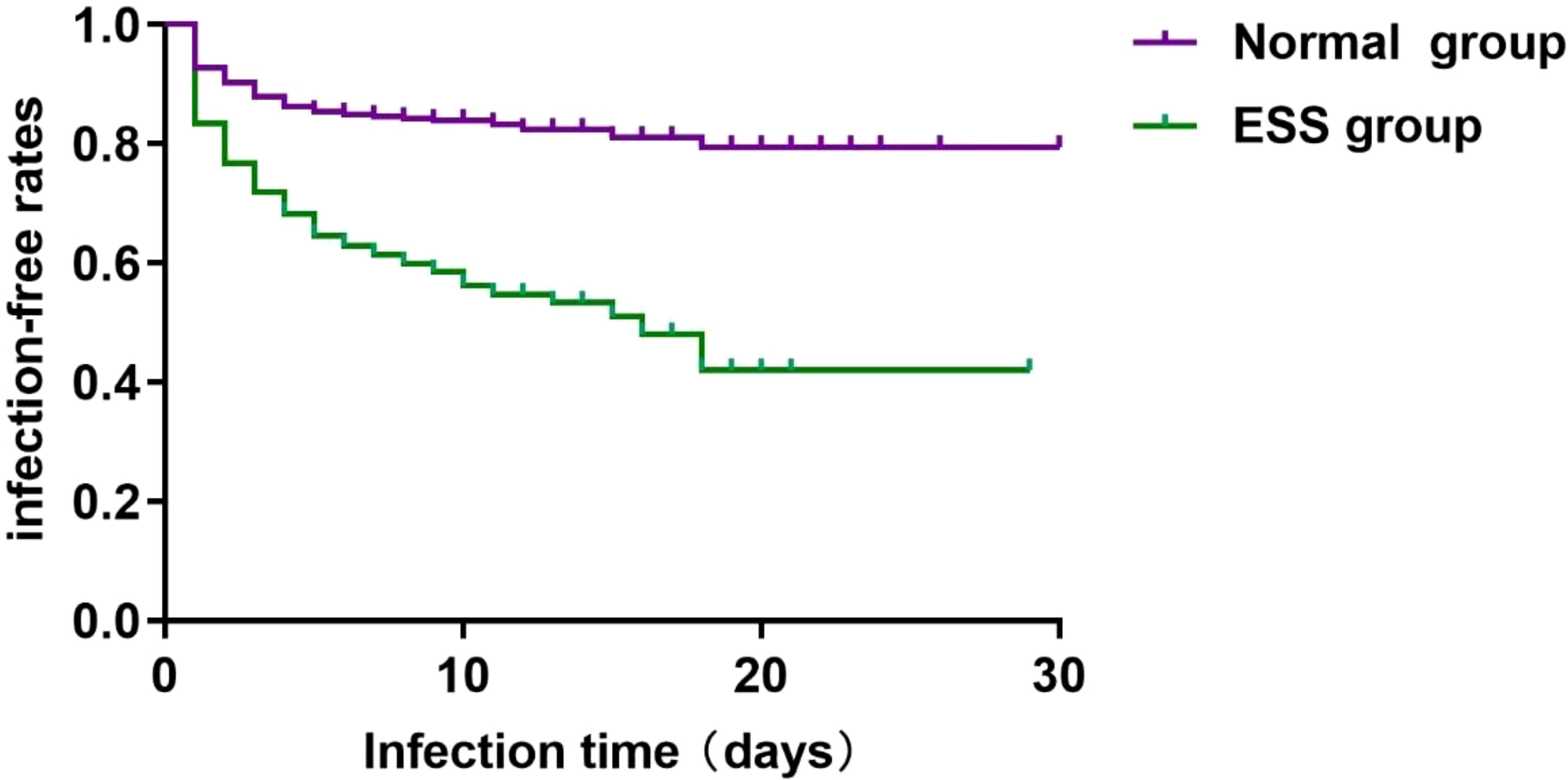
Figure 2. Kaplan-Meier time-event curves and Log-Rank tests for infection time in patients with ESS or Normal group. The median time of infection was 8.0 days (95%CI 3.0-11.0) in ESS group and 9.0 days (95%CI 6.0-11.0) in Normal group (Log Rank P < 0.001).
3.5 The predictive value of various indicators for iSAP
Our analysis of the receiver operating characteristic curve revealed insights into the predictive capabilities of various factors for iSAP. Among these factors, ESS demonstrated a notable area under the curve (AUC) value of 0.618 (95% CI 0.595-0.641), signifying its effectiveness in predicting iSAP. In comparison, COPD (Chronic Obstructive Pulmonary Disease) yielded a lower AUC of 0.558 (95% CI 0.535-0.582, P < 0.05), indicating its comparatively weaker predictive ability for iSAP in this study.
Furthermore, our analysis highlighted several other factors with their respective AUC values and differential effect compared with ESS: eGFR (AUC = 0.566, 95% CI 0.543-0.590, P=0.0109), CRP (C-Reactive Protein, AUC = 0.826, 95% CI 0.807-0.843, P < 0.0001), WBC (White Blood Cell Count, AUC = 0.778, P < 0.0001), A2DS2 (AUC = 0.758, 95% CI 0.737-0.777, P < 0.0001), KWDT (AUC = 0.698, 95% CI 0.676-0.720, P < 0.0001), GCS (Glasgow Coma Scale, AUC = 0.689, 95% CI 0.667-0.711, P=0.0004), and age (AUC = 0.663, 95% CI 0.641-0.685, P=0.0164). Remarkably, ESS’s predictive power was comparable to that of atrial fibrillation, as both exhibited an AUC of 0.618 (95% CI 0.595-0.641, P=0.9907) (Figure 3). These findings underscore the significance of ESS as a predictive factor for iSAP and its competitive performance compared to other variables.
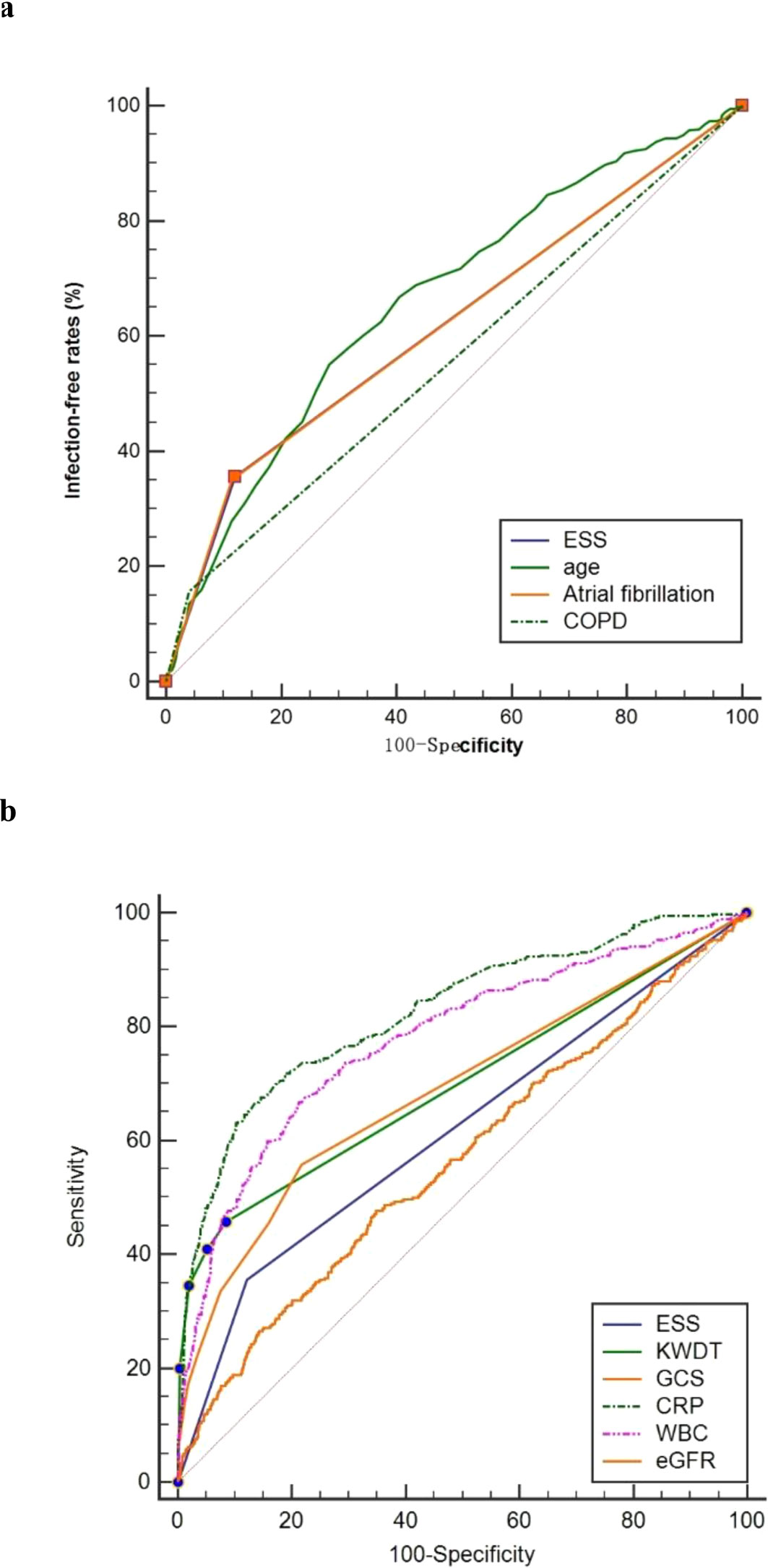
Figure 3. Predictive power comparison between ESS and other factors. ROC curves for ESS, age, Atrial fibrillation, and COPD (A). ROC curves for ESS, KWDT, GCS, CRP, Leukocyte count and eGFR (B). ROC, receiver operating characteristic; AUC, area under the curves.
4 Discussion
This study underscores ESS as a significant predictor of iSAP during the acute stage of ischemic stroke. It confirms several established risk factors for iSAP, such as older age, atrial fibrillation, COPD, and various inflammatory markers, consistent with prior research (7, 31–34). However, hyperhomocysteinemia and NIHSS scores did not independently predict iSAP. This study also identified that ESS, characterized by reduced T3 levels following ischemic stroke, significantly elevates the risk of iSAP independent of stroke severity. This positions ESS as a vital biomarker for early-stage immune function impairment in severe diseases with infection.
ESS patients with ischemic stroke showed significantly higher WBC counts upon admission than those with normal T3 levels, further confirming ESS’s predictive value for iSAP. T3 plays a pivotal role in immune function, impacting various immune cell activities. For example, T3 promotes proliferation and cytotoxicity in T cells, neutrophils, macrophages, and natural killer cells, and increases the production of pro-inflammatory cytokines such as IL-12, IL-6, and TGF-β (35–37). Lower T3 levels compromise immune function, elevating infection risk.
Elevated CRP levels were observed in ischemic stroke patients with ESS. Both ESS and elevated CRP levels independently predict an increased risk of iSAP, even without elevated body temperature (14, 38). Elevated CRP is widely used to monitor acute stress conditions like cardiovascular or cerebrovascular diseases, infections, and surgical procedures. Given that ESS can co-occur with acute stress or chronic disease states, its correlation with elevated CRP levels is not surprising. The predictive value of body temperature at admission for SAP remains uncertain, especially as some elderly patients with pulmonary infections may not exhibit fever symptoms (38, 39). In our study, body temperature upon admission did not independently predict iSAP, possibly due to the low proportion of iSAP patients with fever. Further research is needed to explore its potential utility in specific patient subgroups.
Other studies have confirmed ESS’s ability to predict infection severity and prognosis (40, 41). While a few single-center retrospective studies have reported ESS’s predictive outcomes for SAP or stroke-related infection, they have neglected the temporal aspects and lacked information on the sensitivity and specificity of the diagnostic data (14, 42). In our study, ESS exhibited a specificity of 87.92%, sensitivity of 35.64%, and an AUC of 0.618 for predicting iSAP. ESS’s predictive performance for iSAP paralleled that of atrial fibrillation and surpassed that of COPD and eGFR. Atrial fibrillation has been adopted as a predictor of SAP by several scales (43, 44). Furthermore, hypothyroidism increases the risk of atrial fibrillation (45–49). Although ESS’s predictive performance has a relatively poor sensitivity and a marginally satisfactory AUC, it exhibits strong specificity and performs well in conjunction with other clinical benchmarks.
The inhalation of oropharyngeal flora due to dysphagia is a primary cause of SAP (50). Patients with severe strokes are at higher risk of oropharyngeal flora colonization in the alveoli and have a reduced cough reflex, promoting the development of SAP (51). The KWDT score is a widely used tool for assessing swallowing function with high sensitivity and specificity (52). In our study, the KWDT score’s correlation with iSAP prediction was stronger than the NIHSS score ability to predict iSAP risk. This is likely due to the relatively smaller proportion of the NIHSS focusing on dysphagia at the expense of including factors like motor function or level of consciousness. The relevance of the KWDT score allows it to independently predict the risk of iSAP in our study.
There are several limitations in this study. First, the ability of nested case-control studies to retrospectively evaluate the level of influencing factors depends on the level of influencing factors of the patients included in the cohort. Differences in exposure factors between cohort studies and nested case-control studies may lead to selection bias and result bias. Second, a single test of T3 level on admission may not fully reflect the changes of T3 concentration in different periods of AIS and its influence on the prognosis. Third, a single factor approach was not effective in predicting iSAP risk in this study, as with previous studies. This study did not explore which combination of risk factors could effectively predict iSAP. Although there are limitations, this study is the first to report the efficacy of ESS in predicting stroke-associated pneumonia in the acute stage of ischemic stroke, and confounding factors were well controlled, which provides clear evidence for the effect of ESS in predicting iSAP.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by ethics committee of the Affiliated Suzhou Hospital of Nanjing Medical University (Suzhou Municipal Hospital), Quzhou Affiliated Hospital of Wenzhou Medical University and Affiliated Changshu Hospital of Nantong University. University (2015/KL901045, QE2023R/151,2023/IEC-C-008-A07-V3.0). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JY: Data curation, Formal analysis, Writing – original draft. SY: Data curation, Formal Analysis, Writing – review & editing. RL: Data curation, Writing – original draft. WT: Data curation, Formal Analysis, Writing – original draft. JY: Data curation, Writing – original draft. HF: Data curation, Writing – original draft. MW: Data curation, Writing – original draft. QX: Data curation, Writing – original draft. XJ: Data curation, Writing – original draft. HL: Data curation, Writing – original draft. QG: Data curation, Resources, Writing – review & editing. TD: Data curation, Resources, Writing – review & editing. GW: Data curation, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. G-HW is funded by Top-Notch Talent Foundation of Six Types of Outstanding Personnel from Jiangsu Provincial Health Commission (LGY2019015) and Suzhou Municipal Health Commission (GSWS2019057, LCZX202318). The funders had no role in the conception, design, analysis, interpretation and writing of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Feigin V, Stark B, Johnson C, Roth G, Bisignano C, Abady G, et al. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. (2021) 20:795–820. doi: 10.1016/s1474-4422(21)00252-0
2. Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2019) 394:1145–58. doi: 10.1016/s0140-6736(19)30427-1
3. Andres W, Rothstein A, Elser H, Sloane KL, Gottesman RF, Kasner SE, et al. Trends in the prevalence of stroke among community-dwelling individuals in the US, 1999-2018. JAMA neurology. (2023) 80:646–8. doi: 10.1001/jamaneurol.2023.0742
4. Thrift AG, Howard G, Cadilhac DA, Howard VJ, Rothwell PM, Thayabaranathan T, et al. Global stroke statistics: An update of mortality data from countries using a broad code of “cerebrovascular diseases. Int J Stroke. (2017) 12:796–801. doi: 10.1177/1747493017730782
5. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart disease and stroke statistics-2018 update: A report from the american heart association. Circulation. (2018) 137:e67–e492. doi: 10.1161/cir.0000000000000558
6. Badve MS, Zhou Z, van de Beek D, Anderson CS, Hackett ML. Frequency of post-stroke pneumonia: Systematic review and meta-analysis of observational studies. Int J Stroke. (2019) 14:125–36. doi: 10.1177/1747493018806196
7. Ji R, Shen H, Pan Y, Wang P, Liu G, Wang Y, et al. Novel risk score to predict pneumonia after acute ischemic stroke. Stroke. (2013) 44:1303–9. doi: 10.1161/strokeaha.111.000598
8. Koennecke HC, Belz W, Berfelde D, Endres M, Fitzek S, Hamilton F, et al. Factors influencing in-hospital mortality and morbidity in patients treated on a stroke unit. Neurology. (2011) 77:965–72. doi: 10.1212/WNL.0b013e31822dc795
9. Zhang X, Xiao L, Niu L, Tian Y, Chen K. Comparison of six risk scores for stroke-associated pneumonia in patients with acute ischemic stroke: A systematic review and Bayesian network meta-analysis. Front Med. (2022) 9:964616. doi: 10.3389/fmed.2022.964616
10. Yu Y, Xia T, Tan Z, Xia H, He S, Sun H, et al. A(2)DS(2) score combined with clinical and neuroimaging factors better predicts stroke-associated pneumonia in hyperacute cerebral infarction. Front neurology. (2022) 13:800614. doi: 10.3389/fneur.2022.800614
11. Zapata-Arriaza E, Moniche F, Blanca PG, Bustamante A, Escudero-Martínez I, Uclés O, et al. External validation of the ISAN, A2DS2, and AIS-APS scores for predicting stroke-associated pneumonia. J stroke cerebrovascular diseases: Off J Natl Stroke Assoc. (2018) 27:673–6. doi: 10.1016/j.jstrokecerebrovasdis.2017.09.059
12. Ganesan K, Anastasopoulou C, Wadud K. Euthyroid Sick Syndrome. Treasure Island (FL: StatPearls (2023). ineligible companies. Disclosure: Catherine Anastasopoulou declares no relevant financial relationships with ineligible companies. Disclosure: Khurram Wadud declares no relevant financial relationships with ineligible companies.
13. Müller NA, Kaegi-Braun N, Durmisi M, Gressies C, Tribolet P, Stanga Z, et al. Low T3 syndrome on admission and response to nutritional support in malnourished medical inpatients. J Clin Endocrinol Metab. (2023) 108:e240–8. doi: 10.1210/clinem/dgac743
14. Chen H, Xu M, Huang Y, He J, Ren W. Low triiodothyronine syndrome is associated with stroke-associated pneumonia. Eur J Clin Invest. (2022) 52:e13840. doi: 10.1111/eci.13840
15. Zhang JG, Fu SM, Liu F, Wan JG, Wu SB, Jiang GH, et al. Correlation and prognostic assessment of low T3 syndrome and norepinephrine dosage for patients with sepsis: A retrospective single-center (Cohort) study. Int J Gen Med. (2022) 15:4837–47. doi: 10.2147/ijgm.S362748
16. Świstek M, Broncel M, Gorzelak-Pabiś P, Morawski P, Fabiś M, Woźniak E. Euthyroid sick syndrome as a prognostic indicator of COVID-19 pulmonary involvement, associated with poorer disease prognosis and increased mortality. Endocrine practice: Off J Am Coll Endocrinol Am Assoc Clin Endocrinologists. (2022) 28:494–501. doi: 10.1016/j.eprac.2022.02.006
17. Yuan CX, Ruan YT, Zeng YY, Cheng HR, Cheng QQ, Chen YB, et al. Liver fibrosis is associated with hemorrhagic transformation in patients with acute ischemic stroke. Front neurology. (2020) 11:867. doi: 10.3389/fneur.2020.00867
18. Hoffmann S, Harms H, Ulm L, Nabavi DG, Mackert BM, Schmehl I, et al. Stroke-induced immunodepression and dysphagia independently predict stroke-associated pneumonia - The PREDICT study. J Cereb Blood Flow Metab. (2017) 37:3671–82. doi: 10.1177/0271678x16671964
19. Chiriboga Reyes G, Pallares Vela E, Bernad PG. Cerebellar ataxia in the setting of hashimoto’s thyroiditis: A case report update and review. Cureus. (2023) 15:e45959. doi: 10.7759/cureus.45959
20. Falb V, Costanzo L, Avalos C, Feoktistov A. Autoimmune encephalopathy associated with anti-thyroid antibodies: A case report. Cureus. (2022) 14:e28183. doi: 10.7759/cureus.28183
21. Katagiri N, Ohta R, Yamane F, Sano C. Hashimoto encephalopathy of a middle-aged man with progressive symptoms of dementia. Cureus. (2022) 14:e27518. doi: 10.7759/cureus.27518
22. Foster P, Craig T, Jha P, Dhariwal MS. Lingering effects: hashimoto’s encephalopathy. Cureus. (2022) 14:e26809. doi: 10.7759/cureus.26809
23. Cooper DS, Biondi B. Subclinical thyroid disease. Lancet. (2012) 379:1142–54. doi: 10.1016/s0140-6736(11)60276-6
24. Pearce SH, Brabant G, Duntas LH, Monzani F, Peeters RP, Razvi S, et al. 2013 ETA guideline: management of subclinical hypothyroidism. Eur Thyroid J. (2013) 2:215–28. doi: 10.1159/000356507
25. Madden KP, Karanjia PN, Adams HP Jr., Clarke WR. Accuracy of initial stroke subtype diagnosis in the TOAST study. Trial of ORG 10172 in Acute Stroke Treatment. Neurology. (1995) 45:1975–9. doi: 10.1212/wnl.45.11.1975
26. Qin H, Wang A, Zuo Y, Zhang Y, Yang B, Wei N, et al. Malnutrition could predict 3-month functional prognosis in mild stroke patients: findings from a nationwide stroke registry. Curr neurovascular Res. (2021) 18:489–96. doi: 10.2174/1567202619666211217130221
27. Song X, He Y, Bai J, Zhang J. A nomogram based on nutritional status and A(2)DS(2) score for predicting stroke-associated pneumonia in acute ischemic stroke patients with type 2 diabetes mellitus: A retrospective study. Front Nutr. (2022) 9:1009041. doi: 10.3389/fnut.2022.1009041
28. Argente Del Castillo P, Pastor García MI, Morell-Garcia D, Martinez-Gomez L, Ballesteros MA, Barcelo A. Thyroid panel reference intervals in healthy children and adolescents: A Spanish cohort. Clin Biochem. (2021) 91:39–44. doi: 10.1016/j.clinbiochem.2021.01.011
29. Wu GH, Kong FZ, Cheng QZ, Luo WF, Du XD. Low T3 syndrome predicts severe neurological deficits of cerebral infarction inpatients with large artery artherosclerosis in internal carotid artery system. Neuro Endocrinol letters. (2014) 35:149–53.
30. Smith CJ, Kishore AK, Vail A, Chamorro A, Garau J, Hopkins SJ, et al. Diagnosis of stroke-associated pneumonia: recommendations from the pneumonia in stroke consensus group. Stroke. (2015) 46:2335–40. doi: 10.1161/strokeaha.115.009617
31. Sui R, Zhang L. Risk factors of stroke-associated pneumonia in Chinese patients. Neurological Res. (2011) 33:508–13. doi: 10.1179/016164111x13007856084205
32. Ouyang M, Boaden E, Arima H, Lavados PM, Billot L, Hackett ML, et al. Dysphagia screening and risks of pneumonia and adverse outcomes after acute stroke: An international multicenter study. Int J Stroke. (2020) 15:206–15. doi: 10.1177/1747493019858778
33. Sellars C, Bowie L, Bagg J, Sweeney MP, Miller H, Tilston J, et al. Risk factors for chest infection in acute stroke: a prospective cohort study. Stroke. (2007) 38:2284–91. doi: 10.1161/strokeaha.106.478156
34. Hoffmann S, Malzahn U, Harms H, Koennecke HC, Berger K, Kalic M, et al. Development of a clinical score (A2DS2) to predict pneumonia in acute ischemic stroke. Stroke. (2012) 43:2617–23. doi: 10.1161/strokeaha.112.653055
35. Mascanfroni I, Montesinos Mdel M, Susperreguy S, Cervi L, Ilarregui JM, Ramseyer VD, et al. Control of dendritic cell maturation and function by triiodothyronine. FASEB J. (2008) 22:1032–42. doi: 10.1096/fj.07-8652com
36. Rubingh J, van der Spek A, Fliers E, Boelen A. The role of thyroid hormone in the innate and adaptive immune response during infection. Compr Physiol. (2020) 10:1277–87. doi: 10.1002/cphy.c200003
37. Gigena N, Alamino VA, Montesinos MD, Nazar M, Louzada RA, Wajner SM, et al. Dissecting thyroid hormone transport and metabolism in dendritic cells. J endocrinology. (2017) 232:337–50. doi: 10.1530/joe-16-0423
38. Kalra L, Smith CJ, Hodsoll J, Vail A, Irshad S, Manawadu D. Elevated C-reactive protein increases diagnostic accuracy of algorithm-defined stroke-associated pneumonia in afebrile patients. Int J Stroke. (2019) 14:167–73. doi: 10.1177/1747493018798527
39. Forstner C, Patchev V, Rohde G, Rupp J, Witzenrath M, Welte T, et al. Rate and predictors of bacteremia in afebrile community-acquired pneumonia. Chest. (2020) 157:529–39. doi: 10.1016/j.chest.2019.10.006
40. Schwarz Y, Percik R, Oberman B, Yaffe D, Zimlichman E, Tirosh A. Sick euthyroid syndrome on presentation of patients with COVID-19: A potential marker for disease severity. Endocrine Pract. (2021) 27:101–9. doi: 10.1016/j.eprac.2021.01.001
41. Zou R, Wu C, Zhang S, Wang G, Zhang Q, Yu B, et al. Euthyroid sick syndrome in patients with COVID-19. Front endocrinology. (2020) 11:566439. doi: 10.3389/fendo.2020.566439
42. Suda S, Aoki J, Shimoyama T, Suzuki K, Sakamoto Y, Katano T, et al. Low free triiodothyronine at admission predicts poststroke infection. J stroke cerebrovascular Dis. (2018) 27:397–403. doi: 10.1016/j.jstrokecerebrovasdis.2017.09.012
43. Nam KW, Kim TJ, Lee JS, Kwon HM, Lee YS, Ko SB, et al. High neutrophil-to-lymphocyte ratio predicts stroke-associated pneumonia. Stroke. (2018) 49:1886–92. doi: 10.1161/strokeaha.118.021228
44. Li W, He C. Association of platelet-to-lymphocyte ratio with stroke-associated pneumonia in acute ischemic stroke. J healthcare engineering. (2022) 2022:1033332. doi: 10.1155/2022/1033332
45. Kannan L, Shaw PA, Morley MP, Brandimarto J, Fang JC, Sweitzer NK, et al. Thyroid dysfunction in heart failure and cardiovascular outcomes. Circ Heart failure. (2018) 11:e005266. doi: 10.1161/circheartfailure.118.005266
46. Huang M, Yang S, Ge G, Zhi H, Wang L. Effects of thyroid dysfunction and the thyroid-stimulating hormone levels on the risk of atrial fibrillation: A systematic review and dose-response meta-analysis from cohort studies. Endocrine Pract. (2022) 28:822–31. doi: 10.1016/j.eprac.2022.05.008
47. Wang L, Zhang Y. Role of hyperhomocysteine, thyroid dysfunction and their interaction in ischemic stroke patients with non-valvular atrial fibrillation. Sci Rep. (2020) 10:12419. doi: 10.1038/s41598-020-69449-2
48. Liu LM, Shen LS, Liu SY, Tu B, Li GL, Hu F, et al. Correlations between low thyroid function and incidence of atrial fibrillation in hypertrophic obstructive cardiomyopathy. Chronic Dis Trans Med. (2020) 6:35–45. doi: 10.1016/j.cdtm.2020.02.002
49. Floriani C, Gencer B, Collet TH, Rodondi N. Subclinical thyroid dysfunction and cardiovascular diseases: 2016 update. Eur Heart J. (2018) 39:503–7. doi: 10.1093/eurheartj/ehx050
50. Wagner C, MarChina S, Deveau JA, Frayne C, Sulmonte K, Kumar S. Risk of stroke-associated pneumonia and oral hygiene. Cerebrovascular Dis (Basel Switzerland). (2016) 41:35–9. doi: 10.1159/000440733
51. Perry SE, Huckabee ML, Tompkins G, Milne T. The association between oral bacteria, the cough reflex and pneumonia in patients with acute stroke and suspected dysphagia. J Oral rehabilitation. (2020) 47:386–94. doi: 10.1111/joor.12903
Keywords: euthyroid sick syndrome, ischemic stroke-associated pneumonia, nested case-control study, ischemic stroke, thyroid hormone
Citation: Yu S, Yan J, Logan R, Tang W-T, Ye J-N, Feng H-X, Wang M-X, Xu Q-R, Jiang X-L, Lin H-Y, Wu G-H, Gui Q and Duan T-T (2024) Euthyroid sick syndrome predicts the risk of ischemic stroke-associated pneumonia in the acute stage of ischemic stroke: a nested case-control study. Front. Endocrinol. 15:1438700. doi: 10.3389/fendo.2024.1438700
Received: 26 May 2024; Accepted: 23 October 2024;
Published: 11 November 2024.
Edited by:
Joseph V. Martin, Rutgers University Camden, United StatesReviewed by:
Jorge Francisco Gomez Cerezo, Universidad Europeas de Madrid, SpainXiaolei Li, University of Pennsylvania, United States
Copyright © 2024 Yu, Yan, Logan, Tang, Ye, Feng, Wang, Xu, Jiang, Lin, Wu, Gui and Duan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guan-Hui Wu, Z2h3dXN6MjZAbmptdS5lZHUuY24=; Qian Gui, Z3E4MjEwMTdAc2luYS5jb20=; Ting-Ting Duan, MTM1ODcwMDIyMzBAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Shuai Yu
Shuai Yu Jia Yan1†
Jia Yan1† Robert Logan
Robert Logan Hong-Xuan Feng
Hong-Xuan Feng Mei-Xia Wang
Mei-Xia Wang Guan-Hui Wu
Guan-Hui Wu