- 1Department of Endocrinology and Metabology, The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, Shandong First Medical University, Shandong Key Laboratory of Rheumatic Disease and Translational medicine, Shandong Institute of Nephrology, Jinan, China
- 2School of Clinical Medicine, Jining Medical University, Jining, China
- 3Institute for Literature and Culture of Chinese Medicine, Shandong University of Traditional Chinese Medicine, Jinan, China
- 4Department of Radiology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
Objective: Evaluate the effects of sodium-glucose cotransporter 2 inhibitor (SGLT2i) on cardiovascular and cerebrovascular diseases.
Methods: Articles of SGLT2i on cardiovascular and cerebrovascular diseases were searched. Two authors independently screened the literature, extracted the data, assessed the quality of the study and performed statistical analyses using Review Manager 5.4.
Results: Random-effect model was used to merge the OR values, and the pooled effect showed that SGLT2i had significant preventive effects on cardiovascular death (OR=0.76, 95%CI 0.64 to 0.89), myocardial infarction (OR=0.90, 95%CI 0.84 to 0.96), heart failure (OR=0.69, 95%CI 0.64 to 0.74) and all-cause mortality (OR=0.65, 95%CI 0.58 to 0.73). Empagliflozin, dapagliflozin and canagliflozin all reduced the incidence of heart failure (OR=0.72, 95%CI 0.64 to 0.82; OR=0.56, 95%CI 0.39 to 0.80; OR=0.62, 95%CI 0.53 to 0.73), but only dapagliflozin displayed a favorable effect on inhibiting stroke (OR=0.78, 95%CI 0.63 to 0.98). SGLT2i could prevent stroke (OR=0.86, 95%CI 0.75 to 0.99), heart failure (OR=0.63, 95%CI 0.56 to 0.70) and all-cause mortality (OR=0.64, 95%CI 0.57 to 0.72) compared to DPP-4i. Furthermore, SGLT2i could reduce the incidence of heart failure (OR=0.72, 95%CI 0.67 to 0.77) and cardiovascular death (OR=0.72, 95%CI 0.54 to 0.95) in patients with high-risk factors.
Conclusions: SGLT2i affects cardiovascular death, myocardial infarction, heart failure and all-cause mortality. Only dapagliflozin displayed a favorable effect on inhibiting stroke. SGLT2i could prevent stroke, heart failure and all-cause mortality compared to DPP-4i. In addition, SGLT2i significantly reduced the development of heart failure and cardiovascular death in patients with high-risk factors.
Systematic review registration: https://www.crd.york.ac.uk/prospero, identifier CRD42024532783.
1 Introduction
Diabetes mellitus is a class of metabolic diseases characterized by hyperglycemia. Type 2 diabetes caused by relative insulin deficiency or insulin resistance is prevalent in clinical practice. With the rapid development of the socio-economic conditions, the prevalence of type 2 diabetes has shown an increasing trend with each passing year. According to the study, there will be more than 640 million people with type 2 diabetes in 2024 (1). Hyperglycemia is often associated with disorders of lipid and protein metabolism, which induces and exacerbates oxidative stress and increases the risk of atherosclerotic vascular disease. Patients are highly susceptible to adverse outcomes such as cardiovascular disease, stroke or chronic renal insufficiency if they do not receive effective treatment at an early age (2–5). Cardiopathy and stroke are second only to cancer in terms of death and disability; the hyperglycemic state of the body results in a poor prognosis for cardiovascular disease. Currently, there is a limited range of antihyperglycemic agents (AHAs) available in the clinic and multiple drug loads may have adverse effects on the liver or kidney. So, it is crucial to choose a safe and effective class of glucose-lowering drugs.
Sodium-glucose cotransporter 2 inhibitor (SGLT2i) is a class of prescription drugs approved for the treatment of type 2 diabetes. SGLT2i reduces blood glucose without increasing the risk of hypoglycemia in patients with type 2 diabetes by blocking glucose and sodium reabsorption in renal proximal tubules (6). In addition, the mechanism of promoting urinary sodium excretion and diuresis by SGLT2i may allow it to decrease blood pressure and weight without increasing the heart rate, which has a preventive effect on the progression of atherosclerotic heart disease, heart failure or chronic kidney disease (6–9). Some findings suggested that SGLT2i could reduce the risk of stroke in Asian patients with type 2 diabetes (10); Zhou speculated that this favorable effect may be related to the reduction of atrial fibrillation/atrial flutter by SGLT2i (11). A meta-analysis found that although SGLT2i was more appropriate for type 2 diabetes patients who were at high risk of stroke compared to dipeptidyl peptidase 4 inhibitor (DPP-4i), the results of this study showed that SGLT2i did not reduce the risk of stroke (12). Therefore, we need to confirm the cardiovascular and cerebrovascular effects of SGLT2i in further clinical studies as well as to verify whether the effect is related to diseases or race/ethnicity.
Up to now, several clinical studies have reported the therapeutic effects of SGLT2i on cardiovascular and cerebrovascular diseases (10, 13–60); but the evidence needs to deepen due to the differences in search strategies, interventions, inclusion populations, sample sizes and other factors. In this study, we conducted a meta-analysis of clinical controlled trials on cardiovascular and cerebrovascular diseases with SGLT2i by systematically searching literature at home and abroad.
2 Materials and methods
2.1 Searching progress
We searched of the following databases: PubMed, Cochrane library and Sinomed for clinical controlled trials of SGLT2i on the effects of cardiovascular and cerebrovascular diseases. Reference lists of all eligible articles and related previous review articles were also manually searched. The literature search for this meta-analysis was restricted to published results. Databases were searched from the earliest data to 3 January 2024 with the search terms: ((SGLT2 inhibitors) OR (Sodium-Glucose Transporter 2 Inhibitors) OR (Sodium-glucose cotransporter-2 inhibitors) OR (Dapagliflozin) OR (Canagliflozin) OR (Empagliflozin) OR (Ipragliflozin) OR (Luseogliflozin) OR (Tofogliflozin)) AND ((acute cerebral infarction) OR (acute cerebral stroke) OR (ischemia stroke) OR (cerebral infarction)) AND ((cardiac failure) OR (acute cardiac failure) OR (heart failure) OR (acute heart failure) OR (cardiac insufficiency) OR (congestive cardiac failure) OR (congestive heart failure)) AND ((myocardial infarction) OR (acute myocardial infarction) OR (ST-segment elevation myocardial infarction) OR (ST elevated acute myocardial infarction) OR (non-ST-elevation myocardial infarction) OR (heart attack)).
Eligible studies were screened and selected based on the following criteria: (1) published in English or Chinese language; (2) evaluated the effect of SGLT2i intervention in cardiovascular and cerebrovascular diseases; (3) clinical controlled trial; (4) reported at least one outcome.
2.2 Study selection and data extraction
Two reviewers independently checked all titles and abstracts for studies that could potentially meet the inclusion criteria. We retrieved full reports of these potentially eligible studies for detailed assessment by two reviewers, who then independently extracted information on study design, drug use, study location, characteristics of participants, sample size and relevant outcomes on to a preformatted spreadsheet (10, 13–60). Any uncertainties or discrepancies between the two reviewers were resolved through consensus after rechecking of the source data and consultation with the third reviewer. We also contacted authors if any areas of uncertainty needed clarification.
2.3 Risk of bias in results of included studies
Two reviewers independently assessed the risk of bias in included studies to avoid conflicts of interest of study investigators or funders. Randomized controlled trials (RCTs) were evaluated using the revised version of the Cochrane tool, known as RoB 2. While cohort studies were evaluated using the Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) tool, which is recommended for assessing risk of bias in a non-randomized study of interventions (NRSI) (61, 62). The articles were evaluated separately by two reviewers and disagreements were settled by discussion.
2.4 Statistical analysis
The primary outcomes were the incident rate of stroke, cardiovascular death, myocardial infarction, heart failure or all-cause mortality. The secondary outcomes were the incident rate of ischemic stroke, acute coronary syndrome (ACS) or revascularization. Subgroup analyses were carried out according to differences in interventions and population characteristics. The fixed-model was performed by odds ratio (OR) and 95% confidence intervals (CI) for dichotomous variables. The I2 was calculated as an index of heterogeneity between studies. If a considerable heterogeneity exists, then the fixed-effects model is replaced by the random-effect model. The analyses were performed by Review Manager 5.4 (Cochrane Collaboration, United Kingdom, http://www.cochrane.org).
3 Results
3.1 Search results and characteristics of included studies
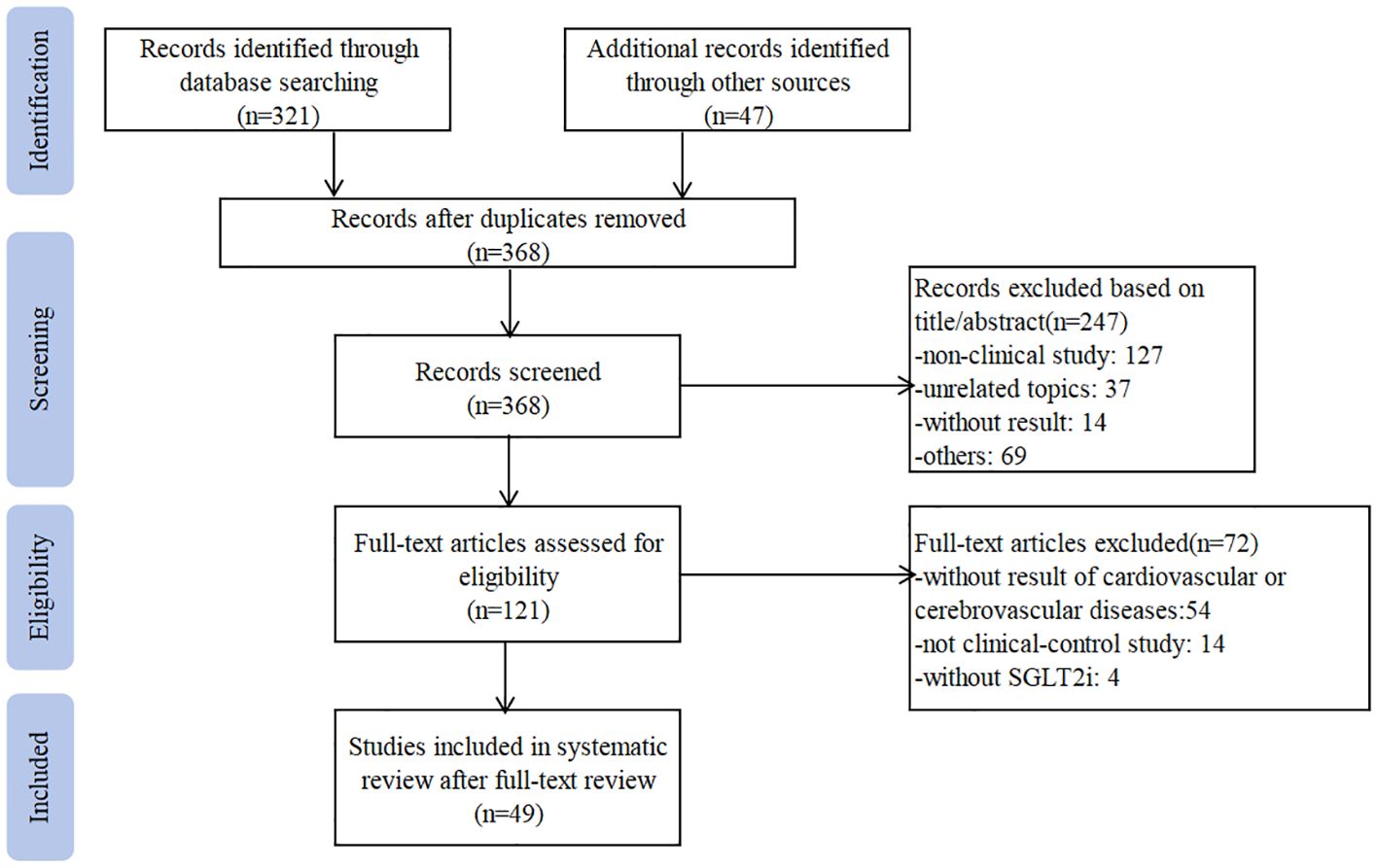
Our research yielded 368 articles in English or Chinese that were potentially relevant to this study. After screening the abstract, 121 articles were selected for full-text review. Of these, 49 articles were eligible and included in this meta-analysis (10, 13–60). Searching progress is shown in Figure 1. Nine of the included studies were RCT (14, 23, 40, 42–45, 48, 60), and the rest 40 trials were cohort studies. Nine trials were multi-center studies (14, 16, 23, 24, 31, 36, 40, 42, 43). Eight studies were published in Chinese (44–49, 59, 60), and the rest were published in English. There are 1270038 patients received SGLT2i treatment (dapagliflozin: 21145 patients (27, 36, 40, 41, 46, 47, 49, 60); empagliflozin: 110227 patients (20, 23, 27, 32, 39, 42, 43); canagliflozin:55950 patients (33, 44, 45, 48, 59) and 1339802 assigned to the control group (glucagon-like peptide 1 (GLP-1RA): 427963 patients (15, 17, 20, 28, 30, 33–35, 37, 39, 53); DPP-4i: 469049 patients (10, 18, 20–22, 26, 27, 31–33, 36, 50, 53, 55–58). The sample size ranges from 30 to 133139 in the SGLT2i treatment group and the control group. Due to the large sample size and complex population characteristics of this meta-analysis, the exact dosage and frequency of treatment regimens were unclear. Only eleven trials reported the precise time of follow-up, and ranged from 1 month to 2 years (16, 20, 29, 37, 44, 45, 48–50, 56, 60). The detailed characteristics of the included studies are summarized in Supplementary Table 1.
3.2 Risk of bias
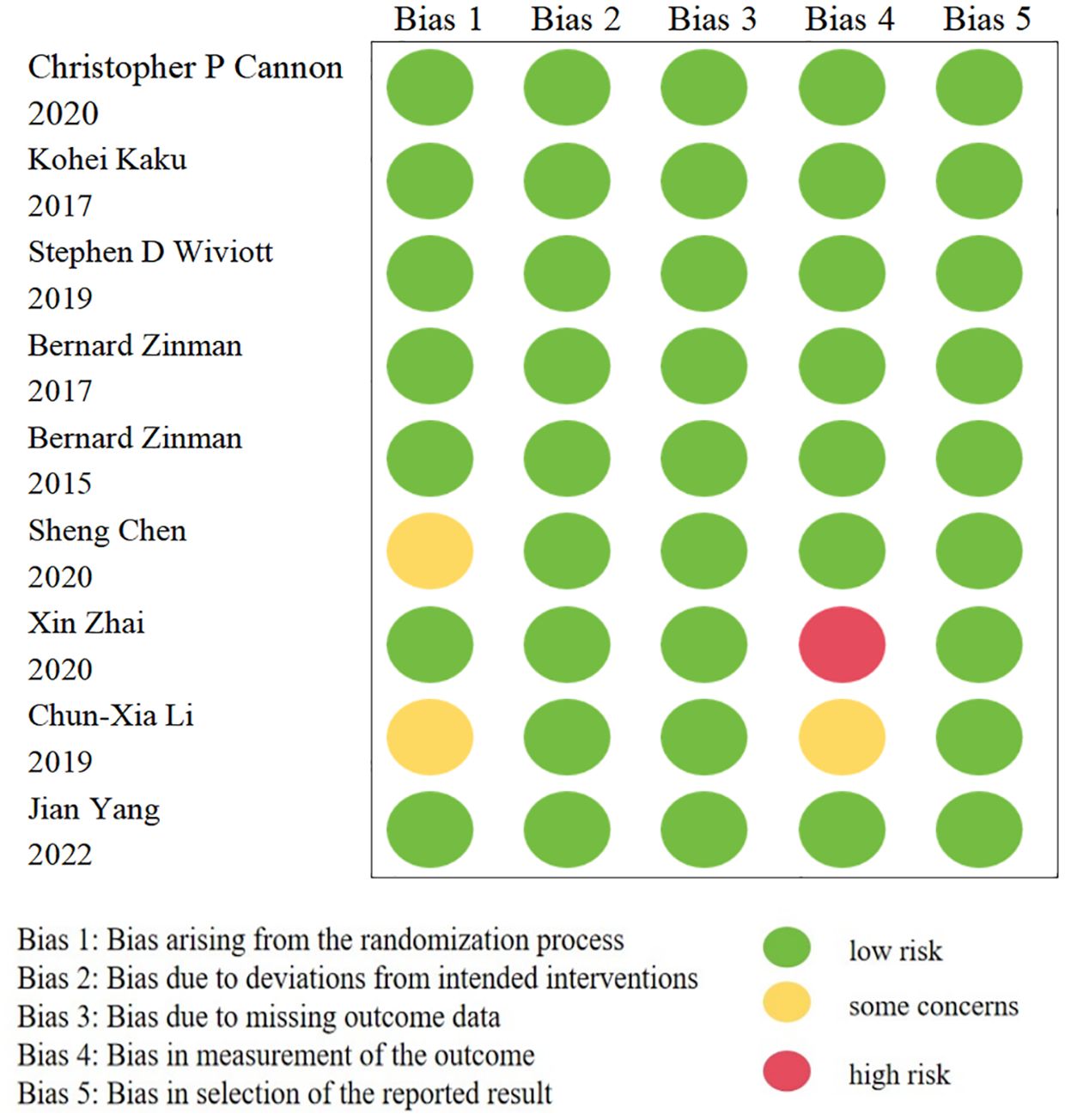
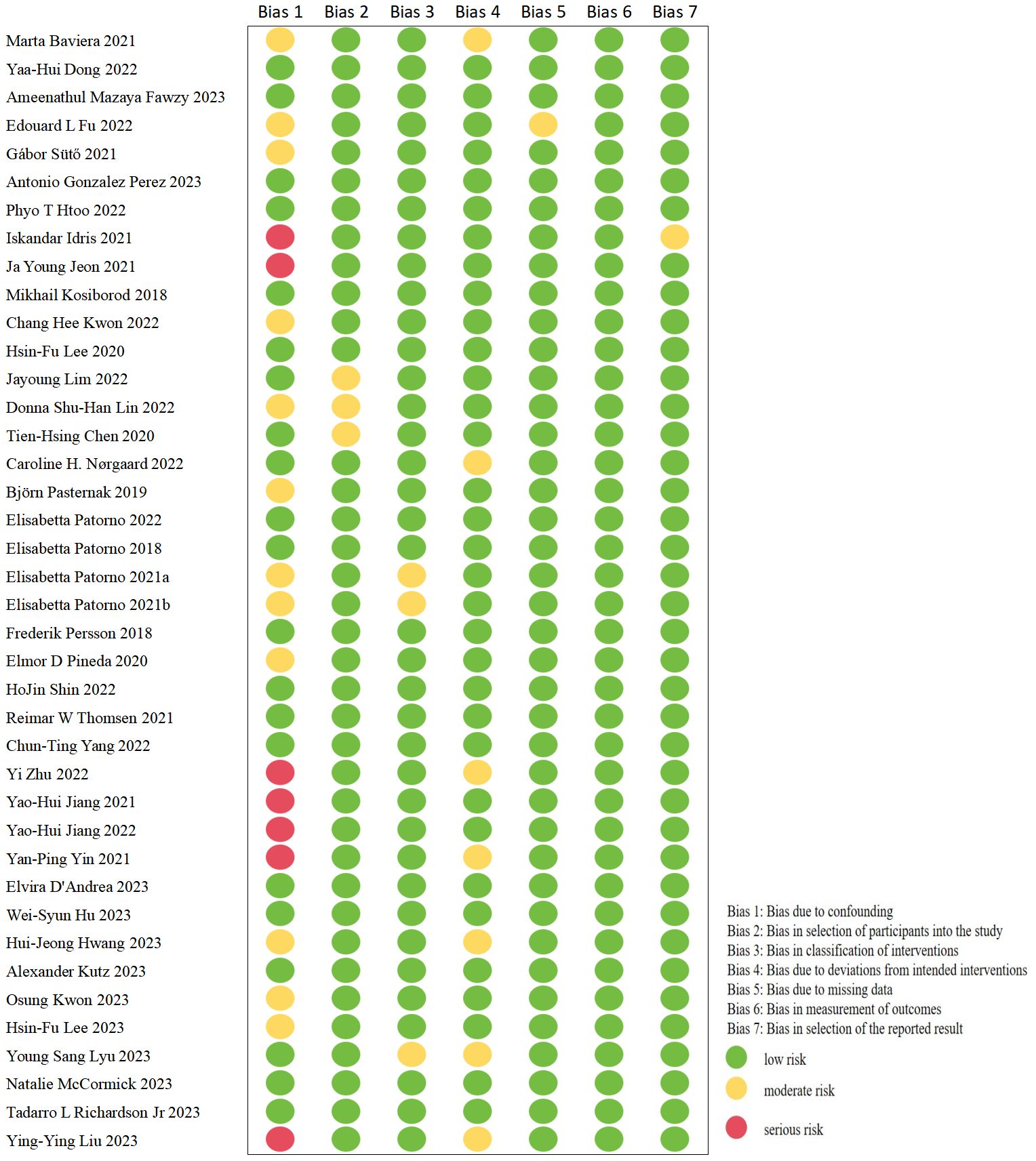
In this meta-analysis, only nine trials were RCTs (14, 23, 40, 42–45, 48, 60); one of which was found to be a high-risk trial after evaluating the quality of these studies with RoB 2 tool (45). The details are illustrated in Figure 2. The remaining cohort studies were evaluated in 7 dimensions for risk of bias using the ROBINS-I tool (10, 13, 15–22, 24–39, 41, 46, 47, 49–59). Figure 3 shows that 17 (42.5%) of the 40 papers had a low risk of bias (10, 15, 16, 19, 20, 24, 26, 32, 33, 36, 38, 39, 50, 51, 53, 57, 58), 16 (40%) had a medium risk of bias (13, 17, 18, 25, 27–31, 34, 35, 37, 52, 54–56) and 7 (17.5%) had a high risk of bias (21, 22, 41, 46, 47, 49, 59).
3.3 Main outcome
3.3.1 Incidence of stroke
Of these 49 included studies, 26 studies of SGLT2i with other AHAs reported the rate of incidence of stroke as an outcome (10, 14, 15, 18–24, 27, 30–33, 35–38, 41–43, 50, 56, 57, 59). A fixed-effect model was used for the pooled effect of these studies, which showed a significant heterogeneity (heterozygosity test, Chi2 = 76.74, P<0.00001, I2 = 67%). Then, we used the random-effect model for comparison, which showed that SGLT2i did not reduce the incidence of stroke (OR=0.92, 95%CI 0.83 to 1.01, P=0.07) (Figure 4).
3.3.2 Incidence of cardiovascular death
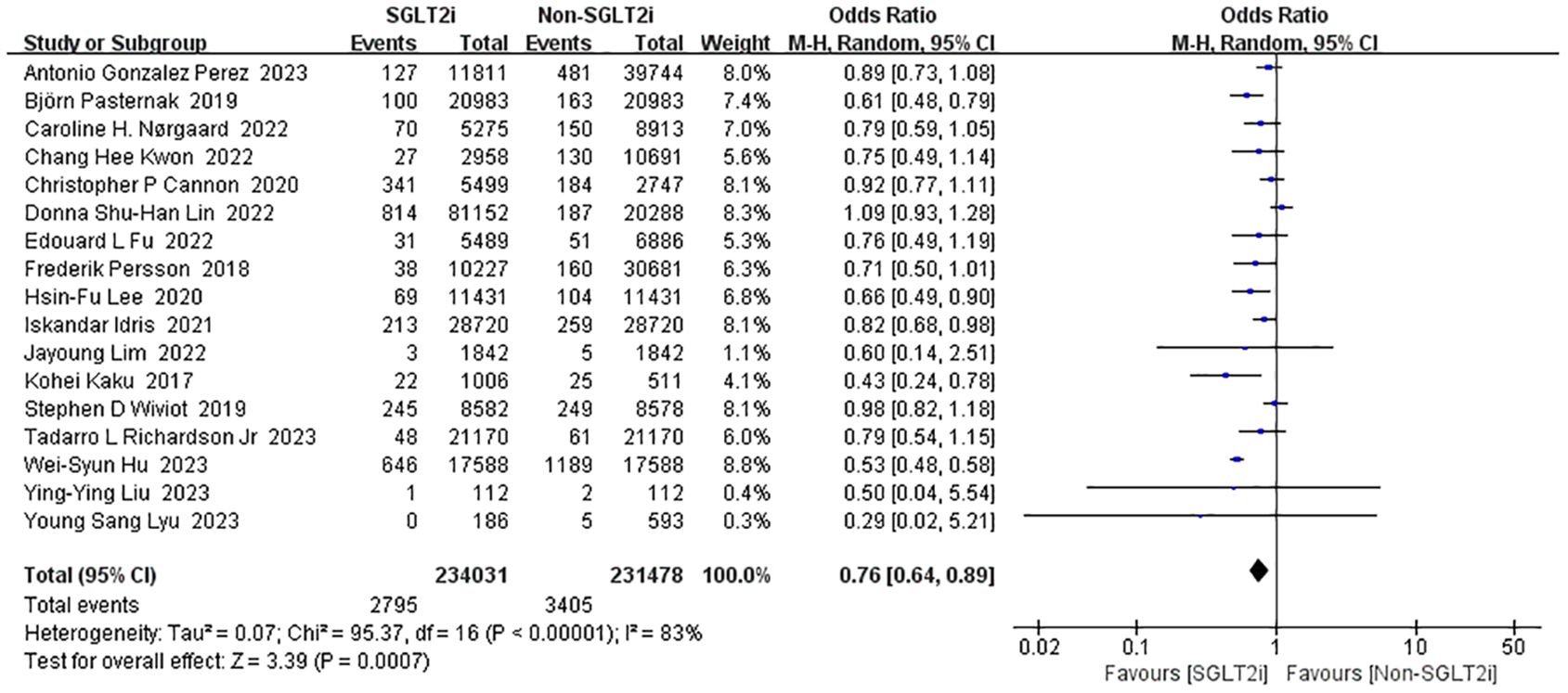
Seventeen studies reported the effect of SGLT2i intervention on cardiovascular death (14, 17, 19, 21, 23, 25–28, 30, 31, 36, 40, 51, 56, 58, 59). Analyses using the fixed-effect model showed enormous heterogeneity (heterozygosity test, Chi2 = 95.37, P<0.00001, I2 = 83%). So, the studies were instead analyzed using random-effect model and the merged OR value of the effect value was 0.76 (95%CI 0.64 to 0.89, P=0.0007) (Figure 5). Thus, the SGLT2i treatment group reduced the incidence of cardiovascular death compared to the non-SGLT2i control group.
3.3.3 Incidence of myocardial infarction
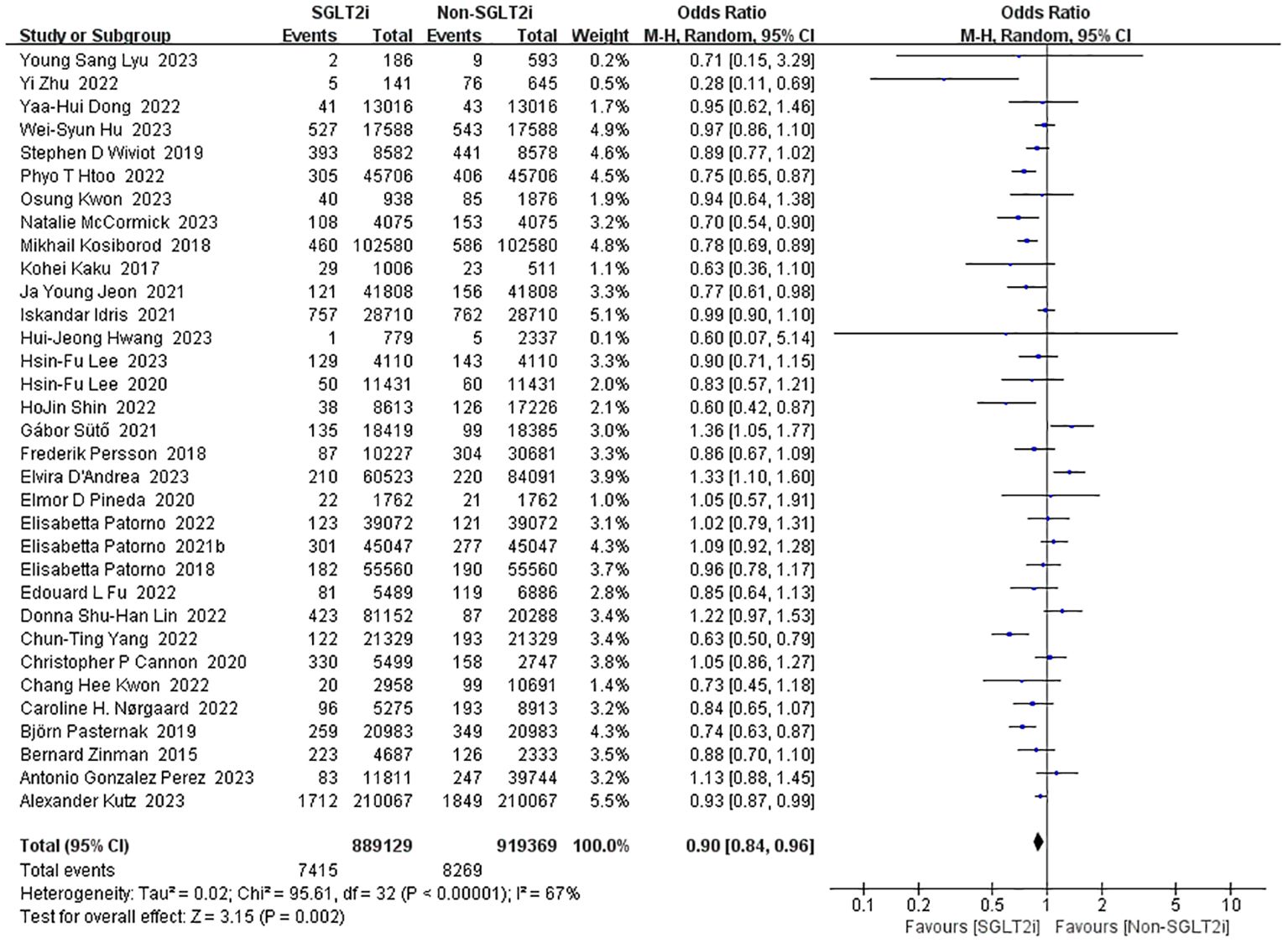
A total of 33 studies reported the incidence of myocardial infarction with SGLT2i intervention (10, 14, 15, 17–26, 28, 30–33, 35–38, 40, 41, 43, 50–57). Heterogeneity was significant in the fixed-effect model analysis of these studies (heterozygosity test, Chi2 = 95.61, P<0.00001, I2 = 67%), after that, a random-effect model was used and pooled effect value was 0.90 (95%CI 0.84 to 0.96, P=0.002) (Figure 6). Thus, SGLT2i could reduce the incidence of myocardial infarction.
3.3.4 Incidence of heart failure
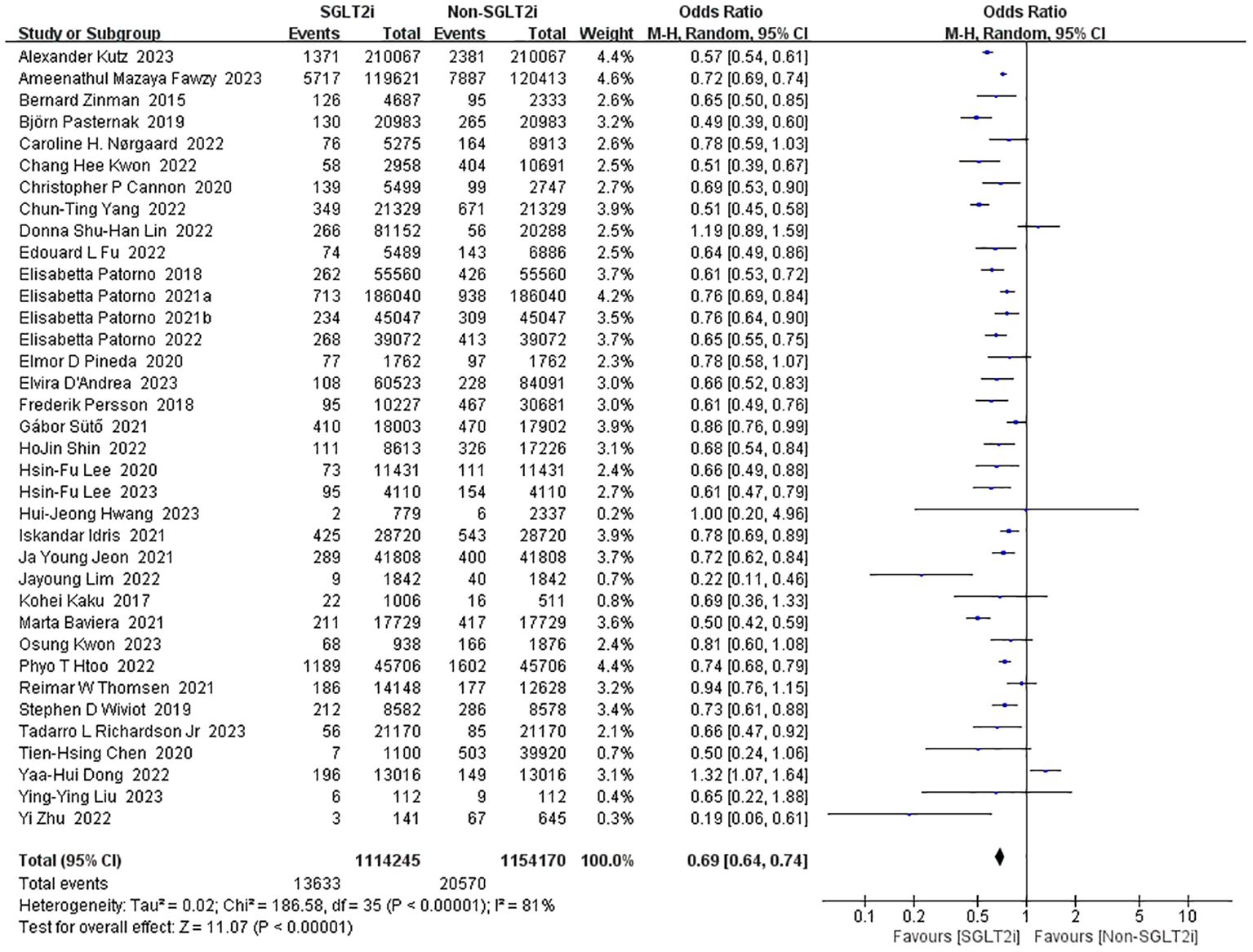
36 studies reported the incidence of heart failure (10, 13–18, 20–23, 25–41, 43, 50, 52–55, 58, 59). Heterogeneity analysis of these studies showed substantial heterogeneity (heterozygosity test, Chi2 = 186.58, P<0.00001, I2 = 81%). Therefore, the analysis was performed using the random-effect model with a pooled effect value of 0.69 (95%CI 0.64 to 0.74, P<0.00001) (Figure 7). It can be indicated that SGLT2i significantly reduced the occurrence of heart failure compared with non-SGLT2i.
3.3.5 Incidence of all-cause mortality
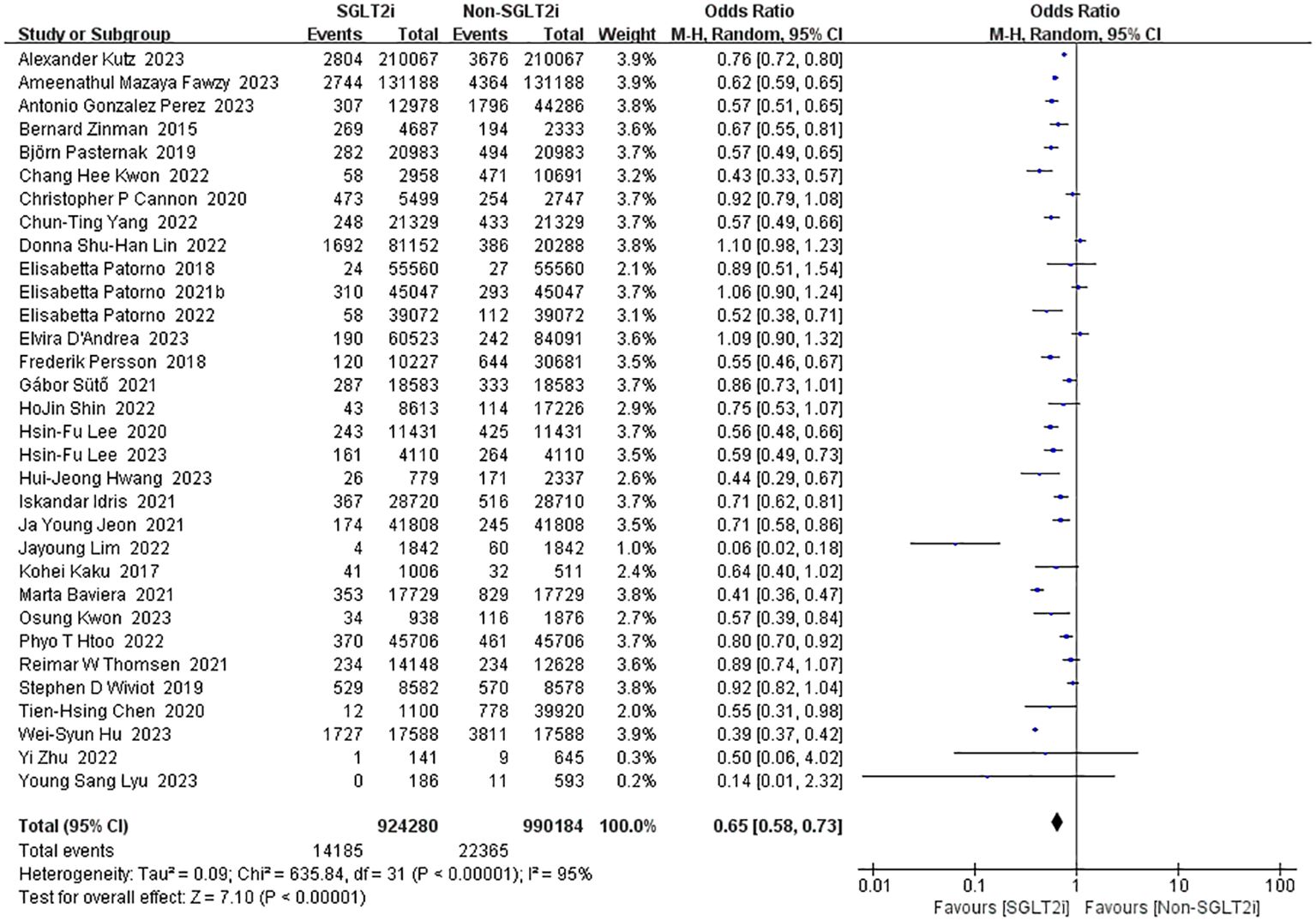
Among the intervention studies of SGLT2i, 32 studies reported all-cause mortality (10, 13, 14, 16, 18–23, 25–29, 31–33, 35, 36, 38–41, 43, 50–56). As there was substantial heterogeneity (heterozygosity test, Chi2 = 635.84, P<0.00001, I2 = 95%), pooled analyses using the random-effect model was instead which resulted in a favorable pooled effect value of 0.65 (95%CI 0.58 to 0.73, P<0.00001) for SGLT2i (Figure 8). In summary, SGLT2i could reduce all-cause mortality and improve survival.
3.4 Secondary outcome
3.4.1 Incidence of ischemic stroke
Fourteen studies reported the incidence of ischemic stroke between SGLT2i group and non-SGLT2i group (13, 15–17, 25, 26, 28, 29, 40, 51–55). Firstly, we pooled the OR value from these studies by fixed-effect model, as a result, a significant heterogeneity was found (heterozygosity test, Chi2 = 47.65, P<0.00001, I2 = 73%). Then the random-effect model was instead, and it was found that SGLT2i could not reduce ischemic stroke in patients (OR=0.95, 95%CI 0.87 to 1.05, P=0.32).
3.4.2 Incidence of revascularization
Only six studies reported the incidence of revascularization as an outcome (27, 33, 37, 43, 55, 56). A pooled analysis of outcome events from these studies using the fixed-effect model revealed tremendous heterogeneity of results (heterozygosity test, Chi2 = 23.96, P=0.0002, I2 = 79%). When analyzed using the random-effect model, the merged OR value was 0.85 (95%CI 0.65 to 1.11, P=0.23). In conclusion, SGLT2i did not reduce the occurrence of revascularization.
3.4.3 Incidence of acute coronary syndrome
Events of ACS in patients with SGLT2i have been reported in four studies (13, 27, 29, 59). However, a significantly and huge heterogeneity was found by fixed-effect model (heterozygosity test, Chi2 = 19.53, P=0.0002, I2 = 85%), then a random-effect model was used and pooled OR value was 0.98(95%CI 0.86 to 1.12, P=0.77). This means that SGLT2i is not beneficial in preventing the development of ACS. Detailed data for the above secondary endpoints are shown in Figure 9.
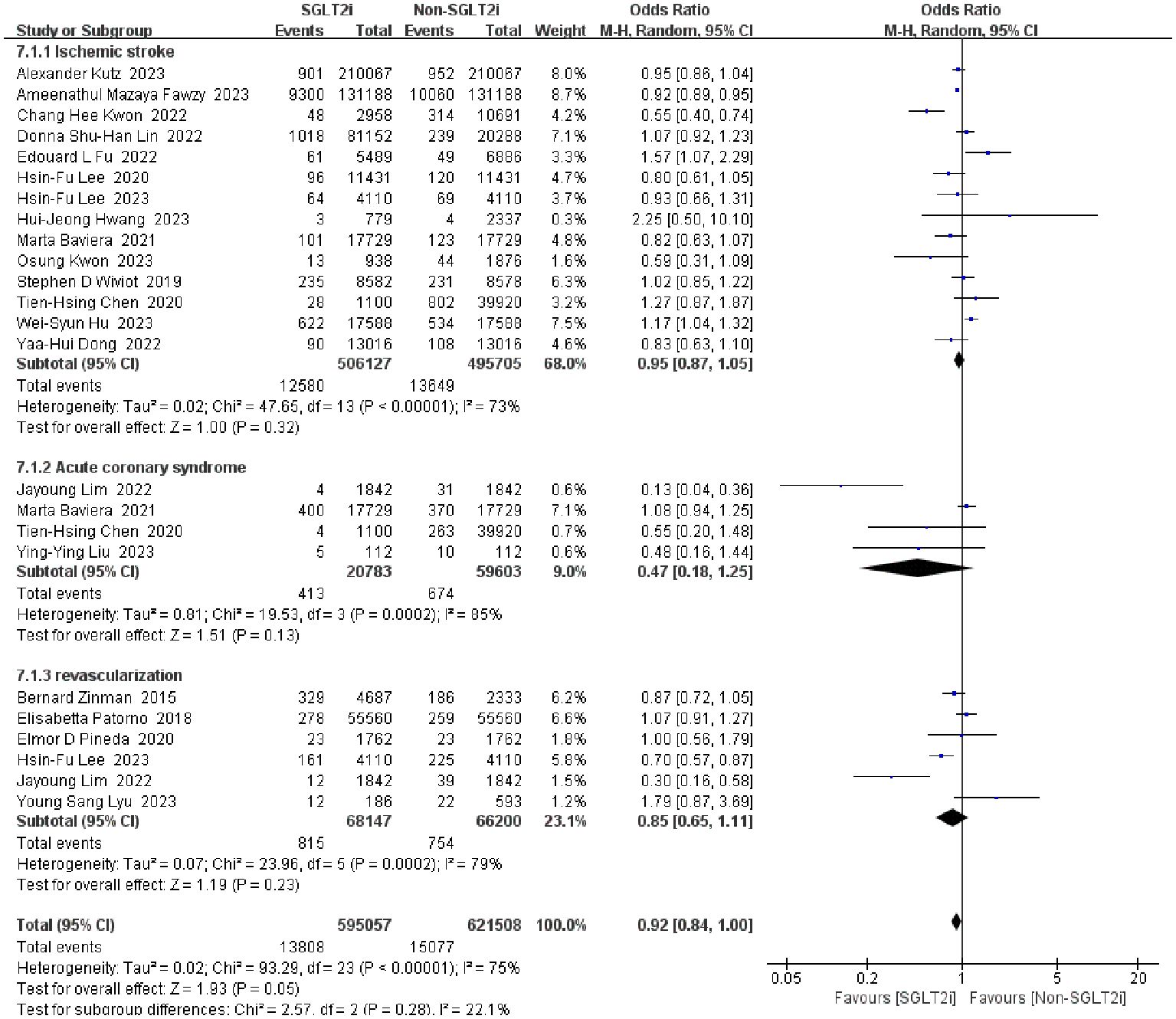
Figure 9. Forest plot of the incidence of secondary outcomes (ischemic stroke, revascularization, ACS).
3.5 Subgroup analyses
3.5.1 Incidence of cardiovascular and cerebrovascular diseases under different interventions
Seven studies analyzed the effect of empagliflozin on cardiovascular and cerebrovascular diseases (20, 23, 27, 32, 39, 42, 43); eight studies analyzed the effect of dapagliflozin on it (27, 36, 40, 41, 46, 47, 49, 60); and only five studies analyzed the effect of canagliflozin on it (33, 44, 45, 48, 59). Appropriate effect models were selected based on the magnitude of heterogeneity. Pooling these studies about different types of SGLT2i revealed that dapagliflozin prevented stroke (OR=0.78, 95%CI 0.63 to 0.98, P=0.03), myocardial infarction (OR=0.83, 95%CI 0.74 to 0.93, P=0.002), heart failure (OR=0.56, 95%CI 0.39 to 0.80, P=0.002), and all-cause mortality (OR=0.50, 95%CI 0.30 to 0.82, P=0.006). At the same time, empagliflozin reduced the incidence of myocardial infarction (OR=0.82, 95%CI 0.73 to 0.91, P=0.0003), heart failure (OR=0.72, 95% CI 0.64 to 0.82, P<0.00001), and all-cause mortality (OR=0.68, 95%CI 0.55 to 0.84, P=0.0004); canagliflozin only had a positive effect on the occurrence of heart failure (OR=0.56, 95%CI 0.39 to 0.80, P=0.002).
Eleven studies reported the therapeutic effects of SGLT2i on cardiovascular and cerebrovascular diseases compared with GLP-1RA. These studies reported four diseases (including stroke, myocardial infarction, heart failure and all-cause mortality) and the details on the occurrence of each disease were provided (15, 17, 20, 28, 30, 33–35, 37, 39, 53). It was found that SGLT2i only had a significant preventive effect on heart failure (OR=0.83, 95%CI 0.74 to 0.93, P=0.002) compared to GLP-1RA.
Four diseases (including stroke, myocardial infarction, heart failure and all-cause mortality) were reported in seventeen studies (10, 18, 20–22, 26, 27, 31–33, 36, 50, 53, 55–58). What a pity, a considerable heterogeneity was found in all four subgroups by the fixed-effect model. Finally, a random-effect model was used and pooled OR value was 0.86 (95%CI 0.75 to 0.99, P=0.04) in the subset of stroke, 0.63 (95%CI 0.56 to 0.70, P<0.00001) in the subset of heart failure, and 0.64 (95%CI 0.57 to 0.72, P<0.00001) in the subset of all-cause mortality.
The details of the above are shown in Table 1. Summarily, in different types of SGLT2i, empagliflozin, dapagliflozin and canagliflozin all reduced the incident rate of heart failure, but only dapagliflozin could reduce the incident rate of stroke. Compared with DPP-4i, SGLT2i had a positive therapeutic effect on stroke, heart failure and all-cause mortality; however, compared with GLP-1RA, it only had a positive impact on heart failure.
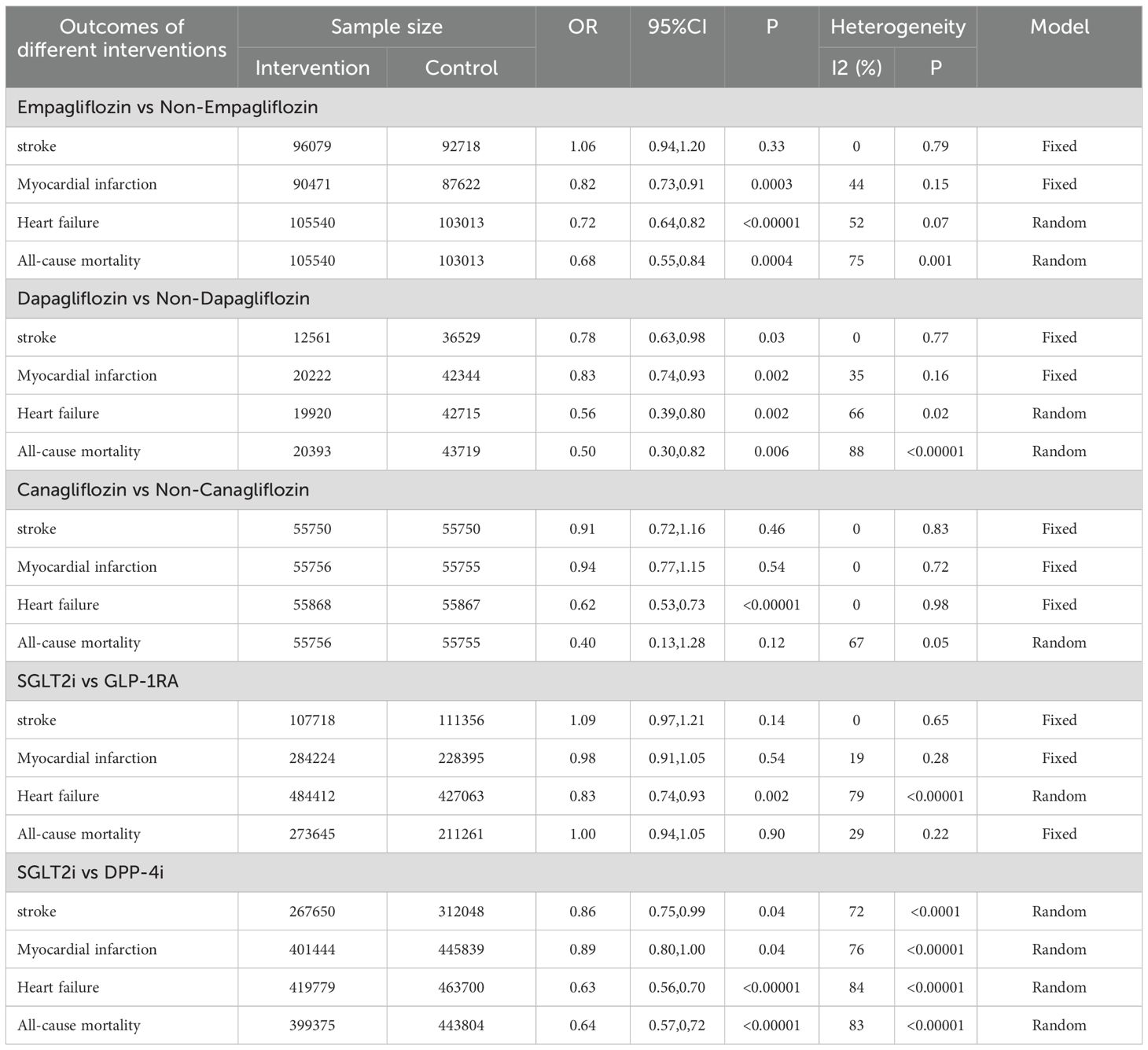
Table 1. The incidence of cardiovascular and cerebrovascular diseases in different intervention measures.
3.5.2 Incidence of cardiovascular and cerebrovascular diseases in different characteristics of patients
Furthermore, fifteen studies explicitly stated whether the subjects had cardiovascular and cerebrovascular diseases or were at other high risk (14, 15, 21, 23, 25, 27, 34, 40, 42, 43, 51, 54–56, 59). Firstly, four outcomes (including stroke, myocardial infarction, heart failure and cardiovascular death) in these studies were analyzed by using the fixed-effect model. However, some significantly and huge heterogeneity were found, then appropriate effect models were selected based on the magnitude of heterogeneity. It was found that SGLT2i demonstrated significant benefits in heart failure (OR=0.72, 95%CI 0.67 to 0.77, P<0.00001) and cardiovascular death (OR=0.72, 95%CI 0.54 to 0.95, P=0.02) in high-risk patients (Table 2).
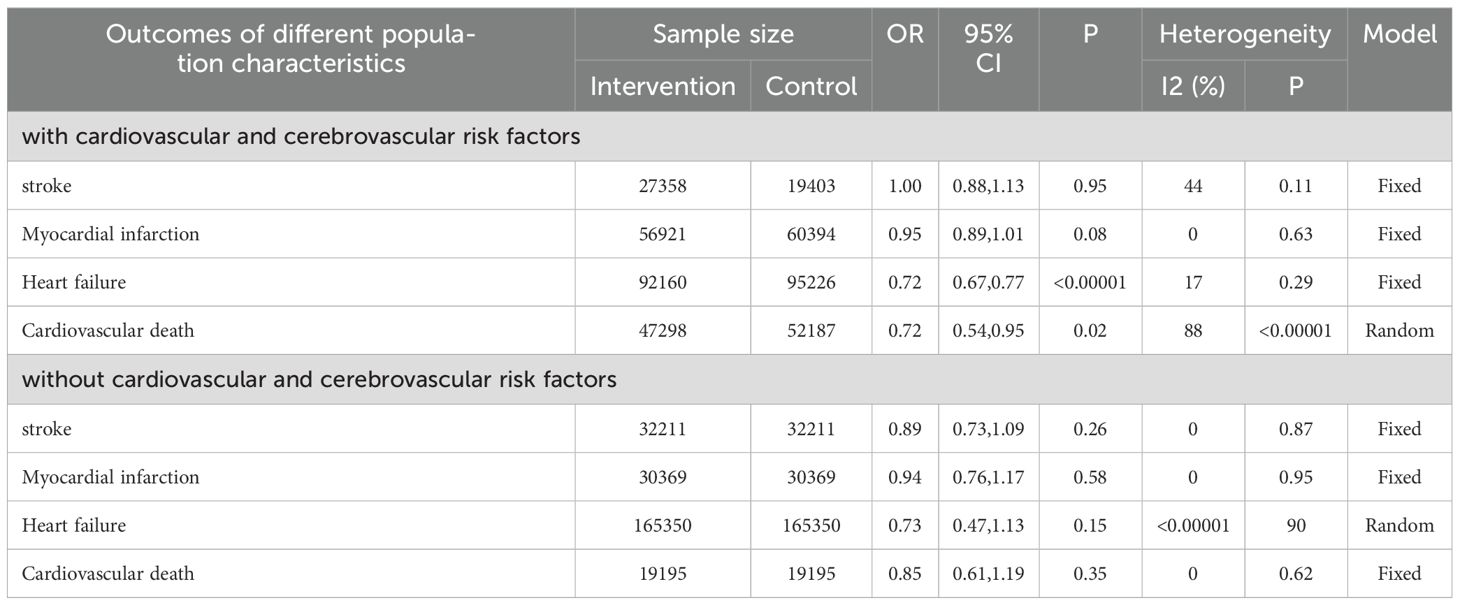
Table 2. The incidence of cardiovascular and cerebrovascular diseases in different characteristics of patients.
3.6 Publication bias
Funnel plot was done to show the publication bias and results were shown in Figures 10, 11. Because of the complexity of population characteristics included in the study and the large gaps in sample sizes, some of the graphs show asymmetry; that is, there is publication bias.
Figure 10. Funnel plot of publication bias on main and secondary outcomes. (A) Funnel plot of publication bias on stroke; (B) Funnel plot of publication bias on cardiovascular death; (C) Funnel plot of publication bias on myocardial Infarction; (D) Funnel plot of publication bias on heart failure; (E) Funnel plot of publication bias on all-cause mortality; (F) Funnel plot of publication bias on secondary outcomes.
Figure 11. Funnel plot of publication bias on subgroup analysis. (A) Funnel plot of publication bias on subgroup analysis of Empagliflozin; (B) Funnel plot of publication bias on subgroup analysis of Empagliflozin Dapagliflozin; (C) Funnel plot of publication bias on subgroup analysis of Canagliflozin; (D) Funnel plot of publication bias on subgroup analysis of SGLT2i VS GLP-1RA; (E) Funnel plot of publication bias on subgroup analysis of SGLT2i VS DPP-4i; (F) Funnel plot of publication bias on subgroup analysis who were at high risk; (G) Funnel plot of publication bias on subgroup analysis who were not at high risk.
4 Discussion
SGLT2i is a new class of insulin-independent drug for type 2 diabetes, which acts highly selectively on renal proximal tubules to block glucose reabsorption and increase the elimination of excess glucose from the body (63). In order to clarify the intervention effect of SGLT2i on cardiovascular and cerebrovascular diseases, researchers have prepared and conducted several clinical trials. EMPA-REG OUTCOME was a multi-center prospective study in which investigators found that the empagliflozin group could significantly reduce the risk of major adverse cardiovascular events in type 2 diabetes patients who were at high risk compared to the placebo group after following up for mean 3.1 years (23). This finding eventually caused SGLT2i was recommended by the American Diabetes Association and the European Association for the Study of Diabetes for the treatment of high-risk type 2 diabetes patients who suffer from arteriosclerotic cardiovascular disease (64). Current studies have found that SGLT2i does not reduce the incidence of stroke (42), and to some extent, it even increases the risk of ischemic stroke (65). The results of our study showed that SGLT2i does have great advantages in the prevention of cardiovascular and cerebrovascular diseases: SGLT2i could significantly reduce the incidence of myocardial infarction, heart failure, cardiovascular death and all-cause mortality; in subgroup analyses, the risk of heart failure was seen to be decreased by SGLT2i regardless of the type of SGLT2i; furthermore, in high-risk patients, SGLT2i exerted a positive effect in preventing the occurrence of heart failure and cardiovascular death. It was interesting to note that although SGLT2i reduced the risk of stroke compared to DPP-4i, but it had no preventative effect on the occurrence of stroke or ischemic stroke when comparing to non-SGLT2i.
At present, the mechanism of SGLT2i intervention in cardiovascular and cerebrovascular diseases is still being discovered and improved. Hemodynamic optimization and renal effect were thought to be the main two mechanisms (23, 66). Osmotic diuresis by SGLT2i reduces blood volume and cardiac load; in turn, sodium excretion decreases intraglomerular pressure by activating tubuloglomerular feedback (23, 67). On the other hand, SGLT2i has been shown to enhance endothelial cell function by reducing inflammation and oxidative stress (41, 68, 69), thereby improving coronary blood flow and myocardial energy metabolism (70). In contrast, SGLT2i is not as effective for stroke. Hypovolemia and elevated hematocrit from osmotic diuresis may be associated with an increased risk of stroke (42, 43), which seems to be a plausible explanation given that a meta-analysis has found that upright hypotension increases the risk of stroke (71).
GLP-1RA enhances insulin secretion by activating the GLP-1 receptor and inhibits glucagon secretion. It is able to delay gastric emptying and reduce the amount of food intake through central appetite suppression, ultimately achieving the effects of lowering blood glucose and body weight (72). In recent years, with the in-depth studies of the drug, researchers have found that in addition to its hypoglycemic and weight-loss effects, it can improve mitochondrial dysfunction, reduce inflammatory mediators and leukocyte-endothelial interactions, which can prevent the onset and progression of atherosclerosis (73). DPP-4i promotes insulin release from pancreatic beta cells by reducing the inactivation of glucagon-producing polypeptide (74). A large number of studies have been conducted on the comparative clinical efficacy of these three classes of drugs, but the conclusions are conflicting (13, 17, 22, 30, 34, 36, 75). In addition, studies have found that empagliflozin improves sympathetic nerve activity and is more favorable for glycemic control and management of cardiometabolic parameters (76, 77); while dapagliflozin shows more benefits in heart failure (78, 79). In previous studies, investigators have found some heterogeneity in outcome comparisons, which depending on the presence of chronic cardiac and renal diseases in patients before inclusion in the study. With the above in mind, this study conducted a number of subgroup analyses to further analyze the clinical effects of SGLT2i from multiple perspectives.
Although a large number of articles have been published on the topic of SGLT2i and cardiovascular diseases, there are some unique aspects of our work. In this study, we added the keyword “stroke” to focus more on the cerebrovascular diseases which are controversial. We included more studies and larger sample than others, and got more results, what is a supplement to the previous meta-analysis. Patients with type 2 diabetes often have multiple comorbidities, such as microvascular disease and renal disease, which has led to high-risk bias when combining statistics. Therefore, researchers should design and carry out trials with high selectivity, high accuracy, rigorous design and large sample size, and conduct in-depth mechanism exploration to provide a more reliable basis for the application of SGLT2i.
The major limitation of this meta-analysis is the complex and diverse population characteristics of the included studies which may induce a racial heterogeneity. Secondly, among the 49 studies, only nine RCTs and the rest trials were cohort studies, this may lead to a reduction in the methodological quality of clinical controlled studies. Furthermore, when analyzing some results, there was a significant heterogeneity and publication bias due to the small number of included studies and the complexity of population characteristics. Therefore, more prospective clinical studies with a larger sample size may strengthen the evidence.
5 Conclusions
In conclusion, our meta-analysis summarized the efficacy of SGLT2i in cardiovascular and cerebrovascular diseases. The incidence of cardiovascular death, myocardial infarction, heart failure and all-cause mortality was reduced with the use of SGLT2i, but no significant preventive effect was seen for the occurrence of stroke, ischemic stroke, acute coronary syndrome and revascularization. Subgroup analyses showed that the different types of SGLT2i reduced the incidence of heart failure, but only dapagliflozin reduced the incident rate of stroke. SGLT2i had a positive preventive effect on the incidence of stroke, heart failure and all-cause mortality compared to DPP-4i. Furthermore, SGLT2i significantly reduced heart failure and cardiovascular mortality in patients who were at high risk. Further, more studies focusing on the mechanism still needs to be done.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
FW: Data curation, Formal analysis, Software, Visualization, Writing – original draft. CL: Data curation, Formal analysis, Writing – original draft. LC: Data curation, Investigation, Writing – original draft. SG: Data curation, Software, Writing – original draft. JZ: Funding acquisition, Project administration, Supervision, Writing – review & editing. HW: Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by the National Natural Science Foundation of China (grant number 82303031), Shandong Provincial Natural Science Foundation of China Grants (grant number ZR2019PH025), Projects of medical and health technology development program in Shandong province (grant number 2016WS0499), Bethune Public Welfare Foundation (grant number Z04JKM2022E036).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1436217/full#supplementary-material
References
1. Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, et al. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Practice. (2017) 128:40–50. doi: 10.1016/j.diabres.2017.03.024
2. Shao HR, Wang ZJ, Shi XF, Yan JC, Yuan W, Li WD. Prognostic impact the time to PCI of non-infarct-related vessels in patients with acute myocardial infarction. Zhongguo Dong Mai Ying Hua Za Zhi. (2020) 28:147–53.
3. Wang J, Xu HB, Zhang HP, Chen JL, Qiao SB, Hu FH, et al. Impact of type 2 diabetes mellitus on the progression and revascularization of coronary non-target lesions in patients with coronary heart disease. Zhonghua Xin Xue Guan Bing Za Zhi. (2020) 48:393–400. doi: 10.3760/cma.j.cn112148-20190425-00204
4. Ahmad FS, Ning H, Rich JD, Yancy CW, Lloyd-Jones DM, Wilkins JT. Hypertension, obesity, diabetes, and heart failure-free survival: the cardiovascular disease lifetime risk pooling project. JACC Heart Failure. (2016) 4:911–9. doi: 10.1016/j.jchf.2016.08.001
5. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Colvin MM, et al. ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the american college of cardiology/american heart association task force on clinical practice guidelines and the heart failure society of america. Circulation. (2017) 136:e137–61. doi: 10.1161/CIR.0000000000000509
6. Zelniker TA, Braunwald E. Cardiac and renal effects of sodium-glucose co-transporter 2 inhibitors in diabetes: JACC state-of-the-art review. J Am Coll Cardiol. (2018) 72:1845–55. doi: 10.1016/j.jacc.2018.06.040
7. Zelniker TA, Braunwald E. Treatment of heart failure with sodium-glucose cotransporter 2 inhibitors and other anti-diabetic drugs. Card Fail Rev. (2019) 5:27–30. doi: 10.15420/cfr
8. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. (2019) 380:2295–306. doi: 10.1056/NEJMoa1811744
9. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet (London England). (2019) 393:31–9. doi: 10.1016/S0140-6736(18)32590-X
10. Yang CT, Peng ZY, Chen YC, Ou HT, Kuo S. Cardiovascular benefits with favorable renal, amputation and hypoglycemic outcomes of SGLT-2 inhibitors in type 2 diabetes from the asian perspective: A population-based cohort study and systematic review. Circulation. (2022) 13:836365. doi: 10.3389/fendo.2022.836365
11. Zhou Z, Jardine MJ, Li Q, Neuen BL, Cannon CP, de Zeeuw D, et al. Effect of SGLT2 inhibitors on stroke and atrial fibrillation in diabetic kidney disease: results from the CREDENCE trial and meta-analysis. Stroke. (2021) 52:1545–56. doi: 10.1161/STROKEAHA.120.031623
12. Dave CV, Kim SC, Goldfine AB, Glynn RJ, Tong A, Patorno E. Risk of cardiovascular outcomes in patients with type 2 diabetes after addition of SGLT2 inhibitors versus sulfonylureas to baseline GLP-1RA therapy. Circulation. (2021) 143:770–9. doi: 10.1161/CIRCULATIONAHA.120.047965
13. Baviera M, Genovese S, Lepore V, Colacioppo P, Robusto F, Tettamanti M, et al. Lower risk of death and cardiovascular events in patients with diabetes initiating glucagon-like peptide-1 receptor agonists or sodium-glucose cotransporter-2 inhibitors: A real-world study in two Italian cohorts. Diabetes Obes Metab. (2021) 23(7):1484–95. doi: 10.1111/dom.14361
14. Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. (2020) 383:1425–35. doi: 10.1056/NEJMoa2004967
15. Dong YH, Chang CH, Lin JW, Yang WS, Wu LC, Toh S. Comparative cardiovascular effectiveness of glucagon-like peptide-1 receptor agonists versus sodium-glucose cotransporter-2 inhibitors in patients with type 2 diabetes: A population-based cohort study. Diabetes Obes Metab. (2022) 24:1623–37. doi: 10.1111/dom.14741
16. Fawzy AM, Rivera-Caravaca JM. Incident heart failure, arrhythmias and cardiovascular outcomes with sodium-glucose cotransporter 2 (SGLT2) inhibitor use in patients with diabetes: Insights from a global federated electronic medical record database. Diabetes Obes Metab. (2023) 25(2):602–10. doi: 10.1111/dom.14854
17. Fu EL, Clase CM, Janse RJ, Lindholm B, Dekker FW, Jardine MJ, et al. Comparative effectiveness of SGLT2i versus GLP1-RA on cardiovascular outcomes in routine clinical practice. Int J Cardiol. (2022) 352:172–9. doi: 10.1016/j.ijcard.2022.01.042
18. Gábor Sütő GAM, Rokszin G, Fábián I, Kiss Z, Szekanecz Z, Poór G, et al. Risk of morbidity and mortality in patients with type 2 diabetes treated with sodium-glucose cotransporter-2 inhibitor and/or dipeptidyl peptidase-4 inhibitor: a nationwide study. BMJ Open Diabetes Res Care. (2021) 9:e001765. doi: 10.1136/bmjdrc-2020-001765
19. Gonzalez Perez A, Vizcaya D, Sáez ME, Lind M, Garcia Rodriguez LA. Cardiovascular and renal outcomes among patients with type 2 diabetes using SGLT2 inhibitors added to metformin: a population-based cohort study from the UK. BMJ Open Diabetes Res Care. (2023) 11:e003072. doi: 10.1136/bmjdrc-2022-003072
20. Htoo PT, Tesfaye H, Schneeweiss S, Wexler DJ, Everett BM, Glynn RJ, et al. Comparative effectiveness of empagliflozin vs liraglutide or sitagliptin in older adults with diverse patient characteristics. JAMA Network Open. (2022) 5:e2237606. doi: 10.1001/jamanetworkopen.2022.37606
21. Idris I, Zhang R, Mamza JB, Ford M, Morris T, Banerjee A, et al. Lower risk of hospitalization for heart failure, kidney disease and death with sodium-glucose co-transporter-2 inhibitors compared with dipeptidyl peptidase-4 inhibitors in type 2 diabetes regardless of prior cardiovascular or kidney disease: A retrospective cohort study in UK primary care. Diabetes Obes Metab. (2021) 23(10):2207–14. doi: 10.1111/dom.14437
22. Jeon JY, Ha KH, Kim DJ. Cardiovascular safety of sodium glucose cotransporter 2 inhibitors as add-on to metformin monotherapy in patients with type 2 diabetes mellitus. Diabetes Metab J. (2021) 45:505–14. doi: 10.4093/dmj.2020.0057
23. Kaku K, Lee J, Mattheus M, Kaspers S, George J, Woerle HJ. Empagliflozin and cardiovascular outcomes in asian patients with type 2 diabetes and established cardiovascular disease - results from EMPA-REG OUTCOME(®). Circ J: Off J Japanese Circ Society. (2017) 81:227–34. doi: 10.1253/circj.CJ-16-1148
24. Kosiborod M, Birkeland KI, Cavender MA, Fu AZ, Wilding JP, Khunti K, et al. Rates of myocardial infarction and stroke in patients initiating treatment with SGLT2-inhibitors versus other glucose-lowering agents in real-world clinical practice: Results from the CVD-REAL study. Diabetes Obes Metab. (2018) 20:1983–7. doi: 10.1111/dom.13299
25. Kwon CH, Kim YJ, Kim MJ, Cha MJ, Cho MS, Nam GB, et al. Effect of sodium-glucose cotransporter inhibitors on major adverse cardiovascular events and hospitalization for heart failure in patients with type 2 diabetes mellitus and atrial fibrillation. Am J Cardiol. (2022) 178:35–42. doi: 10.1016/j.amjcard.2022.05.017
26. Lee HF, Chen SW, Liu JR, Li PR, Wu LS, Chang SH, et al. Major adverse cardiovascular and limb events in patients with diabetes and concomitant peripheral artery disease treated with sodium glucose cotransporter 2 inhibitor versus dipeptidyl peptidase-4 inhibitor. Cardiovasc Diabetol. (2020) 19:160. doi: 10.1186/s12933-020-01118-0
27. Lim J, Hwang IC. Comparison of cardiovascular and renal outcomes between dapagliflozin and empagliflozin in patients with type 2 diabetes without prior cardiovascular or renal disease. PLoS One. (2022) 17(10):e0269414. doi: 10.1371/journal.pone.0269414
28. Lin DS, Yu AL, Lo HY, Lien CW, Lee JK, Chen WJ. Major adverse cardiovascular and limb events in people with diabetes treated with GLP-1 receptor agonists vs SGLT2 inhibitors. Am J Cardiovasc Drugs: Drugs Devices Other Interventions. (2022) 65:2032–43. doi: 10.1007/s00125-022-05772-9
29. Malinowski B, Chen TH, Li YR, Chen SW, Lin YS, Sun CC, et al. Sodium-glucose cotransporter 2 inhibitor versus metformin as first-line therapy in patients with type 2 diabetes mellitus: a multi-institution database study. Pharm (Basel Switzerland). (2020) 19:189. doi: 10.1186/s12933-020-01169-3
30. Nørgaard CH, Starkopf L, Gerds TA, Vestergaard P, Bonde AN, Fosbøl E and Køber L. Cardiovascular outcomes with GLP-1 receptor agonists vs. SGLT-2 inhibitors in patients with type 2 diabetes. Eur Heart J Cardiovasc Pharmacother. (2022) 8:549–56. doi: 10.1093/ehjcvp/pvab053
31. Pasternak B, Ueda P, Eliasson B, Svensson AM, Franzén S, Gudbjörnsdottir S, et al. Use of sodium glucose cotransporter 2 inhibitors and risk of major cardiovascular events and heart failure: Scandinavian register based cohort study. BMJ (Clinical Res ed). (2019) 366:l4772. doi: 10.1136/bmj.l4772
32. Patorno E. Effectiveness and safety of empagliflozin in routine care patients: Results from the EMPagliflozin compaRative effectIveness and SafEty (EMPRISE) study. Cardiovasc Drugs Ther. (2022) 24:442–54. doi: 10.1111/dom.14593
33. Patorno E, Goldfine AB, Schneeweiss S, Everett BM, Glynn RJ, Liu J, et al. Cardiovascular outcomes associated with canagliflozin versus other non-gliflozin antidiabetic drugs: population based cohort study. BMJ (Clinical Res ed). (2018) 360:k119. doi: 10.1136/bmj.k119
34. Patorno E, Htoo PT. Sodium-glucose cotransporter-2 inhibitors versus glucagon-like peptide-1 receptor agonists and the risk for cardiovascular outcomes in routine care patients with diabetes across categories of cardiovascular disease. Ann Intern Med. (2021) 174:1528–41. doi: 10.7326/M21-0893
35. Patorno E, Pawar A. Comparative effectiveness and safety of sodium-glucose cotransporter 2 inhibitors versus glucagon-like peptide 1 receptor agonists in older adults. Diabetes Care. (2021) 44(3):826–35. doi: 10.2337/dc20-1464
36. Persson F, Nystrom T, Jorgensen ME, Carstensen B, Gulseth HL, Thuresson M, et al. Dapagliflozin is associated with lower risk of cardiovascular events and all-cause mortality in people with type 2 diabetes (CVD-REAL Nordic) when compared with dipeptidyl peptidase-4 inhibitor therapy: A multinational observational study. Diabetes Obes Metab. (2018) 20:344–51. doi: 10.1111/dom.13077
37. Pineda ED, Liao IC, Godley PJ, Michel JB, Rascati KL. Cardiovascular outcomes among patients with type 2 diabetes newly initiated on sodium-glucose cotransporter-2 inhibitors, glucagon-like peptide-1 receptor agonists, and other antidiabetic medications. J Managed Care Specialty Pharmacy. (2020) 26:610–8. doi: 10.18553/jmcp.2020.26.5.610
38. Shchekochikhin D, Andreev D, Dyachuk I, Tarasenko S, Poltavskaya M, Mesitskaya D, et al. Cardiovascular outcomes in patients initiating first-line treatment of type 2 diabetes with sodium-glucose cotransporter-2 inhibitors versus metformin: A cohort study. Open Heart. (2022) 175:927–37. doi: 10.7326/M21-4012
39. Thomsen RW, Knudsen JS, Kahlert J, Baggesen LM, Lajer M, Holmgaard PH, et al. Cardiovascular events, acute hospitalizations, and mortality in patients with type 2 diabetes mellitus who initiate empagliflozin versus liraglutide: A comparative effectiveness study. Eur Heart J Cardiovasc Pharmacother. (2021) 10:e019356. doi: 10.1161/JAHA.120.019356
40. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. (2019) 380:347–57. doi: 10.1056/NEJMoa1812389
41. Zhu Y, Zhang JL, Yan XJ, Sun L, Ji Y, Wang FF. Effect of dapagliflozin on the prognosis of patients with acute myocardial infarction undergoing percutaneous coronary intervention. Cardiovasc Diabetol. (2022) 21:186. doi: 10.1186/s12933-022-01627-0
42. Zinman B, Inzucchi SE, Lachin JM, Wanner C, Fitchett D, Kohler S, et al. Empagliflozin and cerebrovascular events in patients with type 2 diabetes mellitus at high cardiovascular risk. Stroke. (2017) 48:1218–25. doi: 10.1161/STROKEAHA.116.015756
43. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. (2015) 373:2117–28. doi: 10.1056/NEJMoa1504720
44. Chen S. The safety of canagliflozin in the treatment of type 2 diabetes mellitus patients with high cardiovascular risk and its effect on related indicators. Tang Niao Bing Xin Shi Jie. (2020) 23:83–5. doi: 10.16658/j.cnki.1672-4062.2020.23.083
45. Zhai X, Luo DQ, Guan P, Jiang F, Feng GQ, Chen J, et al. The safety of canagliflozin in the treatment of type 2 diabetes mellitus patients with high cardiovascular risk and its effect on related indicators. Zhongguo Yao Fang. (2020) 31:2005–9.
46. Jiang YH, Wang Z, Zheng RJ, Sang HQ. Effect on clinical outcomes after drug-eluting stent implantation in type 2 diabetes mellitus with dapagliflozin. Lin Chuang Xin Xue Guan Za Zhi. (2021) 37:1014–9. doi: 10.13201/j.issn.1001-1439.2021.11.009
47. Jiang YH, Wang Z, Zheng RJ, Sang HQ. Effect on clinical outcomes for patients with coronary artery disease combined with type 2 diabetes mellitus by dapagliflozin. Zhongguo Xun Huan Za Zhi. (2022) 37:250–5.
48. Li CX. Clinical efficacy and valorisation of SGLT2 inhibitors and stroke risk in patients with type 2 diabetes mellitus. Tang Niao Bing Xin Shi Jie. (2019) 22:27–8. doi: 10.16658/j.cnki.1672-4062.2019.07.027
49. Yin YP, Lu XB, Zhang YY, Jiang JJ, Mi YF. Effect of dapagliflozin on blood glucose level and MACE in patients with AMI combined with T2DM. Zhongguo Xian Dai Yi Sheng. (2021) 59:49–52.
50. D’Andrea E, Wexler DJ, Kim SC, Paik JM, Alt E, Patorno E. Comparing effectiveness and safety of SGLT2 inhibitors vs DPP-4 inhibitors in patients with type 2 diabetes and varying baseline hbA1c levels. JAMA Internal Med. (2023) 183:242–54. doi: 10.1001/jamainternmed.2022.6664
51. Hu WS, Lin CL. Clinical outcomes in heart failure patients with and without atrial fibrillation receiving sodium-glucose cotransporter-2 inhibitor. Naunyn-Schmiedeberg’s Arch Pharmacol. (2023) 396:1977–86. doi: 10.1007/s00210-023-02425-5
52. Hwang HJ, Kim M, Jun JE, Yon DK. Sodium-glucose cotransporter-2 inhibitors improve clinical outcomes in patients with type 2 diabetes mellitus undergoing anthracycline-containing chemotherapy: an emulated target trial using nationwide cohort data in South Korea. Sci Rep. (2023) 13:21756. doi: 10.1038/s41598-023-48678-1
53. Kutz A, Kim DH, Wexler DJ, Liu J, Schneeweiss S, Glynn RJ, et al. Comparative cardiovascular effectiveness and safety of SGLT-2 inhibitors, GLP-1 receptor agonists, and DPP-4 inhibitors according to frailty in type 2 diabetes. Diabetes Care. (2023) 46:2004–14. doi: 10.2337/dc23-0671
54. Kwon O, Myong JP, Lee Y, Choi YJ, Yi JE, Seo SM, et al. Sodium-glucose cotransporter-2 inhibitors after acute myocardial infarction in patients with type 2 diabetes: A population-based investigation. J Am Heart Assoc. (2023) 12:e027824. doi: 10.1161/JAHA.122.027824
55. Lee HF, Chan YH, Chuang C, Li PR, Yeh YH, Hsiao FC, et al. Cardiovascular, renal, and lower limb outcomes in patients with type 2 diabetes after percutaneous coronary intervention and treated with sodium-glucose cotransporter 2 inhibitors vs. dipeptidyl peptidase-4 inhibitors. Eur Heart J Cardiovasc Pharmacother. (2023) 9:301–10. doi: 10.1093/ehjcvp/pvad004
56. Lyu YS, Oh S, Kim JH, Kim SY, Jeong MH. Comparison of SGLT2 inhibitors with DPP-4 inhibitors combined with metformin in patients with acute myocardial infarction and diabetes mellitus. Cardiovasc Diabetol. (2023) 22:185. doi: 10.1186/s12933-023-01914-4
57. McCormick N, Yokose C, Wei J, Lu N, Wexler DJ, Aviña-Zubieta JA, et al. Comparative effectiveness of sodium-glucose cotransporter-2 inhibitors for recurrent gout flares and gout-primary emergency department visits and hospitalizations: A general population cohort study. Ann Intern Med. (2023) 176:1067–80. doi: 10.7326/M23-0724
58. Richardson TL Jr., Halvorson AE, Hackstadt AJ, Hung AM, Greevy R, Grijalva CG, et al. Primary occurrence of cardiovascular events after adding sodium-glucose cotransporter-2 inhibitors or glucagon-like peptide-1 receptor agonists compared with dipeptidyl peptidase-4 inhibitors: A cohort study in veterans with diabetes. Ann Intern Med. (2023) 176:751–60. doi: 10.7326/M22-2751
59. Liu YY, Tian YL, Wang QL. Effect of canagliflozin on short-term prognosis in elderly patients with type 2 diabetes undergoing biovalvular replacement. Zhongguo Xin Xue Guan Za Zhi. (2022) 27:552–6.
60. Yang J, Zhang SJ, Wang YL, Wang SB, Wang JK, Zhang J, et al. Hypoglycemic and cardiovascular protective effects of metformin combined with dapagliflozin in patients who have acute chest pain and type 2 diabetes mellitus. Yi Yao Qian Yan. (2022) 12:91–3.
61. Boutron I, Page MJ, Higgins JPT, Altman DG, Lundh A, Hróbjartsson A. Chapter 7: Considering bias and conflicts of interest among the included studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane (2023). Available online at: https://training.cochrane.org/handbook/current/chapter-07.
62. Wang Y. Introduction to the cochrane bias risk assessment tool. Zhongguo Quan Ke Yi Xue. (2019) 22:1322.
63. Seufert J. SGLT2 inhibitors - an insulin-independent therapeutic approach for treatment of type 2 diabetes: focus on canagliflozin. Diabetes Metab Syndrome Obesity: Targets Ther. (2015) 8:543–54. doi: 10.2147/DMSO
64. Davies MJ, Aroda VR, Collins BS, Gabbay RA, Green J, Maruthur NM, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the american diabetes association (ADA) and the european association for the study of diabetes (EASD). Diabetes Care. (2022) 45:2753–86. doi: 10.2337/dci22-0034
65. Haloot J, Krokar L, Badin A. Effect of SLGT2 inhibitors on patients with atrial fibrillation. Neurohospitalist. (2021) 14:20200502. doi: 10.4022/jafib.20200502
66. Broome DT. SGLT-2 inhibitors: discrepancy between MACE reduction and incident MI and stroke. J Clin Endocrinol Metab. (2023) 108:e1450–1. doi: 10.1210/clinem/dgad216
67. Sattar N, McLaren J, Kristensen SL, Preiss D, McMurray JJ. SGLT2 Inhibition and cardiovascular events: why did EMPA-REG Outcomes surprise and what were the likely mechanisms? Diabetologia. (2016) 59:1333–9. doi: 10.1007/s00125-016-3956-x
68. Salvatore T, Caturano A, Galiero R, Di Martino A, Albanese G, Vetrano E, et al. Cardiovascular benefits from gliflozins: effects on endothelial function. Biomedicines. (2021) 9:1356. doi: 10.3390/biomedicines9101356
69. Pahud de Mortanges A, Salvador D Jr., Laimer M, Muka T, Wilhelm M, Bano A. The role of SGLT2 inhibitors in atherosclerosis: A narrative mini-review. Front Pharmacol. (2021) 12:751214. doi: 10.3389/fphar.2021.751214
70. Johri N, Matreja PS, John D, Dutta S, Parida AK, Sarma SN. Influence of SGLT2 inhibitors in remodeling, substrate and ion metabolism of myocardium to prevent cardiovascular risks: recent work and advancement. Curr Mol Pharmacol. (2023) 16:580–91. doi: 10.2174/1874467216666221017123333
71. Ricci F, Fedorowski A, Radico F, Romanello M, Tatasciore A, Di Nicola M, et al. Cardiovascular morbidity and mortality related to orthostatic hypotension: a meta-analysis of prospective observational studies. Eur Heart J. (2015) 36:1609–17. doi: 10.1093/eurheartj/ehv093
72. DeFronzo RA, Eldor R, Abdul-Ghani M. Pathophysiologic approach to therapy in patients with newly diagnosed type 2 diabetes. Diabetes Care. (2013) 36 Suppl 2:S127–138. doi: 10.2337/dcS13-2011
73. Luna-Marco C, de Marañon AM, Hermo-Argibay A, Rodriguez-Hernandez Y, Hermenejildo J, Fernandez-Reyes M, et al. Effects of GLP-1 receptor agonists on mitochondrial function, inflammatory markers and leukocyte-endothelium interactions in type 2 diabetes. Redox Biol. (2023) 66:102849. doi: 10.1016/j.redox.2023.102849
74. Mulvihill EE, Drucker DJ. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocrine Rev. (2014) 35:992–1019. doi: 10.1210/er.2014-1035
75. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Furtado RHM, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. (2019) 139:2022–31. doi: 10.1161/CIRCULATIONAHA.118.038868
76. Ku EJ, Lee DH, Jeon HJ, Oh TK. Empagliflozin versus dapagliflozin in patients with type 2 diabetes inadequately controlled with metformin, glimepiride and dipeptidyl peptide 4 inhibitors: A 52-week prospective observational study. Diabetes Res Clin Practice. (2019) 151:65–73. doi: 10.1016/j.diabres.2019.04.008
77. Ku EJ, Lee DH, Jeon HJ, Oh TK. Long-term effectiveness and safety of quadruple combination therapy with empagliflozin versus dapagliflozin in patients with type 2 diabetes: 3-year prospective observational study. Diabetes Res Clin Practice. (2021) 182:109123. doi: 10.1016/j.diabres.2021.109123
78. Shao SC, Chang KC, Hung MJ, Yang NI, Chan YY, Chen HY, et al. Comparative risk evaluation for cardiovascular events associated with dapagliflozin vs. empagliflozin in real-world type 2 diabetes patients: a multi-institutional cohort study. Cardiovasc Diabetol. (2019) 18:120. doi: 10.1186/s12933-019-0919-9
Keywords: sodium-glucose cotransporter 2 inhibitors, stroke, cardiovascular death, myocardial infarction, heart failure, all-cause mortality
Citation: Wang F, Li C, Cui L, Gu S, Zhao J and Wang H (2024) Effects of sodium-glucose cotransporter 2 inhibitors on cardiovascular and cerebrovascular diseases: a meta-analysis of controlled clinical trials. Front. Endocrinol. 15:1436217. doi: 10.3389/fendo.2024.1436217
Received: 21 May 2024; Accepted: 05 August 2024;
Published: 23 August 2024.
Edited by:
Carmine Izzo, University of Salerno, ItalyReviewed by:
Andrea Da Porto, University of Udine, ItalyFernando P. Dominici, University of Buenos Aires, Argentina
Copyright © 2024 Wang, Li, Cui, Gu, Zhao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haipeng Wang, MTM1ODkxMjkwMzBAMTYzLmNvbQ==; Junyu Zhao, emhhb2p1bnl1QHNkZm11LmVkdS5jbg==
 Fei Wang
Fei Wang Chunyu Li
Chunyu Li Lili Cui
Lili Cui Shuo Gu
Shuo Gu Junyu Zhao
Junyu Zhao Haipeng Wang
Haipeng Wang