- 1Department of Orthopedics, Jincheng General Hospital, Jincheng, China
- 2Department of Gynecology, Gaoping People's Hospital, Jincheng, China
Study Design: A systematic review and Meta-analysis
Objective: To compare the efficacy and safety of denosumab and teriparatide versus oral bisphosphonates to treat postmenopausal osteoporosis.
Summary of Background Data: While bisphosphonates have historically been the cornerstone of pharmacological management for bone protection in patients, emerging evidence suggests that teriparatide and denosumab warrant further investigation as potential first-line treatments. The optimal choice among denosumab, teriparatide, and oral bisphosphonates for the treatment of postmenopausal osteoporosis remains a subject of ongoing debate and controversy within the scientific community.
Methods: This systematic review adhered meticulously to the rigorous standards outlined by the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines as well as the Cochrane Collaboration recommendations. Additionally, it employed the AMSTAR (Assessing the methodological quality of systematic reviews) criteria to ensure methodological robustness and enhance the credibility of the findings. A systematic electronic search was conducted across Web of Science, PubMed, and the Cochrane Library databases from their inception dates up to February 2024.
Results: In this meta-analysis of studies, our findings suggest that compared to bisphosphonates, both teriparatide and denosumab demonstrated notable increases in percentage changes in lumbar spine bone mineral density (BMD) among postmenopausal osteoporosis patients. Furthermore, denosumab exhibited superiority over teriparatide and oral bisphosphonates in enhancing percentage changes in both femoral neck and total hip BMD, indicating its potential as a more efficacious option. Regarding safety outcomes, no significant differences were observed in the incidence of serious adverse events among patients treated with teriparatide, denosumab, and bisphosphonates. However, teriparatide showed superiority over oral bisphosphonates in terms of a lower risk of general adverse events, suggesting a favorable safety profile.
Conclusion: In conclusion, our study suggests that teriparatide and denosumab demonstrate comparable or potentially superior efficacy and safety profiles compared to oral bisphosphonates for the treatment of postmenopausal osteoporosis.
Systematic Review Registration: PROSPERO, identifier CRD42024508382.
1 Introduction
Osteoporosis, characterized as a systemic bone disease, primarily entails the depletion of bone mass and degradation of bone tissue microstructure. This process significantly heightens bone fragility, thereby elevating the risk of fractures (1, 2). In women, the decline in estrogen levels post-menopause, a hormone known for its bone-protective effects, contributes to the onset of osteoporosis, substantially augmenting fracture risk (3–5). It has been reported that approximately 30% of women in the United States are predisposed to developing osteoporosis (6). Therefore, postmenopausal osteoporosis imposes a significant burden on both individual patients and society as a whole.
Bisphosphonates is the most commonly prescribed and available drugs worldwide. Nevertheless, the extended usage of these drugs and their potential adverse effects have sparked ongoing debate within the scientific community (7–9). Denosumab, a fully human monoclonal antibody targeting receptor activator of nuclear factor kappa-B ligand (RANKL), demonstrates efficacy in mitigating bone resorption while concurrently enhancing bone mineral density (BMD) (10, 11). Denosumab received its initial approval from the United States Food and Drug Administration (FDA) in 2010 for the treatment of postmenopausal osteoporosis in high-risk individuals prone to fractures (12). Teriparatide is a synthetic form of human parathyroid hormone (1–34), generated through recombinant technology (13, 14). This implies that the medication can trigger bone remodeling by enhancing osteoblast activity, resulting in a significant elevation in the bone formation marker P1NP. Ultimately, this process culminates in heightened bone density, effectively achieving the therapeutic goal of treating osteoporosis (15).
While bisphosphonates have historically served as the cornerstone of bone protective therapy, teriparatide and denosumab are gaining recognition as promising first-line treatments, warranting further investigation. Consequently, there remains a contentious debate regarding the optimal choice between denosumab, teriparatide, or bisphosphonates for postmenopausal osteoporosis management. The objective of this meta-analysis is to comprehensively evaluate the efficacy and safety profiles of denosumab and teriparatide versus oral bisphosphonates in the treatment of postmenopausal osteoporosis.
2 Materials and methods
2.1 Search strategy and selection criteria
This systematic review adhered meticulously to the rigorous standards outlined by the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines as well as the Cochrane Collaboration recommendations. Additionally, it employed the AMSTAR (Assessing the methodological quality of systematic reviews) criteria to ensure methodological robustness and enhance the credibility of the findings (16–18). The methodologies employed in this review were pre-registered with PROSPERO under registration number CRD42024508382. A systematic electronic search was conducted across Web of Science, PubMed, and the Cochrane Library databases from their inception dates up to February 2024. The comprehensive search strategy is detailed in (Supplementary Data). Additionally, manual searches of reference lists from pertinent reviews and included studies were performed to ensure thoroughness in data collection.
2.2 Selection criteria and study design
The inclusion criteria for this review encompassed randomized controlled trials with a minimum duration of 12 months involving postmenopausal osteoporosis patients. Studies were eligible if they compared either denosumab or teriparatide with a single oral bisphosphonate drug. (Due to the current lack of randomized controlled trials comparing denosumab and teriparatide with intravenous bisphosphonates, intravenous bisphosphonates are not included in the scope.) Additionally, the selected literature needed to present at least one relevant outcome of interest.
Percentage changes in lumbar spine, total hip, and femoral neck bone mineral density (BMD) served as efficacy criteria. Firstly, lumbar spine bone mineral density (BMD) refers to the measurement of mineral content within the bones of the lumbar spine, typically assessed through densitometry techniques such as dual-energy X-ray absorptiometry (DXA). It provides information about the density and strength of the vertebrae in the lower back region (19). Then, total hip bone mineral density (BMD) refers to the measurement of mineral content within the bones of the entire hip joint, including the proximal femur and surrounding structures. This measurement is indicative of bone strength and density in the hip region (20). Finally, femoral neck bone mineral density (BMD) refers to the measurement of mineral content specifically within the narrow portion of the thigh bone (femur) known as the femoral neck. This measurement is particularly important as the femoral neck is a common site for hip fractures, and assessing its bone mineral density helps evaluate fracture risk and overall bone health (21).
Assessment of serious adverse events and general adverse events was utilized to evaluate safety outcomes. Serious adverse events denote notable and potentially life-threatening incidents that individuals may encounter during medical interventions or involvement in clinical trials. These events have the capacity to extend hospitalization periods, cause persistent or substantial disability, or in extreme cases, result in mortality (22). General adverse events, on the other hand, are negative health outcomes that are less severe compared to serious adverse events. They encompass a broad range of symptoms, from mild discomfort to moderate issues. General adverse events refer to undesirable outcomes or effects that arise during or after medical treatment, irrespective of the specific treatment or condition. These events may include a variety of issues, such as nausea, headaches, allergic reactions, or other complications, which are not directly related to the primary therapeutic goal of the treatment. For example, articles have described adverse events in the upper gastrointestinal (GI) tract associated with bisphosphonate use, such as nausea, vomiting, epigastric pain, and dyspepsia, reported shortly after the introduction of oral formulations of these drugs for osteoporosis treatment.
2.3 Data extraction and quality assessment
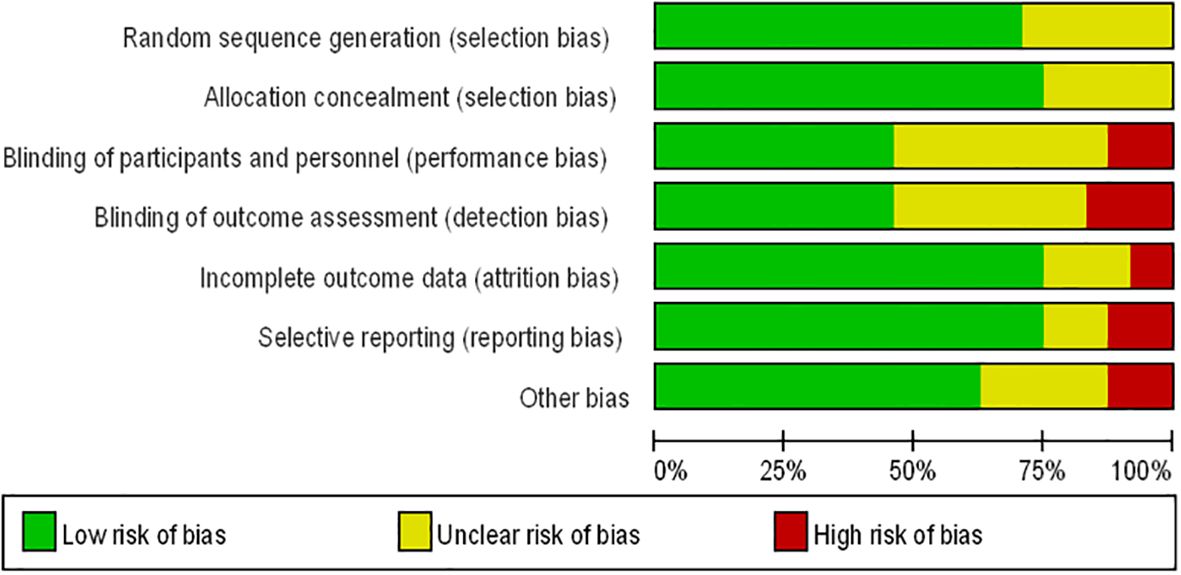
Two authors (JY and XBG) conducted data extraction, documenting details such as the first author’s name, year of publication, sample size, mean age of patients, dosage and interval of comparison, follow-up duration, and study design. Any discrepancies between the two authors were resolved through consensus with a third investigator (JLD). Data were compiled into an electronic database for analysis. The Cochrane Collaboration risk assessment tool for randomized controlled trials (RCTs) was employed to evaluate the quality of the included literature (23, 24). Studies were evaluated for risk of bias based on several criteria including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other potential biases. Ratings of low risk, high risk, or unclear risk were assigned accordingly. Any discrepancies between ratings were resolved through discussion between two independent authors, with a third investigator reviewing and finalizing decisions.
2.4 Data synthesis and statistical analysis
Statistical analyses were conducted using Review Manager software (version 5.3) (25). Data were summarized utilizing odds ratios (OR) for categorical variables and mean differences (MDs) for continuous data (26). Significance was set at P < 0.05. Heterogeneity among studies was evaluated using the I2 test, with interpretation categorized as absent (0%–25%), low (25.1%–50%), moderate (50.1%–75%), or high (75.1%–100%) (27). Funnel plots were utilized to assess publication bias, while forest plots were employed to visually depict individual study results and the corresponding effect sizes of combined estimates.
3 Results
3.1 Systematic review and qualitative assessment
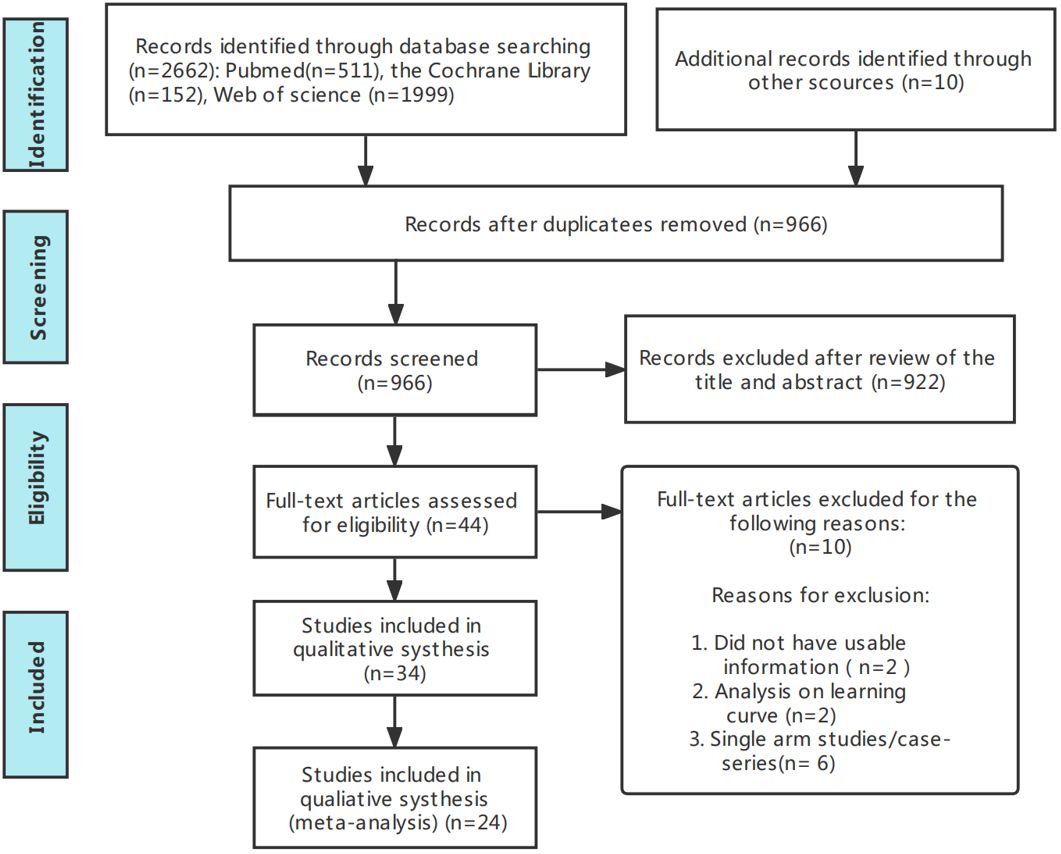
Figure 1 presents the flowchart according to the PRISMA statement, illustrating the study selection process alongside the primary exclusion criteria. Ultimately, our analysis incorporated 24 randomized controlled trials (28–51). Further insights into the risk of bias are encapsulated in Figure 2.

Figure 2. Risk of bias summary for RCTs: Reviewers’ judgments about each risk of bias item per included study.
3.2 Trials characteristics
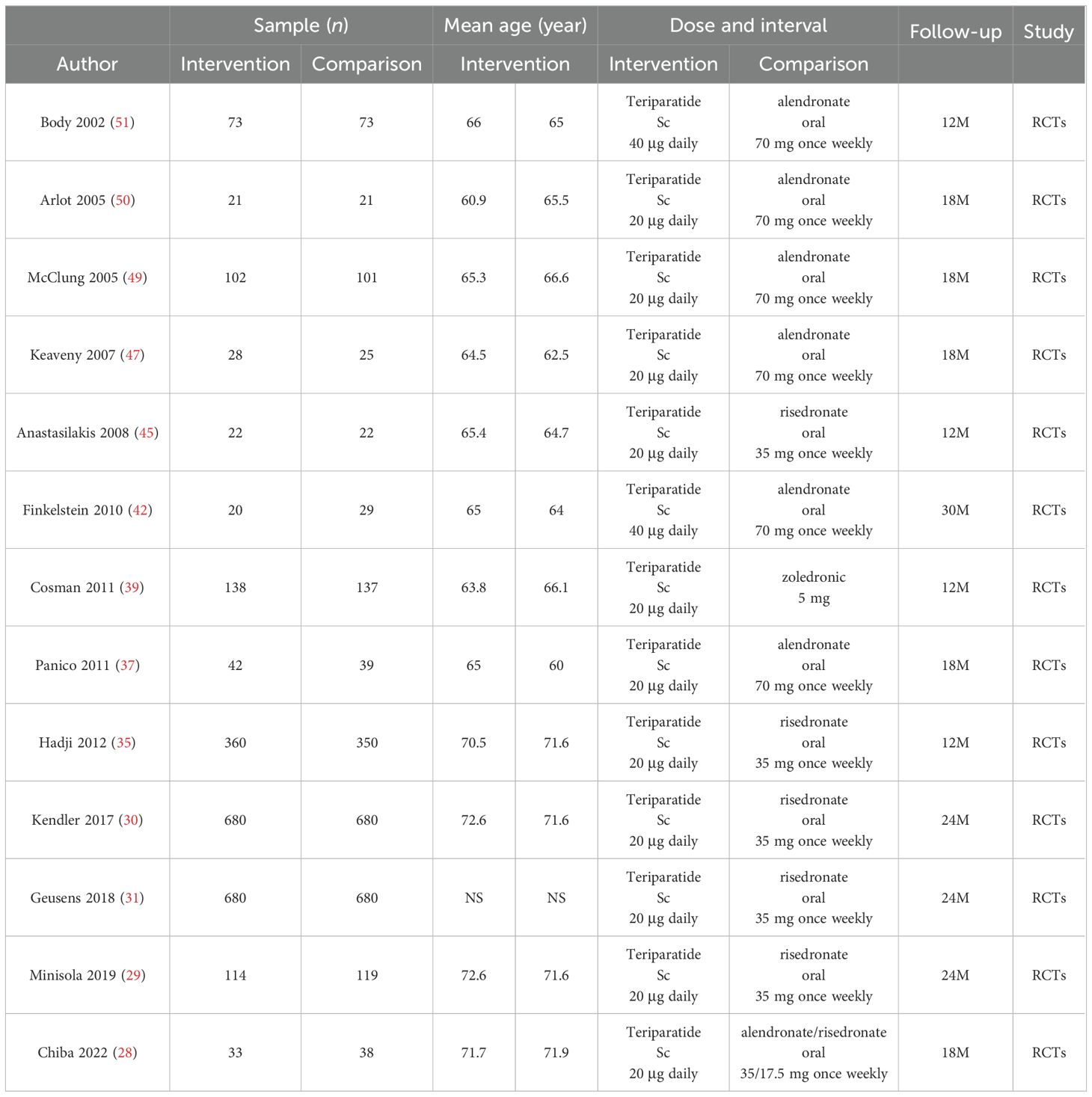
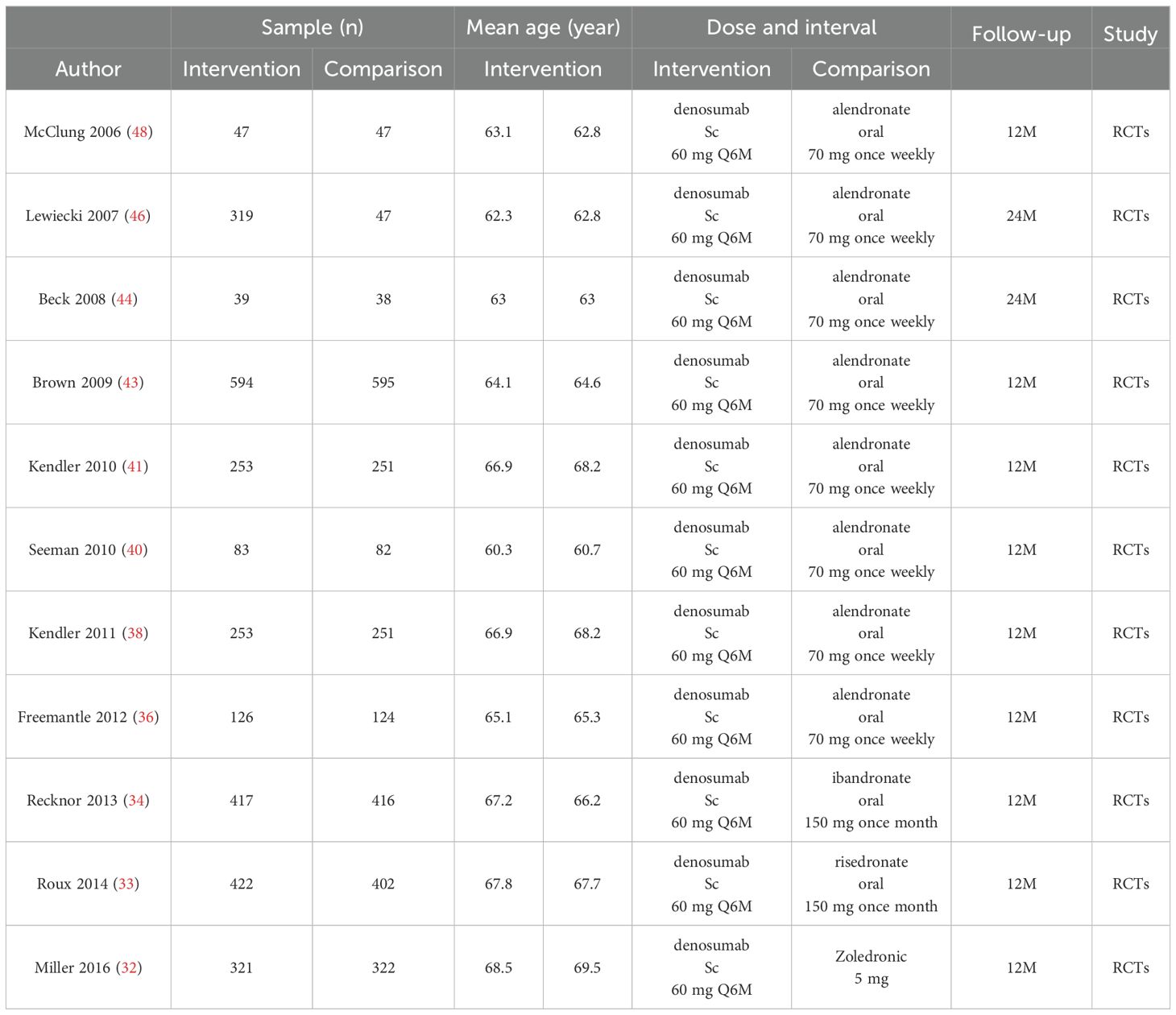
Tables 1, 2 encapsulate the primary characteristics of the trials incorporated in our analysis. These trials span the publication period from 2002 to 2024. Within the teriparatide and bisphosphonates cohort, 5 out of 13 trials conducted comparative assessments between teriparatide and risedronate, while seven trials scrutinized the efficacy of teriparatide against alendronate, and one trial investigated its comparison with zoledronic acid. In the denosumab and oral bisphosphonates subgroup, 1 out of 11 trials compared denosumab with risedronate, eight evaluated denosumab against alendronate, one assessed denosumab versus zoledronic acid, and one investigated denosumab versus ibandronate.
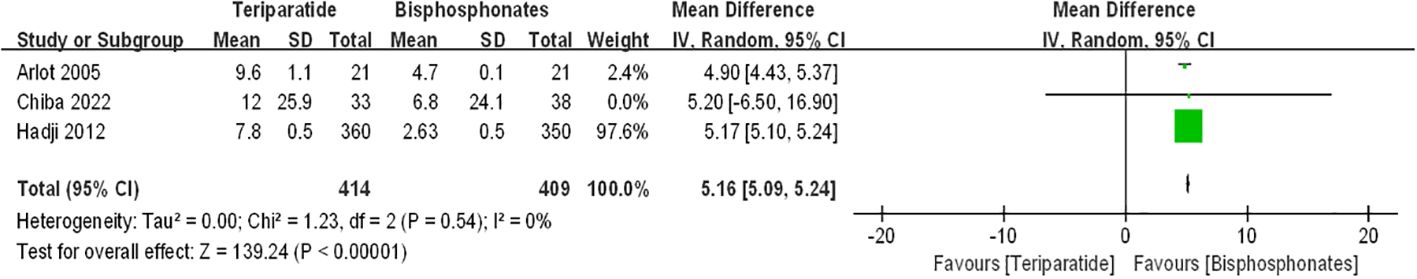
3.3 Percentage changes in the lumbar spine BMD
Figure 3 presents forest plots depicting the percentage changes in lumbar spine bone mineral density (BMD). The data illustrate that compared to bisphosphonates, both teriparatide and denosumab led to further increases in percentage changes in lumbar spine BMD among postmenopausal osteoporosis patients [teriparatide arm: RR=5.16, 95%CI:5.09–5.24, P < 0.00001; denosumab arm: RR=1.21, 95%CI: 0.3–2.11, p=0.009]. Notably, there was no significant statistical heterogeneity observed between the teriparatide and oral bisphosphonates results (I2 = 0%). However, the denosumab and oral bisphosphonates group exhibited substantial heterogeneity (I2 = 97%).
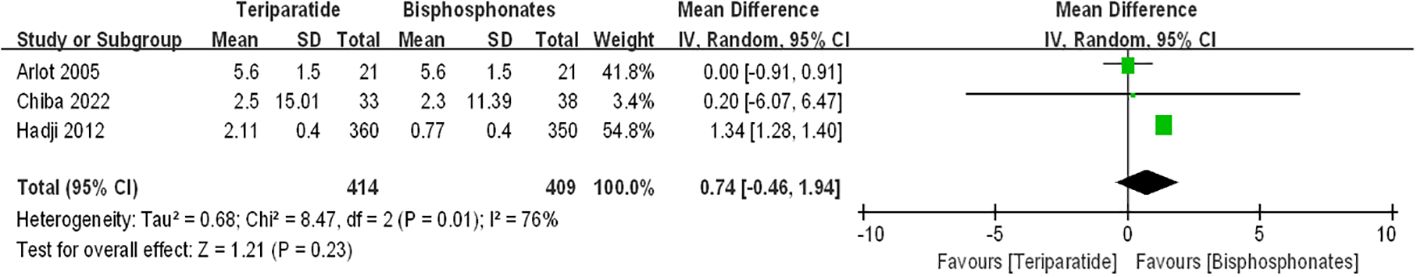
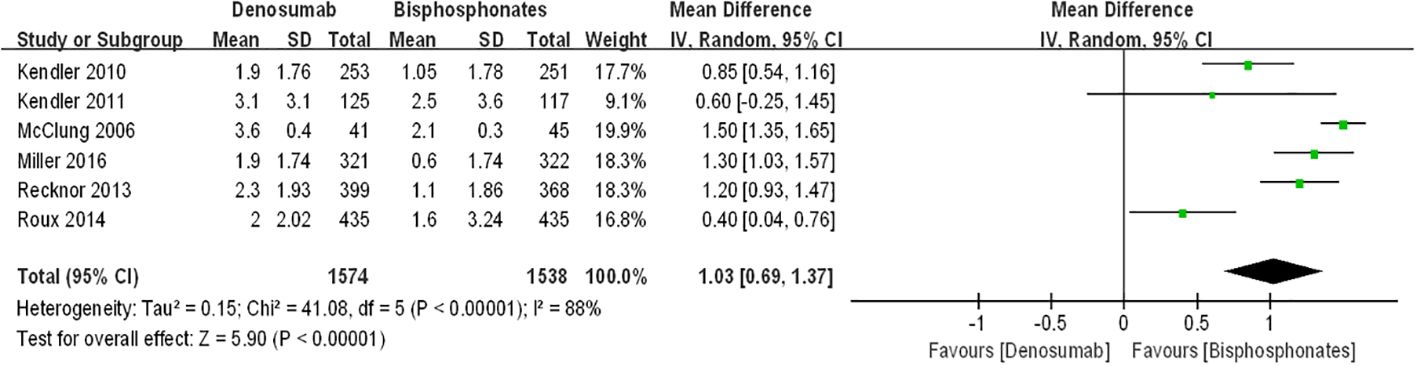
3.4 Percentage changes in the femoral neck BMD
Figure 4 displays forest plots illustrating the percentage changes in femoral neck bone mineral density (BMD). The data indicate that compared to bisphosphonates, denosumab led to a further increase in percentage changes in femoral neck BMD among postmenopausal osteoporosis patients [denosumab arm: RR=1.03, 95%CI: 0.69–1.37, P < 0.00001]. However, the difference in percentage changes in femoral neck BMD between the teriparatide and oral bisphosphonates groups did not reach statistical significance [teriparatide arm: RR=0.74, 95%CI:-0.46–1.94, p=0.23]. Notably, there was substantial statistical heterogeneity observed between the two groups of results (I2 = 76%, I2 = 88%). Thus, we advocate for cautious interpretation of these findings, and we emphasize the necessity for additional randomized controlled trials to validate these results.
3.5 Percentage changes in the total hip BMD
Figure 5 depicts forest plots presenting the percentage changes in total hip bone mineral density (BMD). The data reveal that compared to bisphosphonates, denosumab induced a further increase in percentage changes in total hip BMD among postmenopausal osteoporosis patients [denosumab arm: RR=0.83, 95%CI:0.50–1.17, P < 0.00001]. Conversely, the difference in percentage changes in total hip BMD between the teriparatide and oral bisphosphonates groups did not reach statistical significance [teriparatide arm: RR=0.83, 95%CI:-0.28–1.94, p=0.15]. Noteworthy, there was substantial statistical heterogeneity observed between the denosumab and oral bisphosphonates groups (I2 = 81%), while the teriparatide and oral bisphosphonates group exhibited moderate heterogeneity (I2 = 63%).
3.6 General adverse events
Figure 6 presents forest plots illustrating the incidence of general adverse events. The data indicate that compared to teriparatide, the risk of general adverse events was statistically higher with oral bisphosphonates [teriparatide arm: RR=0.69, 95%CI: 0.49–0.97, p=0.04]. Conversely, there was no significant difference in the incidence of general adverse events between the denosumab and oral bisphosphonates groups [denosumab arm: RR=1.06, 95%CI:0.93–1.21, p=0.37]. Notably, there was no statistical heterogeneity observed between the denosumab and oral bisphosphonates groups (I2 = 0), while the teriparatide and oral bisphosphonates group displayed moderate heterogeneity (I2 = 55%). These findings underscore the importance of cautious interpretation and further investigation into the safety profiles of these treatments.
3.7 Serious adverse events
Figure 7 illustrates forest plots depicting the incidence of serious adverse events. The data reveal that there were no significant differences in the incidence of serious adverse events among patients treated with teriparatide, denosumab, and oral bisphosphonates [teriparatide arm: RR=1.01, 95%CI:0.65–1.57, p=0.95; denosumab arm: RR=1.04, 95%CI:0.79–1.37, p=0.80]. Noteworthy, there was no statistical heterogeneity observed between the denosumab and oral bisphosphonates groups (I2 = 18%), while the teriparatide and oral bisphosphonates group exhibited moderate heterogeneity (I2 = 70%). These findings suggest a comparable safety profile among these treatments in terms of serious adverse events, albeit with some variability that warrants further investigation.
4 Discussion
In this systematic review and meta-analysis, we offer a comprehensive overview of the efficacy and safety profiles of denosumab and teriparatide versus oral bisphosphonates in the treatment of postmenopausal osteoporosis. Our analysis provides valuable insights into the comparative effectiveness and safety considerations of these therapeutic interventions.
In this meta-analysis of studies, our findings suggest that compared to oral bisphosphonates, both teriparatide and denosumab demonstrated notable increases in percentage changes in lumbar spine bone mineral density (BMD) among postmenopausal osteoporosis patients. Furthermore, denosumab exhibited superiority over teriparatide and oral bisphosphonates in enhancing percentage changes in both femoral neck and total hip BMD, indicating its potential as a more efficacious option. Regarding safety outcomes, no significant differences were observed in the incidence of serious adverse events among patients treated with teriparatide, denosumab, and bisphosphonates. However, teriparatide showed superiority over oral bisphosphonates in terms of a lower risk of general adverse events, suggesting a favorable safety profile. This appears to align with the descriptions provided by Chandran et al. And Yuan et al. Chandran et al. described in their article that denosumab can serve as both a first-line agent and an alternative to bisphosphonates for treating postmenopausal osteoporosis (52). Yuan et al. suggested that compared to bisphosphonates, teriparatide can reduce the risk of vertebral fractures and increase changes in lumbar spine and femoral neck BMD (6).
In summary, our analysis suggests that both teriparatide and denosumab exhibit similar or even superior efficacy and safety profiles compared to oral bisphosphonates for the treatment of postmenopausal osteoporosis. One interesting point to consider is that while this study found denosumab to be more effective than oral bisphosphonates overall in increasing bone mineral density (BMD), particularly in the femoral neck and total hip, its effect on lumbar spine BMD appeared to be similar to that of teriparatide. This suggests that different treatment medications may have varying effects on different skeletal regions, highlighting the importance of considering individual patient characteristics and the location of BMD changes when making treatment decisions. Furthermore, despite denosumab and teriparatide showing similar effects in increasing BMD, denosumab seems to exhibit higher heterogeneity in some aspects. This may indicate that the efficacy of denosumab across different studies could be influenced by other factors such as baseline characteristics of patients or duration of treatment. The presence of this heterogeneity may necessitate further research to determine the true effect of denosumab and its optimal application in clinical practice.
These findings provide valuable insights for clinicians in selecting optimal therapeutic options for their patients. Our meta-analysis had some advantages. To the best of our knowledge, we are the first study to combine denosumab, teriparatide and bisphosphonate in a direct comparison study in postmenopausal osteoporosis, which means that unlike the network meta-analysis, the results of the direct comparison will be more reliable. At the same time, a large number of studies (n = 24) including data from more 9000 patients were included, and all these studies were RCTs, which makes the results more credible.
This paper acknowledges several limitations. Firstly, the wide time span of included literature, ranging from 2002 to 2024, may introduce variability in study methodologies and patient characteristics, potentially impacting the quality of the meta-analysis. Secondly, the limited availability of literature specifically addressing serious adverse events and percentage changes in total hip BMD within the teriparatide and oral bisphosphonates group could restrict the robustness of the final analysis results. Finally, This article primarily discusses oral bisphosphonates. This is partly due to insufficient RCTs for intravenous bisphosphonates. On the other hand, oral and intravenous bisphosphonate administrations differ fundamentally. Intravenous treatment is generally preferred over oral bisphosphonates due to its perceived ease, efficacy, reduced burden, lower opportunity costs. However, Abhishek Sharma et al. suggest that intravenous bisphosphonates might increase inflammatory cytokine release more than oral bisphosphonates, potentially increasing the risk of new-onset atrial fibrillation (53). Furthermore, upper gastrointestinal discomfort from oral bisphosphonates and acute reactions from intravenous formulations are significant considerations (54). These factors underscore the need to evaluate both oral and intravenous administration of bisphosphonates. Moving forward, it is imperative to conduct additional studies aimed at generating high-quality randomized controlled trials (RCTs) on these topics. This will facilitate the acquisition of more reliable analysis results, thereby enhancing our understanding of the efficacy and safety profiles of these therapeutic interventions for postmenopausal osteoporosis.
5 Conclusion
In conclusion, our study suggests that teriparatide and denosumab demonstrate comparable or potentially superior efficacy and safety profiles compared to oral bisphosphonates for the treatment of postmenopausal osteoporosis. We believe that they hold promise as potential first-line treatments for this condition. However, given the absence of clear standards and the inherent variability in individual physiological conditions, further high-quality research is warranted to comprehensively explore the efficacy and safety of denosumab, teriparatide, and oral bisphosphonates in the treatment of postmenopausal osteoporosis, ensuring safety under varied clinical circumstances.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
JY: Investigation, Writing – original draft, Methodology. XG: Writing – review & editing, Data curation, Conceptualization. ZC: Writing – review & editing, Methodology, Investigation. HG: Writing – review & editing, Software, Methodology. JD: Writing – review & editing, Supervision, Methodology.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Health Commission of Shanxi Province (No.2021XM39).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1431676/full#supplementary-material
References
1. Qaseem A, Forciea MA, McLean RM, Denberg TD, Barry MJ, Cooke M, et al. Treatment of low bone density or osteoporosis to prevent fractures in men and women: A clinical practice guideline update from the american college of physicians. Ann Intern Med. (2017) 166:818–39. doi: 10.7326/m15-1361
2. Srivastava M, Deal C. Osteoporosis in elderly: prevention and treatment. Clin Geriatr Med. (2002) 18:529–55. doi: 10.1016/s0749-0690(02)00022-8
3. Yuan C, Liang Y, Zhu K, Xie W. Clinical efficacy of denosumab, teriparatide, and oral bisphosphonates in the prevention of glucocorticoid-induced osteoporosis: A systematic review and meta-analysis. J Orthop Surg Res. (2023) 18:447. doi: 10.1186/s13018-023-03920-4
4. Yong EL, Logan S. Menopausal osteoporosis: screening, prevention and treatment. Singapore Med J. (2021) 62:159–66. doi: 10.11622/smedj.2021036
5. Brown JP. Long-term treatment of postmenopausal osteoporosis. Endocrinol Metab (Seoul Korea). (2021) 36:544–52. doi: 10.3803/EnM.2021.301
6. Yuan F, Peng W, Yang C, Zheng J. Teriparatide versus bisphosphonates for treatment of postmenopausal osteoporosis: A meta-analysis. Int J Surg (London England). (2019) 66:1–11. doi: 10.1016/j.ijsu.2019.03.004
7. Fuggle N, Al-Daghri N, Bock O, Branco J, Bruyère O, Casado E, et al. Novel formulations of oral bisphosphonates in the treatment of osteoporosis. Aging Clin Exp Res. (2022) 34:2625–34. doi: 10.1007/s40520-022-02272-z
8. Fernández-Martín S, López-Peña M, Muñoz F, Permuy M, González-Cantalapiedra A. Bisphosphonates as disease-modifying drugs in osteoarthritis preclinical studies: A systematic review from 2000 to 2020. Arthritis Res Ther. (2021) 23:60. doi: 10.1186/s13075-021-02446-6
9. Saito T, Sterbenz JM, Malay S, Zhong L, MacEachern MP, Chung KC. Effectiveness of anti-osteoporotic drugs to prevent secondary fragility fractures: systematic review and meta-analysis. Osteoporosis international: J established as result cooperation between Eur Foundation Osteoporosis Natl Osteoporosis Foundation USA. (2017) 28:3289–300. doi: 10.1007/s00198-017-4175-0
10. Deeks ED. Denosumab: A review in postmenopausal osteoporosis. Drugs Aging. (2018) 35:163–73. doi: 10.1007/s40266-018-0525-7
11. Diab DL, Watts NB. Denosumab in osteoporosis. Expert Opin Drug Saf. (2014) 13:247–53. doi: 10.1517/14740338.2014.860133
12. Lewiecki EM. New and emerging concepts in the use of denosumab for the treatment of osteoporosis. Ther Adv musculoskeletal Dis. (2018) 10:209–23. doi: 10.1177/1759720x18805759
13. Hauser B, Alonso N, Riches PL. Review of current real-world experience with teriparatide as treatment of osteoporosis in different patient groups. J Clin Med. (2021) 10(7):1403. doi: 10.3390/jcm10071403
14. Quattrocchi E, Kourlas H. Teriparatide: A review. Clin Ther. (2004) 26:841–54. doi: 10.1016/s0149-2918(04)90128-2
15. Lindsay R, Nieves J, Formica C, Henneman E, Woelfert L, Shen V, et al. Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet. (1997) 350:550–5. doi: 10.1016/s0140-6736(97)02342-8
16. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (Prisma-P) 2015: elaboration and explanation. BMJ. (2015) 350:g7647. doi: 10.1136/bmj.g7647
17. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The prisma extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. doi: 10.7326/m14-2385
18. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
19. Davenport A. Differences in prevalence of reduced and low bone mineral density between lumbar spine and femoral neck in peritoneal dialysis patients using dual-energy X-ray absorptiometry (Dxa). Peritoneal Dialysis international: J Int Soc Peritoneal Dialysis. (2023) 43:334–8. doi: 10.1177/08968608221146867
20. Kemmler W, Shojaa M, Kohl M, von Stengel S. Effects of different types of exercise on bone mineral density in postmenopausal women: A systematic review and meta-analysis. Calcif Tissue Int. (2020) 107:409–39. doi: 10.1007/s00223-020-00744-w
21. Bouxsein ML, Eastell R, Lui LY, Wu LA, de Papp AE, Grauer A, et al. Change in bone density and reduction in fracture risk: A meta-regression of published trials. J Bone mineral research: Off J Am Soc Bone Mineral Res. (2019) 34:632–42. doi: 10.1002/jbmr.3641
22. Pereira TV, Jüni P, Saadat P, Xing D, Yao L, Bobos P, et al. Viscosupplementation for knee osteoarthritis: systematic review and meta-analysis. BMJ. (2022) 378:e069722. doi: 10.1136/bmj-2022-069722
23. Chen T, Zhou G, Chen Z, Yao X, Liu D. Biportal endoscopic decompression vs. Microscopic decompression for lumbar canal stenosis: A systematic review and meta-analysis. Exp Ther Med. (2020) 20:2743–51. doi: 10.3892/etm.2020.9001
24. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: A new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. (2019) 10:Ed000142. doi: 10.1002/14651858.Ed000142
25. Béliveau A, Boyne DJ, Slater J, Brenner D, Arora P. Bugsnet: an R package to facilitate the conduct and reporting of bayesian network meta-analyses. BMC Med Res Method. (2019) 19:196. doi: 10.1186/s12874-019-0829-2
26. van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta-analysis. Res synthesis Methods. (2012) 3:285–99. doi: 10.1002/jrsm.1054
27. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
28. Chiba K, Okazaki N, Kurogi A, Watanabe T, Mori A, Suzuki N, et al. Randomized controlled trial of daily teriparatide, weekly high-dose teriparatide, or bisphosphonate in patients with postmenopausal osteoporosis: the terabit study. Bone. (2022) 160:116416. doi: 10.1016/j.bone.2022.116416
29. Minisola S, Marin F, Kendler DL, Geusens P, Zerbini CAF, Russo LA, et al. Serum 25-hydroxy-vitamin D and the risk of fractures in the teriparatide versus risedronate vero clinical trial. Arch Osteoporos. (2019) 14:10. doi: 10.1007/s11657-019-0561-x
30. Kendler DL, Marin F, Zerbini CAF, Russo LA, Greenspan SL, Zikan V, et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (Vero): A multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. (2018) 391:230–40. doi: 10.1016/s0140-6736(17)32137-2
31. Geusens P, Marin F, Kendler DL, Russo LA, Zerbini CA, Minisola S, et al. Effects of teriparatide compared with risedronate on the risk of fractures in subgroups of postmenopausal women with severe osteoporosis: the vero trial. J Bone mineral research: Off J Am Soc Bone Mineral Res. (2018) 33:783–94. doi: 10.1002/jbmr.3384
32. Miller PD, Pannacciulli N, Brown JP, Czerwinski E, Nedergaard BS, Bolognese MA, et al. Denosumab or zoledronic acid in postmenopausal women with osteoporosis previously treated with oral bisphosphonates. J Clin Endocrinol Metab. (2016) 101:3163–70. doi: 10.1210/jc.2016-1801
33. Roux C, Hofbauer LC, Ho PR, Wark JD, Zillikens MC, Fahrleitner-Pammer A, et al. Denosumab compared with risedronate in postmenopausal women suboptimally adherent to alendronate therapy: efficacy and safety results from a randomized open-label study. Bone. (2014) 58:48–54. doi: 10.1016/j.bone.2013.10.006
34. Recknor C, Czerwinski E, Bone HG, Bonnick SL, Binkley N, Palacios S, et al. Denosumab compared with ibandronate in postmenopausal women previously treated with bisphosphonate therapy: A randomized open-label trial. Obstet Gynecol. (2013) 121:1291–9. doi: 10.1097/AOG.0b013e318291718c
35. Hadji P, Zanchetta JR, Russo L, Recknor CP, Saag KG, McKiernan FE, et al. The effect of teriparatide compared with risedronate on reduction of back pain in postmenopausal women with osteoporotic vertebral fractures. Osteoporosis international: J established as result cooperation between Eur Foundation Osteoporosis Natl Osteoporosis Foundation USA. (2012) 23:2141–50. doi: 10.1007/s00198-011-1856-y
36. Freemantle N, Satram-Hoang S, Tang ET, Kaur P, Macarios D, Siddhanti S, et al. Final results of the daps (Denosumab adherence preference satisfaction) study: A 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporosis international: J established as result cooperation between Eur Foundation Osteoporosis Natl Osteoporosis Foundation USA. (2012) 23:317–26. doi: 10.1007/s00198-011-1780-1
37. Panico A, Lupoli GA, Marciello F, Lupoli R, Cacciapuoti M, Martinelli A, et al. Teriparatide vs. Alendronate as a treatment for osteoporosis: changes in biochemical markers of bone turnover, bmd and quality of life. Med Sci Monit. (2011) 17:Cr442–8. doi: 10.12659/msm.881905
38. Kendler DL, McClung MR, Freemantle N, Lillestol M, Moffett AH, Borenstein J, et al. Adherence, preference, and satisfaction of postmenopausal women taking denosumab or alendronate. Osteoporosis international: J established as result cooperation between Eur Foundation Osteoporosis Natl Osteoporosis Foundation USA. (2011) 22:1725–35. doi: 10.1007/s00198-010-1378-z
39. Cosman F, Eriksen EF, Recknor C, Miller PD, Guañabens N, Kasperk C, et al. Effects of intravenous zoledronic acid plus subcutaneous teriparatide [Rhpth(1-34)] in postmenopausal osteoporosis. J Bone mineral research: Off J Am Soc Bone Mineral Res. (2011) 26:503–11. doi: 10.1002/jbmr.238
40. Seeman E, Delmas PD, Hanley DA, Sellmeyer D, Cheung AM, Shane E, et al. Microarchitectural deterioration of cortical and trabecular bone: differing effects of denosumab and alendronate. J Bone mineral research: Off J Am Soc Bone Mineral Res. (2010) 25:1886–94. doi: 10.1002/jbmr.81
41. Kendler DL, Roux C, Benhamou CL, Brown JP, Lillestol M, Siddhanti S, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone mineral research: Off J Am Soc Bone Mineral Res. (2010) 25:72–81. doi: 10.1359/jbmr.090716
42. Finkelstein JS, Wyland JJ, Lee H, Neer RM. Effects of teriparatide, alendronate, or both in women with postmenopausal osteoporosis. J Clin Endocrinol Metab. (2010) 95:1838–45. doi: 10.1210/jc.2009-1703
43. Brown JP, Prince RL, Deal C, Recker RR, Kiel DP, de Gregorio LH, et al. Comparison of the effect of denosumab and alendronate on bmd and biochemical markers of bone turnover in postmenopausal women with low bone mass: A randomized, blinded, phase 3 trial. J Bone mineral research: Off J Am Soc Bone Mineral Res. (2009) 24:153–61. doi: 10.1359/jbmr.0809010
44. Beck TJ, Lewiecki EM, Miller PD, Felsenberg D, Liu Y, Ding B, et al. Effects of denosumab on the geometry of the proximal femur in postmenopausal women in comparison with alendronate. J Clin densitometry: Off J Int Soc Clin Densitometry. (2008) 11:351–9. doi: 10.1016/j.jocd.2008.04.001
45. Anastasilakis AD, Goulis DG, Polyzos SA, Gerou S, Koukoulis GN, Efstathiadou Z, et al. Head-to-head comparison of risedronate vs. Teriparatide on bone turnover markers in women with postmenopausal osteoporosis: A randomised trial. Int J Clin Pract. (2008) 62:919–24. doi: 10.1111/j.1742-1241.2008.01768.x
46. Lewiecki EM, Miller PD, McClung MR, Cohen SB, Bolognese MA, Liu Y, et al. Two-year treatment with denosumab (Amg 162) in a randomized phase 2 study of postmenopausal women with low bmd. J Bone mineral research: Off J Am Soc Bone Mineral Res. (2007) 22:1832–41. doi: 10.1359/jbmr.070809
47. Keaveny TM, Donley DW, Hoffmann PF, Mitlak BH, Glass EV, San Martin JA. Effects of teriparatide and alendronate on vertebral strength as assessed by finite element modeling of qct scans in women with osteoporosis. J Bone mineral research: Off J Am Soc Bone Mineral Res. (2007) 22:149–57. doi: 10.1359/jbmr.061011
48. McClung MR, Lewiecki EM, Cohen SB, Bolognese MA, Woodson GC, Moffett AH, et al. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. (2006) 354:821–31. doi: 10.1056/NEJMoa044459
49. McClung MR, San Martin J, Miller PD, Civitelli R, Bandeira F, Omizo M, et al. Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Internal Med. (2005) 165:1762–8. doi: 10.1001/archinte.165.15.1762
50. Arlot M, Meunier PJ, Boivin G, Haddock L, Tamayo J, Correa-Rotter R, et al. Differential effects of teriparatide and alendronate on bone remodeling in postmenopausal women assessed by histomorphometric parameters. J Bone mineral research: Off J Am Soc Bone Mineral Res. (2005) 20:1244–53. doi: 10.1359/jbmr.050309
51. Body JJ, Gaich GA, Scheele WH, Kulkarni PM, Miller PD, Peretz A, et al. A randomized double-blind trial to compare the efficacy of teriparatide [Recombinant human parathyroid hormone (1-34)] with alendronate in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. (2002) 87:4528–35. doi: 10.1210/jc.2002-020334
52. Chandran T, Venkatachalam I. Efficacy and safety of denosumab compared to bisphosphonates in improving bone strength in postmenopausal osteoporosis: A systematic review. Singapore Med J. (2019) 60:364–78. doi: 10.11622/smedj.2019028
53. Sharma A, Einstein AJ, Vallakati A, Arbab-Zadeh A, Walker MD, Mukherjee D, et al. Risk of atrial fibrillation with use of oral and intravenous bisphosphonates. Am J Cardiol. (2014) 113:1815–21. doi: 10.1016/j.amjcard.2014.03.008
Keywords: postmenopausal osteoporosis, denosumab, teriparatide, oral bisphosphonates, efficacy & safety
Citation: Yang J, Guo X, Cui Z, Guo H and Dong J-N (2024) Efficacy and safety of denosumab and teriparatide versus oral bisphosphonates to treat postmenopausal osteoporosis: a systematic review and meta-analysis. Front. Endocrinol. 15:1431676. doi: 10.3389/fendo.2024.1431676
Received: 15 May 2024; Accepted: 14 August 2024;
Published: 02 September 2024.
Edited by:
Robert Daniel Blank, Garvan Institute of Medical Research, AustraliaReviewed by:
Dana Bliuc, Garvan Institute of Medical Research, AustraliaKripa Elizabeth Cherian, Christian Medical College and Hospital, India
Copyright © 2024 Yang, Guo, Cui, Guo and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jia-Nan Dong, Z3Bzcm15eWRqbjIwMjBAMTYzLmNvbQ==
 Jia Yang1
Jia Yang1 Jia-Nan Dong
Jia-Nan Dong