- 1International Medical College, Chongqing Medical University, Chongqing, China
- 2College of Life Sciences, University of Leicester, Leicester, United Kingdom
Background: As the world population recovers from the COVID-19 infection, a series of acute sequelae emerge including new incident diabetes. However, the association between COVID-19 infection and new incident diabetes is not fully understood. We purpose to determine the risk of new incident diabetes after COVID-19 infection.
Methods: PubMed, Embase, and Cochrane Library were used as databases to search for cohort studies published from database inception to February 4, 2024. Two reviewers independently conducted the study screening, data extraction, and risk of bias assessment. A random-effects model was adopted to pool the hazard ratio (HR) with corresponding 95% confidence intervals (CI). Subgroup analysis was conducted to explore the potential influencing factors.
Results: A total of 20 cohort studies with over 60 million individuals were included. The pooling analysis illustrates the association between COVID-19 infection and an increased risk of new incident diabetes (HR = 1.46; 95% CI: 1.38-1.55). In subgroup analysis, the risk of type 1 diabetes was HR=1.44 (95% CI: 1.13-1.82), and type 2 diabetes was HR=1.47 (95% CI: 1.36-1.59). A slightly higher risk of diabetes was found in males (HR=1.37; 95% CI: 1.30-1.45) than in females (HR=1.29; 95% CI: 1.22-1.365). The risk of incident diabetes is associated with hospitalization: non-hospitalized patients have an HR of 1.16 (95% CI: 1.07-1.26), normal hospitalized patients have an HR of 2.15 (95% CI: 1.33-3.49), and patients receiving intensive care have the highest HR of 2.88 (95% CI: 1.73-4.79).
Conclusions: COVID-19 infection is associated with an elevated risk of new incident diabetes. Patients ever infected with COVID-19 should be recognized as a high-risk population with diabetes.
Systematic review registration: https://www.crd.york.ac.uk/prospero, identifier CRD42024522050.
1 Introduction
Diabetes is a chronic non-communicable disease characterized by impaired glucose metabolism that results in persistently raised blood glucose in the context of insufficient insulin caused by autoimmune-mediated destruction of pancreatic β-cells or insulin resistance combined with pancreatic β-cell insufficiency (1). Despite significant process has been made in the exploration of risk factors for diabetes and the implementation of prevention programs, there is a globally increasing incidence and prevalence of the disease (2). Early detection and intensive patient-centered management are expected to optimize the prognosis, reducing morbidity and mortality by preventing or delaying complications (2). A previous study has explored the primary risk factors of diabetes, including BMI, genetics, atmosphere, diet habit, drug use, sedentary way of life, lack of physical exercise, smoking, alcoholic beverages, dyslipidemia, hyperinsulinemia, and improved glucagon activity (3). Recently, the bidirectional interaction between coronavirus disease 2019 (COVID-19) and diabetes has been revealed (4–7). COVID-19 presumably increases the risk of new incident diabetes (8, 9).
The pandemic of COVID-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is recognized as the greatest worldwide public health threat of this century (10). Although the World Health Organization (11) has declared that COVID-19 is no longer a public health emergency of international concern in May 2023, it continues to circulate and evolve, and remains a potentially serious risk to public health. Simultaneously, sequelae after the acute phase of COVID-19 (called long COVID) have aroused wild attention in the medical field (12). Patients with long COVID experience lingering symptoms across multiple organ systems, with common new incident conditions such as diabetes (13). Current reviews revealed an association between COVID and increased incidence of diabetes (14–16), but Zareini et al. (17) indicated an opposite perspective. Therefore, we systematically reviewed the existing cohort studies to clarify the association between COVID-19 and the risk of new incident diabetes.
2 Methods
This systematic review and meta-analysis were conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines (18). The study protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) platform on March 12, 2024 (CRD42024522050).
2.1 Search strategy
We systematically searched PubMed, Embase, and Cochrane Library for studies published up to February 4, 2024. No language restrictions were applied, and the search strategy combined the use of medical subject headings (MeSH) and free text. The search terms were related to COVID-19, Post-Acute COVID-19 Syndrome, Diabetes Mellitus, and risk. The full search strategies are included in Supplementary Tables 1-3. The reference lists of other published meta-analyses were also considered to identify relevant cohort studies.
2.2 Eligibility criteria
Original research studies must meet all the following criteria to be included: (1) the study design was a prospective or retrospective cohort study investigating the association between COVID-19 and the risk of new incident all-type diabetes (no prior history of diabetes); (2) COVID-19 and diabetes were defined based on medical records or International Classification of Diseases (ICD) codes; (3) the hazard ratio (HR) or odds ratio (OR) and its corresponding 95% confidence interval (CI) were reported.
The following were excluded: reviews, study protocols, and commentaries.
2.3 Study selection
Study selection was performed by two reviewers (JYZ and YZW), independently. Titles and abstracts were first screened to exclude duplicate and irrelevant articles. Thereafter, the full texts were examined to identify all eligible studies. If multiple studies conducted assessments from the same database, we include the one with more adequate data based on its sample size and follow-up duration. Any disagreements were resolved by discussing them with the third reviewer (RLX).
2.4 Data extraction
Two reviewers mentioned above (JYZ and YZW) extracted data independently consulting the guidelines on data extraction for systematic reviews and meta-analysis (19). Predesigned forms were used for data extraction, including the first author, year of publication, country, study type, data source, sample size, follow-up duration, mean age, diagnosis criteria of COVID/diabetes, type of diabetes, interval (interval between the first diagnosis of COVID and the onset of diabetes). Disagreements were resolved by consensus with all researchers (JYZ, YZW, and RLX).
2.5 Risk of bias
The quality of the included studies was assessed using the Newcastle-Ottawa scale (NOS) (20). A “star system” was used to judge the studies from three broad perspectives: the selection of participants a measurement of exposure, the comparability of the study groups, and the assessment of outcomes and adequacy of follow-up. Each assessment was carried out by two reviewers (JYZ and YZW) separately and repeatedly. Disagreements were solved by discussion with the third reviewer (RLX).
2.6 Statistical analysis
For this meta-analysis, we sought to identify HRs and 95% CI to assess the association between COVID-19 and the risk of new incident diabetes. Heterogeneity among the studies was evaluated by the χ2 -test and the I2 -values. If I2 > 50%, a random-effects model of analysis was used. We applied a sensitivity analysis by excluding one study each time and rerunning it to verify the robustness of the overall effects. The funnel plot was constructed to inspect and visualize publication bias, and Egger’s regression test was conducted to statically assess it. Subgroup analyses were performed if two or more cohorts were identified. p-values < 0.05 were considered to be statistically significant. All analyses were performed using Stata software (Stata Corp V.14, Texas, USA).
3 Results
3.1 Literature search
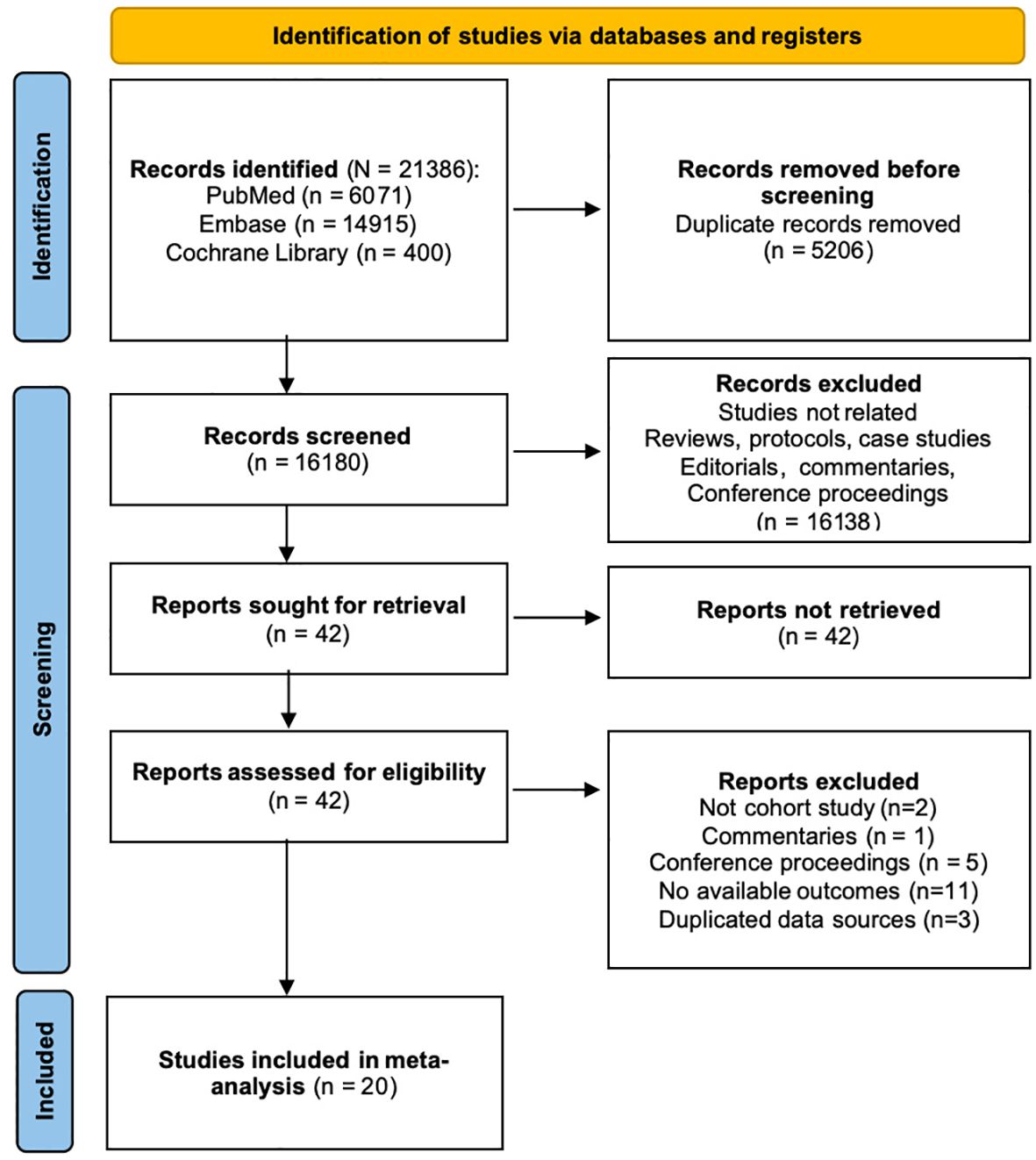
21386 results were obtained after the systematic search. After removing duplicate content and screening the title and abstract, 42 articles were potentially eligible. Full-text articles were all accessible in the remaining 42 studies. Twenty-two studies were excluded after full-text review: 2 were not cohort studies, 1 was commentary, 5 were conference proceedings, 12 did not provide our interested effect sizes and 3 used duplicated data sources. Bowe et al. (21) used the same dataset as Xie and Al-Aly (22) but focused on different outcomes, so we included both. 20 cohort studies (17, 21–39) were included in the meta-analysis. The PRISMA flow diagram illustrating the search and selection process is provided in Figure 1.
3.2 Study characteristics
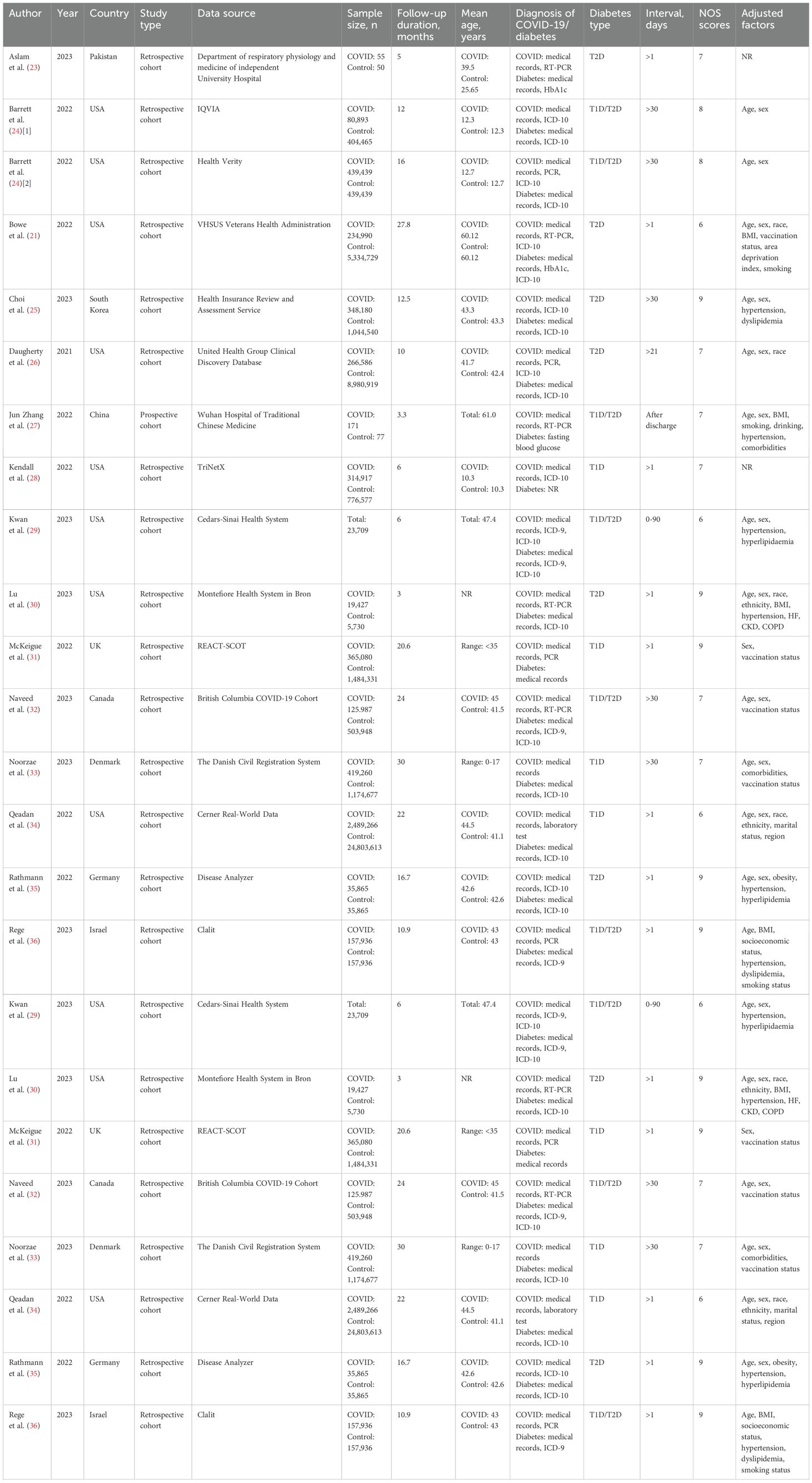
This meta-analysis included 20 cohort studies covering 60,221,176 individuals, which were published between 2021 and 2023. Out of the 20 studies, one was a prospective cohort study, while the other 19 were retrospective studies. Among all the studies included, one reported gestational diabetes mellitus (GDM), five reported type 1 diabetes (T1D), seven reported type 2 diabetes (T2D), and eight reported both T1D and T2D. The follow-up duration of participants ranges from 3 to 84 months. Additional characteristics of the included studies are shown in Table 1.
3.3 Quality assessment
According to the NOS criteria, the average score of all included cohort studies was 8, and the score for five trials (21, 22, 29, 34, 39) was 6 while other 14 trials (17, 23–28, 30–33, 35–38) was 7 or above, indicating that all cohort studies were of relatively high quality in this meta-analysis. The score of each study is shown in Table 1.
3.4 COVID-19 infection and the risk of overall diabetes
We used data from twenty cohort studies (17, 21–39) to explore the association between a history of COVID-19 and the risk of overall diabetes. The pooling analysis reveals that a history of COVID-19 infection is associated with an increased risk of overall diabetes (HR = 1.46; 95% CI: 1.38-1.55; I2 = 92.4%, p < 0.001; Figure 2). The significant heterogeneity in the included studies was interpreted by using a random effect model meta-analysis. Sensitivity analysis shows that none of the individual studies reversed the pool-effect size, indicating that the results are robust (Supplementary Figure 1).
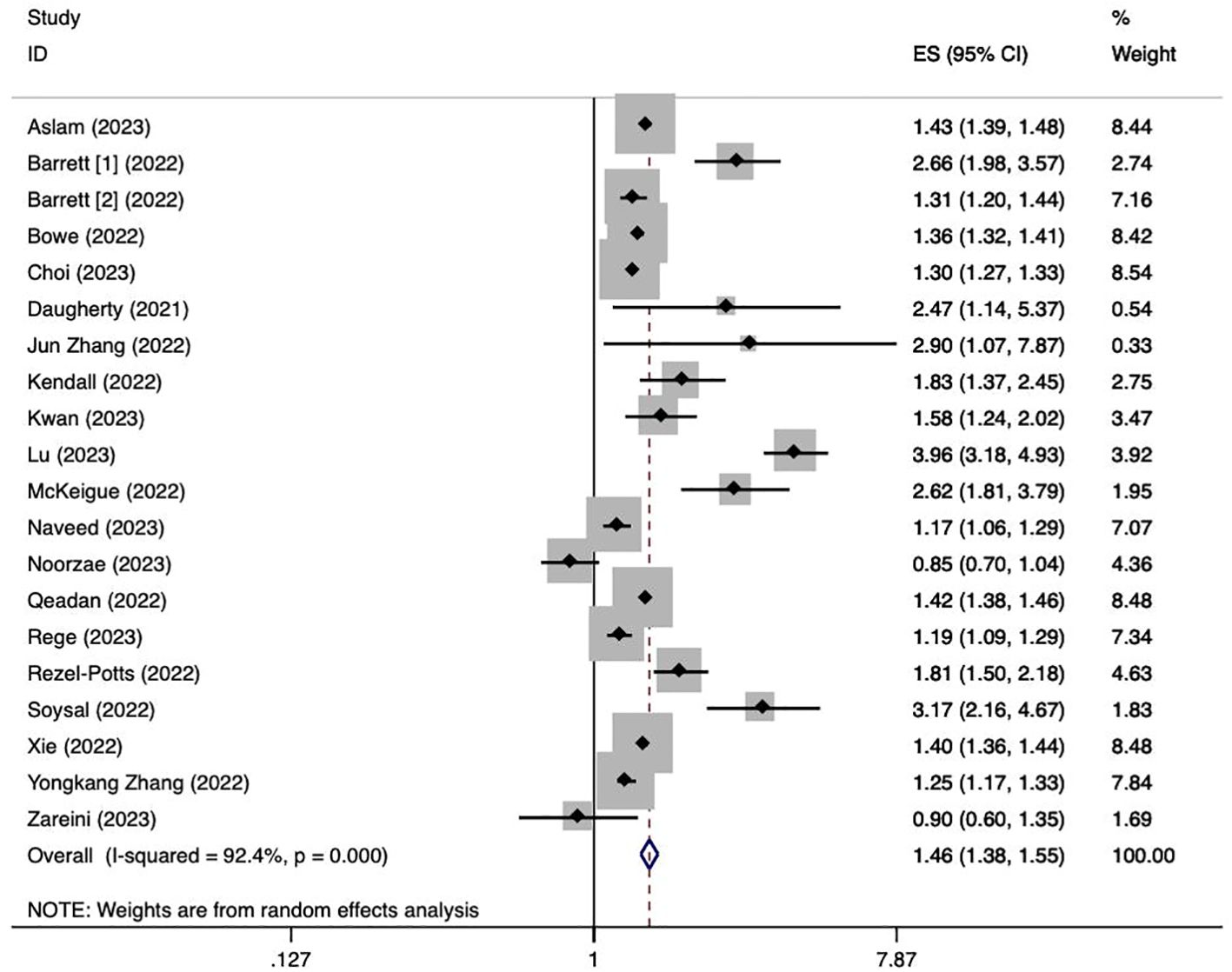
Figure 2. Meta-analysis of the risk of overall diabetes after a history of COVID-19. Barrett [1]: data from IQVIA; Barrett [2]: data from Health Verity.
3.5 Subgroup analysis
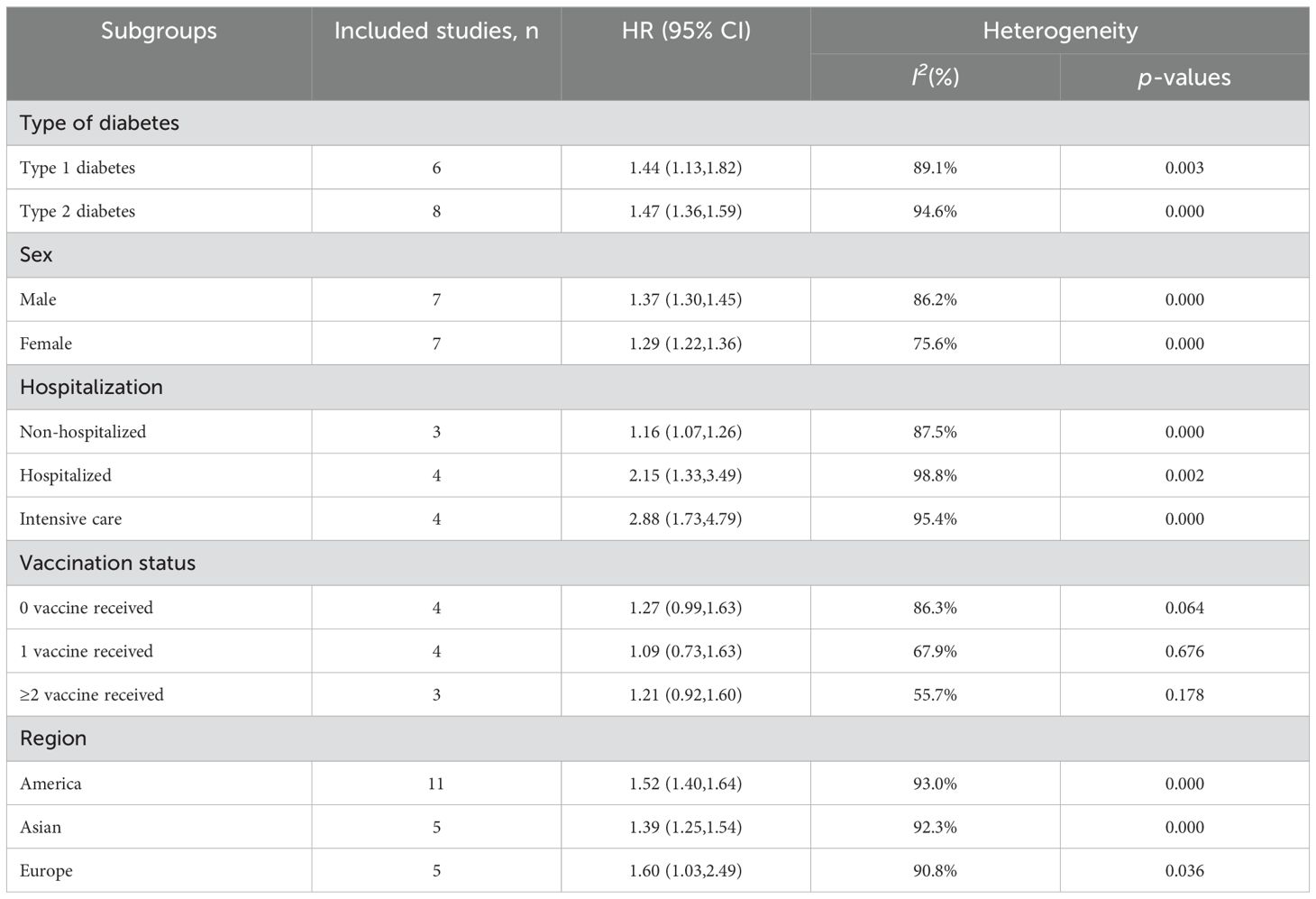
The results of the subgroup analysis are summarized in Table 2. Increased risks of new incident T1D and T2D are associated with COVID-19 infection but there are no significant differences between the two, T1D (HR=1.44; 95% CI: 1.13-1.82; I2 = 89.1%, p = 0.003), and T2D (HR=1.47; 95% CI: 1.36-1.59; I2 = 94.6%, p < 0.001). With stratification by sex, males (HR=1.37; 95% CI: 1.30-1.45; I2 = 86.2%, p < 0.001) are observed with higher risks compared to those for females (HR=1.29; 95% CI: 1.22-1.365; I2 = 75.6%, p < 0.001). In hospitalization-stratified analysis, the pooled risks of new incident diabetes are significantly higher for patients in intensive care (HR=2.88; 95% CI: 1.73-4.79; I2 = 95.4%, p < 0.001) than those for non-hospitalized patients (HR=1.16; 95% CI: 1.07-1.26; I2 = 98.8%, p = 0.002) and normal hospitalized patients (HR=2.15; 95% CI: 1.33-3.49; I2 = 94.6%, p < 0.001). No significant associations are found in the stratification of vaccination status. For populations from different regions, pooled risks were evaluated as America (HR=1.52; 95% CI: 1.40-1.64; I2 = 93.0%, p < 0.001), Asian (HR=1.39; 95% CI: 1.25-1.54; I2 = 92.3%, p < 0.001), and Europe (HR=1.60; 95% CI: 1.03-2.49; I2 = 90.8%, p = 0.036).
3.6 Publication bias
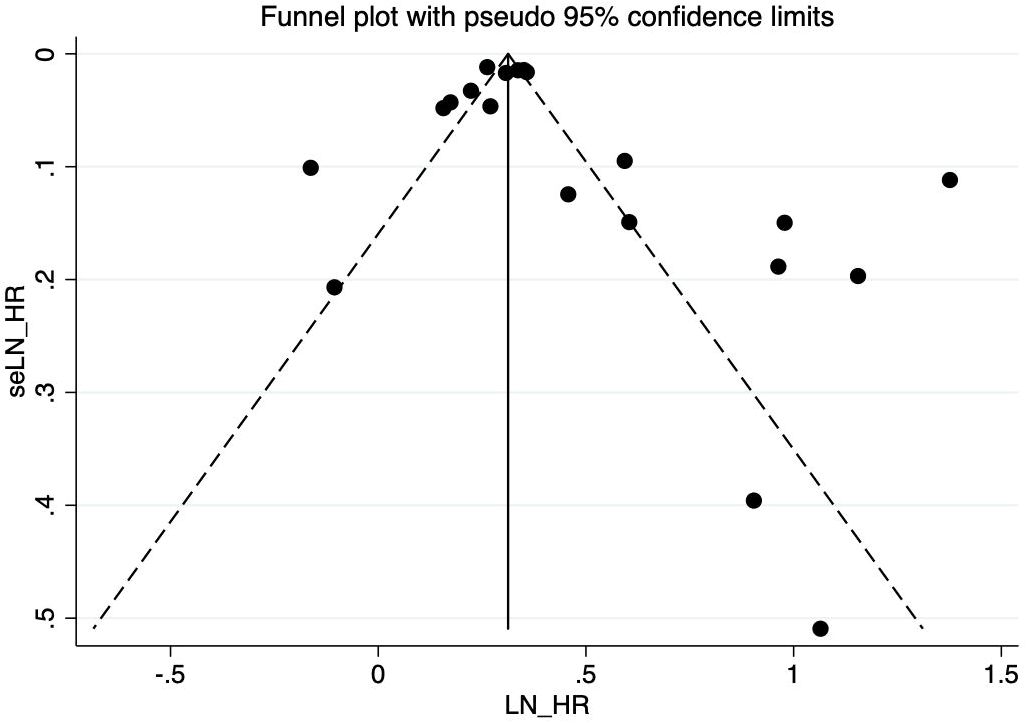
There is no evidence of a significant publication bias in the COVID-19 infection and risk of new incident diabetes revealed from the visual inspection of the funnel plot (Figure 3). Egger’s test (P = 0.166) shows no publication bias in our meta-analysis either.
4 Discussion
4.1 Main findings
We conducted a meta-analysis of 20 cohort studies covering 60,221,176 individuals, which provided a comprehensive evaluation of the association between COVID-19 and new incident diabetes. We find a significant increase in the risk of all-type diabetes among individuals after COVID-19 infection, with an overall 1.46-fold increase in risk. This indicated that COVID-19 infection might be an independent risk factor for new incident diabetes. The importance of screening, prevention, and management of diabetes for patients ever infected with COVID-19 should be emphasized.
4.2 Comparison with previous studies
Our analysis demonstrated a consistent result with previous reviews (14–16), showing that COVID-19 infection increased the risk of all-type diabetes. In addition, Li et al. (40) explored the relationship between new-onset diabetes, hyperglycemia, and COVID-19 infection, showing an elevated incidence and risk. In a review that specifically targeted T2D (41), a higher prevalence of diabetes in people with previous COVID-19 was illustrated, which further corroborated our findings. Compared to prior studies, we added more recent studies and analyzed the data in subgroups, to provide stronger evidence for the association between COVID-19 and diabetes. Simultaneously, we only included data from cases with a confirmed diagnosis of diabetes, contributing to reduced clinical heterogeneity and greater reliability. Although the risk variance between T1D and T2D is not significant in this analysis, a previous study found a higher risk for new incident T2D than T1D for all included cohorts (16). They also indicated that males with COVID-19 were associated with a higher risk of diabetes compared to females, which echoed our conclusions. We assessed the risks between subgroups of hospitalization, vaccination status, and incident diabetes for the first time, and found that an increased risk of diabetes was associated with the exacerbation of hospitalization. However, the risk of new incident diabetes in patients who received vaccination was not statistically significant.
4.3 Interpretation of findings
So far, the pathophysiological mechanism of the association between COVID-19 and diabetes is not entirely clear. It has been suggested that SARS-CoV-2 specifically induces the damage of β-cells, thereby impairing insulin production (42, 43). Angiotensin-converting enzyme 2 (ACE2) is the main receptor of SARS-CoV-2 to gain entry into human cells (44) Several studies have found the ACE2 expression in pancreatic β-cells (42, 45–47), leading to speculation that SARS-CoV-2 may triggers β-cell damage by penetrating the cells using ACE2 (48). In addition to ACE2, other SARS-CoV-2 related entry factors such as TMPRSS2, NRP1, and TRFC are also expressed in pancreatic β-cells, which might play roles in β-cell damage through similar mechanisms (42, 49). However, the expressions of ACE2 and TMPRSS2 in pancreatic β-cells were doubted in other studies (50–52). Therefore, further research is necessary.
ACE2 is a key enzyme in the renin-angiotensin system (RAS). Membrane-bound ACE2 is responsible for catalyzing the conversion from Ang II into Ang-(1-7) (53). Down-regulation of ACE2 is found in patients with COVID-19 that enhances activation of the RAS axis, resulting in decreased insulin and glucose delivery to tissues and impairment of insulin signaling pathways, all of which lead to insulin resistance (54, 55). Additionally, uncontrolled inflammatory response caused by RAS imbalance might account for the potential role in pancreatic dysfunction (53, 54).
Autopsy tissue from deceased COVID-19 patients showed that local inflammation and infiltration of immune cells were associated with impairment of β-cells, causing various degrees of metabolic dysregulation (50). SARS-CoV-2 triggers a macrophage-mediated cytokine storm in which the overactivation of immune cells and persistently increasing cytokines promote excessive inflammation and further induced β-cell damage (56). SARS-CoV-2 induce a decreased chromatin-modifying enzyme SETDB2, causing increased transcription of inflammatory cytokines which impair the pancreas (56).
Steroids are used to treat COVID-19, but their pharmacological effects pose extra burden on blood glucose control (57). Steroid-induced hyperglycemia in patients with COVID-19 may be associated with an increased risk of new incident diabetes (9). A cohort study revealed a higher risk of diabetes in COVID-19 patients using glucocorticoids compared to those without steroid treatments (58), which corroborates this perspective.
Lockdowns during the COVID-19 pandemic slowed the rate of infection but caused negative mental health consequences and adverse health-related behaviors, including reduced physical activities, unhealthy eating, smoking, and binge drinking, which are risk factors for diabetes (59). Symptoms of long COVID such as fatigue, muscle pain, and dyspnea, limit exercise capacity (60), therefore sedentary lifestyles have become common. These changes of lifestyle have a series of pathophysiological effects, including metabolic consequences represented by insulin resistance, which might increase the risks of new incident diabetes (61).
In the subgroup analysis, males with a history of COVID-19 have a higher risk of new incident diabetes than females. A previous study has shown that males infected with COVID-19 are more susceptible to worse outcomes and death, independent of age (62). From another perspective, a study on rats indicated a gender-related difference of ACE2 expression, that ACE2 content was slightly lower in males compared to females (63). This might be attributed to diabetes-related pathophysiological changes. Considering vaccination has shown a potential effectiveness on improvement in long-COVID symptoms (64), it might be also helpful to prevent new incident diabetes in patients ever infected with COVID-19, which accounted for the insignificant association between COVID-19 and incident diabetes.
4.4 Implications and limitations
The pandemic of COVID-19 has placed a tremendous burden on humanity and might co-exist with us for many years. Our meta-analysis summarizes the existing evidence of the association between COVID-19 infection and the risk of new incident diabetes and shows that a history of COVID-19 is a risk factor for all-type diabetes. It suggests that the identification of high-risk groups of diabetes should cover patients with COVID-19, which is conducive to the early detection and management of diabetes. Vaccination is of critical importance for individuals to reduce the risks of adverse outcomes. More studies should be fostered to clarify the potential mechanisms underlying the COVID-related diabetes, given there might be a complex combination of pathophysiological processes behind the COVID-19 infection and new incident diabetes.
Meanwhile, this study has certain limitations. We only included cohort studies of which retrospective cohort studies are the majority. Though there is a broad and deep use of electronic databases based on validated definitions, it still cannot exclude the bias caused by misclassification, particularly for diabetes types. Moreover, the intervals between COVID-19 infection and diabetes diagnosis differ in studies, which might lead to high heterogeneity, and make it hard to discuss the risks of incident diabetes in different phases of COVID-19. Age stratification is diverse among included studies, so we did not pool related data.
5 Conclusions
Patients ever infected with COVID-19 had an elevated incidence and risk of new incident diabetes. However, more studies are necessary to specify the pathophysiological mechanisms underlying this association.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
JZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. YW: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. RX: Data curation, Investigation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1429848/full#supplementary-material
References
1. Harcourt BE, Penfold SA, Forbes JM. Coming full circle in diabetes mellitus: from complications to initiation. Nat Rev Endocrinol. (2013) 9:113–23. doi: 10.1038/nrendo.2012.236
2. Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet (London England). (2017) 389:2239–51. doi: 10.1016/S0140-6736(17)30058-2
3. Alam S, Hasan MK, Neaz S, Hussain N, Hossain MF, Rahman T. Diabetes mellitus: insights from epidemiology, biochemistry, risk factors, diagnosis, complications and comprehensive management. Diabetology. (2021) 2:36–50. doi: 10.3390/diabetology2020004
4. Rey-Reñones C, Martinez-Torres S, Martín-Luján FM, Pericas C, Redondo A, Vilaplana-Carnerero C, et al. Type 2 diabetes mellitus and COVID-19: A narrative review. Biomedicines. (2022) 10:2089. doi: 10.3390/biomedicines10092089
5. Lima-Martínez MM, Carrera Boada C, Madera-Silva MD, Marín W, Contreras M. COVID-19 and diabetes: A bidirectional relationship. Clinica e investigacion en arteriosclerosis: publicacion oficial la Sociedad Espanola Arterioscler. (2021) 33:151–7. doi: 10.1016/j.arteri.2020.10.001
6. Raveendran AV, Misra A. Post COVID-19 syndrome (“Long COVID”) and diabetes: challenges in diagnosis and management. Diabetes Metab syndrome. (2021) 15:102235. doi: 10.1016/j.dsx.2021.102235
7. Cao H, Baranova A, Wei X, Wang C, Zhang F. Bidirectional causal associations between type 2 diabetes and COVID-19. J Med Virol. (2023) 95:e28100. doi: 10.1002/jmv.28100
8. Sathish T, Tapp RJ, Cooper ME, Zimmet P. Potential metabolic and inflammatory pathways between COVID-19 and new-onset diabetes. Diabetes Metab. (2021) 47:101204. doi: 10.1016/j.diabet.2020.10.002
9. Khunti K, Del Prato S, Mathieu C, Kahn SE, Gabbay RA, Buse JB. COVID-19, hyperglycemia, and new-onset diabetes. Diabetes Care. (2021) 44:2645–55. doi: 10.2337/dc21-1318
10. Yuan Y, Jiao B, Qu L, Yang D, Liu R. The development of COVID-19 treatment. Front Immunol. (2023) 14:1125246. doi: 10.3389/fimmu.2023.1125246
11. World Health Organization. Statement on the fifteenth meeting of the IHR, (2005) Emergency Committee on the COVID-19 pandemic (2023). Available online at: https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meeting-of-the-international-health-regulations-%282005%29-emergency-committee-regarding-the-coronavirus-disease-%28covid-19%29-pandemic (Accessed 11 April 2024).
12. Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. (2023) 21:133–46. doi: 10.1038/s41579-022-00846-2
13. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. (2021) 594:259–64. doi: 10.1038/s41586-021-03553-9
14. Sathish T, Kapoor N, Cao Y, Tapp RJ, Zimmet P. Proportion of newly diagnosed diabetes in COVID-19 patients: A systematic review and meta-analysis. Diabetes Obes Metab. (2021) 23:870–4. doi: 10.1111/dom.14269
15. Zhang T, Mei Q, Zhang Z, Walline JH, Liu Y, Zhu H, et al. Risk for newly diagnosed diabetes after COVID-19: a systematic review and meta-analysis. BMC Med. (2022) 20:444. doi: 10.1186/s12916-022-02656-y
16. Lai H, Yang M, Sun M, Pan B, Wang Q, Wang J, et al. Risk of incident diabetes after COVID-19 infection: A systematic review and meta-analysis. Metabolism: Clin Exp. (2022) 137:155330. doi: 10.1016/j.metabol.2022.155330
17. Zareini B, Sørensen KK, Eiken PA, Fischer TK, Kristensen PL, Lendorf ME, et al. Association of COVID-19 and development of type 1 diabetes: A Danish nationwide register study. Diabetes Care. (2023) 46:1477–82. doi: 10.2337/dc23-0428
18. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Systematic Rev. (2021) 10:89. doi: 10.1186/s13643-021-01626-4
19. Taylor KS, Mahtani KR, Aronson JK. Summarising good practice guidelines for data extraction for systematic reviews and meta-analysis. BMJ Evidence-Based Med. (2021) 26:88–90. doi: 10.1136/bmjebm-2020-111651
20. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses (2014). Oxford. Available online at: http://www.evidencebasedpublichealth.de/download/Newcastle_Ottowa_Scale_Pope_Bruce.pdf (Accessed March 12, 2023).
21. Bowe B, Xie Y, Al-Aly Z. Acute and postacute sequelae associated with SARS-CoV-2 reinfection. Nat Med. (2022) 28:2398–405. doi: 10.1038/s41591-022-02051-3
22. Xie Y, Al-Aly Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. (2022) 10:311–21. doi: 10.1016/S2213-8587(22)00044-4
23. Aslam B, Naeem B, Aman S, Majeed M. Association between covid-19 and diabetes mellitus. Pakistan J Med Health Sci. (2023) 17:117–9. doi: 10.53350/pjmhs2023174117
24. Barrett CE, Koyama AK, Alvarez P, Chow W, Lundeen EA, Perrine CG, et al. Risk for newly diagnosed diabetes >30 days after SARS-CoV-2 infection among persons aged <18 years - United States, March 1, 2020-June 28, 2021. MMWR. Morbidity Mortality Weekly Rep. (2022) 71:59–65. doi: 10.15585/mmwr.mm7102e2
25. Choi JH, Kim KM, Song K, Seo GH. Risk for Newly Diagnosed Type 2 Diabetes Mellitus after COVID-19 among Korean Adults: A Nationwide Matched Cohort Study. Endocrinol Metab (Seoul Korea). (2023) 38:245–52. doi: 10.3803/EnM.2023.1662
26. Daugherty SE, Guo Y, Heath K, Dasmariñas MC, Jubilo KG, Samranvedhya J, et al. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ (Clinical Res ed.). (2021) 373:n1098. doi: 10.1136/bmj.n1098
27. Zhang J, Shu T, Zhu R, Yang F, Zhang B, Lai X. The long-term effect of COVID-19 disease severity on risk of diabetes incidence and the near 1-year follow-up outcomes among postdischarge patients in Wuhan. J Clin Med. (2022) 11:3094. doi: 10.3390/jcm11113094
28. Kendall EK, Olaker VR, Kaelber DC, Xu R, Davis PB. Association of SARS-coV-2 infection with new-onset type 1 diabetes among pediatric patients from 2020 to 2021. JAMA Network Open. (2022) 5:e2233014. doi: 10.1001/jamanetworkopen.2022.33014
29. Kwan AC, Ebinger JE, Botting P, Navarrette J, Claggett B, Cheng S. Association of COVID-19 vaccination with risk for incident diabetes after COVID-19 infection. JAMA Network Open. (2023) 6:e2255965. doi: 10.1001/jamanetworkopen.2022.55965
30. Lu JY, Wilson J, Hou W, Fleysher R, Herold BC, Herold KC, et al. Incidence of new-onset in-hospital and persistent diabetes in COVID-19 patients: comparison with influenza. EBioMedicine. (2023) 90:104487. doi: 10.1016/j.ebiom.2023.104487
31. McKeigue PM, McGurnaghan S, Blackbourn L, Bath LE, McAllister DA, Caparrotta TM, et al. Relation of incident type 1 diabetes to recent COVID-19 infection: cohort study using e-health record linkage in Scotland. Diabetes Care. (2023) 46:921–8. doi: 10.2337/dc22-0385
32. Naveed Z, Velásquez García HA, Wong S, Wilton J, McKee G, Mahmood B, et al. Association of COVID-19 infection with incident diabetes. JAMA Network Open. (2023) 6:e238866. doi: 10.1001/jamanetworkopen.2023.8866
33. Noorzae R, Junker TG, Hviid AP, Wohlfahrt J, Olsen SF. Risk of type 1 diabetes in children is not increased after SARS-coV-2 infection: A nationwide prospective study in Denmark. Diabetes Care. (2023) 46:1261–4. doi: 10.2337/dc22-2351
34. Qeadan F, Tingey B, Egbert J, Pezzolesi MG, Burge MR, Peterson KA, et al. The associations between COVID-19 diagnosis, type 1 diabetes, and the risk of diabetic ketoacidosis: A nationwide cohort from the US using the Cerner Real-World Data. PloS One. (2022) 17:e0266809. doi: 10.1371/journal.pone.0266809
35. Rathmann W, Kuss O, Kostev K. Incidence of newly diagnosed diabetes after Covid-19. Diabetologia. (2022) 65:949–54. doi: 10.1007/s00125-022-05670-0
36. Reges O, Test T, Hoshen M, Cicurel A, Saliba W, Greenland P, et al. Time-varying association of acute and post-acute COVID-19 with new-onset diabetes mellitus among hospitalized and non-hospitalized patients. BMJ Open Diabetes Res Care. (2023) 11:e003052. doi: 10.1136/bmjdrc-2022-003052
37. Rezel-Potts E, Douiri A, Sun X, Chowienczyk PJ, Shah AM, Gulliford MC. Cardiometabolic outcomes up to 12 months after COVID-19 infection: A matched cohort study in the UK. PloS Med. (2022) 19:e1004052. doi: 10.1371/journal.pmed.1004052
38. Soysal Ç, Yılmaz E. The effect of previous covid-19 infection on gestational diabetes mellitus. Gazi Med J. (2022) 33:397–400. https://medicaljournal.gazi.edu.tr/index.php/GMJ/article/view/3594.
39. Zhang Y, Romieu-Hernandez A, Boehmer TK, Azziz-Baumgartner E, Carton TW, Gundlapalli AV, et al. Association between SARS-CoV-2 infection and select symptoms and conditions 31 to 150 days after testing among children and adults. BMC Infect Dis. (2024) 24:181. doi: 10.1186/s12879-024-09076-8
40. Li J, Li Y, Wang Z, Liu N, He L, Zhang H. Increased risk of new-onset diabetes in patients with COVID-19: a systematic review and meta-analysis. Front Public Health. (2023) 11:1170156. doi: 10.3389/fpubh.2023.1170156
41. Bellia C, Andreadi A, D’Ippolito I, Scola L, Barraco S, Meloni M, et al. Prevalence and risk of new-onset diabetes mellitus after COVID-19: a systematic review and meta-analysis. Front Endocrinol. (2023) 14:1215879. doi: 10.3389/fendo.2023.1215879
42. Wu CT, Lidsky PV, Xiao Y, Lee IT, Cheng R, Nakayama T, et al. SARS-CoV-2 infects human pancreatic β cells and elicits β cell impairment. Cell Metab. (2021) 33:1565–1576.e5. doi: 10.1016/j.cmet.2021.05.013
43. Accili D. Can COVID-19 cause diabetes? Nat Metab. (2021) 3:123–5. doi: 10.1038/s42255-020-00339-7
44. Gusev E, Sarapultsev A, Solomatina L, Chereshnev V. SARS-coV-2-specific immune response and the pathogenesis of COVID-19. Int J Mol Sci. (2022) 23:1716. doi: 10.3390/ijms23031716
45. Fignani D, Licata G, Brusco N, Nigi L, Grieco GE, Marselli L, et al. SARS-coV-2 receptor angiotensin I-converting enzyme type 2 (ACE2) is expressed in human pancreatic β-cells and in the human pancreas microvasculature. Front Endocrinol. (2020) 11:596898. doi: 10.3389/fendo.2020.596898
46. Liu F, Long X, Zhang B, Zhang W, Chen X, Zhang Z. ACE2 expression in pancreas may cause pancreatic damage after SARS-coV-2 infection. Clin Gastroenterol Hepatol. (2020) 18:2128–2130.e2. doi: 10.1016/j.cgh.2020.04.040
47. Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta diabetologica. (2010) 47:193–9. doi: 10.1007/s00592-009-0109-4
48. Wang Y, Guo H, Wang G, Zhai J, Du B. COVID-19 as a trigger for type 1 diabetes. J Clin Endocrinol Metab. (2023) 108:2176–83. doi: 10.1210/clinem/dgad165
49. Müller JA, Groß R, Conzelmann C, Krüger J, Merle U, Steinhart J, et al. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat Metab. (2021) 3:149–65. doi: 10.1038/s42255-021-00347-1
50. Steenblock C, Richter S, Berger I, Barovic M, Schmid J, Schubert U, et al. Viral infiltration of pancreatic islets in patients with COVID-19. Nat Commun. (2021) 12:3534. doi: 10.1038/s41467-021-23886-3
51. Coate KC, Cha J, Shrestha S, Wang W, Gonçalves LM, Almaça J, et al. SARS-coV-2 cell entry factors ACE2 and TMPRSS2 are expressed in the microvasculature and ducts of human pancreas but are not enriched in β Cells. Cell Metab. (2020) 32:1028–1040.e4. doi: 10.1016/j.cmet.2020.11.006
52. Kusmartseva I, Wu W, Syed F, van der Heide V, Jorgensen M, Joseph P, et al. Expression of SARS-coV-2 entry factors in the pancreas of normal organ donors and individuals with COVID-19. Cell Metab. (2020) 32:1041–1051.e6. doi: 10.1016/j.cmet.2020.11.005
53. Lanza K, Perez LG, Costa LB, Cordeiro TM, Palmeira VA, Ribeiro VT, et al. Covid-19: the renin-angiotensin system imbalance hypothesis. Clin Sci (London England: 1979). (2020) 134:1259–64. doi: 10.1042/CS20200492
54. Jandeleit-Dahm KA, Tikellis C, Reid CM, Johnston CI, Cooper ME. Why blockade of the renin-angiotensin system reduces the incidence of new-onset diabetes. J hypertension. (2005) 23:463–73. doi: 10.1097/01.hjh.0000160198.05416.72
55. Mehrabadi ME, Hemmati R, Tashakor A, Homaei A, Yousefzadeh M, Hemati K, et al. Induced dysregulation of ACE2 by SARS-CoV-2 plays a key role in COVID-19 severity. Biomedicine pharmacotherapy = Biomedecine pharmacotherapie. (2021) 137:111363. doi: 10.1016/j.biopha.2021.111363
56. Melvin WJ, Audu CO, Davis FM, Sharma SB, Joshi A, DenDekker A, et al. Coronavirus induces diabetic macrophage-mediated inflammation via SETDB2. Proc Natl Acad Sci United States America. (2021) 118:e2101071118. doi: 10.1073/pnas.2101071118
57. Sosale A, Sosale B, Kesavadev J, Chawla M, Reddy S, Saboo B, et al. Steroid use during COVID-19 infection and hyperglycemia - What a physician should know. Diabetes Metab syndrome. (2021) 15:102167. doi: 10.1016/j.dsx.2021.06.004
58. Jabbour RM, Hallit S, Saliby R, Baydoun AEK, Nakhoul N. Is COVID-19 incriminated in new onset type 2 diabetes mellitus in Lebanese adults? BMC Res Notes. (2023) 16:176. doi: 10.1186/s13104-023-06454-4
59. Keng SL, Stanton MV, Haskins LB, Almenara CA, Ickovics J, Jones A, et al. COVID-19 stressors and health behaviors: A multilevel longitudinal study across 86 countries. Prev Med Rep. (2022) 27:101764. doi: 10.1016/j.pmedr.2022.101764
60. Koc HC, Xiao J, Liu W, Li Y, Chen G. Long COVID and its management. Int J Biol Sci. (2022) 18:4768–80. doi: 10.7150/ijbs.75056
61. Martinez-Ferran M, de la Guía-Galipienso F, Sanchis-Gomar F, Pareja-Galeano H. Metabolic impacts of confinement during the COVID-19 pandemic due to modified diet and physical activity habits. Nutrients. (2020) 12:1549. doi: 10.3390/nu12061549
62. Jin JM, Bai P, He W, Wu F, Liu XF, Han DM, et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health. (2020) 8:152. doi: 10.3389/fpubh.2020.00152
63. Xie X, Chen J, Wang X, Zhang F, Liu Y. Age- and gender-related difference of ACE2 expression in rat lung. Life Sci. (2006) 78:2166–71. doi: 10.1016/j.lfs.2005.09.038
Keywords: COVID-19, long COVID, SARS-CoV-2, diabetes mellitus, public health
Citation: Zhou J, Wang Y and Xu R (2024) Association of COVID-19 infection and the risk of new incident diabetes: a systematic review and meta-analysis. Front. Endocrinol. 15:1429848. doi: 10.3389/fendo.2024.1429848
Received: 08 May 2024; Accepted: 08 August 2024;
Published: 26 August 2024.
Edited by:
Jeff M. P. Holly, University of Bristol, United KingdomReviewed by:
Mohammed Amir Rais, University of Algiers, AlgeriaEva Szabo, University of Pécs, Hungary
Copyright © 2024 Zhou, Wang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingye Zhou, amluZ3llemhvdTExNkBnbWFpbC5jb20=
 Jingye Zhou
Jingye Zhou Yuzhu Wang
Yuzhu Wang Ruolan Xu
Ruolan Xu