- 1Department of Orthopedics, The First People’s Hospital of Kunshan, Gusu School, Nanjing Medical University, Suzhou, Jiangsu, China
- 2Department of Orthopedics, Affiliated Kunshan Hospital of Jiangsu University, Suzhou, Jiangsu, China
- 3Information Department, Affiliated Kunshan Hospital of Jiangsu University, Suzhou, Jiangsu, China
Objective: To evaluate the correlation between the triglyceride-glucose (TyG) index and bone turnover markers (BTMs) in osteoporotic fractures (OPFs) patients hospitalized for surgical intervention.
Methods: A retrospective cross-sectional study was conducted on 3558 OPFs patients hospitalized for surgical intervention between January 2017 and July 2022. The study obtained baseline values for various biomarkers and covariates, including fasting blood glucose, β-C-terminal telopeptide of type I collagen (β-CTX), procollagen type 1 N-terminal propeptide (P1NP), triglycerides, age, sex, body mass index, smoking, drinking, low-density lipoprotein, high-density lipoprotein, aspartate aminotransferase, uric acid, the score of American society of anesthesiologists, homocysteine, parathyroid hormone, apolipoprotein B, apolipoprotein A, magnesium, phosphorus and calcium. Multiple linear regression, curve fitting, threshold effects, and subgroup analyses were also applied.
Results: After adjusting for covariates in the regression analysis, the results revealed a negative correlation between β-CTX and P1NP levels and the baseline TyG index. Specifically, a one-unit increase in the TyG index was associated with a reduction in β-CTX levels of -0.06 (95% CI: -0.10, -0.01; P-value = 0.012) and a reduction in P1NP levels of -4.70 (95% CI: -9.30, -0.09; P-value = 0.046). Additionally, the inflection points for the nonlinear correlation between the TyG index and β-CTX and P1NP were found to be K = 6.31 and K = 6.63, respectively.
Conclusion: The study demonstrated a negative, non-linear relationship among the TyG index, β-CTX and P1NP in OPFs patients hospitalized for surgical intervention. These findings suggest that elevated TyG index levels may adversely affect bone turnover, potentially contributing to the progression of OP.
Introduction
Osteoporosis (OP) is a prevalent metabolic bone disorder in the elderly, characterized by a gradual and continuous reduction in bone density and increased susceptibility to skeletal fragility (1). It is characterized by a bone mineral density (BMD) that is ≤ 2.5 standard deviations (SD) beneath the average peak of bone weight of normal adults (2, 3). Fragility fractures associated with OP are estimated to affect one-fifth of males and one-third of females aged 50 and above (4, 5). Currently, OP imposes an annual economic burden of $650 billion in the United States, Canada, and Europe, and this number is rapidly increasing (4, 6). Therefore, identifying and mitigating factors contributing to OP and improving overall bone health is crucial for public health and socioeconomic efforts.
Aging, obesity, and insulin resistance have been shown to cause pathogenic bone turnover and disruption of bone homeostasis (7, 8). The hyperinsulinemic-euglycemic clamp (HIEC) is usually recognized as the best method for assessing insulin resistance (IR); yet, its time-consuming and complex design limits its use in large clinical settings (9). To address these constraints, the triglyceride-glucose (TyG) index, which is measured as Ln [triglyceride (TG) (mg/dL) × fasting blood glucose (FBG) (mg/dL)/2] (7), has been recently recognized as a promising alternative biomarker for IR (10, 11). Recent studies have revealed a link between the TyG index and several conditions, including coronary artery disease (12), depression (13), hypertension (14), ischemic stroke (9), heart failure (15), liver fibrosis (16), kidney stone (17) and erectile dysfunction in men (18). Nevertheless, the correlation between this index and OP fractures (OPFs) patients hospitalized for surgical intervention remains unexplored.
Bone turnover markers (BTMs) are indicators of bone remodeling rates. They are byproducts of the bone remodeling process and can be measured in urine or serum (19). The International Osteoporosis Foundation (IOF) and the International Federation of Clinical Chemistry (IFCC) have identified serum β-C-terminal telopeptide of type I collagen (β-CTX) and procollagen type 1 N-terminal propeptide (P1NP) as reference indicators for bone development and resorption. These indicators are suggested for assessing fracture risk and tracking treatment for OP, as they can change with the progression of the disease (19). Both β-CTX and P1NP are degradation products of type I collagen. P1NP is released by osteoblasts during bone formation, while β-CTX is secreted by osteoclasts during bone resorption. As such, both markers are valuable for evaluating bone development and resorption (20).
Previous research has explored insulin resistance-related dysregulation of bone homeostasis in various populations, including patients with type 2 diabetes (7), non-diabetic Koreans (10), adult Americans (21), middle-aged and elderly non-diabetic Chinese individuals (22), and postmenopausal females (23). These studies have also investigated the relationship between the TyG index and bone mineral density (BMD). However, the connection between the TyG index and BTMs in OPFs patients hospitalized for surgical intervention has not been thoroughly investigated or supported by evidence. Therefore, the primary aim of the current study was to examine the potential correlation between the TyG index and BTMs.
Materials and methods
Ethical consideration
The study was carried out according to the Declaration of Helsinki and was approved by the Affiliated Kunshan Hospital of Jiangsu University (AKHJU) (2021-06-015-K01). During data analysis, patient information was kept confidential from the investigators. Before the study’s commencement, written consent was obtained from all participants.
Research participants and design
This study was carried out at the AKHJU in Jiangsu Province, China. Electronic patient records were obtained for all individuals aged 50 and above who had recently been diagnosed with osteoporotic fractures (OPFs) requiring hospitalization between January 1, 2017, and July 27, 2022. An OPF was defined as a fragility fracture of the wrist, proximal humerus, hip, or vertebra that required hospitalization for surgical intervention and was diagnosed using the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10), specifically codes starting with S22, S32, S42, S52, or S72 (24). A total of 3,558 OPFs patients hospitalized for surgical intervention were included in the study. Exclusions were made for patients who: 1) did not provide data on the TyG index or BTMs, 2) had extreme values for TyG index or BTMs, 3) were under 50 years of age, 4) had a history of malignant tumors or psychiatric disorders, 5) had experienced myocardial infarction, cerebral hemorrhage, severe hepatic or renal insufficiency, acute infection, or stress within the past 3 months, or 6) were taking medications that could impact bone metabolism (7). A total of 578 patients met the inclusion criteria for the current analysis (refer to Figure 1).
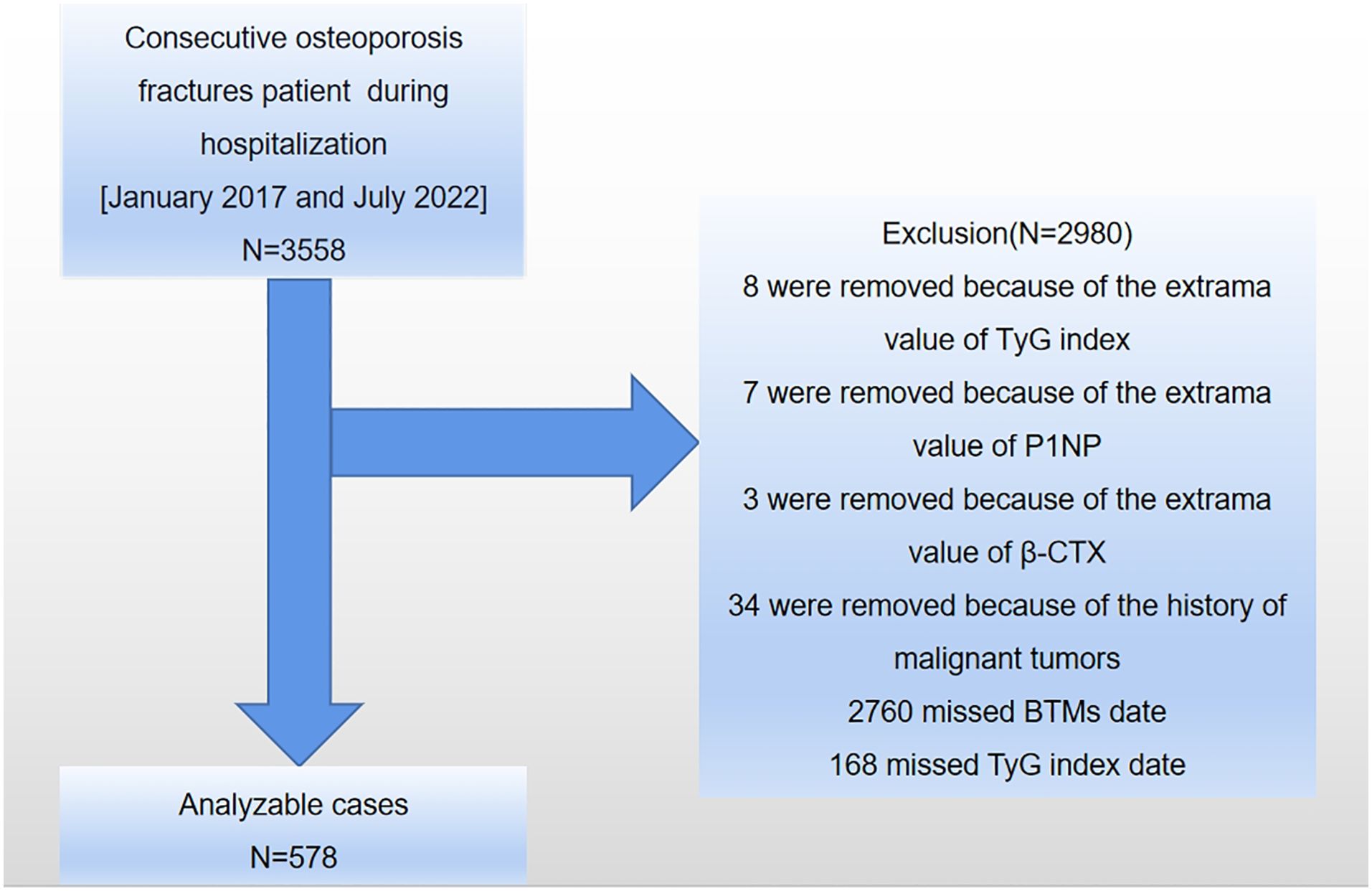
Figure 1. A diagrammatic representation of the study’s design. TyG index, triglyceride-glucose index; P1NP, type I procollagen N-terminal propeptide; β-CTX, type I collagen β-C-terminal telopeptide; BTMs, bone turnover markers.
Study variables
The mentioned Ln [TG (mg/dL) × FBG (mg/dL)/2] (7) formula was used to determine the TyG index. A Beckman AU5800 automated biochemistry analyzer was employed to observe FBG using the hexokinase method and TG via the GPO-POD method. An electrochemiluminescence immunoassay was conducted on a Roche Cobas 8000 analyzer to quantify β-CTX and P1NP. The following covariates were included in the analysis: body mass index (BMI), sex, age, smoking, drinking, homocysteine, American Society of Anesthesiologists (ASA) score, aspartate aminotransferase (AST), uric acid (UA), magnesium (Mg), phosphorus (P), calcium (Ca), triglycerides (TG), fasting blood glucose (FBG), low-density lipoprotein (LDL), high-density lipoprotein (HDL), parathyroid hormone (PTH), apolipoprotein B (Apo B), and apolipoprotein A (Apo A). The Beckman AU5800 automated biochemical analyzer was used for further analysis. AST and homocysteine levels were measured using the enzymatic method, while UA was assessed with the uricase-peroxidase method. Ca was measured using the arsenazo III method, Mg with the dimethylaminoazobenzene method, and P using the phosphomolybdate method. HDL and LDL were measured using the direct method, and Apo A and Apo B were assessed using the immunoturbidimetric method. PTH was measured with the Beckman DXI800 analyzer using the chemiluminescent immunoassay method.
For BMI measurement, weight (kg) was divided by the square of height ^2 (m). The current or former smokers within the previous 12 months were identified as having engaged in smoking. Weekly alcohol consumption over the past 12 months was used to identify drinking habits. All patients underwent an 8-hour fasting period before blood collection, during which all experimental measurements were taken.
Statistics
In the study, categorical data were represented as frequencies (percentages), whereas continuous demographic, laboratory, and clinical information were displayed as means ± SD or medians (25th, 75th). Univariate analyses of categorical data were performed using Pearson’s chi-square tests or Fisher’s exact tests. For continuous data, independent sample t-tests and Mann-Whitney U tests were used, depending on whether the data followed a normal or non-normal distribution. Furthermore, the univariate analysis was performed to explore the correlation between BTMs and the characteristics of OPFs patients hospitalized for surgical intervention.
Appropriate covariate adjustments have been implemented using the generalized estimating equation (GEE) to examine the independent connection between TyG index levels and BTMs in OPFs patients hospitalized for surgical intervention. The models that were developed comprised three variations: Model 1 (unadjusted), Model 2 (minimally adjusted), and Model 3 (completely adjusted). Initially, to identify collinearity among these covariates, a variance inflation factor (VIF) analysis was executed. Afterward, adjustments for these covariates were determined using the following standards: (1) the standardized regression coefficient (β) or matched odds ratio (OR) changed by ≥ 10% when a covariate was included or excluded from the baseline or full model; and (2) if the P-value for the covariate in model 1 or the univariate model was ≤ 0.1.
The identification of possible non-linear correlations was accomplished using a generalized additive model (GAM). In cases where an apparent correlation existed, the threshold effect of the resultant smoothed curve was determined using a two-segment linear regression model. A recursive method was employed to independently compute the inflection point applying a maximum likelihood model in situations where these curves displayed a distinct ratio. Subgroup analyses were also conducted on clinically relevant subsets of study participants to enhance the reliability of the findings. A two-tailed test revealed a significant threshold at P-value ≤ 0.05.
This study utilizes GPT-3.5 as a language refinement tool. A threshold level for all evaluations was noted via a two-sided P-value ≤ 0.05 obtained from the R packages (http://www.R-project.org, The R Foundation) and Empower Stats (http://www.empowerstats.com, X&Y Solutions, Inc, MA, USA).
Results
Patient details
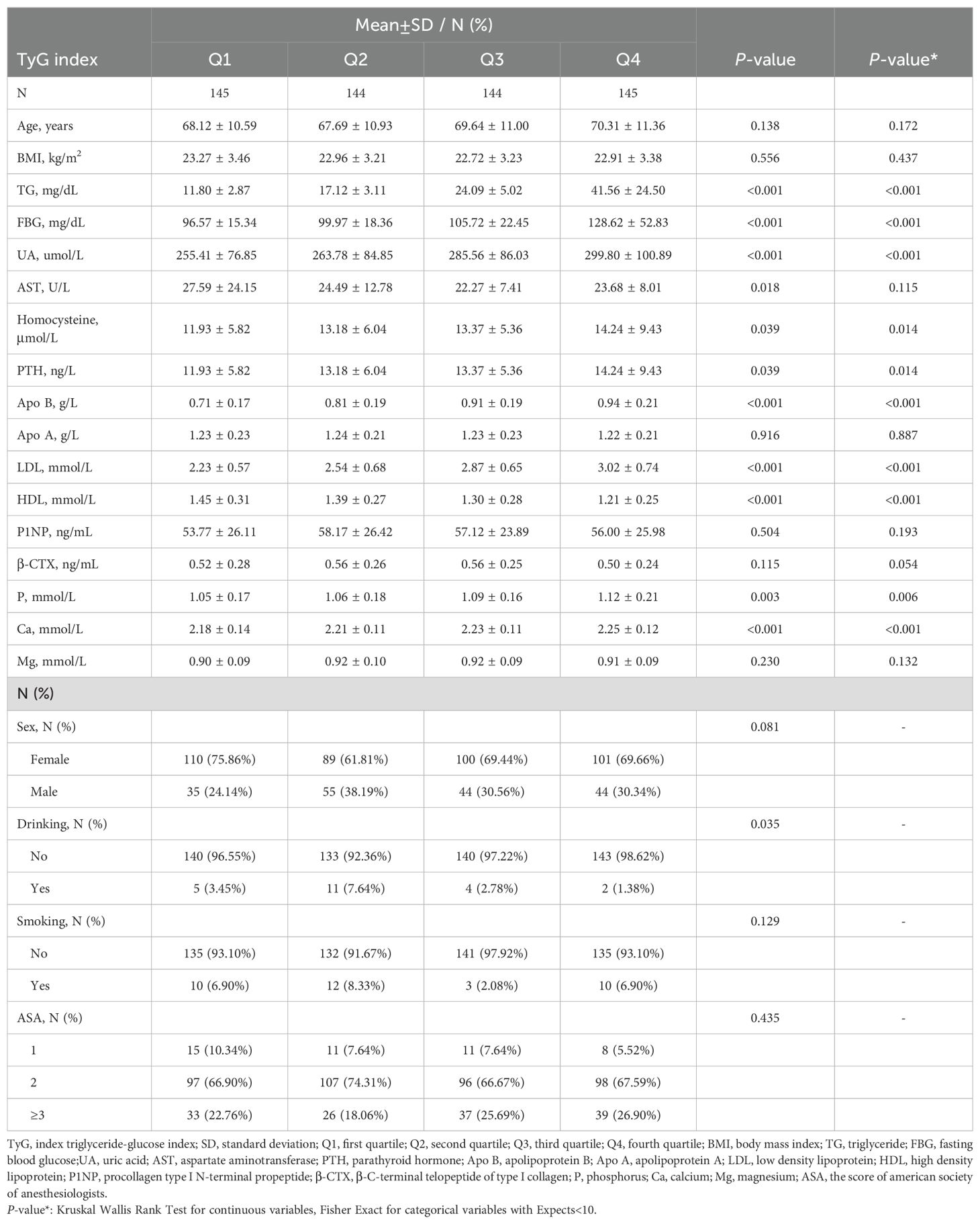
The baseline characteristics of 578 OPFs patients hospitalized for surgical intervention between January 1, 2017, and July 27, 2022 (n = 578) are summarized in Table 1. The baseline characteristics are presented in the established TyG quartiles. The average age of these patients was 68.94 ± 11.00 years, with 30.80% being male and 69.20% being female. The mean concentrations of P1NP and β-CTX in these patients were measured at 56.26 ± 25.61 ng/mL and 0.53 ± 0.26 ng/mL, respectively. In comparison, the overall patient population had a mean TyG index of 6.96 ± 0.58. The population’s baseline characteristics were analyzed using ascending quartiles of the TyG index (Q1-Q4). There were remarkable variations (P-value ≤ 0.05) noted in the levels of TG, FBG, homocysteine, Apo B, P, Ca, UA, LDL, and PTH as the TyG index elevated. In contrast, HDL levels reduced as the TyG index increased.
Univariate analyses of factors linked with BTMs
The univariate analyses (Supplementary Table S1) revealed a distinct correlation between P1NP and the variables FBG, UA, and P. There were no other variables that showed a correlation with P1NP in OPFs patients hospitalized for surgical intervention. A significant link was also identified in univariate analyses between β-CTX and the following variables: FBG, AST, HDL, UA, P, and BMI ≥ 28. Besides, no other variables exhibited a correlation with BTMs in OPFs patients hospitalized for surgical intervention.
Exploration of the connection between the TyG index and BTMs
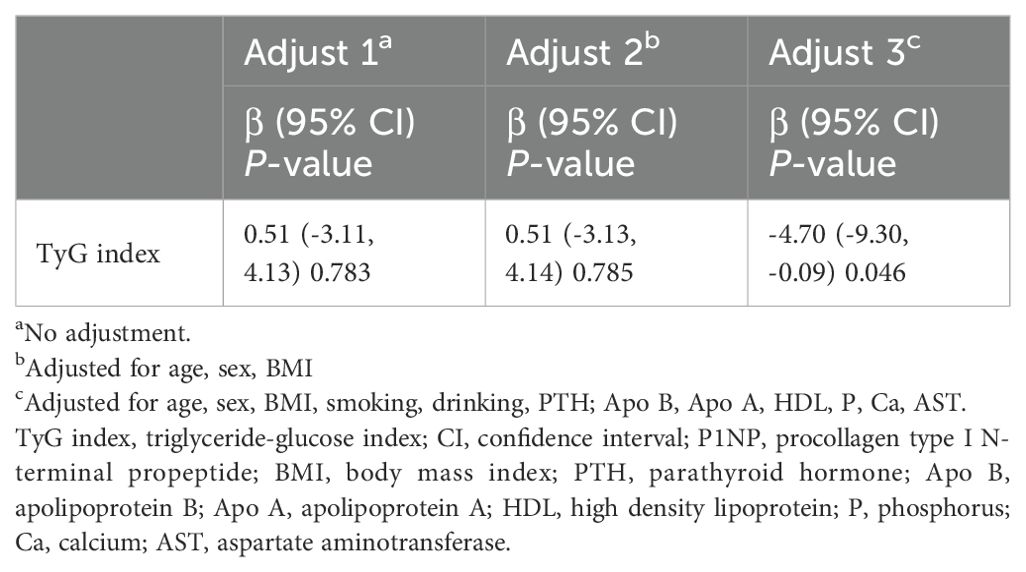
The relationship between the TyG index and P1NP in OPFs patients hospitalized for surgical intervention was further studied using three models (Table 2). Based on the findings, it can be observed that the TyG index and P1NP in model 1 do not show a statistically significant correlation (β = 0.51, 95% CI: -3.11 to 4.13, P-value = 0.783). Moreover, the link between the TyG index and P1NP remained insignificant (β = 0.51, 95% CI: -3.13 to 4.14, P-value = 0.785) despite controlling for age, gender, and BMI in model 2. Nevertheless, after conducting further modifications to adjust during potential confounding variables, including age, sex, smoking, drinking, BMI, PTH, Apo B, Apo A, HDL, P, Ca, and AST, model 3 identified a statistically significant inverse relationship between TyG index and P1NP (β = -4.70, 95% CI: -9.30 to -0.09, P-value = 0.046).
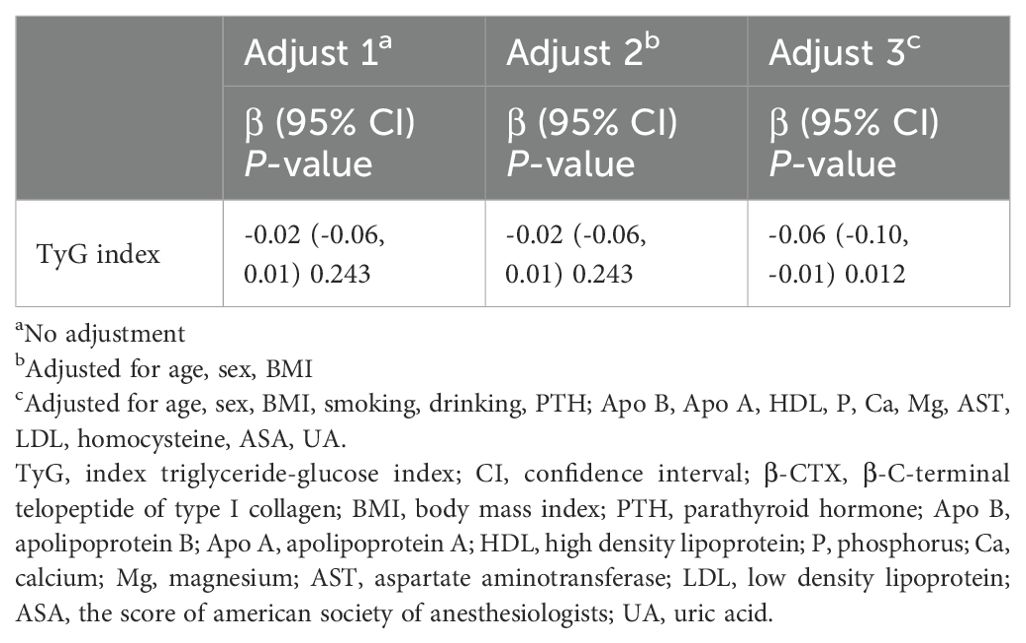
The possible relationship between β-CTX and TyG was examined using the defined models, as detailed in Table 3. There was no significant relationship between the two variables in model 1 (β = -0.02, 95% CI: -0.06 to 0.01, P-value = 0.243). The link between the TyG index and β-CTX remained insignificant despite controlling for gender, age, and BMI in model 2 (β = -0.02, 95% CI: -0.06 to 0.01, P-value = 0.243). Nevertheless, after accounting for other confounding variables, including age, sex, BMI, smoking, drinking, PTH, Apo B, Apo A, HDL, P, Ca, Mg, AST, LDL, homocysteine, ASA, and UA, model 3 listed a significant inverse relation (β = -0.06, 95% CI: -0.10 to -0.01, P-value = 0.012) between TyG index and β-CTX.
Spline smoothing plot and threshold analyses
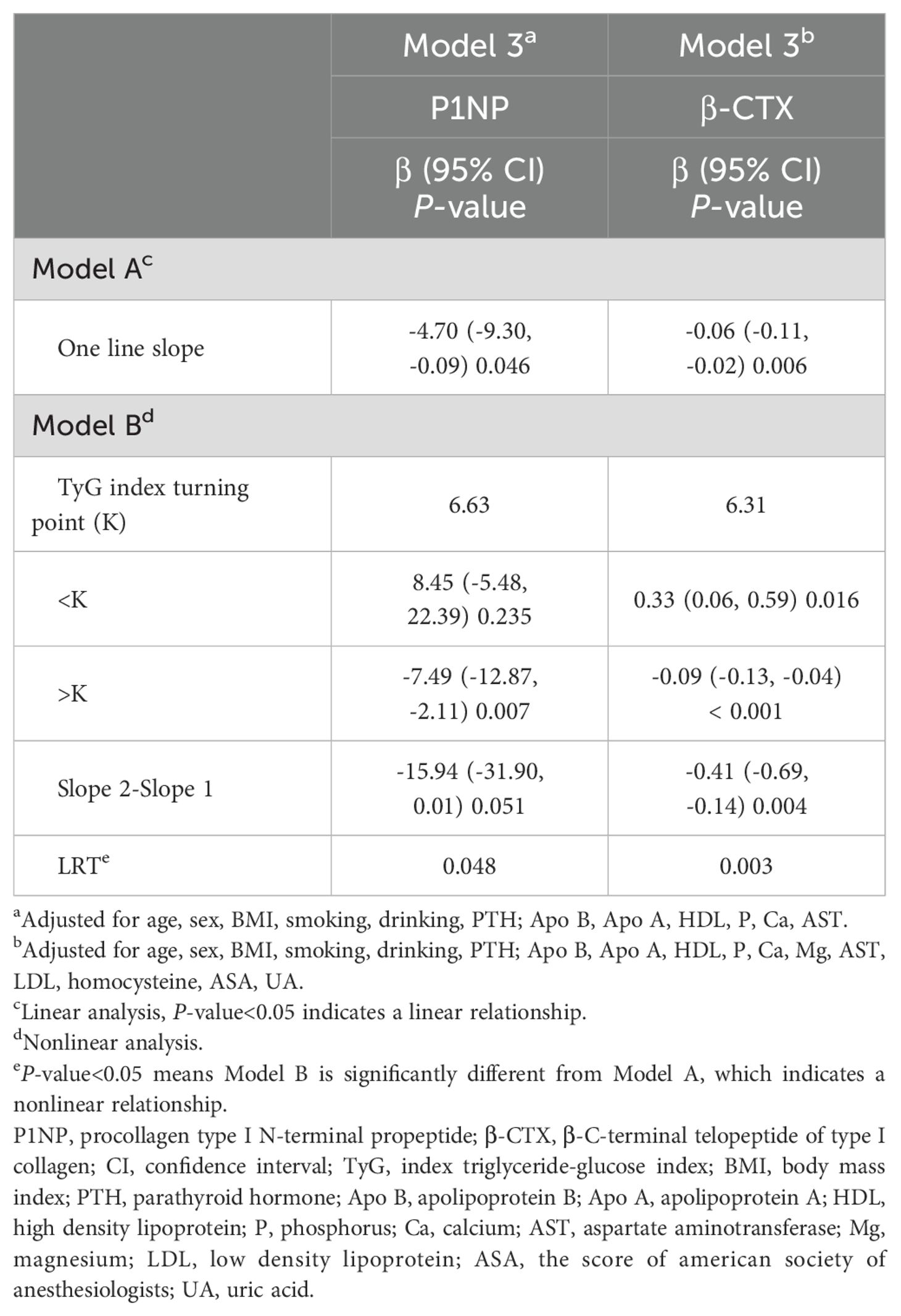
A threshold effect analysis was performed (Table 4) to explore the nature of the relationship between the TyG index and P1NP. After adjusting for potential confounders such as age, sex, BMI, smoking, drinking, PTH, Apo B, Apo A, HDL, P, Ca, and AST, the results revealed a non-linear correlation between the TyG index and P1NP. On the left side of the inflection point (K = 6.63), there was a positive link between the TyG index and P1NP, with an effect size, 95% CI, and P-value of 8.45 (-5.48, 22.39) and 0.235, respectively. Conversely, at the inflection point (K = 6.63) on the right side, a negative association was noted between the TyG index and P1NP, with effect size, 95% CI, and P-value of -7.49 (-12.87, -2.11) and 0.007, respectively. (refer to Figure 2).
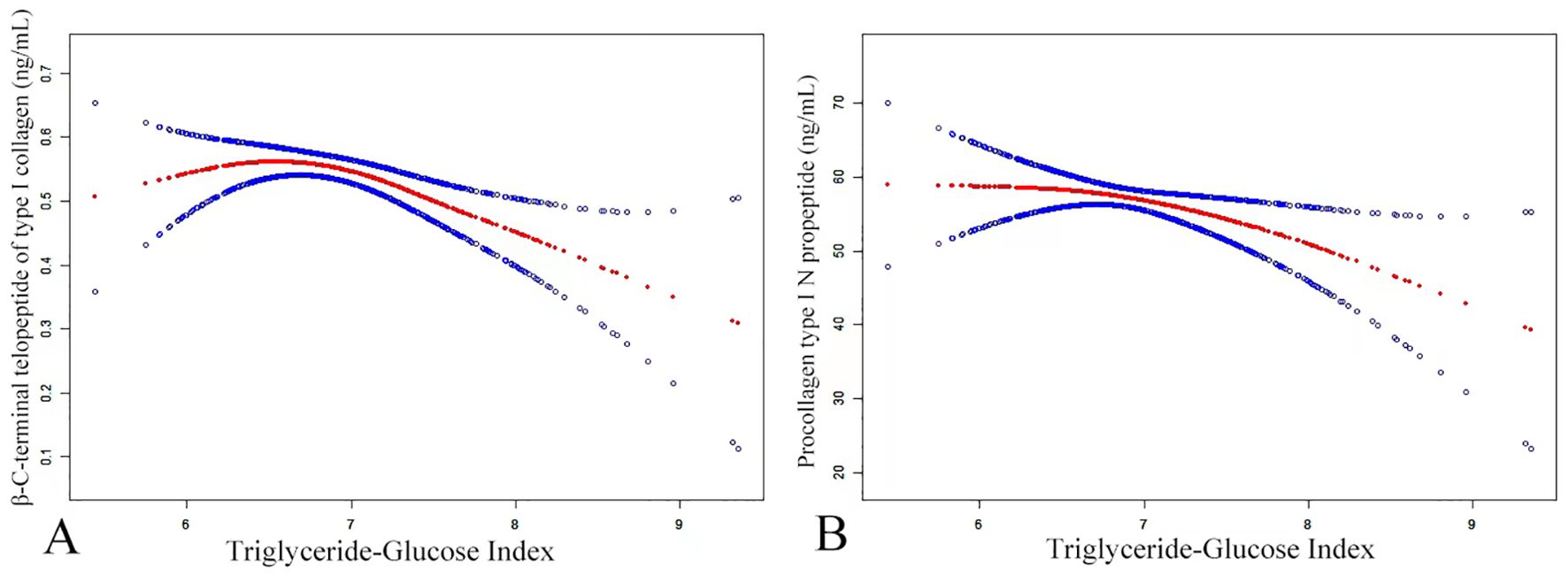
Figure 2. The smoothed adjusted curves for the TyG index, β-CTX (A), and P1NP (B). The red line denotes the non-linear connection between the TyG index and both β-CTX and P1NP, whereas the blue line signifies the CI at 95%. (A) A non-linear correlation was identified even after controlling for variables such as age, sex, smoking, drinking, BMI, PTH, Apo B, Apo A, HDL, P, Ca, Mg, AST, LDL, homocysteine, ASA, and UA. (B) After controlling for age, sex, smoking, drinking, BMI, PTH, Apo B, Apo A, HDL, P, Ca, and AST, a non-linear connection was noticed.
Similarly, a threshold effect analysis was performed to examine the relationship between the TyG index and β-CTX, aiming to determine if it was linear or non-linear. The analysis, adjusted for confounding variables such as age, sex, BMI, smoking, drinking, PTH, Apo B, Apo A, HDL, P, Ca, Mg, AST, LDL, homocysteine, ASA, UA, revealed a non-linear relationship, with an inflection point at K = 6.31. On the left side of this inflection point, a positive association between the TyG index and β-CTX was observed, with an effect size of 0.33 (95% CI: 0.06, 0.59) and a P-value of 0.016. Conversely, on the right side of the inflection point, a negative correlation was detected, with an effect size of -0.09 (95% CI: -0.13, -0.04) and a P-value of ≤ 0.001.
Subgroup analysis
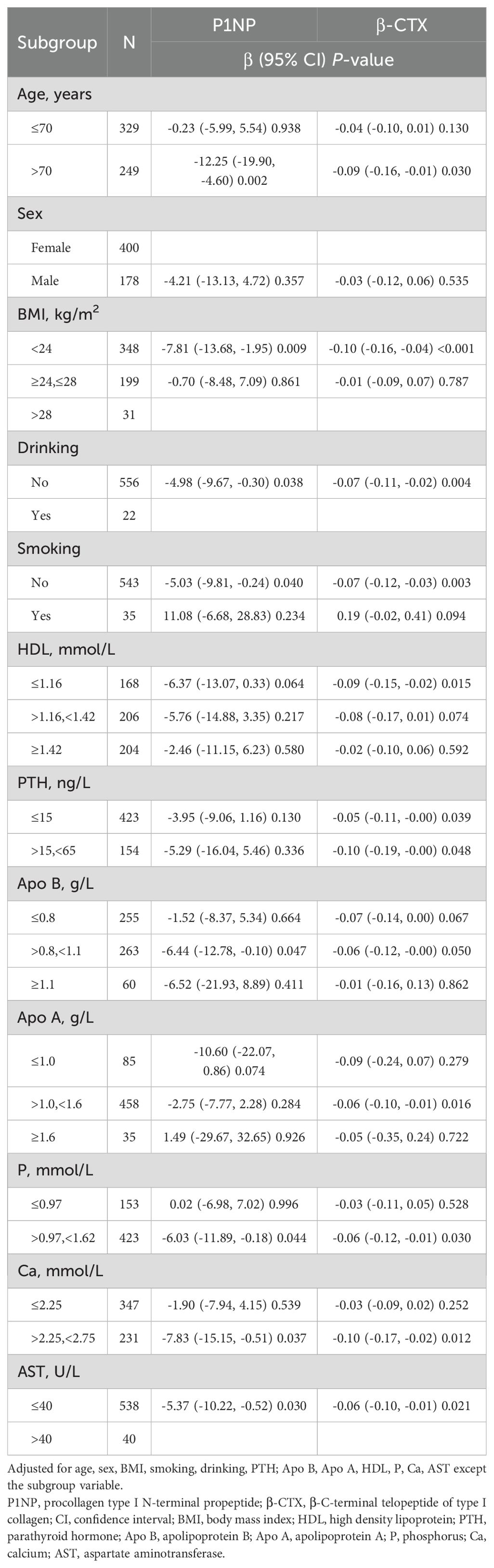
To improve the reliability of the findings following the final adjustment in model 3 for potential confounding variables, subgroup analyses were conducted with stratification by age, sex, BMI, smoking, drinking, PTH, Apo B, Apo A, HDL, P, Ca, and AST. Consistent patterns were observed across all strata (Table 5), except for the subgroup variables. All analyses were adjusted for the 11 covariates previously listed.
Discussion
OP is particularly common among the elderly, and the aging process contributes to an increase in OP cases (25). Previous research has shown a link between these elevated levels and an increased risk of OP (22). The primary aim of this study was to investigate the relationship between the TyG index and BTMs in OPFs patients hospitalized for surgical intervention. The study also sought to control for potential confounding variables that might affect the observed relationship. The results revealed a significant negative association between the TyG index and both P1NP and β-CTX. Furthermore, a non-linear relationship was identified, with inflection points at 6.63 and 6.31, respectively.
A previous study examined a connection between bone density and TyG index. The increased TyG index has been linked to decreased bone density in diabetic patients (7), non-diabetic individuals in Korea (10), adult Americans (21), healthy Chinese younger aged and non-diabetic individuals (22), and postmenopausal women (23). Even though bone density is the highest priority for OP diagnosis (26). Dual-energy X-ray absorptiometry (DXA) is considered a promising candidate for quantifying BMD (27). The BMD measurement via DXA may be influenced by osteophytes, degenerative alterations in minor joints, and vertebral abnormalities (27). Consequently, BTMs have appeared as a highly beneficial tool for evaluating bone quality and metabolism, and their applications in the detection of OP and fractures are constantly increasing (28, 29). Previous research has also investigated the link between the TyG index and BTMs in patients with type 2 diabetes, revealing a negative relationship between them (7). In the influence of multiple confounding variables, this study evaluated the further connection between the TyG index and BTMs in OPFs patients hospitalized for surgical intervention and found an unfavorable relationship between these two variables. Moreover, a threshold effect and a non-linear connection were observed between the TyG index and BTMs.
Insulin resistance impacts bone cells and disrupts bone formation, likely by inhibiting the Wnt/β-catenin signaling pathway (30). Studies in rodent models have shown that obesity, coupled with insulin resistance, leads to reduced osteoblastic proliferation, increased osteoblastic apoptosis, heightened osteoblastic insulin resistance, and greater bone porosity. These changes result in impaired bone quality, particularly in trabecular bone areas (30).
It has demonstrated that elevated TG and blood glucose levels influence a patient’s bone metabolism in distinct ways, ultimately resulting in reduced bone density and a higher susceptibility to OP. Previous findings have revealed a positive association between triglyceride levels and OP (31), and high TG levels may likely affect bone density via various mechanisms. One possible mechanism involves the activation of peroxisome proliferator-activated receptor γ (PPARγ), a key factor in the relationship between lipid indicators and BMD. The PPARγ is activated by lipid metabolites, and elevated levels of these metabolites can lead to increased PPARγ activity. This, in turn, inhibits bone formation while promoting bone resorption. Another possible mechanism entails the strong correlation between elevated levels of triglycerides in the blood and enhanced fat content in the bone marrow; this correlation may result in a reduction in trabecular bone density (32–34). It can be attributed to the detrimental consequences of oxidative stress, which simultaneously hinder the differentiation of osteoblasts and stimulate the adipocyte differentiation (35, 36).
Hyperglycemia has also been associated with reduced bone density via multiple pathways. Prolonged hyperglycemia in these mechanisms inhibits osteoblast proliferation and stimulates osteoclast differentiation (37). It is currently believed that high glucose levels in the bone marrow microenvironment can lead to an increase in osteoclast differentiation, resulting in the development of osteoporosis (37). Furthermore, elevated blood glucose levels may contribute to a rise in advanced glycation end-products (AGEs), which can bind with the receptor for AGEs (RAGE) on bone cells and react to collagen in the bone. This may result in the generation of oxidative and inflammatory mediators, including nuclear factor κB (NF-κB), which can impair bone biomechanical characteristics and reduce its mass, thereby elevating the susceptibility to fractures. Finally, the differentiation of mesenchymal stem cells (MSCs) and bone development can be influenced by oxidative stress, hyperglycemia (38), and the generation of reactive oxygen species (ROS) (39).
These findings have significant clinical implications. First, a negative connection was identified between BTMs and the TyG index, characterized by a threshold effect and a non-linear relationship. Specifically, markers of bone formation declined when the TyG index exceeded 6.63, while markers of bone resorption decreased when the TyG index surpassed 6.31. These results collectively suggest that elevated TyG index levels have an adverse impact on bone turnover. Therefore, monitoring TyG index levels in patients can provide valuable insights into bone metabolism in OPFs patients hospitalized for surgical intervention. Interventions targeting patients with TyG index levels above these thresholds could potentially improve their bone health and overall quality of life. Furthermore, multiple studies have been carried out to examine the link between the TyG index and BTMs in various populations, including diabetic patients (7), non-diabetic Koreans (10), American adults (21), young-aged non-diabetic Chinese individuals (22), and postmenopausal females (23). Nevertheless, to the best of our understanding, no previous study has examined the link between the TyG index and BTMs in OPFs patients hospitalized for surgical intervention; thus, this study holds considerable therapeutic value in this population. Consistent with previous studies, these findings indicate that an elevated TyG index has an adverse effect on bone turnover.
This study also possesses certain strengths. Initially, three distinct models were established to explore the connection between the TyG index and BTMs with potential confounding variables. The results showed a negative relationship between the TyG index and BTMs in OPFs patients hospitalized for surgical intervention, indicating that a high TyG index level may impair BTMs in this population. Thus, to reduce the high incidence and death rates related to OP and fractures, it may be useful to monitor the TyG index levels of patients for screening and assessing their risk. Moreover, compared to laboratory and imaging tests related to bone density, obtaining the TyG index is simpler, more convenient, and more cost-effective. Nonetheless, the present study has some limitations. To commence, as a result of its cross-sectional design, finding the temporal correlation between the TyG index and the onset of OP unveils a difficult challenge. Secondly, as many genetic and non-genetic factors are linked with the development of OP, the presented data analysis mainly adjusted for certain demographic, lifestyle, and laboratory variables. However, it is important to note that individuals with diseases or those taking medications that affect lipid and glucose metabolism were excluded from the study. As a result, the applicability of these findings to such populations may be limited. Moreover, the sample size was comparatively low, consisting of no more than 578 identifiable samples. Finally, these results may not be applicable to individuals of other races and are only applicable to OPFs patients hospitalized for surgical intervention, as the study was carried out at a single institute on a comparatively small patient population. To ensure the consistency and validity of these findings, it will be necessary to conduct extensive follow-up studies that incorporate multi-center randomized designs, a wider range of biochemical markers, and diverse ethnic populations.
Conclusion
In conclusion, this study identified a significant correlation and non-linear relationship between BTMs and the TyG index in OPFs patients hospitalized for surgical intervention. The analysis revealed threshold effects at TyG index values of 6.63 and 6.31. These findings suggest that elevated TyG index levels may impair bone turnover and contribute to the development of osteoporosis. However, it is important to acknowledge the limitations of this study, such as the small sample size, lack of mechanistic investigations, and restricted population selection. Therefore, further research is needed to explore the causal relationship between the TyG index and osteoporosis and to validate the accuracy of the TyG index in larger and more diverse patient populations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The IRB of Affiliated Kunshan Hospital of Jiangsu University. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from All patients with ≥ 50 age were hospitalized between January 1, 2017, and July 27, 2022, due to an OP fracture (fracture of the wrist, hip, vertebra, proximal humerus) and reported through retrieving their online records. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JX: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. S-hG: Data curation, Formal analysis, Investigation, Writing – review & editing. M-zX: Data curation, Investigation, Writing – review & editing. CL: Data curation, Methodology, Resources, Software, Supervision, Validation, Funding acquisition, Project administration, Writing – review & editing. Y-qG: Data curation, Investigation, Writing – review & editing. KL: Data curation, Methodology, Resources, Software, Supervision, Validation, Project administration, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study was supported by National Natural Science Foundation of China (CN) (82172441), Suzhou City Major Disease Multicenter Clinical Research Project (CN) (DZXYJ202312), Special Funding for Jiangsu Province Science and Technology Plan (Key Research and Development Program for Social Development) (CN) (BE2023737) and Gusu Health Talent Plan Scientific Research Project (CN) (GSWS2022109).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1418271/full#supplementary-material.
References
1. Ioannidis G, Papaioannou A, Thabane L, Gafni A, Hodsman A, Kvern B, et al. Family physicians’ personal and practice characteristics that are associated with improved utilization of bone mineral density testing and osteoporosis medication prescribing. Popul Health Manag. (2009) 12:131–8. doi: 10.1089/pop.2008.0025
3. Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. (2011) 377:1276–87. doi: 10.1016/S0140-6736(10)62349-5
4. Adejuyigbe B, Kallini J, Chiou D, Kallini JR. Osteoporosis: molecular pathology, diagnostics, and therapeutics. Int J Mol Sci. (2023) 24:14583. doi: 10.3390/ijms241914583
5. Shen Y, Huang X, Wu J, Lin X, Zhou X, Zhu Z, et al. The global burden of osteoporosis, low bone mass, and its related fracture in 204 countries and territories, 1990-2019. Front Endocrinol (Lausanne). (2022) 13:882241. doi: 10.3389/fendo.2022.882241
6. Rashki Kemmak A, Rezapour A, Jahangiri R, Nikjoo S, Farabi H, Soleimanpour S. Economic burden of osteoporosis in the world: A systematic review. Med J Islam Repub Iran. (2020) 34:154. doi: 10.34171/mjiri.34.154
7. Sun W, Xing Y, Zhou F, Ma Y, Wan X, Ma H. Association analysis of triglyceride glucose-body mass index and bone turnover markers in patients with type 2 diabetes mellitus. Diabetes Metab Syndr Obes. (2023) 16:1435–47. doi: 10.2147/DMSO.S406849
8. Kwon S, Kim W, Yang S, Choi KH. Influence of the type of occupation on osteoarthritis of the knee in men: The Korean National Health and Nutrition Examination Survey 2010-2012. J Occup Health. (2019) 61:54–62. doi: 10.1002/1348-9585.12022
9. Yang Y, Huang X, Wang Y, Leng L, Xu J, Feng L, et al. The impact of triglyceride-glucose index on ischemic stroke: a systematic review and meta-analysis. Cardiovasc Diabetol. (2023) 22:2. doi: 10.1186/s12933-022-01732-0
10. Yoon JH, Hong AR, Choi W, Park JY, Kim HK, Kang H-C. Association of triglyceride-glucose index with bone mineral density in non-diabetic koreans: KNHANES 2008-2011. Calcif Tissue Int. (2021) 108:176–87. doi: 10.1007/s00223-020-00761-9
11. Locateli JC, Lopes WA, Simões CF, de Oliveira GH, Oltramari K, Bim RH, et al. Triglyceride/glucose index is a reliable alternative marker for insulin resistance in South American overweight and obese children and adolescents. J Pediatr Endocrinol Metab. (2019) 32:1163–70. doi: 10.1515/jpem-2019-0037
12. Wang X, Xu W, Song Q, Zhao Z, Meng X, Xia C, et al. Association between the triglyceride-glucose index and severity of coronary artery disease. Cardiovasc Diabetol. (2022) 21:168. doi: 10.1186/s12933-022-01606-5
13. Shi YY, Zheng R, Cai J-J, Qian S-Z. The association between triglyceride glucose index and depression: data from NHANES 2005-2018. BMC Psychiatry. (2021) 21:267. doi: 10.1186/s12888-021-03275-2
14. Xu J, Xu W, Chen G, Hu Q, Jiang J. Association of TyG index with prehypertension or hypertension: a retrospective study in Japanese normoglycemia subjects. Front Endocrinol (Lausanne). (2023) 14:1288693. doi: 10.3389/fendo.2023.1288693
15. Zhou Q, Yang J, Tang H, Guo Z, Dong W, Wang Y, et al. High triglyceride-glucose (TyG) index is associated with poor prognosis of heart failure with preserved ejection fraction. Cardiovasc Diabetol. (2023) 22:263. doi: 10.1186/s12933-023-02001-4
16. Tutunchi H, Naeini F, Mobasseri M, Ostadrahimi A. Triglyceride glucose (TyG) index and the progression of liver fibrosis: A cross-sectional study. Clin Nutr ESPEN. (2021) 44:483–7. doi: 10.1016/j.clnesp.2021.04.025
17. Qin Z, Zhao J, Geng J, Chang K, Liao R, Su B. Higher triglyceride-glucose index is associated with increased likelihood of kidney stones. Front Endocrinol (Lausanne). (2021) 12:774567. doi: 10.3389/fendo.2021.774567
18. Yilmaz M, Karaaslan M, Tonyali S, Celik M, Toprak T, Odabas O. Triglyceride-Glucose Index (TyG) is associated with erectile dysfunction: A cross-sectional study. Andrology. (2021) 9:238–44. doi: 10.1111/andr.12904
19. Williams C, Sapra A. “Osteoporosis Markers”, In: StatPearls. Treasure Island (FL): StatPearls Publishing (2024).
20. Ahn TK, Kim K-T, Joshi HP, Park KH, Kyung JW, Choi U-Y, et al. Therapeutic potential of tauroursodeoxycholic acid for the treatment of osteoporosis. Int J Mol Sci. (2020) 21:4274. doi: 10.3390/ijms21124274
21. Zhan H, Liu X, Piao S, Rong X, Guo J. Association between triglyceride-glucose index and bone mineral density in US adults: a cross sectional study. J Orthop Surg Res. (2023) 18:810. doi: 10.1186/s13018-023-04275-6
22. Wen Z, Li Y, Xu L, Yue C, Wang Q, Chen R, et al. Triglyceride glucose-body mass index is a reliable indicator of bone mineral density and risk of osteoporotic fracture in middle-aged and elderly nondiabetic chinese individuals. J Clin Med. (2022) 11:5694. doi: 10.3390/jcm11195694
23. Gu P, Pu B, Xin Q, Yue D, Luo L, Tao J, et al. The metabolic score of insulin resistance is positively correlated with bone mineral density in postmenopausal patients with type 2 diabetes mellitus. Sci Rep. (2023) 13:8796. doi: 10.1038/s41598-023-32931-8
24. Lu K, Wu Y-M, Shi Q, Gong Y-Q, Zhang T, Li C. A novel fracture liaison service using digital health: impact on mortality in hospitalized elderly osteoporotic fracture patients. Osteoporos Int. (2024) 35:53–67. doi: 10.1007/s00198-023-06905-5
25. Xun J, Li C, Liu M, Mei Y, Zhou Q, Wu B, et al. Serum exosomes from young rats improve the reduced osteogenic differentiation of BMSCs in aged rats with osteoporosis after fatigue loading in vivo. Stem Cell Res Ther. (2021) 12:424. doi: 10.1186/s13287-021-02449-9
26. Zhang C, Wang Y, Zhang C-L, Wu H-R. Prioritization of candidate metabolites for postmenopausal osteoporosis using multi-omics composite network. Exp Ther Med. (2019) 17:3155–61. doi: 10.3892/etm
27. Zhao Y, Zhao T, Chen S, Zhang X, Serrano Sosa M, Liu J, et al. Fully automated radiomic screening pipeline for osteoporosis and abnormal bone density with a deep learning-based segmentation using a short lumbar mDixon sequence. Quant Imaging Med Surg. (2022) 12:1198–213. doi: 10.21037/qims
28. LeBoff MS, Greenspan SL, Insogna KL, Lewiecki EM, Saag KG, Singer AJ, et al. The clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. (2022) 33:2049–102. doi: 10.1007/s00198-021-05900-y
29. Lane NE, Saag K, O’Neill TJ, Manion M, Shah R, Klause U, et al. Real-world bone turnover marker use: impact on treatment decisions and fracture. Osteoporos Int. (2021) 32:831–40. doi: 10.1007/s00198-020-05734-0
30. Viljakainen HT, Koistinen HA, Tervahartiala T, Sorsa T, Andersson S, Mäkitie O. Metabolic milieu associates with impaired skeletal characteristics in obesity. PloS One. (2017) 12:e0179660. doi: 10.1371/journal.pone.0179660
31. Kan B, Zhao Q, Wang L, Xue S, Cai H, Yang S. Association between lipid biomarkers and osteoporosis: a cross-sectional study. BMC Musculoskelet Disord. (2021) 22:759. doi: 10.1186/s12891-021-04643-5
32. Bredella MA, Gerweck AV, Landa MG, Kumar V, Daley SM, et al. Ectopic and serum lipid levels are positively associated with bone marrow fat in obesity. Radiology. (2013) 269:534–41. doi: 10.1148/radiol.13130375
33. Chang PY, Gold EB, Cauley JA, Johnson WO, Karvonen-Gutierrez C, Jackson EA, et al. Triglyceride levels and fracture risk in midlife women: study of women’s health across the nation (SWAN). J Clin Endocrinol Metab. (2016) 101:3297–305. doi: 10.1210/jc.2016-1366
34. Burian E, Syväri J, Dieckmeyer M, Holzapfel C, Drabsch T, Sollmann N, et al. Age- and BMI-related variations of fat distribution in sacral and lumbar bone marrow and their association with local muscle fat content. Sci Rep. (2020) 10:9686. doi: 10.1038/s41598-020-66649-8
35. Yamaguchi T, Sugimoto T, Yano S, Yamauchi M, Sowa H, Chen Q, et al. Plasma lipids and osteoporosis in postmenopausal women. Endocr J. (2002) 49:211–7. doi: 10.1507/endocrj.49.211
36. Parhami F, Jackson SM, Tintut Y, Le V, Balucan JP, Territo M, et al. Atherogenic diet and minimally oxidized low density lipoprotein inhibit osteogenic and promote adipogenic differentiation of marrow stromal cells. J Bone Miner Res. (1999) 14:2067–78. doi: 10.1359/jbmr.1999.14.12.2067
37. Qi S, Zheng H, Chen C, Lan S. Icariin prevents diabetes-induced bone loss in rats by reducing blood glucose and suppressing bone turnover. Molecules. (2019) 24:1871. doi: 10.3390/molecules24101871
38. Waqas K, Chen J, Koromani F, Trajanoska K, van der Eerden BC, Uitterlinden AG, et al. Skin autofluorescence, a noninvasive biomarker for advanced glycation end-products, is associated with prevalent vertebral and major osteoporotic fractures: the rotterdam study. J Bone Miner Res. (2020) 35:1904–13. doi: 10.1002/jbmr.4096
Keywords: P1NP, β-CTX, BTMs, osteoporotic fractures, TyG index
Citation: Xu J, Guo S-h, Xu M-z, Li C, Gong Y-q and Lu K (2024) The association between the triglyceride-glucose index and bone turnover markers in osteoporotic fractures patients aged 50 and above who are hospitalized for surgical intervention: a retrospective cross-sectional study. Front. Endocrinol. 15:1418271. doi: 10.3389/fendo.2024.1418271
Received: 16 April 2024; Accepted: 30 August 2024;
Published: 18 September 2024.
Edited by:
An Song, Peking Union Medical College Hospital (CAMS), ChinaReviewed by:
Rong Chen, Beijing Jishuitan Hospital, ChinaYiyang Gao, Peking Union Medical College Hospital (CAMS), China
Luping Ren, Hebei General Hospital, China
Copyright © 2024 Xu, Guo, Xu, Li, Gong and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ke Lu, c2d1ODQzNEBzaW5hLmNvbQ==
†ORCID: Jian Xu, orcid.org/0009-0000-7692-3125
Shao-han Guo, orcid.org/0009-0003-6894-2926
Min-zhe Xu, orcid.org/0000-0002-7094-2189
Chong Li, orcid.org/0000-0002-1526-221X
Ya-qin Gong, orcid.org/0000-0001-8695-4048
Ke Lu, orcid.org/0000-0002-0029-7874
 Jian Xu
Jian Xu Shao-han Guo2†
Shao-han Guo2† Min-zhe Xu
Min-zhe Xu Chong Li
Chong Li Ke Lu
Ke Lu