- 1Department of Pain, The First People’s Hospital of Chenzhou, The First Affiliated Hospital of Xiangnan University, Chenzhou, Hunan, China
- 2School of Basic Medicine, Xiangnan University, Chenzhou, Hunan, China
- 3Department of Metabolism and Endocrinology, The First People’s Hospital of Chenzhou, The First Affiliated Hospital of Xiangnan University, Chenzhou, Hunan, China
Background: The prevalence of papillary thyroid cancer is gradually increasing and the trend of youthfulness is obvious. Some patients may not be able to undergo surgery, which is the mainstay of treatment, due to physical or financial reasons. Therefore, the prediction of cancer-specific survival (CSS) in patients with non-operated papillary thyroid cancer is necessary.
Methods: Patients’ demographic and clinical information was extracted from the Surveillance, Epidemiology, and End Results database. SPSS software was used to perform Cox regression analyses as well as propensity score matching analyses. R software was used to construct and validate the nomogram. X-tile software was used to select the best cutoff point for patient risk stratification.
Results: A total of 1319 patients were included in this retrospective study. After Cox regression analysis, age, grade, T stage, M stage, radiotherapy, and chemotherapy were used to construct the nomogram. C-index, calibration curves, and receiver operating characteristic curves all verified the high predictive accuracy of the nomogram. The decision curve analysis demonstrated that patients could gain clinical benefit from this predictive model. Survival curve analysis after propensity score matching demonstrated the positive effects of radiotherapy on CSS in non-operated patients.
Conclusion: Our retrospective study successfully established a nomogram that accurately predicts CSS in patients with non-operated papillary thyroid cancer and demonstrated that radiotherapy for operated patients can still help improve prognosis. These findings can help clinicians make better choices.
Introduction
In recent years, the incidence of papillary thyroid cancer has been increasing every year (1). According to the 2020 WHO Cancer Incidence and Mortality Database, thyroid malignant tumors rank as the ninth most common cancer worldwide (2). Females show a higher prevalence, with a ratio of female patients to male patients of approximately 3:1 (3, 4). Thyroid cancer can be categorized into many histological types with great prognostic differences, including papillary thyroid cancer, medullary carcinoma, and interstitial thyroid carcinoma, of which the most common malignant type is papillary thyroid cancer, accounting for about 80% of cases, so this study discusses papillary thyroid cancer (3). Thyroid cancer, as the most common malignant tumor in adolescents and adults aged 16-33, poses a serious threat to patients’ lives and health (5).
Many malignant thyroid tumors require surgical removal (6, 7). The extent of the surgery depends on the size and spread of the tumor and includes the removal of a lobe or the entire thyroid gland. For patients with high-risk thyroid cancer, postoperative radiation therapy can reduce the risk of recurrence, and this is usually done after total thyroidectomy. Radiation therapy can also be used to manage residual thyroid tissue after surgery or local recurrence of thyroid cancer (8, 9). This helps to reduce the size of the tumor and control the disease. While surgical treatment is often considered the first option for thyroid cancer patients, it is undeniable that surgical treatment may not be appropriate for all thyroid cancer patients. First, those with thyroid cancer with fatal disease may not be candidates for surgical treatment because they cannot tolerate surgery. Secondly, the cost of surgical treatment may not be affordable for all thyroid cancer patients, who may choose to decline surgery for financial reasons. Finally, some thyroid cancer patients may have lost the opportunity for surgery, and thus clinicians may not recommend surgery. Therefore, we need to pay more attention to those thyroid cancer patients who are not treated with surgery.
Nomogram is an emerging event prediction model that has been widely used to predict the prognosis of patients with a variety of cancers (10, 11). It can give an accurate prognostic evaluation based on different disease-related information for each individual. Therefore, for the first time, we have developed a nomogram that can predict the prognosis of patients with papillary thyroid cancer who have not undergone surgery, which can help clinicians provide the best treatment options for patients.
Method
Data collection and demographics
Patient data from the retrospective study came from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database, which covers cancer information for 28 percent of the total US population. Patient consent is not required because our study is retrospective and does not involve patients’ personally identifiable information.
The inclusion and exclusion criteria for extracting and screening data from the SEER database were as follows: The inclusion criteria: (1) Primary papillary thyroid cancer (International Classification of Disease for Oncology histopathology codes: 8050, 8260, 8340, 8341, 8344, 8347); (2) For patients who refuse to undergo the surgery or who are not recommended by their clinician to undergo the surgery; (3) Diagnosed between 2010 and 2015. The exclusion criteria: (1) Incomplete information regarding patient ID and survival status; (2) Lack of information on follow-up information; (3) Carcinoma in situ. Clinical pathological data and demographic data were extracted from patients including age, sex, race, tumor grade, TNM stage, radiotherapy, and chemotherapy status. Outcome factors include survival time and cause-specific death.
Statistical analysis
The total cohort was randomly assigned into a training set and a validation set in a ratio of 7:3. A Cox proportional hazards regression model was used for multivariate analysis to determine independent prognostic factors for thyroid cancer patients without surgery. The training cohort involved employing univariate and multivariate Cox regression analyses to identify risk variables affecting postoperative cancer-specific survival (CSS). Using R software, a predictive model for postoperative CSS was then established from the results of the multivariate Cox regression analysis. Additionally, receiver operating characteristic curves (ROC) were generated, and the area under the curve (AUC) was computed to evaluate the model’s discriminatory capacity. Calibration curves and decision curve analysis (DCA) were developed for 3, 5, and 8 years to assess predictive accuracy and clinical utility. X-tile software was used to select the best cutoff point for the nomogram score. Based on this score cutoff point patients were categorized into three risk strata and Kaplan-Meier survival curves were plotted. Finally, patients were propensity score matched (PSM) in a 1:1 ratio based on radiotherapy, with a caliper of 0.01, and Kaplan-Meier survival curves were plotted to assess outcome differences. All statistical analysis and charting were carried out by using SPSS (25.0) and R software version 4.3.1. A two-tailed P<0.05 was considered statistically significant.
Results
Inclusion of patients
A total of 200950 patients with primary papillary thyroid cancer diagnosed between 2010 and 2015 were identified from the SEER database. Among them, 9072 patients were included because of lack of surgery. After excluding patients whose ID, survival information, and variables were unknown, 1319 patients were finally included in our study. Demographic and clinical characteristics of the patients are shown in Table 1.
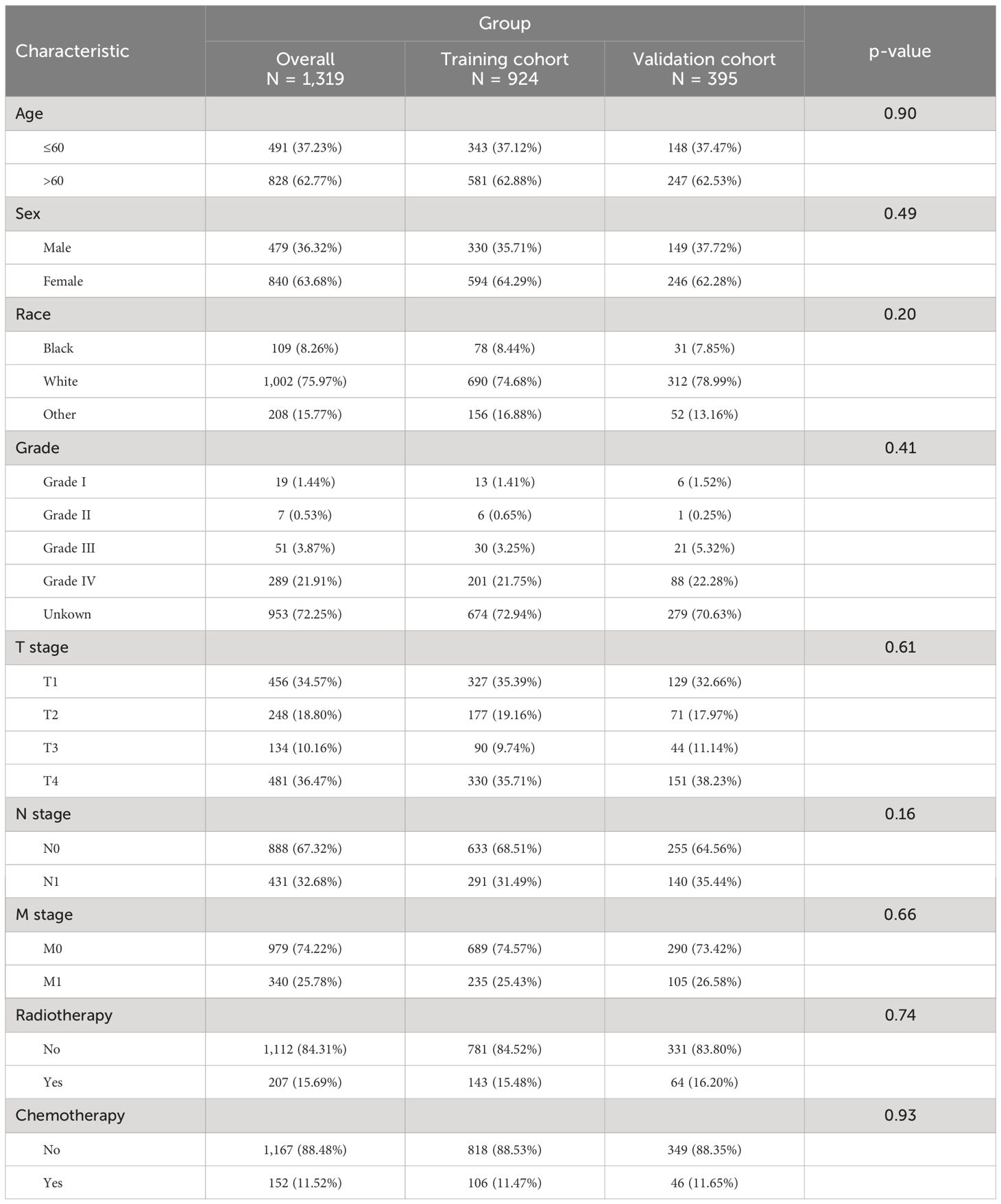
Table 1. Clinical and pathological characteristics of patients with non-operative papillary thyroid cancer.
Independent predictors of cancer-specific survival
We conducted univariable Cox regression analyses to recognize the factors significantly associated with CSS of thyroid cancer patients without surgery. As shown in Table 2, age, race, T stage, N stage, M stage, radiotherapy, chemotherapy, and grade were significantly associated with poor CSS (P<0.05).
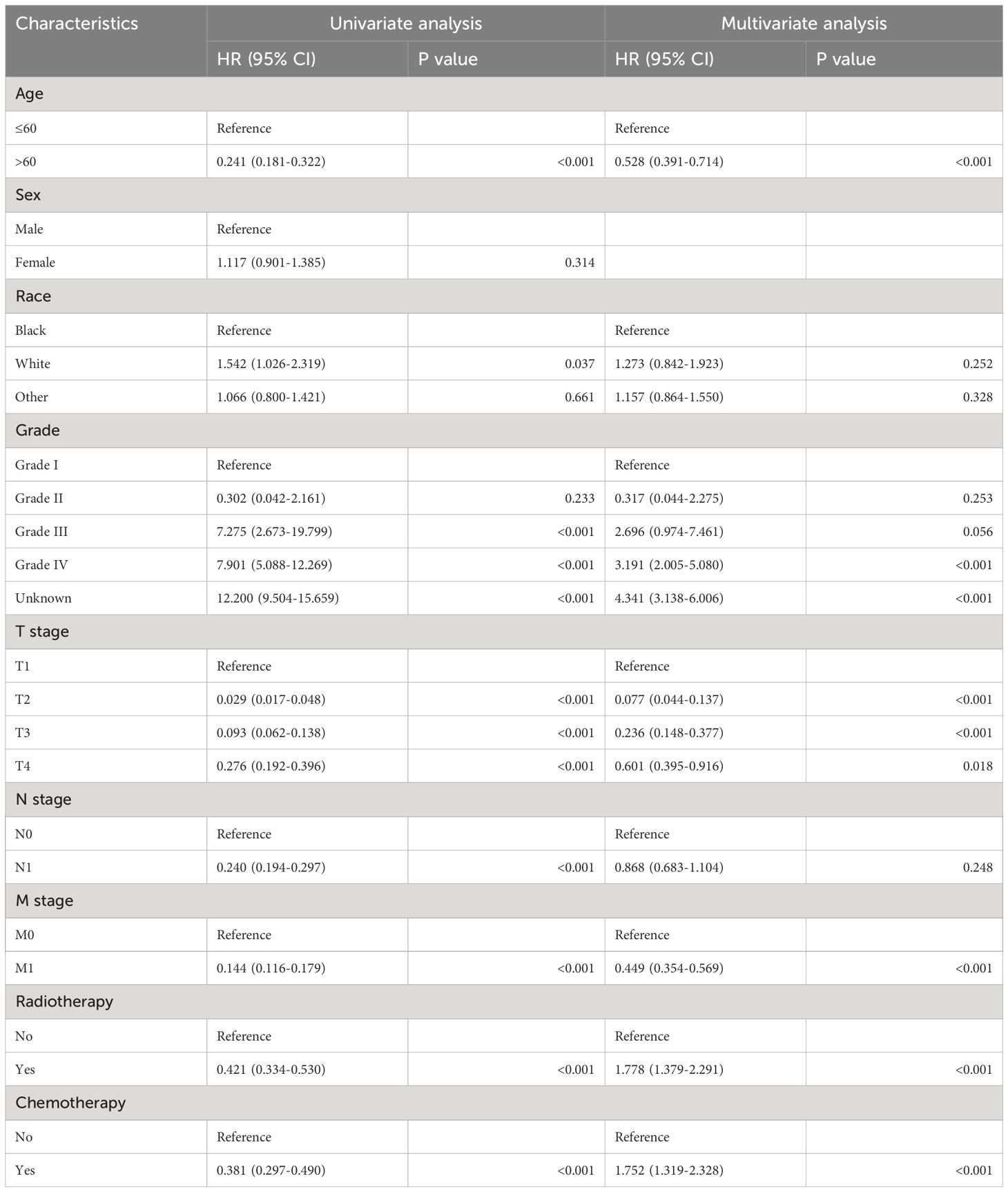
Table 2. Analysis of univariate and multivariate Cox regression in patients with non-operative papillary thyroid cancer.
We conducted a multivariable Cox regression analysis, including the significant variables by univariate analyses, to explore independent predictors of the CSS. As shown in Table 2, age, grade, T stage, M stage, radiotherapy, and chemotherapy were independent predictors of poor CSS (P<0.05).
Construction and validation of a nomogram
We combined four independent prognostic factors for CSS and developed a nomogram for predicting the CSS of patients (Figure 1). The C-index in the training and validation queues are 0.885 and 0.874, respectively. The AUC values at 3, 5, and 8 years were 0.944, 0.936, and 0.957 in the training cohort and 0.942, 0.968, and 0.968 in the validation cohort (Figure 2). The C-index and AUC values indicate robust predictive performance in both cohorts. The calibration curve closely mirrors the 45° line, signifying strong agreement between the nomogram’s predictions and actual outcomes (Figure 3). In both training and validation cohorts, the DCA underscores the nomogram’s valuable clinical utility for predicting CSS in thyroid cancer patients without surgery (Figure 4). Kaplan-Meier survival curves demonstrated significant differences in survival outcomes for patients in different risk strata (Figure 5).
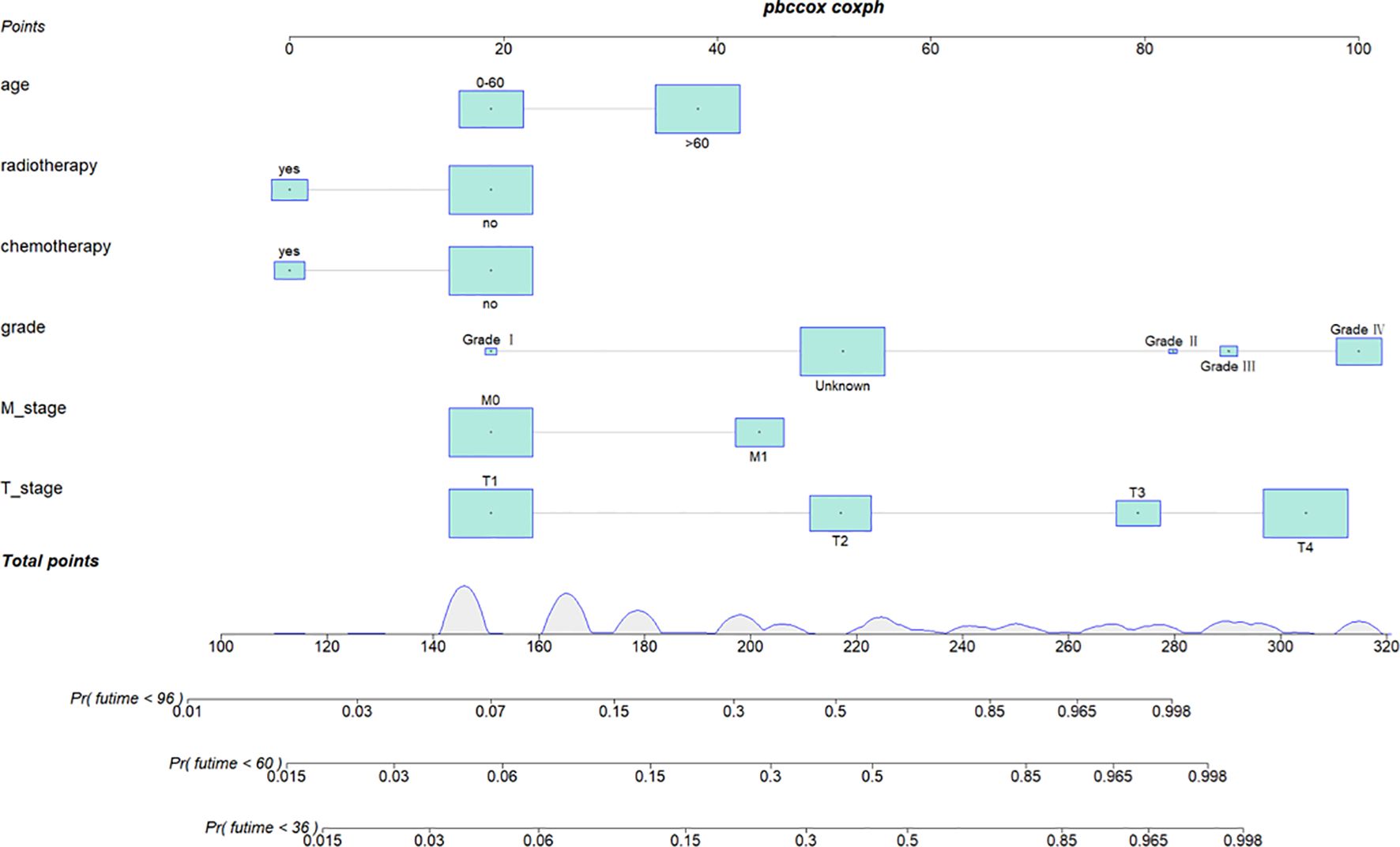
Figure 1. Nomogram for predicting 3-year, 5-year, and 8-year cancer-specific survival of patients with non-operative papillary thyroid cancer.
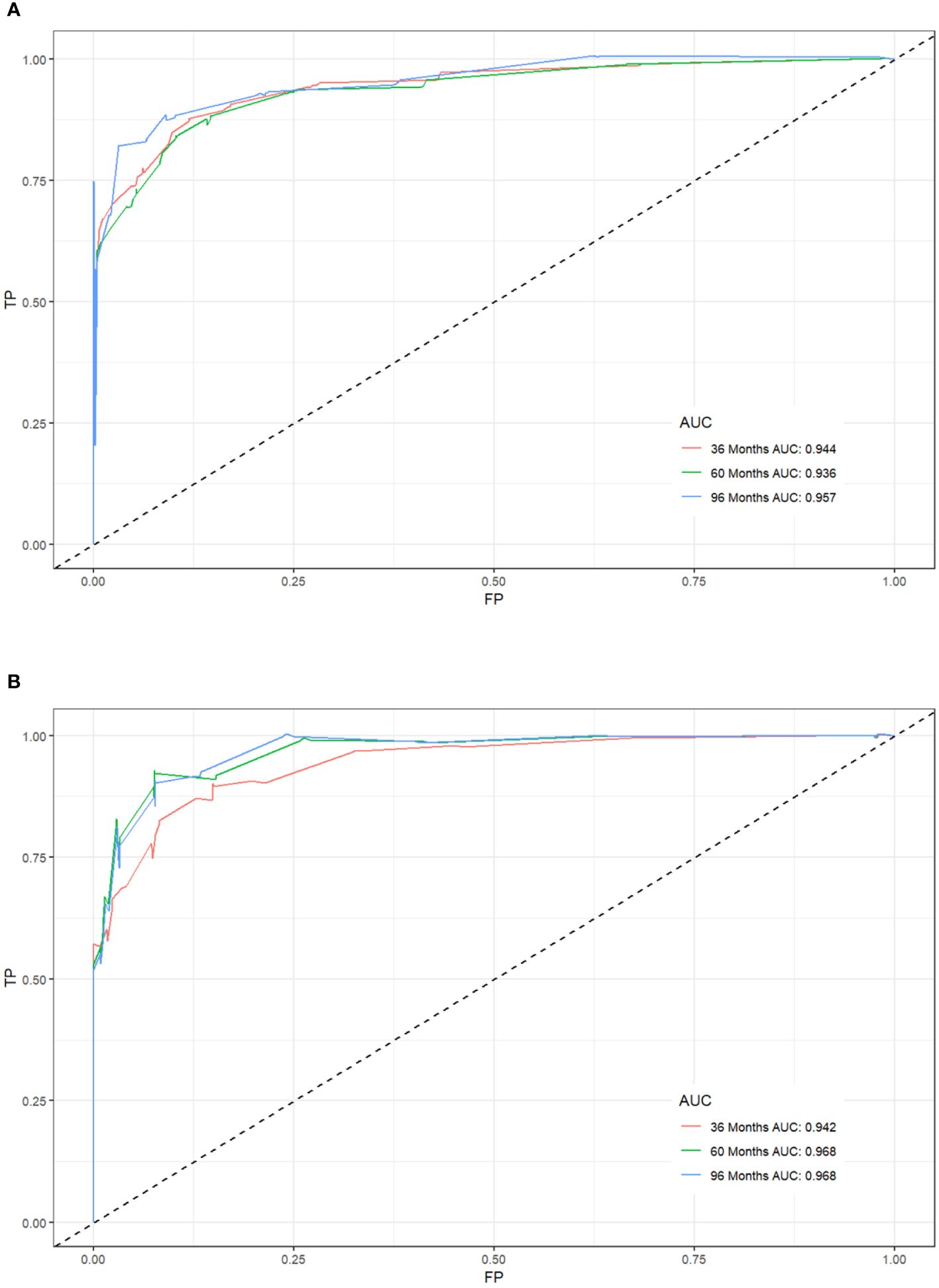
Figure 2. Receiver operating characteristic (ROC) curves for postoperative cancer-specific survival prediction of patients with non-operative papillary thyroid cancer. (A) Training cohort, (B) Validation cohort.

Figure 3. Calibration of the nomogram model in the training cohort (A-C), and validation cohort (D-F).
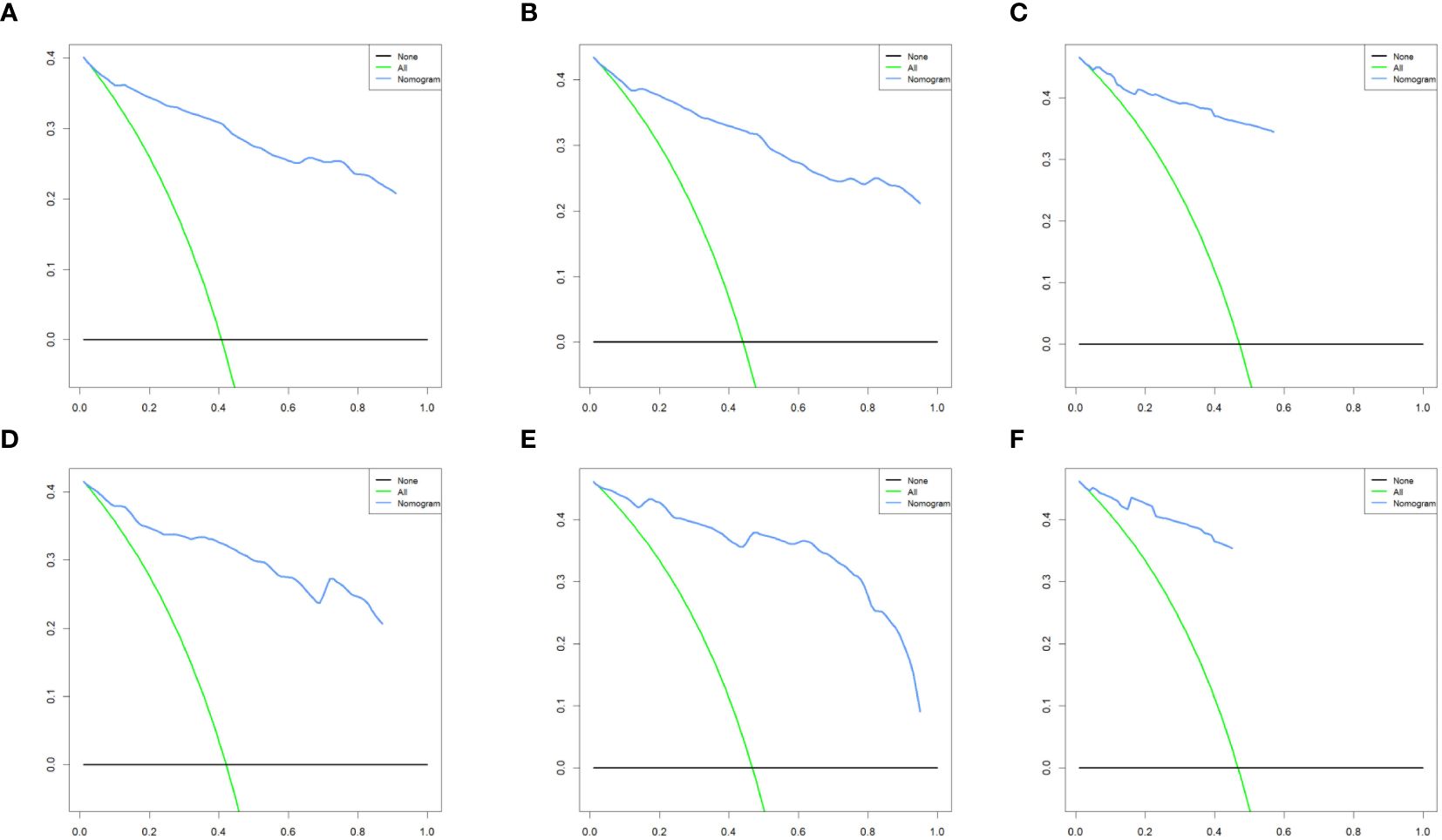
Figure 4. Decision curve analysis of the nomogram model in the training cohort (A-C), and validation cohort (D-F).
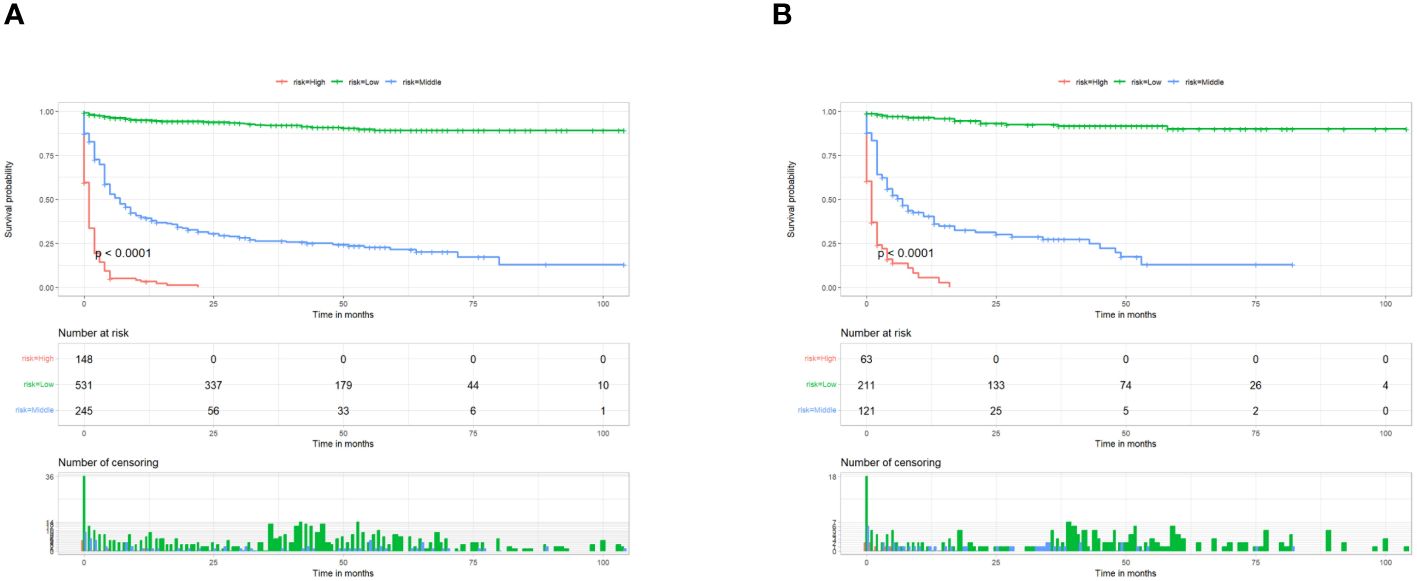
Figure 5. Kaplan–Meier survival analyses were performed to compare postoperative cancer-specific survival in the low risk, middle risk, and high risk subgroups of all patients in the training cohort (A) and validation cohort (B).
PSM and survival curve based on radiotherapy
A 1:1 PSM analysis was performed on the training cohort based on whether they received radiotherapy. It can be seen that after PSM analysis, the baseline information of patients in the matched cohort reached equilibrium (P>0.05) (Table 3). The Kaplan-Meier survival curves instructed that unoperated patients with thyroid cancer who received radiotherapy could produce a trend of significant change in the survival outcome (P< 0.05), as shown in Figure 6.
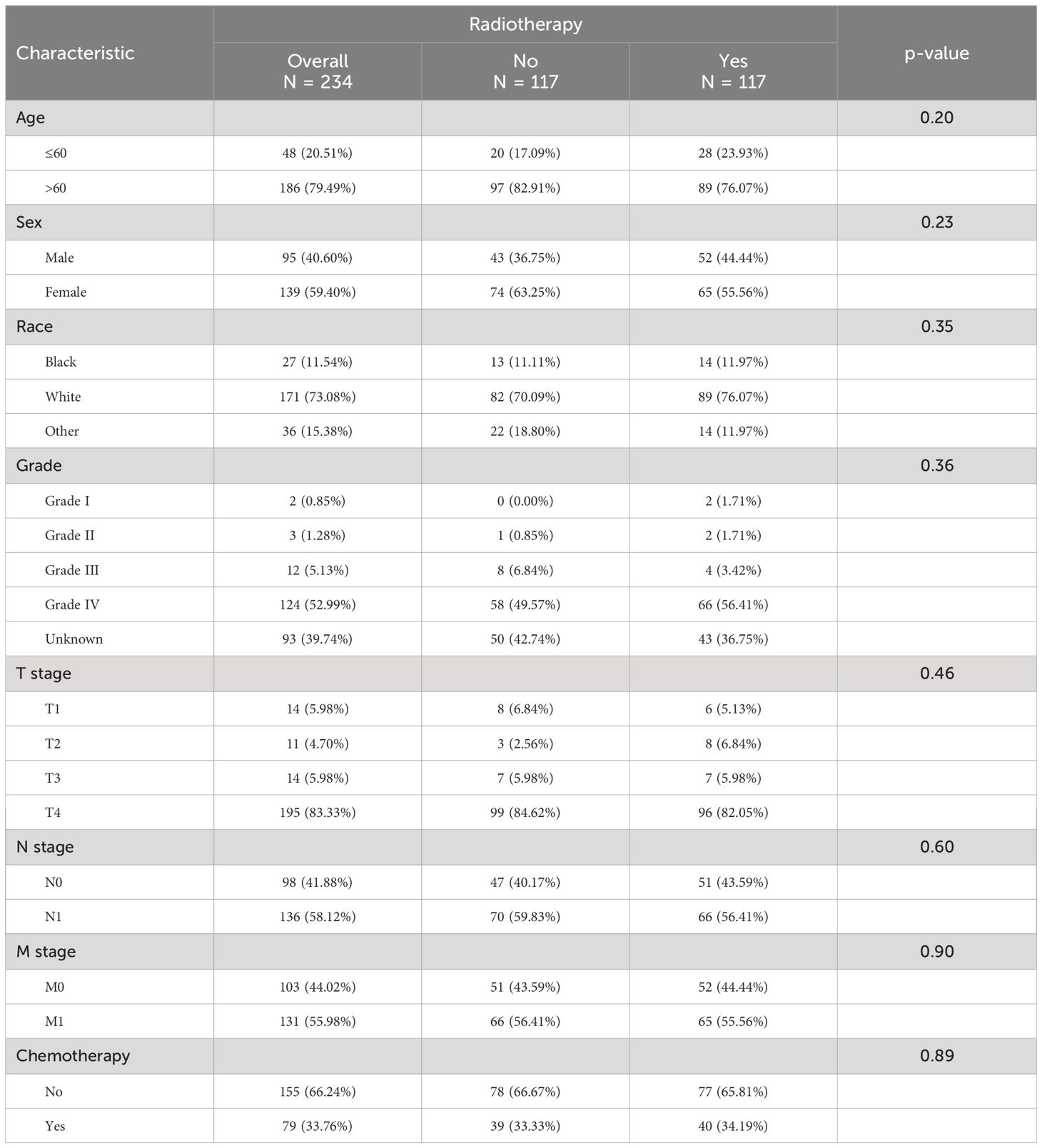
Table 3. Baseline table of the population after PSM analysis using radiotherapy receipt as a subgrouping criterion.

Figure 6. Kaplan–Meier survival analyses were performed to compare postoperative cancer-specific survival for patients receiving radiotherapy and those not receiving radiotherapy after PSM analysis.
Discussion
In this study, we successfully developed a nomogram that can predict the prognosis of unoperated papillary thyroid cancer patients from the clinical information of the patients, and this nomogram was shown to have a good predictive effect in both the training cohort and the validation cohort. We found that the prognosis of patients with unoperated papillary thyroid cancer was correlated with age, grade, T stage, M stage, radiotherapy, and chemotherapy. In addition, we emphasize that radiotherapy remains an independent risk factor for patient survival after PSM analysis, demonstrating that receiving radiotherapy if a patient fails to undergo surgery remains a positive influence on long-term survival.
Age as one of the major high-risk predisposing factors for thyroid is also recognized as a factor affecting the prognostic survival of patients. A retrospective study noted that age AJCC/TNM staging showed consistency (12). This means that older patients are more likely to receive a late diagnosis of the tumor as well as a poorer prognosis compared to younger patients. Interestingly, our study points out that although gender is an important risk factor for thyroid cancer incidence, gender does not seem to affect patient prognosis. In a systematic review, researchers noted that male patients with papillary thyroid cancer were significantly more likely to develop lymph node metastases than female patients (13). However, there is a lack of reports demonstrating the correlation between gender and survival in thyroid cancer patients (14). This question still needs to be confirmed by more relevant prospective studies.
Consistent with previous findings grade, T stage, and M stage were identified as risk factors affecting patient prognosis (15–17). Among them, T stage and M stage showed a significant trend of worse prognosis with tumor progression, but Grade II showed a higher nomogram score than Grade III. We believe that this result may be due to the bias generated by the small sample size. Also of interest is that after Cox survival analysis, the N stage was excluded as a variable affecting the prognosis of patients with unoperated thyroid cancer (Table 2). Previously, it was well recognized that lymph node metastasis increased locoregional recurrence and mortality in thyroid cancer patients (13, 18). However, these are retrospective studies based on those who received surgery to remove the cancerous lesions. Therefore, we believe that for thyroid cancer patients who have not received surgery, whether lymph node metastasis occurs or not is no longer the main factor affecting the survival rate, and what should be emphasized more is whether distant metastasis occurs or not.
The priority of surgery for the management of non-inert thyroid cancer is indisputable (3). One study noted that almost all thyroid cancer patients in Korea underwent total or subtotal thyroidectomy (19). However, there is a lack of adequate clinical evidence for treatment strategies for patients who cannot undergo surgery due to medical conditions or financial reasons. In such cases, radiation therapy is usually the treatment of choice for non-operated patients. After PSM analysis to maximize the effect of confounding factors, a significant difference in survival prognosis between patients who received radiation therapy and those who did not (P<0.05) can be seen in Figure 6. This result suggests that radiation therapy for non-surgical thyroid cancer patients can still have a positive impact on long-term survival. Radioiodine therapy (RIT) is the most common treatment modality and has been used in clinical care for more than 60 years (20, 21). The treatment of papillary thyroid cancer with radiation therapy primarily involves external beam radiation therapy (EBRT) and radioisotope therapy (RAI), each with distinct applications. EBRT is primarily employed for local recurrence or residual disease, particularly when surgery or RAI is ineffective. It effectively controls local tumor growth and reduces recurrence, though it may cause side effects such as skin reactions and difficulty swallowing (22). In contrast, RAI is used postoperatively to ablate residual thyroid tissue and treat metastatic papillary thyroid cancer by administering radioactive iodine, which destroys cancer cells. This significantly reduces recurrence risk, though it may cause temporary side effects such as salivary gland swelling and dry mouth. In summary, EBRT is suitable for local treatment, while RAI offers systemic therapeutic effects (23). Both modalities can be used independently or in combination, depending on the specific clinical scenario, to achieve optimal treatment outcomes. For patients who cannot be surgically removed or for whom surgery is inappropriate, RIT can be used to control the growth of localized lesions, relieve symptoms, and improve patients’ quality of life (24–26). If there are metastases of cancer cells in the lymph node area using RIT may also control cancer cells in the lymph node area (27). However, in the treatment of low-risk thyroid cancer patients, the positive impact of RIT on long-term patient survival has not been demonstrated (21). Therefore, the results of our study may be able to serve as a reference for researchers to demonstrate the favorable role of radiation therapy in improving the prognosis of patients.
In addition, we believe that chemotherapy is also a potential risk factor that may affect the prognosis of patients with non-operated thyroid cancer. However, in the latest guidelines for the management of thyroid cancer, chemotherapy is still mainly used as an individualized treatment modality adjuvant to radiation therapy to enhance the anti-tumor effect (28, 29). In recent years, research on molecularly targeted therapies for thyroid cancer, such as tyrosine kinase inhibitors and anti-angiogenic drugs, is progressing at a rapid pace. These chemotherapeutic agents that act on cell proliferation, immunosuppression, and angiogenesis to inhibit tumors may become a new strategy for the treatment of thyroid cancer in the future (30).
Conclusion
After Cox survival analysis, age, grade, T stage, M stage, radiotherapy, and chemotherapy were considered risk factors to construct a nomogram. We successfully developed and validated the nomogram that predicts CSS in patients with non-operated papillary thyroid cancer. After PSM analysis, radiotherapy was shown to have a positive effect on CSS in all patients with non-operated papillary thyroid cancer.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
SC: Conceptualization, Data curation, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. YmT: Conceptualization, Formal analysis, Writing – original draft. XH: Investigation, Methodology, Writing – original draft. YfT: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Hunan Provincial Health and Wellness Commission Scientific Research Program (D202303067556) and the Intramural Technology Program of the First People’s Hospital of Chenzhou (2020B28).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CSS, cancer-specific survival; ROC, receiver operating characteristic; DCA, decision curve analysis; SEER, Surveillance, Epidemiology, and End Results; PSM, propensity score matching.
References
1. Kaliszewski K, Diakowska D, Miciak M, Jurkiewicz K, Kisiel M, Makles S, et al. The incidence trend and management of thyroid cancer-what has changed in the past years: own experience and literature review. Cancers. (2023) 15(20):4941. doi: 10.3390/cancers15204941
2. Pizzato M, Li M, Vignat J, Laversanne M, Singh D, La Vecchia C, et al. The epidemiological landscape of thyroid cancer worldwide: GLOBOCAN estimates for incidence and mortality rates in 2020. Lancet Diabetes Endocrinol. (2022) 10:264–72. doi: 10.1016/S2213-8587(22)00035-3
3. Chen DW, Lang BHH, McLeod DSA, Newbold K, Haymart MR. Thyroid cancer. Lancet (London England). (2023) 401:1531–44. doi: 10.1016/S0140-6736(23)00020-X
4. Rahbari R, Zhang L, Kebebew E. Thyroid cancer gender disparity. Future Oncol (London England). (2010) 6:1771–9. doi: 10.2217/fon.10.127
5. La Vecchia C, Malvezzi M, Bosetti C, Garavello W, Bertuccio P, Levi F, et al. Thyroid cancer mortality and incidence: a global overview. Int J Cancer. (2015) 136:2187–95. doi: 10.1002/ijc.29251
6. Nguyen QT, Lee EJ, Huang MG, Park YI, Khullar A, Plodkowski RA. Diagnosis and treatment of patients with thyroid cancer. Am Health Drug benefits. (2015) 8:30–40.
7. Tuttle RM. Controversial issues in thyroid cancer management. J Nucl Med. (2018) 59:1187–94. doi: 10.2967/jnumed.117.192559
8. Lancellotta V, Fanetti G, Monari F, Mangoni M, Mazzarotto R, Tagliaferri L, et al. Stereotactic radiotherapy (SRT) for differentiated thyroid cancer (DTC) oligometastases: an AIRO (Italian association of radiotherapy and clinical oncology) systematic review. La Radiologia Med. (2022) 127:681–9. doi: 10.1007/s11547-022-01489-2
9. Inskip PD. Thyroid cancer after radiotherapy for childhood cancer. Med Pediatr Oncol. (2001) 36:568–73. doi: 10.1002/mpo.1132
10. Tong C, Miao Q, Zheng J, Wu J. A novel nomogram for predicting the decision to delayed extubation after thoracoscopic lung cancer surgery. Ann Med. (2023) 55:800–7. doi: 10.1080/07853890.2022.2160490
11. Cai Z, Lin H, Li Z, Chen W, Zhou J, Wu H, et al. A prediction nomogram for postoperative gastroparesis syndrome in right colon cancer: a retrospective study. Langenbeck's Arch Surg. (2023) 408:148. doi: 10.1007/s00423-023-02885-6
12. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA. (2017) 317:1338–48. doi: 10.1001/jama.2017.2719
13. Mao J, Zhang Q, Zhang H, Zheng K, Wang R, Wang G. Risk factors for lymph node metastasis in papillary thyroid carcinoma: A systematic review and meta-analysis. Front Endocrinol. (2020) 11:265. doi: 10.3389/fendo.2020.00265
14. Kim DH, Kim SW, Hwang SH. Predictive value of Delphian lymph node metastasis in the thyroid cancer. Laryngoscope. (2021) 131:1990–6. doi: 10.1002/lary.29426
15. van Velsen EFS, Peeters RP, Stegenga MT, van Kemenade FJ, van Ginhoven TM, van Balkum M, et al. Evaluating disease-specific survival prediction of risk stratification and TNM systems in differentiated thyroid cancer. J Clin Endocrinol Metab. (2023) 108:e267–74. doi: 10.1210/clinem/dgac721
16. Manzardo OA, Cellini M, Indirli R, Dolci A, Colombo P, Carrone F, et al. TNM 8th edition in thyroid cancer staging: is there an improvement in predicting recurrence? Endocrine-related Cancer. (2020) 27:325–36. doi: 10.1530/ERC-19-0412
17. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. (2016) 26:1–133. doi: 10.1089/thy.2015.0020
18. Patron V, Hitier M, Bedfert C, Le Clech G, Jégoux F. Occult lymph node metastases increase locoregional recurrence in differentiated thyroid carcinoma. Ann otology rhinology laryngology. (2012) 121:283–90. doi: 10.1177/000348941212100501
19. Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol. (2016) 12:646–53. doi: 10.1038/nrendo.2016.110
20. Verburg FA, Hänscheid H, Luster M. Radioactive iodine (RAI) therapy for metastatic differentiated thyroid cancer. Best Pract Res Clin Endocrinol Metab. (2017) 31:279–90. doi: 10.1016/j.beem.2017.04.010
21. Schmidbauer B, Menhart K, Hellwig D, Grosse J. Differentiated thyroid cancer-treatment: state of the art. Int J Mol Sci. (2017) 18(6):1292. doi: 10.3390/ijms18061292
22. Sarah N H, Eric T, Eric B, Jonn W. The role of external beam radiation therapy in well-differentiated thyroid cancer. Expert Rev Anticancer Ther. (2017) 17(10):905–10. doi: 10.1080/14737140.2017.1361324
23. Michael TR, Genevieve R, Nancy Y L. A risk-adapted approach to the use of radioactive iodine and external beam radiation in the treatment of well-differentiated thyroid cancer. Cancer Control. (2011) 18.
24. Goffredo P, Robinson TJ, Youngwirth LM, Roman SA, Sosa JA. Intensity-modulated radiation therapy use for the localized treatment of thyroid cancer: Nationwide practice patterns and outcomes. Endocrine. (2016) 53:761–73. doi: 10.1007/s12020-016-0937-2
25. Tam S, Amit M, Boonsripitayanon M, Cabanillas ME, Busaidy NL, Gunn GB, et al. Adjuvant external beam radiotherapy in locally advanced differentiated thyroid cancer. JAMA otolaryngology– Head Neck Surg. (2017) 143:1244–51. doi: 10.1001/jamaoto.2017.2077
26. Valerio L, Maino F, Castagna MG, Pacini F. Radioiodine therapy in the different stages of differentiated thyroid cancer. Best practice & research. Clin Endocrinol Metab. (2023) 37:101703. doi: 10.1016/j.beem.2022.101703
27. van den Bosch S, Vogel WV, Raaijmakers CP, Dijkema T, Terhaard CHJ, Al-Mamgani A, et al. Implications of improved diagnostic imaging of small nodal metastases in head and neck cancer: Radiotherapy target volume transformation and dose de-escalation. Radiotherapy Oncol. (2018) 128:472–8. doi: 10.1016/j.radonc.2018.04.020
28. Filetti S, Durante C, Hartl DM, Leboulleux S, Locati LD, Newbold K, et al. ESMO Clinical Practice Guideline update on the use of systemic therapy in advanced thyroid cancer. Ann Oncol. (2022) 33:674–84. doi: 10.1016/j.annonc.2022.04.009
29. Bible KC, Kebebew E, Brierley J, Brito JP, Cabanillas ME, Clark TJ Jr., et al. 2021 American thyroid association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. (2021) 31:337–86. doi: 10.1089/thy.2020.0944
Keywords: papillary thyroid cancer, non-operative, PSM, nomogram, SEER
Citation: Chen S, Tan Y, Huang X and Tan Y (2024) Construction of a new tool for predicting cancer-specific survival in papillary thyroid cancer patients who have not received surgery. Front. Endocrinol. 15:1417528. doi: 10.3389/fendo.2024.1417528
Received: 15 April 2024; Accepted: 16 July 2024;
Published: 16 August 2024.
Edited by:
Cristina Alina Silaghi, University of Medicine and Pharmacy Iuliu Hatieganu, RomaniaReviewed by:
Otilia Kimpel, University Hospital of Wuerzburg, GermanyWen-Wu Dong, The First Affiliated Hospital of China Medical University, China
Copyright © 2024 Chen, Tan, Huang and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanfei Tan, dGFueWFuZmVpMjExQDE2My5jb20=
†These authors have contributed equally to this work
 Sanjun Chen1†
Sanjun Chen1† Yanfei Tan
Yanfei Tan