
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 18 July 2024
Sec. Reproduction
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1416841
 Lin Sun1†
Lin Sun1† Beining Yin1†
Beining Yin1† Zhiyi Yao1
Zhiyi Yao1 Congli Zhang1
Congli Zhang1 Jinyu Li1
Jinyu Li1 Sichen Li1
Sichen Li1 Yueyue Cui1
Yueyue Cui1 Fang Wang1
Fang Wang1 Wei Dai1
Wei Dai1 Zhiqin Bu1
Zhiqin Bu1 Yile Zhang1,2*
Yile Zhang1,2*Purpose: To investigate potential differences in pregnancy outcomes among patients with regular menstruation who underwent frozen-thawed embryo transfer using natural cycle (NC) or hormone replacement therapy (HRT).
Methods: This study retrospectively analyzed 2672 patients with regular menstruation who underwent FET from November 2015 to June 2021 at the single reproductive medical center. A one-to-one match was performed applying a 0.02 caliper with propensity score matching. Independent factors influencing the live birth and clinical pregnancy rates were screened and developed in the nomogram by logistic regression analysis. The efficacy of live birth rate and clinical pregnancy rate prediction models was assessed with the area under the ROC curve, and the live birth rate prediction model was internally validated within the bootstrap method.
Results: The NC protocol outperformed the HRT protocol in terms of clinical pregnancy and live birth rates. The stratified analysis revealed consistently higher live birth and clinical pregnancy rates with the NC protocol across different variable strata compared to the HRT protocol. However, compared to the HRT treatment, perinatal outcomes indicated that the NC protocol was related to a higher probability of gestational diabetes. Multifactorial logistic regression analysis demonstrated independent risk factors for live birth rate and clinical pregnancy rate. To predict the two rates, nomogram prediction models were constructed based on these influencing factors. The receiver operating characteristic curve demonstrated moderate predictive ability with an area under curve (AUC) of 0.646 and 0.656 respectively. The internal validation of the model for live birth rate yielded an average AUC of 0.646 implying the stability of the nomogram model.
Conclusion: This study highlighted that NC yielded higher live birth and clinical pregnancy rates in comparison to HRT in women with regular menstruation who achieved successful pregnancies through frozen-thawed embryo transfer. However, it might incur a higher risk of developing gestational diabetes.
The publication of the initial case of frozen-thawed embryo transfer (FET) in 1983 marked a significant milestone (1). Over the past four decades, the utilization of embryo freezing has steadily increased in China, Europe, and the United States, with frozen cycles accounting for over 40% of total cycles (2). Moreover, a comprehensive clinical multi-center study has discovered that FET substantially improves the live birth rate and reduces the probability of ovarian hyperstimulation syndrome (OHSS) versus fresh-cycle transfer (3). OHSS is an iatrogenic disease caused by overstimulation of the ovaries with exogenous gonadotropins during ovulation induction.
Endometrial preparation plays a crucial role in FET as it determines endometrial receptivity and coordinates the development of both the endometrium and embryo (4). Currently, numerous kinds of endometrial preparation protocols are employed for FET including the natural cycle, hormone replacement therapy, and promoting ovulation cycle with the NC and HRT being the most commonly used. Consequently, our objective was to discover the most beneficial endometrial preparation method for patients utilizing frozen embryos.
To date, multiple studies have endeavored to explore the clinical results of various endometrial preparation protocols, yet the conclusions remain inconsistent and controversial. Notably, when compared to HRT, NC has been revealed to offer a higher opportunity of live birth in young patients with regular cycles of menstruation (5, 6). However, several have reported that NC and HRT have similar outcomes and are equally effective (7, 8). Meanwhile, the repercussions of these two procedures on prenatal and neonatal outcomes have been the subject of a multitude of research. Retrospective investigations have indicated that there is a higher chance of adverse perinatal outcomes when receiving HRT (9–12). Conversely, Saito et al. demonstrated that the risk of gestational diabetes mellitus (GDM) was lower in HRT pregnancies (11). Consequently, there is no consensus regarding the safety and efficiency of the two endometrial preparation protocols.
In summary, the objective of this research project was to examine, the clinical and perinatal results between the NC and HRT protocols after FET in infertile patients with regular menstruation. The findings might provide valuable guidance to clinicians in selecting individualized protocols for FET patients.
This cohort study reviewed clinical information of patients receiving frozen-thawed cycles from November 2015 to June 2021 at the Reproductive Medicine Center of the First Affiliated Hospital of Zhengzhou University. The anonymous data were gathered from our center’s data entry systems. This research was approved by the hospital’s Institutional Review Board and Ethics Committee (reference number: 2023-KY-1115–002). On account of the study being retrospective, informed permission was not required. Every procedure was executed in compliance with applicable rules and legislation.
The research included individuals who fulfilled the subsequent criteria: 1) adoption of either the HRT or NC protocol; 2) compliance with the 2017 American Society for Reproductive Medicine consensus diagnostic criteria for infertility (13). The following were criteria for exclusion: 1) patients with other factors affecting pregnancy like ovarian insufficiency, adenomyosis, endometriosis, uterine abnormalities, uterine adhesions, cervical insufficiency or hydrosalpinx; 2) chromosomal abnormalities in the patient or spouse; 3) recurrent implantation failures; 4) abnormal male reproductive function; 5) irregular menstrual cycles (<21 or >35 days); 6) incomplete clinical information.
Every method performed to prepare the endometrium for this research was meticulously documented. Patients were allocated to either the NC group or the HRT group based on their individual circumstances and the experience of the clinician.
For patients in the NC group, on the eighth and ninth day of the menstrual cycle, transvaginal ultrasounds were implemented to monitor follicular development and endometrial growth. Once the dominant follicle reached 14 mm in mean diameter, transvaginal ultrasounds and the level of urinary luteinizing hormone (LH) level were monitored every day. On the day of ovulation (Day 1, D1), 400mg of vaginal progesterone soft capsules (Utrogestan, Cyndea Pharma, S.L, Spain) was administered once daily, and three days later, oral dydrogesterone (Duphaston; Abbott, Netherlands) was implemented. In accordance with the fact that the optimal endometrial receptivity usually occurs between the fourth and sixth days after ovulation, cleavage-stage embryos and blastocysts were implanted on D4 and D5 respectively (14).
For patients undergoing the HRT protocol, estradiol valerate 4mg (Progynova®; Bayer, Leverkusen, Germany) was taken every day beginning on the menstrual cycle’s third day. Every four days, the oral dosage was modified based on the thickness of the endometrium. Once the endometrium thickness reached 7mm, intramuscular progesterone (60mg) was administered to the protocol to transform the endometrium (Day 1, D1). The following day, a dose of 10 mg/day of oral dydrogesterone was applied and the dose was raised to 30 mg/day after three days. Cleavage-stage embryos and blastocysts were transferred on D5 and D6 respectively.
We selected one or two good-quality blastocyst and cleavage embryos to transfer for each patient. The following are the criteria for transfer. Embryos were evaluated according to Peter’s criteria: grade I and grade II were considered high quality (15). Blastocysts were scored according to the Gardner criteria: high-quality embryos are those with 2 scores of B and above for inner cell mass and trophectoderm (16).
Under the supervision of an ultrasonography, up to two embryos were implanted into the uterus. Starting from the day of embryo transplantation, daily administration of 90mg progesterone sustained-release vaginal gel (Crinone 8%; Merck Serono, Switzerland) or 400mg progesterone soft capsules and 20mg of oral dydrogesterone was initiated. But in HRT protocol, 10mg estradiol valerate is required in addition to the above medications. The serum human chorionic gonadotropin (hCG) levels were tested two weeks following the transfer of the embryo. Upon surpassing 50 IU/L in blood hCG, the luteal phase was continued. In the fifth week following the embryo transfer, transvaginal ultrasound was conducted to clinically confirm pregnancy. If pregnancy occurred, the luteal phase was still continued. Progesterone sustained-release vaginal gel or progesterone soft capsules was discontinued in the 45th day after transportation and oral dydrogesterone was discontinued in the 65th day after transportation.
Every patient received follow-up for no fewer than a year. Live birth was defined as the delivery of at least one live child beyond 22 weeks of pregnancy. Clinical pregnancy was defined as the presence of one or more gestational sacs observed by ultrasonography or the presence of clear clinical indicators of pregnancy. A preterm birth occurred after 22 weeks but before the full 37 weeks of gestation. Low-birth-weight infants and macrosomia were defined as those with a birth weight of less than 2,500 g and a weight of more than 4,000 g respectively.
This study was statistically analyzed using SPSS 25.0 software and the R language software statistical package (R version 4.1.3). Dichotomous variables were expressed as percentages (%), while mean ± standard deviation is the presentation format for continuous variables with a normally distributed distribution. Comparisons of two independent samples of dichotomous variables were evaluated using the chi-square test, and Fisher’s exact test or chi-square test was employed to assess the count data. For normally distributed continuous variables that met the assumption of equal variances, the independent t-test was employed. Propensity score matching (PSM) was employed to 1:1 match baseline data with statistically significant differences within either of the groups with a caliper value of 0.02. The variables used for matching included female age, infertility duration, infertility type, gravity, parity, NO. of miscarriages, BMI, basal serum FSH, AMH, AFC, type of embryo transferred and NO. of embryo transferred. The study performed univariate logistic regression analysis to ascertain independent and confounding factors. Moreover, the multivariate logistic regression analysis incorporated variables from the univariate study that were correlated to the two clinical outcomes. The study conducted stratified analyses based on female age, infertility duration, BMI, AFC, the type of embryo transferred, number of embryos transferred, endometrial thickness, and triple-line endometrial pattern on the day of progesterone administration, to observe the effects of the two protocols in different subgroup.
The independent variables affecting the rates of clinical pregnancy and live births were identified by the multifactorial logistic regression analysis. Incorporating these factors as modeling variables, predictive nomogram models were built using the R statistical software. The bootstrap sampling method was employed for internal validation of the model, and the predictive power of the model was assessed by calculating the area under the curve (AUC) and the receiver operating characteristic (ROC) curve. The statistical significance was identified by applying a two-sided significance criterion of 0.05. Supplementary Figure 1 illustrated the study’s data collection methodology.
The research comprised 3,569 patients who underwent FET between November 2015 and June 2021. Of those, 1,914 patients were in the HRT group and 1,655 individuals belonged to the NC group. After conducting propensity score-matched (PSM), 1,336 infertile patients with regular menstruation were included in each group.
Baseline characteristics were illustrated in Table 1. Before matching, the NC and HRT groups had statistically significant differences in terms of female age, infertility duration, infertility type, gravity, parity, number of miscarriages, BMI, AMH, number of AFC, number of embryos transferred, and type of embryos transferred. Following PSM, all these variables were balanced between the two groups. The clinical outcomes before and after matching were also presented in Table 1. Before PSM, the NC group exhibited better endometrial thickness and higher incidence of live birth and clinical pregnancy and single pregnancy. After PSM, these differences remained significant, with the NC group consistently exhibiting higher rates of live birth, clinical pregnancy and thicker endometrium versus the HRT group.
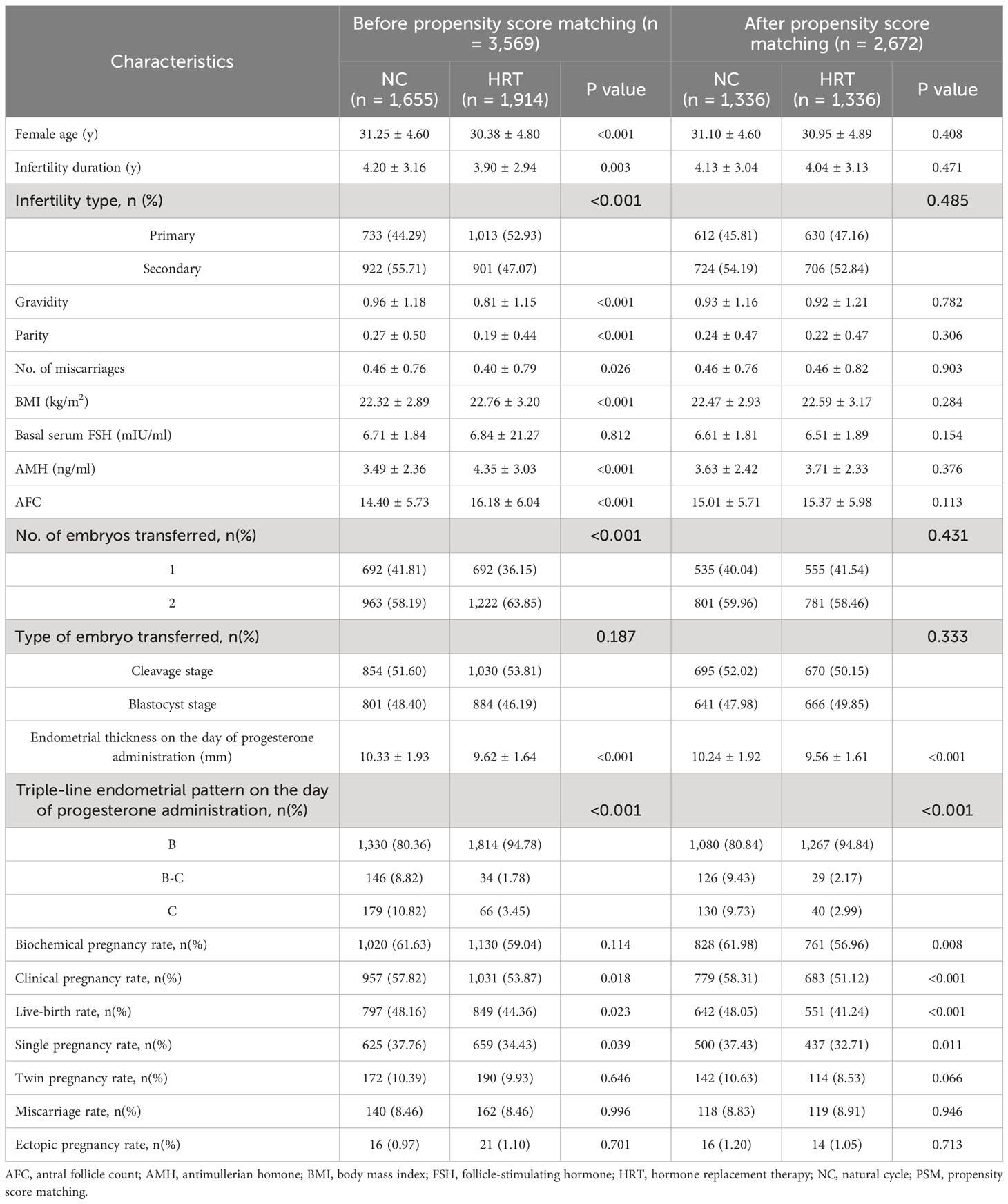
Table 1 Comparison of baseline characteristics and pregnancy outcomes between NC and HRT before and after PSM.
The influence of different variables was evaluated utilizing univariate and multivariate analysis (Supplementary Table 1). The results revealed that female age, infertility duration, and the endometrial preparation protocol were associated with the live birth rate and clinical pregnancy rate negatively. Conversely, AFC, NO. of embryos transferred, type of embryos transferred, and endometrial thickness on the day of progesterone administration were positively associated with them. To ascertain whether there was a consistent correlation across several subgroups between the live birth rate and various endometrial preparation protocols, stratified analyses were performed based on female age, infertility duration, BMI, AFC, the type of embryo transferred, number of embryo transferred, endometrial thickness and triple-line endometrial pattern on the day of progesterone administration (Table 2). The NC group had an increased chance of achieving clinical pregnancy and live births in the subgroup that female age ≤35 years, BMI ≤24kg/m2, AFC >15, the number of embryos transferred was one, transferred any embryo type, the endometrial thickness <10mm, and B endometrial pattern. It appeared that there was no interaction between any of the subgroups (p > 0.05).
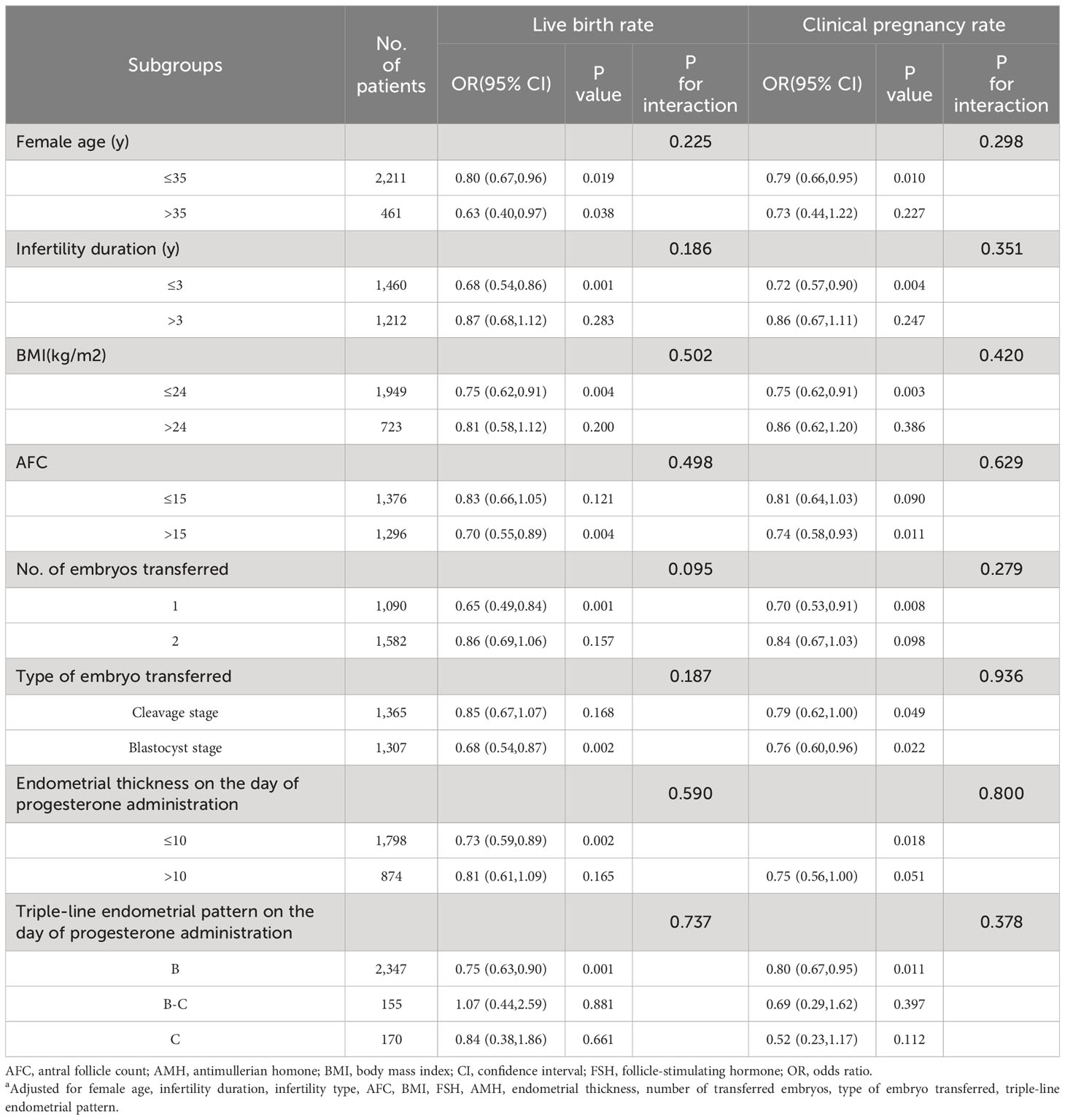
Table 2 Impact of two endometrial preparation protocols on live birth rate and clinical pregnancy rate in each subgroupa.
There was a substantial increase in the likelihood of gestational diabetes when comparing the two protocols (Table 3). However, there were no statistical differences observed in gestational hypertension, premature rupture of membranes, or other adverse outcomes, including anemia during pregnancy, oligohydramnios, meconium stained amniotic fluid, fetal distress, and placental abruption. Among the 1,193 newborns involved in the research, no statistically significant differences existed within the two groups in preterm birth rate, newborn weights, cesarean section rate, or weights of single and twin pregnancies.
The independent variables influencing the live birth rate were uncovered by the multifactorial logistic regression analysis. The results revealed that the endometrial preparation protocol, female age, infertility duration, AFC, type of embryos transferred and number of embryos transferred were significant factors (Figure 1A). The predictive model for the live birth rate was evaluated using the ROC curve, and an area under the curve of 0.646 (95% CI: 0.626–0.667) was obtained, indicating a moderate predictive power. Moreover, the predictive ability of the nomogram model with that of each individual indicator was also compared using ROC curves (Figure 1B). The nomogram model outperformed each indicator in terms of predictive ability. In addition, A computer simulation of repeated sampling was executed to further validate the model internally. The ROC curve was employed for calculating the model’s predictive performance after 1000 repeated samples, resulting in an average AUC = 0.646 (95% CI: 0.629–0.663) (Figure 1C). The AUC remained essentially unchanged after internal validation, indicating the stability of the model. Additionally, we also constructed the nomogram prediction model for the clinical pregnancy rate and leveraged the ROC curve with the AUC of 0.656 (95% CI: 0.635–0.677). (Supplementary Figure 2)

Figure 1 Construction and internal validation of the predictive model for live birth rate. (A) The nomogram exhibited six characteristics of a patient (NO. of transferrable embryo = 2, Type of embryo transferred = Blastocyst stage, AFC = 18, Infertility duration = 9, Female age = 31, Endometrial Preparation Protocol = HRT), with a total score of 399 points, and the predicted probability of live birth was 53.7%. (B) The area under the curve of the nomogram model was 0.646 (95% CI: 0.626–0.667), indicating medium predictive power. (C) Internal validation with 1000 repeated sampling was carried out to predict the effectiveness of the model, resulting in an average AUC = 0.646 (95% CI: 0.629–0.663). AFC, antral follicle count; AUC, area under the curve; CI, confidence interval; HRT, hormone replacement therapy; NC, natural cycle; ROC, receiver operating characteristic curve.
The FET cycle has grown in favor all around the world in light of its ability to reduce the incidence of ovarian stimulation syndrome, preserve female fertility, and provide other advantages (17, 18). Consequently, research on the effects of various endometrial preparation protocols on the result of pregnancy is becoming more and more popular. Our investigation demonstrated that the NC protocol produced greater rates of clinical pregnancy and live births.
Currently, the most appropriate protocol for endometrial preparation is still no consensus. Our observations are substantiated by recent published articles. Within the patient subgroup with D5/D6 blastocyst embryo transfer, the NC group revealed a trend toward greater clinical pregnancy and live birth rates, according to a large retrospective cohort analysis (19). Two other studies reached similar conclusions (20, 21). Another study with low-quality evidence elucidated that for double embryo transfer, the modified NC group had dramatically superior clinical outcomes compared to the HRT group (22). However, several relevant studies displayed no distinction in clinical outcomes within the two protocols (23–26). Consistent conclusions have also been presented in two high-quality Cochrane analyses. Ghobara et al. presumed that in patients with regular menstruation but low fertility, there was inadequate data to prove the superiority of one endometrial preparation protocol over another (27). Similarly, Glujovsky et al. indicated a lack of evidence regarding specific interventions for endometrial preparation in patients receiving frozen embryo transfers (28). Nevertheless, in our research, all FET cycles were included for embryo transfer, and women with low fertility were excluded to minimize the interference of confounding factors. This might explain why the NC group in our cohort achieved optimal clinical outcomes.
In light of the underlying mechanism, it might be related to the type of embryo transferred. It has been warranted that fresh blastocyst transfers had a higher probability of live birth and clinical pregnancy than fresh cleavage stage transfers (29). While many studies prefer blastocyst transfers, the optimal endometrial preparation protocol for blastocyst transfers remains inconsistent (7, 20). Researchers are convinced that only surviving embryos will undergo self-selection, and blastocysts undergo a reselection program during the developmental block at the eight-cell stage to eliminate embryos with inadequate potential for development (30). Therefore, blastocyst transfer might be a superior choice for research purposes. However, there have also been cohort studies that revealed no disparity in cumulative live birth rate regarding the transfer of blastocysts and cleavage (31). In our study, no distinction was made between the cleavage stage and blastocyst embryos, but stratified analysis revealed that the NC protocol was inclined to achieve remarkable clinical benefits in both groups.
In addition, the embryo must be implanted in the endometrium at the “window of implantation”, which represents the period of highest receptivity for trophoblast-endometrium interactions (4). A study similar to ours transferred cleavage embryos one day earlier than our center but reached the same conclusion that the live birth rate increased as a result of the NC protocol (21). Another low-quality analysis performed with both cleavage and blastocyst transfers one day earlier than our center found no distinction between the two protocols about the clinical pregnancy outcomes (25). Other research has indicated that prolonged progesterone supplementation is linked to an increased incidence of biochemical pregnancy (32). However, the ideal length of time to use progesterone supplements remains a subject of debate, as prolonged supplementation might narrow the window of implantation, while a too-short period could raise the chance of losing a pregnancy too soon (32–35). Taken together, the intricate process of implantation comprises both intercellular and extracellular matrix interactions as well as spatiotemporally regulated endocrine, paracrine, and autocrine interactions (36). Furthermore, every center has a varied choice of when to schedule the transfer day depending on the width of the implantation window. At our center, despite developing two days apart, the blastocysts and cleavage stage embryos were transferred one day apart. This is due to the fact that our center accidentally discovered that transplanting one day apart may resulted in better pregnancy outcomes in 2016 (37–39).
Furthermore, endometrial preparation protocols might also impact obstetric and perinatal outcomes. Administering the HRT protocol might raise the risk of hypertensive disorders in pregnant women with preeclampsia and other hypertensive diseases (10, 40–42). According to a high-quality review, the NC protocol was related to lower rates of macrosomia, hypertensive disorders of pregnancy, and early pregnancy loss (43). Nevertheless, no differences in neonatal and perinatal outcomes were found within either of the groups in our research, which could be brought about by the tiny sample size. Additionally, we observed a higher risk of developing gestational diabetes mellitus (GDM) in the NC group, consistent with a study by Saito et al. (11). A review of the data has demonstrated that maternal peripheral insulin resistance is a critical event in the occurrence of GDM. The HRT protocol might reduce the release of insulin-resistant hormones from the placenta, potentially reducing the incidence of GDM (44). However, other studies have displayed no difference in the risk of developing GDM between the two groups (45, 46). Therefore, the placenta may also play an indispensable role in adverse neonatal and perinatal outcomes, necessitating further exploration in future large-scale studies.
Our study possesses several noteworthy strengths. Firstly, this study is the first PSM cohort study to analyze the effects of natural versus hormone replacement cycles on pregnancy outcomes and perinatal outcomes with a huge sample size used to guarantee robust statistical power. Additionally, the study population included people whose average was thirty years and was not limited by age, thereby enhancing the generalizability of the findings to a wide range of patients. Moreover, patients with regular menstruation were specifically selected to minimize selection bias. In addition, to control confounding factors, we implemented stratified analysis and logistic regression making conclusions more dependable. Finally, we internally validated the nomogram prediction model, and the ROC curves and AUC supported the model’s sensitivity and accuracy.
However, this research does have its limitations. To begin with, given that the study was a retrospective cohort, confounders besides the variables collected in the study could not be investigated, such as lifestyle habits (smoking or non-smoking) and adjustments made by clinicians during the study period. Secondly, the data were solely derived from the same center, and future studies should involve multiple centers for broader analysis and further prospective randomized controlled trials. Thirdly, the protocol criteria exclude most gynecological conditions that could potentially influence endometrial receptivity. Therefore, these results might not encompass patients with impaired endometrial receptivity. Fourthly, this analysis didn’t perform preimplantation genetic testing for aneuploidies (PGT-A) because of the restricted data accessibility, thus transfer failure due to aneuploid embryos could not be excluded. If euploid embryos were being transferred, the findings would be more robust.
This study demonstrated that natural cycles yield higher rates of live birth and clinical pregnancy by contrasting hormone replacement cycles in women with regular menstruation undergoing frozen-thawed embryo transfer. However, it was vital to note that natural cycles were linked to a greater incidence of gestational diabetes. Based on our findings, we recommend the use of natural cycles as the preferred option for performing FET. However, it will take more superior prospective randomized controlled studies to identify and validate the most appropriate endometrial preparation strategy.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethics Committee of Scientific Research and Clinical Trials of the First Affiliated Hospital of Zhengzhou University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
LS: Data curation, Formal analysis, Software, Writing – original draft. BY: Data curation, Software, Visualization, Writing – original draft. ZY: Writing – review & editing. CZ: Writing – review & editing. JL: Writing – review & editing. SL: Writing – review & editing. YC: Writing – review & editing. FW: Writing – review & editing. WD: Writing – review & editing. ZB: Writing – review & editing. YZ: Conceptualization, Data curation, Funding acquisition, Resources, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This project was supported by grant 32271169 from the National Natural Science Foundation of China.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1416841/full#supplementary-material
Supplementary Figure 1 | The flowchart of participants. HRT, hormone replacement therapy; NC, natural cycle.
Supplementary Figure 2 | Construction of the predictive model for clinical pregnancy rate. The nomogram revealed five characteristics of a patient (NO. of transferrable embryo = 2, Type of embryo transferred = Blastocyst stage, AFC = 18, Infertility duration = 9, Female age = 31, Endometrial Preparation Protocol = HRT), with a total score of 329 points, and the predicted probability of clinical pregnancy rate was 70.5%. The area under the curve of ROC for the nomogram model was 0.656 (95% CI: 0.635–0.677). AFC, antral follicle count; CI, confidence interval; HRT, hormone replacement therapy; NC, natural cycle; ROC, receiver operating characteristic curve.
AFC, antral follicle count; AMH, antimul1lerian hormone; AUC, area under the curve; BMI, body mass index; CI, confidence interval; FET, frozen-thawed embryo transfer; FSH, follicle-stimulating hormone; GDM, gestational diabetes mellitus; HDP, hypertensive disorders of pregnancy; HRT, hormone replacement therapy; LBW, low birth weight; NC, natural cycle; OR, odds ratio; PGT-A, preimplantation genetic testing for aneuploidies; pPROM, preterm premature rupture of the membrane; PSM, propensity score matching; PTB, preterm birth.
1. Trounson A, Mohr L. Human pregnancy following cryopreservation, thawing and transfer of an eight-cell embryo. Nature. (1983) 305:707–9. doi: 10.1038/305707a0
2. Jewett A, Mardovich S, Zhang Y, Sunderam M, DeSantis C, Cofie A, et al. Assisted Reproductive Technology Fertility Clinic and National Summary Report (2020). Available online at: https://www.cdc.gov/art/reports/2020/index.html (Accessed January 1, 2020).
3. He Y, Lu Y, Zhu Q, Wang Y, Lindheim SR, Qi J, et al. Influence of metabolic syndrome on female fertility and in vitro fertilization outcomes in PCOS women. Am J Obstet Gynecol. (2019) 221:138.e1–138.e12. doi: 10.1016/j.ajog.2019.03.011
4. Casper RF, Yanushpolsky EH. Optimal endometrial preparation for frozen embryo transfer cycles: window of implantation and progesterone support. Fertil Steril. (2016) 105:867–72. doi: 10.1016/j.fertnstert.2016.01.006
5. Li J, Sun Q, Zhang M, Fu X, Zhang Y, Gao S, et al. Natural cycles achieve better pregnancy outcomes than artificial cycles in non-PCOS women undergoing vitrified single-blastocyst transfer: a retrospective cohort study of 6840 cycles. J Assist Reprod Genet. (2022) 39:639–46. doi: 10.1007/s10815-022-02424-0
6. Liu J, Zheng J, Lei YL, Wen XF. Effects of endometrial preparations and transferred embryo types on pregnancy outcome from patients with advanced maternal age. Syst Biol Reprod Med. (2019) 65:181–6. doi: 10.1080/19396368.2018.1501114
7. Jin Z, Shi H, Bu Z, Guo Y, Su Y, Song H, et al. Live birth rates after natural cycle versus hormone replacement therapy for single euploid blastocyst transfers: a retrospective cohort study. Reprod BioMed Online. (2021) 43:1002–10. doi: 10.1016/j.rbmo.2021.09.003
8. Carosso AR, Brunod N, Filippini C, Revelli A, Evangelisti B, Cosma S, et al. Reproductive and obstetric outcomes following a natural cycle vs. Artificial endometrial preparation for frozen-thawed embryo transfer: A retrospective cohort study. J Clin Med. (2023) 12:4032. doi: 10.3390/jcm12124032
9. von Versen-Höynck F, Schaub AM, Chi YY, Chiu KH, Liu J, Lingis M, et al. Increased preeclampsia risk and reduced aortic compliance with in vitro fertilization cycles in the absence of a corpus luteum. Hypertension. (2019) 73:640–9. doi: 10.1161/HYPERTENSIONAHA.118.12043
10. Ginström Ernstad E, Wennerholm UB, Khatibi A, Petzold M, Bergh C. Neonatal and maternal outcome after frozen embryo transfer: Increased risks in programmed cycles. Am J Obstet Gynecol. (2019) 221:126.e1–126.e18. doi: 10.1016/j.ajog.2019.03.010
11. Saito K, Kuwahara A, Ishikawa T, Morisaki N, Miyado M, Miyado K, et al. Endometrial preparation methods for frozen-thawed embryo transfer are associated with altered risks of hypertensive disorders of pregnancy, placenta accreta, and gestational diabetes mellitus. Hum Reprod. (2019) 34:1567–75. doi: 10.1093/humrep/dez079
12. Gu F, Wu Y, Tan M, Hu R, Chen Y, Li X, et al. Programmed frozen embryo transfer cycle increased risk of hypertensive disorders of pregnancy: a multicenter cohort study in ovulatory women. Am J Obstet Gynecol MFM. (2023) 5:100752. doi: 10.1016/j.ajogmf.2022.100752
13. Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, et al. The international glossary on infertility and fertility care, 2017. Hum Reprod. (2017) 32:1786–801. doi: 10.1093/humrep/dex234
14. Lessey BA, Young SL. What exactly is endometrial receptivity? Fertil Steril. (2019) 111:611–7. doi: 10.1016/j.fertnstert.2019.02.009
15. Fragouli E, Alfarawati S, Spath K, Wells D. Morphological and cytogenetic assessment of cleavage and blastocyst stage embryos. Mol Hum Reprod. (2014) 20:117–26. doi: 10.1093/molehr/gat073
16. Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. (2000) 73:1155–8. doi: 10.1016/S0015-0282(00)00518-5
17. Shi Y, Sun Y, Hao C, Zhang H, Wei D, Zhang Y, et al. Transfer of fresh versus frozen embryos in ovulatory women. N Engl J Med. (2018) 378:126–36. doi: 10.1056/NEJMoa1705334
18. Roque M, Haahr T, Geber S, Esteves SC, Humaidan P. Fresh versus elective frozen embryo transfer in IVF/ICSI cycles: a systematic review and meta-analysis of reproductive outcomes. Hum Reprod Update. (2019) 25:2–14. doi: 10.1093/humupd/dmy033
19. Qian Y, Wan Q, Bu X-Q, Li T, Tang X-J, Jia Y, et al. Pregnancy outcomes of four different cycle protocols for frozen embryo transfer: a large retrospective cohort study. Reprod Dev Med. (2023) 7:135–41. doi: 10.1097/RD9.0000000000000052
20. Li X, Gao Y, Shi J, Shi W, Bai H. Natural cycle increases the live-birth rate compared with hormone replacement treatment for frozen-thawed single euploid blastocyst transfer. Front Endocrinol (Lausanne). (2022) 13:969379. doi: 10.3389/fendo.2022.969379
21. Liu X, Shi W, Shi J. Natural cycle frozen-thawed embryo transfer in young women with regular menstrual cycles increases the live-birth rates compared with hormone replacement treatment: a retrospective cohort study. Fertil Steril. (2020) 113:811–7. doi: 10.1016/j.fertnstert.2019.11.023
22. Guan Y, Fan H, Styer AK, Xiao Z, Li Z, Zhang J, et al. A modified natural cycle results in higher live birth rate in vitrified-thawed embryo transfer for women with regular menstruation. Syst Biol Reprod Med. (2016) 62:335–42. doi: 10.1080/19396368.2016.1199064
23. Agha-Hosseini M, Hashemi L, Aleyasin A, Ghasemi M, Sarvi F, Shabani Nashtaei M, et al. Natural cycle versus artificial cycle in frozen-thawed embryo transfer: A randomized prospective trial. Turk J Obstet Gynecol. (2018) 15:12–7. doi: 10.4274/tjod.47855
24. Sahin G, Acet F, Calimlioglu N, Meseri R, Tavmergen Goker EN, Tavmergen E. Live birth after frozen-thawed embryo transfer: which endometrial preparation protocol is better? J Gynecol Obstet Hum Reprod. (2020) 49:101782. doi: 10.1016/j.jogoh.2020.101782
25. Cardenas Armas DF, Peñarrubia J, Goday A, Guimerá M, Vidal E, Manau D, et al. Frozen-thawed blastocyst transfer in natural cycle increase implantation rates compared artificial cycle. Gynecol Endocrinol. (2019) 35:873–7. doi: 10.1080/09513590.2019.1600668
26. Kalem Z, Namlı Kalem M, Bakırarar B, Kent E, Gurgan T. Natural cycle versus hormone replacement therapy cycle in frozen-thawed embryo transfer. Saudi Med J. (2018) 39:1102–8. doi: 10.15537/smj.2018.11.23299
27. Ghobara T, Gelbaya TA, Ayeleke RO. Cycle regimens for frozen-thawed embryo transfer. Cochrane Database Syst Rev. (2017) 7:CD003414. doi: 10.1002/14651858.CD003414.pub3
28. Glujovsky D, Pesce R, Sueldo C, Quinteiro Retamar AM, Hart RJ, Ciapponi A. Endometrial preparation for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes. Cochrane Database Syst Rev. (2020) 10:CD006359. doi: 10.1002/14651858.CD006359.pub3
29. Glujovsky D, Farquhar C, Quinteiro Retamar AM, Alvarez Sedo CR, Blake D. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev. (2016) 6:CD002118. doi: 10.1002/14651858.CD002118.pub5
30. Glujovsky D, Quinteiro Retamar AM, Alvarez Sedo CR, Ciapponi A, Cornelisse S, Blake D. Cleavage-stage versus blastocyst-stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev. (2022) 5:CD002118. doi: 10.1002/14651858.CD002118.pub6
31. De Croo I, Colman R, De Sutter P, Stoop D, Tilleman K. No difference in cumulative live birth rates between cleavage versus blastocyst transfer in patients with four or fewer zygotes: results from a retrospective study. Hum Reprod Open. (2022) 2022:hoac031. doi: 10.1093/hropen/hoac031
32. Escribá MJ, Bellver J, Bosch E, Sánchez M, Pellicer A, Remohí J. Delaying the initiation of progesterone supplementation until the day of fertilization does not compromise cycle outcome in patients receiving donated oocytes: a randomized study. Fertil Steril. (2006) 86:92–7. doi: 10.1016/j.fertnstert.2005.12.048
33. Roelens C, Santos-Ribeiro S, Becu L, Mackens S, Van Landuyt L, Racca A, et al. Frozen-warmed blastocyst transfer after 6 or 7 days of progesterone administration: impact on live birth rate in hormone replacement therapy cycles. Fertil Steril. (2020) 114:125–32. doi: 10.1016/j.fertnstert.2020.03.017
34. Yang X, Bu Z, Hu L. Live birth rate of frozen-thawed single blastocyst transfer after 6 or 7 days of progesterone administration in hormone replacement therapy cycles: A propensity score-matched cohort study. Front Endocrinol (Lausanne). (2021) 12:706427. doi: 10.3389/fendo.2021.706427
35. Liu L, Zhou H, Hu J, Sun X, Liu D, Huang G. Association between duration of progesterone supplementation and clinical outcomes in artificial frozen-thawed embryo transfer cycles. Front Endocrinol (Lausanne). (2023) 14:1193826. doi: 10.3389/fendo.2023.1193826
36. Tranguch S, Daikoku T, Guo Y, Wang H, Dey SK. Molecular complexity in establishing uterine receptivity and implantation. Cell Mol Life Sci. (2005) 62:1964–73. doi: 10.1007/s00018-005-5230-0
37. Song L, Bu Z, Sun Y. Endometrial thickness and early pregnancy complications after frozen-thawed embryo transfers. Front Endocrinol (Lausanne). (2023) 14:1066922. doi: 10.3389/fendo.2023.1066922
38. Song W, Zhang F, Wang Y, Shi H, Sun N, Jin H, et al. Effective protection: the embryonic development and clinical outcomes of emergency vitrification of 1246 oocytes and Day 0-Day 5 embryos in a natural disaster. Hum Reprod. (2023) 38:2412–21. doi: 10.1093/humrep/dead210
39. Bu Z, Wang K, Dai W, Sun Y. Endometrial thickness significantly affects clinical pregnancy and live birth rates in frozen-thawed embryo transfer cycles. Gynecol Endocrinol. (2016) 32:524–8. doi: 10.3109/09513590.2015.1136616
40. Zong L, Liu P, Zhou L, Wei D, Ding L, Qin Y. Increased risk of maternal and neonatal complications in hormone replacement therapy cycles in frozen embryo transfer. Reprod Biol Endocrinol. (2020) 18:36. doi: 10.1186/s12958-020-00601-3
41. Asserhøj LL, Spangmose AL, Aaris Henningsen AK, Clausen TD, Ziebe S, Jensen RB, et al. Adverse obstetric and perinatal outcomes in 1,136 singleton pregnancies conceived after programmed frozen embryo transfer (FET) compared with natural cycle FET. Fertil Steril. (2021) 115:947–56. doi: 10.1016/j.fertnstert.2020.10.039
42. Busnelli A, Schirripa I, Fedele F, Bulfoni A, Levi-Setti PE. Obstetric and perinatal outcomes following programmed compared to natural frozen-thawed embryo transfer cycles: a systematic review and meta-analysis. Hum Reprod. (2022) 37:1619–41. doi: 10.1093/humrep/deac073
43. Busnelli A, Di Simone N, Levi-Setti PE. Artificial cycle frozen embryo transfer and obstetric adverse outcomes: association or causation? Hum Reprod Update. (2023) 29:694–6. doi: 10.1093/humupd/dmad020
44. Olmos-Ortiz A, Flores-Espinosa P, Díaz L, Velázquez P, Ramírez-Isarraraz C, Zaga-Clavellina V. Immunoendocrine dysregulation during gestational diabetes mellitus: the central role of the placenta. Int J Mol Sci. (2021) 22:8087. doi: 10.3390/ijms22158087
45. Zhao Z, Chen Y, Deng H, Huang L, Lu D, Shen X, et al. The influence of embryo stage on obstetric complications and perinatal outcomes following programmed compared to natural frozen-thawed embryo transfer cycles: a systematic review and meta-analysis. Front Endocrinol (Lausanne). (2023) 14:1186068. doi: 10.3389/fendo.2023.1186068
46. Xu J, Zhou H, Zhou T, Guo Y, Liang S, Jia Y, et al. The impact of different endometrial preparation protocols on obstetric and neonatal complications in frozen-thawed embryo transfer: a retrospective cohort study of 3,458 singleton deliveries. Reprod Biol Endocrinol. (2022) 20:141. doi: 10.1186/s12958-022-01009-x
Keywords: hormone replacement therapy, natural cycle, pregnancy outcomes, propensity score matching analysis, predictive model
Citation: Sun L, Yin B, Yao Z, Zhang C, Li J, Li S, Cui Y, Wang F, Dai W, Bu Z and Zhang Y (2024) Comparison of clinical outcomes and perinatal outcomes between natural cycle and hormone replacement therapy of frozen-thawed embryo transfer in patients with regular menstruation: a propensity score-matched analysis. Front. Endocrinol. 15:1416841. doi: 10.3389/fendo.2024.1416841
Received: 13 April 2024; Accepted: 01 July 2024;
Published: 18 July 2024.
Edited by:
Wei Wang, Second Hospital of Hebei Medical University, ChinaReviewed by:
Qin Qin, Shanxi Provincial People’s Hospital, ChinaCopyright © 2024 Sun, Yin, Yao, Zhang, Li, Li, Cui, Wang, Dai, Bu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yile Zhang, luna020996@126.com
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.