- Department of Andrology, Ganzhou People’s Hospital, Ganzhou, Jiangxi, China
Background: Antidiabetic drugs are widely used in clinical practice as essential drugs for the treatment of diabetes. The effect of hypoglycemic drugs on erectile dysfunction has not been fully proven due to the presence of multiple confounding factors.
Methods: Two-sample Mendelian randomization (TSMR) was used to examine the causal effect of antidiabetic drugs (including metformin, insulin and gliclazide) on erectile dysfunction. We used five robust analytic methods, of which the inverse variance weighting (IVW) method was the primary method, and also assessed factors such as sensitivity, pleiotropy, and heterogeneity. Effect statistics for exposures and outcomes were downloaded from publicly available data sets, including open Genome-Wide Association Studies (GWAS) and the UK Biobank (UKB).
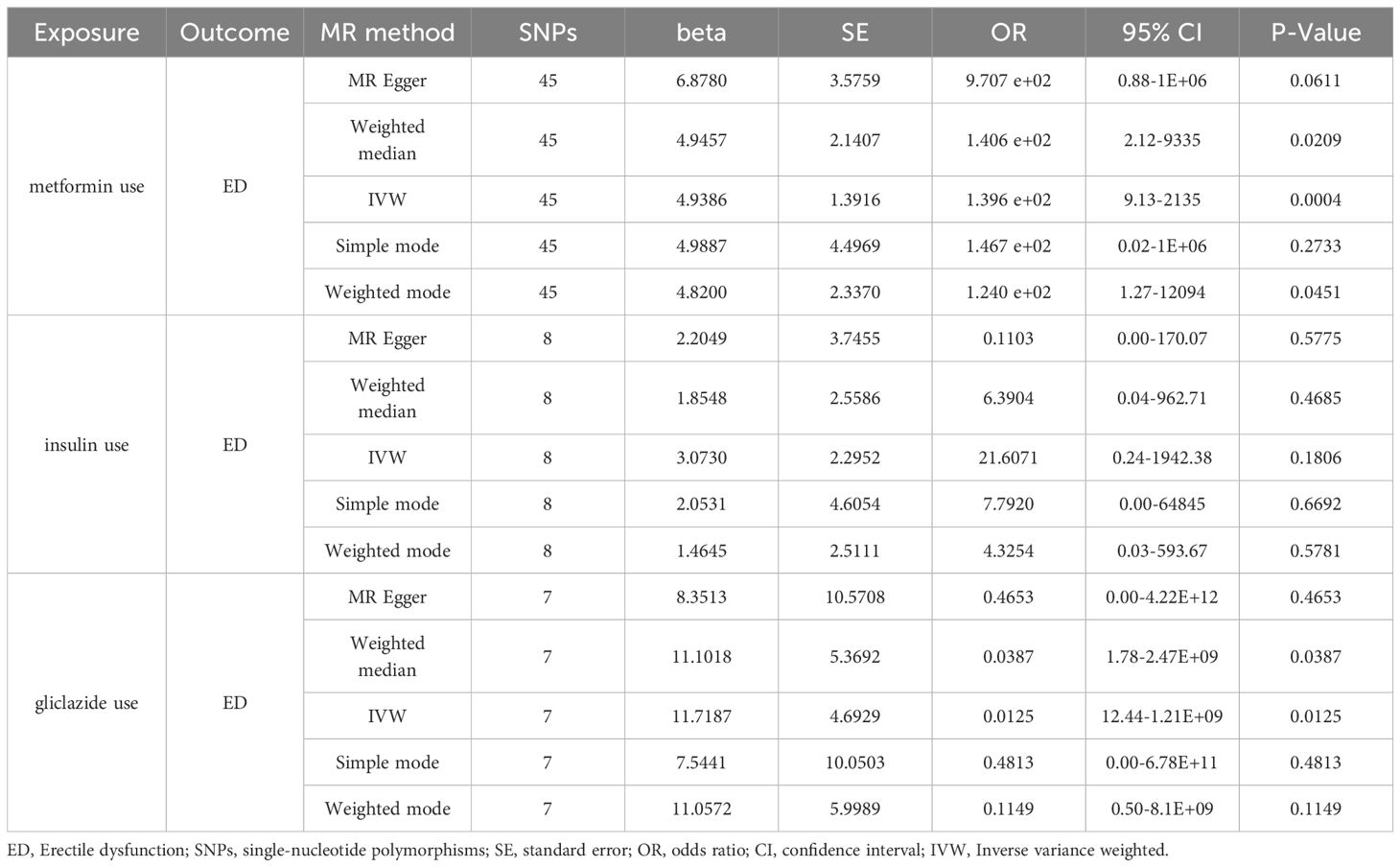
Results: In some of the hypoglycemic drug use, there was a significant causal relationship between metformin use and erectile dysfunction [Beta: 4.9386; OR:1.396E+02 (95% CI:9.13-2135); p-value: 0.0004), suggesting that metformin increased the risk of erectile dysfunction development. Also, we saw that gliclazide use also increased the risk of erectile dysfunction [Beta: 11.7187; OR:0.0125 (95% CI:12.44-1.21E+09); P value: 0.0125). There was no significant causal relationship between insulin use and erectile dysfunction [Beta: 3.0730; OR:21.6071 (95% CI:0.24-1942.38); p-value: 0.1806).Leave-one-out, MR-Egger, and MR-PRESSO analyses produced consistent results.
Conclusion: The use of metformin and gliclazide have the potential to increase the risk of erectile dysfunction. There is no causal relationship between the use of insulin and erectile dysfunction.
1 Introduction
Erectile dysfunction (ED) is one of the most common sexual dysfunctions in men, which is defined as the inability of a men to consistently obtain and maintain sufficient to accomplish a satisfactory sexual life. It is a chronic disease that seriously affects physical and mental health (1, 2). In recent years, it has a high incidence in middle-aged and elderly men, and its prevalence in young men is also on the rise (3–6). The risk factors related to ED include educational level (such as low education), socioeconomic status, sexual function status, health status (such as age, BMI, etc.), and psychiatric disorders, with diabetes mellitus being a risk factor for ED (7).
At present, the incidence of diabetes is increasing year by year, and tends to be younger (8, 9). There is a wide variety of drugs for the treatment of diabetes, among which insulin therapy is the most effective method, and other antidiabetic drugs including biguanides, sulfonylureas, α-glycosidase inhibitors, non-sulfonylureas insulin secretogens, thiazolidinediones and other hypoglycemic drugs are also widely used in clinical practice (10). These antidiabetic drugs can control and stabilize blood glucose through different mechanisms, improve microvascular lesions and neurological complications, which will have a beneficial effect on diabetic sexual function (11). However, whether antidiabetic drugs themselves will have a direct beneficial or adverse effect on diabetic sexual function has been paid more and more attention by doctors. Previous clinical studies are all based on observational data and may be affected by biases such as confounding factors and reverse causality (12). It is not possible to determine whether there is a causal relationship between the observed use of antidiabetic drugs and ED.
MR Has been widely used as an etiological inference method in the field of genetic epidemiology (13–15). It uses comprehensive statistics from GWAS to infer causal relationships between certain diseases and exposure factors, thereby identifying potential risk factors. The effect of confounding factors was eliminated by using single nucleotide polymorphism SNPs as instrumental variables. In this study, we conducted a two-sample MR Analysis using pooled data from large-scale GWAS to explore the association between the use of metformin, insulin, gliclazide and ED, to overcome the limitations in basic experimental and clinical research, so as to facilitate the rational use of drugs in clinical practice.
2 Materials and methods
2.1 Study design
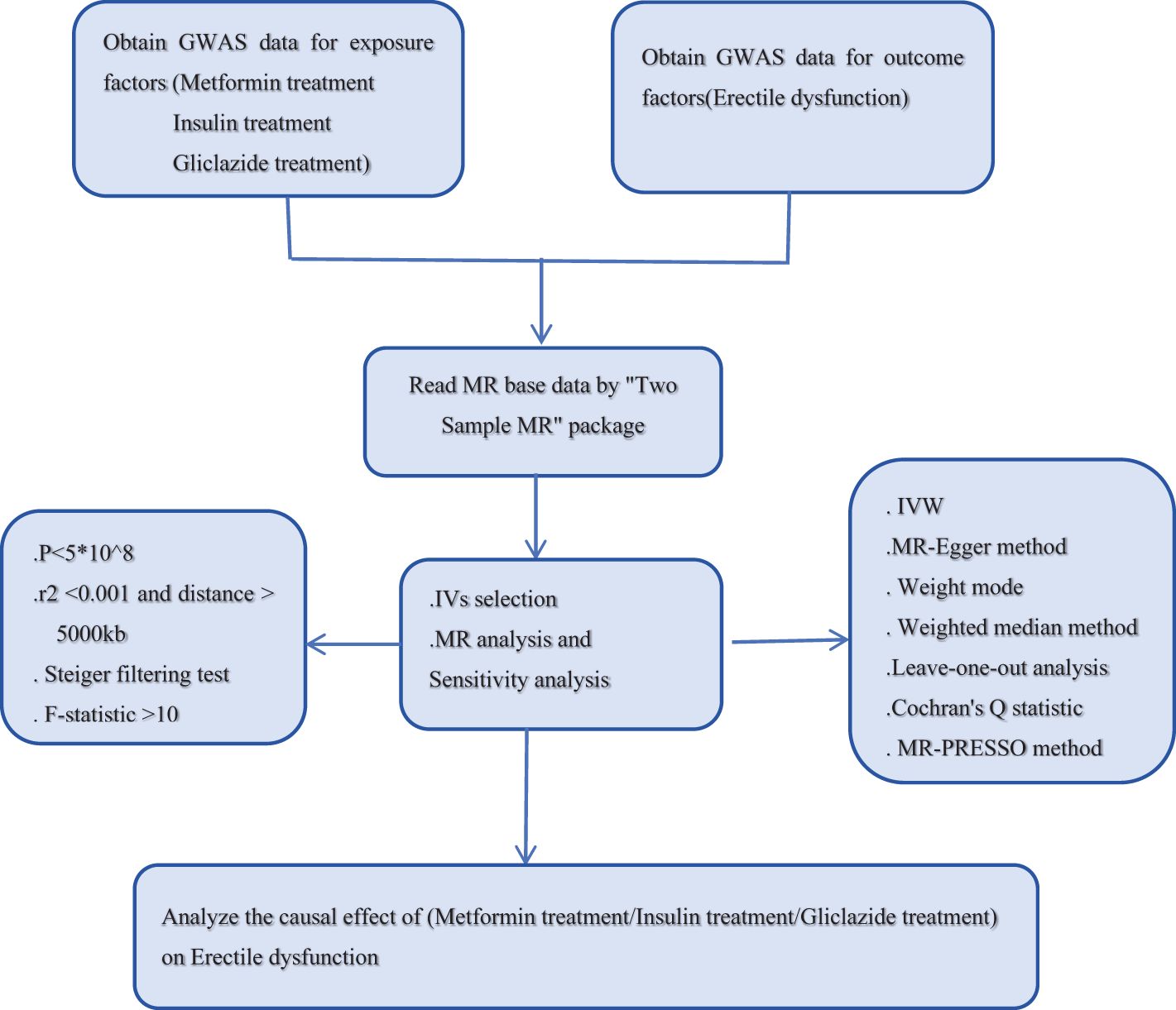
To explore the potential causal relationship between antidiabetic drugs and ED, we used SNPs from the GWAS database as instrumental variables (IVs). The data set “antidiabetic drugs (metformin, insulin, gliclazide)” as exposure factor was from the UK-Biobank. The data set of ED as an outcome variable was obtained from the ebi. Two-sample MR Analysis was used in this study. The MR Study design process is shown in Figure 1. MR Analysis relies on three basic assumptions (16). (1) hypothesis of association, IVs is closely related to exposure factors; (2) the assumption of independence: IVs is independent of the confounding factors affecting “exposure and outcome”; (3) The exclusivity hypothesis that IVs can only affect the outcome variables through exposure factors. It is important to note that because these databases are publicly available, no additional ethical approval or informed consent from participants is required.
2.2 Data sources
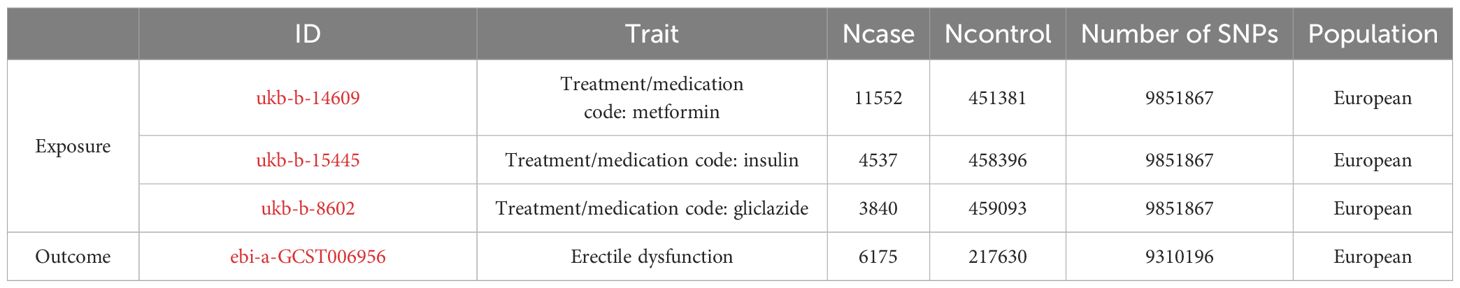
From the IEU Open GWAS Project database (https://gwas.mrcieu.ac.uk/) to retrieve the metformin, insulin, gliclazide and ED data, we tend to choose include SNPs and larger sample sizes, GWAS data. In addition, to reduce potential errors arising from stratification effects related to factors such as ancestry and population, we deliberately selected individuals of European ancestry as our cohort sample. Exposure factors were obtained from the genetic information associated with metformin in the UK Biobank. SNPS associated with metformin were selected from GASS-ID: ukb-b-14609, which included 11,552 metformin-treated patients and 451,381 healthy controls; SNPS associated with insulin were selected in GWAS-id: ukb-b-15445, which included 4537 insulin-treated patients and 458396 healthy controls; GWAS-id: ukb-b-8602 was used to identify SNPs associated with gliclazide in 3840 insulin-treated patients and 459093 healthy controls, resulting in 9,851,867 SNPS. The outcome variable ED was derived from the ebi, GWAS-id: EBI-A-GCST006956, with 9310,196 SNPs in 6175 positive cases and 217,630 controls. Details are provided in Table 1 below.
2.3 SNP selection
In this study, SNPs should meet the genome-wide significance threshold (P < 5e-08), which indicates a genetic association with exposure. Linkage disequilibrium r2 > 0.001 was excluded, and the clustered genetic distance was set at 10000kb. SNPs related to antidiabetic drug treatment were screened, and the independence of SNPS was guaranteed (17). To exclude the influence of weak bias, the formula F = beta2/se2 was used, and the statistical power F was used. When the F-statistic is > 10, the SNPs screened by this tool are considered as strong instrumental variables (18). Subsequently, the exposure and outcome data sets were reconciled to exclude SNPS with palindromic properties and intermediate allele frequencies to ensure consistency of effect alleles (19). See Supplementary Material, Supplementary Table for specific information.
2.4 Statistical analysis
In this study, the main statistical analysis was performed using TwoSample MR Package (version 0.5.11) and R software (version R-4.3.3) for a two-sample MR Study with a total of five different methods. Among them, inverse variance weighting (IVW) method was used as the main analysis method (20) to analyze the causal relationship between metformin, insulin, gliclazide and ED. Secondary methods MR-Egger regression, weighted median (21), simple model (22) and weighted model were used for auxiliary analysis. To elucidate the genetic basis linking exposure and outcome phenomena. Visual representations of the results are provided through scatter plots and funnel plots. To assess horizontal pleiotropy, MR-Egger regression (23) and the MR-PRESSO method (24) were used, where MR-PRESSO not only detects outliers but also provides recalibrating estimates after excluding outliers. Cochran’s Q statistic was used to assess heterogeneity and to exclude any MR Results with heterogeneity (25). Leave-one-out analysis was used to test the sensitivity of the findings and evaluate the robustness of the results by exploring the impact of individual SNPS and revealing the residual collective influence of genetic instruments. Pleiotropy was tested using MR-Egger intercept method.
3 Results
3.1 Results of metformin use
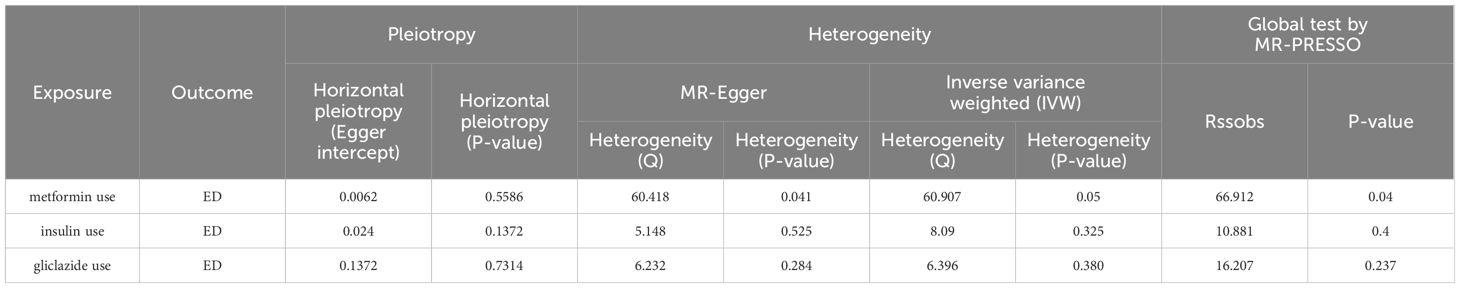
In the selection of genetic tool variants (IVs), we strictly followed the above criteria for selection. Therefore, from 9,851,867 SNPs in the UKB results, 46 SNPS were significantly associated with metformin treatment. After excluding the palindromic SNPS with effect allele frequencies between 0.3 and 0.7, and excluding rs11658063, we found 45 SNPS that supported the causal relationship between metformin treatment and ED. Additional details are provided in Supplementary Table 1 in the Supplementary Material. The F-statistic (also see Appendix Material) indicates no evidence of instrumental variation. The IVW approach revealed a statistically significant causal effect of metformin use on the risk of ED (Beta: 4.9386; OR:1.396E+02(95%CI:9.13-2135); P value: 0.0004). Weighted model (Beta: 4.82; OR: e+02 1.240 (95% CI: 1.27-12094); P-value: 0.0451) and the weighted median method observed similar trends (Beta: 4.9457; OR: e+02 1.406 (95% CI: 2.12-9335); P value: 0.0209). These results are visually presented in the forest plot (Figure 2A) and scatter plot (Figure 2B). The forest plot shows the effect estimate for each SNP and its confidence interval, whereas the scatter plot depicts the association between exposure (metformin use) and outcome (ED) using instrumental variables. To ensure reliability of the results, we performed a series of assessments. The pleiotropy of IVs was investigated by applying MR-Egger regression and MR-PRESSO global test. The results of MR-Egger regression inference showed that there was no pleiotropy in IVs (P = 0.5586, Table 2). Cochran’s Q test showed that there was heterogeneity (Q=60.907; P=0.0463) (Table 2), indicating that there was variation in the estimated causal effect among different SNPs. However, this heterogeneity did not impair our ability to draw meaningful conclusions, because neither the MR-Egger intercept test (MR-Egger intercept=–0.0062; P=0.5586) or MR-PRESSO, confirming the reliability of the results. Sensitivity analysis using the leave-one-out method (Figure 2C) confirmed the reliability of our findings. See Table 3 and Supplementary Table 1 for details of the results of the analyses related to metformin and ED.
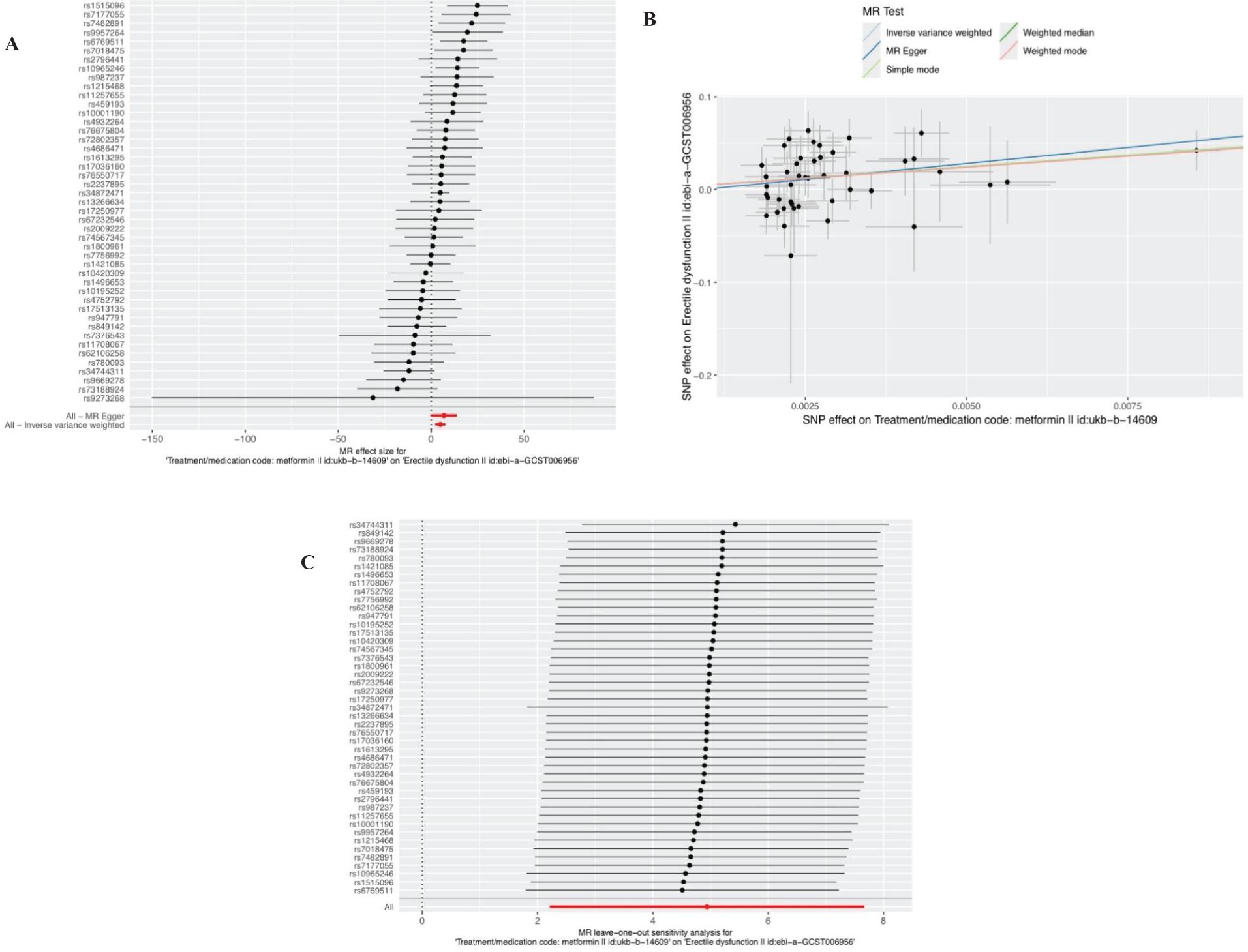
Figure 2. Plots of causal estimates of genetically predicted metformin use on ED. (A) The forest plot. (B) The scatter plot. (C) The leave-one-out plot.
3.2 Insulin treatment outcomes
After removal of linkage disequilibrium screening, 9 SNPs were obtained. After screening of palindromic SNPS of effect alleles, rs689 was excluded, and 8 SNPS were finally determined as instrumental variables with F values >10. Horizontal pleiotropy test showed that there was no horizontal pleiotropy in the instrumental variables (MR-Egger intercept=-0.024; se=-0.014,P=0.1372). The results of MR Analysis showed that the IVW method suggested that there was no causal relationship between insulin use and the increased risk of ED (Beta: 3.073; OR:21.6071 (95%CI:0.24-1942.38); P value: 0.1806) (Figures 3A, B). In the heterogeneity test (Table 2), MR-Egger regression showed Cochran’s Q = 5.148, P=0.525, indicating that there was no heterogeneity among the instrumental variables. IVW fixed effects model was used to estimate causal effects (P=0.15) because MR-Egger regression method suggested no heterogeneity among instrumental variables. MR-PRESSO test showed that there was no horizontal pleiotropy in the instrumental variables (Global test Rssobs = 10.881, P=0.4), and no abnormal values were found. Sensitivity analysis showed that the results of MR Analysis were reliable (Figure 3C). Overall, we conclude that the use of insulin does not appear to have a significant effect on ED. See Table 3 and Supplementary Table 2 for details of the results of the analyses related to insulin and ED.
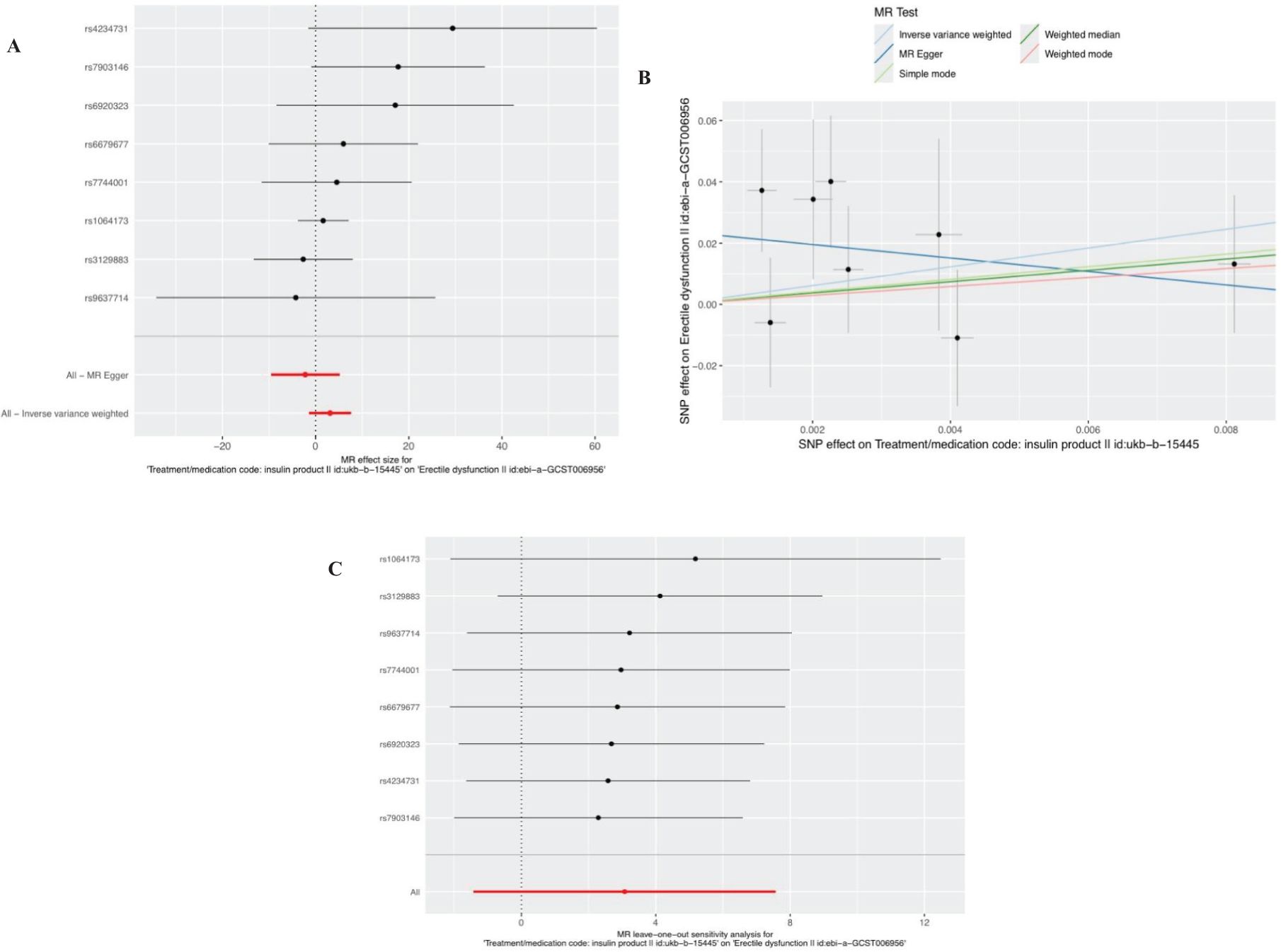
Figure 3. Plots of causal estimates of genetically predicted insulin use on ED. (A) The forest plot. (B) The scatter plot. (C) The leave-one-out plot.
3.3 Results of gliclazide use
Ten SNPS significantly associated with gliclazide treatment were screened. After the palindrome SNPS with effect allele frequencies between 0.3 and 0.7 were screened, rs2411884, rs59442809 and rs6780171 were excluded, and 7 SNPS were finally selected as instrumental variables, with F values >10. The intercept of MR-Egger regression was close to 0 (MR-Egger intercept=-0.1372,P=0.7314), indicating that there was no horizontal pleiotropy of instrumental variables, which had little effect on the results of MR Analysis. MR Analysis using IVW as the primary analysis method showed a causal relationship between gliclazide use and an increased risk of ED (Beta: 11.7187; OR:0.0125(95%CI: 12.44-1.21e +09); P value: 0.0125). MR-Egger regression and IVW method were used to detect the heterogeneity among instrumental variables. MR-Egger regression results showed that Cochran’s Q = 6.232, P=0.284; IVW results showed that Cochran’s Q = 6.396,P=0.380; This indicated that there was no heterogeneity among the instrumental variables. MR-PRESSO test showed that there was no horizontal pleiotropy in the instrumental variables (global test RSSobs =16.207, P=0.237), and no outlying values were found. (See Table 2 for details). Sensitivity analysis was performed using the leave-one-out method, in which SNPS were removed one by one, and the causal effects of the remaining SNPS were compared with the MR Analysis results of all SNPS to determine whether the causal association was caused by a single instrumental variable. Sensitivity analyses showed that the results of the MR Analysis were robust (Figure 4). See Table 3 and Supplementary Table 3 for details of the analysis results related to gliclazide and ED.
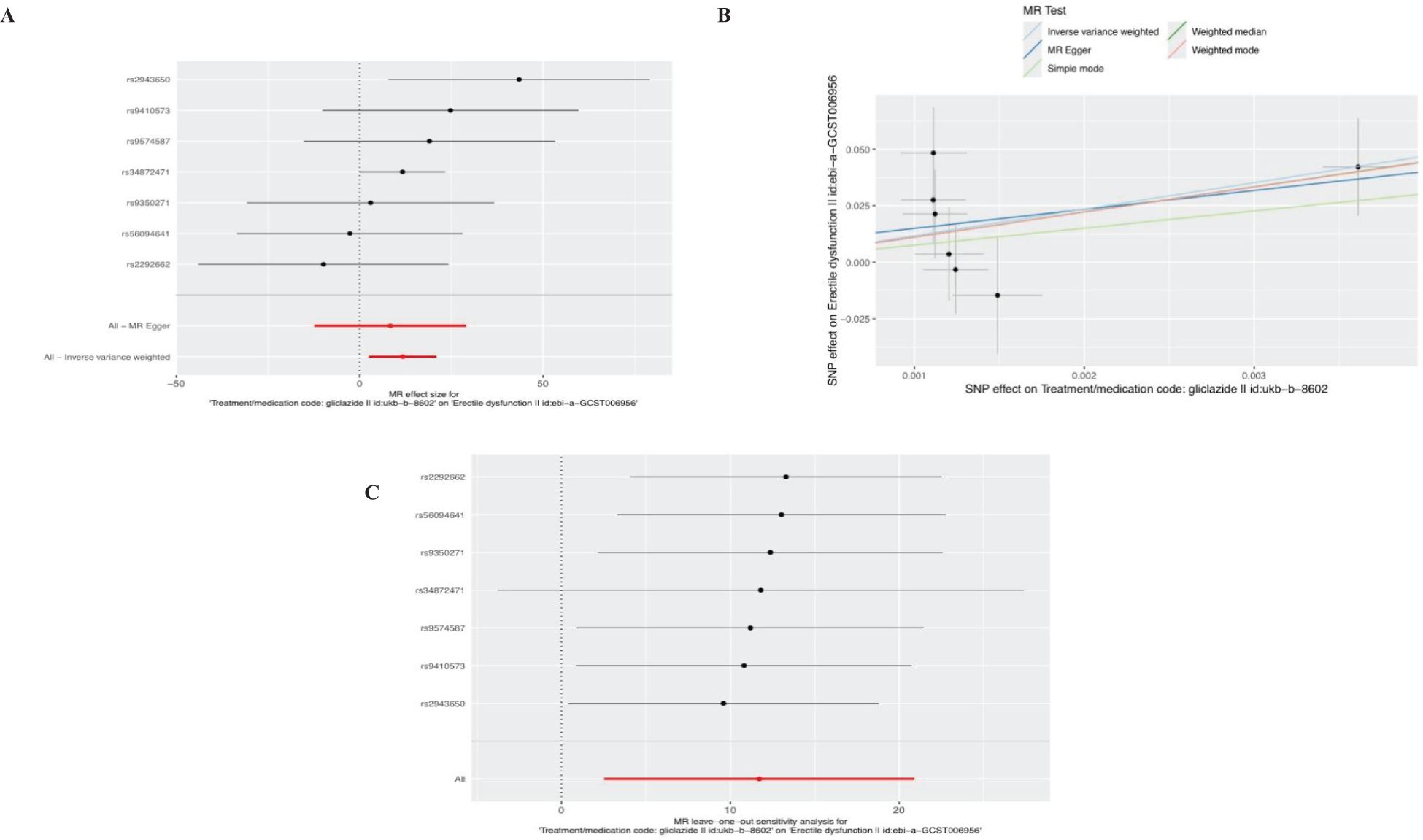
Figure 4. Plots of causal estimates of genetically predicted gliclazide use on ED. (A) The forest plot. (B) The scatter plot. (C) The leave-one-out plot.
4 Discussion
Diabetes mellitus is a risk factor for ED, and its pathophysiological pathogenesis is complex. Hyperglycemia can lead to vascular endothelial dysfunction and atherosclerosis through the activation of advanced glycation end metabolites (AGEs pathway, protein kinase C pathway and hexosamine pathway leading to neural, vascular, endocrine and other changes) (26). In addition, hyperglycemia and insulin resistance can affect the function of the hypothalamic-pituitary-gonadal axis, leading to hypogonadism and decreased libido. Whether the use of antidiabetic drugs will affect erectile function has attracted more and more attention. Our study is the first to examine the causal association between multiple antidiabetic drugs and ED outcomes using two-sample Mendelian randomization. This study revealed that although insulin use can treat diabetes, it does not relieve ED in patients, and it may increase the risk of ED, but there is no causal association in itself. However, the use of metformin and gliclazide in the treatment of diabetes increases the risk of ED.
Since metformin has been recommended as the first-line treatment for patients with type 2 diabetes mellitus (T2DM), it has become the most prescribed oral antidiabetic drug (27). Several studies have been conducted to determine whether metformin can improve erectile function while controlling blood glucose in patients with diabetes mellitus complicated with ED. In animal experiments, Labazi et al. (28) used an experimental rat ED model to study the effect of metformin on ED. After adding metformin, it can up-regulate the expression of nitric oxide synthase (eNOS) in the corpus cavernosum, increase NO production, improve vascular endothelial relaxation function, and thus improve the ED of rats. Another basic study (29) also confirmed that metformin could not only increase NO-mediated vasodilation, but also reduce the over-activated sympathetic nerve activity in hypertensive rats. In the clinical trial, Rey-Valzacchi et al (30) conducted a small sample, randomized, double-blind, placebo-controlled prospective cohort study that evaluated nondiabetic ED patients with insulin resistance who did not respond well to sildenafil. After 4 months of combined treatment with metformin, erectile function was improved, while BMI was reduced. However, the sample size of this study was too small, and the conclusions have certain limitations. However, in two studies by Al-Kuraishy et al. (31) and Abdul-Hadi et al. (32), opposite conclusions were reached. In patients with diabetes who used metformin, low testosterone levels, decreased libido, and an increased incidence of ED caused by low testosterone were observed. This is in good agreement with the results of our study. It should be noted, however, that the small sample study by Rey-Valzacchi et al. (30) recruited non-diabetic patients with insulin resistance, whereas in the latter two studies Al-Kuraishy et al. (31) and Abdul-Hadi et al. (32) recruited patients with pre-existing diabetes. Therefore, the different effects of metformin on ED in patients with or without diabetes need to be further verified (33).
Gliclazide is a sulfonylurea oral hypoglycemic drug, which acts on adenosine triphosphate (ATP) -sensitive potassium (K) channel and stimulates insulin release from pancreatic cells to reduce glucose (34, 35). At present, there are few reports on the use of gliclazide in ED, and the effects of sulfonylureas on sexual function are mainly based on animal experiments. Benelli (36) treated mouse hypothalamic preoptic tissue blocks with tolbutamide and found that it could significantly increase GnRH secretion; Intraperitoneal injection of glyburide in male rats can increase their sexual behavior, while intraventricular injection of glyburide can open ATP-sensitive potassium (K-ATP) channels and inhibit their sexual behavior (37). also found in animal studies that sulfonylureas may play a role in relaxation of the corpus cavernosus due to their effects on K-ATP channels and penile resistance arteries. Another scholar Insuk (38) confirmed that sulfonylureas such as gliclazide or glibenclamide can bind and close potassium channels, thereby inhibiting smooth muscle relaxation, and this mechanism of action seems to aggravate ED. However, when glibenclamide and sildenafil were administered in combination, glibenclamide did not affect the cgMsmediated smooth muscle relaxation of sildenafil. In the previously mentioned study described by Al-Kuraishy (31), patients treated with glimepiride had elevated testosterone levels and libido, as well as ED improvement. However, our results were contrary to those obtained in previous studies, showing that the use of gliclazide increased the risk of ED while improving diabetes in patients. This may be because there are still some differences in the mechanism of action between gliclazide and glimepiride.
Insulin has been in use for more than 100 years now. It has been confirmed in animal experiments that both vascular endothelial cells and vascular smooth muscle cells express insulin receptors, which promote the release of nitric oxide (NO) through PI3K-Akt-eNOS pathway and vasodilation of blood vessels (39, 40). However, another in vivo experiment in rats (41) showed that insulin therapy alone could not alleviate ED in type I diabetic rats. In addition, in clinical studies, insulin therapy can lead to an increase in body fat percentage, and iatrogenic hyperinsulinemia can also induce hypertension, abnormal lipid metabolism and atherosclerosis, which are risk factors for ED (42, 43). Therefore, whether insulin has an effect on ED independent of lowering blood glucose has been controversial. This study reveals that there is no causal relationship between insulin use and ED by MR.
Mendelian randomization studies use genetic variants closely related to the underlying risk factor as instrumental variables to determine whether the risk factor is the cause of the disease. Compared with traditional observational studies, MR Studies are less susceptible to reverse causality and potential confounding factors. The limitations of this study are as follows: (1) The results of MR Analysis are based on European population, and the causal relationship obtained may be biased by race, which limits the extrapolation of the results; (2) The SNPS used in the analysis may be associated with other traits due to genetic polymorphisms, which may cause confounding bias, which may affect causal inference; (3) This study was limited to a single exposure and outcome data, and the results need to be further verified by other GWAS pooled data.
5 Conclusion
In conclusion, this two-sample Mendelian randomization analysis revealed that the use of metformin and gliclazide could potentially increase the risk of ED, and there was no causal relationship between the use of insulin and ED. However, more work is needed to determine the rationale for the use of these antidiabetic agents in clinical practice.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval were not required for this study in human subjects in accordance with the local laws and institutional requirements.
Author contributions
LF: Conceptualization, Writing – original draft, Methodology. WJ: Writing – review & editing, Data curation, Project administration. GS: Resources, Writing – review & editing, Conceptualization. XJ: Formal analysis, Investigation, Writing – review & editing, Visualization. LZ: Formal analysis, Writing – review & editing, Investigation, Visualization. LX: Writing – review & editing, Software, Visualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Ganzhou City guiding science and technology project (GZ2022ZSF084).
Acknowledgments
We express our gratitude to the IEU database, UKB and ebi for providing publicly available summarized data from GWAS studies for our study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1414958/full#supplementary-material
References
1. Lewis RW, Fugl-Meyer KS, Corona G, Hayes RD, Laumann EO, Moreira ED Jr., et al. Definitions/epidemiology/risk factors for sexual dysfunction. J sexual Med. (2010) 7:1598–607. doi: 10.1111/j.1743-6109.2010.01778.x
2. Burnett AL, Nehra A, Breau RH, Culkin DJ, Faraday MM, Hakim LS, et al. Erectile dysfunction: AUA guideline. J Urol. (2018) 200:633–41. doi: 10.1016/j.juro.2018.05.004
3. Krzastek SC, Bopp J, Smith RP, Kovac JR. Recent advances in the understanding and management of erectile dysfunction. F1000Res. (2019) 8:F1000. doi: 10.12688/f1000research
4. Yafi FA, Jenkins L, Albersen M, Corona G, Isidori AM, Goldfarb S, et al. Erectile dysfunction. Nat Rev Dis Primers. (2016) 2:16003. doi: 10.1038/nrdp.2016.3
5. Chew KK, Stuckey B, Bremner A, Earle C, Jamrozik K. Male erectile dysfunction: its prevalence in Western Australia and associated sociodemographic factors. J sexual Med. (2008) 5:60–9. doi: 10.1111/j.1743-6109.2007.00548.x
6. Caskurlu T, Tasci AI, Resim S, Sahinkanat T, Ergenekon E. The etiology of erectile dysfunction and contributing factors in different age groups in Turkey. Int J urology: Off J Japanese Urological Assoc. (2004) 11:525–9. doi: 10.1111/j.1442-2042.2004.00837.x
7. Kouidrat Y, Pizzol D, Cosco T, Thompson T, Carnaghi M, Bertoldo A, et al. High prevalence of erectile dysfunction in diabetes: a systematic review and meta-analysis of 145 studies. Diabet Med. (2017) 34(9):1185–92. doi: 10.1111/dme.13403
8. Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. (2014) 103:137–49. doi: 10.1016/j.diabres.2013.11.002
9. Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. (2018) 138:271–81. doi: 10.1016/j.diabres.2018.02.023
10. American Diabetes Association Professional Practice Committee. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2022. Diabetes Care. (2022) 45:S125–s143. doi: 10.2337/dc22-S009
11. Pappachan JM, Fernandez CJ, Chacko EC. Diabesity and antidiabetic drugs. Mol aspects Med. (2019) 66:3–12. doi: 10.1016/j.mam.2018.10.004
12. Batty GD, Li Q, Czernichow S, Neal B, Zoungas S, Huxley R, et al. Erectile dysfunction and later cardiovascular disease in men with type 2 diabetes: prospective cohort study based on the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified-Release Controlled Evaluation) trial. J Am Coll Cardiol. (2010) 56:1908–13. doi: 10.1016/j.jacc.2010.04.067
13. Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. Jama. (2017) 318:1925–6. doi: 10.1001/jama.2017.17219
14. Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. (2014) 23:R89–98. doi: 10.1093/hmg/ddu328
15. Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. Bmj. (2018) 362:k601. doi: 10.1136/bmj.k601
16. Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. (2008) 27:1133–63. doi: 10.1002/sim.3034
17. Machiela MJ, Chanock SJ. LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinf (Oxford England). (2015) 31:3555–7. doi: 10.1093/bioinformatics/btv402
18. Pierce BL, Ahsan H, Vanderweele TJ. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int J Epidemiol. (2011) 40:740–52. doi: 10.1093/ije/dyq151
19. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. (2018) 7:e34408. doi: 10.7554/eLife.34408
20. Burgess S, Scott RA, Timpson NJ, Davey Smith G, Thompson SG. Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol. (2015) 30:543–52. doi: 10.1007/s10654-015-0011-z
21. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
22. Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. (2017) 46:1985–98. doi: 10.1093/ije/dyx102
23. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
24. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50(5):693–8. doi: 10.1038/s41588-018-0099-7
25. Bowden J, Del Greco MF, Minelli C, Zhao Q, Lawlor DA, Sheehan NA, et al. Improving the accuracy of two-sample summary-data Mendelian randomization: moving beyond the NOME assumption. Int J Epidemiol. (2019) 48:728–42. doi: 10.1093/ije/dyy258
26. Russo V, Chen R, Armamento-Villareal R. Hypogonadism, type-2 diabetes mellitus, and bone health: A narrative review. Front Endocrinol. (2020) 11:607240. doi: 10.3389/fendo.2020.607240
27. Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. (2012) 35:1364–79. doi: 10.2337/dc12-0413
28. Labazi H, Wynne BM, Tostes R, Webb RC. Metformin treatment improves erectile function in an angiotensin II model of erectile dysfunction. J sexual Med. (2013) 10:2154–64. doi: 10.1111/jsm.12245
29. Patel JP, Lee EH, Mena CI, Walker CN. Effects of metformin on endothelial health and erectile dysfunction. Trans andrology Urol. (2017) 6:556–65. doi: 10.21037/tau
30. Rey-Valzacchi GJ, Costanzo PR, Finger LA, Layus AO, Gueglio GM, Litwak LE, et al. Addition of metformin to sildenafil treatment for erectile dysfunction in eugonadal nondiabetic men with insulin resistance. A prospective, randomized, double-blind pilot study. J Androl. (2012) 33(4):608–14. doi: 10.2164/jandrol.111.013714
31. Al-Kuraishy HM, Al-Gareeb AI. Erectile dysfunction and low sex drive in men with type 2 DM: the potential role of diabetic pharmacotherapy. J Clin Diagn research: JCDR. (2016) 10:FC21–6. doi: 10.7860/JCDR/2016/19971.8996
32. Abdul-Hadi MH, Naji MT, Shams HA, Sami OM, Al-Kuraishy HM, Al-Gareeb AI. Erectile dysfunction and type 2 diabetes mellitus: A new twist. Int J Nutrition Pharmacology Neurological Dis. (2020) 10:43–9. doi: 10.4103/ijnpnd.ijnpnd_83_19
33. Tseng CH. Metformin’s effects on varicocele, erectile dysfunction, infertility and prostate-related diseases: A retrospective cohort study. Front Pharmacol. (2022) 13:799290. doi: 10.3389/fphar.2022.799290
34. Wang L, Xu Y, Li H, Lei H, Guan R, Gao Z, et al. Antioxidant icariside II combined with insulin restores erectile function in streptozotocin-induced type 1 diabetic rats. J Cell Mol Med. (2015) 19:960–9. doi: 10.1111/jcmm.12480
35. Lv W, Wang X, Xu Q, Lu W. Mechanisms and characteristics of sulfonylureas and glinides. Curr topics medicinal Chem. (2020) 20:37–56. doi: 10.2174/1568026620666191224141617
36. Benelli A, Arletti R, Poggioli R, Cavazzuti E, Mameli M, Bertolini A. Effect of potassium channel modulators on male sexual behavior. Life Sci. (1997) 60:263–7. doi: 10.1016/S0024-3205(96)00626-1
37. Ruiz Rubio JL, Hernández M, Rivera de los Arcos L, Benedito S, Recio P, García P, et al. Role of ATP-sensitive K+ channels in relaxation of penile resistance arteries. Urology. (2004) 63:800–5. doi: 10.1016/j.urology.2003.10.071
38. Insuk SO, Chae MR, Choi JW, Yang DK, Sim JH, Lee SW. Molecular basis and characteristics of KATP channel in human corporal smooth muscle cells. Int J impotence Res. (2003) 15:258–66. doi: 10.1038/sj.ijir.3901013
39. Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA. (2003) 290(16):2159–67. doi: 10.1001/jama.290.16.2159
40. Cho SY, Chai JS, Lee SH, Park K, Paick JS, Kim SW. Investigation of the effects of the level of glycemic control on erectile function and pathophysiological mechanisms in diabetic rats. J sexual Med. (2012) 9:1550–8. doi: 10.1111/j.1743-6109.2012.02720.x
41. Cignarelli A, Genchi VA, D’Oria R, Giordano F, Caruso I, Perrini S, et al. Role of glucose-lowering medications in erectile dysfunction. J Clin Med (2021) 10(11):2501. doi: 10.3390/jcm10112501
42. Zhou F, Li GY, Gao ZZ, Liu J, Liu T, Li WR, et al. The TGF-β1/Smad/CTGF pathway and corpus cavernosum fibrous-muscular alterations in rats with streptozotocin-induced diabetes. J andrology. (2012) 33:651–9. doi: 10.2164/jandrol.111.014456
Keywords: antidiabetic drugs, metformin, insulin, gliclazide, erectile dysfunction, Mendelian randomization
Citation: Feng L, Jinhua W, Shulin G, Jiangping X, Zhongxiang L and Xiaohong L (2024) Causal association between antidiabetic drugs and erectile dysfunction: evidence from Mendelian randomization. Front. Endocrinol. 15:1414958. doi: 10.3389/fendo.2024.1414958
Received: 09 April 2024; Accepted: 06 August 2024;
Published: 23 August 2024.
Edited by:
Adriana Coppola, Clinical Institute Beato Matteo, ItalyReviewed by:
Giuseppe Murdaca, University of Genoa, ItalyFusong Jiang, Shanghai Jiao Tong University, China
Copyright © 2024 Feng, Jinhua, Shulin, Jiangping, Zhongxiang and Xiaohong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wu Jinhua, MTg3NzA3ODkyOTlAMTYzLmNvbQ==
 Lin Feng
Lin Feng Wu Jinhua*
Wu Jinhua*