- 1Division of Cardiology, The First Affiliated Hospital of Soochow University, Suzhou, China
- 2Department of Ultrasound, Shanghai Chest Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
Background: The association between Estradiol (E2) levels and abdominal aortic calcification (AAC) in postmenopausal women remains unclear.
Methods: 614 postmenopausal women from the 2013-2014 NHANES survey cycle were included in this study. The study population was divided into 3 groups according to E2 tertiles: Tertile1 (2.12-3.57pg/mL), Tertile2 (3.60-7.04pg/mL), and Tertile3 (7.06-38.4pg/mL). Estrogen concentration data were natural logarithmically transformed. A Kauppila score > 5 was regarded as prominent arterial calcification and was used to define (EAAC). Logistic regression models were used to assess the association between E2 levels and EAAC prevalence. Subgroup analyses were performed to test whether the association between E2 levels and EAAC prevalence was consistent in different groups. Sensitivity analyses tested the stability of the model in women older than 45 years.
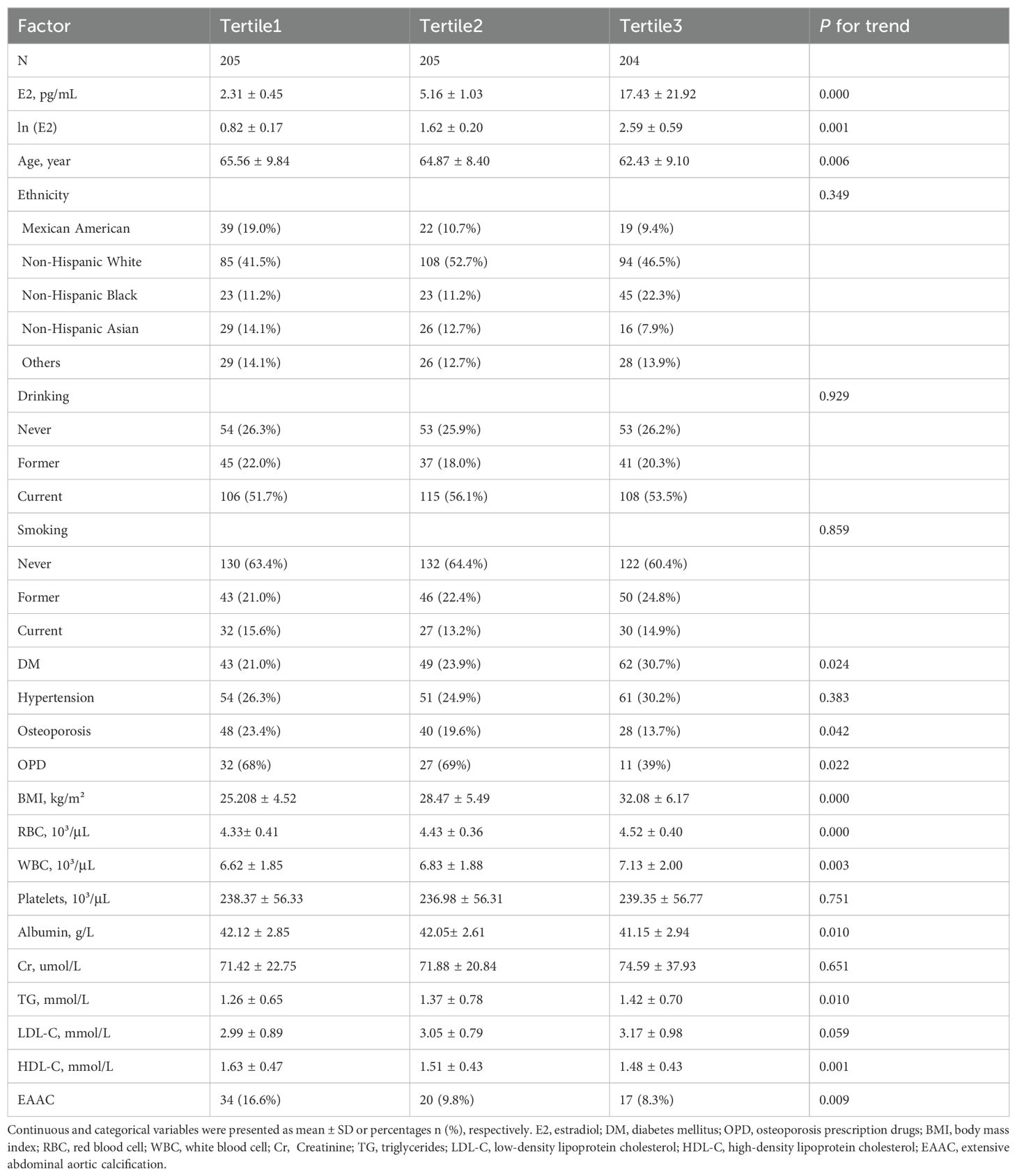
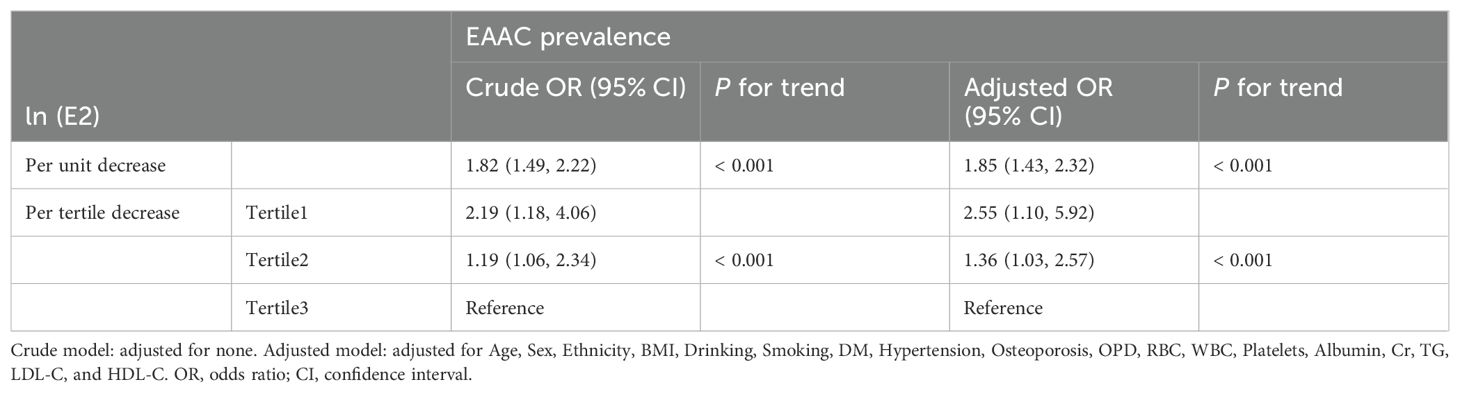
Results: EAAC prevalence was significantly higher in Tertile1 (16.6%) than in Tertile2 (9.8%) and Tertile3 (8.3%). On a continuous scale, the adjusted model showed a 58% [OR (95%CI), 1.58 (1.02, 2.54)] increase in the risk of EAAC prevalence for per unit decrease in ln(E2). On a categorical scale, the adjusted model showed that Tertile1 and Tertile2 were 2.55 [OR (95%CI), 2.55 (1.10, 5.92)] and 1.31[OR (95%CI), 1.31(1.03, 2.57)] times higher risk of suffering from EAAC than Tertile3, respectively.
Conclusion: This study found that a higher prevalence of AAC in postmenopausal women is closely associated with lower serum E2 levels. Our research further underscores the importance of E2 in maintaining cardiovascular health in postmenopausal women and suggests that monitoring E2 levels may aid in the early prevention and management of AAC and related cardiovascular diseases.
Introduction
Postmenopausal women often experience a range of menopausal symptoms, primarily including hot flashes, night sweats, sleep disturbances, and genitourinary discomfort (1). Other common symptoms and conditions include bone loss, obesity, low libido, mood swings, and cognitive changes (2). These effects are largely attributed to the decline in estrogen levels (3). Estradiol (E2) is a female hormone and the most biologically active form of estrogen. It is primarily secreted by the ovaries, but also by the adrenal glands, fat tissue, and liver, and it circulates in the bloodstream (4). Numerous studies have highlighted the benefits of E2 for the human body, including the prevention of bone loss, protection against ischemia-reperfusion injury, relief of menopausal symptoms, prevention of neuronal degeneration, maintenance of vaginal health, and assistance in treating ovarian failure or hypogonadism. Over the years, a significant amount of anecdotal evidence has accumulated, supporting the view that postmenopausal E2 may reduce the risk of cardiovascular disease (5).
Abdominal aortic calcification (AAC) is the process in which calcium and phosphate-based minerals deposit in the walls of the abdominal aorta. Driven by various risk factors such as aging, prolonged dialysis, osteoporosis, diabetes, and hypertension, the aortic walls undergo irreversible calcification and become brittle (6). This condition is a significant warning sign for cardiovascular disease. AAC is an important predictor of cardiovascular disease risk, including conditions such as carotid atherosclerosis, myocardial infarction, congestive heart failure, stroke, and intermittent claudication (7–10). It is also a significant indicator of both all-cause mortality and cardiovascular mortality (11). AAC can be easily assessed and semi-quantified using lateral X-rays of the lumbar spine and lateral scans from DXA (Dual-energy X-ray Absorptiometry) (12). Consequently, in recent years, AAC’s role in predicting cardiovascular risk has garnered increasing attention.
The specific mechanisms linking the decline in estradiol levels to increased cardiovascular risk, particularly in relation to AAC, remain unclear. Previous studies have indicated that the incidence and severity of AAC are significantly higher in postmenopausal women (13–16). This study aims to explore the relationship between E2 levels and AAC in postmenopausal women, which could provide valuable insights for the prevention and management of cardiovascular disease in this population.
Methods
Study population
The subjects for this study were selected from the National Health and Nutrition Examination Survey (NHANES). NHNES is a major program of the National Center for Health Statistics that uses stratified and probability sampling principles to select a representative portion of the U.S. non-institutionalized population for the purpose of assessing their health and nutritional status. The NHANES survey protocol was approved by the National Institutes of Health Research Ethics Review Board prior to implementation of the survey, and all participants signed and provided informed consent. The absence of regular menstruation for the past 12 months was considered a postmenopausal state. Exclusion criteria for study population: i) Male; ii) Age < 45; iii) Regular menstruation for the past 12 months; iii) Irregular menstruation due to hysterectomy or other reasons; and iii) Missing data on menstruation, AAC and E2. The study population was divided into 3 groups according to E2 tertiles: Tertile1(2.12-3.57pg/mL), Tertile2 (3.60-7.04pg/mL), and Tertile3 (7.06-38.4pg/mL).
Natural logarithmic conversion of E2
In this study, we explored the relationship between estrogen levels and vascular calcification. The estrogen level data exhibited a significant skewed distribution on the original scale. To meet the normality assumption required for statistical analysis and to mitigate the effects of skewness, we applied a natural logarithmic transformation to the estrogen concentration data.
Specifically, the estrogen concentration (measured in pg/mL) was log-transformed using the following formula:
where E2 represents the serum estrogen concentration, and ln denotes the natural logarithm. The log-transformed variable ln(E2) was subsequently used in the statistical analysis.
Definition and measurement of exposure, study outcome and covariates
The outcome of this study was the prevalence of extensive AAC (EAAC). The degree and extent of AAC was assessed using the AAC-24 semiquantitative technique (Kauppila 1997) (17). A Kauppila score > 5 was regarded as prominent arterial calcification and was used to define EAAC (18–21). Age, Sex, and Ethnicity were obtained from the demographics of the National Center for Health Statistics.
Smoking, Drinking, Hypertension, and Diabetes Mellitus (DM) were obtained through questionnaires. The definitions of smoking and drinking referred to the latest standards on the New Zealand Ministry of Health website (22). Hypertension was defined as self-reported by asking the question, “Have you been told by a doctor that you have hypertension?”. Osteoporosis was defined by self-reported question “Ever told had osteoporosis?”. Osteoporosis prescription drugs were defined by self-reported question “If diagnosed with osteoporosis, whether the participant has been treated by prescription medicine (Fosamax, Boniva, Actonel, Reclast, Miacalcin, Fortical, Evista or Forteo)”. DM diagnosis referred to the most recent ADA criteria (FPG≥7.0 mmol/L or A1C ≥ 6.5% or 2-h OGTT ≥11.1mmol/L or a random plasma glucose≥11.1 mmol/L) (23). Body mass index (BMI) was obtained by physical examination, where BMI was evaluated by body mass (kilograms) and body height (m2). Red blood cell (RBC), White blood cell (WBC), Platelets, Albumin, Creatinine (Cr), Triglycerides (TG), Low-density lipoprotein cholesterol (LDL-C), High-density lipoprotein cholesterol (HDL-C) were obtained by laboratory measurements. Blood cell counts and were analyzed with a Beckman Coulter MAXM or DXH 800. Albumin and Cr were measured using the DcX800 method. TG and HDL-C were analyzed by the Roche/Hitachi Modular P Chemistry Analyzer (Mod P) in Mobile Examination Centers (MECs). LDL-C was calculated from measured values of TG, HDL-C, and total cholesterol according to the Friedewald calculation (24). Measurements of E2 in serum are performed using isotope dilution liquid chromatography tandem mass spectrometry (ID-LC-MS/MS) method for routine analysis developed by CDC. Details on the methods are publicly available on the official NHANES website (25).
Statistical analyses
The Shapiro-Wilk test was used to examine the normality of the data. The Cochran-Armitage test was used to test for between-group trends. Logistic regression model was used to assess the association between E2 levels and EAAC prevalence. The multivariate model was adjusted for Age, Sex, Ethnicity, BMI, Drinking, Smoking, DM, Hypertension, RBC, WBC, Platelets, Albumin, Cr, TG, LDL-C, and HDL-C. Subgroup analyses were performed to test whether the association between E2 levels and EAAC prevalence was consistent in different groups. Continuous variables in subgroups were grouped by median. Sensitivity analyses tested the stability of the model in women older than 45 years. To handle missing data in our analysis, we employed the multiple imputation (MI) method. Specifically, we used the Stata 17 (Stata Corp, TX, US) to perform the imputations. This approach involves creating multiple datasets (25 times in our case) where the missing values are replaced by plausible data points estimated based on observed data patterns. The imputation model included all relevant covariates to account for potential correlations with the missing data. After creating the imputed datasets, we combined the results from each dataset using Rubin’s rules to obtain the final estimates and confidence intervals. All tests were two-sided. Statistical significance was considered when a P < 0.05.
Results
Baseline characteristics
A total of 10,175 participants were included in the potential analyses during the 2013-2014 NHANES survey cycle, and 614 postmenopausal women were ultimately enrolled in this study after exclusion of participants who were male, older than 45 years, had regular menstruation or irregular menstruation not due to menopause, and had missing AAC and E2 data (Figure 1). ln(E2) levels showed a normal distribution, and EAAC prevalence was significantly higher in Tertile1 (16.6%) than in Tertile2 (9.8%) and Tertile3 (8.3%) (Figure 2). In addition, participants in the lower tertile were older, more likely to have diabetes mellitus, had higher TG levels and lower HDL-C levels (Table 1).
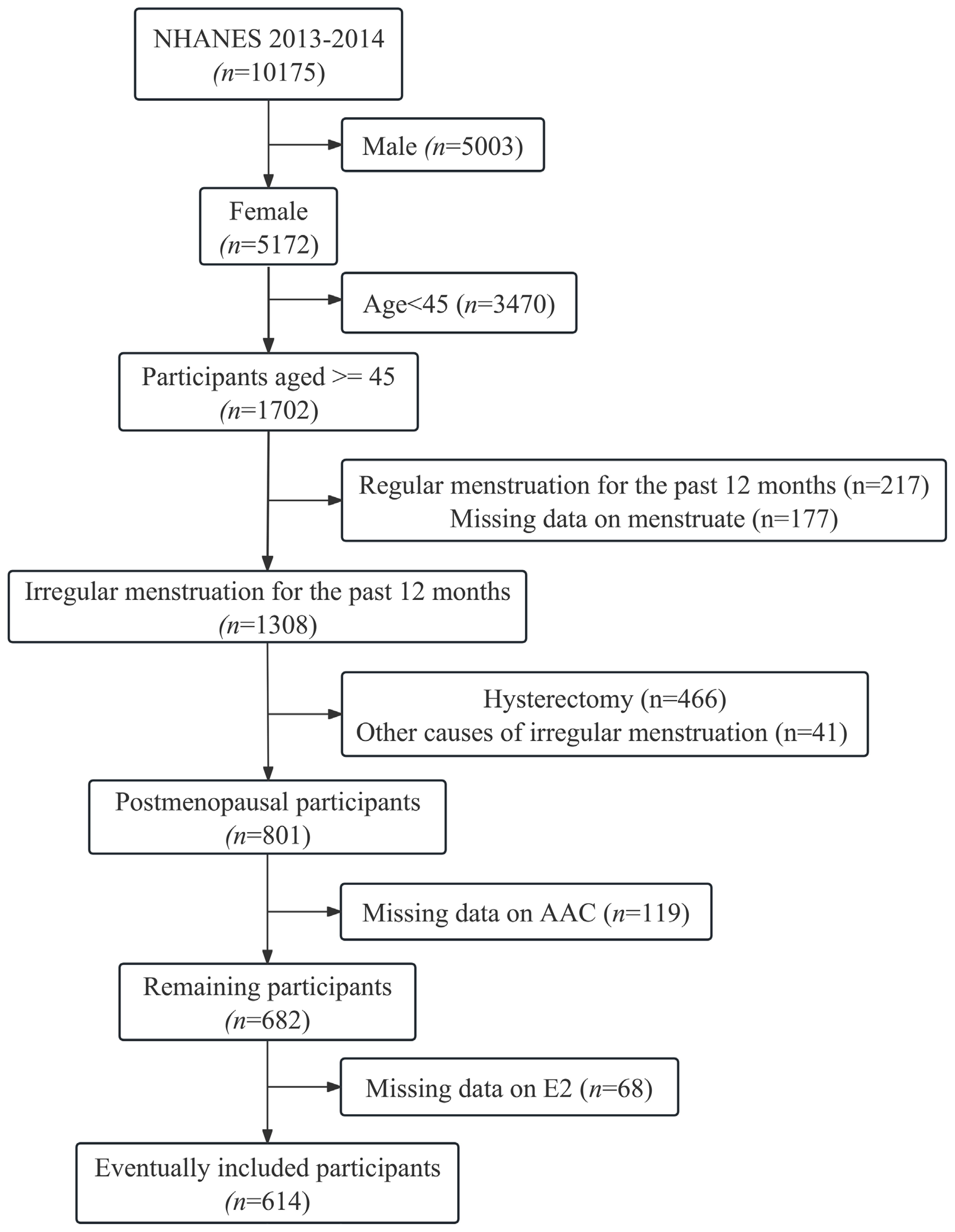
Figure 1. Flowchart for inclusion of participants. E2, Estradiol; AAC, abdominal aortic calcification.
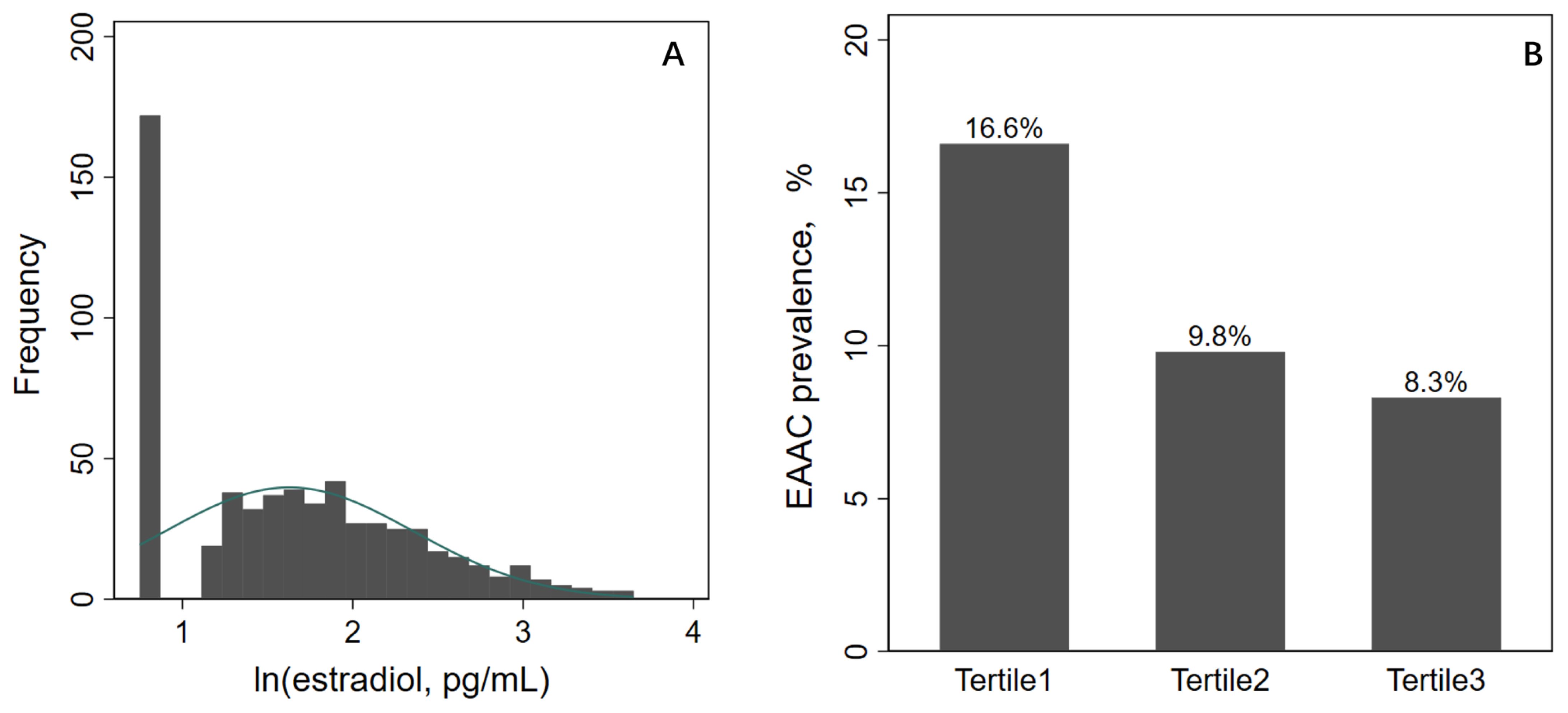
Figure 2. ln(E2) distribution and EAAC prevalence. E2, estradiol; AAC, abdominal aortic calcification.
Association between E2 and EAAC
On a continuous scale, the crude model showed a 54% [OR (95%CI), 1.54(1.09, 2.18)] increase in the risk of EAAC prevalence for per unit decrease in ln(E2), which was a 58% [OR (95%CI), 1.58(1.02, 2.54)] increase after adjusting for all possible confounders (Table 2).
On a categorical scale, the crude model showed that Tertile1 and Tertile2 were 2.19[OR (95%CI), 2.19(1.18, 4.06)] and 1.19[OR (95%CI), 1.19(1.07, 2.34)] times higher risk of suffering from EAAC than Tertile3, respectively, and the adjusted model showed 2.55[OR (95%CI), 2.55(1.10, 5.92)] and 1.31[OR (95%CI), 1.31(1.03, 2.57)] times higher risk, respectively (Table 2).
Subgroup and sensitivity analysis
Subgroup analysis largely confirmed the associations between E2 levels and EAAC prevalence in postmenopausal women revealed in the current study across a broad spectrum of risk factors (Figure 3). Sensitivity analyses showed that the models in this study still yielded similar results when analyzed in women older than 45 years (Table 3).
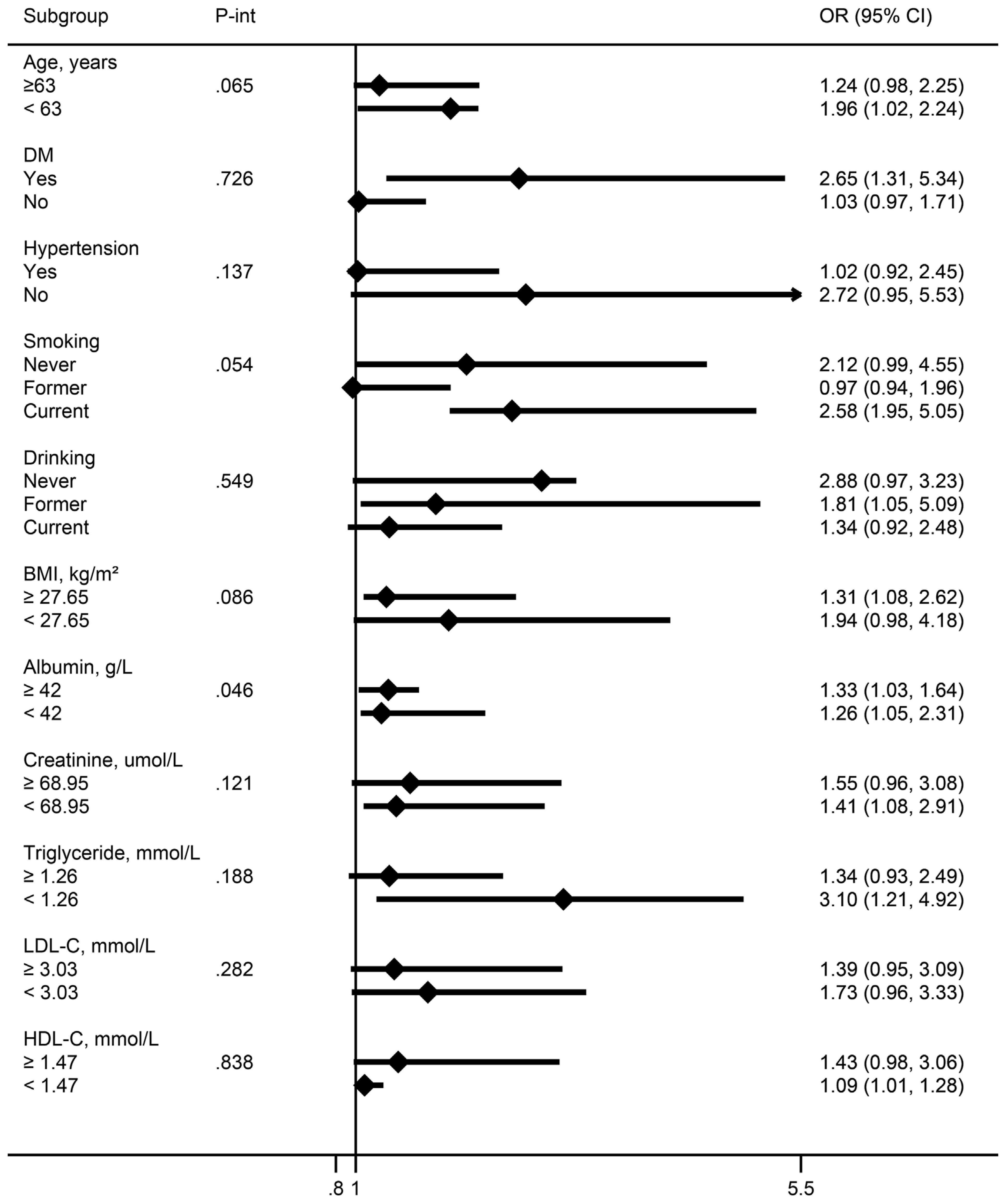
Figure 3. Association between E2 levels and EAAC prevalence in menopausal females in different subgroups [OR (95%CI), per unit ln(E2) decrease]. Continuous variables were grouped by median. All models were adjusted as in Table 2. OR, odds ratio; CI, confidence interval; P-int, P-interaction.
Discussion
This study found that after adjusting for possible confounders, for per unit decrease in ln(E2), the risk of EAAC prevalence increased by 58%, and the risk of EAAC prevalence in tertile1 and tertile2 of the E2 level was 2.55 and 1.31 times higher than that in tertile3, respectively. To summarize, lower E2 levels were associated with a higher prevalence of EAAC.
Several previous studies have supported the findings of this study. J Nakao et al. found that serum E2 levels were lower in postmenopausal women with iliac artery calcification (8.4 ± 1.4 pg/mL) than in the control group (16.1 ± 1.6 pg/mL) (26), while the present study found that E2 levels were also significantly lower in the EAAC group (5.5 ± 4.6pg/mL) than in the non-EAAC group (8.6 ± 15pg/mL). Gyun-Ho Jeon et al. found higher coronary artery calcium accumulation (CACS) in postmenopausal women with lower serum E2 levels, independent of age and other coronary risk factors. Their results showed that after adjusting for possible confounders, the risk of CACS (>100) in subjects with E2 levels less than 20 pg/mL was 4-fold higher than in subjects with E2 levels greater than 20 pg/mL (27), while our multivariate analysis showed that subjects with an E2 range of 2.12-3.57 pg/mL had a 2.19-fold higher risk of EAAC than subjects with an E2 range of 7.06-38.4 pg/mL. The risk multiplier was higher than in the present study probably because the effects of diabetes mellitus and chronic kidney disease on arterial calcification were not taken into account. Samar R El Khoudary et al. found that E2 levels were negatively correlated with the extent of CAC in obese participants, but showed a significant positive correlation with the extent of CAC in non-obese participants (28). The conclusions of the above study are controversial because the included population was not postmenopausal and the potential E2 fluctuating effect from the menstrual cycle was not eliminated, but the interaction between E2 and obesity found in the study was consistent with that of the present study in the subgroup analyses. Although the study endpoints of the aforementioned studies were coronary artery calcification and iliac artery calcification rather than abdominal aortic calcification, arterial calcification has a common pathogenesis and is often systemic and segmental in nature. Thus, both our and previous studies have demonstrated a connection between E2 levels and arterial calcification, but the differences in the associations between E2 levels and different sites of calcification still need to be further demonstrated.
Two other studies showed findings that contradicted the present study and those mentioned above.
Erin D. Michos et al. found that there was no association between E2 levels and the presence or absence of AAC in postmenopausal women with subclinical atherosclerosis. The conflicting findings may be related to the selection of the study population and the definition of the study endpoints being different from the present study. Erin D. Michos et al. studied patients with subclinical atherosclerosis, who often have co-morbid chronic diseases such as diabetes mellitus, chronic kidney disease, hypertension, and vascular and valvular lesions, which are causally related to arterial calcification, potentially masking the true effect of E2 levels on AAC. In addition, the study’s definition of the presence (AAC>0) or absence (AAC=0) of AAC as a study endpoint may have attenuated the association between E2 levels and AAC. Because arterial calcification is a natural manifestation of aging, an AAC score <5 is considered to be the absence of significant calcification (19–21). The study may have included a large sample of subjects with AAC<5 who were considered to have undergone significant calcification. Pamela Ouyang et al. did a similar study in patients with subclinical atherosclerosis as did Erin D. Michos et al. The difference is that they did the association between E2 levels and carotid intima-media thickness (CIMT) and coronary artery calcification (CAC), so the same limitations as in the study by Erin D. Michos et al. exist.
Basic studies have shown that the cardiovascular protective effects of estrogen are attributable to estrogen-induced lipid changes on the one hand and to direct estrogen effects on the vasculature on the other hand (29–31). The mechanisms mediating the effects of estrogen on the vascular wall are not fully understood, but it has been reported that estrogen induces vasodilation by increasing the bioavailability of nitric oxide to endothelial cells (32). In addition, estrogen also prevents atherosclerosis by long-term inhibition of the vascular injury response through estrogen receptor-mediated changes in gene expression (32).
Another important aspect is that E2 plays a critical role in regulating bone metabolism and significantly impacts vascular calcification by influencing both osteogenesis and osteoclastogenesis processes. Traditionally, osteoporosis has been recognized as a condition primarily affecting postmenopausal women, largely due to the deficiency of E2 (33, 34). E2 regulates bone remodeling by modulating the production of cytokines and growth factors in the bone marrow and osteoblasts, a process mediated by the basic multicellular unit (BMU) (35). When E2 levels are deficient, BMU activity increases, leading to higher rates of osteoblast apoptosis and reduced osteoclast apoptosis. This imbalance shortens the bone formation period, preventing new bone from adequately filling the spaces left by resorbed old bone, resulting in significant bone loss and thus promoting the onset and progression of osteoporosis (36–38). A substantial body of evidence indicates that vascular calcification is a central event linking bone loss to cardiovascular risk (39). In particular, prospective epidemiological studies have shown a significant association between aortic calcification and lower bone density (40–42). Indeed, vascular calcification and osteoporosis share common risk factors and pathophysiological mechanisms, including the relationship between bone-derived proteins (e.g., osteoprotegerin and osteopontin) and vascular pathology, as well as the involvement of intercellular protein systems like the RANK/RANKL/OPG axis and Wnt signaling pathways (43). However, the interactions between bone loss and vascular calcification are complex, and their precise mechanisms and clinical significance remain unclear.
Estrone(E1), the predominant estrogen form in postmenopausal women, is known to be less potent than estradiol but still plays a critical role in maintaining bone density (44). The decline in E1 levels post-menopause is strongly associated with the onset and progression of osteoporosis (45). As estrone is integral to bone metabolism, its deficiency could contribute to both osteoporosis and the calcification of the abdominal aorta. This suggests that in postmenopausal women, low E1 levels might not only predispose them to osteoporosis but also exacerbate the risk of developing AAC (34, 46). On the other hand, studies suggest that E1 may be more likely than E2 to induce platelet aggregation, possibly due to its different regulatory effects on platelet surface receptors or cellular signaling pathways (47, 48). This characteristic makes E1 particularly significant in research related to cardiovascular diseases, especially when investigating the risk of thrombosis in postmenopausal women. Therefore, while our study primarily focused on the association between E2 levels and AAC, the role of E1 and its relationship with both osteoporosis and vascular calcification should not be overlooked. Future research should consider E1 levels as a variable of interest when exploring the links between bone health and cardiovascular outcomes in postmenopausal women. Such studies could provide deeper insights into the comprehensive role of estrogenic hormones in vascular health and further clarify the potential benefits of hormone replacement therapy that includes both E2 and E1 in preventing AAC and related cardiovascular diseases.
Several clinical trials have demonstrated a significant benefit of hormone replacement therapy for vascular calcification. Postmenopausal female participants treated with E2 got regressive CIMT (49). CIMT tended to progress in non-estrogen-treated users, whereas CIMT tended to regress in estrogen-treated users (50). Manson et al. recently showed in a randomized clinical trial that estrogen therapy reduced CACS as measured by cardiac CT (51). Lipid levels are considered important factors influencing vascular calcification (52). In subjects receiving estrogen/progestin therapy, HDL-C levels were elevated, while LDL-C levels were reduced (53, 54). However, another clinical trial showed that starting hormone replacement therapy many years after menopause (an average of 15 years) increases the risk of coronary heart disease (55).Thus, there is still controversy about whether estradiol has a beneficial effect on arterial calcification, and further confirmation from additional studies is needed.
The innovation of this study lies in its systematic exploration of the relationship between serum E2 levels and AAC, with a specific focus on postmenopausal women. While previous research has established a link between declining estrogen levels and increased cardiovascular risk, studies specifically addressing AAC as a subclinical marker have been relatively scarce. This study fills a critical gap in the literature and provides foundational data to further investigate the potential of estrogen replacement therapy in preventing AAC and associated cardiovascular diseases. However, this study has certain limitations. First, as a cross-sectional study, it can only demonstrate an association between E2 levels and AAC, without establishing a causal relationship. Second, the sample size is limited, which may affect the generalizability of the results. Third, not including osteoporosis as an inclusion criterion may limit our comprehensive understanding of the relationship between estrogen levels and AAC. Additionally, the study did not account for other factors that could influence AAC, such as lifestyle, dietary habits, other hormone levels, or long-term medication use, all of which could introduce potential confounding effects. Future research should consider longitudinal study designs with larger sample sizes to further validate these findings and explore the underlying mechanisms.
Conclusion
This study found that a higher prevalence of AAC in postmenopausal women is closely associated with lower serum E2 levels. This finding supports the potential link between declining estrogen levels and increased cardiovascular risk, particularly in relation to the development of AAC. Our research further underscores the importance of E2 in maintaining cardiovascular health in postmenopausal women and suggests that monitoring E2 levels may aid in the early prevention and management of AAC and related cardiovascular diseases.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: the National Health and Nutrition Examination Survey (NHANES).
Ethics statement
The studies involving humans were approved by the NCHS Research Ethics Review Board Approval Code: Protocol #2011-17. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LH: Data curation, Formal analysis, Investigation, Methodology, Resources, Writing – original draft. XL: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Resources. ES: Formal analysis, Project administration, Resources, Supervision, Writing – review & editing. Y-MH: Methodology, Project administration, Resources, Software, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to thank the NHANES administration for providing publicly available data and reports that enabled us to prepare this paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Santoro N, Epperson CN, Mathews SB. Menopausal symptoms and their management. Endocrinol Metab Clin North Am. (2015) 44:497–515. doi: 10.1016/j.ecl.2015.05.001
2. Duralde ER, Sobel TH, Manson JE. Management of perimenopausal and menopausal symptoms. Bmj. (2023) 382:e072612. doi: 10.1136/bmj-2022-072612
3. Takahashi TA, Johnson KM. Menopause. Med Clin North Am. (2015) 99:521–34. doi: 10.1016/j.mcna.2015.01.006
4. MohanKumar SMJ, Balasubramanian P, Subramanian M, MohanKumar PS. Chronic estradiol exposure - harmful effects on behavior, cardiovascular and reproductive functions. Reproduction. (2018) 156:R169–r186. doi: 10.1530/REP-18-0116
5. Gersh F, O'Keefe JH, Elagizi A, Lavie CJ, Laukkanen JA. Estrogen and cardiovascular disease. Prog Cardiovasc Dis. (2024) 84:60–7. doi: 10.1016/j.pcad.2024.01.015
6. Golledge J. Abdominal aortic calcification: clinical significance, mechanisms and therapies. Curr Pharm Des. (2014) 20:5834–8. doi: 10.2174/1381612820666140212195309
7. Chen HC, Wang WT, Hsi CN, Chou CY, Lin HJ, Huang CC, et al. Abdominal aortic calcification score can predict future coronary artery disease in hemodialysis patients: a 5-year prospective cohort study. BMC Nephrol. (2018) 19:313. doi: 10.1186/s12882-018-1124-x
8. Levitzky YS, Cupples LA, Murabito JM, Kannel WB, Kiel DP, Wilson PW, et al. Prediction of intermittent claudication, ischemic stroke, and other cardiovascular disease by detection of abdominal aortic calcific deposits by plain lumbar radiographs. Am J Cardiol. (2008) 101:326–31. doi: 10.1016/j.amjcard.2007.08.032
9. O’Connor SD, Graffy PM, Zea R, Pickhardt PJ. Does nonenhanced CT-based quantification of abdominal aortic calcification outperform the Framingham risk score in predicting cardiovascular events in asymptomatic adults? Radiology. (2019) 290:108–15. doi: 10.1148/radiol.2018180562
10. van der Meer IM, Bots ML, Hofman A, del Sol AI, van der Kuip DA, Witteman JC. Predictive value of noninvasive measures of atherosclerosis for incident myocardial infarction: the Rotterdam Study. Circulation. (2004) 109:1089–94. doi: 10.1161/01.CIR.0000120708.59903.1B
11. Bartstra JW, Mali WPTM, Spiering W, de Jong PA. Abdominal aortic calcification: from ancient friend to modern foe. Eur J Prev Cardiol. (2021) 28:1386–91. doi: 10.1177/2047487320919895
12. Szulc P. Abdominal aortic calcification: A reappraisal of epidemiological and pathophysiological data. Bone. (2016) 84:25–37. doi: 10.1016/j.bone.2015.12.004
13. Bristow SM, Gamble GD, Horne AM, Reid IR. Longitudinal changes in bone mineral density, bone mineral content and bone area at the lumbar spine and hip in postmenopausal women, and the influence of abdominal aortic calcification. Bone Rep. (2019) 10:100190. doi: 10.1016/j.bonr.2018.100190
14. El Maghraoui A, Hamza T, Sadni S, El Maataoui A, Majjad A, Rezqi A, et al. Vitamin D status and abdominal aortic calcification in postmenopausal women. J Bone Miner Metab. (2018) 36:229–37. doi: 10.1007/s00774-017-0832-9
15. El Maghraoui A, Rezqi A, Mounach A, Achemlal L, Bezza A, Dehhaoui M, et al. Vertebral fractures and abdominal aortic calcification in postmenopausal women. A cohort study. Bone. (2013) 56:213–9. doi: 10.1016/j.bone.2013.05.022
16. Lampropoulos CE, Kalamara P, Konsta M, Papaioannou I, Papadima E, Antoniou Z, et al. Osteoporosis and vascular calcification in postmenopausal women: a cross-sectional study. Climacteric. (2016) 19:303–7. doi: 10.3109/13697137.2016.1164134
17. Kauppila LI, Polak JF, Cupples LA, Hannan MT, Kiel DP, Wilson PW. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: a 25-year follow-up study. Atherosclerosis. (1997) 132:245–50. doi: 10.1016/S0021-9150(97)00106-8
18. Chen Y, Chang Z, Zhao Y, Liu Y, Fu J, Zhang Y, et al. Association between the triglyceride-glucose index and abdominal aortic calcification in adults: A cross-sectional study. Nutr Metab Cardiovasc Dis. (2021) 31:2068–76. doi: 10.1016/j.numecd.2021.04.010
19. Lu YY, Chen WL. Clinical relevance of serum selenium levels and abdominal aortic calcification. Biol Trace Elem Res. (2021) 199:2803–10. doi: 10.1007/s12011-020-02405-3
20. Szulc P, Blackwell T, Kiel DP, Schousboe JT, Cauley J, Hillier T, et al. Abdominal aortic calcification and risk of fracture among older women - The SOF study. Bone. (2015) 81:16–23. doi: 10.1016/j.bone.2015.06.019
21. Wilson PW, Kauppila LI, O'Donnell CJ, Kiel DP, Hannan M, Polak JM, et al. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation. (2001) 103:1529–34. doi: 10.1161/01.CIR.103.11.1529
22. Definitions of smoking and drinking status. Available online at: https://www.health.govt.nz/ (accessed February 19, 2024).
23. Classification and diagnosis of diabetes: standards of medical care in diabetes—2022. Available online at: https://www.diabetes.org/ (accessed February 19, 2024).
24. Sampson M, Ling C, Sun Q, Harb R, Ashmaig M, Warnick R, et al. A new equation for calculation of low-density lipoprotein cholesterol in patients with normolipidemia and/or hypertriglyceridemia. JAMA Cardiol. (2020) 5:540–8. doi: 10.1001/jamacardio.2020.0013
25. Survey data and documentation. Available online at: https://www.cdc.gov/nchs/nhanes/index.htm (accessed February 15, 2024).
26. Nakao J, Orimo H, Ooyama T, Shiraki M. Low serum estradiol levels in subjects with arterial calcification. Atherosclerosis. (1979) 34:469–74. doi: 10.1016/0021-9150(79)90071-6
27. Jeon GH, Kim SH, Yun SC, Chae HD, Kim CH, Kang BM. Association between serum estradiol level and coronary artery calcification in postmenopausal women. Menopause. (2010) 17:902–7. doi: 10.1097/gme.0b013e3181d76768
28. El Khoudary SR, Wildman RP, Matthews K, Powell L, Hollenberg SM, Edmundowicz D, et al. Effect modification of obesity on associations between endogenous steroid sex hormones and arterial calcification in women at midlife. Menopause. (2011) 18:906–14. doi: 10.1097/gme.0b013e3182099dd2
29. Grodstein F, Stampfer M. The epidemiology of coronary heart disease and estrogen replacement in postmenopausal women. Prog Cardiovasc Dis. (1995) 38:199–210. doi: 10.1016/S0033-0620(95)80012-3
30. Bush TL, Barrett-Connor E, Cowan LD, Criqui MH, Wallace RB, Suchindran CM, et al. Cardiovascular mortality and noncontraceptive use of estrogen in women: results from the Lipid Research Clinics Program Follow-up Study. Circulation. (1987) 75:1102–9. doi: 10.1161/01.CIR.75.6.1102
31. Farhat MY, Lavigne MC, Ramwell PW. The vascular protective effects of estrogen. FASEB J. (1996) 10:615–24. doi: 10.1096/fasebj.10.5.8621060
32. Mikkola TS, Clarkson TB. Estrogen replacement therapy, atherosclerosis, and vascular function. Cardiovasc Res. (2002) 53:605–19. doi: 10.1016/S0008-6363(01)00466-7
33. Pietschmann P, Kerschan-Schindl K. Osteoporosis: gender-specific aspects. Wien Med Wochenschr. (2004) 154:411–5. doi: 10.1007/s10354-004-0100-1
34. Lu L, Tian L. Postmenopausal osteoporosis coexisting with sarcopenia: the role and mechanisms of estrogen. J Endocrinol. (2023) 259:e230116. doi: 10.1530/JOE-23-0116
35. Bolamperti S, Villa I, Rubinacci A. Bone remodeling: an operational process ensuring survival and bone mechanical competence. Bone Res. (2022) 10:48. doi: 10.1038/s41413-022-00219-8
36. Raehtz S, Bierhalter H, Schoenherr D, Parameswaran N, McCabe LR. Estrogen deficiency exacerbates type 1 diabetes-induced bone TNF-α Expression and osteoporosis in female mice. Endocrinology. (2017) 158:2086–101. doi: 10.1210/en.2016-1821
37. Karlamangla AS, Shieh A, Greendale GA. Hormones and bone loss across the menopause transition. Vitam Horm. (2021) 115:401–17. doi: 10.1016/bs.vh.2020.12.016
38. Wang Y, Mei R, Hao S, Luo P, Wang P, Almatari Y, et al. Up-regulation of SIRT1 induced by 17beta-estradiol promotes autophagy and inhibits apoptosis in osteoblasts. Aging (Albany NY). (2021) 13:23652–71. doi: 10.18632/aging.203639
39. Vassalle C, Mazzone A. Bone loss and vascular calcification: A bi-directional interplay? Vascul Pharmacol. (2016) 86:77–86. doi: 10.1016/j.vph.2016.07.003
40. Bagger YZ, Tankó LB, Alexandersen P, Qin G, Christiansen C, Prospective Epidemiological Risk Factors Study Group. Radiographic measure of aorta calcification is a site-specific predictor of bone loss and fracture risk at the hip. J Intern Med. (2006) 259:598–605. doi: 10.1111/j.1365-2796.2006.01640.x
41. Hyder JA, Allison MA, Wong N, Papa A, Lang TF, Sirlin C, et al. Association of coronary artery and aortic calcium with lumbar bone density: the MESA Abdominal Aortic Calcium Study. Am J Epidemiol. (2009) 169:186–94. doi: 10.1093/aje/kwn303
42. Schousboe JT, Taylor BC, Kiel DP, Ensrud KE, Wilson KE, McCloskey EV. Abdominal aortic calcification detected on lateral spine images from a bone densitometer predicts incident myocardial infarction or stroke in older women. J Bone Miner Res. (2008) 23:409–16. doi: 10.1359/jbmr.071024
43. García-Gómez MC, Vilahur G. Osteoporosis and vascular calcification: A shared scenario. Clin Investig Arterioscler. (2020) 32:33–42. doi: 10.1016/j.artere.2019.03.008
44. Väänänen HK, Härkönen PL. Estrogen and bone metabolism. Maturitas. (1996) 23 Suppl:S65–9. doi: 10.1016/0378-5122(96)01015-8
46. Iorga A, Cunningham CM, Moazeni S, Ruffenach G, Umar S, Eghbali M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol Sex Differ. (2017) 8:33. doi: 10.1186/s13293-017-0152-8
47. Hashemzadeh M, Haseefa F, Peyton L, Park S, Movahed MR. The effects of estrogen and hormone replacement therapy on platelet activity: a review. Am J Blood Res. (2022) 12:33–42.
48. Akarasereenont P, Tripatara P, Chotewuttakorn S, Palo T, Thaworn A. The effects of estrone, estradiol and estriol on platelet aggregation induced by adrenaline and adenosine diphosphate. Platelets. (2006) 17:441–7. doi: 10.1080/09537100600745302
49. Hodis HN, Mack WJ, Lobo RA, Shoupe D, Sevanian A, Mahrer PR, et al. Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. (2001) 135:939–53. doi: 10.7326/0003-4819-135-11-200112040-00005
50. Espeland MA, Applegate W, Furberg CD, Lefkowitz D, Rice L, Hunninghake D. Estrogen replacement therapy and progression of intimal-medial thickness in the carotid arteries of postmenopausal women. ACAPS Investigators. Asymptomatic Carotid Atherosclerosis Progression Study. Am J Epidemiol. (1995) 142:1011–9. doi: 10.1093/oxfordjournals.aje.a117553
51. Manson JE, Allison MA, Rossouw JE, Carr JJ, Langer RD, Hsia J, et al. Estrogen therapy and coronary-artery calcification. N Engl J Med. (2007) 356:2591–602. doi: 10.1056/NEJMoa071513
52. Boutouyrie P, Chowienczyk P, Humphrey JD, Mitchell GF. Arterial stiffness and cardiovascular risk in hypertension. Circ Res. (2021) 128:864–86. doi: 10.1161/CIRCRESAHA.121.318061
53. Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The Writing Group for the PEPI Trial. Jama. (1995) 273:199–208. doi: 10.1001/jama.273.3.199
54. Miller VT, Byington RL, Espeland MA, Langer R, Marcus R, Shumaker S, et al. Baseline characteristics of the PEPI participants. Postmenopausal Estrogen/Progestin Interventions. Control Clin Trials. (1995) 16:54s–65s. doi: 10.1016/0197-2456(94)00113-H
55. Vickers MR, MacLennan AH, Lawton B, Ford D, Martin J, Meredith SK, et al. Main morbidities recorded in the women’s international study of long duration oestrogen after menopause (WISDOM): a randomised controlled trial of hormone replacement therapy in postmenopausal women. Bmj. (2007) 335:239. doi: 10.1136/bmj.39266.425069.AD
Keywords: abdominal aortic calcification, estradiol, NHANES, postmenopausal woman, cross-sectional study
Citation: He L, Li X, Shen E and He Y-M (2024) Association between serum estradiol levels and abdominal aortic calcification in postmenopausal woman: a cross-sectional study. Front. Endocrinol. 15:1411803. doi: 10.3389/fendo.2024.1411803
Received: 03 April 2024; Accepted: 03 September 2024;
Published: 20 September 2024.
Edited by:
Hong Liu, UC Davis Health, United StatesReviewed by:
Barbara Ciastek, Nicolaus Copernicus University in Toruń, PolandShivshankar Thanigaimani, James Cook University, Australia
Copyright © 2024 He, Li, Shen and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: E. Shen, c2hlbmUyMDA4QDE2My5jb20=; Yong-Ming He, aGV5b25nbWluZ0BzdWRhLmVkdS5jbg==
†These authors have contributed equally to this work
 Lan He
Lan He Xu Li
Xu Li E Shen
E Shen Yong-Ming He
Yong-Ming He