- 1Department of Endocrinology, First Hospital of Shanxi Medical University, Shanxi Medical University, Taiyuan, Shanxi, China
- 2First Clinical Medical College, Shanxi Medical University, Taiyuan, Shanxi, China
- 3Department of Endocrinology, Changzhi Second People's Hospital, Changzhi, Shanxi, China
- 4Shanxi Innovation Center for Integrated Management of Hypertension, Hyperlipidemia and Hyperglycemia Correlated with Cardiovascular and Cerebrovascular Diseases, Taiyuan, Shanxi, China
Objectives: This study aimed to investigate the link between 25-hydroxy vitamin D and serum asprosin in individuals with type 2 diabetes within the community. The goal was to provide a foundation for clinical interventions.
Methods: Between November 2019 and July 2021, data from 463 patients with type 2 diabetes were consistently gathered at a community health service station in Southeast Shanxi Province. General information and laboratory metrics were compiled, including serum asprosin levels. The participants were categorized based on three serum asprosin quantiles, allowing for a comparison of various factors among the groups. The correlation between serum asprosin levels and other factors was analyzed. Employing a general linear model, the connection between 25-hydroxy vitamin D and serum asprosin levels was studied. Utilizing three quantiles of 25-hydroxy vitamin D, serum asprosin was treated as the dependent variable, while 25-hydroxy vitamin D served as the independent variable for linear regression analysis.
Results: As serum asprosin increased, there were gradual increments in age, disease duration, SBP, BMI, WC, creatinine, and SUA levels (P<0.05). Conversely, HbA1c, HDL-C, GFR, and 25-hydroxy vitamin D levels exhibited gradual declines (P<0.05). Age, 25-hydroxy vitamin D, SUA, creatinine, and LDL-C emerged as independent influencing factors for serum asprosin. Across the 1st to 3rd 25-hydroxy vitamin D quantiles, elevated 25-hydroxy vitamin D levels correlated with a gradual reduction in mean serum asprosin (P<0.05).
Conclusion: Serum asprosin levels demonstrate an inverse correlation with 25-hydroxy vitamin D levels in community-dwelling individuals with type 2 diabetes. Serum asprosin levels might independently contribute to 25-hydroxy vitamin D levels.
Introduction
In 2016, Romere et al. made the initial discovery of asprosin, encoded by two exons (exon 65 and exon 66) of the fibrillin 1 (FBN1) gene, as a novel adipokine protein involved in glucose production (1). This finding emerged from a study on patients with Neonatal Progeroid Syndrome (NPS). Individuals with NPS, who exhibit asprosin deficiency due to truncated FBN1 mutations, maintain normal blood glucose levels despite reduced plasma insulin levels (1). Extensive research has shown a relationship between asprosin concentration and insulin resistance (IR) as well as diabetes in both mice and humans. A cross-sectional analysis involving 143 subjects demonstrated elevated asprosin levels in Impaired Glucose Regulation (IGR) and Type 2 Diabetes Mellitus (T2DM) groups in comparison to the Normal Glucose Regulation (NGR) group. Asprosin exhibited positive correlation with insulin resistance homeostatic model assessment (HOMA-IR) and negative correlation with β-cell function (HOMA-β) (2). Moreover, a case-control study encompassing 170 subjects identified higher T2DM-associated asprosin levels in adults compared to the control group. Notably, an independent relationship between fasting plasma glucose and asprosin in T2DM patients was observed, in alignment with numerous other studies (3). Animal investigations have also indicated heightened liver asprosin levels in Type 1 Diabetes Mellitus (T1DM) mice (4). Consequently, serum asprosin could serve as a biomarker for early diabetes diagnosis.
The principal form of vitamin D in the body, 25-hydroxy vitamin D, contributes to preserving pancreatic islet β-cell function and inhibiting β-cell apoptosis (5). Studies have revealed that deficiency in 25-hydroxy vitamin D impacts glucose metabolism in individuals with Type 2 Diabetes Mellitus (T2DM) and is presumed to associate with insulin resistance (6). A link between adipocytokines and blood 25-hydroxy vitamin D levels has also been established. Among patients with T2DM, fasting serum asprosin levels increase and demonstrate a strong association with bone mineral density (3). Additionally, research involving prenatal screening for Trisomy 21 demonstrated an inverse correlation between amniotic fluid 25-hydroxy vitamin D and asprosin levels (7). Despite these findings, the intricate relationship among 25-hydroxy vitamin D, asprosin, and T2DM necessitates further elucidation. One plausible hypothesis is that 25-hydroxy vitamin D might influence the production and/or secretion of adipocytokines. Whether a linear negative correlation exists between these factors in T2DM patients remains uncertain. This study thus collected clinical data from community-based T2DM patients to analyze the connection between 25-hydroxy vitamin D and serum asprosin levels.
Materials and methods
Research participants
The subjects of the present report were those of the previous study (Xu et al, 2022) with the same inclusion criteria. Clinical and biochemical data were taken from the previous study (methods outlined in Xu et al, 2022) (8), possession of complete clinical data, and willingness to cooperate with the study. Exclusion criteria encompassed patients with renal insufficiency characterized by creatinine levels exceeding 178 μmol/L, type 1 diabetes, diabetes resulting from other endocrine disorders, diabetic ketoacidosis, diabetic hyperosmolar coma, severe liver or kidney dysfunction, severe infection, malignant tumors, individuals with communication disorders like mental illness, and those unable to collaborate with the study’s requirements. After excluding 35 participants due to incomplete clinical data, this investigation analyzed clinical information from 463 patients.
Research methods
General data collection
General data collection involved measuring patients’ height and weight to calculate the body mass index (BMI). Patient demographic information, such as gender, age, abdominal circumference, systolic and diastolic blood pressure, and the usage of hypoglycemic medications and insulin, was gathered. Fasting venous blood samples were obtained to measure fasting plasma glucose (FPG), 2-hour postprandial plasma glucose (2hPG), serum uric acid (SUA), creatinine (CRE), total cholesterol (TC), triglyceride (TG), high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) using Beckman Automatic Biochemical Analyzer (USA, BK-200). High-pressure liquid phase assessment (Roche 501, Switzerland) was conducted to determine glycated hemoglobin A1c (HbA1c) levels. Roche Diagnostic Products (Shanghai) Co. Ltd provided serum 25-hydroxy vitamin D concentration determination kits, utilizing chemiluminescence methods with within-batch CV < 8% and between-batch CV < 10%.
Measurement of serum asprosin
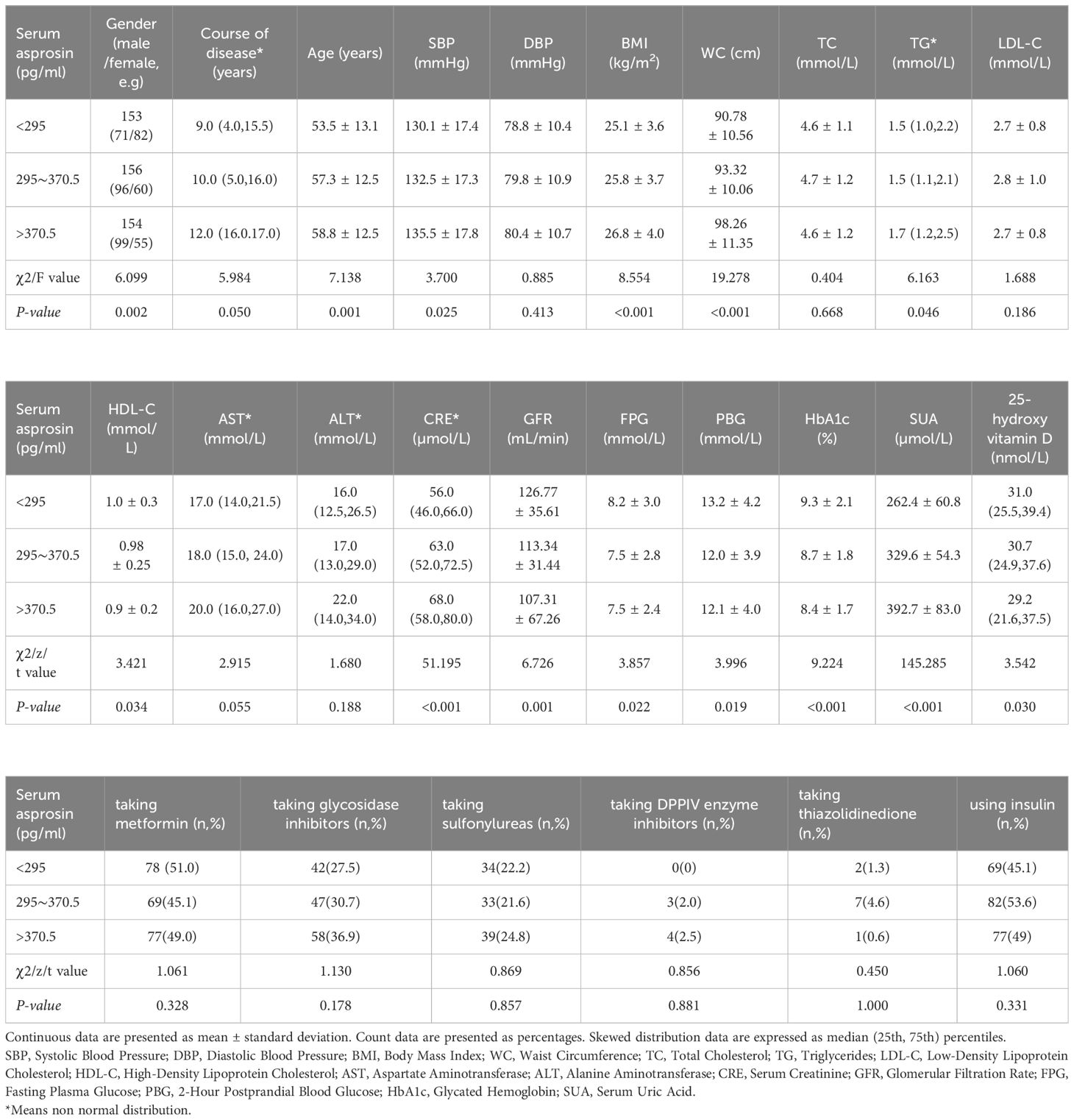
After fasting for 8-10 hours, venous blood samples were collected the next morning, followed by centrifugation at 3000 rpm for 15 minutes. Serum asprosin levels were determined using enzyme-linked immunosorbent assay (ELISA) provided by Hepeng (Shanghai) Biotechnology Co. Ltd. All samples were stored at -80°C. Double-well duplication was employed for measurement, ensuring batch-to-batch difference <11% and intra-batch difference <8%. Strict adherence to kit and instrument manuals was maintained. Patients with T2DM were categorized into three groups based on serum asprosin tertiles: T1 (asprosin < 295.0 pg/ml, 153 cases), T2 (asprosin 295.0~370.5 pg/ml, 153 patients), and T3 (asprosin > 370.5 pg/ml, 157 cases).
Statistical processing
Statistical analysis was performed using SPSS 22.0 software. Normally distributed metric data were expressed as Mean ± SD. One-way ANOVA was used for group comparisons, followed by LSD tests for pairwise comparisons. Non-normally distributed measurement data were expressed as M(Q1, Q3), and Mann-Whitney rank sum tests were applied for intergroup comparisons. Pearson correlation analysis was conducted for normally distributed data, while Spearman correlation analysis was used for data not conforming to bivariate normal distribution. The relationship between serum asprosin and 25-hydroxyvitamin D tertiles was evaluated using a general linear model, with statistical significance defined as P < 0.05.
Results
The clinical characteristics of the patient
Among the 463 patients with type 2 diabetes, 266 were male with a mean age of 54.3 ± 13.0 years, and 197 were female with a mean age of 59.6 ± 12.1 years. The median duration of diabetes was 10 years, and the average glycated hemoglobin was 8.8%. Patients were categorized into three groups based on serum asprosin quantiles. As serum asprosin levels increased, patient age, disease duration, SBP, BMI, WC, creatinine, and SUA levels also increased, while levels of HbA1c, HDL-C, GFR, and 25-hydroxy vitamin D decreased (Table 1).
The correlation between serum asprosin and other variables
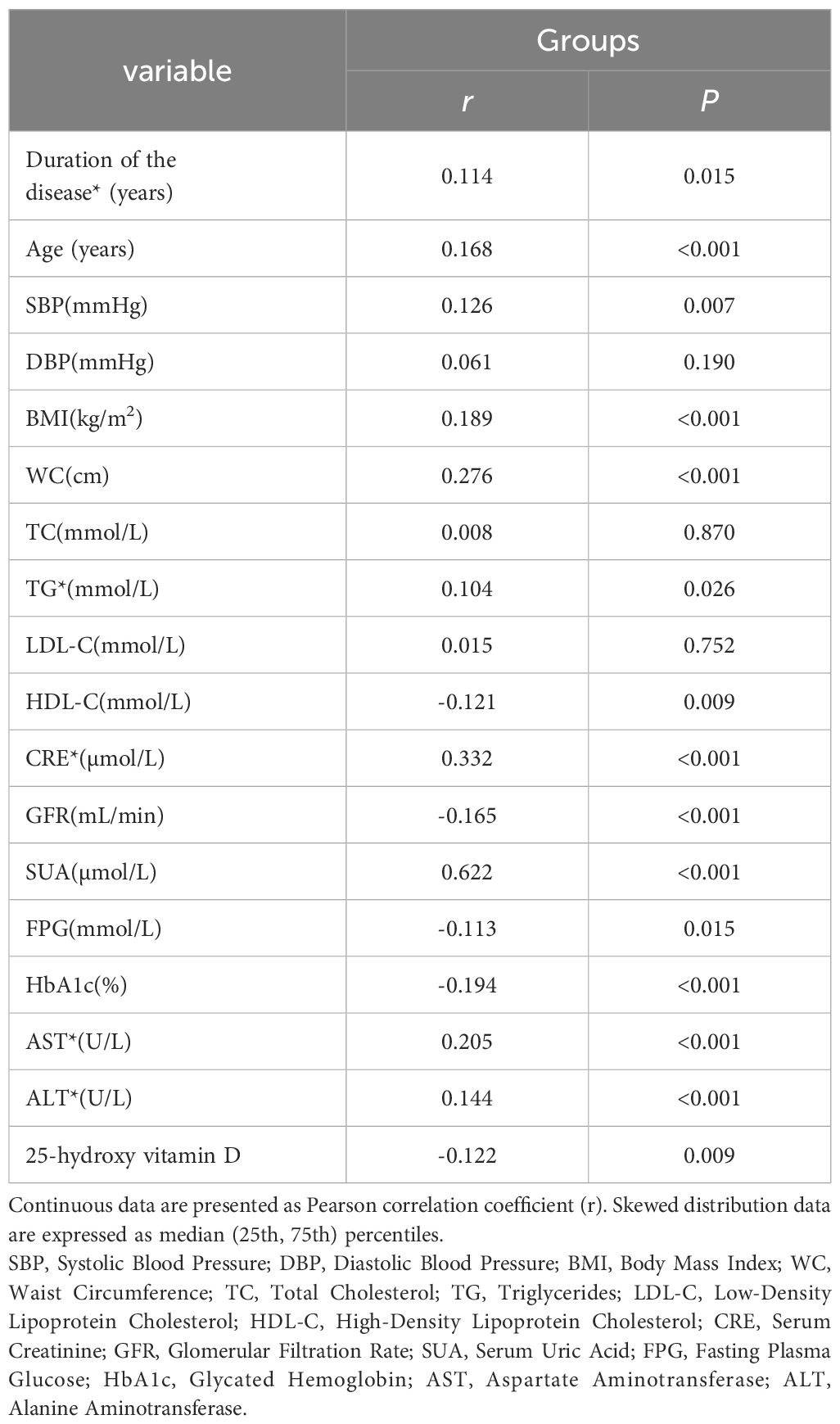
Serum asprosin displayed positive correlations with disease duration, age, SBP, BMI, WC, TG, creatinine, SUA, AST, and ALT (r = 0.114, 0.168, 0.126, 0.189, 0.276, 0.104, 0.332, 0.622, 0.205, and 0.144, respectively; P < 0.05). Conversely, serum asprosin exhibited negative correlations with HDL-C, GFR, FPG, HbA1c, and 25-hydroxy vitamin D (r = -0.121, -0.165, -0.113, -0.194, and -0.122, respectively; P < 0.05) (Table 2).
The results of multiple stepwise linear regression analysis of serum asprosin level and related indexes
Utilizing multiple stepwise linear regression analysis with serum asprosin as the dependent variable and age, BMI, SBP, HbA1c, creatinine, LDL-C, 25-hydroxy vitamin D, and SUA as independent variables, the study found that age, 25-hydroxy vitamin D, SUA, creatinine, and LDL-C were independent influencing factors for serum asprosin (P < 0.05, Table 3).
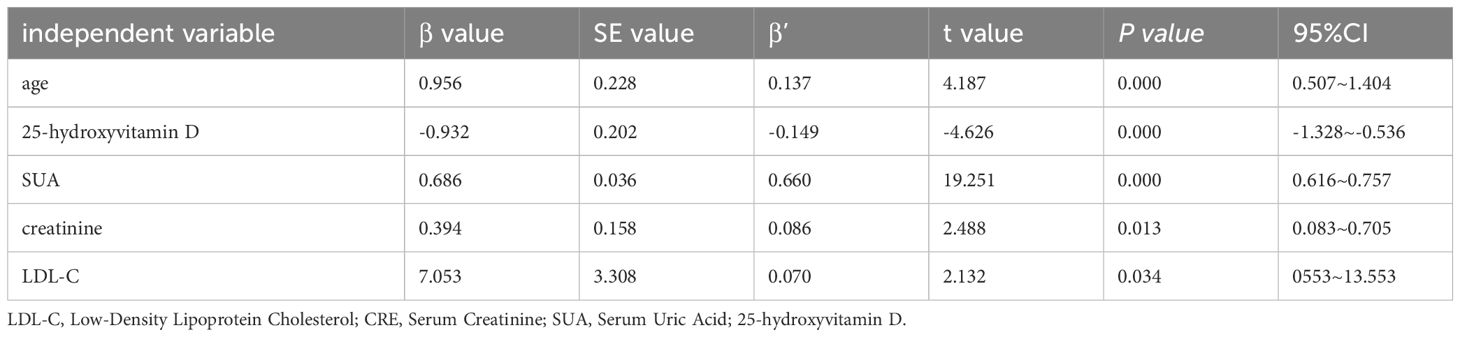
Table 3 Multiple stepwise linear regression analysis of factors influencing serum asprosin in patients with type 2 diabetes.
Correlation analysis of 25-hydroxy vitamin D and serum asprosin levels indifferent groups
By grouping patients into three quantiles of 25-hydroxy vitamin D (All patients: T1 < 26.2 nmol/L, T2 26.2~35.5 nmol/L, T3 > 35.5 nmol/L; Male: T1 < 263.3nmol/L, T2 23.3~36.7 nmol/L, T3 > 36.7 nmol/L;Female:T1 < 25.3 nmol/L, T2 25.3~40.8 nmol/L, T3 > 40.8 nmol/L);, a general linear regression model was used with serum asprosin as the dependent variable. Mean serum asprosin levels (95% CI) for T1, T2, and T3 were 360.6 (346.2, 375.5), 328.4 (315.5, 341.3), and 324.0 (309.8, 338.2) in all patients, Mean serum asprosin levels (95% CI) for T1, T2, and T3 were 374.1 (352.1, 396.0, 337.1 (323.4, 350.8), and 351.6 (330.5 372.6) in male patients, Mean serum asprosin levels (95% CI) for T1, T2, and T3 were 354.8 (324.2, 385.4), 322.8 (306.6, 338.9), and 284.6 (259.1, 309.9) in female patients, respectively. Compared to the T1 group, serum asprosin levels were significantly reduced in the other two groups between all patients and female patients (P < 0.01). As 25-hydroxy vitamin D levels increased from T1 to T3, mean serum asprosin gradually decreased (Figure 1). Compared to T2 groups, serum asprosin levels were significantly increased in male patients (Figure 1).
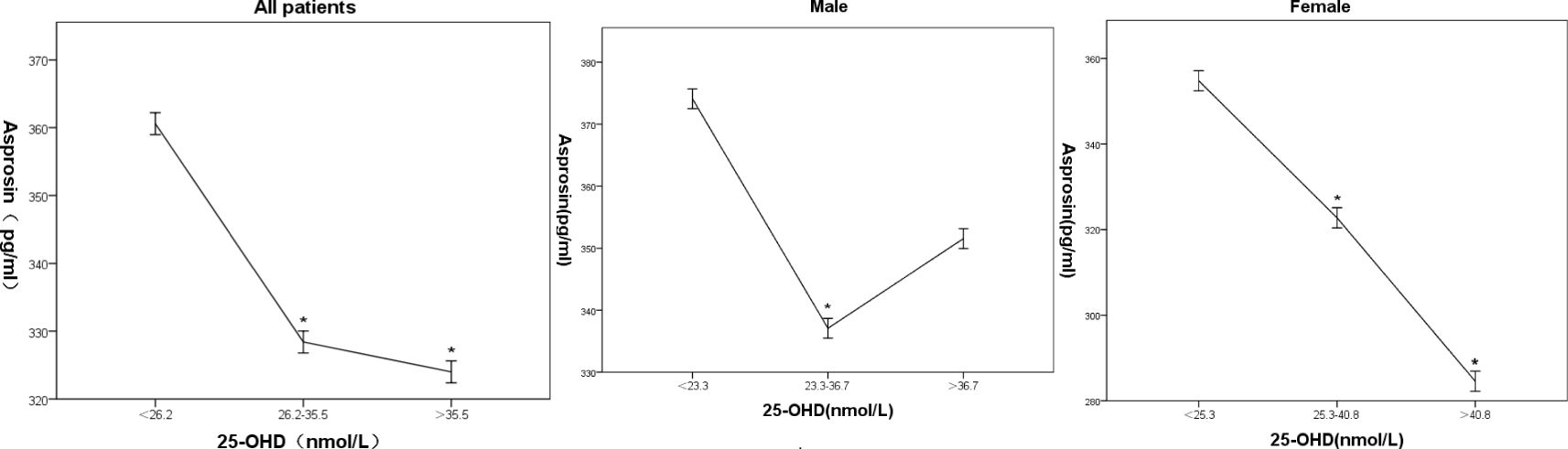
Figure 1 Association of 25-hydroxy Vitamin D with Serum Asprosin in Patients with Type 2 Diabetes in all, male and female patients. Mean serum asprosin and 95% confidence intervals corresponding to three quantiles of 25-hydroxyvitamin D; * indicates statistical significance compared to the first quantile array (*P < 0.05).
Discussion
The findings of this study demonstrated that in community-dwelling patients with type 2 diabetes, there was a gradual increase in age, disease duration, SBP, BMI, WC, creatinine, and SUA levels with the rise in serum asprosin levels. Conversely, serum asprosin displayed an inverse correlation with 25-hydroxy vitamin D levels, indicating a gradual decrease in serum asprosin as 25-hydroxy vitamin D levels increased.
25-hydroxy vitamin D deficiency has been identified as a common underlying factor for metabolic disorders like obesity, type 2 diabetes, and hypertension (9–12). Asprosin, a novel protein hormone found in white adipose tissue, has been shown in animal studies to exhibit elevated serum levels in mammals with insulin resistance (1, 13). In population-based investigations involving polycystic ovary syndrome patients and women with type 2 diabetes, higher serum asprosin levels were identified compared to healthy individuals (14). The present study revealed that heightened serum asprosin levels were associated with indicators linked to insulin resistance, including elevated BMI and WC levels, as well as decreased blood glucose and 25-hydroxy vitamin D levels. Correlation analysis further indicated a positive association between serum asprosin and BMI, as well as TG, while a negative correlation existed between serum asprosin and 25-hydroxy vitamin D. These findings suggest a potential connection between increased serum asprosin levels, decreased 25-hydroxy vitamin D, and insulin resistance in community-based patients with type 2 diabetes. Notably, patients with serum asprosin > 370.5 pg/ml displayed a higher proportion receiving oral hypoglycemic drugs and insulin-lowering therapy, possibly contributing to lower blood glucose levels, which aligns with findings by Wang (15).
The potential mechanism underlying the link between 25-hydroxy vitamin D and asprosin can be understood as follows (16–21). Firstly, Adipokines and adipose tissue may be a direct target for vitamin D. The expression of both the vitamin D receptor and 25-hydroxyvitamin D 1α-hydroxylase (CYP27B1) genes has been demonstrated in murine and human adipocytes. Secondly, Vitamin D metabolites might influence an increased asprosin production. Furthermore, Vitamin D may also be involved in the regulation of asprosin levels through modification of insulin sensitivity. The study’s findings revealed a negative correlation between serum asprosin and 25-hydroxy vitamin D levels in patients with type 2 diabetes, but this correlation was observed only within a certain range of 25-hydroxy vitamin D levels. The underlying cause remains uncertain and necessitates confirmation through further investigations. One hypothesis is that low stimulation of fat cells by 25-hydroxy vitamin D might contribute to conditions like obesity and insulin resistance. This could lead to elevated inflammatory factors like tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1), ultimately causing an imbalance in adipocytokine levels (22).
Type 2 diabetes and obesity have a comorbidity mechanism, which is related to 25 hydroxyvitamin D deficiency, and may affect the synthesis and metabolism of 25 hydroxyvitamin D through inflammation and oxidative stress. White adipose tissue is mainly produced by white adipocytes, and we speculate that obesity may indirectly affect the level of 25 hydroxyvitamin D by affecting the production of white adipose tissue.
Several limitations should be acknowledged in this study. Firstly, geographic factors, sample size, and population characteristics could have introduced confounding influences, and there might be unaccounted variables. Additionally, this study has a cross-sectional design, the relationship between 25-hydroxy vitamin D and serum asprosin might be bidirectional and interacting, necessitating further confirmation through prospective studies. Therefore, causal relationships between 25-hydroxy vitamin D and serum asprosin cannot be established solely from this study. Secondly, the assessment of islet function and insulin resistance index was lacking in this community-based study of patients with type 2 diabetes. Lastly, diet control and sunlight conditions during the research period were not evaluated in this study, so the effect on asprosin could not be more clearly reflected, which is also a limitation of this study”.
In addition, we found that with an increase in 25-OHD levels, the trend of serum asprosin levels in males and females was different. Currently, there are no reports on 25-OHD and asprosin levels, and we believe it may be related to changes in estrogen levels. Further confirmation is needed in other studies to determine whether there is a gender difference in the effect of 25-OHD on asprosin levels.
In conclusion, further investigation is warranted to understand the intricate regulatory role of 25-hydroxy vitamin D on adipocytokines, particularly its potential variations between health and disease states. This study underscored an inverse correlation between serum asprosin and 25-hydroxy vitamin D levels among patients with type 2 diabetes in the community.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the First Hospital of Shanxi Medical University (Approval number: 2019 [K056]). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JC: Conceptualization, Formal analysis, Methodology, Software, Validation, Writing – original draft. ZW: Data curation, Formal analysis, Methodology, Software, Writing – original draft. JYi: Data curation, Writing – original draft. MLi: Methodology, Writing – original draft. QW: Investigation, Writing – original draft. MLiu: Investigation, Writing – original draft. HS: Validation, Writing – original draft. HR: Visualization, Writing – original draft. MX: Investigation, Writing – original draft. JYa: Funding acquisition, Supervision, Writing – review & editing. LX: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the China Diabetes Research Fund, China Foundation for International Medical Exchanges (Z-2017-26-2202-4). Shanxi Provincial Health(2021050).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Romere C, Duerrschmid C, Bournat J, Constable P, Jain M, Xia F, et al. Asprosin, a fasting-induced glucogenic protein hormone. Cell. (2016) 165:566–79. doi: 10.1016/j.cell.2016.02.063
2. Wang Y, Qu H, Xiong X, Qiu Y, Liao Y, Chen Y, et al. Plasma asprosin concentrations are increased in individuals with glucose dysregulation and correlated with insulin resistance and first-phase insulin secretion. Med Inflam. (2018) 2018:9471583. doi: 10.1155/2018/9471583
3. Zhang L, Chen C, Zhou N, Fu Y, Cheng X. Circulating asprosin concentrations are increased in type 2 diabetes mellitus and independently associated with fasting glucose and triglyceride. Clin Chim Acta. (2019) 489:183–8. doi: 10.1016/j.cca.2017.10.034
4. Ko JR, Seo DY, Kim TN, Park SH, Kwak HB, Ko KS, et al. Aerobic exercise training decreases hepatic asprosin in diabetic rats. J Clin Med. (2019) 8:666. doi: 10.3390/jcm8050666
5. Gao Y, Wu X, Fu Q, Li Y, Yang T, Tang W. The relationship between serum 25-hydroxy vitamin D and insulin sensitivity and beta-cell function in newly diagnosed type 2 diabetes. J Diabetes Res. (2015) 2015:636891. doi: 10.1155/2015/636891
6. Pittas AG, Sun Q, Manson JE, Dawson-Hughes B, Hu FB. Plasma 25-hydroxyvitamin D concentration and risk of incident type 2 diabetes in women. Diabetes Care. (2010) 33:2021–3. doi: 10.2337/dc10-0790
7. Buczyńska A, Sidorkiewicz I, Ławicki S, Krętowska AJ, Zbucka-Krętowska M. Prenatal screening of trisomy 21: could oxidative stress markers play a role? J Clin Med. (2021) 10:2382. doi: 10.3390/jcm10112382
8. Xu L, Cui J, Li M, Wu Q, Liu M, Xu M, et al. Mellitus in the community: A cross-sectional nephropathy in patients with type 2 association between serum asprosin and diabetic diabetes study. Diabetes Metab Syndr Obes: Targets Ther. (2022) 15:1877–84. doi: 10.2147/DMSO.S361808
9. Defronzo RA. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. (2009) 58:773–95. doi: 10.2337/db09-9028
10. Czech MP. Insulin action and resistance in obesity and type 2 diabetes. Nat Med. (2017) 23:804–14. doi: 10.1038/nm.4350
11. Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. (2004) 89:2548–56. doi: 10.1210/jc.2004-0395
12. Piya MK, McTernan PG, Kumar S. Adipokine inflammation and insulin resistance: the role of glucose, lipids and endotoxin. J Endocrinol. (2013) 216:T1–T15. doi: 10.1530/JOE-12-0498
13. Duerrschmid C, He Y, Wang C, Li C, Bournat JC, Romere C, et al. Asprosin is a centrally acting orexigenic hormone. Nat Med. (2017) 23:1444–53. doi: 10.1038/nm.4432
14. Li X, Liao M, Shen R, Zhang L, Hu H, Wu J, et al. Plasma Asprosin Levels are associated with glucose metabolism, lipid, and sex hormone profiles in females with metabolic-related diseases. Mediators Inflammation. (2018) 2018:7375294. doi: 10.1155/2018/7375294
15. Wang R, Lin P, Sun H, Hu W. Increased serum asprosin is correlated with diabetic nephropathy. Diabetol Metab Syndr. (2021) 13:51. doi: 10.1186/s13098-021-00668-x
16. Ching S, Kashinkunti S, Niehaus MD, Zinser GM. Mammary adipocytes bioactivate 25-hydroxyvitamin D and signal via vitamin D receptor, modulating mammary epithelial cell growth. J Cell Biochem. (2011) 112:3393–405. doi: 10.1002/jcb.23273
17. Li J, Byrne ME, Chang E, Jiang Y, Donkin SS, Buhman KK, et al. 1alpha,25-Dihydroxyvitamin D hydroxylase in adipocytes. J Steroid Biochem Mol Biol. (2008) 112:122–6. doi: 10.1016/j.jsbmb.2008.09.006
18. Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. (2005) 115:911–20. doi: 10.1016/j.jaci.2005.02.023
19. Al-Daghri NM, Al-Attas OS, Alokail MS, Alkharfy KM, Al-Othman A, Draz HM, et al. Hypovitaminosis D associations with adverse metabolic parameters are accentuated in patients with Type 2 diabetes mellitus: a body mass index-independent role of adiponectin? J Endocrinol Invest. (2013) 36:1–6. doi: 10.3275/8183
20. Gannagé-Yared MH, Chedid R, Khalife S, Azzi E, Zoghbi F, Halaby G. Vitamin D in relation to metabolic risk factors, insulin sensitivity and adiponectin in a young middle-eastern population. Eur J Endocrinol. (2009) 160:965–71. doi: 10.1530/EJE-08-0952
21. Szymczak-Pajor I, Drzewoski J, Śliwińska A. The molecular mechanisms by which Vitamin D Prevents insulin resistance and associated disorders. Int J Mol Sci. (2020) 21:6644. doi: 10.3390/ijms21186644
Keywords: diabetes, type 2, asprosin, 25-hydroxy vitamin D, community, insulin resistance
Citation: Cui J, Wang Z, Yin J, Li M, Wu Q, Liu M, Su H, Ren H, Xu M, Yang J and Xu L (2024) The relationship between 25-hydroxy vitamin D and serum asprosin in patients with type 2 diabetes in the community. Front. Endocrinol. 15:1409156. doi: 10.3389/fendo.2024.1409156
Received: 29 March 2024; Accepted: 16 July 2024;
Published: 31 July 2024.
Edited by:
Mohammed S. Razzaque, The University of Texas Rio Grande Valley, United StatesReviewed by:
Haluk Kelestimur, Firat University, TürkiyeMagdalena Wiecek, University School of Physical Education in Krakow, Poland
Copyright © 2024 Cui, Wang, Yin, Li, Wu, Liu, Su, Ren, Xu, Yang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Yang, eWFuZ2psbUAxMjYuY29t; Linxin Xu, eHVsaW54aW5fNTE4QDE2My5jb20=
†These authors have contributed equally to this work
 Junfang Cui1,2†
Junfang Cui1,2† Jing Yang
Jing Yang Linxin Xu
Linxin Xu