
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol., 11 June 2024
Sec. Pituitary Endocrinology
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1407615
This article is part of the Research TopicCancer Risk in Patients with Acromegaly – is Extensive Screening Needed?View all 6 articles
Acromegaly is a rare endocrine disorder caused by hypersecretion of growth hormone (GH) from a pituitary adenoma. Elevated GH levels stimulate excess production of insulin-like growth factor 1 (IGF-1) which leads to the insidious onset of clinical manifestations. The most common primary central nervous system (CNS) tumors, meningiomas originate from the arachnoid layer of the meninges and are typically benign and slow-growing. Meningiomas are over twice as common in women as in men, with age-adjusted incidence (per 100,000 individuals) of 10.66 and 4.75, respectively. Several reports describe co-occurrence of meningiomas and acromegaly. We aimed to determine whether patients with acromegaly are at elevated risk for meningioma. Investigation of the literature showed that co-occurrence of a pituitary adenoma and a meningioma is a rare phenomenon, and the majority of cases involve GH-secreting adenomas. To the best of our knowledge, a systematic review examining the association between meningiomas and elevated GH levels (due to GH-secreting adenomas in acromegaly or exposure to exogenous GH) has never been conducted. The nature of the observed coexistence between acromegaly and meningioma -whether it reflects causation or mere co-association -is unclear, as is the pathophysiologic etiology.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42022376998.
Acromegaly is a rare endocrine disorder caused by hypersecretion of growth hormone (GH) from a pituitary adenoma. Elevated GH levels stimulate excess production of insulin-like growth factor 1 (IGF-1) which leads to the insidious onset of clinical manifestations (1).
The most common primary central nervous system (CNS) tumors, meningiomas originate from the arachnoid layer of the meninges and are typically benign and slow-growing. Meningiomas are over twice as common in women as in men, with age-adjusted incidence (per 100,000 individuals) of 10.66 and 4.75, respectively (2).
Several reports describe co-occurrence of meningiomas and acromegaly (3). We aimed to determine whether patients with acromegaly are at an elevated risk for meningioma. Investigation of the literature showed that co-occurrence of a pituitary adenoma and a meningioma is a rare phenomenon, and the majority of cases involve GH-secreting adenomas. To the best of our knowledge, a systematic review examining the association between meningiomas and elevated GH levels (due to GH-secreting adenomas in acromegaly or exposure to exogenous GH) has never been conducted.
The nature of the observed coexistence between acromegaly and meningioma – whether it reflects causation or mere co-association – is unclear, as is the pathophysiologic etiology. Meningiomas express both GH and IGF-1 receptors as indicated by in vitro and in vivo studies in mice (4). These receptors could potentially be therapeutic targets for GH antagonists such as pegvisomant (5, 6). In addition, somatostatin receptors – particularly subtype-2 (SSTR2) – are highly expressed in nearly all human meningiomas (7). I am editing the texting again as I did in my draft I sent back – please make these changes.
The objective of this systematic review was to study the prevalence of intracranial meningiomas in patients with GH-secreting pituitary adenomas or exposure to exogenous GH therapy. Furthermore, this review aimed to identify patient and tumor factors that predict the development of metachronous or synchronous intracranial meningiomas in patients with acromegaly.
This systematic review was registered on Prospero (ID CRD42022376998) and conducted with adherence to PRISMA statement guidelines.
Studies eligible for inclusion met the following criteria: 1) discussion of both GH (e.g., secreting tumors, replacement, treatment) and meningioma, 2) written in English or with available English translation, and 3) published in a peer-reviewed journal. There was no time limitation for included studies. Animal studies, studies published in non-English languages, and systematic reviews were excluded from the review.
Searches were conducted using PubMed, EMBASE, and Web of Science. The following search terms were used: “growth hormone” OR “acromegaly” OR “growth hormone-secreting pituitary adenoma” OR “growth hormone secreting” OR “growth hormone producing” OR “growth hormone hypersecretion” OR “GH secreting” OR “GH producing” OR “GH hypersecretion” AND “meningioma” OR “meningiomas” OR “meningeal tumor” OR “meningeal tumors” OR “meningeal neoplasm” OR “meningeal neoplasms”.
All abstracts identified in the initial literature search were screened for relevance by two independent investigators. The full text of selected articles was then reviewed to assess the eligibility of the study for inclusion in this systematic review. Data extraction was performed using Microsoft Excel. Information extracted included study location, study design, years including follow up, number (n) of individuals, % male, mean age. Patient factors extracted included genetics, family predisposition, comorbidities, radiation history, symptomatology, biochemical levels (e.g., GH, IGF-1), imaging modality and findings, surgical intervention, tumor remission/recurrence, and pathology. Data were synthesized and analyzed using linear regression analysis functions in Microsoft Excel and are presented in Table 1 an elevated risk.
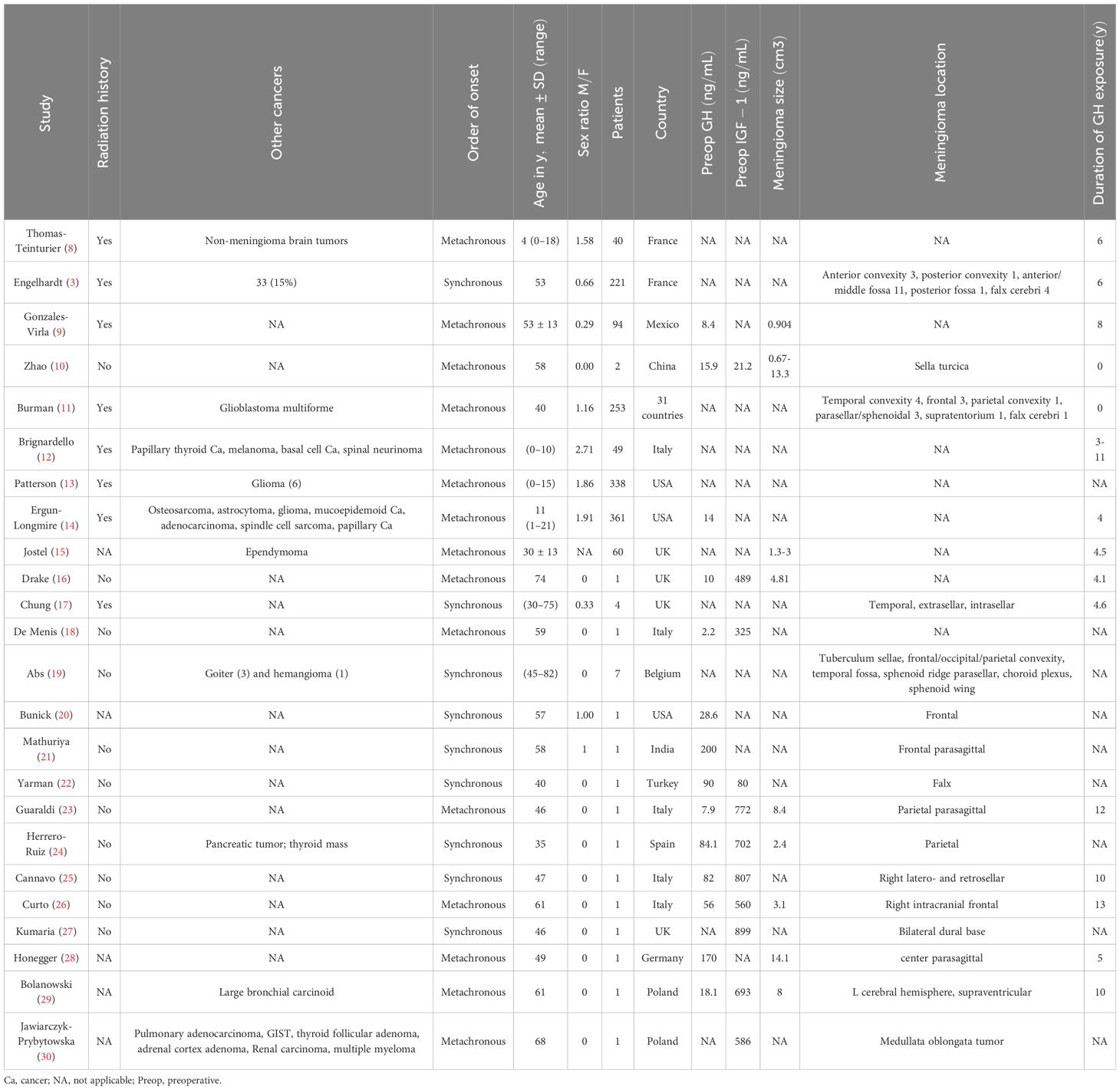
Table 1 Study information and patient factors for the 24 studies included in this systematic review.
Additional patients were included in a separate analysis using unpublished data from the investigators’ affiliated medical institution, NYU Langone Medical Center in Manhattan, New York. In conducting this analysis, we focused solely on patients with a diagnosis of both acromegaly and intracranial meningioma. Relevant cases were identified by searching the Department of Neurosurgery’s database of consecutive patients who had undergone resection of GH-secreting pituitary adenomas at NYU from December 2013 through March 2022. The electronic medical records (EMRs) of these patients were screened for imaging or chart evidence of meningioma. Additional cases were identified by searching through the database of patients with acromegaly currently or previously managed in NYU’s outpatient endocrinology clinics. The EMRs of these patients were screened for imaging or chart evidence of meningioma. We assessed 54 patients in the NYU system and identified 4 as having a diagnosis of both acromegaly and intracranial meningioma (Table 2). This portion of the study was approved by the IRB of NYU.
Analysis of data from literature review showed that GH and IGF-1 levels did not have strong correlations with meningioma size (Figure 1). There was a weak positive correlation between IGF-1 level and GH basal level and meningioma size, but the sample size was relatively small even with integration of NYU patient data (Figures 2, 3).
Roughly half of the patients included in the review developed meningioma after acromegaly (metachronous), and the other half were diagnosed with meningioma and acromegaly simultaneously (synchronous). (Table 1). For the metachronous patients in the studies that also reported their meningioma sizes and GH exposure years, we explored the correlation between the years elapsed between the two diagnoses and the size of the meningioma but discovered no clear correlation (Figure 4). However, this analysis was also limited by the small size of the study.
The locations of the meningiomas that co-occurred with acromegaly did not follow an obvious pattern (Tables 1, 2). The location of the development of meningioma seemed to randomly occur from frontal to parietal lobe and from falx to sphenoid wing.
Data from NYU patients (Table 2) followed the same pattern observed in the literature; half of the patients had synchronous meningioma and acromegaly and half had metachronous presentation. The locations of meningiomas did not follow any specific pattern. Due to the scarcity of data, not many conclusions can be drawn regarding IGF-1 and GH levels and meningioma size.
Researching the incidence of meningioma in a rare cohort of acromegaly patients is a challenging task. This systematic review studies the prevalence of meningiomas in patients with GH excess. The review is limited by -patient case reports, making it difficult to identify a causal association. Assessment of possible causality is further limited by the use of radiation in a large cohort of aggressive GH-producing pituitary adenomas, which poses a confounding risk for the development of intracranial meningiomas. While previous case reports comment on a synchronous and metachronous development of meningiomas, the timing of onset is difficult to verify given the insidious disease course and delay in acromegaly diagnosis.
Surgery is the first line of treatment in patients with symptomatic or compressive meningiomas. However, tumors deemed surgically unresectable owing to location or extension into vital neurovascular structures may require radiotherapy (31). While there are currently no approved medications for treatment of meningiomas, the use of targeted therapy to help with recurrent or extensive meningiomas is certainly an attractive concept.
The strong expression of SSTR2 is well documented in intracranial meningiomas. Another pathway with a dominant role in meningioma development is that of Pi3K/Akt/mTOR (32, 33). Loss of function of the Merlin protein – a negative regular of mTOR encoded by the NF2 gene – leads to overactivation of this pathway and subsequent meningioma oncogenesis. Inactivation of the NF2 gene is one of the primary chromosomal changes observed in meningioma cells (34). Activation of SSTR2 can downregulate the Pi3K/Akt pathway. One agent with such action is octreotide, a somatostatin analog currently used to suppress tumor activity in acromegaly and other neuroendocrine tumors. Pasireotide is a newer agent with greater affinity for the SSTR5 receptor (35). In a study by Graillon el al.comparingthe effects of pasireotide to those of octreotide in vitro on meningioma primary cell cultures, combining an SST analog with an mTOR inhibitor like everolimus has demonstrated efficacy in treating aggressive meningiomas. These two agents together impose a strong synergistic inhibitory effect on oncogenic pathways in meningioma cells (36).
This phenomenon raises an important confounding variable in our study: exposure to somatostatin analogs in our study populations. Somatostatin Receptor Analogs (SRLs) are commonly used as adjuvant therapy in patients with acromegaly experiencing residual disease following neurosurgical resection of the adenoma. SRLs are even used as first-line therapy in certain cases of high surgical risk or low likelihood of surgical cure due to extrasellar tumor extension (37). The use of SRL, octreotide was not disclosed in any of the articles studied in the review, but it is highly likely that several of the patients in these studies were treated with octreotide, given how commonly this agent is used in acromegaly therapy. This possibility is important to note because SRLs can interfere with the natural history of meningiomas by downregulating the Pi3K/Akt pathway. One of the four NYU patients received a trial of octreotide for treatment of acromegaly approximately 24 years prior to meningioma diagnosis. However, we cannot draw meaningful conclusions regarding the effect of SRLs on meningioma development on the basis of a single patient.
Our review suggests that hereditary cancer syndromes might have a role in the co-occurrence of acromegaly and meningiomas. In nearly half of the articles studied, patients reported additional tumors (separate from pituitary adenoma and meningioma) including papillary thyroid cancer, osteosarcoma, hemangioma, skin tumors, and other CNS tumors such as glioblastoma, astrocytoma, and ependymoma. The presence of multiple tumor diagnoses raises the possibility that patients in this cohort were afflicted by familial cancer syndromes. A study by Engelhardt et al. showed that acromegaly patients with multiple endocrine neoplasia type 1 (MEN-1) were at significantly higher risk of developing a meningioma (11.7% vs 7%) compared to patients without this inherited disorder (3). A separate study by Asgharian demonstrated that meningiomas were 11 times more common in patients with Zollinger-Ellison syndrome (ZES) and MEN-1 than in patients with ZES alone (38). These findings suggest that hereditary cancer syndromes such as MEN-1 may have a potentiating effect on the relationship between meningiomas and GH-secreting pituitary adenomas.
In this literature review, no significant correlation was found between GH or IGF-1 level and meningioma size. GH and IGF-1 receptors are expressed in 88-94% of human meningiomas, across all histological grades (4). For this reason, IGF-1 upregulation is thought to be the primary mechanism underlying the co-occurrence of GH-secreting pituitary adenomas and intracranial meningiomas. However, the findings of this study raise the possibility that a different biologic mechanism might be at play. The various other receptors commonly expressed in meningioma cells – and their interplay with the metabolic consequences of acromegaly – should also be considered. These immunohistochemical markers include progesterone receptors, vascular endothelial growth factor, and caspase-3, which are detected in 87%, 69%, and 30% of meningiomas, respectively (4).
While no clear correlation between GH or IGF-1 level and meningioma size was identified in our review, previous studies have suggested that these hormones play a dominant role in the pathogenesis of meningiomas. Puduvalli et al. examined the in vitro action of fenretinide, a synthetic retinoid, against primary meningioma cultures and discovered that one mechanism by which this agent induces apoptosis in tumor cells is via inhibition of IGF-1-induced proliferation (39). In that study, meningioma cells were treated with IGF-1 alone, fenretinide alone, or a combination of both agents. A dose-dependent increase in cell proliferation was seen with IGF-1 compared to untreated controls. In the combination cultures, fenretinide suppressed the proliferative effect of IGF-1 on tumor cells. Thus, it is feasible that the correlation between IGF-1 level and meningioma size – a relationship well-established in prior literature – is dampened in our review by the effect of agents like retinoids and other undisclosed chemicals in our pooled patient population. This possibility highlights the importance of exploring the potential role of other pathophysiologic mechanisms.
Conflicting reports of the role of GH and IGF-1 receptors in meningioma oncogenesis are found in the literature. Blockage of these receptors (e.g., with the GH receptor antagonist pegvisomant) slows the growth of meningioma cells in both in vitro and in vivo mice studies (5, 6). This observation suggests that upregulation of IGF-1 due to GH hypersecretion in acromegaly may induce the appearance and growth of meningiomas. However, in one case report of a patient with acromegaly and meningioma (metachronously diagnosed one decade apart), volume of the meningioma increased over 10-fold during a 5-year period of treatment with pegvisomant (16).
Radiation-induced meningiomas are a very delayed adverse effect of brain irradiation. One study of 253 patients identified a mean latency period of 36 years between initial radiation exposure and meningioma diagnosis (40). The vast majority of patients were exposed in early childhood. Another study of 49 survivors of childhood leukemia treated with cranial irradiation found that 22% developed meningiomas as adults, with a mean latency period of 26 years (41). In the two metachronous NYU patients, one was diagnosed with the two tumors 8 years apart and the other was diagnosed 24 years apart. Neither patient had any reported history of radiation exposure. Acromegaly is typically diagnosed in the fifth decade of life, and meningioma is most often diagnosed in the sixth decade of life. Given the long latency period, it is unlikely that use of radiation to treat a pituitary tumor would lead to growth of meningioma a decade later. Thus, the effect of this confounding factor can be presumed to be minimal at most however larger studies are required to answer this question definitively.
Implications of a larger-scale replication study could be far-reaching, extending from screening guidelines to pharmacologic breakthroughs. Given that other studies have demonstrated higher incidence of intracranial meningiomas in patients with acromegaly compared to the general population, it is reasonable to adjust current clinical practice guidelines for acromegaly to include screening for meningioma at time of diagnosis and regular intervals thereafter (3). As magnetic resonance imaging of the brain is already a common part of the diagnostic workup for acromegaly, adding this recommendation to the guidelines should not add a significant cost burden. Still to be determined, however, is an appropriate frequency and duration (even after remission is achieved) of screening for meningioma in patients with acromegaly. Although this study was unable to ascertain the pathophysiologic etiology of the co-occurrence of these two tumors, further investigation into these mechanisms could revolutionize treatment for both conditions. A potential consideration is the use of SRLs and GH receptor antagonists for treatment of intracranial meningiomas based on future prospective studies.
An important limitation of our study pertains to radiation exposure Ionizing radiation is a strong risk factor for meningiomas and other CNS neoplasms (35). For patients with metachronous patterns of acromegaly and meningioma, interim exposure to cranial irradiation may confound the relationship between these two tumors. Though not the primary treatment modality, pituitary irradiation is often used in the management of GH-secreting pituitary adenomas, particularly in cases that are refractory to first-line surgical and pharmacologic interventions (42). Few of the studies in our review disclosed information regarding radiation exposure, making it difficult to ascertain how substantial a role this factor played in the findings we observed.
The main limitation of this study is the relatively small sample size of pooled patients in our systematic analysis, which is due in part to the overall rarity of co-occurrence of these two tumors in the general population. The correlation between the level of GH and size of meningioma is difficult to study in such a small sample. There is a lack of high-quality, large cohort studies examining how GH and GH inhibitors affect meningioma growth. Such studies could provide valuable data to study the biological relationship between these two conditions.
Despite the limitations of the small sample size of the study there could be a positive correlation between GH and IGF-1 levels and meningiomas in patients with coexisting tumors. Future prospective studies in larger groups of patients can help delineate the mechanism of this correlation and helping with management of this rare condition.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
AG: Data curation, Methodology, Writing – original draft, Writing – review & editing, Investigation, Formal analysis. AJ: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. DP: Data curation, Validation, Resources, Project administration, Writing – review & editing. NA: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing, Conceptualization, Project administration, Resources, Supervision, Validation.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Principal investigator NA is on the advisory board for Xeris, Amryt, Camurus and on research trials with Amryt, Recordati, Ascendis.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Lavrentaki A, Paluzzi A, Wass JAH, Karavitaki N. Epidemiology of acromegaly: review of population studies. Pituitary. (2017) 20:4–9. doi: 10.1007/s11102-016-0754-x
2. Lin D, Lin J, Deng X, Li W, Li D, Yin B, et al. Trends in intracranial meningioma incidence in the United States, 2004-2015. Cancer Med. (2019) 8:6458–67. doi: 10.1002/cam4.2516
3. Engelhardt J, Nunes ML, Pouchieu C, Ferrière A, San-Galli F, Gimbert E, et al. Increased incidence of intracranial meningiomas in patients with acromegaly. Neurosurgery. (2020) 87:639–46. doi: 10.1093/neuros/nyz438
4. Baxter DS, Orrego A, Rosenfeld JV, Mathiesen T. An audit of immunohistochemical marker patterns in meningioma. J Clin Neurosci Off J Neurosurg Soc Australas. (2014) 21:421–6. doi: 10.1016/j.jocn.2013.06.008
5. Friend KE, Radinsky R, McCutcheon IE. Growth hormone receptor expression and function in meningiomas: effect of a specific receptor antagonist. J Neurosurg. (1999) 91:93–9. doi: 10.3171/jns.1999.91.1.0093
6. McCutcheon IE, Flyvbjerg A, Hill H, Li J, Bennett WF, Scarlett JA, et al. Antitumor activity of the growth hormone receptor antagonist pegvisomant against human meningiomas in nude mice. J Neurosurg. (2001) 94:487–92. doi: 10.3171/jns.2001.94.3.0487
7. Wu W, Zhou Y, Wang Y, Liu L, Lou J, Deng Y, et al. Clinical significance of somatostatin receptor (SSTR) 2 in meningioma. Front Oncol. (2020) 10:1633. doi: 10.3389/fonc.2020.01633
8. Thomas-Teinturier C, Oliver-Petit I, Pacquement H, Fresneau B, Allodji RS, Veres C, et al. Influence of growth hormone therapy on the occurrence of a second neoplasm in survivors of childhood cancer. Eur J Endocrinol. (2020) 183:471–80. doi: 10.1530/EJE-20-0369
9. Gonzales-Virla B, Vargas-Ortega G, Martínez-Vázquez KB, de lo Monteros ALE, Sosa-Erosa E, López-Félix B, et al. Efficacy and safety of fractionated conformal radiation therapy in acromegaly: a long-term follow-up study. Endocrine. (2019) 65:386–92. doi: 10.1007/s12020-019-01955-4
10. Zhao Y, Zhang H, Lian W, Xing B, Feng M, Liu X, et al. Collision tumors composed of meningioma and growth hormone-secreting pituitary adenoma in the sellar region: Case reports and a literature review. Med (Baltimore). (2017) 96:e9139. doi: 10.1097/MD.0000000000009139
11. Burman P, van Beek AP, Biller BMK, Camacho-Hübner C, Mattsson AF. Radiotherapy, especially at young age, increases the risk for de novo brain tumors in patients treated for pituitary/sellar lesions. J Clin Endocrinol Metab. (2017) 102:1051–8. doi: 10.1210/jc.2016-3402
12. Brignardello E, Felicetti F, Castiglione A, Fortunati N, Matarazzo P, Biasin E, et al. GH replacement therapy and second neoplasms in adult survivors of childhood cancer: a retrospective study from a single institution. J Endocrinol Invest. (2015) 38:171–6. doi: 10.1007/s40618-014-0179-1
13. Patterson BC, Chen Y, Sklar CA, Neglia J, Yasui Y, Mertens A, et al. Growth hormone exposure as a risk factor for the development of subsequent neoplasms of the central nervous system: a report from the childhood cancer survivor study. J Clin Endocrinol Metab. (2014) 99:2030–7. doi: 10.1210/jc.2013-4159
14. Ergun-Longmire B, Mertens AC, Mitby P, Qin J, Heller G, Shi W, et al. Growth hormone treatment and risk of second neoplasms in the childhood cancer survivor. J Clin Endocrinol Metab. (2006) 91:3494–8. doi: 10.1210/jc.2006-0656
15. Jostel A, Mukherjee A, Hulse PA, Shalet SM. Adult growth hormone replacement therapy and neuroimaging surveillance in brain tumour survivors. Clin Endocrinol (Oxf). (2005) 62:698–705. doi: 10.1111/j.1365-2265.2005.02282.x
16. Drake WM, Grossman AB, Hutson RK. Effect of treatment with pegvisomant on meningioma growth in vivo. Eur J Endocrinol. (2005) 152:161–2. doi: 10.1530/eje.1.01825
17. Chung TT, Drake WM, Evanson J, Walker D, Plowman PN, Chew SL, et al. Tumour surveillance imaging in patients with extrapituitary tumours receiving growth hormone replacement. Clin Endocrinol (Oxf). (2005) 63:274–9. doi: 10.1111/j.1365-2265.2005.02338.x
18. De Menis E, Tulipano G, Villa S, Billeci D, Bonfanti C, Pollara P, et al. Development of a meningioma in a patient with acromegaly during octreotide treatment: are there any causal relationships? J Endocrinol Invest. (2003) 26:359–63. doi: 10.1007/BF03345185
19. Abs R, Parizel PM, Willems PJ, Van de Kelft E, Verlooy J, Mahler C, et al. The association of meningioma and pituitary adenoma: report of seven cases and review of the literature. Eur Neurol. (1993) 33:416–22. doi: 10.1159/000116986
20. Bunick EM, Mills LC, Rose LI. Association of acromegaly and meningiomas. JAMA. (1978) 240:1267–8. doi: 10.1001/jama.240.12.1267
21. Mathuriya SN, Vasishta RK, Dash RJ, Kak VK. Pituitary adenoma and parasagittal meningioma: an unusual association. Neurol India. (2000) 48:72–4.
22. Yarman S, Minareci O. Value of petrosal sinus sampling: coexisting acromegaly, empty sella and meningioma. Neuroradiology. (2004) 46:1027–30. doi: 10.1007/s00234-003-0993-1
23. Guaraldi F, Corazzini V, Gallia GL, Grottoli S, Stals K, Dalantaeva N, et al. Genetic analysis in a patient presenting with meningioma and familial isolated pituitary adenoma (FIPA) reveals selective involvement of the R81X mutation of the AIP gene in the pathogenesis of the pituitary tumor. Pituitary. (2012) 15 Suppl 1:S61–67. doi: 10.1007/s11102-012-0391-y
24. Herrero-Ruiz A, Villanueva-Alvarado HS, Corrales-Hernández JJ, Higueruela-Mínguez C, Feito-Pérez J, Recio-Cordova JM. Coexistence of GH-producing pituitary macroadenoma and meningioma in a patient with multiple endocrine neoplasia type 1 with hyperglycemia and ketosis as first clinical sign. Case Rep Endocrinol. (2017) 2017:2390797. doi: 10.1155/2017/2390797
25. Cannavò S, Curtò L, Fazio R, Paterniti S, Blandino A, Marafioti T, et al. Coexistence of growth hormone-secreting pituitary adenoma and intracranial meningioma: a case report and review of the literature. J Endocrinol Invest. (1993) 16:703–8. doi: 10.1007/BF03348915
26. Curto L, Squadrito S, Almoto B, Longo M, Granata F, Salpietro F, et al. MRI finding of simultaneous coexistence of growth hormone-secreting pituitary adenoma with intracranial meningioma and carotid artery aneurysms: report of a case. Pituitary. (2007) 10:299–305. doi: 10.1007/s11102-007-0011-4
27. Kumaria A, Scott IS, Robertson IJ. An unusual pituitary adenoma coexistent with bilateral meningiomas: case report. Br J Neurosurg. (2019) 33:579–80. doi: 10.1080/02688697.2017.1386283
28. Honegger J, Buchfelder M, Schrell U, Adams EF, Fahlbusch R. The coexistence of pituitary adenomas and meningiomas: three case reports and a review of the literature. Br J Neurosurg. (1989) 3:59–69. doi: 10.3109/02688698909001027
29. Bolanowski M, Schopohl J, Marciniak M, Rzeszutko M, Zatonska K, Daroszewski J, et al. Acromegaly due to GHRH-secreting large bronchial carcinoid. Complete recovery following tumor surgery. Exp Clin Endocrinol Diabetes Off J Ger Soc Endocrinol Ger Diabetes Assoc. (2002) 110:188–92. doi: 10.1055/s-2002-32151
30. Jawiarczyk-Przybyłowska A, Wojtczak B, Whitworth J, Sutkowski K, Bidlingmaier M, Korbonits M, et al. Acromegaly associated with GIST, non-small cell lung carcinoma, clear cell renal carcinoma, multiple myeloma, medulla oblongata tumour, adrenal adenoma, and follicular thyroid nodules. Endokrynol Pol. (2019) 70:213–7. doi: 10.5603/EP.a2019.0005
31. Rogers L, Barani I, Chamberlain M, Kaley TJ, McDermott M, Raizer J, et al. Meningiomas: knowledge base, treatment outcomes, and uncertainties. A RANO review. J Neurosurg. (2015) 122:4–23. doi: 10.3171/2014.7.JNS131644
32. Xiao Z, Xiao P, Wang Y, Fang C, Li Y. Risk of cancer in acromegaly patients: An updated meta-analysis and systematic review. PloS One. (2023) 18:e0285335. doi: 10.1371/journal.pone.0285335
33. Johnson M, Toms S. Mitogenic signal transduction pathways in meningiomas: novel targets for meningioma chemotherapy? J Neuropathol Exp Neurol. (2005) 64:1029–36. doi: 10.1097/01.jnen.0000189834.63951.81
34. Peyre M, Kalamarides M. Molecular genetics of meningiomas: Building the roadmap towards personalized therapy. Neurochirurgie. (2018) 64:22–8. doi: 10.1016/j.neuchi.2014.06.007
35. Graillon T, Romano D, Defilles C, Lisbonis C, Saveanu A, Figarella-Branger D, et al. Pasireotide is more effective than octreotide, alone or combined with everolimus on human meningioma in vitro. Oncotarget. (2017) 8:55361–73. doi: 10.18632/oncotarget.v8i33
36. Graillon T, Defilles C, Mohamed A, Lisbonis C, Germanetti AL, Chinot O, et al. Combined treatment by octreotide and everolimus: Octreotide enhances inhibitory effect of everolimus in aggressive meningiomas. J Neurooncol. (2015) 124:33–43. doi: 10.1007/s11060-015-1812-3
37. Gariani K, Meyer P, Philippe J. Implications of somatostatin analogues in the treatment of acromegaly. Eur Endocrinol. (2013) 9:132–5. doi: 10.17925/EE.2013.09.02.132
38. Asgharian B, Chen YJ, Patronas NJ, Peghini PL, Reynolds JC, Vortmeyer A, et al. Meningiomas may be a component tumor of multiple endocrine neoplasia type 1. Clin Cancer Res Off J Am Assoc Cancer Res. (2004) 10:869–80. doi: 10.1158/1078-0432.CCR-0938-3
39. Puduvalli VK, Li JT, Chen L, McCutcheon IE. Induction of apoptosis in primary meningioma cultures by fenretinide. Cancer Res. (2005) 65:1547–53. doi: 10.1158/0008-5472.CAN-04-0786
40. Sadetzki S, Flint-Richter P, Ben-Tal T, Nass D. Radiation-induced meningioma: a descriptive study of 253 cases. J Neurosurg. (2002) 97:1078–82. doi: 10.3171/jns.2002.97.5.1078
41. Banerjee J, Pääkkö E, Harila M, Herva R, Tuominen J, Koivula A, et al. Radiation-induced meningiomas: A shadow in the success story of childhood leukemia. Neuro-Oncol. (2009) 11:543–9. doi: 10.1215/15228517-2008-122
Keywords: GH, acromegaly, acromegaly and cancer, meningioma, IGF - I
Citation: Guo AX, Job A, Pacione D and Agrawal N (2024) Risk of intracranial meningioma in patients with acromegaly: a systematic review. Front. Endocrinol. 15:1407615. doi: 10.3389/fendo.2024.1407615
Received: 27 March 2024; Accepted: 21 May 2024;
Published: 11 June 2024.
Edited by:
Monica Livia Gheorghiu, Carol Davila University of Medicine and Pharmacy, RomaniaReviewed by:
Marek Bolanowski, Wroclaw Medical University, PolandCopyright © 2024 Guo, Job, Pacione and Agrawal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nidhi Agrawal, bmlkaGkuYWdyYXdhbEBueXVsYW5nb25lLm9yZw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.