
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 11 June 2024
Sec. Clinical Diabetes
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1407408
This article is part of the Research Topic Digital Technology in the Management and Prevention of Diabetes View all 11 articles
Objective: We aimed to explore the relationship between remote resistance exercise programs delivered via a smartphone application and skeletal muscle mass among elderly patients with type 2 diabetes, utilizing real-world data.
Methods: The resistance exercises were provided through Joymotion®, a web-based telerehabilitation smartphone application (Shanghai Medmotion Medical Management Co., Ltd). The primary outcome was the changes in skeletal muscle index (SMI) before and after the remote resistance exercises programs. The secondary outcomes were changes in skeletal muscle cross-sectional area (SMA), skeletal muscle radiodensity (SMD) and intermuscular adipose tissue (IMAT).
Results: A total of 101 elderly patients with type 2 diabetes were analyzed. The participants had an average age of 72.9 ± 6.11 years for males and 74.4 ± 4.39 years for females. The pre- and post-intervention SMI mean (± SE) was 31.64 ± 4.14 vs. 33.25 ± 4.22 cm2/m2 in male, and 22.72 ± 3.24 vs. 24.28 ± 3.60 cm2/m2 in female respectively (all P < 0.001). Similarly, a statistically significant improvement in SMA, IMAT, and SMD for both male and female groups were also observed respectively (P < 0.001). Multiple linear regression models showed potential confounding factors of baseline hemoglobin A1c and duration of diabetes with changes in SMI in male, while hemoglobin A1c and high density lipoprotein cholesterol with changes in SMI in female.
Conclusion: Remote resistance exercises programs delivered by a smartphone application were feasible and effective in helping elderly patients with type 2 diabetes to improve their skeletal muscle mass.
Elderly patients with type 2 diabetes (T2D) are a distinct and rapidly expanding group, confronting a wide array of health challenges, including a heightened risk of developing sarcopenia (1). Sarcopenia, defined by the loss of muscle mass, strength, and function, significantly diminishes quality of life, functional independence, and healthcare outcomes in this demographic (2). The relationship between T2D and sarcopenia in elderly adults is intricate and reciprocal. T2D propels the aging process via several mechanisms, such as chronic inflammation, oxidative stress, and endothelial dysfunction, all of which contribute to the development and progression of sarcopenia. In turn, the presence of sarcopenia can exacerbate diabetes management, complicating glycemic control, increasing the likelihood of hypoglycemia, and elevating the risk of hospital admissions, falls and fractures, and even death (3, 4). Additionally, there is an increasing body of evidence indicating that both sarcopenia and T2D independently and together play a role in cognitive deterioration, elevating the risk of developing dementia, including Alzheimer’s disease (5–7).
Resistance exercise, also known as strength training, is crucial in managing T2D and addressing sarcopenia, especially among the elderly population (8–10). This physical activity entails exercises that force muscles to contract against an external force, aiming for improvements in strength, tone, mass, and/or endurance. The advent of remote resistance exercise programs available through smartphone applications marks a significant progression in healthcare technology (11, 12). These innovations provide accessible, personalized, and scalable exercise solutions, featuring real-time feedback and instructions, some of which can integrate with wearable technology. While several clinical trials have shed light on the effectiveness of such interventions in clinical scenarios (13–15), home-based real-world evidence, particularly within the Chinese population, remains scarce. Our objective is to explore the relationship between remote resistance exercise programs delivered via a smartphone application and skeletal muscle mass among elderly patients with T2D, utilizing real-world data.
The Department of Rehabilitation at the Third Affiliated Hospital of Jinzhou Medical University offers a comprehensive array of services, including a rehabilitation medicine clinic and specialized clinics. It is recognized as the most expansive and technologically advanced rehabilitation facility among the large comprehensive hospitals in western Liaoning, boasting expertise in several key areas such as exercise prescriptions, neurological rehabilitation, trauma rehabilitation, and traditional medicine rehabilitation. We extracted data from the electronic health records (EHRs) from the Third Affiliated Hospital of Jinzhou Medical University. Eligible patients were (1) aged 65 years or older; (2) confirmed diagnosis of T2D; (3) using remote resistance exercises programs for at least 3 months; (3) available data on clinical characteristics and medical history. Exclusion criteria included: (1) patients without available images in the CT scan or with poor image quality; (2) patients with loss of rehabilitation record; (3) without complete clinical data (see Figure 1 in detail). The analytic plan was approved by the Institutional Review Board of the Third Affiliated Hospital of Jinzhou Medical University (JYDSY-KXYJ-IEC-2021–009). Our study did not require obtaining informed consent from individual participants as it utilized retrospective anonymized data extracted from EHRs.
Using the Chinese government-issued unique personal identification numbers, we developed an electronic database. This database compiled information such as date of birth, sex, age at diabetes diagnosis, smoking status, and medication use, including antihypertensive, glucose-lowering, lipid-lowering, and antiplatelet or anticoagulant drugs. Data was gathered via a standardized form for collecting electronic inpatient and outpatient medical record information. Additionally, at every inpatient admission or outpatient visit, standardized methods were used to measure patients’ height, weight, blood pressure, plasma glucose levels, C-peptide, total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, hemoglobin A1c (HbA1c), serum creatinine, and C-reactive protein. The body mass index (BMI) was calculated using the formula weight in kilograms (kg) divided by the square of height in meters (m²). The estimated glomerular filtration rate (eGFR) was determined using the formula from the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) (16). Comorbidities and history of diseases were coded using the International Classification of Diseases 10th version (ICD-10).
The resistance exercises were provided through Joymotion®, a web-based telerehabilitation smartphone application (Shanghai Medmotion Medical Management Co., Ltd) (17). Joymotion®; is a remote rehabilitation and exercise prescription monitoring device designed for treating musculoskeletal diseases. It integrates wearable sensor hardware with an app that features a library of standardized exercise prescriptions based on evidence-based practices. These can be tailored by doctors to create personalized treatment plans. The app guides patients through exercises with video and written instructions, monitors performance in real-time, and offers interactive communication with online physiotherapists. It also pushes educational content tailored to patients’ conditions, enhancing understanding and engagement in their rehabilitation. Key features include the ability to facilitate easy follow-ups for doctors and automatically push tele-rehabilitation as “daily tasks” and targeted educational videos, improving patient education and compliance. Through real-time tracking, messaging, and video consultations, Joymotion® facilitates continuous personalized care and supports comprehensive disease management, even after hospital discharge. Within the app, immediate feedback on the completion of exercises within a session is recorded, determining success based on whether the patient fully viewed or skipped the instructional videos.
In this study, a 12-week progressive elastic band resistance training program was delivered via the Joymotion®. Participants engaged in a structured series of resistance exercises employing elastic bands (Thera-Band®, The Hygenic Corporation, Akron, OH, USA). These bands, differentiated by color (yellow, red, green, blue, black, or silver), provided varying levels of resistance, with about 20% increase in resistance intensity. The training protocol was meticulously designed to encompass all major muscle groups, incrementally elevating both the volume and intensity of the exercises. Training intensities were meticulously tailored to the individual’s clinical profile and historical health data, ensuring a bespoke rehabilitation process. Adherence to the resistance training guidelines established by the American College of Sports Medicine for elderly adults was ensured (18).
Participant compliance and safety were paramount; thus, participants were instructed to report the perceived exertion and any difficulties encountered during each session through the application. Weekly virtual consultations with the physical therapist were mandated to evaluate training load, ascertain the presence of any adverse reactions, and adjust training intensity and exercises progressively optimizing the intervention protocol based on these evaluations. A series of elastic bands with varying levels of resistance were provided, allowing for progression based on participants’ training capacity. In instances where participants were unable to tolerate the increased intensity of the next resistance level, the resistance intensity of the elastic band was maintained at the previous level for an additional session. However, in such cases, the number of repetitions per set might be increased to continue challenging the participants and promoting strength development. This maintenance continued until participants could successfully achieve a 20% increase in resistance intensity, signaling readiness to progress to the next color-coded elastic band. The program stipulated a thrice-weekly engagement over 12 weeks, culminating in 36 sessions. Each session was structured to last 50 minutes, comprising a 5-minute general warm-up, 40 minutes of targeted elastic band resistance exercises, and a concluding 5-minute cool-down and stretching phase. The exercise component was designed to target specific muscle groups—including the lower limbs, core, arms, and shoulders—through 1 or 2 exercise variations. For each exercise, participants were instructed to perform 2–3 sets of 8–12 repetitions, focusing on controlled concentric and eccentric muscle contractions across the full movement range, facilitated by the resistance offered by the elastic bands.
We used computed tomography (CT) images at the thoracic 12 (T12) vertebral level to assess the muscle area and quality. This method is commonly used in many other studies for assessing sarcopenia or the loss of skeletal muscle mass and function (19–21). Since the COVID-19 pandemic in 2019, chest CT scanning is routine at every clinical visit and the reports and images were stored in the EHR systems. The T12 vertebra is identified on the CT scan by locating the last rib’s attachment point, as T12 is the last thoracic vertebra to which ribs attach. We used SliceOmatic 6 (TomoVision, Magog, Canada) to analyze the images. The skeletal muscle cross-sectional area (SMA) at the T12 level is delineated manually. The muscles analyzed include the paraspinal muscles (erector spinae and multifidus), and intercostal muscles. The software calculated the total cross-sectional area of skeletal muscle in square centimeters (cm²) and we further adjusted these measurements for height to derive a skeletal muscle index (SMI). Skeletal muscle radiodensity (SMD) and intermuscular adipose tissue (IMAT) were determined using the images at T12 level based on the Hounsfield Unit (HU) values. The SMD and IMAT were reported as the mean HU value within the erector spinae muscle area.
The primary outcome was the changes in SMI before and after the remote resistance exercises programs. The secondary outcomes included the changes in SMA, SMD and IMAT.
For the primary outcome measure of SMI, we determined that a sample size of 44 patients in each gender would grant the study 90% statistical power, with a two-sided alpha level of 0.05, in a two-sided paired t-test, to detect a treatment difference of 2 cm2/m2 in male and 1.5 cm2/m2 in female, assuming a standard deviation of 4 in male and 3 in female. G*power version 3.1 (Heinrich-Heine-Universität Düsseldorf Universitätsstr, Germany) was employed in the calculation (22). To accommodate potential exclusion, we increased the target enrollment to 133 patients.
Data with a normal distribution were described using the mean and standard deviation, while skewed data were presented as the median alongside interquartile ranges. Frequencies and percentages were used to describe categorical variables. To compare between groups, Student’s t-tests were applied to normally distributed data, the Wilcoxon rank-sum test was used for skewed data, and the χ2 test was utilized for categorical variables. Baseline variables that were considered clinically relevant or that showed a univariate relationship with outcome were entered into multiple regression model (Table 1). Variables for inclusion were carefully chosen, given the number of events available, to ensure parsimony of the final model. Stepwise multiple linear regression models (lmtest, caret in R) were used to estimate the confounding factors associated with the outcomes (Table 2). The primary analysis was performed according to sexes separately. The model employed the Breusch-Pagan test to assess the homoscedasticity of the residuals for the validity of Ordinary Least Squares (OLS) regression. A P-value of less than 0.05 was considered of statistical significance. The R software version 4.3.3 (R Foundation for Statistical Computing, Vienna, Austria) was utilized to carry out all the statistical analysis.
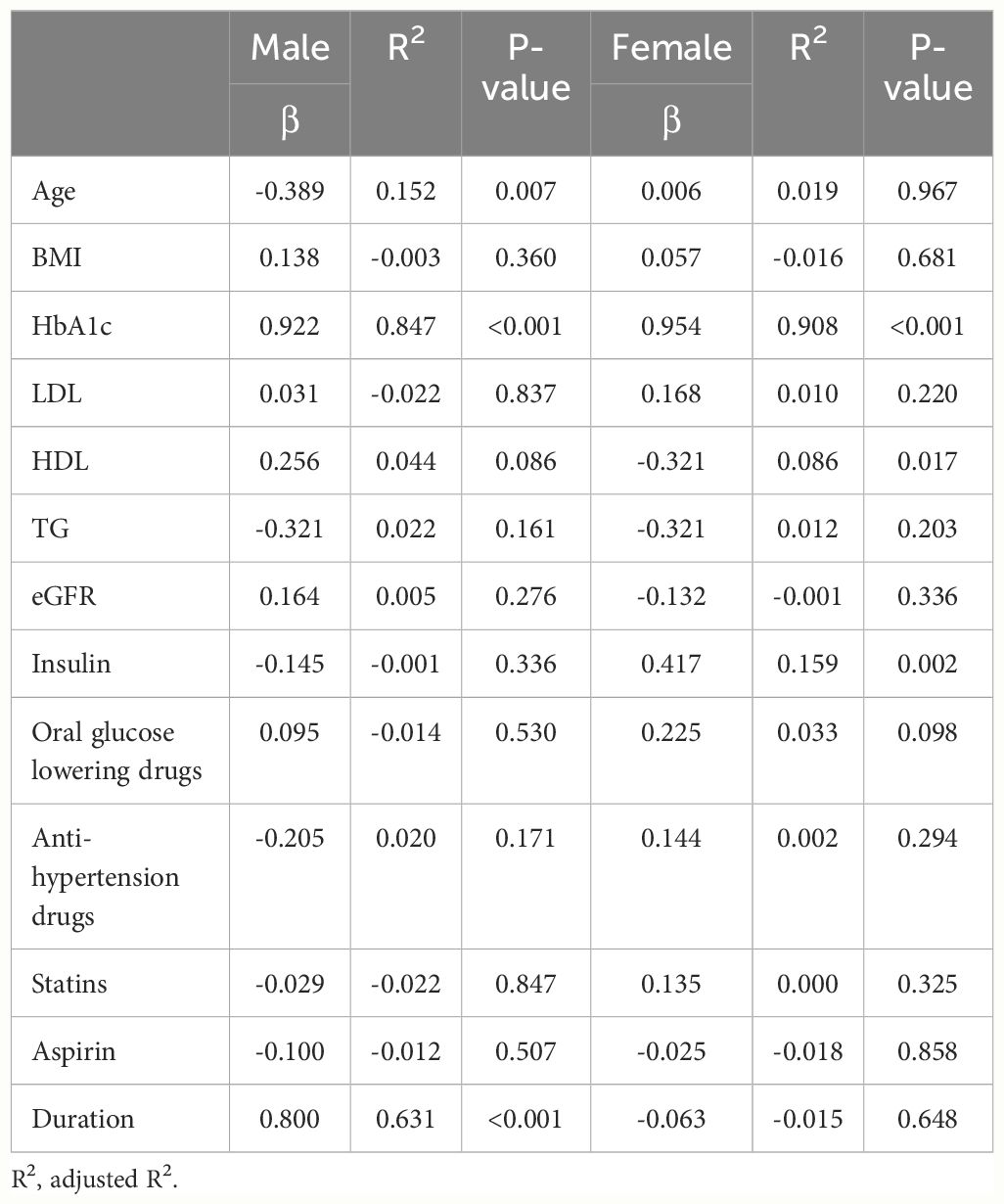
Table 1 Univariable regression analysis to explore the associations of baseline characteristics with ΔSMI.
From November 2022 to December 2023, 133 patients were screened for eligibility, and 101 were analyzed, with 46 male (45.5%) and 55 female (54.5%) patients (Figure 1). The demographic and clinical characteristics of the patients at baseline are shown in Table 3. The participants had an average age of 72.9 ± 6.11 years for males and 74.4 ± 4.39 years for females (P < 0.05). The mean diastolic blood pressure (DBP) was 72.3 ± 5.62 mmHg for males and 70.8 ± 5.60 mmHg for females. Systolic blood pressure (SBP) was 132 ± 9.08 mmHg for males and 136 ± 10.8 mmHg for females. BMI was balanced across genders, with males at 28.0 ± 3.76 kg/m2 and females at 28.2 ± 4.43 kg/m2. Medication usage varied across the cohort, with higher percentages using insulin (male 50%, female 47.2%) in male and metformin (male 54.3%, female 70.9%) in female. Anti-hypertensive drug use was prevalent, with over 89% of each gender using these medications. Statin use was also high, with 76.1% of males and 78.2% of females on these cholesterol-lowering drugs. Smoking status was reported by 34.8% of males and 27.3% of females (P < 0.05).
The primary outcome assessed in this study was the change in SMI following participation in a remote resistance exercise program (Figure 2). A statistically significant improvement in SMI for both male and female groups were observed after the program when compared to the SMI at baseline (P < 0.001). The pre- and post-intervention mean SMI was 31.64 ± 4.14 vs. 33.25 ± 4.22 cm2/m2 in male, and 22.72 ± 3.24 vs. 24.28 ± 3.60 cm2/m2 in female respectively (all P < 0.001). The secondary outcomes were the changes in SMA, IMAT, and SMD (Figure 2). Similarly, a statistically significant improvement in SMA, IMAT, and SMD for both male and female groups was also found respectively (P < 0.001).
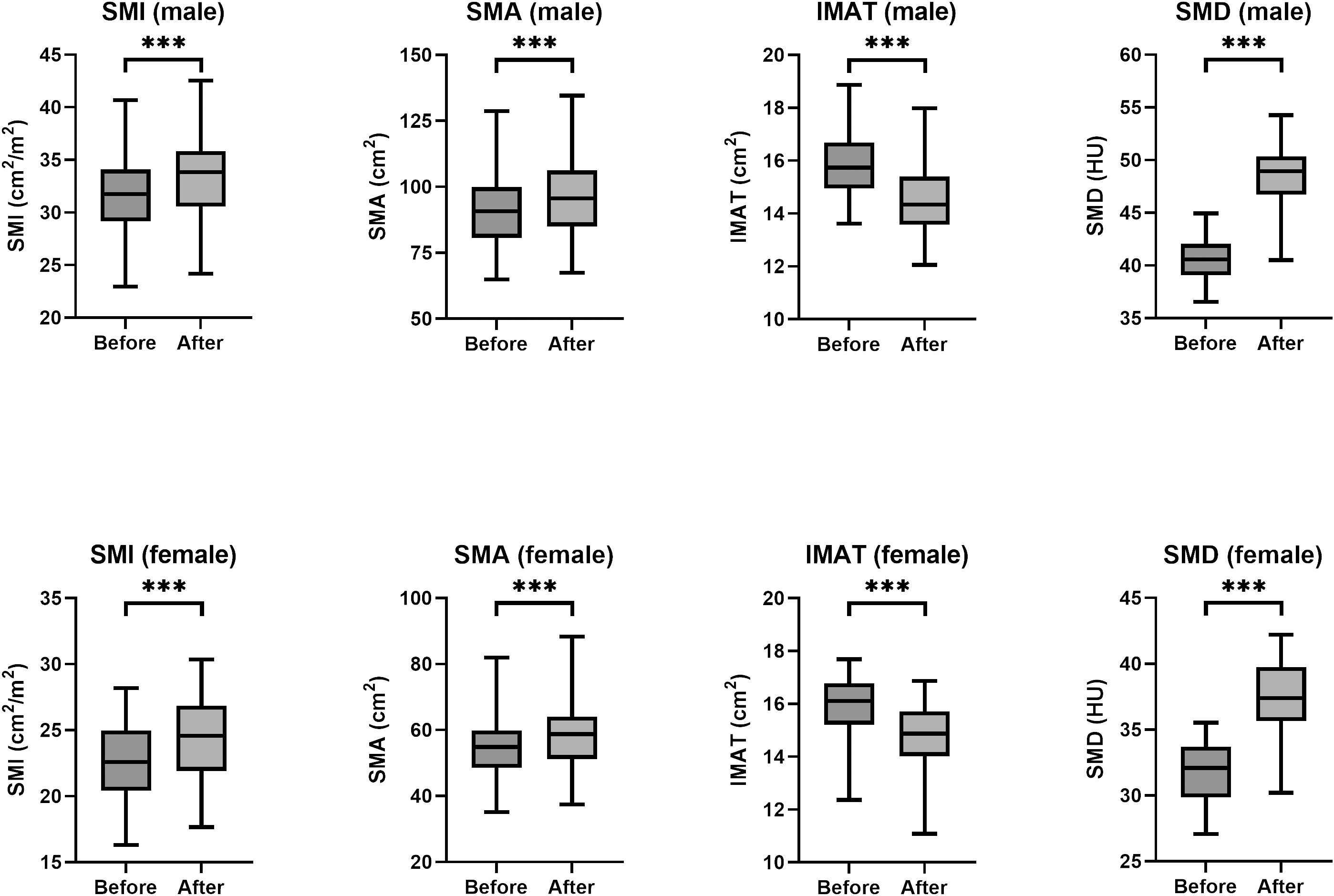
Figure 2 Box plots of SMI, SMA, IMAT, and SMD values before and after training by sex. HU, Hounsfield unit. (*** P < 0.001).
We further explored the correlations between the changes in SMI before and after the resistance training program (ΔSMI), and various baseline characteristics of patients were analyzed separately in univariate models (Table 1), then a forward stepwise approach used to inform the final variables for inclusion into the multiple regression model (Table 2). The analysis was conducted separately for male and female groups to identify variables significantly associated with ΔSMI within each gender. In the male group, HbA1c and duration of diabetes showed significant positive association with ΔSMI (HbA1c, coefficient 0.208 (0.164, 0.251), P < 0.001; duration of diabetes, coefficient 0.030 (0.014, 0.047), P < 0.001). In the female group, HbA1c was also found to have a significant positive correlation with ΔSMI (coefficient 0.349 (0.320, 0.378), P <0.001). HDL cholesterol levels were negatively associated with ΔSMI (coefficient -0.004 (-0.006, -0.002), P <0.001).
In this study, we demonstrated the significant improvements in SMI, SMA, IMAT, and SMD for both males and females by a remote resistance exercise program. Multiple linear regression analysis highlighted significant correlations between post-interventional SMI and various factors. These findings underscore the effectiveness of resistance training in enhancing muscle metrics among older adults, along with the complex interplay of metabolic health factors in modulating these outcomes.
The effectiveness of resistance exercises in addressing sarcopenia, particularly among elderly patients with T2D, has been substantiated by some clinical trials and studies. One prospective study (23) aimed to assess the impact of combined strength and aerobic exercises on frailty levels in elderly diabetic patients over 70 years of age with relatively high functional and cognitive capacities. The study demonstrated that a 6-month regimen of strength exercises with elastic bands and aerobic activities can effectively reduce frailty prevalence among elderly diabetic patients. Another randomized clinical trial (24) assessed the impact of high-protein diets both with and without resistance exercise training on weight loss, body composition, and cardiovascular disease risk factors over a 16-week period. The study found significant group effects for body weight, fat mass, and waist circumference, with the most substantial reductions observed in the low fact hypocaloric diet + resistance exercise group. Specifically, this group experienced the largest decrease in body weight (-13.8 ± 6.0 kg), fat mass (-11.1 ± 3.7 kg), and waist circumference (-13.7 ± 4.6 cm). Bouchi et al (25) also assessed the impact of combining resistance training, with the sodium-glucose cotransporter 2 inhibitor dapagliflozin on body composition and metabolic health in type 2 diabetes patients over a 24-week period of intervention. While this trial revealed no significant difference between the two groups in terms of changes in fat-free mass and SMI, a notable reduction in trunk fat mass was observed in the combination group. Our study showed notable improvements in the SMI following participation in remote resistance exercise programs, alongside a reduction in IMAT. These findings indicate the effectiveness of such a remote program, though further research is required to substantiate these results.
Certain gaps between clinical trials and real-world evidence may limit the interpretation of the findings above. Differences in population diversity, intervention adherence, data collection and outcomes, and study design and flexibility were huge (26). Evidence from real world setting of resistance exercises on skeletal muscle mass is few. Remote resistance exercise programs thus offer a series of advantages for real world use (27). Remote programs can be accessed from any location at any time, eliminating the need for transportation to a gym or fitness center. This is especially beneficial for individuals with mobility issues or those living in areas with limited access to fitness facilities. Remote exercise programs can be highly personalized to meet the specific needs and limitations of elderly individuals. This ensures that exercises are both safe and effective, contributing to improvements in strength, balance, and functional ability without risking injury. By reducing or eliminating the need for clinical visits, personal trainers, or specialized equipment, remote resistance exercises can provide a cost-effective way to combat sarcopenia. This is particularly important for those on a fixed income, such as many elderly individuals. The Joymotion®; incorporates elements of gamification, social interaction, and progress tracking, which can significantly enhance motivation and adherence. Being part of a virtual community or having the ability to share achievements with friends and family can provide additional encouragement to continue with the program. The programs and instructions within the Joymotion®; are designed based on the Standards of Care in Diabetes by the American Diabetes Association (28), and the consensus statement on exercises/physical activity in individuals with T2D from the American College of Sports Medicine (18), which provides interventions that are scientifically proven to be effective in improving strength, mobility, and frailty among elderly patients with T2D. This study with real world evidence provided a flexible, personalized, and accessible means of engaging in remote resistance exercises, representing a valuable tool in the fight against frailty, empowering individuals to maintain and improve their health and independence.
In our study, we also analyzed the potential confounding factors associated with the improvement of skeletal muscle mass. We found that elderly patients with long duration of diabetes may have a great improvement in SMI. Interestingly, high hemoglobin A1c level may also contribute to a significant improvement in SMI. Although we did not observe any association between insulin use and changes in skeletal muscle mass, insulin plays a critical role in muscle protein synthesis and glucose uptake in muscle tissues (29, 30). Therefore, individuals who manage diabetes without insulin might have a metabolic environment more conducive to gaining muscle mass when engaged in resistance training. The positive correlation between the duration of diabetes, as well as the hemoglobin A1c levels at baseline and SMI improvement might seem counterintuitive, as long-term diabetes along with poor glycemic control is often associated with complications that could impair muscle function. However, this finding could indicate that individuals with a longer history of diabetes and poorer glycemic control have more to gain from engaging in a resistance exercise program. It’s possible that these individuals had greater initial muscle loss or more pronounced muscle quality deterioration, thereby experiencing more significant gains when exposed to resistance training. Engaging in resistance exercise can lead to various metabolic adaptations that improve muscle protein synthesis, increase glucose uptake by muscles, and enhance insulin sensitivity (31, 32). These adaptations could be more pronounced in individuals who start with higher levels of metabolic dysfunction. Further studies are needed to validate these hypotheses.
The association between resistance exercises and sarcopenia is underpinned by several biological mechanisms and physiological adaptations that contribute to improved health outcomes and a reduction in frailty indicators. As aging is associated with sarcopenia, resistance training can stimulate muscle protein synthesis and inhibits protein degradation through the activation of the mammalian target of rapamycin (mTOR) pathway (33), leading to increases in muscle mass and strength. Resistance exercises also enhance neuromuscular efficiency, improving the recruitment of muscle fibers, and increasing motor unit activation. The mechanical stress applied to bones during resistance training stimulates osteogenesis (34), helping to prevent or reverse osteopenia and osteoporosis, which contributes to sarcopenia through increased fracture risk. Resistance exercises have been shown to reduce systemic inflammation (35), through the downregulation of pro-inflammatory cytokines and the enhancement of anti-inflammatory mediators. Meanwhile, AMP-activated protein kinase (AMPK) can be activated by resistance training in response to changes in cellular energy status (36). AMPK activation further promotes glucose uptake, fatty acid oxidation, and mitochondrial biogenesis by upregulating peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) (37). Research also supports the hypothesis that resistance exercises can increase the levels of IGF-1 (38), which binds to its receptor on muscle cells, activating the Akt pathway. This also leads to increased protein synthesis and muscle cell growth, as well as decreased protein degradation. Other benefits from resistance exercises may include the reduction of myostatin and modulating the activity of NF-kB, further reducing muscle loss and dysfunction associated with sarcopenia (39).
This study underscores the potential of emerging digital technologies in managing T2D in the elderly population, along with precise quantification of skeletal muscle mass through CT imaging. However, several limitations should be fully addressed. Firstly, the study’s reliance on a self-control design may restrict the strength of the evidence provided. Secondly, it was conducted with a small participant group at a single center, suggesting the need for a larger, multi-center study to validate the findings. Lastly, the omission of dietary data, a significant confounding factor, was noted, although all participants were local residents likely sharing similar dietary habits. In future research, we plan to employ food recall questionnaires to meticulously document nutrient intake, with a focus on protein consumption, to address this limitation.
Remote resistance exercises programs delivered by a smartphone application were feasible and effective in helping elderly patients with type 2 diabetes to improve their skeletal muscle mass. Future prospective randomized controlled trials are warranted to provide further evidence on this digital technology-based intervention.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Institutional Review Board of the Third Affiliated Hospital of Jinzhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
JY: Writing – original draft, Data curation. HT: Writing – original draft. HY: Writing – original draft, Software, Formal analysis. JL: Writing – review & editing, Data curation. YC: Writing – review & editing, Data curation. YL: Writing – review & editing. XL: Writing – review & editing. QC: Writing – review & editing. DZ: Writing – review & editing, Supervision, Methodology, Funding acquisition, Conceptualization.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the China Stroke Association Whole Course Management of Cerebrovascular Disease Sailing Funding (2020017). The funding source had no role in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. de Luis Román D, Gómez JC, García-Almeida JM, Vallo FG, Rolo GG, Gómez JJL, et al. Diabetic Sarcopenia. A proposed muscle screening protocol in people with diabetes: Expert document. Rev Endocr Metab Disord. (2024). doi: 10.1007/s11154-023-09871-9. Epub ahead of print.
2. Hou Y, Xiang J, Wang B, Duan S, Song R, Zhou W, et al. Pathogenesis and comprehensive treatment strategies of sarcopenia in elderly patients with type 2 diabetes mellitus. Front Endocrinol (Lausanne). (2023) 14:1263650. doi: 10.3389/fendo.2023.1263650
3. Lisco G, Disoteo OE, De Tullio A, De Geronimo V, Giagulli VA, Monzani F, et al. Sarcopenia and diabetes: A detrimental liaison of advancing age. Nutrients. (2023) 16(1):63. doi: 10.3390/nu16010063
4. Lopez-Pedrosa JM, Camprubi-Robles M, Guzman-Rolo G, Lopez-Gonzalez A, Garcia-Almeida JM, Sanz-Paris A, et al. The vicious cycle of type 2 diabetes mellitus and skeletal muscle atrophy: clinical, biochemical, and nutritional bases. Nutrients. (2024) 16(1):172. doi: 10.3390/nu16010172
5. Liu YH, Ma LL, Hu LK, Cui L, Li YL, Chen N, et al. The joint effects of sarcopenia and cardiometabolic risk factors on declined cognitive function: Evidence from a 7-year cohort study. J Affect Disord. (2024) 344:644–52. doi: 10.1016/j.jad.2023.10.056
6. Low S, Goh KS, Ng TP, Moh A, Ang SF, Khoo J, et al. Decline in skeletal muscle mass is associated with cognitive decline in type 2 diabetes mellitus. J Diabetes Complications. (2022) 36:108258. doi: 10.1016/j.jdiacomp.2022.108258
7. Low S, Ng TP, Lim CL, Moh A, Ang SF, Wang J, et al. Association between lower extremity skeletal muscle mass and impaired cognitive function in type 2 diabetes. Sci Rep. (2020) 10:2956. doi: 10.1038/s41598-020-59914-3
8. Yamamoto Y, Nagai Y, Kawanabe S, Hishida Y, Hiraki K, Sone M, et al. Effects of resistance training using elastic bands on muscle strength with or without a leucine supplement for 48 weeks in elderly patients with type 2 diabetes. Endocr J. (2021) 68:291–8. doi: 10.1507/endocrj.EJ20-0550
9. Kobayashi Y, Long J, Dan S, Johannsen NM, Talamoa R, Raghuram S, et al. Strength training is more effective than aerobic exercise for improving glycaemic control and body composition in people with normal-weight type 2 diabetes: a randomised controlled trial. Diabetologia. (2023) 66:1897–907. doi: 10.1007/s00125-023-05958-9
10. Consitt LA, Dudley C, Saxena G. Impact of endurance and resistance training on skeletal muscle glucose metabolism in older adults. Nutrients. (2019) 11(11):2636. doi: 10.3390/nu11112636
11. Sosa-Pedreschi A, Donadio MVF, Iturriaga-Ramírez T, Yvert T, Pérez-Salazar F, Santiago-Dorrego C, et al. Effects of a remotely supervised resistance training program on muscle strength and body composition in adults with cystic fibrosis: Randomized controlled trial. Scand J Med Sci Sports. (2024) 34:e14564. doi: 10.1111/sms.14564
12. Andonian B, Ross LM, Zidek AM, Fos LB, Piner LW, Johnson JL, et al. Remotely supervised weight loss and exercise training to improve rheumatoid arthritis cardiovascular risk: rationale and design of the supervised weight loss plus exercise training-rheumatoid arthritis trial. ACR Open Rheumatol. (2023) 5:252–63. doi: 10.1002/acr2.11536
13. Suzuki Y, Shimizu Y, Soma Y, Matsuda T, Hada Y, Koda M. Effectiveness of a remote monitoring-based home training system for preventing frailty in older adults in Japan: A preliminary randomized controlled trial. Geriatr (Basel). (2024) 9(1):20. doi: 10.3390/geriatrics9010020
14. Kompf J, Whiteley J, Wright J, Brenner P, Camhi S. Resistance training behavior is enhanced with digital behavior change coaching: A randomized controlled trial with novice adults. J Phys Act Health. (2023) 20:531–7. doi: 10.1123/jpah.2022-0367
15. Fyfe JJ, Dalla Via J, Jansons P, Scott D, Daly RM. Feasibility and acceptability of a remotely delivered, home-based, pragmatic resistance ‘exercise snacking’ intervention in community-dwelling older adults: a pilot randomised controlled trial. BMC Geriatr. (2022) 22:521. doi: 10.1186/s12877-022-03207-z
16. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
17. Hu C, Zhu B, Wang Y, Yang F, Zhang J, Zhong W, et al. Effectiveness of blood flow restriction versus traditional weight-bearing training in rehabilitation of knee osteoarthritis patients with MASLD: a multicenter randomized controlled trial. Front Endocrinol (Lausanne). (2023) 14:1220758. doi: 10.3389/fendo.2023.1220758
18. Kanaley JA, Colberg SR, Corcoran MH, Malin SK, Rodriguez NR, Crespo CJ, et al. Exercise/physical activity in individuals with type 2 diabetes: A consensus statement from the American College of Sports Medicine. Med Sci Sports Exerc. (2022) 54:353–68. doi: 10.1249/MSS.0000000000002800
19. Sun L, Ma H, Du G, Fan D, Liu J, Wang X, et al. Low skeletal muscle area at the T12 paravertebral level as a prognostic marker for community-acquired pneumonia. Acad Radiol. (2022) 29:e205–10. doi: 10.1016/j.acra.2021.12.026
20. McSweeney DM, Raby S, Radhakrishna G, Weaver J, Green A, Bromiley PA, et al. Low muscle mass measured at T12 is a prognostic biomarker in unresectable oesophageal cancers receiving chemoradiotherapy. Radiother Oncol. (2023) 186:109764. doi: 10.1016/j.radonc.2023.109764
21. Çinkooğlu A, Bayraktaroğlu S, Ufuk F, Unat Ö S, Köse T, Savaş R, et al. Reduced CT-derived erector spinae muscle area: a poor prognostic factor for short- and long-term outcomes in idiopathic pulmonary fibrosis patients. Clin Radiol. (2023) 78:904–11. doi: 10.1016/j.crad.2023.08.011
22. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. (2007) 39:175–91. doi: 10.3758/BF03193146
23. García Díaz E, Alonso Ramírez J, Herrera Fernández N, Peinado Gallego C, Pérez Hernández DG. Effect of strength exercise with elastic bands and aerobic exercise in the treatment of frailty of the elderly patient with type 2 diabetes mellitus. Endocrinol Diabetes Nutr (Engl Ed). (2019) 66:563–70. doi: 10.1016/j.endien.2019.01.008
24. Wycherley TP, Noakes M, Clifton PM, Cleanthous X, Keogh JB, Brinkworth GD. A high-protein diet with resistance exercise training improves weight loss and body composition in overweight and obese patients with type 2 diabetes. Diabetes Care. (2010) 33:969–76. doi: 10.2337/dc09-1974
25. Bouchi R, Sonoda N, Itoh J, Ono Y, Fukuda T, Takeuchi T, et al. Effects of intensive exercise combined with dapagliflozin on body composition in patients with type 2 diabetes: a randomized controlled trial. Endocr J. (2021) 68:329–43. doi: 10.1507/endocrj.EJ20-0599
26. SChad F, Thronicke A. Real-world evidence-current developments and perspectives. Int J Environ Res Public Health. (2022) 19(16):10159. doi: 10.3390/ijerph191610159
27. Hutchison MG, Di Battista AP, Loenhart MM. A continuous aerobic resistance exercise protocol for concussion rehabilitation delivered remotely via a mobile app: feasibility study. JMIR Form Res. (2023) 7:e45321. doi: 10.2196/45321
28. American Diabetes Association Professional Practice Committee. 13. Older adults: standards of care in diabetes-2024. Diabetes Care. (2024) 47:S244–s257. doi: 10.2337/dc24-S013
29. Tipton KD, Wolfe RR. Exercise, protein metabolism, and muscle growth. Int J Sport Nutr Exerc Metab. (2001) 11:109–32. doi: 10.1123/ijsnem.11.1.109
30. Abdulla H, Smith K, Atherton PJ, Idris I. Role of insulin in the regulation of human skeletal muscle protein synthesis and breakdown: a systematic review and meta-analysis. Diabetologia. (2016) 59:44–55. doi: 10.1007/s00125-015-3751-0
31. Lim C, Nunes EA, Currier BS, McLeod JC, Thomas ACQ, Phillips SM. An evidence-based narrative review of mechanisms of resistance exercise-induced human skeletal muscle hypertrophy. Med Sci Sports Exerc. (2022) 54:1546–59. doi: 10.1249/MSS.0000000000002929
32. Moghetti P, Bacchi E, Brangani C, Donà S, Negri C. Metabolic effects of exercise. Front Horm Res. (2016) 47:44–57. doi: 10.1159/000445156
33. Levitt DE, Luk HY, Vingren JL. Alcohol, resistance exercise, and mTOR pathway signaling: an evidence-based narrative review. Biomolecules. (2022) 13(1):2. doi: 10.3390/biom13010002
34. Benedetti MG, Furlini G, Zati A, Letizia Mauro G. The effectiveness of physical exercise on bone density in osteoporotic patients. BioMed Res Int. (2018) 2018:4840531. doi: 10.1155/2018/4840531
35. El Assar M, Álvarez-Bustos A, Sosa P, Angulo J, Rodríguez-Mañas L. Effect of physical activity/exercise on oxidative stress and inflammation in muscle and vascular aging. Int J Mol Sci. (2022) 23(15):8713. doi: 10.3390/ijms23158713
36. Kjøbsted R, Wojtaszewski JF, Treebak JT. Role of AMP-activated protein kinase for regulating post-exercise insulin sensitivity. Exp Suppl. (2016) 107:81–126. doi: 10.1007/978-3-319-43589-3_5
37. Ruas JL, White JP, Rao RR, Kleiner S, Brannan KT, Harrison BC, et al. A PGC-1α isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell. (2012) 151:1319–31. doi: 10.1016/j.cell.2012.10.050
38. Feng L, Li B, Xi Y, Cai M, Tian Z. Aerobic exercise and resistance exercise alleviate skeletal muscle atrophy through IGF-1/IGF-1R-PI3K/Akt pathway in mice with myocardial infarction. Am J Physiol Cell Physiol. (2022) 322:C164–c176. doi: 10.1152/ajpcell.00344.2021
Keywords: resistance exercises, smart phone application, skeletal muscle mass, diabetes mellitus, remote rehabilitation, elderly
Citation: Yang J, Tan H, Yu H, Li J, Cui Y, Lu Y, Liu X, Chen Q and Zhou D (2024) Association between remote resistance exercises programs delivered by a smartphone application and skeletal muscle mass among elderly patients with type 2 diabetes– a retrospective real-world study. Front. Endocrinol. 15:1407408. doi: 10.3389/fendo.2024.1407408
Received: 26 March 2024; Accepted: 29 May 2024;
Published: 11 June 2024.
Edited by:
Yun Shen, Pennington Biomedical Research Center, United StatesReviewed by:
Linyang Ju, Novartis Institutes for BioMedical Research, United StatesCopyright © 2024 Yang, Tan, Yu, Li, Cui, Lu, Liu, Chen and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daan Zhou, ZG9jemhvdWRhYW5AMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.