- 1Guang’anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 2School of Management, Fudan University, Shanghai, China
Objective: The progression of carotid intima-media thickness (cIMT) can partially predict the occurrence of future cardiovascular events. This network meta-analysis compared the effects of 14 antidiabetic drugs (acarbose, alogliptin, exenatide, glibenclamide, glimepiride, ipragliflozin, metformin, nateglinide, pioglitazone, rosiglitazone, sitagliptin, tofoglifozin, troglitazone, voglibose) on the progression of cIMT.
Method: PubMed, EMBASE, Cochrane Library, and Web of Science were searched to screen all clinical trials of treatment of cIMT with hypoglycemic agents before March 1, 2024. The differences in the changes in cIMT between the treatment group and control group were evaluated.
Result: After screening 8395 citations, 25 studies (6675 patients) were included. The results indicated that exenatide had the best efficacy in slowing down cIMT progress, and exenatide [MD=-0.13,95%CI (-0.25, -0.01)], alogliptin [MD=-0.08,95%CI (-0.13, -0.02)] and metformin [MD=-0.05, 95%CI (-0.09, -0.02)] are more effective than placebo.
Conclusion: Long-term treatment of exenatide, alogliptin, and metformin may be more effective than other hypoglycemic drugs in slowing the progression of cIMT.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42024519474.
Introduction
Atherosclerosis (AS) plays a crucial role in the pathogenesis of cardiovascular diseases, which may ultimately lead to atherosclerotic cardiovascular disease (ASCVD), posing significant threats to human health. In this pathological process of atherosclerosis formation, excessive lipid deposition and macrophage transformation into foam cells lead to the increase in intima-media thickness (IMT) (1). In fact, IMT can assess the extent of atherosclerosis in the whole human body (2), especially the carotid intima-media thickness (cIMT), serves as a crucial indicator for cardiovascular disease, whose progression can predict future cardiovascular events to a certain extent (3–5). Dysglycemia is generally considered to be an important risk factor for AS (6). Some traditional hypoglycemic drugs(HD) have shown good cardiovascular safety (7–9), especially the clinical application of novel types of hypoglycemic drugs, including dipeptidyl peptidase-4 inhibitor (DPP-4i), glucagon like peptide-1 receptor agonists (GLP-1 RA), sodium-glucose cotransporter-2 inhibitor (SGLT-2i), peroxisome proliferator-activated receptor gamma (PPARγ) agonist, has ushered in a new era of diabetes treatment. These drugs are increasingly recognized to have multiple effects beyond lowering blood glucose, some of which may contribute to clinical practice in the treatment of cardiovascular diseases (10–13), SGLT-2i and GLP-1 RA are also included in the guidelines as a recommended drug for patients with diabetes and at high risk for cardiovascular events (14, 15). Some clinical trials have conducted to explore the efficacy of one or more HDs and reported the changes of cIMT, however, the impact of different HDs on cIMT has not yet been identified. A network meta-analysis (NMA) can simultaneously compare the effects of multiple interventions because of the lack of direct comparisons between these drugs (16). Therefore, we extracted data from relevant studies to conduct a NMA to assess the relative effectiveness of different HDs in cIMT changes, and to provide evidence for clinical application.
Methods
Study design and registration
We developed a detailed search strategy following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (17), the research protocol was registered with PROSPERO(CRD42024519474).
Study selection
A comprehensive search was conducted using four databases (PubMed, Embase, Cochrane Library, and Web of Science), for all studies on the relationship between HDs and cIMT. The search strategy was implemented by multiplying the search formulas for HDs and carotid intima-media thickness/atherosclerosis. The search period was from the establishment of the database to March 1, 2024. The complete search strategy is given in additional file: Appendix S1.
Inclusion and exclusion criteria
Identified studies were enrolled based on the following inclusion criteria: (a) to evaluate the therapeutic effect of various HDs in retarding cIMT; (b) recruited patients with Type 1 diabetes mellitus(T1DM), type 2 diabetes mellitus(T2DM) or impaired glucose tolerance (IGT) or impaired fasting glucose(IFG) or coronary heart disease (CVD) or metabolic syndrome (MS) or abnormal glucose metabolism(AGM);(c) recruited patients were at least 18 years of age; (d) the duration of treatment is at least one year; (e) had a placebo (PLC) or comparative drug control group to compare the outcomes.
Studies containing the following items were excluded: (a) was not a randomized controlled trial (RCT) trial; (b) a combination of drugs was included in the treatment or control group; (c) treatment duration is less than one year; (d) the study did not provide sufficient information about the dataset; (e) animal experiments, review, protocol, case report, post hoc analysis; (f) non-English language literature.
Data extraction and quality assessment
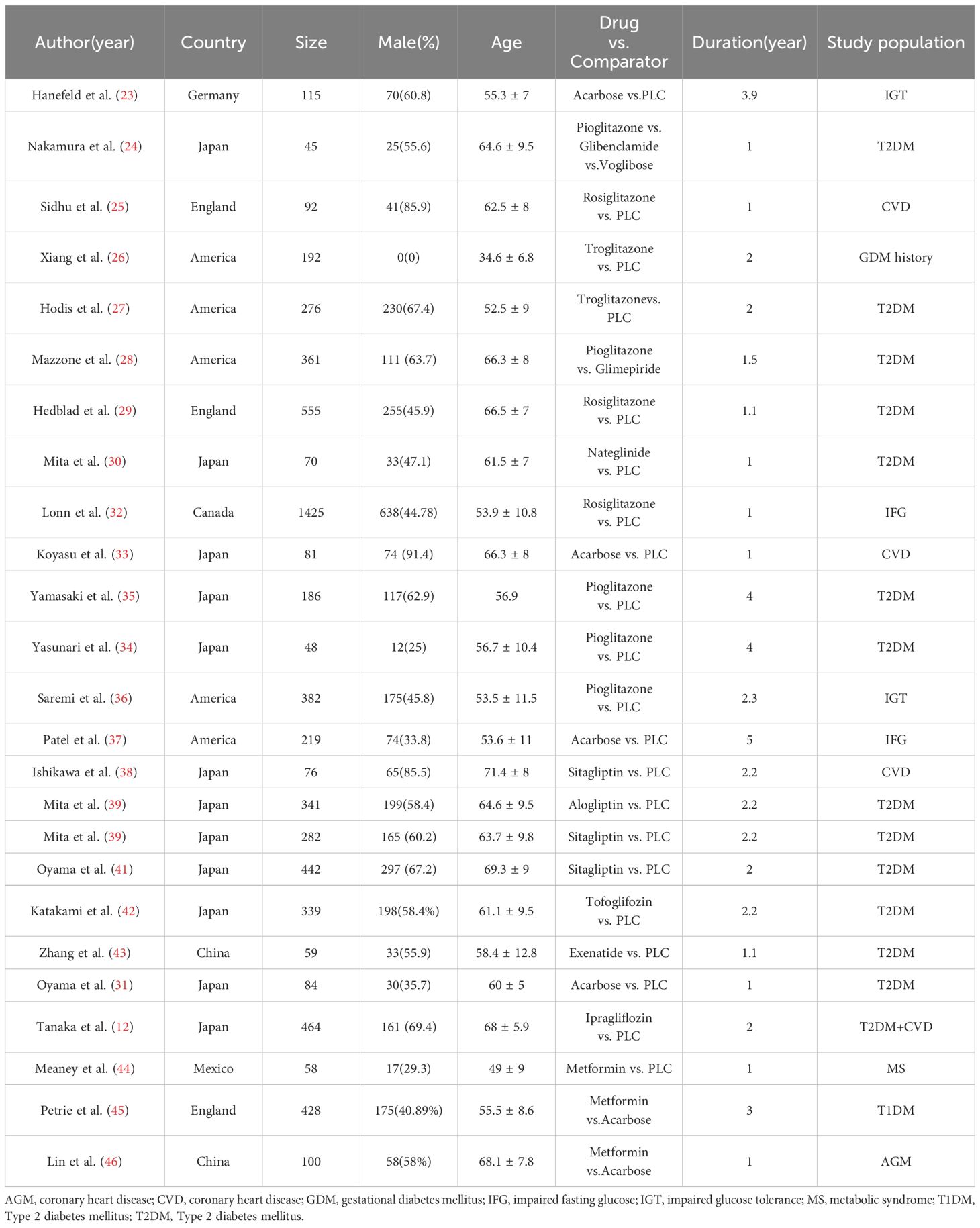
Firstly, the possibly relevant research was imported into EndNote X9, after removing duplicates, independent literature data extraction is conducted by two reviewers, the title and abstract are reviewed to exclude unqualified literature, and then the full text evaluation is conducted. This NMA mainly extracted the basic information of the included literature (name and country of the main author, publication year), basic characteristics of the study population (number of cases, age, sex, disease situation, basic treatment), and outcome indicators (changes of the cIMT).
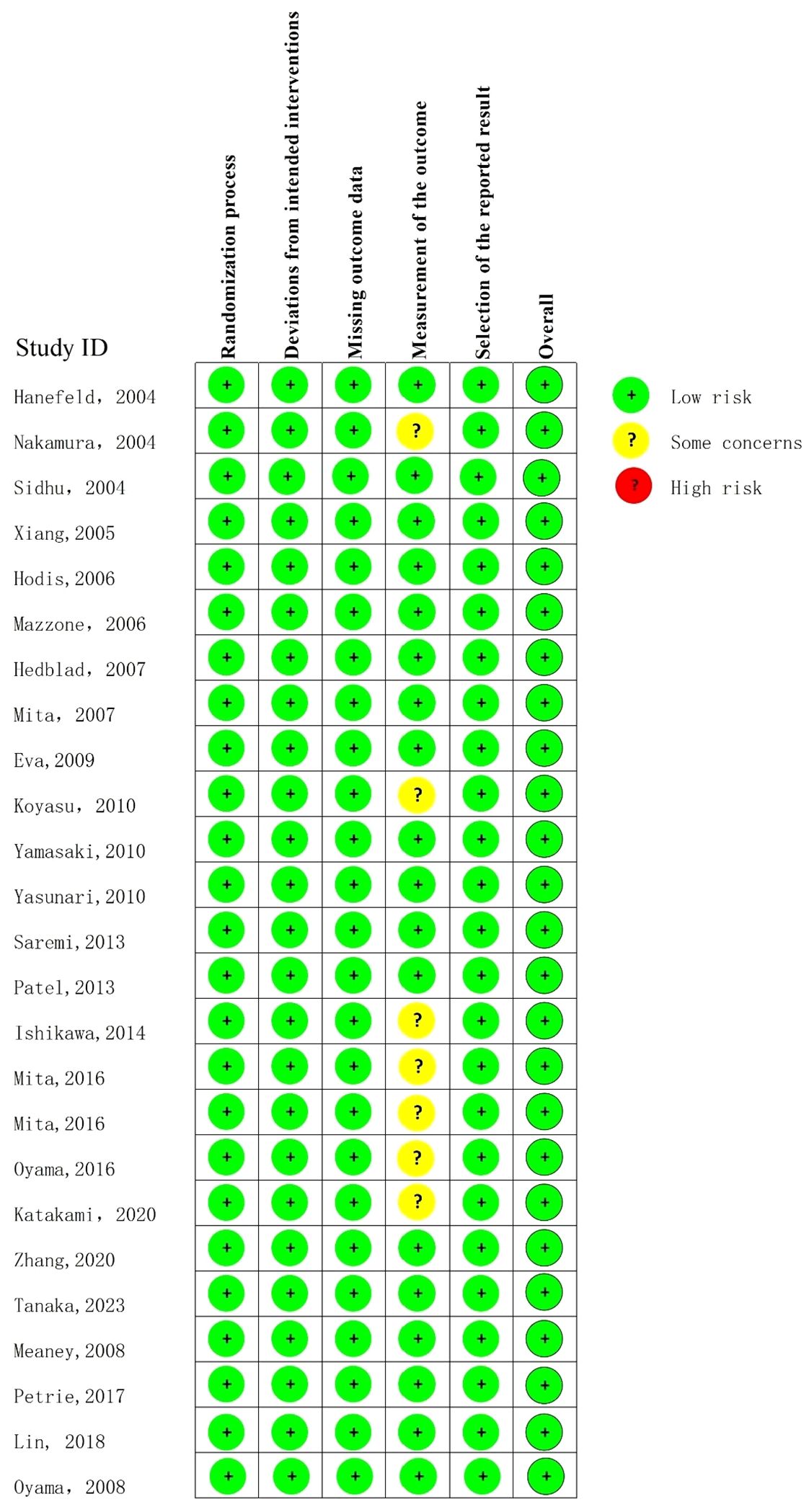
Literature quality was assessed using the RoB (18) in the following five bias domains: randomization process, allocation concealment, intervention blinding of participants and investigators, missing outcome data, outcome measurement and selection of reported results. An algorithm was employed to estimate the overall risk of bias, i.e., low risk, some concerns, or high risk. The above bias risk assessment was independently conducted and cross-checked by two reviewers. In case of disagreement, a third reviewer was involved to discuss and determine the evaluation result.
Data analysis
The statistical model of Bayesian framework was constructed using JAGS software 4.3.1 (gemtc package 0.8–2 and rjags package 4–10) (Rstudio, Boston, MA, USA), cIMT(mm) was used as continuous variable, while mean difference (MD) and 95% credible interval (CI) were used as effect indexes. A random effects model with four Markov chains, each chain generating 50,000 iterations (burn-in period of 20,000 iterations). was used for the outcome indicator. The convergence of iterations was monitored using plots and the Gelman–Rubin–Brooks statistic (19). Model consistency was assessed using the deviation information criterion (DIC), with differences in DIC < 5 points indicating good consistency and consistency modeling was used (20). Heterogeneity was estimated using the I2 statistic with I2 values < 25% indicating low heterogeneity, 25 to 75%, moderate heterogeneity, and > 75%, high heterogeneity (21). Publication bias was assessed by funnel plots. Network plots and funnel plots were drawn by Stata SE 15.0 (Stata Corp, College Station, Texas, USA). In addition, we also comparing the therapeutic effect of HDs using a Surface Under the Cumulative Ranking curve (SUCRA) (22), the closer SUCRA was to 100, the better the therapeutic effect. What is more, we performed sensitivity analyses to examine the influence of individual studies on the total merged effects to assess the stability of the conclusion, and publication bias was analyzed by funnel plot using Stata16.0 software.
Results
Data characteristics
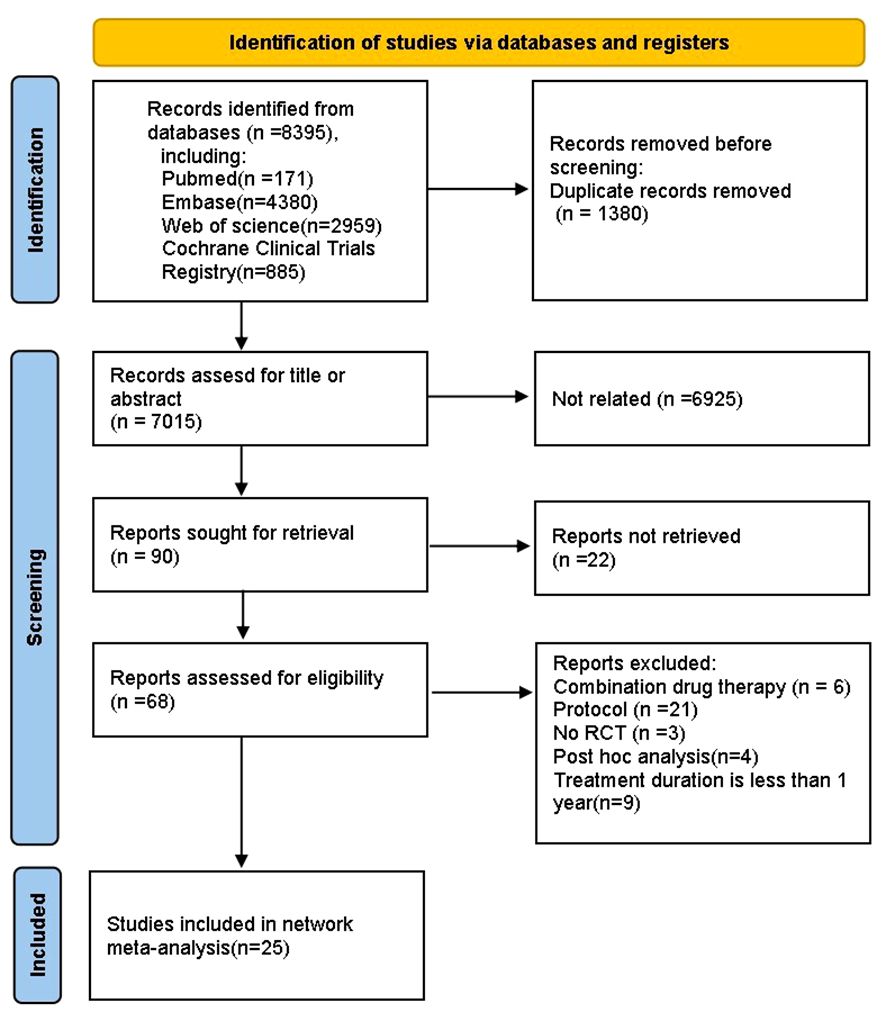
We finally identified 8395 studies through an extensive search, after removing 1380 duplicates, we reviewed the titles and abstracts of the remaining articles based on eligibility criteria. 6959 studies were excluded because they were not relevant to our NMA, original articles were not found for 22 articles, 6 papers reported the combined use of drugs, 21 protocols, 3 studies were not RCT, 4 were post hoc analyses, 9 studies had a treatment duration less than one year. Ultimately, 25 studies (12, 23–46) were included in the NMA, and the literature screening process is shown in the (Figure 1). Our NMA included 14 HDs (acarbose, alogliptin, exenatide, glibenclamide, glimepiride, ipragliflozin, metformin, nateglinide, pioglitazone, rosiglitazone, sitagliptin, tofoglifozin, troglitazone, voglibose and PLC). A total of 6720 patients with complete outcome data were included in the NMA. Among them, there were 2491 patients with pre-diabetes, while the number of patients with diabetes was 3980. The main basic characteristics of the included patients are shown in Table 1.
Quality assessment
The quality assessment results showed that the overall quality of the included literatures was high (Figure 2).
NMA outcomes
The NMA network diagram involved conducting direct and indirect comparisons among a total of 14 drugs (Figure 3), DIC comparison results showed good agreement (DIC, 102.6 VS. 103.0). According to the NMA, exenatide [MD=-0.13,95%CI (-0.25, -0.01)] was found to be the most promising drug for slowing the progression of cIMT comparing to PLC, followed by alogliptin [MD=-0.08,95%CI (-0.13, -0.02)] and metformin [MD=-0.05, 95%CI (-0.09, -0.02)], the forest diagram is shown in Figure 4.

Figure 3 Network plot of clinical trials on HDs or PLC patients. Nodes stand for the comparison between treatments and the size is proportional to the number of subjects. The width of the lines is proportional to the number of trials per pair of interventions.

Figure 4 Forest plot showing the outcomes of NMA (relative differences of various HDs in reducing cIMT compared with PLC).
What’s more, the results of our pairwise comparison analysis among HDs indicate that exenatide[MD=-0.23, 95%CI (-0.39, -0.08)], alogliptin[MD=-0.18,95%CI (-0.3, -0.06)], metformin[MD=-0.16, 95%CI (-0.27, -0.05)], nateglinide[MD=-0.14,95%CI (-0.27, -0.01), pioglitazone[MD=-0.11, 95%CI (-0.21, -0.01)], rosiglitazone[MD=-0.12, 95%CI (-0.23, -0.02)], sitagliptin[MD=-0.13, 95%CI (-0.24, -0.02)] was more effective than glibenclamide; exenatide[MD=-0.14, 95%CI (-0.27, -0.01)] and alogliptin[MD= -0.08, 95%CI (-0.16, -0.01)] was superior to that of tofoglifozin. However, voglibose demonstrated inferior efficacy compared to exenatide [MD=0.23, 95%CI (0.39, 0.07), alogliptine[MD=0.17, 95%CI (0.3, 0.05)], metformin [MD=0.15, 95%CI (0.27, 0.04)] and sitagliptin[MD=0.13, 95%CI (0.24, 0.01)].
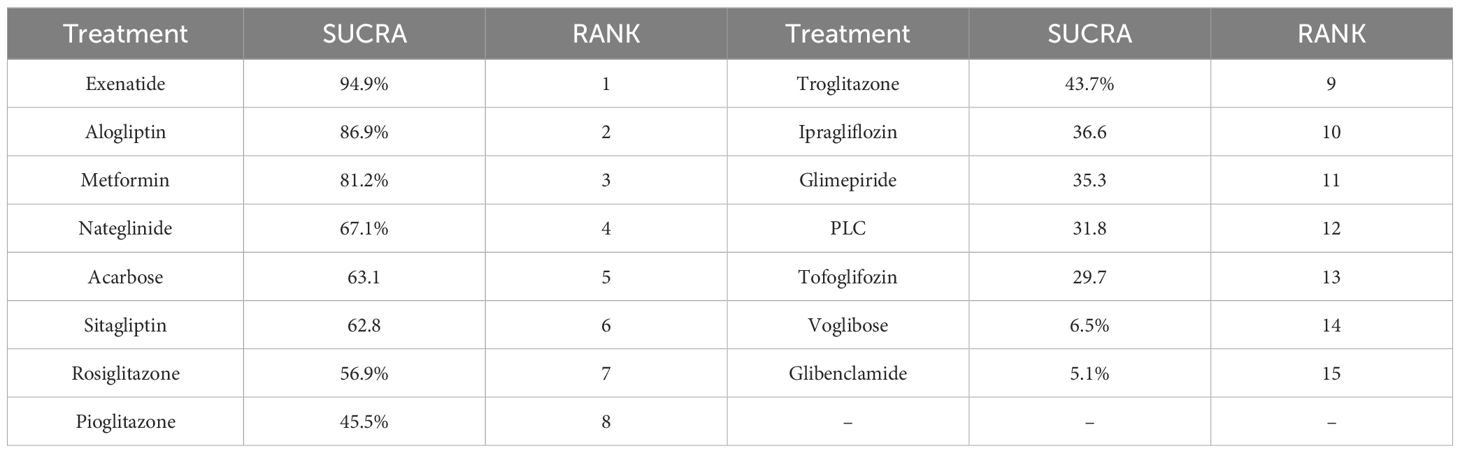
The drug with the highest SUCRA value was exenatide(SUCRA 94.9%), followed by alogliptin(SUCRA 86.9%) and metformin(SUCRA 81.2%), PLC has a SUCRA values with 31.7%, Table 2 shows the results of the SUCRA analysis.
Sensitivity analysis
A sensitivity analyses was conducted by systematically excluding individual studies and performing an additional meta-analysis with each study removed. The impact of each exclusion on the pooled MD was evaluated. Based on the results of the sensitivity analysis, we found that none of the studies significantly influenced the overall effect, indicating the robustness of our NMA. Results of sensitivity analyses are provided in Appendix S2.
Bias of publication
There was no significant publication bias in the funnel plot of bias analysis drawn for cIMT (Figure 5).
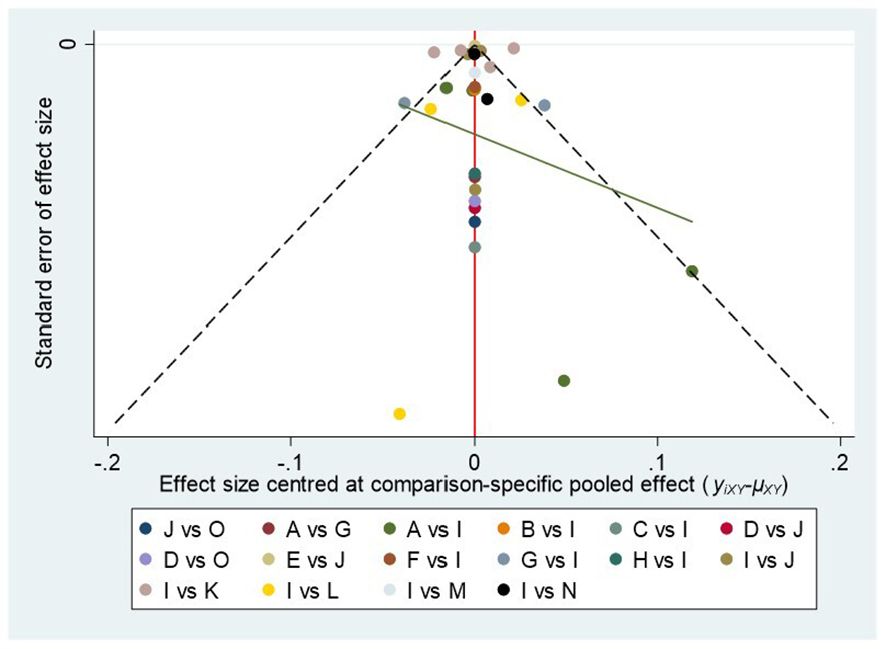
Figure 5 Plot of funnel (A, acarbose; B, alogliptin; C, exenatide; D, glibenclamide; E, glimepiride; F, ipragliflozin; G, metformin; H, nateglinide; I, PLC; J, pioglitazone; K, rosiglitaxone; L, sitagliptin; M, tofoglifozin; N, roglitaxone; O, voglibose).
Discussion
The presence of abnormal blood glucose levels significantly exacerbates the morbidity and mortality associated with atherosclerosis (47, 48). It is of great interest to reduce the occurrence of cardiovascular events through controlling the risk factors. We compared the treatment effects of long-term usage of 14 types of HD on cIMT in individuals with and at high risk for diabetes in our NMA. The results of our NMA indicate that exenatide may be the optimal treatment option, followed by alogliptin and metformin.
Exenatide is a short-acting analogue of the Glucagon-like peptide-1(GLP-1). This type of medications worked by binding to the GLP-1 receptor, improving insulin sensitivity and insulin secretion function, and help to lower blood glucose levels (49–51). Recently, it has been discovered that the utilization of GLP-1 RA can not only effectively regulates blood glucose levels but also exerts significant effects on the modulation of blood lipids, blood pressure, and adipose content (52–54), all of which are recognized risk factors for AS (55–57). Thus, he protective effect of GLP-1 RA on the cardiovascular system may, in part, be attributed to its capacity for mitigating these risk factors. A large meta-analysis, involving 60,080 patients, reported that GLP-1 RA can reduce major adverse cardiovascular events (MACE) in type 2 diabetes patients with ASCVD by 14%, and lowers the risk of hospitalization due to heart failure (58). Our NMA suggests that exenatide, among the 14 drugs included, may be the best treatment for retarding the progression of cIMT. Dyslipidemia has been demonstrated to be a significant risk factor for the development of AS. Various studies have shown that exenatide significantly reduces serum total cholesterol(TC), triglyceride (TG), and low-density lipoprotein cholesterol(LDL-C) levels while increasing high density lipoprotein cholesterol (HDL-C) levels in patients with T2DM, and exhibiting favorable long-term effects (59–61). Koska et al. reported that once weekly administration of exenatide resulted in positive effects on blood glucose and body weight, as well as an appropriate increase in heart rate, which is also a risk factor for carotid atherosclerosis (62, 63). Several in vivo experiments suggest the potential mechanism by which exenatide improves IMT. Yang et al. (64) found that exenatide could inhibit oxidative stress and inflammation in mice, and improve the accumulation of plaque macrophages and osteopontin expression. Jansen et al. (65) demonstrated that exenatide improved abdominal fat deposition in high-fat fed mice. At the same time, there was study reported no statistically significant difference in the incidence of major adverse cardiovascular events with once-weekly exenatide versus PLC (66).
Only one GLP-A RA, exenatide, was included in our study. However, at the same time, some other types of GLP-A RA have also attracted our attention because of their cardiovascular protective effects (67, 68). Related to our study, a real-world study in patients with T2DM showed that the addition of liraglutide to metformin monotherapy significantly reduced cIMT, as well as serum TC, TG, and LDL-C (69). Another clinical study reported that 4 months of semaglutide injection treatment could also reduce cIMT to some extent (70). Unfortunately, these studies were not included in the NMA because they lacked a control group.
The NMA findings also indicate that alogliptin, a DDP-4i, achieved good performance in intervening cIMT progression. DPP-4i, belong to the group of incretin-based medications that act by stimulating the insulin secretion and inhibiting glucagon secretion in a glucose-dependent manner (71). A prospective clinical study observed that a 10-month treatment of alogliptin intervention can significantly reduce the volume of AS plaque and necrosis, as well as an increase in fibrosis volume compared to PLC (72). In addition, a mechanistic study had shown that alogliptin can inhibit the expression of inflammatory factors interleukin-1(IL-1) and Interleukin-6(IL-6) through a glucose-dependent or independent way (73). However, the extended study based on the SPEAD-A trial followed T2DM patients for 520 weeks and found that early use of alogliptin was not associated with a reduction in the risk of developing cardiovascular disease, which may be related to a relatively small number of events during follow-up (74). A clinical trial conducted by Barbieri et al. (75) showed that both 3 months of sitagliptin and vildagliptin treatment may possible to prevent AS progression in patients with T2DM by reducing inflammation and oxidative stress, moreover, compared with baseline, vildagliptin was more effective in reducing IMT than sitagliptin. It has also reported that vildagliptin is more effective than sitagliptin in controlling the mean 24-hour blood glucose level at a same dose (76), and vildagliptin also shows a superior effectiveness in improving oxidative stress and inflammatory markers, the variations in effectiveness among these drugs are likely attributed to their structural heterogeneity (77).
Our study also included three PPARγ agonists, pioglitazone, rosiglitazone and troglitazone, but no significant improvement was observed for any of them compared with PLC. Contrary to our study, a previous meta-analysis reported a statistically significant reduction in the progression of cIMT with the use of pioglitazone (78). At the same time, clinical studies and meta-analyses (79, 80) have reported that long-term use of rosiglitazone may increase the risk of cardiovascular mortality. Indeed, the administration of pioglitazone and rosiglitazone was associated with the adverse effect of fluid retention, which potentially contributed to or exacerbated congestive heart failure (81).
Despite numerous reports demonstrating the cardiovascular benefits of SGLT-2i (82–84), we did not find significant effect of SGLT-2i on the progression of cIMT. This may be related to the limited number of corresponding clinical studies, as we were only able to retrieve data on two SGLT-2i, ipragliflozin and tofogliflozin.
So far, metformin is still the first-line drug for people with diabetes (85). The results of our NMA also demonstrated a significant improvement in cIMT with the long-term administration of metformin. This finding aligns with a meta-analysis conducted by Chen et al. (86), their study also showed that metformin exhibited a significant impact on cIMT only when the intervention exceeded 12 months. Therefore, we speculate that longer duration of drug therapy is likely to result in a more pronounced therapeutic effect.
Limitation
This study may represent the first NMA to compare and rank the effects of long-term use of different HDs on cIMT progression. This NMA may serve as a valuable reference for the utilization of cardiovascular protective drugs in individuals with diabetes or at risk of diabetes. However, the present NMA has some limitations. Firstly, limited number of studies and some small sample sizes may potentially affect the accuracy and applicability of the obtained results. Secondly, the research population in this NMA is based on type 1 diabetes patients, type 2 diabetes patients, pre-diabetes patients and coronary heart disease patients, which may lead to heterogeneity between the studies, so the interpretation of the results should be cautious. At last, we expect to see the performance of a variety of emerging HDs, such as DDP-4i of SGTL-2i, GLP-1 RA and PPARγ agonist. However, the available literature is currently limited. In conclusion, we anticipate to see more kinds of drugs and higher quality RCTs to verify our result in the future.
Conclusions
We employed a Bayesian NMA to assess the impact of prolonged utilization of various HD methods on retarding the progression of carotid intima-media thickness (cIMT). Long-term administration of exenatide, alogliptin and metformin may be most effective in retarding the progression of cIMT.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
QL: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. YY: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. YL: Data curation, Writing – original draft. QW: Data curation, Writing – original draft. XH: Formal Analysis, Writing – original draft. LL: Formal Analysis, Writing – original draft. XY: Data curation, Writing – original draft. CY: Data curation, Writing – original draft. SW: Conceptualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by Beijing Natural Science Foundation (7232311); Science and Technology Innovation Project of China Academy of Chinese Medical Sciences (NO.CI2021A00921).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1403606/full#supplementary-material
Abbreviations
AS, atherosclerosis; ASCVD, atherosclerotic cardiovascular disease; AGM, abnormal glucose metabolism; CI, confidence interval; cIMT, carotid intima-media thickness; DDP-4i, dipeptidyl peptidase-4 inhibitor; DIC, deviation information criterion; GDM, gestational diabetes mellitus; GLP-1 RA, Glucagon like peptide-1 receptor agonists; HD, hypoglycemic drugs; HDL-C, high density lipoprotein cholesterol; IFG; Impaired fasting glucose; IGT, Impaired glucose tolerance; IL-1, interleukin-1; IL-6:interleukin-6;IMT, intima-media thickness; LDL-C, low-density lipoprotein cholesterol; MD, mean difference; MS, Metabolic syndrome; NMA, network meta-analysis; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses; PPARγ, peroxisome proliferator-activated receptor gamma; RCT, randomized controlled trial; SGLT-2i, sodium-glucose cotransporter -2 inhibitor; SUCRA, Surface Under the Cumulative Ranking curve. TC, total cholesterol; TG, triglyceride.
References
1. Frostegård J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. (2013) 11:117. doi: 10.1186/1741–7015-11–117
2. Lundby-Christensen L, Almdal TP, Carstensen B, Tarnow L, Wiinberg N. Carotid intima-media thickness in individuals with and without type 2 diabetes: a reproducibility study. Cardiovasc diabetol. (2010) 9:40. doi: 10.1186/1475–2840-9–40
3. Lorenz MW, Polak JF, Kavousi M, Mathiesen EB, Völzke H, Tuomainen TP, et al. Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): a meta-analysis of individual participant data. Lancet. (2012) 379:2053–62. doi: 10.1016/s0140–6736(12)60441–3
4. Geroulakos G, O’Gorman DJ, Kalodiki E, Sheridan DJ, Nicolaides AN. The carotid intima-media thickness as a marker of the presence of severe symptomatic coronary artery disease. Eur Heart J. (1994) 15:781–5. doi: 10.1093/oxfordjournals.eurheartj.a060585
5. Willeit P, Tschiderer L, Allara E, Reuber K, Seekircher L, Gao L, et al. Carotid intima-media thickness progression as surrogate marker for cardiovascular risk: meta-analysis of 119 clinical trials involving 100 667 patients. Circulation. (2020) 142:621–42. doi: 10.1161/circulationaha.120.046361
6. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. (2009) 120:1640–5. doi: 10.1161/circulationaha.109.192644
7. Wang H, Cordiner RLM, Huang Y, Donnelly L, Hapca S, Collier A, et al. Cardiovascular safety in type 2 diabetes with sulfonylureas as second-line drugs: A nationwide population-based comparative safety study. Diabetes Care. (2023) 46:967–77. doi: 10.2337/dc22–1238
8. Holman RR, Coleman RL, Chan JCN, Chiasson JL, Feng H, Ge J, et al. Effects of acarbose on cardiovascular and diabetes outcomes in patients with coronary heart disease and impaired glucose tolerance (ACE): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes endocrinol. (2017) 5:877–86. doi: 10.1016/s2213–8587(17)30309–1
9. Roumie CL, Chipman J, Min JY, Hackstadt AJ, Hung AM, Greevy RA Jr., et al. Association of treatment with metformin vs sulfonylurea with major adverse cardiovascular events among patients with diabetes and reduced kidney function. Jama. (2019) 322:1167–77. doi: 10.1001/jama.2019.13206
10. Dicker D. DPP-4 inhibitors: impact on glycemic control and cardiovascular risk factors. Diabetes Care. (2011) 34 Suppl 2:S276–8. doi: 10.2337/dc11-s229
11. Krishnan A, Schneider CV, Hadi Y, Mukherjee D, AlShehri B, Alqahtani SA. Cardiovascular and mortality outcomes with GLP-1 receptor agonists vs other glucose-lowering drugs in individuals with NAFLD and type 2 diabetes: a large population-based matched cohort study. Diabetologia. (2024) 67:483–93. doi: 10.1007/s00125–023-06057–5
12. Tanaka A, Sata M, Okada Y, Teragawa H, Eguchi K, Shimabukuro M, et al. Effect of ipragliflozin on carotid intima-media thickness in patients with type 2 diabetes: a multicenter, randomized, controlled trial. Eur Heart J Cardiovasc pharmacotherapy. (2023) 9:165–72. doi: 10.1093/ehjcvp/pvac059
13. Green JB, Everett BM, Ghosh A, Younes N, Krause-Steinrauf H, Barzilay J, et al. Cardiovascular outcomes in GRADE (Glycemia reduction approaches in type 2 diabetes: A comparative effectiveness study). Circulation. (2024) 149:993–1003. doi: 10.1161/circulationaha.123.066604
14. Marx N, Federici M, Schütt K, Müller-Wieland D, Ajjan RA, Antunes MJ, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. (2023) 44:4043–140. doi: 10.1093/eurheartj/ehad192
15. American Diabetes Association Professional Practice Committee, Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2024. Diabetes Care. (2024) 47:S158–s78. doi: 10.2337/dc24-S009
16. Hoaglin DC, Hawkins N, Jansen JP, Scott DA, Itzler R, Cappelleri JC, et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 2. Value health: J Int Soc Pharmacoeconomics Outcomes Res. (2011) 14:429–37. doi: 10.1016/j.jval.2011.01.011
17. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. [The PRISMA 2020 statement: an updated guideline for reporting systematic reviewsDeclaración PRISMA 2020: una guía actualizada para la publicación de revisiones sistemáticas]. Rev panamericana salud publica = Pan Am J Public Health. (2022) 46:e112. doi: 10.26633/rpsp.2022.112
18. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj. (2019) 366:l4898. doi: 10.1136/bmj.l4898
19. Brooks S, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graphi Stat. (1998) 7:434–55. doi: 10.1080/10618600.1998.10474787
20. Dempster AP. The direct use of likelihood for significance testing. Stat Computing. (1997) 7:247–52. doi: 10.1023/a:1018598421607
21. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database systematic Rev. (2019) 10:Ed000142. doi: 10.1002/14651858.Ed000142
22. Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PloS One. (2013) 8:e76654. doi: 10.1371/journal.pone.0076654
23. Hanefeld M, Chiasson JL, Koehler C, Henkel E, Schaper F, Temelkova-Kurktschiev T. Acarbose slows progression of intima-media thickness of the carotid arteries in subjects with impaired glucose tolerance. Stroke. (2004) 35:1073–8. doi: 10.1161/01.STR.0000125864.01546.f2
24. Nakamura T, Matsuda T, Kawagoe Y, Ogawa H, Takahashi Y, Sekizuka K, et al. Effect of pioglitazone on carotid intima-media thickness and arterial stiffness in type 2 diabetic nephropathy patients. Metabolism: Clin experimental. (2004) 53:1382–6. doi: 10.1016/j.metabol.2004.05.013
25. Sidhu JS, Kaposzta Z, Markus HS, Kaski JC. Effect of rosiglitazone on common carotid intima-media thickness progression in coronary artery disease patients without diabetes mellitus. Arteriosclerosis thrombosis Vasc Biol. (2004) 24:930–4. doi: 10.1161/01.Atv.0000124890.40436.77
26. Xiang AH, Peters RK, Kjos SL, Ochoa C, Marroquin A, Goico J, et al. Effect of thiazolidinedione treatment on progression of subclinical atherosclerosis in premenopausal women at high risk for type 2 diabetes. J Clin Endocrinol Metab. (2005) 90:1986–91. doi: 10.1210/jc.2004–1685
27. Hodis HN, Mack WJ, Zheng L, Li Y, Torres M, Sevilla D, et al. Effect of peroxisome proliferator-activated receptor gamma agonist treatment on subclinical atherosclerosis in patients with insulin-requiring type 2 diabetes. Diabetes Care. (2006) 29:1545–53. doi: 10.2337/dc05–2462
28. Mazzone T, Meyer PM, Feinstein SB, Davidson MH, Kondos GT, D’Agostino RB, et al. Effect of pioglitazone compared with glimepiride on carotid intima-media thickness in type 2 diabetes: a randomized trial. Jama. (2006) 296:2572–81. doi: 10.1001/jama.296.21.joc60158
29. Hedblad B, Zambanini A, Nilsson P, Janzon L, Berglund G. Rosiglitazone and carotid IMT progression rate in a mixed cohort of patients with type 2 diabetes and the insulin resistance syndrome: main results from the Rosiglitazone Atherosclerosis Study. J Intern Med. (2007) 261:293–305. doi: 10.1111/j.1365-2796.2007.01767.x
30. Mita T, Watada H, Shimizu T, Tamura Y, Sato F, Watanabe T, et al. Nateglinide reduces carotid intima-media thickening in type 2 diabetic patients under good glycemic control. Arteriosclerosis thrombosis Vasc Biol. (2007) 27:2456–62. doi: 10.1161/atvbaha.107.152835
31. Oyama T, Saiki A, Endoh K, Ban N, Nagayama D, Ohhira M, et al. Effect of acarbose, an alpha-glucosidase inhibitor, on serum lipoprotein lipase mass levels and common carotid artery intima-media thickness in type 2 diabetes mellitus treated by sulfonylurea. J Atheroscl thrombosis. (2008) 15:154–9. doi: 10.5551/jat.e549
32. Lonn EM, Gerstein HC, Sheridan P, Smith S, Diaz R, Mohan V, et al. Effect of ramipril and of rosiglitazone on carotid intima-media thickness in people with impaired glucose tolerance or impaired fasting glucose: STARR (STudy of Atherosclerosis with Ramipril and Rosiglitazone). J Am Coll Cardiol. (2009) 53:2028–35. doi: 10.1016/j.jacc.2008.12.072
33. Koyasu M, Ishii H, Watarai M, Takemoto K, Inden Y, Takeshita K, et al. Impact of acarbose on carotid intima-media thickness in patients with newly diagnosed impaired glucose tolerance or mild type 2 diabetes mellitus: A one-year, prospective, randomized, open-label, parallel-group study in Japanese adults with established coronary artery disease. Clin Ther. (2010) 32:1610–7. doi: 10.1016/j.clinthera.2010.07.015
34. Yasunari E, Takeno K, Funayama H, Tomioka S, Tamaki M, Fujitani Y, et al. Efficacy of pioglitazone on glycemic control and carotid intima-media thickness in type 2 diabetes patients with inadequate insulin therapy. J Diabetes Invest. (2011) 2:56–62. doi: 10.1111/j.2040-1124.2010.00064.x
35. Yamasaki Y, Katakami N, Furukado S, Kitagawa K, Nagatsuka K, Kashiwagi A, et al. Long-term effects of pioglitazone on carotid atherosclerosis in Japanese patients with type 2 diabetes without a recent history of macrovascular morbidity. J Atheroscl thrombosis. (2010) 17:1132–40. doi: 10.5551/jat.4663
36. Saremi A, Schwenke DC, Buchanan TA, Hodis HN, Mack WJ, Banerji M, et al. Pioglitazone slows progression of atherosclerosis in prediabetes independent of changes in cardiovascular risk factors. Arteriosclerosis thrombosis Vasc Biol. (2013) 33:393–9. doi: 10.1161/atvbaha.112.300346
37. Patel YR, Kirkman MS, Considine RV, Hannon TS, Mather KJ. Effect of acarbose to delay progression of carotid intima-media thickness in early diabetes. Diabetes/metabolism Res Rev. (2013) 29:582–91. doi: 10.1002/dmrr.2433
38. Ishikawa S, Shimano M, Watarai M, Koyasu M, Uchikawa T, Ishii H, et al. Impact of sitagliptin on carotid intima-media thickness in patients with coronary artery disease and impaired glucose tolerance or mild diabetes mellitus. Am J Cardiol. (2014) 114:384–8. doi: 10.1016/j.amjcard.2014.04.050
39. Mita T, Katakami N, Yoshii H, Onuma T, Kaneto H, Osonoi T, et al. Alogliptin, a dipeptidyl peptidase 4 inhibitor, prevents the progression of carotid atherosclerosis in patients with type 2 diabetes: the study of preventive effects of alogliptin on diabetic atherosclerosis (SPEAD-A). Diabetes Care. (2016) 39:139–48. doi: 10.2337/dc15–0781
40. Mita T, Katakami N, Shiraiwa T, Yoshii H, Onuma T, Kuribayashi N, et al. Sitagliptin attenuates the progression of carotid intima-media thickening in insulin-treated patients with type 2 diabetes: the sitagliptin preventive study of intima-media thickness evaluation (SPIKE): A randomized controlled trial. Diabetes Care. (2016) 39:455–64. doi: 10.2337/dc15–2145
41. Oyama J, Murohara T, Kitakaze M, Ishizu T, Sato Y, Kitagawa K, et al. The effect of sitagliptin on carotid artery atherosclerosis in type 2 diabetes: the PROLOGUE randomized controlled trial. PloS Med. (2016) 13:e1002051. doi: 10.1371/journal.pmed.1002051
42. Katakami N, Mita T, Yoshii H, Shiraiwa T, Yasuda T, Okada Y, et al. Tofogliflozin does not delay progression of carotid atherosclerosis in patients with type 2 diabetes: a prospective, randomized, open-label, parallel-group comparative study. Cardiovasc diabetol. (2020) 19:110. doi: 10.1186/s12933–020-01079–4
43. Zhang J, Xian TZ, Wu MX, Li C, Pan Q, Guo LX. Comparison of the effects of twice-daily exenatide and insulin on carotid intima-media thickness in type 2 diabetes mellitus patients: a 52-week randomized, open-label, controlled trial. Cardiovasc diabetol. (2020) 19:48. doi: 10.1186/s12933–020-01014–7
44. Meaney E, Vela A, Samaniego V, Meaney A, Asbún J, Zempoalteca JC, et al. Metformin, arterial function, intima-media thickness and nitroxidation in metabolic syndrome: the mefisto study. Clin Exp Pharmacol Physiol. (2008) 35:895–903. doi: 10.1111/j.1440-1681.2008.04920.x
45. Petrie JR, Chaturvedi N, Ford I, Brouwers M, Greenlaw N, Tillin T, et al. Cardiovascular and metabolic effects of metformin in patients with type 1 diabetes (REMOVAL): a double-blind, randomised, placebo-controlled trial. Lancet Diabetes endocrinol. (2017) 5:597–609. doi: 10.1016/s2213–8587(17)30194–8
46. Lin Y, Wang K, Ma C, Wang X, Gong Z, Zhang R, et al. Corrigendum: evaluation of metformin on cognitive improvement in patients with non-dementia vascular cognitive impairment and abnormal glucose metabolism. Front Aging Neurosci. (2018) 10:322. doi: 10.3389/fnagi.2018.00322
47. Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, et al. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. (2007) 30:753–9. doi: 10.2337/dc07–9920
48. Nelson AJ, Peterson ED, Pagidipati NJ. Atherosclerotic cardiovascular disease and heart failure: Determinants of risk and outcomes in patients with diabetes. Prog Cardiovasc Dis. (2019) 62:306–14. doi: 10.1016/j.pcad.2019.07.001
49. Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. (2006) 368:1696–705. doi: 10.1016/s0140–6736(06)69705–5
50. Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinology. (2012) 8:728–42. doi: 10.1038/nrendo.2012.140
51. Ding X, Saxena NK, Lin S, Gupta NA, Anania FA. Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology. (2006) 43:173–81. doi: 10.1002/hep.21006
52. Li H, Yu G, Huang Q, Yang B, Nie J, Liu Y, et al. Efficacy and safety of GLP-1RAs for people with obesity: A systematic review based on RCT and Bayesian network meta-analysis. Biomedicine pharmacotherapy = Biomedecine pharmacotherapie. (2024) 171:116150. doi: 10.1016/j.biopha.2024.116150
53. Wang B, Zhong J, Lin H, Zhao Z, Yan Z, He H, et al. Blood pressure-lowering effects of GLP-1 receptor agonists exenatide and liraglutide: a meta-analysis of clinical trials. Diabetes Obes Metab. (2013) 15:737–49. doi: 10.1111/dom.12085
54. Bunck MC, Diamant M, Eliasson B, Cornér A, Shaginian RM, Heine RJ, et al. Exenatide affects circulating cardiovascular risk biomarkers independently of changes in body composition. Diabetes Care. (2010) 33:1734–7. doi: 10.2337/dc09–2361
55. Meigs JB, Nathan DM, D’Agostino RB, Wilson PW. Fasting and postchallenge glycemia and cardiovascular disease risk: the Framingham Offspring Study. Diabetes Care. (2002) 25:1845–50. doi: 10.2337/diacare.25.10.1845
56. Capellini VK, Celotto AC, Baldo CF, Olivon VC, Viaro F, Rodrigues AJ, et al. Diabetes and vascular disease: basic concepts of nitric oxide physiology, endothelial dysfunction, oxidative stress and therapeutic possibilities. Curr Vasc Pharmacol. (2010) 8:526–44. doi: 10.2174/157016110791330834
57. Chen J, Li W, Cao J, Lu Y, Wang C, Lu J. Risk factors for carotid plaque formation in type 2 diabetes mellitus. J Trans Med. (2024) 22:18. doi: 10.1186/s12967–023-04836–7
58. Sattar N, Lee MMY, Kristensen SL, Branch KRH, Del Prato S, Khurmi NS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes endocrinol. (2021) 9:653–62. doi: 10.1016/s2213–8587(21)00203–5
59. Liang Y, Vetrano DL, Qiu C. Serum total cholesterol and risk of cardiovascular and non-cardiovascular mortality in old age: a population-based study. BMC geriatrics. (2017) 17:294. doi: 10.1186/s12877-017-0685-z
60. Vallejo-Vaz AJ, Robertson M, Catapano AL, Watts GF, Kastelein JJ, Packard CJ, et al. Low-density lipoprotein cholesterol lowering for the primary prevention of cardiovascular disease among men with primary elevations of low-density lipoprotein cholesterol levels of 190 mg/dL or above: analyses from the WOSCOPS (West of scotland coronary prevention study) 5-year randomized trial and 20-year observational follow-up. Circulation. (2017) 136:1878–91. doi: 10.1161/circulationaha.117.027966
61. Yu XH, Zhang DW, Zheng XL, Tang CK. Cholesterol transport system: An integrated cholesterol transport model involved in atherosclerosis. Prog Lipid Res. (2019) 73:65–91. doi: 10.1016/j.plipres.2018.12.002
62. Fox K, Borer JS, Camm AJ, Danchin N, Ferrari R, Lopez Sendon JL, et al. Resting heart rate in cardiovascular disease. J Am Coll Cardiol. (2007) 50:823–30. doi: 10.1016/j.jacc.2007.04.079
63. Whelton SP, Blankstein R, Al-Mallah MH, Lima JA, Bluemke DA, Hundley WG, et al. Association of resting heart rate with carotid and aortic arterial stiffness: multi-ethnic study of atherosclerosis. Hypertension. (2013) 62:477–84. doi: 10.1161/hypertensionaha.113.01605
64. Yang G, Lei Y, Inoue A, Piao L, Hu L, Jiang H, et al. Exenatide mitigated diet-induced vascular aging and atherosclerotic plaque growth in ApoE-deficient mice under chronic stress. Atherosclerosis. (2017) 264:1–10. doi: 10.1016/j.atherosclerosis.2017.07.014
65. Jansen KM, Moreno S, Garcia-Roves PM, Larsen TS. Dietary Calanus oil recovers metabolic flexibility and rescues postischemic cardiac function in obese female mice. Am J Physiol Heart Circulatory Physiol. (2019) 317:H290–h9. doi: 10.1152/ajpheart.00191.2019
66. Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. New Engl J Med. (2017) 377:1228–39. doi: 10.1056/NEJMoa1612917
67. Williams TC, Stewart E. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. New Engl J Med. (2017) 376:891. doi: 10.1056/NEJMc1615712
68. Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. New Engl J Med. (2016) 375:311–22. doi: 10.1056/NEJMoa1603827
69. Nikolic D, Giglio RV, Rizvi AA, Patti AM, Montalto G, Maranta F, et al. Liraglutide reduces carotid intima-media thickness by reducing small dense low-density lipoproteins in a real-world setting of patients with type 2 diabetes: A novel anti-atherogenic effect. Diabetes therapy: research Treat Educ Diabetes related Disord. (2021) 12:261–74. doi: 10.1007/s13300–020-00962–3
70. Patti AM, Giglio RV, Allotta A, Bruno A, Di Bella T, Pantea Stoian A, et al. Effect of semaglutide on subclinical atherosclerosis and cardiometabolic compensation: A real-world study in patients with type 2 diabetes. Biomedicines. (2023) 11(5):1362. doi: 10.3390/biomedicines11051362
71. Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. (2013) 17:819–37. doi: 10.1016/j.cmet.2013.04.008
72. Okada K, Kikuchi S, Kuji S, Nakayama N, Maejima N, Matsuzawa Y, et al. Impact of early intervention with alogliptin on coronary plaque regression and stabilization in patients with acute coronary syndromes. Atherosclerosis. (2022) 360:1–7. doi: 10.1016/j.atherosclerosis.2022.09.005
73. Ta NN, Schuyler CA, Li Y, Lopes-Virella MF, Huang Y. DPP-4 (CD26) inhibitor alogliptin inhibits atherosclerosis in diabetic apolipoprotein E-deficient mice. J Cardiovasc Pharmacol. (2011) 58:157–66. doi: 10.1097/FJC.0b013e31821e5626
74. Mita T, Katakami N, Yoshii H, Onuma T, Kaneto H, Osonoi T, et al. Long-term efficacy and safety of early alogliptin initiation in subjects with type 2 diabetes: an extension of the SPEAD-A study. Sci Rep. (2023) 13:14649. doi: 10.1038/s41598–023-41036–1
75. Barbieri M, Rizzo MR, Marfella R, Boccardi V, Esposito A, Pansini A, et al. Decreased carotid atherosclerotic process by control of daily acute glucose fluctuations in diabetic patients treated by DPP-IV inhibitors. Atherosclerosis. (2013) 227:349–54. doi: 10.1016/j.atherosclerosis.2012.12.018
76. Sakamoto M, Nishimura R, Irako T, Tsujino D, Ando K, Utsunomiya K. Comparison of vildagliptin twice daily vs. sitagliptin once daily using continuous glucose monitoring (CGM): crossover pilot study (J-VICTORIA study). Cardiovasc diabetol. (2012) 11:92. doi: 10.1186/1475–2840-11–92
77. Rizzo MR, Barbieri M, Marfella R, Paolisso G. Reduction of oxidative stress and inflammation by blunting daily acute glucose fluctuations in patients with type 2 diabetes: role of dipeptidyl peptidase-IV inhibition. Diabetes Care. (2012) 35:2076–82. doi: 10.2337/dc12–0199
78. Webb DR, Davies MJ, Gray LJ, Abrams KR, Srinivasan B, Das S, et al. Searching for the right outcome? A systematic review and meta-analysis of controlled trials using carotid intima-media thickness or pulse wave velocity to infer antiatherogenic properties of thiazolidinediones. Diabetes Obes Metab. (2010) 12:124–32. doi: 10.1111/j.1463-1326.2009.01122.x
79. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. New Engl J Med. (2007) 356:2457–71. doi: 10.1056/NEJMoa072761
80. Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. Jama. (2007) 298:1189–95. doi: 10.1001/jama.298.10.1189
81. Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, et al. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. Circulation. (2003) 108:2941–8. doi: 10.1161/01.CIR.0000103683.99399.7E
82. Yoshihara F, Imazu M, Sakuma I, Hiroi Y, Hara H, Okazaki O, et al. DAPagliflozin for the attenuation of albuminuria in Patients with hEaRt failure and type 2 diabetes (DAPPER study): a multicentre, randomised, open-label, parallel-group, standard treatment-controlled trial. EClinicalMedicine. (2023) 66:102334. doi: 10.1016/j.eclinm.2023.102334
83. Kutz A, Kim DH, Wexler DJ, Liu J, Schneeweiss S, Glynn RJ, et al. Comparative cardiovascular effectiveness and safety of SGLT-2 inhibitors, GLP-1 receptor agonists, and DPP-4 inhibitors according to frailty in type 2 diabetes. Diabetes Care. (2023) 46:2004–14. doi: 10.2337/dc23–0671
84. Biegus J, Fudim M, Salah HM, Heerspink HJL, Voors AA, Ponikowski P. Sodium-glucose cotransporter-2 inhibitors in heart failure: Potential decongestive mechanisms and current clinical studies. Eur J Heart Fail. (2023) 25:1526–36. doi: 10.1002/ejhf.2967
85. Gnesin F, Thuesen ACB, Kähler LKA, Madsbad S, Hemmingsen B. Metformin monotherapy for adults with type 2 diabetes mellitus. Cochrane Database systematic Rev. (2020) 6:Cd012906. doi: 10.1002/14651858.CD012906.pub2
Keywords: atherosclerosis, intima-media thickness, antidiabetic drug, cardiovascular, diabetes
Citation: Lv Q, Yang Y, Lv Y, Wu Q, Hou X, Li L, Ye X, Yang C and Wang S (2024) Long-term effects of different hypoglycemic drugs on carotid intima-media thickness progression: a systematic review and network meta-analysis. Front. Endocrinol. 15:1403606. doi: 10.3389/fendo.2024.1403606
Received: 19 March 2024; Accepted: 15 May 2024;
Published: 31 May 2024.
Edited by:
Gabor Czibik, Queen Mary University of London, United KingdomReviewed by:
Aleksandra Jotic, University of Belgrade, SerbiaAlina Yu Babenko, Almazov National Medical Research Centre, Russia
Copyright © 2024 Lv, Yang, Lv, Wu, Hou, Li, Ye, Yang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shihan Wang, d2FuZ3NoaWhhbjkxQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 Qianyu Lv
Qianyu Lv Yingtian Yang
Yingtian Yang Yanfei Lv2
Yanfei Lv2 Qian Wu
Qian Wu Xinzheng Hou
Xinzheng Hou Lanlan Li
Lanlan Li Xuejiao Ye
Xuejiao Ye Chenyan Yang
Chenyan Yang Shihan Wang
Shihan Wang