
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 28 May 2024
Sec. Pituitary Endocrinology
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1400671
 Atsushi Ishida1*
Atsushi Ishida1* Naoko Inoshita2
Naoko Inoshita2 Noriaki Tanabe2
Noriaki Tanabe2 Koji Takano3
Koji Takano3 Sachiko Tanaka-Mizuno4
Sachiko Tanaka-Mizuno4 Masataka Kato1
Masataka Kato1 Haruko Yoshimoto1
Haruko Yoshimoto1 Hideki Shiramizu1
Hideki Shiramizu1 Go Matsuoka1
Go Matsuoka1 Shozo Yamada1
Shozo Yamada1Objective: Postoperative nonfunctioning pituitary tumor (NFPT) regrowth is a significant concern, but its predictive factors are not well established. This study aimed to elucidate the pathological characteristics of NFPTs indicated for reoperation for tumor regrowth.
Methods: Pathological, radiological, and clinical data were collected from patients who underwent repeat operation for NFPT at Moriyama Memorial Hospital (MMH) between April 2018 and September 2023. For comparison, we also gathered data from patients who underwent initial surgery for NFPT during the same period at MMH.
Results: Overall, 61 and 244 NFPT patients who respectively underwent reoperation and initial operation were evaluated. The mean period between the previous operation and reoperation was 113 months. Immunonegativity for any adenohypophyseal hormone was significantly more frequent in the reoperation group than in the initial operation group. In addition, the rate of hormone-negative but transcription factor–positive (H-/TF+) tumors among silent gonadotroph tumors was significantly higher in the reoperation group than in the initial operation group. Furthermore, seven silent corticotroph tumors (SCTs) in the reoperation group were ACTH-negative but TPIT-positive. Because most of the previous surgeries were performed in other hospitals a long time ago, we could procure the previous pathological results with immunohistochemistry (IHC) only from 21 patients. IHC for TF had not been performed in all the previous specimens. IHC for adenohypophyseal hormone was almost the same as the current results, and many H-/TF+ tumors were previously diagnosed as NCT. In addition, the reoperated patients were classified into 3 groups on the basis of the condition of the previous operation: gross total resection (GTR), 12 patients; subtotal resection (STR), 17 patients; and partial resection (PR), 32 patients. The mean Ki-67 LI in the GTR, STR, and PR subgroups were 1.82, 1.37, and 0.84, respectively, with the value being significantly higher in the GTR subgroup than in the PR subgroup (P < 0.05).
Conclusions: The ratio of H-/TF+ tumors is significantly higher in symptomatically regrown tumors than in the initial cases, which used to be diagnosed as NCT. PR cases tend to grow symptomatically in a shorter period, even with lower Ki-67 LI than GTR cases.
Nonfunctioning pituitary tumors (NFPTs) are mostly benign and lack the clinical symptoms of excessive pituitary hormone levels. NFPTs usually show symptoms owing to the mass effect of the tumor, such as hypopituitarism and/or visual disturbance (1–3). Transsphenoidal surgery (TSS) is the gold standard for NFPT treatment (4, 5). After TSS for NFPT, recurrence is defined as the re-emergence of tumors from the absence of residual tumors on postoperative magnetic resonance imaging (MRI), whereas regrowth indicates that the remaining tumors after operation show enlargement during follow-up. The complete resection of NFPT at the cellular level is difficult, and theoretically, some remaining tumors usually exist and may regrow. Therefore, we used the term “regrowth” for “recurrence or regrowth” in this report. The symptomatic regrowth of NFPTs is not uncommon, but management is generally challenging because reoperation is more complicated and technically difficult than the initial operation (6). Furthermore, there is no effective medical therapy for most NFPTs. Thus, the characteristics of tumors that tend to regrow after operation are of great interest to pituitary surgeons. However, no histological predictive factors for NFPT regrowth have been identified.
The 4th edition of the World Health Organization (WHO) classification was updated to include immunohistochemistry (IHC) for transcription factors (TFs), such as steroidogenic factor 1 (SF1), T-box transcription factor (TPIT), and pituitary transcription factor 1 (PIT1), to classify NFPT [7]. The null cell tumor (NCT) was defined as immune-negative NFPT (i.e., negative transcription factors) in the 5th edition of the WHO classification (7–9). Since then, several studies have reported a relationship between these histological characteristics and regrowth rates (10–16). However, evidence is still controversial owing to the technical difficulty of IHC and the lack of a sufficient number of reoperated NFPT cases (10–16). Previous studies emphasized that gross total resection (GTR) in the initial operation was essential to prevent regrowth (17, 18). Thus, the better the surgical skills of the surgeon, the fewer regrowth occur. However, there are no reports of many reoperated cases, especially those with the abovementioned pathological characteristics. In addition, NFPTs with Ki-67 labeling index (LI) > 3% are at high risk of regrowth (19, 20). However, the Ki-67 LI of most NFPTs is <3% (21, 22); thus, the majority of regrowing NFPTs should have a low Ki-67 LI.
We encountered many cases of NFPT that regrew and became symptomatic after previous surgery. The senior author (S.Y.) reoperated on these cases, and an endocrine pathologist (N.I.) examined the extracted specimens and those from the initial surgical cases. Thus, we took advantage of this situation and studied the characteristics of reoperated NFPT specimens in a large population, and compared them with those of initial surgical cases.
This retrospective study was approved by the ethical review board of Moriyama Memorial Hospital (MMH) (Approval No. 23013) and was conducted according to the tenets of the Declaration of Helsinki. All patients with NFPT who underwent TSS for MMH between April 2018 and September 2023 were evaluated. Data, including radiographic, operative, and pathological findings, were obtained from medical records. Patients with information from previous hospitals or sufficient pathological examinations at MMH were included.
The patients were divided into two groups: the reoperation group and the initial operation group. The reoperation patients underwent one or more surgeries, mostly in other hospitals. These patients were referred to our senior author for repeat operation to ameliorate the relapsed symptoms years after the previous operation. The reoperation group was further classified into three subgroups on the basis of the extent of resection (EOR) in the previous operation assessed by postoperative MRI and surgical record. Those with no detectable residual tumor on MRI were categorized into the GTR subgroup. Most of the tumors were resected in the subtotal resection (STR) subgroup, but a residue of <10% was present on postsurgical MRI. For the partial resection (PR) subgroup, ≥10% of the tumor remained after the operation (23).
The tissue specimens were evaluated using routine pathological and immunohistochemical examinations. Sections were incubated with the following antibodies for immunohistochemical evaluation: cytokeratin (CAM 5.2, Roche Diagnostic, Basel, Switzerland), growth hormone (MU925–5UC, BioGenex, Fremont, CA, USA), Ki-67 (MIB-1, Dako, Carpinteria, CA, USA), prolactin (PRL/2644, BioGenex, Fremont, CA, USA), adrenocorticotrophic hormone (ACTH) (02A3, Dako, Carpinteria, CA, USA), luteinizing hormone (C93, Dako, Carpinteria, CA, USA), follicle-stimulating hormone (C10, Dako, Carpinteria, CA, USA), thyrotropin stimulating hormone (0042, Dako, Carpinteria, CA, USA), PIT1 (D-7, Santa Cruz Biotechnology, Dallas, TX, USA), TPIT (CL6251; Atlas Antibodies, Bromma, Sweden), and SF1 (N1665; Perseus Proteomics, Tokyo, Japan). IHC studies were performed using a BenchMark GX automated slide preparation system (Ventana, Roche, Basel, Switzerland) according to the manufacturer’s protocols, except for SF1, in which samples were incubated overnight. Staining results were considered positive when >1% of the cells were positive for adenohypophyseal hormones; other patients were classified as hormone-negative tumors (HNTs) and examined also for TFs. Among them, tumors with immunoreactivity for TFs were named as hormone-negative but TF–positive (H-/TF+) tumors. In addition, the Ki-67 LI was measured in 1000 tumor cells, and the positivity rate was noted.
Clinicodemographic patient factors were described using summary statistics. Categorical variables were presented as numbers and proportions, whereas continuous variables were presented as means and ranges. The immunohistochemical results were compared between the reoperation and initial operation groups by using Pearson’s chi-square test. The mean of Ki-67 LI and time until reoperation were compared among the three subgroups. Comparisons were made for each of the two subgroups by using Tukey’s test, with consideration to the multiplicity of statistical tests. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). P-value < 0.05 was considered significant.
The reoperation group involved 61 patients; 12, 17, and 32 patients were classified into the GTR, STR, and PR subgroups, respectively, based on the EOR in the previous operation. A total of 13/61 patients had undergone previous surgeries performed by the senior author (11 GTR patients and 2 STR patients). Previous surgeries of the remaining 48 patients were performed in other hospitals, and they were referred to us for repeat surgery to ameliorate imminent symptoms. We could procure the previous pathological results with reliable IHC from 21 patients among the reoperated 61 patients. IHC for TF was not studied in those patients because the initial surgeries were performed a long time ago. Meanwhile, the initial operation group performed in our hospital consisted of 244 patients. All surgeries of both the reoperation and the initial operation groups were performed by the same senior author. Demographics of patients in reoperation and initial operation groups were comparable and summarized in Table 1.
Figure 1 demonstrates the histological classification of the reoperation (Figure 1A) and initial (Figure 1B) groups based on IHC results. There were 35 silent gonadotroph tumors (SGTs) (H-/TF+: 21), 24 silent corticotroph tumors (SCTs) (H-/TF+: 7), and 1 HNT but PIT1-positive tumor in the reoperation group. Meanwhile, there were 174 SGTs (H-/TF+: 68), 55 SCTs (H-/TF+: 15), and 3 HNT but PIT1-positive tumors in the initial operation group. The comparison of IHC results between the two groups is shown in Table 2. The HNT rate was significantly higher in the reoperation group. Additionally, the proportion of SCTs was significantly higher in the reoperated group than in the initial operation group. The rate of gonadotropin-negative, SF1-positive tumors among all SGTs was significantly higher in the reoperation group (Table 2). In the reoperation group, the mean Ki-67 LI of SCTs was slightly higher (1.35) than that of SGTs (1.09) and others (0.90), but the difference was not significant. The characteristics of the 21 patients in which the previous pathological results were available are also shown in Table 3. IHC was only done for adenohypophyseal hormones and not for TFs as mentioned previously. We compared the immunoreactivity between the current and the previous specimen to prove the validity of this study. There were only three patients who had discordant results. IHC for adenohypophyseal hormones was negative previously but positive this time in those three cases (Table 3). As a result, 12 patients were diagnosed as NCT previously (Table 3).
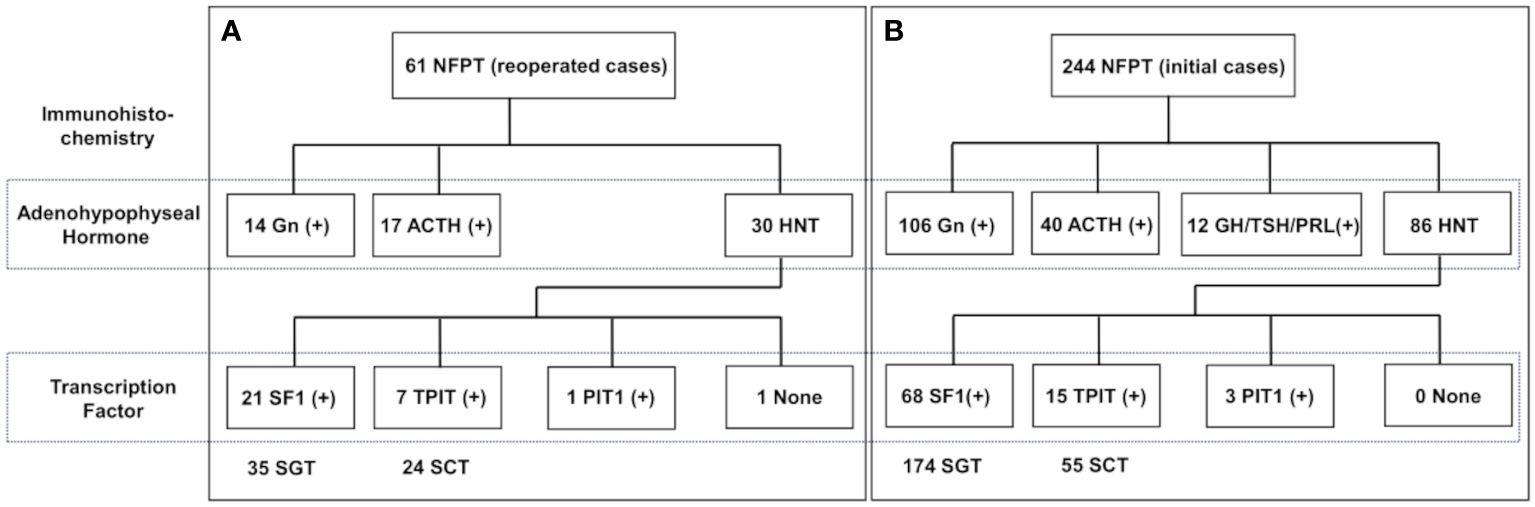
Figure 1 Histological classification of NFPT divided into 2 groups: (A) reoperation (n = 61) and (B) initial operation (n = 244). Histological classification is according to the results of immunohistochemistry. NFPT, nonfunctioning pituitary tumor; Gn, gonadotropin; ACTH, adrenocorticotrophic hormone; GH, growth hormone; TSH, thyrotropin stimulating hormone; PRL, prolactin; HNT, hormone-negative tumor; SF1, steroidogenic factor 1; TPIT, T-box transcription factor; PIT1, pituitary transcription factor 1; SGT, silent gonadotroph tumor; SCT, silent corticotroph tumor.
The demographics of the subgroups classified by EOR of the previous TSS are shown in Table 4. Some patients with STR and PR had already undergone two or more surgeries and/or radiotherapy (RT) at the previous hospitals. The Ki-67 LI of the 7 patients who had previously undergone RT in the reoperation group was 1.27, and it was not elevated after RT. Two patients in the reoperation group had Ki-67 LI > 3%, but the majority had Ki-67 LI < 2%. Ki-67 LI was higher in the GTR subgroup than in the PR and STR subgroups, although the difference was only significant for the PR subgroup (Table 4). Ki-67 LI values were also higher in the STR subgroup than in the PR group, although the difference was not significant. The period from the previous operation to our repeat operation was longer in the GTR subgroup than in the PR subgroup, although the difference was not significant (Table 4). Representative comparisons of regrown NFPTs between GTR and PR patients are shown in Figure 2. Enhanced T1-weighted MR images the GTR case (Figures 2A–C) and PR case (Figures 2D–F), respectively, illustrate tumor regrowth and compression of the optic nerve. The GTR case with Ki-67 LI: 2.2 regrew symptomatically in 137 months, while the PR case with Ki-67 LI: 0.4 showed symptomatic regrowth in 80 months.
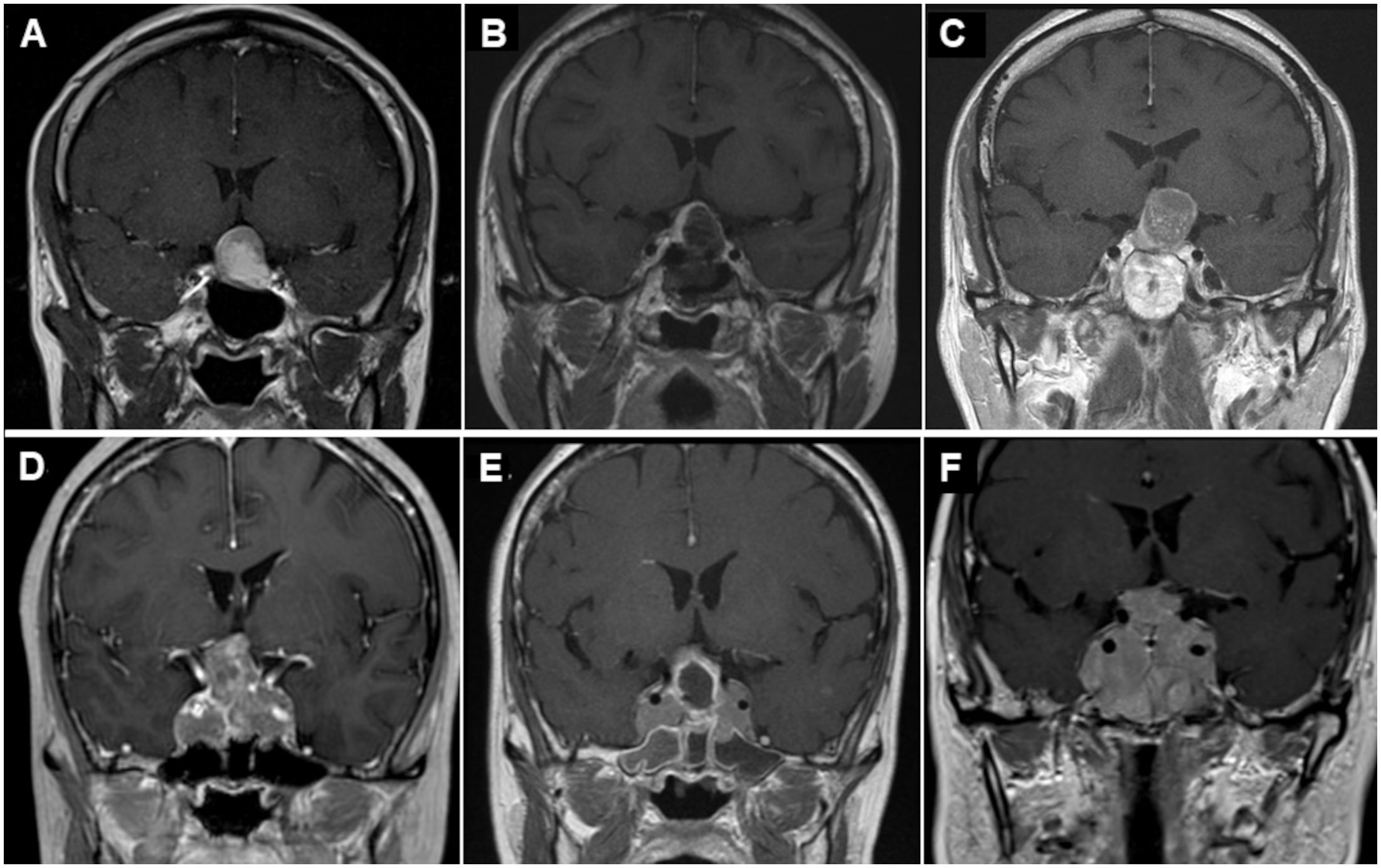
Figure 2 Representative case of regrowing nonfunctioning pituitary tumors after prior TSS. GTR case (A–C) and PR case (D–F). Preoperative (A) and postoperative (B) enhanced T1-weighted MR images showing GTR after initial surgery. However, enhanced T1-weighted MR image before reoperation (C) showing tumor regrowth and compression of the optic nerve again 137 months after the primary surgery. Preoperative (D) and postoperative (E) enhanced T1-weighted MR images showing PR after initial TSS. Preoperative enhanced T1-weighted MR image before reoperation (F) showing tumor regrowth and compression of the optic nerve again 80 months after the primary surgery. TSS, transsphenoidal surgery; GTR, gross total resection; PR, partial resection; MR, magnetic resonance.
This study found the characteristics of NFPT with symptomatic regrowth and provides further insight into how these cases should be followed. NFPT regrowth is a medical challenge in the management of pituitary tumors. The number of recurrent or regrowing tumors must be analyzed to predict the type of NFPT regrowth postoperatively. In the 4th edition of the WHO classification, immunostaining for TFs was newly added to achieve a more confident pathological diagnosis of NFPT; tumors that were negative for both conventional adenohypophyseal hormones and TFs were only diagnosed as NCT (7–9). We thoroughly reviewed previous literature that investigated the relationship between histopathological classification and recurrence or regrowth in NFPT, but the results were inconsistent and debatable. These data are summarized in Table 5 (10–16, 24, 25). Notably, there were many studies in which the NCT accounted for more than 10% of the entire NFPT. The possible causes of this inconsistency include IHC without TFs and the small number of surgical cases of regrowth. In addition, the long-term recurrence rate after GTR is low (6, 11). The reoperation rate is lower in high-volume centers with experienced surgeons, and the data on pathological results are limited to be statistically significant (11). Thus, academic discussion is difficult. At our institution, we encounter many cases of regrown tumors requiring reoperation. In the past 5 years, we have obtained solid pathological results, in all 61 cases of reoperation including immunostaining for TFs. We compared the characteristics of the reoperated cases with those of the initial surgical cases (n = 244). We also compared the immunoreactivity between the current and the previous specimen to prove the validity of this study. There were only three patients who had discordant results, in which IHC for adenohypophyseal hormones was negative previously but positive this time. This may be due to better antibodies and technical improvement from the previous pathological study which was conducted a long time ago. Thus, our strategy to use the current pathological results should be optimal.
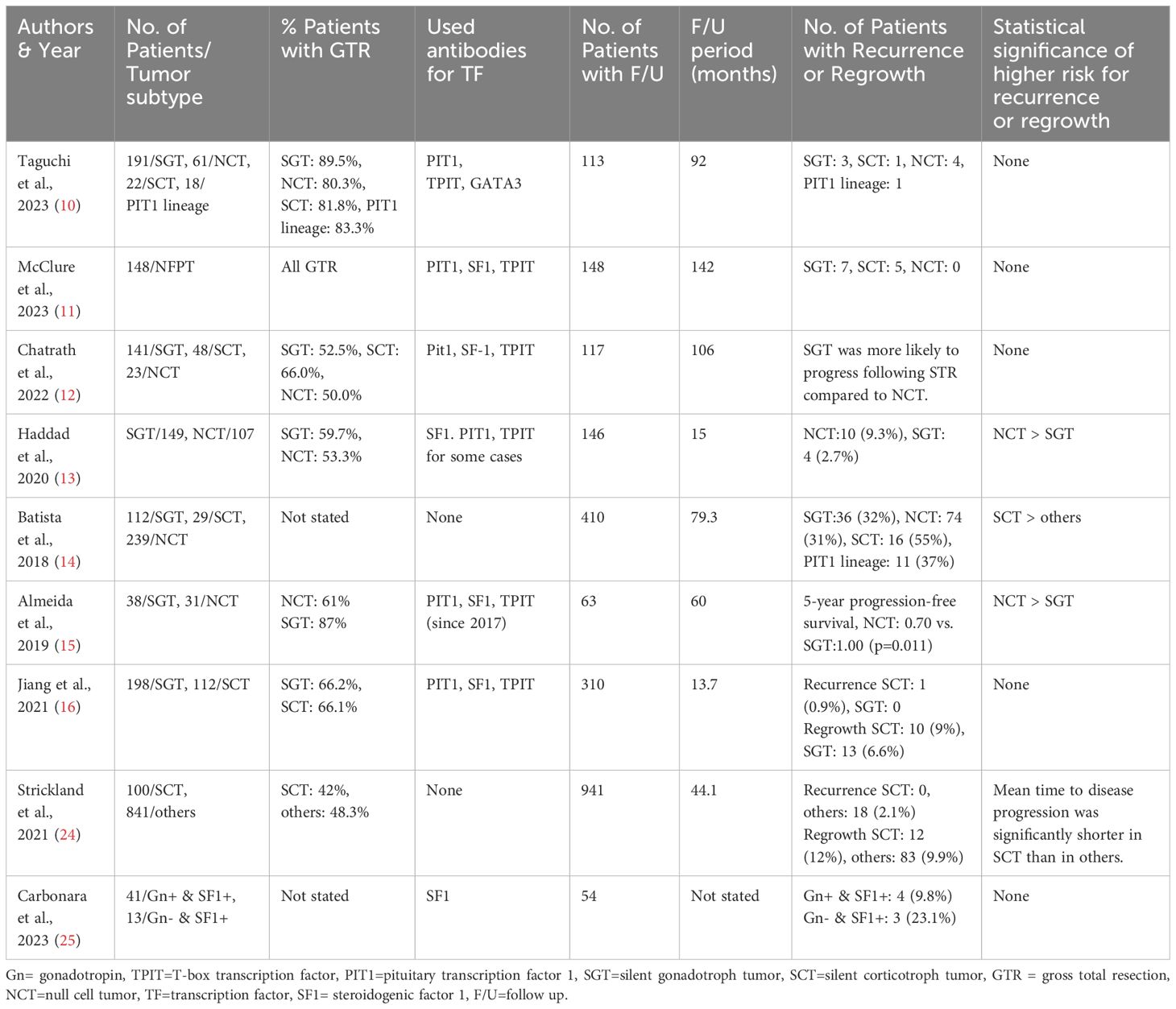
Table 5 Previous literature on the relationship between histopathological classification and recurrence or regrowth in nonfunctioning pituitary tumor (NFPT).
In our recent study, no “true NCT” was detected among 1071 pituitary neuroendocrine tumors (PitNETs), and we emphasized the importance of precise diagnosis following the most recent criteria to improve therapeutic success (21, 26). There are several NCT cases in other reports (Table 5). IHC is an ambiguous technique, and the results are susceptible to specimen degeneration due to events such as extended time spent at room temperature or damage via surgical manipulation. In our hospital, according to Tayler and Shi (27), tumor samples are immediately placed in 10% neutral-buffered formalin instead of immersing it in physiological saline. Similarly, Ki-67 LI must be measured carefully. To eliminate vascular endothelial cells and lymphocytes that exhibit false positives, Ki-67 LI is calculated by visual measurement under a high-magnification microscope rather than calculated using an automatic analyzer. Epigenomic analysis also suggests that the incidence of TF–negative and hormone-negative PitNETs has markedly decreased owing to advanced diagnostic techniques (28).
The Ki-67 LI of GTR patients in the current study was lower than previously reported, including the proportion of patients with LI ≥ 3 (6). Tumors with relatively high Ki-67 LI and those with LI < 3 have a higher risk of regrowth in the long term, as observed in GTR cases. Interestingly, our 12 GTR patients had similar immunohistochemical characteristics to those of the 12 patients with recurrence after GTR reported by McClure et al., with 7 and 5 of them having SGT and SCT, respectively. Meanwhile, 21 of the 35 SGTs (60%) in our study were diagnosed as H-/TF (SF1) + tumors. The case without immunoreactivity to any pituitary hormones in NFPT may indicate a higher risk of regrowth after STR and PR and even after GTR. This may be one of the reasons for the higher rate of recurrence in NCT when an IHC analysis of TFs is not available. Similarly in our study, the previous pathological results diagnosed 12/21 patients as NCT, which mostly turned out to be H-/TF+ tumors (Table 3).
Silva-Ortega et al. reported that using SF1 IHC enabled the detection of a substantial portion of gonadotroph tumors and reduced the estimated prevalence of NCTs to less than 5% (29). Thus, the many NCTs reported to have more recurrence and regrowth could be reclassified as hormone-negative, SF1-positive SGTs. Carbonara et al. recently reported that hormone-negative SGTs were not significantly different from other SGTs concerning invasion or proliferation patterns (25). The recurrence rate was higher in hormone-negative SGTs than in other SGTs, although the difference was not significant due to their small number of cases (25). In the current study, the rate of H-/TF+ tumors among SGTs was significantly higher in the reoperation group than in the initial operation group. The reason for this is unknown, but H-/TF+ tumors may be less differentiated than anterior pituitary hormone-positive tumors, as reported for PIT1 lineage tumors (30).
SCTs are considered a “high-risk” subtype of pituitary tumors in the 2017 WHO classification system (24, 31, 32). However, this is controversial, and conflicting findings have been reported (33–35). For example, Chatrath et al. recently reported that compared to both corticotroph and null cell adenomas classified using the 2017 WHO guidelines, gonadotroph adenomas were likely to progress following STR (12). However, these differences did not reach significance (12). Again, the inconsistency may be due to the lack of accuracy in IHC and/or the small number of samples. Strickland et al. defined SCT only as ACTH immunoreactivity and did not include TPIT IHC (24). There were several ACTH-negative but TPIT-positive SCTs in our study (7/24 SCT). Thus, ACTH-negative but TPIT-positive tumors were not categorized as SCT in their report (24). To the best of our knowledge, this is the first report in which a large number of reoperation patients with reliable IHC results for both ACTH and TPIT immunostaining were compared with patients who only underwent initial surgery. Pathological examination showed significantly more SCTs in the reoperation group than in the initial operation group. Surprisingly, nearly 40% of the 61 patients with symptomatic recurrence had SCT, and this proportion was remarkably higher than that in the initial operation group (22.5%) like in other reports (10, 12, 21).
Surgeons should aim for GTR in NFPT (36–39), but it is neither easy nor safe (6). In the real world, countless TSS for NFPT ends up with PR (6, 14). We found that NFPT, after PR operation, regrows and becomes symptomatic again in a much shorter time, even with a significantly lower Ki-67 LI than in GTR. The reason is quite understandable: a small number of residual tumors after GTR only becomes large enough to be symptomatic with a relatively large Ki-67 LI and an extended period. In contrast, many residual tumors regrow after a PR and become symptomatic, even with a low Ki-67 LI, in a shorter time. Some studies (6, 10, 22) concluded that cavernous sinus invasion (CSI) significantly predicts postoperative progression in NFPT. CSI causes incomplete resection and increases the number of residual tumor cells. In our recent report, 57% of functioning pituitary tumors with direct contact with the medial wall of the cavernous sinus had histological CSI (40). Therefore, there may be many residual tumor cells even with GTR. Furthermore, it is technically impossible to resect all tumor cells with complete CSI (40), especially those with Knosp grade 4, resulting in PR. Thus, we classified reoperation patients according to EOR instead of CSI.
The characteristics of regrowing NFPTs after operation have long been discussed. A Ki-67 LI of >3% has been reported to predict recurrence and regrowth in NFPTs (19, 20). However, in the real world, most regrowing NFPTs have a low Ki-67 LI (21, 22). Chiloiro et al. suggested that Ki-67 LI ≥ 1.5% might be useful as a prognostic marker for pituitary tumors after radical removal (41, 42). The results of this study also indicate that the reference values of Ki-67 LI regarding the risks of regrowing depend on the EOR of the previous surgeries. NFPTs with low Ki-67 LI should be carefully monitored as they may regrow to be symptomatic, especially after incomplete removal.
We used the results of the pathological examination of the reoperated samples. Although this should be valid, regrowing tumors from patients who underwent an initial operation in our hospital needs to be further analyzed to validate our results. However, this is time-consuming because only one patient underwent reoperation in our own cases.
The ratio of H-/TF+ SGT is significantly higher in symptomatically regrown tumors than in the initial cases, which used to be diagnosed as NCT before the introduction of immunostaining for TF. With meticulous IHC of TPIT, we also confirmed that the ratio of SCT is significantly higher in cases with symptomatic tumor regrowth than in initial cases. PR cases tend to grow symptomatically in a shorter period of time, even with lower Ki-67 LI than GTR cases. On the contrary, we should follow GTR cases for a longer. especially with relatively high Ki-67 LI.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by The ethical review board of Moriyama Memorial Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
AI: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. NI: Data curation, Methodology, Supervision, Writing – review & editing. NT: Data curation, Writing – review & editing. KT: Supervision, Writing – review & editing, Conceptualization. ST-M: Data curation, Writing – review & editing, Methodology. MK: Data curation, Writing – review & editing. HY: Data curation, Writing – review & editing. HS: Data curation, Writing – review & editing. GM: Data curation, Writing – review & editing. SY: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We would like to thank Dr. Ko Nakase and Ms. Akiko Jo for assisting in the data collection and Editage for the English language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ntali G, Wass JA. Epidemiology, clinical presentation and diagnosis of non-functioning pituitary adenomas. Pituitary. (2018) 21:111–8. doi: 10.1007/s11102–018-0869–3
2. Huang W, Molitch ME. Management of nonfunctioning pituitary adenomas (NFAs): observation. Pituitary. (2018) 21:162–7. doi: 10.1007/s11102–017-0856–0
3. Mercado M, Melgar V, Salame L, Cuenca D. Clinically non-functioning pituitary adenomas: pathogenic, diagnostic and therapeutic aspects. Endocrinol Diabetes Nutr. (2017) 64:384–95. doi: 10.1016/j.endinu.2017.05.009
4. Freda PU, Beckers AM, Katznelson L, Molitch ME, Montori VM, Post KD, et al. Endocrine Society. Pituitary incidentaloma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2011) 96:894–904. doi: 10.1210/jc.2010–1048
5. Chen L, White WL, Spetzler RF, Xu B. A prospective study of nonfunctioning pituitary adenomas: presentation, management, and clinical outcome. J Neurooncol. (2011) 102:129–38. doi: 10.1007/s11060–010-0302-x
6. Gerges MM, Rumalla K, Godil SS, Younus I, Elshamy W, Dobri GA, et al. Long-term outcomes after endoscopic endonasal surgery for nonfunctioning pituitary macroadenomas. J Neurosurg. (2020) 134:535–46. doi: 10.3171/2019.11.JNS192457
7. Lloyd RV, Osamura RY, Klöppel G, Rosai J eds. Pituitary adenoma. In: WHO Classification of Tumours of Endocrine Organs, 4th Edition, vol. 10. Lyon, France: IARC. p. 12–40.
8. Asa SL, Mete O, Perry A, Osamura RY. Overview of the 2022 WHO classification of pituitary tumors. Endocr Pathol. (2022) 33:6–26. doi: 10.1007/s12022–022-09703–7
9. Kovacs K, Horvath E, Ryan N, Ezrin C. Null cell adenoma of the human pituitary. Virchows Archiv A Pathol Anat Histol. (1980) 387:165–74. doi: 10.1007/BF00430697
10. Taguchi A, Kinoshita Y, Amatya VJ, Onishi S, Go Y, Tominaga A, et al. Differences in invasiveness and recurrence rate among nonfunctioning pituitary neuroendocrine tumors depending on tumor subtype. Neurosurg Rev. (2023) 46:317. doi: 10.1007/s10143–023-02234–7
11. McClure JJ, Chatrath A, Robison TR, Jane JA. Conditioned recurrence-free survival following gross-total resection of nonfunctioning pituitary adenoma: a single-surgeon, single-center retrospective study. J Neurosurg. (2023) 8:1–6. doi: 10.3171/2023.10.JNS23754
12. Chatrath A, Kosyakovsky J, Patel P, Ahn J, Elsarrag M, Young LC, et al. Impact of histopathological classification of non-functioning adenomas on long term outcomes: comparison of the 2004 and 2017 WHO classifications. Pituitary. (2022) 25:988–96. doi: 10.1007/s11102–022-01281–5
13. Haddad AF, Young JS, Oh T, Pereira MP, Joshi RS, Pereira KM, et al. Clinical characteristics and outcomes of null-cell versus silent gonadotroph adenomas in a series of 1166 pituitary adenomas from a single institution. Neurosurg Focus. (2020) 48:E13. doi: 10.3171/2020.3.FOCUS20114
14. Batista RL, Trarbach EB, Marques MD, Cescato VA, da Silva GO, Herkenhoff CGB, et al. Nonfunctioning pituitary adenoma recurrence and its relationship with sex, size, and hormonal immunohistochemical profile. World Neurosurg. (2018) 120:e241–6. doi: 10.1016/j.wneu.2018.08.043
15. Almeida JP, Stephens CC, Eschbacher JM, Felicella MM, Yuen KCJ, White WL, et al. Clinical, pathologic, and imaging characteristics of pituitary null cell adenomas as defined according to the 2017 World Health Organization criteria: a case series from two pituitary centers. Pituitary. (2019) 22:514–9. doi: 10.1007/s11102–019-00981–9
16. Jiang S, Zhu J, Feng M, Yao Y, Deng K, Xing B, et al. Clinical profiles of silent corticotroph adenomas compared with silent gonadotroph adenomas after adopting the 2017 WHO pituitary classification system. Pituitary. (2021) 24:564–73. doi: 10.1007/s11102–021-01133–8
17. Dallapiazza RF, Grober Y, Starke RM, Laws ER Jr, Jane JA Jr. Long-term results of endonasal endoscopic transsphenoidal resection of nonfunctioning pituitary macroadenomas. Neurosurgery. (2015) 76:42–52; discussion 52–53. doi: 10.1227/NEU.0000000000000563
18. Chen Y, Cai F, Cao J, Gao F, Lv Y, Tang Y, et al. Analysis of related factors of tumor recurrence or progression after transnasal sphenoidal surgical treatment of large and giant pituitary adenomas and establish a nomogram to predict tumor prognosis. Front Endocrinol (Lausanne). (2021) 12:793337. doi: 10.3389/fendo.2021.793337
19. Trouillas J, Jaffrain-Rea ML, Vasiljevic A, Raverot G, Roncaroli F, Villa C. How to classify the pituitary neuroendocrine tumors (PitNET)s in 2020. Cancers (Basel). (2020) 12:514. doi: 10.3390/cancers12020514
20. Nishioka H. Aggressive pituitary tumors (PitNETs). Endocr J. (2023) 70:241–8. doi: 10.1507/endocrj.EJ23–0007
21. Nishioka H, Inoshita N, Mete O, Asa SL, Hayashi K, Takeshita A, et al. The complementary role of transcription factors in the accurate diagnosis of clinically nonfunctioning pituitary adenomas. Endocr Pathol. (2015) 26:349–55. doi: 10.1007/s12022–015-9398-z
22. Hussein Z, Grieve J, Dorward N, Miszkiel K, Kosmin M, Fersht N, et al. Non-functioning pituitary macroadenoma following surgery: long-term outcomes and development of an optimal follow-up strategy. Front Surg. (2023) 10:1129387. doi: 10.3389/fsurg.2023.1129387
23. Florea SM, Graillon T, Cuny T, Gras R, Brue T, Dufour H. Ophthalmoplegic complications in transsphenoidal pituitary surgery. J Neurosurg. (2019) 26:1–9. doi: 10.3171/2019.5.JNS19782
24. Strickland BA, Shahrestani S, Briggs RG, Jackanich A, Tavakol S, Hurth K, et al. Silent corticotroph pituitary adenomas: clinical characteristics, longterm outcomes, and management of disease recurrence. J Neurosurg. (2021) 135:1706–13. doi: 10.3171/2020.10.JNS203236
25. Carbonara F, Feola T, Gianno F, Polidoro MA, Di Crescenzo RM, Arcella A, et al. Clinical and molecular characteristics of gonadotroph pituitary tumors according to the WHO classification. Endocr Pathol. (2024) 35(1):1–13. doi: 10.1007/s12022–023-09794-w
26. Inoshita N, Yoshimoto T, Takazawa Y, Fukuhara N, Okada M, Nishioka H, et al. Immunohistochemical and ultrastructural review of six cases previously diagnosed as null cell PitNETs. Brain Tumor Pathol. (2023) 40:158–62. doi: 10.1007/s10014–023-00462–9
27. Taylor CR, Shi SR. Techniques of immunohistochemistry: principles, pitfalls, and standardization. In: Diagnostic Immunohistochemistry: Theranostic and Genomic Applications, 6th Edition. Amsterdam, Netherlands: Elsevier (2021). p. 1–38.
28. Dottermusch M, Schüller U, Hagel C, Saeger W. Unveiling the identities of null cell tumours: Epigenomics corroborate subtle histological cues in pituitary neuroendocrine tumour/adenoma classification. Neuropathol Appl Neurobiol. (2023) 49:e12870. doi: 10.1111/nan.12870
29. Silva-Ortega S, García-Martinez A, Niveiro de Jaime M, Torregrosa ME, Abarca J, Monjas I, et al. Proposal of a clinically relevant working classification of pituitary neuroendocrine tumors based on pituitary transcription factors. Hum Pathol. (2021) 110:20–30. doi: 10.1016/j.humpath.2020.12.001
30. Zhao J, Ji C, Cheng H, Ye Z, Yao B, Shen M, et al. Digital image analysis allows objective stratification of patients with silent PIT1-lineage pituitary neuroendocrine tumors. J Pathol Clin Res. (2023) 9:488–97. doi: 10.1002/cjp2.340
31. Vuong HG, Dunn IF. The clinicopathological features and prognosis of silent corticotroph tumors: an updated systematic review and meta-analysis. Endocrine. (2023) 82:527–35. doi: 10.1007/s12020–023-03449-w
32. Fountas A, Lavrentaki A, Subramanian A, Toulis KA, Nirantharakumar K, Karavitaki N. Recurrence in silent corticotroph adenomas after primary treatment: A systematic review and meta-analysis. J Clin Endocrinol Metab. (2019) 104(4):1039–48. doi: 10.1210/jc.2018–01956
33. Liu J, He Y, Zhang X, Yan X, Huang Y. Clinicopathological analysis of 250 cases of pituitary adenoma under the new WHO classification. Oncol Lett. (2020) 19:1890–8. doi: 10.3892/ol.2020.11263
34. Trouillas J, Roy P, Sturm N, Dantony E, Cortet-Rudelli C, Viennet G, et al. A new prognostic clinicopathological classification of pituitary adenomas: a multicentric case–control study of 410 patients with 8 years post-operative follow-up. Acta Neuropathol. (2013) 126:123–35. doi: 10.1007/s00401–013-1084-y
35. Roelfsema F, Biermasz NR, Pereira AM. Clinical factors involved in the recurrence of pituitary adenomas after surgical remission: a structured review and meta-analysis. Pituitary. (2012) 15:71–83. doi: 10.1007/s11102–011-0347–7
36. Constantinescu SM, Duprez T, Fomekong E, Raftopoulos C, Alexopoulou O, Maiter D. Natural history and surgical outcome of incidentally discovered clinically nonfunctioning pituitary macroadenomas. Endocr Connect. (2023) 12:e230224. doi: 10.1530/EC-23–0224
37. Fong KY, Lim MJR, Fu S, Low CE, Chan YH, Deepak DS, et al. Postsurgical outcomes of nonfunctioning pituitary adenomas: a patient-level meta-analysis. Pituitary. (2023) 26:461–73. doi: 10.1007/s11102–023-01335–2
38. Micko A, Agam MS, Brunswick A, Strickland BA, Rutkowski MJ, Carmichael JD, et al. Treatment strategies for giant pituitary adenomas in the era of endoscopic transsphenoidal surgery: a multicenter series. J Neurosurg. (2022) 136:776–85. doi: 10.3171/2021.1.JNS203982
39. Caulley L, Dijk SW, Krijkamp E, Dong SX, Alkherayf F, Amrani L, et al. Cost-effectiveness of postoperative imaging surveillance strategies for nonfunctional pituitary adenomas after resection with curative intent. J Neurosurg. (2023) 139:1207–15. doi: 10.3171/2023.2.JNS221903
40. Ishida A, Shiramizu H, Yoshimoto H, Kato M, Inoshita N, Miki N, et al. Resection of the cavernous sinus medial wall improves remission rate in functioning pituitary tumors: retrospective analysis of 248 consecutive cases. Neurosurgery. (2022) 91:775–81. doi: 10.1227/neu.0000000000002109
41. Chiloiro S, Doglietto F, Trapasso B, Iacovazzo D, Giampietro A, Di Nardo F, et al. Typical and atypical pituitary adenomas: a single-center analysis of outcome and prognosis. Neuroendocrinology. (2015) 101:143–50. doi: 10.1159/000375448
Keywords: nonfunctioning pituitary tumor, regrowth, gross total resection, transcription factor, silent gonadotroph tumors, silent corticotroph tumor, null cell tumor
Citation: Ishida A, Inoshita N, Tanabe N, Takano K, Tanaka-Mizuno S, Kato M, Yoshimoto H, Shiramizu H, Matsuoka G and Yamada S (2024) Pathological characteristics of reoperated regrowing clinically nonfunctioning pituitary tumor cases in comparison with initial surgical cases. Front. Endocrinol. 15:1400671. doi: 10.3389/fendo.2024.1400671
Received: 14 March 2024; Accepted: 09 May 2024;
Published: 28 May 2024.
Edited by:
Xiang’En Shi, Capital Medical University, ChinaReviewed by:
Martin Weiss, University of Southern California, United StatesCopyright © 2024 Ishida, Inoshita, Tanabe, Takano, Tanaka-Mizuno, Kato, Yoshimoto, Shiramizu, Matsuoka and Yamada. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Atsushi Ishida, djJkYW55b25AZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.