
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol. , 25 June 2024
Sec. Thyroid Endocrinology
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1399236
This article is part of the Research Topic Vascular Dysfunction and Endocrine Disorders View all 7 articles
Background: Subclinical hypothyroidism (SCH) is a common endocrine subclinical disorder, the main adverse consequences of which are the development of clinical hypothyroidism and the promotion of ischemic heart disease. Metabolic syndrome (MetS) is a collection of metabolic problems. The goal of this meta-analysis was to evaluate the relationship between MetS and SCH.
Methods: Suitable publications were identified using PubMed, Embase, and the Cochrane Library. The meta-analysis included only studies in English that reported odds ratio (OR) data for MetS and SCH. Two researchers combined data using a random-effects model. OR and 95% confidence intervals (CIs) were used to present the results.
Results: MetS was associated with an elevated risk of developing SCH (OR 2.56, 95% CI 1.44–4.55). However, the individual components of MetS were not associated with the risk of SCH. Subgroup analysis revealed that different definitions of MetS had varying effects on SCH. Sensitivity analysis confirmed that our results were robust.
Conclusions: This meta-analysis indicates that patients with MetS have an increased risk of SCH, while there is no significant association between the five individual components of MetS and the risk of SCH.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42023454415.
Metabolic syndrome (MetS) is a pathological state of a variety of metabolic disorders, including obesity, hyperglycemia, hypertension, and dyslipidemia (1, 2). It is recognized as one of the clinical syndromes that significantly impact human health (1, 3–6), affecting an estimated 25% of the world’s population (4, 6, 7). The risk of cardiovascular disease would be significantly increased when these metabolic abnormalities co-exist in an individual.
Subclinical hypothyroidism (SCH) is a metabolic disease that has no obvious clinical symptoms and signs, and the thyroid hormone level is normal and the thyroid-stimulating hormone (TSH) in the blood is elevated (8, 9). A growing body of research shows that SCH is associated with lipid abnormalities, increased cardiovascular risk, and metabolic disorders such as high blood pressure, chronic inflammation, and a hypercoagulable state of the blood, especially in older women (10). Many studies have also pointed to thyroid disorders as being complications of MetS and type 2 diabetes (11, 12). Numerous studies have shown a connection between thyroid hormone and TSH levels in serum and elements of the MetS (13–16). For instance, in a study by Kim et al., serum free thyroxine 4 (FT4) concentrations were found to be positively correlated with blood pressure, fasting glucose, high-density lipoprotein cholesterol (HDL-C), and triglyceride (TG) levels (13). Furthermore, MetS and SCH have been frequently linked in the studies (17). In a study of 2,119 people aged 70 to 79 years, Antika et al. found that elevated TSH levels increased the risk of MetS (1). However, the relationship between MetS and its five components with SCH remains a subject of debate (18, 19). Consequently, we conducted this systematic review and meta-analysis to explore whether MetS and its components are associated with an increased risk of SCH.
The study has been reported according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses), and the registration number is CRD42023454415 (PROSPERO registration platform).
In the PubMed, Cochrane Library, and EMBASE databases, two researchers (SL and LZ) independently looked for publications published from January 1988 to March 2023. The following were searched for words: (“Subclinical hypothyroidism” OR “SCH” OR “hyperthyroidism” OR “sub-clinical thyroid deficiency”) AND (“Metabolic Syndrome” OR “Metabolic Cardiovascular” OR “Dysmetabolic” OR “Metabolic X”). We only considered research that was written in English. To find prospective acceptable articles, we also looked for and read the complete contents of references from the original studies.
The following were the research’s inclusion requirements: (1) observational studies (cohort studies, case–control studies, and cross-sectional studies) published in English; (2) the primary outcome was the effect of MetS on SCH prevalence; and (3) there were sufficient data to do a comprehensive analysis. Studies, however, were instantly disqualified when they met any of the following requirements: (1) publications are studies such as case reports, animal experiments, or conference abstracts that do not provide critical data; and (2) diagnostic criteria for MetS and SCH were not provided.
The following information was gathered by SL and LZ: initially, the basics (first author’s name, publication year, and place of publishing); second, participant data (size of the sample, mean age, and sex ratio); and third, MetS and SCH diagnostic standards. Two examiners independently extracted the data and cross-checked them and individually evaluated the quality of every research according to the Newcastle–Ottawa Scale (NOS) and the Agency for Healthcare Research and Quality (AHRQ). Every study was given a rating based on whether it was low (<4), medium, or high quality (>8). All disagreements were worked out by mutual consent and discussion with another author (JFL).
Odds ratios (ORs) and its 95% CI were used to assess the correlation between MetS and SCH. Furthermore, we evaluated the effects of each MetS factor on the risk of SCH. The I2 was applied to evaluate statistical heterogeneity for each study. An I2 statistic of less than 25% indicates low heterogeneity, a score greater than 75% indicates high heterogeneity, and a score between the two indicates moderate heterogeneity. When the heterogeneity of the study is large, subgroup analysis and/or meta-regression will be used to find the source of heterogeneity. Sensitivity analysis was used to measure the stability of the study. All data were combined using a random-effects model. Publication bias was evaluated through Egger’s test and Begg’s test. All analyses were performed by Stata, and p < 0.05 denotes statistical significance.
A flowchart that depicts the literature screening procedure is shown in Figure 1. A total of 701 studies were found in the database. Studies that failed to meet the inclusion criteria and all duplicate articles were removed. Nine studies (2, 18–25) in total met the inclusion requirements for the present analyses. On the chosen literature, we performed a meta-analysis and systematic review.
The study comprises research published between 2007 and 2022, and it received an average quality rating of 8.7 stars (Supplementary Tables S1–S3). There were three different kinds of investigations, including a cohort study, four case–control studies, and four cross-sectional studies. Table 1 provides a summary of the fundamental characteristics of each study included in this meta-analysis.
Figure 2 presents the forest plots from the meta-analysis of SCH and MetS. In contrast to non-MetS patients, those with MetS had a higher prevalence of SCH (OR 2.56, 95% CI 1.44–4.55). However, our study did not find an association between the various components of MetS and the incidence of SCH (Figure 3).
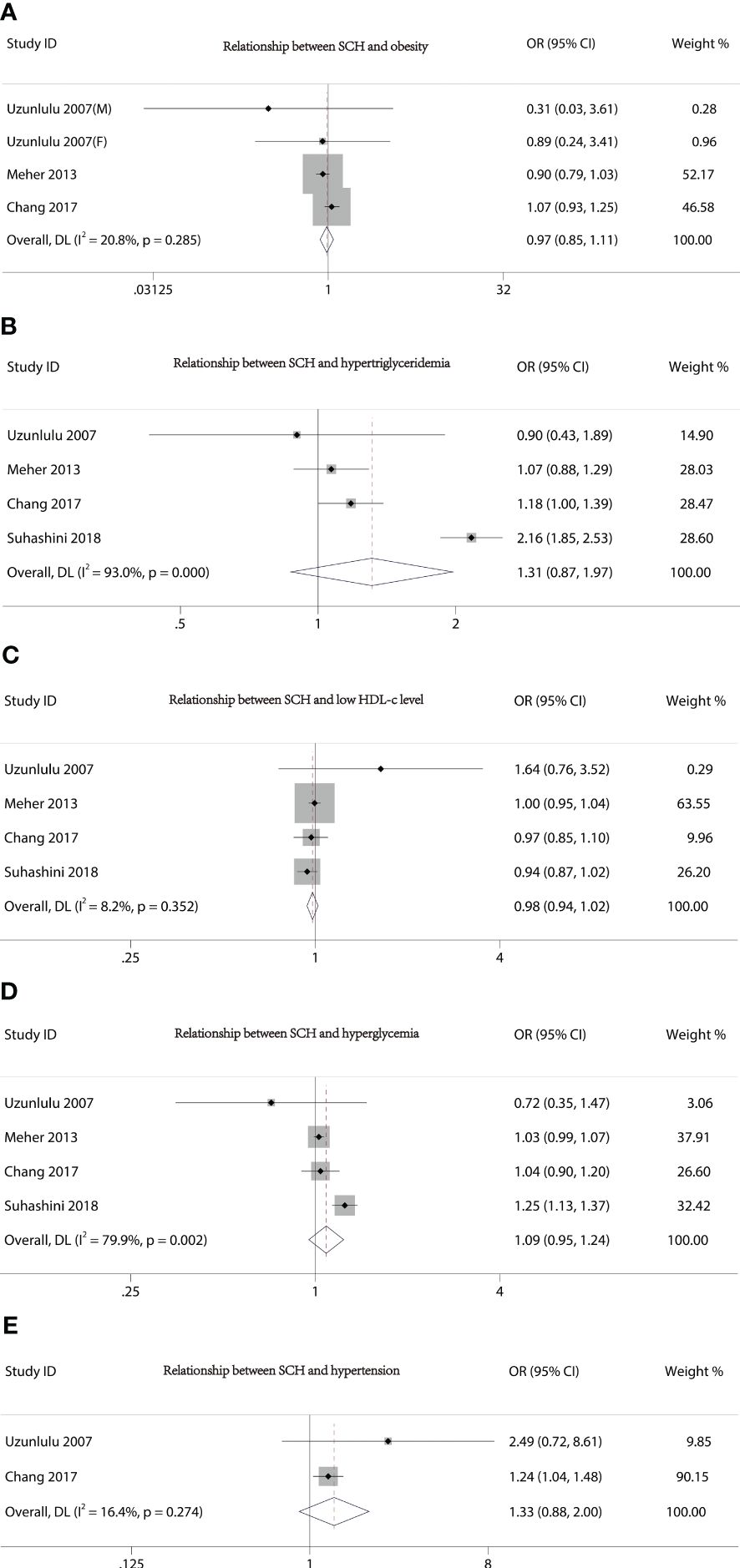
Figure 3 Forest plot of the relationship between MetS components and SCH. (A) Relationship between SCH and obesity. (B) Relationship between SCH and hypertriglyceridemia. (C) Relationship between SCH and low HDL-c level. (D) Relationship between SCH and hyperglycemia. (E) Relationship between SCH and hypertension.
Sensitivity analyses of nine articles showed that arbitrary deletion of the literature in this study will not affect the results of this study, meaning that the above results are stable and reliable (Figure 4). Either Egger regression analysis or funnel plots (Figure 5) indicated the presence of no publication bias for our analyses.
Obesity, hypertension, hyperlipidemia, and hyperglycemia are among the metabolic risk factors that can occur together to form MetS. It is significant to note that multiple cross-sectional studies revealed a connection between SCH and MetS and its components (26–28). Our meta-analysis comprehensively assessed the association between SCH and MetS by taking into account and evaluating the results of nine independent observational studies. Our results were in agreement with the majority of previous studies in that MetS would increase the risk of developing SCH. Surprisingly, our study found no significant association between the individual components of MetS and the risk of SCH. Analysis of sensitivity and the detection of publication bias supported the stability of our findings.
Although hypothyroidism is frequently thought to be secondary to weight gain (7), more recent arguments have been made that hypothyroidism may be secondary to obesity (26, 29–31). A possible explanation was that leptin, cytokines, and other inflammatory markers are produced by excessive adipose tissue (31), which may inhibit sodium/iodide symporter mRNA expression and disrupt iodide uptake activity in thyroid cells (32, 33) or modulate the expression and activity of deiodinases (34, 35). Additionally, evidence from people and a mouse model suggests that obesity causes fat to build up in the thyroid gland. Studies on obese mice suggest that this may have an impact on the thyroid’s ability to produce hormones and cause SCH (36). The cause of the link between fat and hypothyroidism still has to be clarified, though.
Numerous studies have revealed that people with diabetes may have different serum concentrations of thyroid hormones (37). Type 2 diabetes mellitus has been proven to be negatively correlated with serum TSH38 levels (37), and it has been shown that poorly controlled diabetes removed the nocturnal TSH peak because the TSH response to TRH was disturbed (38). In a few studies, it has also been shown that SCH leads to insulin resistance (39, 40). This connection between hypothyroidism and insulin resistance, which refers to one such scenario where insulin resistance plays a key role in the clustering of risk factors for cardiovascular disease, can help to explain why people with MetS experience an elevated incidence of hypothyroidism.
Although it is generally accepted that there is a strong relationship between hypercholesterolemia and clinical hypothyroidism (41), Chang et al.’s analysis suggests that high serum triglycerides may be an important independent factor in increasing SCH risk (18). Meanwhile, Shao and colleagues also discovered that rats fed a high-fat lard diet for 24 weeks had significantly higher serum triglyceride levels in both the serum and thyroid tissue, lower serum total and free T4 levels in conjunction with higher serum TSH levels, and altered macro- and micromorphology of the thyroid gland (42). Furthermore, Han and colleagues showed in a study on animals that a high-fat diet could harm mice’s thyroid glands and result in a thyroid hormone disorder (43). In our investigation, no correlation between hypertriglyceridemia and SCH was found. It is necessary to conduct more research to determine whether dietary factors may play a role in SCH incidence in those who are at risk.
In a recent study, Cai et al. (44) examined the connection between thyroid function and various forms of hypertension. They discovered that patients with clinical hypertension had higher serum TSH levels than patients with clinical normal blood pressure, and that people with ambulatory hypertension frequently had higher serum TSH levels than people with ambulatory normal blood pressure (44). Another study carried out in India revealed that individuals with high blood pressure had much higher average TSH than the general population (45). In addition, the study found that in new cases of hypothyroidism across all cohorts, SCH was more common than overt hypothyroidism (45). This is due to the fact that SCH is indicative of the early or beginning phases of thyroid illness, which, if left untreated, can result in severe hypothyroidism (45). Furthermore, among all the pathogenic pathways that might result in hypertension, a number of them are linked to hypothyroidism (46). These pathways include altered catecholamine levels in the blood, perturbations to the renin–angiotensin–aldosterone system, and elevated peripheral vascular resistance (47, 48). Therefore, we believe that there may be an interactive relationship between hypertension and SCH. However, since most of these studies are cross-sectional studies, more clinical trials and basic research are needed to confirm them.
Furthermore, there was strong heterogeneity in the results of our study. The reasons for this may be the differences in the diagnostic criteria of MetS and SCH, study population, and epidemiological study methods included in the study. Further prospective, multicenter, large cohort studies are needed to confirm this.
Additionally, the study contains some flaws. First of all, since the majority of the literature used in this investigation was observational, it might be challenging to differentiate between cause and effect from the correlation between SCH and MetS. Secondly, there have not been as many investigations on the connection between MetS and SCH, which might make the findings less trustworthy. Thirdly, the results of our study have strong heterogeneity, and we were unable to conduct subgroup analyses for additional characteristics, such as sex and age, due to a lack of data, which prohibited us from exploring the potential relationship between MetS and SCH in greater detail. Moreover, most of the articles selected for our study were based on people from China and India, which may have influenced the results. Therefore, it is necessary to conduct more longitudinal large-scale prospective cohort studies to determine whether MetS may play a role in SCH incidence.
In our meta-analysis of nine studies, the patients with MetS were found to be associated with an increased incidence of SCH. However, no significant association was found between the five components of MetS and the risk of SCH.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
LZ: Conceptualization, Data curation, Investigation, Writing – original draft, Writing – review & editing. SL: Conceptualization, Methodology, Software, Writing – original draft, Writing – review & editing. YY: Writing – original draft, Data curation, Validation, Investigation. TX: Writing – original draft, Data curation, Validation, Investigation. JL: Conceptualization, Investigation, Methodology, Writing – review & editing. HZ: Conceptualization, Methodology, Writing – review & editing. GT: Conceptualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1399236/full#supplementary-material
Supplementary Figure 1 | Different MetS diagnosis criteria.
MetS, metabolic syndrome; OR, odds ratio; CI, confidence interval; SCH, subclinical hypothyroidism; HDL-c, high-density lipoprotein cholesterol; TSH, thyroid-stimulating hormone; TG, triglyceride; NCEP-ATP III, National Cholesterol Education Program Adult Treatment Panel III; IDF, International Diabetes Federation; AHA, American Heart Association; FT4, free tetraiodothyronine; FT3, free triiodothyronine.
1. Waring AC, Rodondi N, Harrison S, Kanaya AM, Simonsick EM, Miljkovic I, et al. Thyroid function and prevalent and incident metabolic syndrome in older adults: the Health, Ageing and Body Composition Study. Clin Endocrinol (Oxf). (2012) 76:911–8. doi: 10.1111/j.1365-2265.2011.04328.x
2. Gyawali P, Takanche JS, Shrestha RK, Bhattarai P, Khanal K, Risal P, et al. Pattern of thyroid dysfunction in patients with metabolic syndrome and its relationship with components of metabolic syndrome. Diabetes Metab J. (2015) 39:66–73. doi: 10.4093/dmj.2015.39.1.66
3. Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. (2005) 28:1769–78. doi: 10.2337/diacare.28.7.1769
4. Zabetian A, Hadaegh F, Azizi F. Prevalence of metabolic syndrome in Iranian adult population, concordance between the IDF with the ATPIII and the WHO definitions. Diabetes Res Clin Pract. (2007) 77:251–7. doi: 10.1016/j.diabres.2006.12.001
6. Ansarimoghaddam A, Adineh HA, Zareban I, Iranpour S, HosseinZadeh A, Kh F. Prevalence of metabolic syndrome in Middle-East countries: Meta-analysis of cross-sectional studies. Diabetes Metab Syndr. (2018) 12:195–201. doi: 10.1016/j.dsx.2017.11.004
7. Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. (2018) 20:12. doi: 10.1007/s11906-018-0812-z
8. Surks MI, Ortiz E, Daniels GH, Sawin CT, Col NF, Cobin RH, et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. Jama. (2004) 291:228–38. doi: 10.1001/jama.291.2.228
9. Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. (2008) 29:76–131. doi: 10.1210/er.2006-0043
10. Fatourechi V. Subclinical hypothyroidism: an update for primary care physicians. Mayo Clin Proc. (2009) 84:65–71. doi: 10.4065/84.1.65
11. Swamy R, Kumar N, Srinivasa K, Manjunath G, DS PB, Venkatesh G. Evaluation of hypothyroidism as a complication in Type II Diabetes Mellitus. Biomed Res. (2012) 23:170–2.
12. Raghuwanshi PK, Rajput DPS, Ratre BK, Jain R, Patel N, Jain S. Evaluation of thyroid dysfunction among type 2 diabetic patients. Asian J Med Sci. (2015) 6:33–7. doi: 10.3126/ajms.v6i3.10814
13. Kim BJ, Kim TY, Koh JM, Kim HK, Park JY, Lee KU, et al. Relationship between serum free T4 (FT4) levels and metabolic syndrome (MS) and its components in healthy euthyroid subjects. Clin Endocrinol (Oxf). (2009) 70:152–60. doi: 10.1111/j.1365-2265.2008.03304.x
14. Park SB, Choi HC, Joo NS. The relation of thyroid function to components of the metabolic syndrome in Korean men and women. J Korean Med Sci. (2011) 26:540–5. doi: 10.3346/jkms.2011.26.4.540
15. Chugh K, Goyal S, Shankar V, Chugh SN. Thyroid function tests in metabolic syndrome. Indian J Endocrinol Metab. (2012) 16:958–61. doi: 10.4103/2230-8210.102999
16. Oh JY, Sung YA, Lee HJ. Elevated thyroid stimulating hormone levels are associated with metabolic syndrome in euthyroid young women. Korean J Intern Med. (2013) 28:180–6. doi: 10.3904/kjim.2013.28.2.180
17. Deng L, Wang L, Zheng X, Shuai P, Liu Y. Women with Subclinical Hypothyroidism are at Higher Prevalence of Metabolic Syndrome and Its Components Compared to Men in an Older Chinese Population. Endocrine research. (2021) 46:186–95. doi: 10.1080/07435800.2021.1928177
18. Chang CH, Yeh YC, Caffrey JL, Shih SR, Chuang LM, Tu YK. Metabolic syndrome is associated with an increased incidence of subclinical hypothyroidism - A Cohort Study. Sci Rep. (2017) 7:6754. doi: 10.1038/s41598-017-07004-2
19. Mehran L, Amouzegar A, Abdi H, Delbari N, Madreseh E, Tohidi M, et al. Incidence of thyroid dysfunction facing metabolic syndrome: A prospective comparative study with 9 years of follow-up. Eur Thyroid J. (2021) 10:390–8. doi: 10.1159/000512665
20. Uzunlulu M, Yorulmaz E, Oguz A. Prevalence of subclinical hypothyroidism in patients with metabolic syndrome. Endocr J. (2007) 54:71–6. doi: 10.1507/endocrj.k06-124
21. Udenze I, Nnaji I, Oshodi T. Thyroid function in adult Nigerians with metabolic syndrome. Pan Afr Med J. (2014) 18:352. doi: 10.11604/pamj.2014.18.352.4551
22. Saluja M, Pyarsabadi P, Jelia S, Chittora S, Swami Y, Vimlani H. Study of thyroid dysfunction in metabolic syndrome and association with its components. Curr Med Res Practice. (2018) 8:3–7. doi: 10.1016/j.cmrp.2017.11.010
23. Suhashini JS, Savitha G. Association of subclinical hypothyroidism in metabolic syndrome patients. Asian J Pharm Clin Res. (2018) 11:188–91. doi: 10.22159/ajpcr.2018.v11i9.26734
24. Rao M, Malik N, Singla S, Kumar V. Prevalence of subclinical hypothyroidism in metabolic syndrome and correlation with its components. Indian J Public Health Res Dev. (2022) 13:31–5. doi: 10.37506/ijphrd.v13i2.17889
25. Meher LK, Raveendranathan SK, Kota SK, Sarangi J, Jali SN. Prevalence of hypothyroidism in patients with metabolic syndrome. Thyroid Res Practice. (2013) 10:60–4. doi: 10.4103/0973-0354.110583
26. Knudsen N, Laurberg P, Rasmussen LB, Bülow I, Perrild H, Ovesen L, et al. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J Clin Endocrinol Metab. (2005) 90:4019–24. doi: 10.1210/jc.2004-2225
27. Roos A, Bakker SJ, Links TP, Gans RO, Wolffenbuttel BH. Thyroid function is associated with components of the metabolic syndrome in euthyroid subjects. J Clin Endocrinol Metab. (2007) 92:491–6. doi: 10.1210/jc.2006-1718
28. Ruhla S, Weickert MO, Arafat AM, Osterhoff M, Isken F, Spranger J, et al. A high normal TSH is associated with the metabolic syndrome. Clin Endocrinol (Oxf). (2010) 72:696–701. doi: 10.1111/j.1365-2265.2009.03698.x
29. Marzullo P, Minocci A, Tagliaferri MA, Guzzaloni G, Di Blasio A, De Medici C, et al. Investigations of thyroid hormones and antibodies in obesity: leptin levels are associated with thyroid autoimmunity independent of bioanthropometric, hormonal, and weight-related determinants. J Clin Endocrinol Metab. (2010) 95:3965–72. doi: 10.1210/jc.2009-2798
30. Marwaha RK, Tandon N, Garg MK, Ganie MA, Narang A, Mehan N, et al. Impact of body mass index on thyroid functions in Indian children. Clin Endocrinol (Oxf). (2013) 79:424–8. doi: 10.1111/cen.12148
31. Fontenelle LC, Feitosa MM, Severo JS, Freitas TE, Morais JB, Torres-Leal FL, et al. Thyroid function in human obesity: underlying mechanisms. Horm Metab Res. (2016) 48:787–94. doi: 10.1055/s-0042-121421
32. Isozaki O, Tsushima T, Nozoe Y, Miyakawa M, Takano K. Leptin regulation of the thyroids: negative regulation on thyroid hormone levels in euthyroid subjects and inhibitory effects on iodide uptake and Na+/I- symporter mRNA expression in rat FRTL-5 cells. Endocr J. (2004) 51:415–23. doi: 10.1507/endocrj.51.415
33. Longhi S, Radetti G. Thyroid function and obesity. J Clin Res Pediatr Endocrinol. (2013) 5 Suppl 1:40–4. doi: 10.4274/jcrpe.856
34. Jakobs TC, Mentrup B, Schmutzler C, Dreher I, Köhrle J. Proinflammatory cytokines inhibit the expression and function of human type I 5'-deiodinase in HepG2 hepatocarcinoma cells. Eur J Endocrinol. (2002) 146:559–66. doi: 10.1530/eje.0.1460559
35. Kwakkel J, Surovtseva OV, de Vries EM, Stap J, Fliers E, Boelen A. A novel role for the thyroid hormone-activating enzyme type 2 deiodinase in the inflammatory response of macrophages. Endocrinology. (2014) 155:2725–34. doi: 10.1210/en.2013-2066
36. Lee MH, Lee JU, Joung KH, Kim YK, Ryu MJ, Lee SE, et al. Thyroid dysfunction associated with follicular cell steatosis in obese male mice and humans. Endocrinology. (2015) 156:1181–93. doi: 10.1210/en.2014-1670
37. Hage M, Zantout MS, Azar ST. Thyroid disorders and diabetes mellitus. J Thyroid Res. (2011) 2011:439463. doi: 10.4061/2011/439463
38. Gursoy N, Tuncel E, Erturk E, Imamoglu S, Arinik A. The relationship between the glycemic control and the hypothalamus-pituitary-thyroid axis in diabetic patients. Turkish J Endocrinol Metab. (1999) 4:163–8.
39. Dessein PH, Joffe BI, Stanwix AE. Subclinical hypothyroidism is associated with insulin resistance in rheumatoid arthritis. Thyroid. (2004) 14:443–6. doi: 10.1089/105072504323150750
40. Maratou E, Hadjidakis DJ, Peppa M, Alevizaki M, Tsegka K, Lambadiari V, et al. Studies of insulin resistance in patients with clinical and subclinical hyperthyroidism. Eur J Endocrinol. (2010) 163:625–30. doi: 10.1530/EJE-10-0246
41. Tagami T, Kimura H, Ohtani S, Tanaka T, Tanaka T, Hata S, et al. Multi-center study on the prevalence of hypothyroidism in patients with hypercholesterolemia. Endocr J. (2011) 58:449–57. doi: 10.1507/endocrj.k11e-012
42. Shao SS, Zhao YF, Song YF, Xu C, Yang JM, Xuan SM, et al. Dietary high-fat lard intake induces thyroid dysfunction and abnormal morphology in rats. Acta Pharmacol Sin. (2014) 35:1411–20. doi: 10.1038/aps.2014.82
43. Han H, Xin P, Zhao L, Xu J, Xia Y, Yang X, et al. Excess iodine and high-fat diet combination modulates lipid profile, thyroid hormone, and hepatic LDLr expression values in mice. Biol Trace Elem Res. (2012) 147:233–9. doi: 10.1007/s12011-011-9300-x
44. Cai P, Peng Y, Chen Y, Li L, Chu W, Wang Y, et al. Association of thyroid function with white coat hypertension and sustained hypertension. J Clin hypertension (Greenwich Conn). (2019) 21:674–83. doi: 10.1111/jch.13536
45. Talwalkar P, Deshmukh V, Bhole M. Prevalence of hypothyroidism in patients with type 2 diabetes mellitus and hypertension in India: a cross-sectional observational study. Diabetes Metab syndrome Obes Targets Ther. (2019) 12:369–76. doi: 10.2147/DMSO.S181470
46. Dzau VJ, Hodgkinson CP. Precision hypertension. Hypertension (Dallas Tex 1979). (2024) 81:702–8. doi: 10.1161/HYPERTENSIONAHA.123.21710
47. Fletcher AK, Weetman AP. Hypertension and hypothyroidism. J Hum hypertension. (1998) 12:79–82. doi: 10.1038/sj.jhh.1000574
Keywords: subclinical hypothyroidism, thyroid, metabolic syndrome, metabolic component, meta-analysis
Citation: Zhong L, Liu S, Yang Y, Xie T, Liu J, Zhao H and Tan G (2024) Metabolic syndrome and risk of subclinical hypothyroidism: a systematic review and meta-analysis. Front. Endocrinol. 15:1399236. doi: 10.3389/fendo.2024.1399236
Received: 11 March 2024; Accepted: 28 May 2024;
Published: 25 June 2024.
Edited by:
Zhice Xu, Wuxi Maternity and Child Health Care Hospital, ChinaReviewed by:
Joao D. T. S. Anselmo, Hospital do Divino Espírito Santo, PortugalCopyright © 2024 Zhong, Liu, Yang, Xie, Liu, Zhao and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guang Tan, Z3Vhbmd0YW5AZG11LmVkdS5jbg==; Huahui Zhao, emhhb2hoMDMyOUAxNjMuY29t; Jifeng Liu, amlmZW5nMDIxM0AxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.