
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 05 July 2024
Sec. Reproduction
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1397869
 Tarek Ziad Arabi1†
Tarek Ziad Arabi1† Hana M. A. Fakhoury1†
Hana M. A. Fakhoury1† Hani Tamim2
Hani Tamim2 Rene F. Chun3
Rene F. Chun3 Martin Hewison4
Martin Hewison4 Fatme AlAnouti5,6
Fatme AlAnouti5,6 Stefan Pilz7
Stefan Pilz7 Cedric Annweiler8
Cedric Annweiler8 Georgios Tzimagiorgis9
Georgios Tzimagiorgis9 Costas Haitoglou9
Costas Haitoglou9 Spyridon N. Karras9*
Spyridon N. Karras9*Background: Apart from the well-established skeletal effects, vitamin D has been explored as a secretagogue influencing various adipokines, including adiponectin and irisin. Recent evidence suggests that specific forms of 25-Hydroxyvitamin D (25(OHD), such as free and bioavailable 25(OH)D, may provide more accurate measurements of vitamin D status. The relationship between vitamin D status and serum irisin and adiponectin concentrations remains largely unexplored, particularly during pregnancy.
Methods: We analyzed data from 67 healthy maternal-neonatal pairs from Northern Greece at birth. Biochemical and hormonal tests were conducted on each maternal-neonatal pair. The vitamin D forms were estimated using validated mathematical models. Subsequently, regression analyses were conducted to determine the association between the vitamin D forms and adipokine levels.
Results: Bioavailable maternal 25(OH)D was inversely associated with neonatal irisin concentrations [β=-73.46 (-140.573 to -6.341), p=0.034]. No other associations were observed between maternal vitamin D status and neonatal adipokine concentrations.
Conclusion: In conclusion, maternal bioavailable vitamin D concentrations are inversely associated with neonatal serum irisin concentrations, warranting further studies to evaluate the underlying mechanisms for this finding.
Adipokines, hormones produced by adipocytes, exert diverse effects throughout the human body (1). Among these, adiponectin plays a pivotal role in promoting fatty acid oxidation, blunting gluconeogenesis, and mediating various anti-inflammatory effects (2). Adiponectin levels are influenced by gender and BMI, typically ranging between 2–20µg/ml (3). Numerous studies have underscored the significance of adiponectin levels in influencing neonatal outcomes. Yeung et al. reported a significant association between lower quartiles of neonatal adiponectin levels and an increased likelihood of preterm birth (4). Conversely, investigations of the association between maternal adiponectin concentrations and neonatal outcomes have yielded limited results (5, 6).
Irisin, a myokine and adipokine, is responsible for mediating several exercise-related metabolic changes and, potentially, exhibiting antineoplastic effects (7–12). Circulating irisin levels in humans are highly variable (0.01–2000 ng/ml) and influenced by various factors, including exercise, obesity, diet, diseases, and exposure to certain medications (13). Similar to the case of adiponectin, reduced cord blood concentrations of irisin have been associated with lower birthweight, but this correlation is not observed with maternal irisin levels (14). Additionally, diminished plasma irisin is independently linked with increased endothelial microparticles and endothelial progenitor cells, both of which are early markers of endothelial dysfunction, in preterm-born children (15). Conversely, elevated neonatal irisin levels have been linked to an increased risk of fetal macrosomia in a Chinese cohort (16).
It has been hypothesized that vitamin D may exert metabolic effects mediated through its effects on adipokines, including adiponectin (17). In a meta-analysis, Nikooyeh et al. concluded that vitamin D supplementation increases adiponectin concentrations in diabetic patients, hinting at its role as an adiponectin secretagogue (17). Similarly, vitamin D supplementation has been shown to increase irisin concentrations (18, 19), supporting the notion that vitamin D may have a broad effect in the regulation of adipokine secretion.
While total 25-hydroxyvitamin D [25(OH)D] is the most commonly used clinical measure of vitamin D status, debates still persist whether more accurate and clinically impactful measures are available (20). Existing in three forms in the body (21)—free, albumin-bound, and vitamin D binding protein-bound (VDBP)—25(OH)D’s biological activity is hypothesized to be primarily attributed to the free form (22). Hence, the free form could be a more accurate marker of vitamin D status in specific conditions, such as pregnancy (20). Our previous research has revealed a strong positive correlation of maternal VDBP with maternal adiponectin and irisin, while VDBP is associated with adiponectin but not irisin in neonates (23). Furthermore, we have demonstrated a maternal-neonatal relationship with free 25(OH)D levels, although no link to neonatal birth weight or anthropometry was identified (24).
To our knowledge, the associations between free and bioavailable (free with albumin-bound) forms of 25(OH)D with adiponectin and irisin levels have not been investigated. Unraveling these relationships holds the potential to shed light on the nature of vitamin D as a secretagogue of adipokines. Thus, our study aimed to analyze the associations between various forms of vitamin D and adipokine levels in maternal-neonatal pairs from the Mediterranean region.
Our study is a prospective study with a cross-sectional design consisting of 67 pairs of mothers and their newborns, as detailed previously (25). The study recruited fair-skinned women who delivered at term (between 37 and 42 weeks). Exclusion criteria encompassed conditions such as primary hyperparathyroidism, secondary osteoporosis, alcohol addiction, hyperthyroidism, nephritic syndrome, inflammatory bowel disease, rheumatoid arthritis, osteomalacia, obesity, gestational diabetes, or medication influencing calcium or vitamin D levels. Newborns classified as small for their gestational age or with severe congenital anomalies were also excluded. Ethical approval was obtained from the Bioethics Committee of Aristotle University in Thessaloniki, Greece (approval number 1/19–12-2011).
All participating mothers provided written consent prior to the study.
Upon registration, demographic and social attributes of mothers were recorded, including age, pre-pregnancy weight, gestational weeks, and number of prior live births. Maternal alcohol use during pregnancy was treated as a dichotomous variable, defined either as none (subdivided in never drinking alcohol or drinking alcohol but not during pregnancy) or light (1–2 units per week before pregnancy), moderate (3–6 units per week), or heavy (≥7 units per week or at any time during pregnancy).Tobacco consumption was recorded according by multiplying the number of packs of cigarettes smoked per day by the number of years the person has smoked.
Blood samples from mothers were collected 30–60 minutes before delivery, and umbilical cord blood was collected post-clamping. Biochemical analyses included substances such as albumin, conducted as previously described (25, 26). The concentrations of 1,25(OH)2D, 25(OH)D2, and 25(OH)D3 were determined using liquid chromatography–tandem mass spectrometry, with a lower limit of quantification set at 0.5 ng/mL for each form. The total 25(OH)D was calculated by adding the concentrations of both forms, i.e. 25(OH)D2 plus 25(OH)D3. VDBP, irisin, and adiponectin were measured with enzyme-linked immunosorbent assay (ELISA) on a Synergy H1 Hybrid reader and Gen5 software (BioTek, Winooski, VT, USA). Detection limits for assays were 0.098 μg/ml for VDBP, 3.12 ng/ml for irisin, 0.039 μg/ml for adiponectin, and 5–100 ng/ml for 25(OH)D. Intra-assay and inter-assay variance were <8% and <10% for adiponectin and irisin, respectively.
Free and bioavailable 25(OH)D levels in both mothers and newborns were estimated using a mathematical model based on prior research by Chun et al. (27). Observed values of VDBP (μM), albumin (μM), 25(OH)D (nM), and 1,25(OH)2D (nM) from the samples were fed into a MATLAB script, which produced estimates of free and bioavailable 25(OH)D. Serum free 25(OH)D levels were converted from nM to pg/ml by multiplying 0.4166 × 10^3 (20).
Data was analyzed using the Statistical Package for Social Sciences (SPSS, version 28, IBM Corp., Armonk, NY). Descriptive statistics included numbers, percentages, means ± standard deviations for categorical and continuous variables, respectively. Associations between measures of vitamin D status and adipokines were calculated by univariate and multivariate linear regression analyses considering potential confounding factors such as maternal height, pre-pregnancy BMI, at-term BMI, and weeks of gestation (28). Results were expressed as beta coefficients (β) and 95% confidence intervals (CI). A p-value < 0.05 was considered statistically significant.
A total of 67 pairs of mothers and their newborns are included in the current analysis. The average maternal age in our study cohort was 31.75 ± 6.11 years, and the gestational age was 38.83 ± 1.59 weeks. Of the participating mothers, 43 (69.4%) were nulliparous prior to the study, 14 (22.2%) had attained advanced education, 7 (11.1%) reported alcohol consumption during pregnancy, and 10 (15.9%) acknowledged smoking during pregnancy. A comprehensive overview of the study population is presented in Table 1.
Initially, we investigated the relationship between various maternal 25(OH)D forms and neonatal adipokine levels. Univariate and multivariate analyses revealed no associations between any 25(OH)D forms and neonatal adiponectin concentrations (Table 2). Similarly, maternal total and free 25(OH)D levels were not associated with neonatal irisin levels. However, bioavailable 25(OH)D, showed an inverse association with neonatal irisin in univariate analysis [β = -56.257 (-106.227 to -6.288), p = 0.028]. This relationship became more pronounced in multivariate analysis [β = -73.457 (-140.573 to -6.341), p = 0.034] (Figure 1).
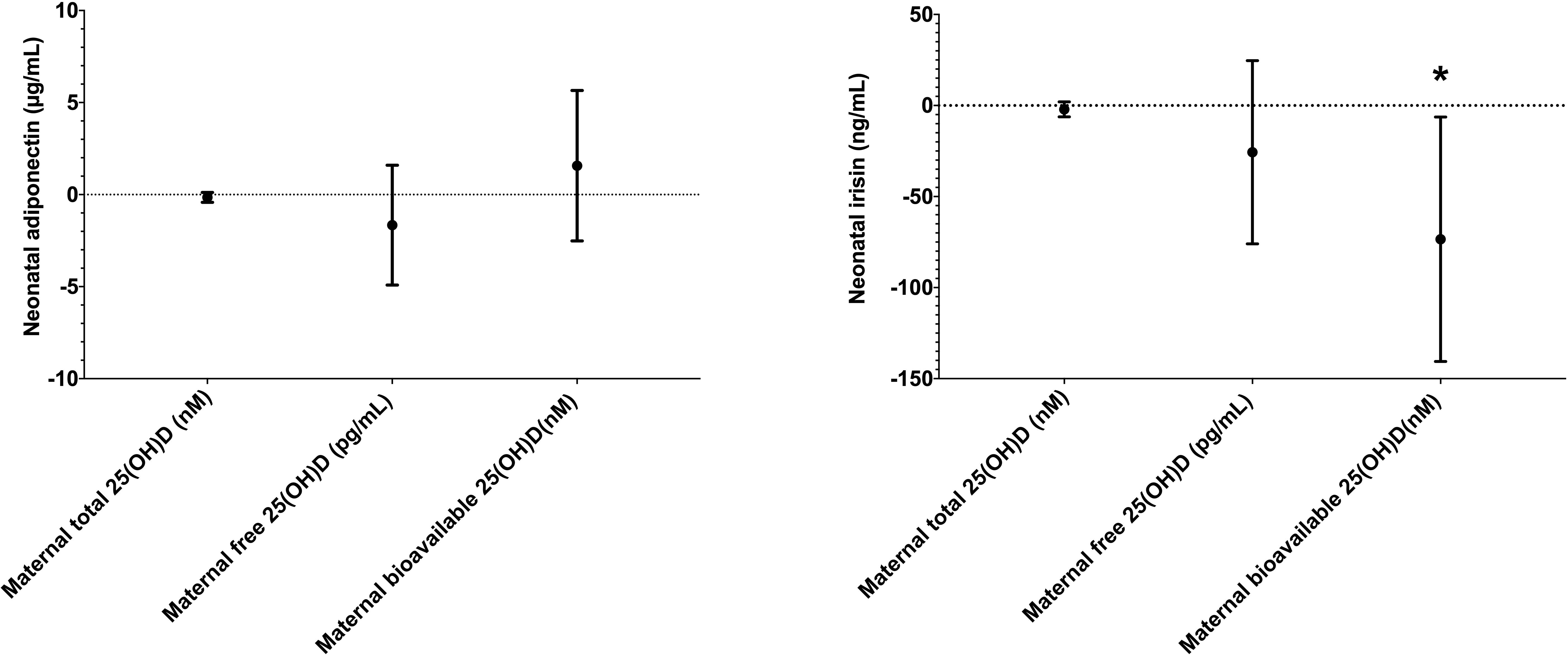
Figure 1 The association between maternal 25(OH)D forms and neonatal adiponectin (left) and irisin (right) in multivariate analysis. * indicates statistical significance.
Subsequently, we analyzed the intrarelationships between 25(OH)D forms and adipokines in the mothers and neonates. We found no association between the levels of maternal 25(OH)D forms with maternal adipokines. Similar results were also seen between neonatal 25(OH)D and adipokine concentrations Table 3.
In this study, we investigated the associations between various forms of 25(OH)D and adipokine levels in maternal-neonatal pairs. Interestingly, we observed a significant inverse association between maternal bioavailable 25(OH)D and neonatal irisin levels, highlighting a potential role of this specific form of vitamin D in regulating neonatal adipokines. We observed no intrarelationships between maternal and neonatal 25(OH)D forms and adipokine levels.
The interplay between vitamin D and adipokines remains a topic of considerable debate. In the general population, vitamin D supplementation generally does not lead to increased adiponectin levels (17). However, subgroup analyses have suggested a positive effect among diabetic individuals and those undergoing daily supplementation (17). To our knowledge, the influence of maternal vitamin D on neonatal adiponectin has not been previously studied, and our findings revealed no association between maternal vitamin D forms and neonatal adiponectin.
Similarly, the connection between vitamin D and irisin is not well-established, with conflicting findings in various studies. For example, Prader–Willi syndrome patients who do not receive vitamin D supplementations have significantly lower irisin levels than those who do (29). In a double-blind, randomized clinical trial, vitamin D supplementation significantly increased irisin serum levels in overweight and obese type 2 diabetics (30). However, serum irisin levels are negatively correlated with 25(OH)D in Charcot-Marie-Tooth patients (31). Similarly, type 1 diabetes mellitus patients demonstrate a similar negative association (32). Other studies have also revealed no association between the two parameters (33). Our study found no association of total and free 25(OH)D with neonatal irisin levels. However, maternal bioavailable 25(OH)D is independently and inversely associated with neonatal irisin concentrations. This observation warrants further investigation, as this study is the first to explore the relationship between maternal vitamin D status and neonatal irisin. The reason why bioavailable 25(OH)D is negatively associated with neonatal irisin concentrations while the free form is not, remains unclear. However, this finding is in accordance with our previous results, where maternal VDBP manifested significant associations with maternal adiponectin and irisin, whereas neonatal VDBP was associated with neonatal adiponectin and irisin, although the later association was not significant. It becomes evident that non-bounds 25(OH)D forms, in this case maternal bioavailable forms, may exert reverse regulatory effects compared to bound forms and VBDP, implying a diverse metabolic role of free and bound vitamin D forms on adipokine concentrations. Further studies are needed to verify our findings and understand the relationship between bioavailable vitamin D forms and irisin.
The impact of irisin levels on neonatal outcomes presents divergent findings in the literature. In our study, we observed a correlation between maternal bioavailable 25(OH)D and reduced neonatal irisin. Some studies have reported that elevated irisin levels are linked to endothelial dysfunction in pre-term children (15). It could be hypothesized that maternal bioavailable 25(OH)D may provide a protective effect against endothelial dysfunction. However, contrasting results have been reported, with other studies indicating an inverse association between irisin levels and the risk of fetal macrosomia (16). The conflicting evidence in existing literature highlights the imperative need for future studies to untangle the intricate role of adipokines, particularly irisin, in neonatal outcomes.
This study has some limitations. A significant constraint is its single-center nature, which potentially limits the generalizability of our findings, i.e. a limited external validity. Variations in 25(OH)D levels due to differences in geographic locations and seasonal changes are well-documented (20). Due to its homogenous population and single location, these factors were not taken into consideration. Furthermore, there are several factors that can influence adipokine levels, such as exercise levels (34), that were not examined in our study. Our observational design also precludes inferring causality from the data. Additionally, the study might be susceptible to statistical type I errors (false positive findings) due to multiple testing. As our statistical analysis plan was however based on hypotheses derived from previous investigations by others, we refrained from adjusting for multiple testing.
In conclusion, our findings indicate that maternal bioavailable 25(OH)D is associated with reduced neonatal irisin concentrations, while total and free maternal 25(OH)D have no impact on neonatal adipokines. Intrarelationships between vitamin D forms and adipokine levels were not observed. The potential protective effects of bioavailable 25(OH)D against harmful irisin effects warrant further investigations. Future studies should explore the underlying mechanisms by which maternal bioavailable 25(OH)D regulates neonatal irisin levels. Additionally, investigating this interplay in diverse populations or at-risk groups like mothers with vitamin D deficiency could provide valuable insights.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Bioethics Committee of Aristotle University in Thessaloniki. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
TA: Methodology, Validation, Writing – original draft, Writing – review & editing. HF: Data curation, Visualization, Writing – original draft, Writing – review & editing. HT: Formal analysis, Visualization, Writing – original draft, Writing – review & editing. RC: Data curation, Validation, Writing – original draft, Writing – review & editing. MH: Investigation, Validation, Writing – review & editing. FA: Resources, Validation, Writing – review & editing. SP: Resources, Validation, Writing – review & editing. CA: Resources, Validation, Writing – review & editing. GT: Resources, Validation, Writing – review & editing. CH: Resources, Validation, Writing – review & editing. SK: Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Arabi T, Shafqat A, Sabbah BN, Fawzy NA, Shah H, Abdulkader H, et al. Obesity-related kidney disease: beyond hypertension and insulin-resistance. Front Endocrinol. (2023) 13:1095211. doi: 10.3389/fendo.2022.1095211
2. Fang H, Judd RL. Adiponectin regulation and function. Compr Physiol. (2018) 8:1031–63. doi: 10.1002/cphy.c170046
3. da Silva Rosa SC, Liu M, Sweeney G. Adiponectin synthesis, secretion and extravasation from circulation to interstitial space. Physiology. (2021) 36:134–49. doi: 10.1152/physiol.00031.2020
4. Yeung EH, McLain AC, Anderson N, Lawrence D, Boghossian NS, Druschel C, et al. Newborn adipokines and birth outcomes. Paediatr Perinat Epidemiol. (2015) 29:317–25. doi: 10.1111/ppe.12203
5. Zhang ZQ, Lu QG, Huang J, Jiao CY, Huang SM, Mao LM. Maternal and cord blood adiponectin levels in relation to post-natal body size in infants in the first year of life: A prospective study. BMC Pregnancy Childbirth. (2016) 16:189. doi: 10.1186/s12884-016-0978-9
6. Goto E. Maternal and cord blood adiponectin concentrations in small for gestational age: A meta-analysis. Ann Nutr Metab. (2018) 72:57–64. doi: 10.1159/000485748
7. Roca-Rivada A, Castelao C, Senin LL, Landrove MO, Baltar J, Belén Crujeiras A, et al. Fndc5/irisin is not only a myokine but also an adipokine. PloS One. (2013) 8:e60563. doi: 10.1371/journal.pone.0060563
8. Bao JF, She QY, Hu PP, Jia N, Li A. Irisin, a fascinating field in our times. Trends Endocrinol Metab. (2022) 33:601–13. doi: 10.1016/j.tem.2022.06.003
9. Maalouf GE, El Khoury D. Exercise-induced irisin, the fat browning myokine, as a potential anticancer agent. J Obes. (2019) 2019:6561726. doi: 10.1155/2019/6561726
10. Demirel S, Sahinturk S, Isbil N, Ozyener F. Physiological role of K(+) channels in irisin-induced vasodilation in rat thoracic aorta. Peptides. (2022) 147:170685. doi: 10.1016/j.peptides.2021.170685
11. Demirel S, Ozyener F. Irisin relaxes rat trachea via K(V) channels, K(Atp) channels, and bk(Ca) channels. Protein Pept Lett. (2022) 29:760–8. doi: 10.2174/0929866529666220729115541
12. Demirel S, Şahintürk S, İşbil N, Özyener F. Irisin relaxes rat thoracic aorta through inhibiting signaling pathways implicating protein kinase C. Turk J Med Sci. (2022) 52:514–21. doi: 10.55730/1300-0144.5340
13. Luo Y, Qiao X, Xu L, Huang G. Irisin: circulating levels in serum and its relation to gonadal axis. Endocrine. (2022) 75:663–71. doi: 10.1007/s12020-022-02981-5
14. Shan D, Liu X, Cai Y, Qiao X, Xu L, Zou L. Irisin level and neonatal birthweight: A systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. (2020) 254:25–32. doi: 10.1016/j.ejogrb.2020.08.045
15. Markopoulou P, Koutroumpa A, Mantzou A, Margeli A, Papanikolaou E, Siahanidou T. Association of plasma irisin levels with circulating endothelial microparticles (Emps) and endothelial progenitor cells (Epcs) in children born prematurely. Metabolites. (2023) 13:120. doi: 10.3390/metabo13010120
16. Zheng S, Guo W, Wang X. Study on the relationship between the levels of irisin in umbilical cord blood and neonatal growth in China. J Matern Fetal Neonatal Med. (2020) 33:4133–8. doi: 10.1080/14767058.2019.1597843
17. Nikooyeh B, Neyestani TR. Can vitamin D be considered an adiponectin secretagogue? A systematic review and meta-analysis. J Steroid Biochem Mol Biol. (2021) 212:105925. doi: 10.1016/j.jsbmb.2021.105925
18. Sanesi L, Dicarlo M, Pignataro P, Zerlotin R, Pugliese F, Columbu C, et al. Vitamin D increases irisin serum levels and the expression of its precursor in skeletal muscle. Int J Mol Sci. (2023) 24:4129. doi: 10.3390/ijms24044129
19. Safari S, Rafraf M, Malekian M, Molani-Gol R, Asghari-Jafarabadi M, Mobasseri M. Effects of vitamin D supplementation on metabolic parameters, serum irisin and obesity values in women with subclinical hypothyroidism: A double-blind randomized controlled trial. Front Endocrinol. (2023) 14:1306470. doi: 10.3389/fendo.2023.1306470
20. Fernando M, Coster TG, Ellery SJ, Guingand DD, Lim S, Harrison CL, et al. Relationships between total, free and bioavailable vitamin D and vitamin D binding protein in early pregnancy with neonatal outcomes: A retrospective cohort study. Nutrients. (2020) 12:2495. doi: 10.3390/nu12092495
21. Turkes GF, Uysal S, Demir T, Demiral Y, Pamuk BO, Yılmaz H, et al. Associations between bioavailable vitamin D and remnant cholesterol in patients with type 2 diabetes mellitus. Cureus. (2021). doi: 10.7759/cureus.13248
22. Karras SN, Koufakis T, Fakhoury H, Kotsa K. Deconvoluting the biological roles of vitamin D-binding protein during pregnancy: A both clinical and theoretical challenge. Front Endocrinol. (2018) 9:259. doi: 10.3389/fendo.2018.00259
23. Karras SN, Polyzos SA, Newton DA, Wagner CL, Hollis BW, Ouweland JVD, et al. Adiponectin and vitamin D-binding protein are independently associated at birth in both mothers and neonates. Endocrine. (2018) 59:164–74. doi: 10.1007/s12020-017-1475-2
24. AlAnouti F, Annweiler C, Arabi TZ, Chun RF, Fakhoury HMA, Grant WB, et al. Associations of free, bioavailable and total 25-hydroxyvitamin D with neonatal birth anthropometry and calcium homoeostasis in mother–child pairs in a sunny mediterranean region. Br J Nutr. (2023) 131, 1–8. doi: 10.1017/S000711452300243X
25. Karras SN, Shah I, Petroczi A, Goulis DG, Bili H, Papadopoulou F, et al. An observational study reveals that neonatal vitamin D is primarily determined by maternal contributions: implications of a new assay on the roles of vitamin D forms. Nutr J. (2013) 12:1–8. doi: 10.1186/1475-2891-12-77
26. Shah I, James R, Barker J, Petroczi A, Naughton DP. Misleading measures in vitamin D analysis: A novel lc-ms/ms assay to account for epimers and isobars. Nutr J. (2011) 10:1–9. doi: 10.1186/1475-2891-10-46
27. Chun RF, Peercy BE, Adams JS, Hewison M. Vitamin D binding protein and monocyte response to 25-hydroxyvitamin D and 1, 25-dihydroxyvitamin D: analysis by mathematical modeling. PloS One. (2012) 7:e30773. doi: 10.1371/journal.pone.0030773
28. Karras SN, Dursun E, Alaylıoglu M, Gezen-Ak D, Al Anouti F, Pilz S, et al. Upregulation of irisin and vitamin D-binding protein concentrations by increasing maternal 25-hydrovitamin D concentrations in combination with specific genotypes of vitamin D-binding protein polymorphisms. Nutrients. (2021) 14:90. doi: 10.3390/nu14010090
29. Faienza MF, Brunetti G, Grugni G, Fintini D, Convertino A, Pignataro P, et al. The genetic background and vitamin D supplementation can affect irisin levels in prader–willi syndrome. J Endocrinological Invest. (2021) 44:2261–71. doi: 10.1007/s40618-021-01533-4
30. Safarpour P, Daneshi-Maskooni M, Vafa M, Nourbakhsh M, Janani L, Maddah M, et al. Vitamin D supplementation improves sirt1, irisin, and glucose indices in overweight or obese type 2 diabetic patients: A double-blind randomized placebo-controlled clinical trial. BMC Family Pract. (2020) 21:26. doi: 10.1186/s12875-020-1096-3
31. Colaianni G, Oranger A, Dicarlo M, Lovero R, Storlino G, Pignataro P, et al. Irisin serum levels and skeletal muscle assessment in a cohort of charcot-marie-tooth patients. Front Endocrinol. (2022) 13:886243. doi: 10.3389/fendo.2022.886243
32. Faienza MF, Brunetti G, Sanesi L, Colaianni G, Celi M, Piacente L, et al. High irisin levels are associated with better glycemic control and bone health in children with type 1 diabetes. Diabetes Res Clin Pract. (2018) 141:10–7. doi: 10.1016/j.diabres.2018.03.046
33. Wang Y, Gu Y, Huang J, Wu H, Meng G, Zhang Q, et al. Serum vitamin D status and circulating irisin levels in older adults with sarcopenia. Front Nutr. (2022) 9:1051870. doi: 10.3389/fnut.2022.1051870
Keywords: vitamin D, adiponectin, irisin, neonatal, maternal, free vitamin D, bioavailable vitamin D2
Citation: Arabi TZ, Fakhoury HMA, Tamim H, Chun RF, Hewison M, AlAnouti F, Pilz S, Annweiler C, Tzimagiorgis G, Haitoglou C and Karras SN (2024) Associations between total, free and bioavailable 25-hydroxyvitamin D forms with adiponectin and irisin in maternal-neonatal pairs at birth from Greece. Front. Endocrinol. 15:1397869. doi: 10.3389/fendo.2024.1397869
Received: 08 March 2024; Accepted: 17 June 2024;
Published: 05 July 2024.
Edited by:
Bassem Refaat, Umm Al-Qura University, Saudi ArabiaReviewed by:
Rosaura Leis, University of Santiago de Compostela, SpainCopyright © 2024 Arabi, Fakhoury, Tamim, Chun, Hewison, AlAnouti, Pilz, Annweiler, Tzimagiorgis, Haitoglou and Karras. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Spyridon N. Karras, a2FycmFzcGlyb3NAeWFob28uZ3I=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.