
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 21 May 2024
Sec. Cardiovascular Endocrinology
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1397062
This article is part of the Research TopicEndocrine insights into heart diseaseView all 18 articles
 Gabriele Brosolo1†
Gabriele Brosolo1† Andrea Da Porto2†
Andrea Da Porto2† Luca Bulfone1
Luca Bulfone1 Antonio Vacca1
Antonio Vacca1 Nicole Bertin3
Nicole Bertin3 Cinzia Vivarelli1
Cinzia Vivarelli1 Cristiana Catena1‡
Cristiana Catena1‡ Leonardo A. Sechi1,2,3*‡
Leonardo A. Sechi1,2,3*‡Background and aims: A prothrombotic state was demonstrated in patients with Cushing’s syndrome and is involved in the development and progression of cardiovascular and renal damage in hypertensive patients. This study was designed to examine the relationships between cortisol secretion and the hemostatic and fibrinolytic systems in hypertension.
Methods: In 149 middle-aged, nondiabetic, essential hypertensive patients free of cardiovascular and renal complications, we measured hemostatic markers that express the spontaneous activation of the coagulation and fibrinolytic systems and assessed daily cortisol levels (8 AM, 3 PM, 12 AM; area under the curve, AUC-cortisol) together with the cortisol response to dexamethasone overnight suppression (DST-cortisol).
Results: Plasma levels of D-dimer (D-dim), prothrombin fragment 1 + 2 (F1 + 2), and von Willebrand factor (vWF) were progressively and significantly higher across tertiles of AUC-cortisol and DST-cortisol, whereas no differences were observed in fibrinogen, tissue plasminogen activator, plasminogen activator inhibitor-1, antithrombin III, protein C, and protein S. D-dim, F1 + 2, and vWF were significantly and directly correlated with age and both AUC-cortisol and DST-cortisol. Multivariate regression analysis showed that both AUC-cortisol and DST-cortisol were related to plasma D-dim, F1 + 2, and vWF independently of age, body mass index, blood pressure, and renal function.
Conclusion: Greater daily cortisol profile and cortisol response to overnight suppression are independently associated with a prothrombotic state in hypertensive patients and might contribute to the development of organ damage and higher risk of cardiovascular complications.
Epidemiological evidence indicates that the coagulation system plays an important role in the pathophysiology of atherosclerosis (1), and increased incidence of cardiovascular events has been associated with elevated circulating levels of markers of hemostatic activation (2–4). A prothrombotic state is characterized by intrinsic subclinical activation of the hemostatic system and is a well-recognized risk factor for cardiovascular events in the general population (5, 6) and in patients with hypertension (7, 8) and early renal failure (9). In hypertension, a prothrombotic state is associated with subclinical changes of the arterial tree (10–12), impaired left ventricular diastolic properties (13), and intrarenal hemodynamic changes (14) that are associated with decreased glomerular filtration rate (15). Furthermore, a link of the prothrombotic state with an activated renin-angiotensin-aldosterone system has been demonstrated in hypertension (16, 17) suggesting interaction of the hemostatic system with blood pressure regulatory mechanisms.
A prothrombotic state can be detected in many disease states including those characterized by exogenous or endogenous hypercortisolism (18). In patients with Cushing’s syndrome, elevated circulating levels of a multitude of hemostatic markers were demonstrated, together with evidence of an impaired fibrinolytic system (19–21). Interestingly, initial studies reported activation of the hemostatic system also in patients with subclinical Cushing’s syndrome (22, 23) such as those with incidentally detected adrenal masses in the absence of overt clinical features of hypercortisolism (24). Along these lines, studies conducted in other groups of patients have reported observations that might suggest the existence of a relationship between circulating glucocorticoids and different components of the hemostatic and fibrinolytic systems (25–27).
Although patients with essential hypertension have cortisol secretory rates and response to overnight dexamethasone suppression (DST) within the physiologic range, minor differences in circulating cortisol levels might have some impact on the coagulation system. To date, no information is available on the possible relationship of circulating cortisol with the hemostatic-fibrinolytic balance in patients with hypertension. This information would be relevant for better understanding of mechanisms that contribute to the development and progression of hypertensive organ damage.
The aim of this study was to test the hypothesis that cortisol secretory rates and response to DST are associated with changes in the activity of the coagulation system. We examined the relationship of cortisol daily production and response to DST with a broad panel of hemostatic markers in nondiabetic patients with hypertension who were free of major cardiovascular and renal complications.
Patients with grade 1/grade 2 essential hypertension who consecutively presented at the Hypertension Clinic of our department from January 2021 to December 2021 were included in a cross-sectional study. All patients were white Caucasian, lived in the North-East of Italy, and were representative of the hypertensive population of this regional territory (28). Blood pressure was measured in patients who remained supine for at least 15 minutes using an automatic tool equipped with appropriately sized cuffs (Omron M6, OMRON Healthcare Co., Kyoto, Japan), obtaining 3 separate readings. Diagnosis of hypertension was done after measurements obtained in at least 3 separate visits, according to current guidelines (29). We excluded patients with: age <18 or >80 years; body mass index (BMI) >40 kg/m2; pregnancy or use of estrogens; any type of treatment with corticosteroids; history of alcohol abuse; grade 3 hypertension and secondary hypertension; diabetes; major depressive disorders; 24-hour creatinine clearance <60 ml/min/1,73 m2; use of any type of drugs that could interfere with the hemostatic system; history of recent illness and acute or chronic inflammatory conditions; history of cerebrovascular, ischemic heart, or peripheral artery disease. Causes of secondary hypertension were ruled out according to current guidelines (29) as previously reported (30). Cushing’s syndrome was excluded by measurement of daily plasma cortisol (8 AM, 3 PM, 12 AM), 24-hour free urinary cortisol, and plasma cortisol after an overnight DST with 1 mg dexamethasone, according to guidelines (31). When plasma cortisol after DST was >50 nmol/L (15 patients) a 2 mg/48-hour dexamethasone test was done to confirm suppression of cortisol below that level. All patients underwent either MRI or CT of adrenals to exclude presence of incidentalomas. Diabetes was excluded by measurement of fasting blood glucose and glycated hemoglobin, and by a standard oral glucose tolerance test (32). Smokers were defined if they smoked for more than 5 years and did not quit more than 1 year before examination. A standardized questionnaire was used to assess daily alcohol intake (33). The study was conducted following the statements of the Declaration of Helsinki and was approved by the Institutional Review Board of the Department of Medicine. All patients gave their informed consent.
Blood was collected by venipuncture without venous stasis in the early morning after an overnight fast. Plasma was separated and stored at -80°C until processing. Glucose, total and high-density lipoprotein cholesterol, triglycerides were measured with chemical methods in automated devices, as previously reported (34). Low-density lipoprotein level was calculated by the Friedewald formula. Glomerular filtration rate was assessed by duplicate measurements of 24-hour creatinine clearance. Coagulation parameters were measured in plasma as previously described (35). In brief, fibrinogen was assayed in an automatic coagulometer by a functional test, D-dimer was assayed immunoenzymatically, prothrombin fragment 1 + 2 (F1 + 2) and tissue-plasminogen activator (tPA) by an enzyme-linked immunosorbent assay, plasminogen activator inhibitor-1 (PAI-1) by immunoassay, antithrombin III (AT-III), protein C, protein S, and von Willebrand factor (vWF) by functional chromogenic assays. Plasma cortisol was measured by immunoassay (Electro Chemiluminescence ECLIA, Elecsys cortisol II, Roche Diagnostics, Basel, Switzerland) with an intraassay and interassay coefficient of variation of 1.7% and 2.3%, respectively, and the lowest detection limit of 1.5 nmol/L. The area under the curve of daily cortisol (8 AM, 3 PM, 12 AM; AUC-cortisol) was calculated by the trapezoidal rule (36). Duplicate 24-hour urinary collections were obtained for measurement of free cortisol excretion with a direct chemiluminescence technique (ADVIA Centaur Cortisol Immunoassay System, Siemens Healthcare, Milan, Italy; detection range, 14–2069 nmol/l) and the average value was considered.
The Kolmogorov-Smirnov test was used to determine normality of distribution of the variables included in the study. Normally distributed variables are expressed as mean ± standard deviation and skewed variables as median [interquartile range]. Categorical data are expressed as absolute number and percentage. For statistical reasons patients were grouped in tertiles of either AUC-cortisol or DST-cortisol and two-way ANOVA and the Kruskal-Wallis test were used for comparisons among groups with normal or skewed variable distribution, respectively. The Pearson’s chi-square test was used to compare frequency distributions. The relationships between different variables were examined by linear regression analysis, and correlation was expressed by the correlation coefficient r. In this analysis, variables with skewed distribution were log transformed. Multivariate regression analysis was performed to determine which variables were independently associated with hemostatic markers. A P value of less than 5% was considered to indicate statistical significance. All data analyses were performed using Stata 12.1 (StataCorp LP, College Station, TX, USA).
One-hundred-forty-nine patients (age, 48±13 years; 77 males, 72 females) with essential hypertension were recruited for data analysis. Forty (27%) patients had BMI >30 of whom 21 (14%) had grade 1 (BMI 30–35) and 19 (13%) grade 2 obesity. Sixty-two (42%) patients had never been treated with antihypertensive agents and the remaining 87 (58%) who were taking an average of 1.3 antihypertensive agents (angiotensin-converting enzyme inhibitors or angiotensin receptor antagonists, 39%; calcium-channel blockers, 38%; diuretics, 19%; beta-blockers, 19%; alpha-blockers, 7%) had their drugs withdrawn for at least two weeks before the study.
For statistical reasons, patients were grouped according to tertiles of AUC-cortisol and DST-cortisol. The clinical characteristics of the study patients are summarized in Table 1 together with general biochemistries. Across AUC-cortisol and DST-cortisol tertiles, no significant differences were observed in demographic and anthropometric characteristics, systolic and diastolic blood pressure, plasma glucose and glycated hemoglobin, plasma lipids, electrolyte levels, renal function, and C-reactive protein. No differences were also observed in frequency of previous use of antihypertensive drugs. In particular, no significant differences were observed from patients previously treated with diuretics and the remaining patients. Table 2 summarizes hormonal measurements of the study patients showing that plasma cortisol levels measured at 8 AM, 3 PM, and 12 AM increased significantly across AUC-cortisol and DST-cortisol tertiles, whereas plasma ACTH, active renin and aldosterone, and daily urinary excretion of epinephrine, norepinephrine, and dopamine did not differ among groups. Table 3 shows the hemostatic variables showing that plasma D-dimer, F1 + 2, and vWF levels were significantly and progressively higher across both AUC-cortisol and DST-cortisol tertiles. No significant differences among groups were observed in fibrinogen, t-PA, PAI-1, AT-III, and protein C and protein S.
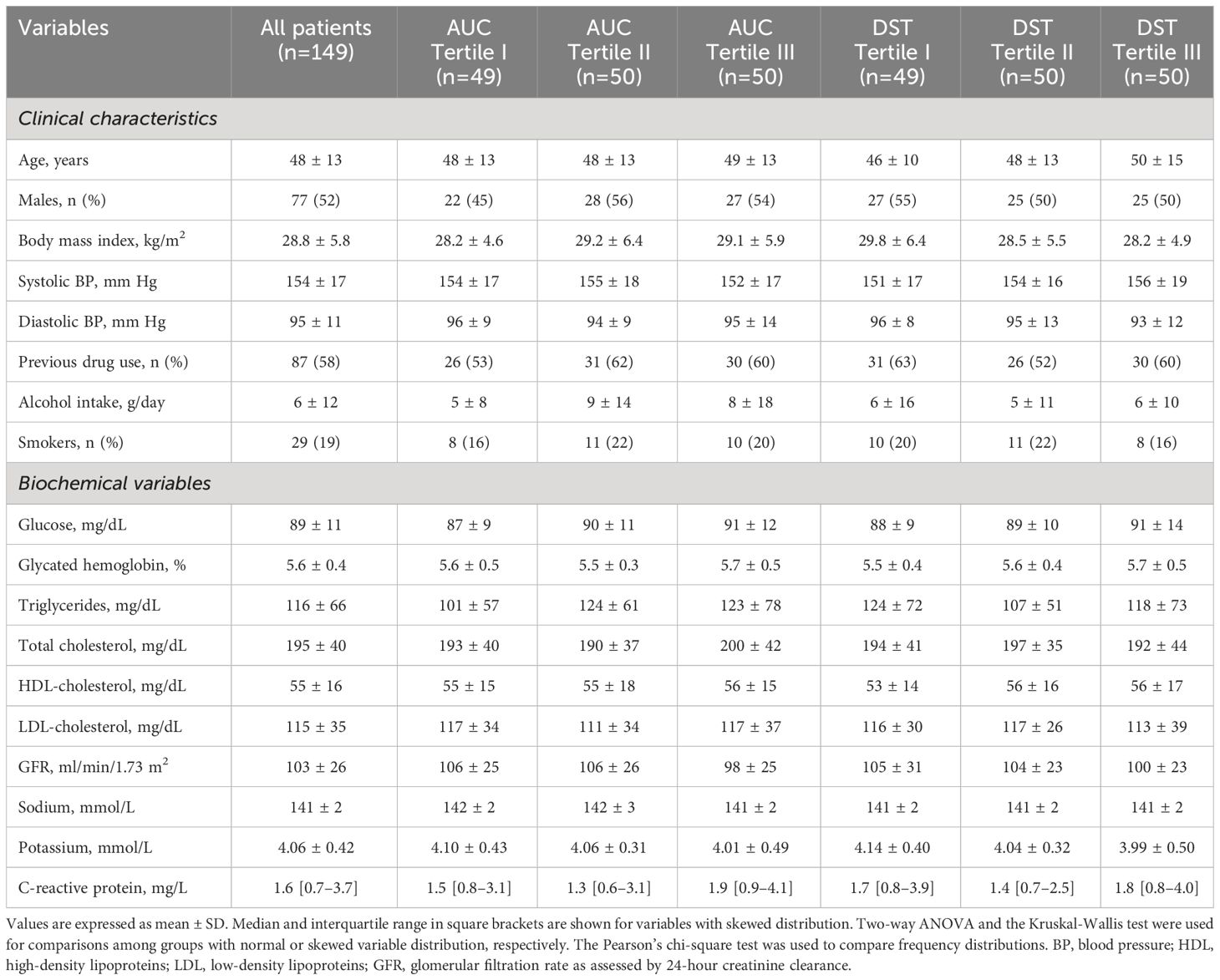
Table 1 Clinical Characteristics and biochemical variables of hypertensive patients who were grouped according to either tertiles of the area under the curve (AUC) of daily plasma cortisol or plasma cortisol after dexamethasone suppression (DST).
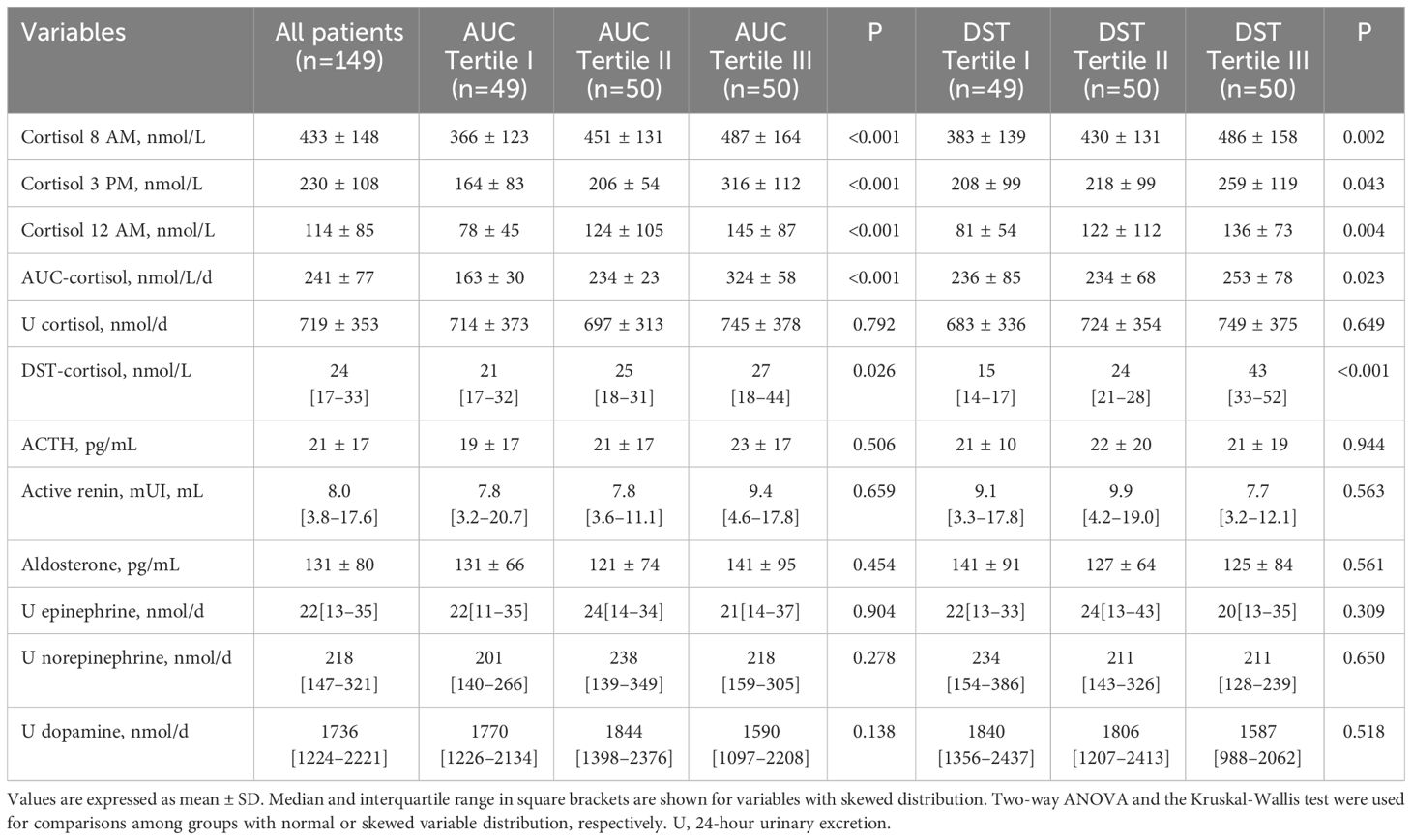
Table 2 Hormonal variables of hypertensive patients who were grouped according to either tertiles of the area under the curve (AUC) of daily plasma cortisol or plasma cortisol after dexamethasone suppression (DST).
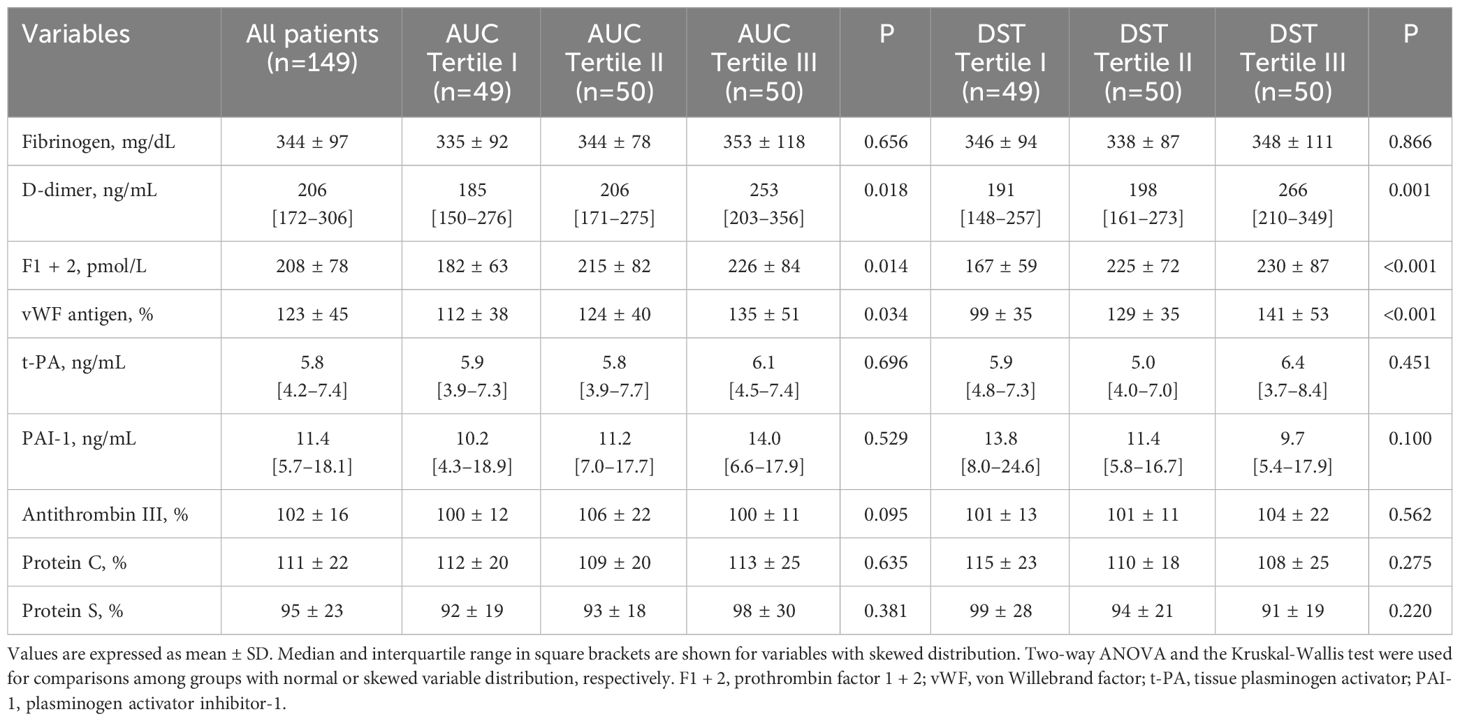
Table 3 Hemostatic variables of hypertensive patients who were grouped according to either tertiles of the area under the curve (AUC) of daily plasma cortisol or plasma cortisol after dexamethasone suppression (DST).
Analysis of univariate correlations (Table 4) showed that D-dimer, F1 + 2, and vWF were significantly and directly correlated with patients’ age, AUC-cortisol (Figure 1) and DST-cortisol (Figure 2). No further significant relationship of plasma cortisol levels was observed with the other hemostatic markers. F1 + 2 was also inversely correlated with 24-hour creatinine clearance while fibrinogen, tPA and PAI-1 were directly correlated with BMI. Only fibrinogen was directly correlated with systolic blood pressure. None of the cortisol and hemostatic variables was correlated with C-reactive protein.
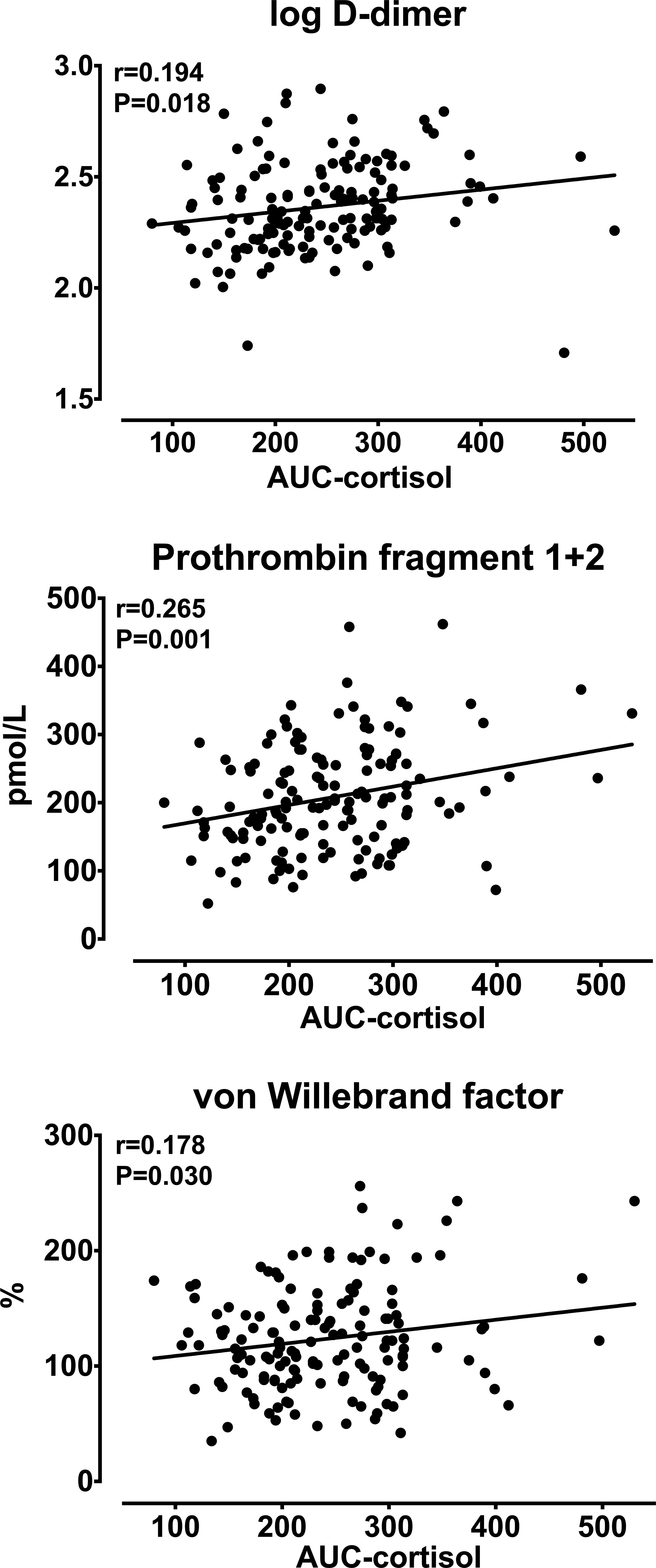
Figure 1 Relationships between the area under the curve (AUC) of daily (8 AM, 3 PM, 12 AM) plasma cortisol and log-transformed D-dimer, prothrombin fragment 1 + 2, and von Willebrand factor.
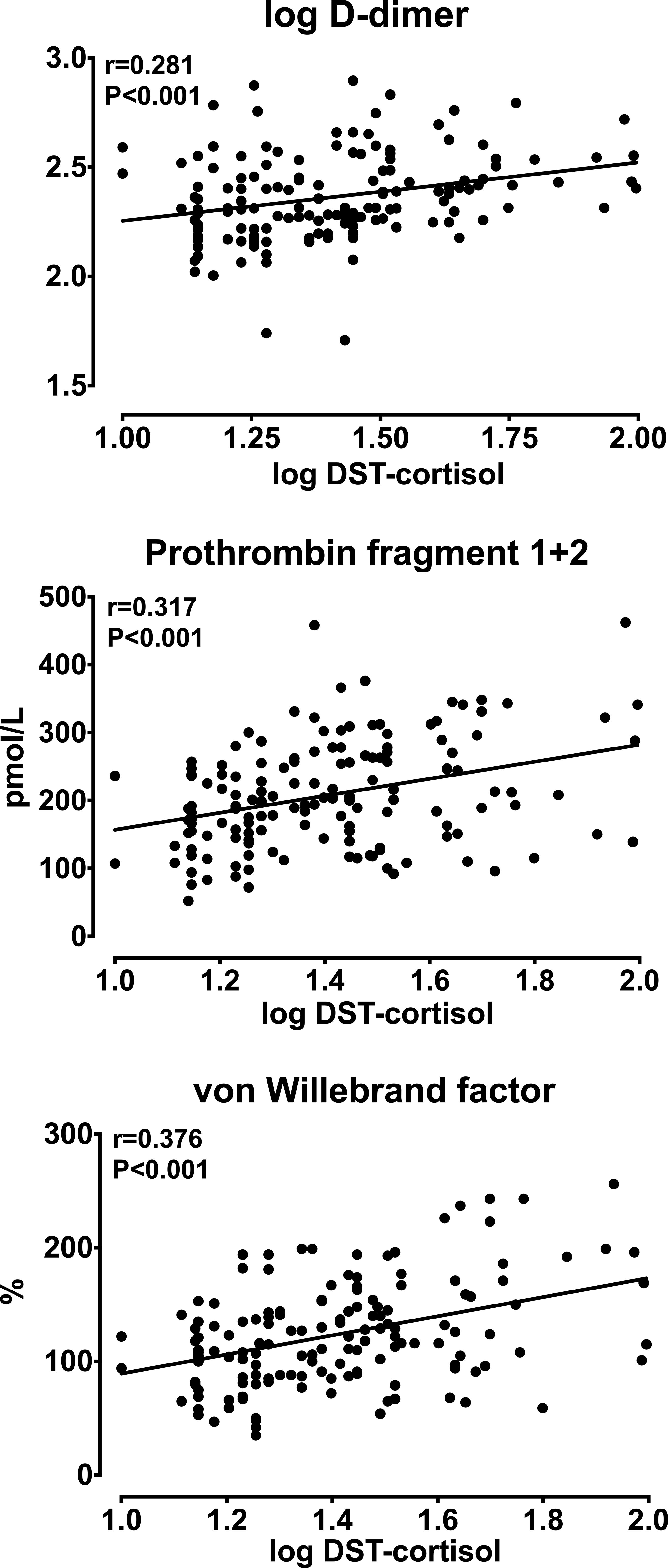
Figure 2 Relationships between log-transformed plasma cortisol measured after a 1 mg overnight dexamethasone suppression test (DST-cortisol) and log-transformed D-dimer, prothrombin fragment 1 + 2, and von Willebrand factor.
Multivariate regression analysis was conducted including different hemostatic markers as the dependent variables and age, sex, systolic blood pressure, creatinine clearance, and either AUC-cortisol (Model 1) or DST-cortisol (Model 2) as the independent variables, respectively (Supplementary Material). In both models, age was independently and directly related with F1 + 2 and vWF, and sex was independently related with log D-dimer. Both AUC-cortisol and DST-cortisol were significantly and independently correlated with log D-dimer (β-coefficient 0.001, P=0.029; β-coefficient 0.003, P<0.001; respectively), F1 + 2 (β-coefficient 0.267, P=0.002; β-coefficient 1.217, P<0.001; respectively), and vWF (β-coefficient 0.106, P=0.035; β-coefficient 0.877, P<0.001; respectively).
A prothrombotic state is associated with major cardiovascular events in hypertension, and many factors can contribute to hemostatic activation in hypertensive patients (37). Evidence previously obtained in patients with Cushing’s syndrome indicates that excess cortisol could contribute to a hypercoagulable state. We tested the hypothesis that even minor differences in regulation of cortisol secretion are associated with a prothrombotic state in patients with essential hypertension. Results show that plasma levels of D-dimer, F1 + 2, and vWF are progressively greater with increasing levels of plasma cortisol daily profile and response to DST. Both AUC-cortisol and DST-cortisol are significantly correlated with plasma D-dimer, F1 + 2, and vWF levels independently of age, BMI, blood pressure, and renal function. These findings indicate that differences in regulation of cortisol production within the physiologic range might contribute to a prothrombotic state in patients with hypertension.
Hypercortisolism is associated with high risk of cardiovascular morbidity and mortality that might be related to a multiplicity of factors (38) including a prothrombotic state. After initial studies that suggested presence of a hypercoagulable state in patients with Cushing’s syndrome (39), spontaneous activation of the hemostatic system was consistently shown in patients with hypercortisolism. Increased circulating levels of fibrinogen (19, 20, 40, 41), D-dimer (20, 23, 40), vWF (20, 42–44), AT-III (19–21, 44, 45), protein C-protein S complex (21, 22, 41, 45), and PAI-1 (19, 20, 41) were reported together with changes of additional coagulation factors and hemostatic tests (19–21, 41, 43, 46) in several cross-sectional comparisons of patients with Cushing’s syndrome with healthy subjects. Some studies suggested that these changes could be more relevant in patients with ACTH-producing adenomas (44) and increased levels of procoagulants and antifibrinolytics were reported also in children with Cushing’s syndrome that resolved after surgical treatment (45). Reversal of hypercoagulability was reported also in adults after surgical resolution of disease (43, 46), although this was not confirmed in other studies (40, 41). Interestingly, hemostatic changes were reported also in patients with excess plasma cortisol or incidentally detected adrenal masses in the absence of overt clinical features of hypercortisolism (22, 23), further supporting the hypothesis of a contribution of cortisol levels to regulation of coagulation and fibrinolysis (18). In our highly selected hypertensive patients who had physiologic suppression of plasma cortisol after DST, cortisol levels were significantly and independently associated with markers of intrinsic hemostatic activation. This study expands to a physiologic range of hormonal secretion the evidence of the potential role of cortisol in causing a prothrombotic state.
Due to its high prevalence in the general population, arterial hypertension is considered the leading cardiovascular risk factor. In patients with essential hypertension, we have previously demonstrated that a prothrombotic state contributes to the development of target organ damage and thereby might increase cardiovascular morbidity and mortality (7, 9–15). In fact, substantial experimental and clinical data indicate that platelets and coagulation cascade are important determinants of both atherogenesis and atherothrombosis (1). The hemostatic system exerts several actions on the vasculature that could influence the structure of the arterial wall and presumably the progression of atherosclerotic lesions. Hemostatic components have been implicated in causing disruption of endothelial lining, leukocyte recruitment, oxidative stress, vascular inflammation, migration of vascular smooth muscle cells, apoptosis, and angiogenesis (47, 48). For these reasons, identification of conditions that may contribute to a prothrombotic state in hypertension would be relevant as an issue for possible preventive interventions.
Many factors could cause activation of the hemostatic system in hypertension and the present study demonstrates that even minor differences in regulation of cortisol secretion are associated with a prothrombotic state as defined by higher levels of D-dimer, F1 + 2, and vWF. Measurements of fibrin D-dimer, the principal breakdown fragment of fibrin, and F1 + 2 that is released when coagulation factor Xa converts prothrombin to thrombin provide a reliable estimation of the overall state of activation of the coagulation pathways. On the other hand, vWF enhances adhesion of platelets to subendothelial collagen and increases the stability of coagulation factor VIII in the hemostatic cascade. Older age, obesity, and impaired renal function are associated with a prothrombotic state, and this is why statistical analysis was corrected for these variables showing that D-dimer, F1 + 2, and vWF were all independently related to both AUC-cortisol and DST-cortisol. Thus, differences in cortisol secretion within a physiological range could contribute to a prothrombotic state and thereby to hypertensive organ damage. Also, to this point it should be noticed that minor differences in regulation of cortisol secretion were previously found to be associated with impaired glucose metabolism in nondiabetic hypertensive patients (36) and greater left ventricular mass (30), suggesting additional mechanisms that might mediate detrimental cardiovascular effects of cortisol in essential hypertension.
Mechanisms that could link excess cortisol secretion to the hypercoagulable state are mostly speculative. Cortisol-induced up-regulation of gene transcription of multiple coagulation factors with intrinsic activation of the hemostatic cascade is the most likely mechanism. Activation of markers of the overall activity of the coagulation system such as D-dimer and F1 + 2 would support this possibility. On the other hand, observation of significantly increased levels of vWF might suggest that the prothrombotic state caused by increased circulating cortisol could be related to an enhanced metabolic function of endothelial cells. This was also suggested by Fatti et al. who reported increased levels of additional markers of endothelial activation (thrombin-antithrombin complex and plasmin-antiplasmin complex) in patients with Cushing’s syndrome (43). Hypercortisolism may also affect the multimeric structure of vWF causing an overexpression of abnormally high molecular weight multimers, capable of inducing spontaneous platelet aggregation (42).
Limitations to the present study need to be considered. First, the cross-sectional design limits the possibility to establish causality of the relationship between cortisol secretion and the prothrombotic state, although independence of this relationship from confounders in the multivariate analysis would suggest so. However, statistical adjustments cannot account for all biological and pathophysiological variables. Also, the possibility that plasma cortisol levels and prothrombotic markers causally affect one another cannot be excluded. Second, use of a selected clinic sample of white Caucasian hypertensive patients might limit the possibility to extend the present findings to a broader context. Third, inclusion of a significant proportion of patients who had been treated with antihypertensive drugs might have affected the results. However, it must be noticed that no differences were found in either hemostatic markers or cortisol measurements between these patients and those who were treatment naïve, nor differences were observed among patients who were treated with different categories of antihypertensive drugs. Last, due to the relatively small size of this study, the possibility that associations are merely due to chance should be considered.
This study demonstrates for the first time that even minor differences in daily cortisol secretion and cortisol levels after overnight suppression are independently associated with markers of a prothrombotic state in patients with essential hypertension who are free of major cardiovascular and renal complications. These findings expand this evidence beyond the boundary of clinical and subclinical Cushing’s syndrome. Results also provide better knowledge of factors that might contribute to develop a prothrombotic state in hypertension, thereby increasing target organ damage and, in turn, the risk of cardiovascular events. This study might have important clinical implications opening new paths to the possibility to identify those hypertensive patients at higher risk of development of subclinical organ damage. Detection of cortisol levels in the upper limit of normality in conjunction with markers of hemostatic activation could be useful to guide physicians toward more aggressive treatment and control of blood pressure and additional risk factors. Also, these findings open a window to the possibility to effectively prevent hypertensive organ damage through specific interventions on cortisol production and hemostatic system. Possible benefits of these interventions will have to be tested in future studies.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Institutional Review Board - Department of Medicine - University of Udine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
GB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Writing – original draft. AD: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Writing – original draft. LB: Data curation, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – review & editing. AV: Data curation, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – review & editing. NB: Data curation, Investigation, Supervision, Validation, Visualization, Writing – review & editing. CV: Data curation, Investigation, Supervision, Validation, Visualization, Writing – review & editing. CC: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. LS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by a research grant of the PierSilverio Nassimbeni Foundation to LS and CC.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1397062/full#supplementary-material
1. Borissoff JI, Spronk HMH, ten Cate H. The hemostatic system as a modulator of atherosclerosis. New Engl J Med. (2011) 364:1746–60. doi: 10.1056/NEJMra1011670
2. Cushman M, Lemaitre RN, Kuller LH, Psaty BM, Macy EM, Sharrett AR, et al. Fibrinolytic activation markers predict myocardial infarction in the elderly. The Cardiovascular Healthy Study. Arterioscler Thromb Vasc Biol. (1999) 19:493–8. doi: 10.1161/01.ATV.19.3.493
3. Folsom AR. Hemostatic risk factors for atherosclerotic disease: an epidemiologic view. Thromb Haemost. (2001) 86:366–73.
5. Ridker PM, Hennekens CH, Cerkus A, Stampfer MJ. Plasma concentrations of cross-linked fibrin degradation product (D-dimer) and the risk of future myocardial infarction among apparently healthy men. Circulation. (1994) 90:2236–40. doi: 10.1161/01.CIR.90.5.2236
6. Smith FB, Lee AJ, Fowkes FG, Price JF, Rumley A, Lowe GD. Hemostatic factors as predictors of ischemic heart disease and stroke in the Edinburgh Artery Study. Artherioscler Thromb Vasc Biol. (1997) 17:3321–5. doi: 10.1161/01.ATV.17.11.3321
7. Sechi LA, Zingaro L, Catena C, Casaccio D, De Marchi S. Relationship of fibrinogen levels and hemostatic abnormalities with organ damage in hypertension. Hypertension. (2000) 36:978–85. doi: 10.1161/01.HYP.36.6.978
8. Varugese GI, Lip GYH. Is hypertension a prothrombotic state? Curr Hypertens Rep. (2005) 7:168–73. doi: 10.1007/s11906-005-0005-4
9. Sechi LA, Zingaro L, Catena C, De Marchi S. Increased fibrinogen levels and hemostatic abnormalities in patients with arteriolar nephrosclerosis: association with cardiovascular events. Thromb Haemost. (2000) 84:565–70.
10. Catena C, Colussi G, Brosolo G, Sechi LA. A prothrombotic state is associated with early arterial damage in hypertensive patients. J Artheroscler Thromb. (2012) 19:471–8. doi: 10.5551/jat.10819
11. Catena C, Colussi G, Fagotto V, Sechi LA. Decreased fibrinolitic activity is associated with carotid artery stiffening in arterial hypertension. J Res Med Sci. (2017) 22:57. doi: 10.4103/jrms.JRMS_619_16
12. Brosolo G, Da Porto A, Bulfone L, Vacca A, Bertin N, Vivarelli C, et al. Association of arterial stiffness with a prothrombotic state in uncomplicated nondiabetic hypertensive patients. Front Cardiovasc Med. (2023) 10:1119516. doi: 10.3389/fcvm.2023.1119516
13. Catena C, Colussi G, Fedrizzi S, Sechi LA. Association of a prothrombotic state with left-ventricular diastolic dysfunction in hypertension: a tissue-Doppler imaging study. J Hypertens. (2013) 31:2077–84. doi: 10.1097/HJH.0b013e328362d951
14. Catena C, Colussi G, Novello M, Fagotto V, Sechi LA. Intrarenal vascular resistance is associated with a prothrombotic state in hypertensive patients. Kidney Blood Press Res. (2016) 41:929–36. doi: 10.1159/000452594
15. Catena C, Zingaro L, Casaccio D, Sechi LA. Abnormalities of coagulation in hypertensive patients with reduced creatinine clearance. Am J Med. (2000) 109:556–61. doi: 10.1016/S0002-9343(00)00567-2
16. Sechi LA, Novello M, Colussi GL, Di Fabio A, Chiuch A, Nadalini E, et al. Relationship of plasma renin with a prothrombotic state in hypertension: relevance for organ damage. Am J Hypertens. (2008) 21:1347–53. doi: 10.1038/ajh.2008.293
17. Tay K-H, Lip GYH. What drives the link between the renin-angiotensin-aldosterone system and the prothrombotic state in hypertension? Am J Hypertens. (2008) 21:1278–9. doi: 10.1038/ajh.2008.315
18. Van Zaane B, Nur E, Squizzato A, Dekkers OM, Twickler MT, Fliers E, et al. Hypercoagulable state in Cushing’s syndrome: a systematic review. J Clin Endocrinol Metab. (2009) 94:2743–50. doi: 10.1210/jc.2009-0290
19. Erem C, Nuhoglu I, Yilmaz M, Kocak M, Demirel A, Ucuncu O, et al. Blood coagulation and fibrinolysis in patients with Cushing’s syndrome: increased plasminogen activator inhibitor-1, decreased tissue factor pathway inhibitor, and unchanged thrombin-activated fibrinolysis inhibitor levels. J Endocrinol Invest. (2009) 32:169–74. doi: 10.1007/BF03345709
20. Manetti L, Bogazzi F, Giovannetti C, Raffaelli V, Genovesi M, Pellegrini G, et al. Changes in coagulation indexes and occurrence of venous thromboembolism in patients with Cushing’s syndrome: results from a prospective study before and after surgery. Eur J Endocrinol. (2010) 163:783–91. doi: 10.1530/EJE-10-0583
21. Kastelan D, Dusek T, Kraljevic I, Polasek O, Giljevic Z, Solak M, et al. Hypercoagulability in Cushing’s syndrome: the role of specific haemostatic and fibrinolytic markers. Endocrine. (2009) 36:70–4. doi: 10.1007/s12020-009-9186-y
22. Świątkowska-Stodulska R, Kaniuka-Jakubowska S, Wiśniewski P, Skibowska-Bielińska A, Sworczak K. The estimation of selected endogenous anticoagulation system parameters in patients with subclinical Cushing’s syndrome. Eur J Endocrinol. (2011) 165:865–71. doi: 10.1530/EJE-11-0535
23. Fukuoka H, Takeuchi T, Matsumoto R, Bando H, Suda K, Nishizawa H, et al. D-dimer as a significant marker of deep vein thrombosis in patients with subclinical or overt Cushing’s syndrome. Endocr J. (2014) 61:1003–10. doi: 10.1507/endocrj.EJ14-0102
24. Di Dalmazi G, Pasquali R, Beuschlein F, Reinke M. Subclinical hypercortisolism: a state, a syndrome, a disease? Eur J Endocrinol. (2015) 173:M61–71. doi: 10.1530/EJE-15-0272
25. Lippi G, Franchini M, Salvagno GL, Montagnana M, Guidi GC. Higher morning serum cortisol level predicts increased fibrinogen but not shortened aPTT. J Thromb Thrombolysis. (2008) 26:103–5. doi: 10.1007/s11239-007-0074-0
26. Dover AR, Hadoke PW, Walker BR, Newby DE. Acute effects of glucocorticoids on endothelial fibrinolytic and vasodilator function in humans. J Cardiovasc Pharmacol. (2007) 50:321–6. doi: 10.1097/FJC.0b013e3180cab148
27. De Pergola G, De Mitrio V, Cignarelli M, Garruti G, Giorgino F, Meola M, et al. Inverse relationship betrween cortisol excretion rate and plasminogen activator inhibitor-1 (PAI-1) antigen and activity in premenopausal obese women. Int J Obes. (1991) 15:619–22.
28. Catena C, Colussi GL, Capobianco F, Brosolo G, Sechi LA. Uricaemia and left ventricular mass in hypertensive patients. Eur J Clin Invest. (2014) 44:972–81. doi: 10.1111/eci.12331
29. Mancia G, Kreutz R, Brunstrom M, Burnier M, Grassi G, Januszewicz A, et al. 2023 ESH guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension. J Hypertens. (2023) 41:1874–2071. doi: 10.1097/HJH.0000000000003480
30. Brosolo G, Catena C, Da Porto A, Bulfone L, Vacca A, Verheyen ND, et al. Differences in regulation of cortisol secretion contribute to left ventricular abnormalities in patients with essential hypertension. Hypertension. (2022) 79:1435–44. doi: 10.1161/HYPERTENSIONAHA.122.19472
31. Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. (2008) 93:1526–40. doi: 10.1210/jc.2008-0125
32. Brosolo G, Da Porto A, Bulfone L, Scandolin L, Vacca A, Bertin N, et al. Vitamin D deficiency is associated with glycometabolic changes in nondiabetic patients with arterial hypertension. Nutrients. (2022) 14:311. doi: 10.3390/nu14020311
33. Catena C, Brosolo G, Da Porto A, Donnini D, Bulfone L, Vacca A, et al. Association of non-alcoholic fatty liver disease with left ventricular changes in treatment-naïve patients with uncomplicated hypertension. Front Cardiovasc Med. (2022) 9:1030968. doi: 10.3389/fcvm.2022.1030968
34. Catena C, Colussi GL, Brosolo G, Verheyen N, Novello M, Bertin N, et al. Long-term renal and cardiac outcomes after stenting in patients with resistant hypertension and atherosclerotic renal artery stenosis. Kidney Blood Press Res. (2018) 42:774–83. doi: 10.1159/000484299
35. Sechi LA, Catena C, Casaccio D, Zingaro L. Lipoprotein(a), haemostativc variables and cardiovascular damage in hypertensive patients. J Hypertens. (2000) 18:709–16. doi: 10.1097/00004872-200018060-00008
36. Brosolo G, Da Porto A, Bulfone L, Vacca A, Bertin N, Catena C, et al. Cortisol secretion and abnormalities of glucose metabolism in nondiabetic patients with hypertension. J Hypertens. (2024) 42:227–35. doi: 10.1097/HJH.0000000000003590
37. Lip GY, Blann AD. Endothelium and fibrinolysis in hypertension: important facets of a prothrombotic state. Hypertension. (2008) 52:218–9. doi: 10.1161/HYPERTENSIONAHA.108.114876
38. Withworth JA, Mangos GJ, Kelly JJ. Cushing, cortisol, and cardiovascular disease. Hypertension. (2000) 36:912–6. doi: 10.1161/01.HYP.36.5.912
39. Patrassi GM, Dal Bo Zanon R, Boscaro M, Martinelli S, Girolami A. Further studies on the hypercoagulable state of patients with Cushing’s syndrome. Thromb Haemost. (1985) 54:518–20.
40. Witek P, Zielinski G, Szamotulska K, Witek J, Kaminski G. Cushing’s disease: fibrinogen and D-dimer levels fall to normalize despite early postoperative remission-a prospective, controlled study. Endokrynol Pol. (2016) 67:283–91. doi: 10.5603/EP.a2016.0034
41. van der Pas R, de Bruin C, Leebeek FWG, de MAAT MPM, Rijken DC, Perreira AM, et al. The hypercoagulable state in Cushing’s disease is associated with increased levels of procoagulant factor and impaired fibrinolysis, but is not reversible after short-term biochemical remission induced by medical therapy. J Clin Endocrinol Metab. (2012) 97:1303–10. doi: 10.1210/jc.2011-2753
42. Casonato A, Pontara E, Boscaro M, Sonino N, Sartorello F, Ferasin S, et al. Abnormalities of von Willbrand factor are also part of the prothrombotic state of Cushing’s syndrome. Blood Coagul Fibrinolysis. (1999) 10:145–51. doi: 10.1097/00001721-199904000-00006
43. Fatti LM, Bottasso B, Invitti C, Coppola R, Cavagnini F, Mannucci PM. Markers of activation of coagulation and fibrinolysis in patients with Cushing’s syndrome. Endocrinol Invest. (2000) 23:145–50. doi: 10.1007/BF03343697
44. Tirosh A, Lodish M, Lyssikatos C, Belyavskaya E, Feelders RA, Stratakis CA. Coagulation profile in patients with different etiologies for Cushing syndrome: a prospective observational study. Horm Metab Res. (2017) 49:365–71. doi: 10.1055/s-0043-100113
45. Birdwell L, Lodish M, Tirosh A, Chittiboina P, Keil M, Lyssikatos C, et al. Coagulation profile dynamics in pediatric patients with Cushing's syndrome: a prospective, observational comparative study. J Pediatr. (2016) 177:227–31. doi: 10.1016/j.jpeds.2016.06.087
46. Ferrante E, Serban AL, Clerici M, Indirli M, Scalambrino E, Carosi G, et al. Evaluation of procoagulant imbalance in Cushing’s syndrome after short- and long-term remission of disease. J Endocrinol Invest. (2022) 45:9–16. doi: 10.1007/s40618-021-01605-5
47. Gailani D, Renne T. Intrinsic pathway of coagulation and arterial thrombosis. Arterioscler Thromb Vasc Biol. (2007) 27:2507–13. doi: 10.1161/ATVBAHA.107.155952
Keywords: coagulation, cortisol, d-dimer, dexamethasone, fibrinogen, fibrinolysis, prothrombin fragment 1 + 2, von Willebrand factor
Citation: Brosolo G, Da Porto A, Bulfone L, Vacca A, Bertin N, Vivarelli C, Catena C and Sechi LA (2024) Daytime plasma cortisol and cortisol response to dexamethasone suppression are associated with a prothrombotic state in hypertension. Front. Endocrinol. 15:1397062. doi: 10.3389/fendo.2024.1397062
Received: 06 March 2024; Accepted: 30 April 2024;
Published: 21 May 2024.
Edited by:
Eduardo Hertel Ribeiro, Escola Superior de Ciências da Santa Casa de Misericórdia de Vitória (EMESCAM), BrazilReviewed by:
Alberto Maino, Azienda Provinciale per i Servizi Sanitari (APSS), ItalyCopyright © 2024 Brosolo, Da Porto, Bulfone, Vacca, Bertin, Vivarelli, Catena and Sechi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Leonardo A. Sechi, c2VjaGlAdW5pdWQuaXQ=
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.