- The Department of Endocrinology Children’s Hospital of Capital Institute of Pediatrics, Beijing, China
Background: The association between 25(OH)D and pubertal timing has not been well studied. The aim of this study was to assess the relationship between 25(OH)D levels and pubertal timing in children.
Methods: Participants aged 6–14 years who had available nutritional and serum sex hormone (total testosterone (TT) and estradiol (E2)) information (n =1318) were included. We conducted a cross-sectional analysis of the associations between 25(OH)D and sex steroid hormones among children in the National Health and Nutrition Examination Survey, 2015–2016. Puberty was indicated by high levels of steroid hormones (TT≥50 ng/dL in men, E2≥20 pg/ml in women) or menarche.
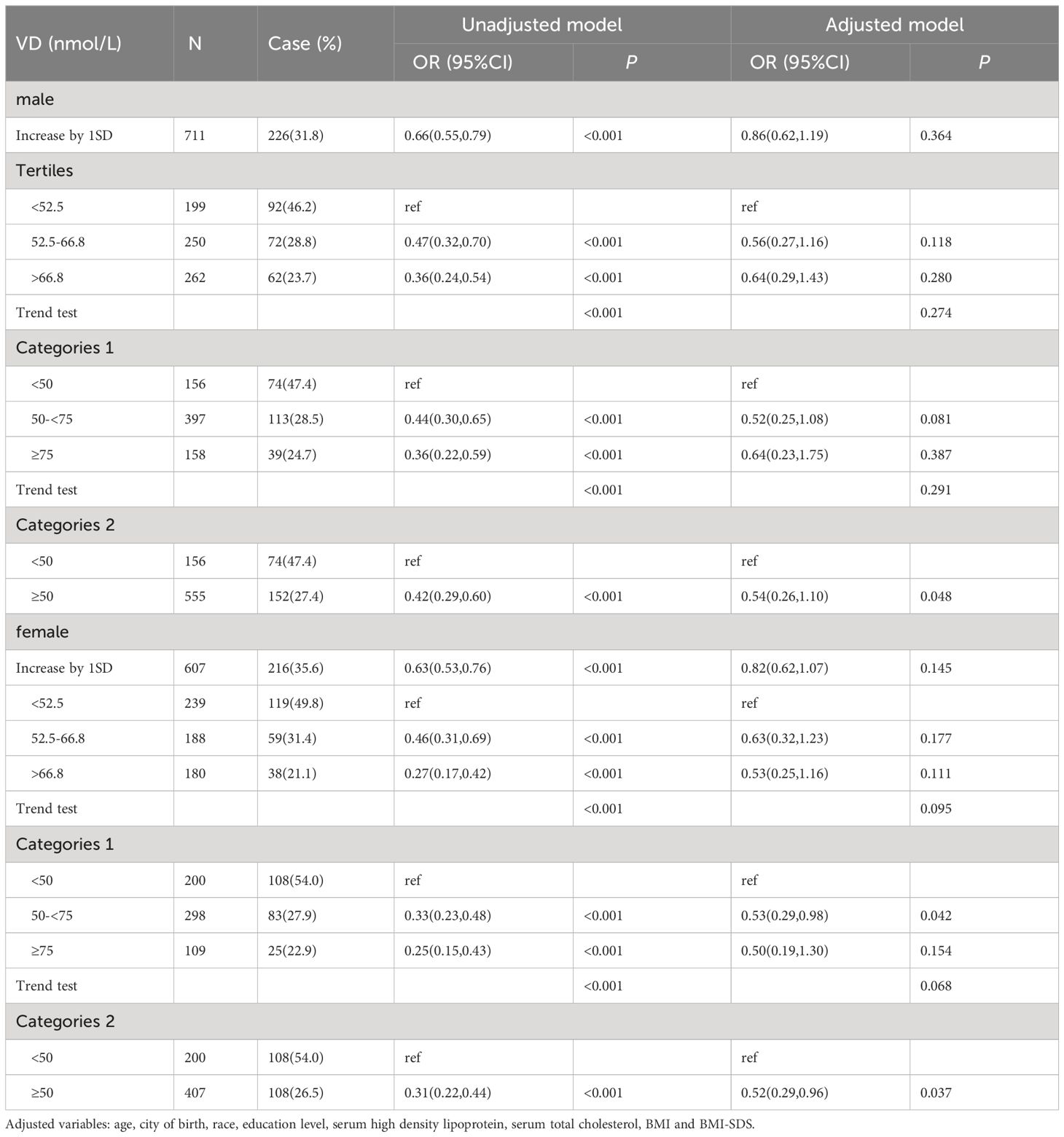
Results: Serum 25(OH)D and pubertal status showed the same trend in both males and females. In the male population, the OR values of serum 25(OH)D between 50 and <75 and ≥75 nmol/L were 0.52 (0.25, 1.08) and 0.64 (0.23, 1.75), respectively, compared with serum 25(OH)D<50 nmol/L. The OR of serum 25(OH)D ≥50 nmol/L compared with <50 nmol/L was 0.54 (0.26, 1.10), and the P value was statistically significant (P=0.048). In the female population, when the serum 25(OH)D concentration was <50 nmol/L, the ORs corresponding to a serum 25(OH)D concentration between 50 and <75 and ≥75 nmol/L were 0.53 (0.29, 0.98) and 0.50 (0.19, 1.30), respectively. The OR of serum 25(OH)D≥50 nmol/L compared with <50 nmol/L was 0.52 (0.19, 0.96), and the P value was statistically significant (P=0.037).
Conclusions: A lower 25(OH)D level was associated with earlier puberty in both girls and boys. There was a negative association between 25(OH)D concentrations and pubertal timing.
1 Introduction
Vitamin D and vitamin D receptor (VDR)-activating enzymes are expressed throughout the hypothalamus–pituitary–gonadal (HPG) axis (1, 2), and vitamin D is metabolized in the developing gonads (1, 3), suggesting a local role for vitamin D during development. The serum 25-hydroxyvitamin D (25(OH)D) concentration is used to evaluate individual vitamin D status and is the best indicator of vitamin D stores; indeed, it is the main circulating form of vitamin D and has a half-life of 2–3 weeks (4). The biological actions of vitamin D are mediated through the VDR, which is distributed across various tissues, including the skeleton and parathyroid glands, as well as reproductive tissues. The rich presence of the VDR in the hypothalamus is consistent with the distribution of other neurosteroids (5), and the VDR has been found in the human pituitary gland (6) as well as in the human endometrium (7). In women, VDR mRNA has been shown to be expressed in the ovaries (8). Peripubertal vitamin D3 sufficiency is important for an appropriately timed pubertal transition and maintenance of normal female reproductive physiology, and vitamin D3 is a key regulator of neuroendocrine and ovarian physiology (9). In men, VDR was detected in human testicular tissue homogenates using titrated vitamin D (10), and VDR was detected in human sperm, with binding sites in the nucleus and the midpiece of the sperm (11). The role of 25(OH)D in the activation of the HPG axis and in influencing the timing of puberty has been reported (12, 13). Furthermore, 25(OH)D has been found to be involved in the functioning of the reproductive system in several studies (1, 14, 15).
Puberty activates the HPG axis, leading to psychological and physical maturation, accelerated linear growth, the development of secondary sexual characteristics, and gonadal maturation (16). Common factors affecting pubertal development include genetics, environment, diet, and nutrition (17). Among the nutritional factors, vitamin D is very important. Vitamin D deficiency and insufficiency are global health issues that affect more than one billion children and adults worldwide (18). The role of vitamin D deficiency in puberty or precocious puberty remains controversial.
Considering that there are only a few relevant studies, which had small sample sizes and mostly included girls but not boys, we performed a literature search to provide evidence for the association between vitamin D and pubertal timing. To fill these knowledge gaps, we analyzed data on 6- to 14-year-old participants from the National Health and Nutrition Examination Survey (NHANES) and explored the effects of vitamin D status on puberty.
2 Methods
The datasets generated and analyzed in the present study are available at the NHANES website (https://www.cdc.gov/nchs/nhanes/index.htm). The NHANES is a nationally representative survey of the civilian, noninstitutionalized US population that was conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC). We downloaded data from one cycle of the NHANES from 2015 to 2016. The data contained five parts: demographic data, dietary data, examination data, laboratory data, and questionnaire data. All procedures and study procedures were approved by the NCHS Ethics Review Board, and written informed consent was obtained from all participants (19).
2.1 Study design and population
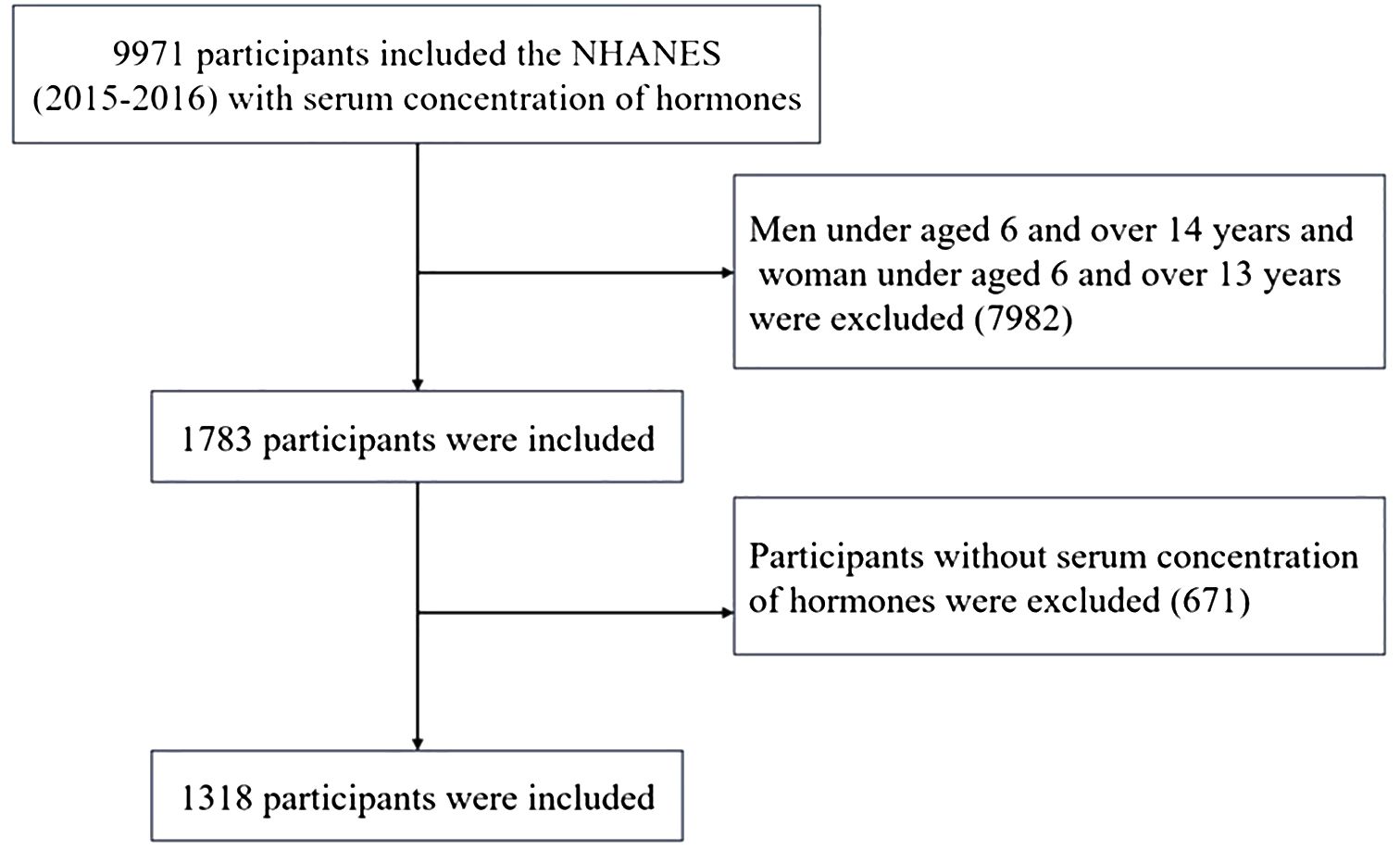
A total of 1318 participants were included in this study. Participants who were younger than 6 years or older than 14 years of age (n = 7982) or who had missing data (n = 671) were excluded, while 1318 participants from the 2015–2016 NHANES who had available data on serum total testosterone (TT), estradiol (E2) and sex hormone binding globulin (SHBG) were included. The flowchart of the participant selection process is presented in Figure 1.
2.2 Measurement of sex hormone indicators
The test principle for the CDC method utilizes high-performance liquid chromatography−tandem mass spectrometry (HPLC−MS/MS) for the quantitative detection of 25(OH)D. Serum 25(OH)D levels were categorized according to the Endocrine Society Clinical Practice guidelines (17) as follows: <49.99 nmol/L was considered to indicate vitamin D deficiency; 50.00–74.99 nmol/L was considered to indicate vitamin D insufficiency; and ≥75.00 nmol/L was considered to indicate vitamin D sufficiency (20). Serum TT and E2 levels were measured using isotope dilution high-performance liquid chromatography tandem mass spectrometry (ID-LC−MS/MS), while the concentrations of SHBG were quantified based on the reaction of SHBG with antibodies and chemiluminescence measurements of the reaction products via a photomultiplier tube. The lower limits of detection (LLODs) for TT, E2 and SHBG were 0.75 ng/mL, 2.994 pg/m and 0.800 nmol/l, respectively. Puberty was indicated by high levels of steroid hormones (TT≥50 ng/dL in men, E2≥20 pg/ml in women) or menarche (21).
2.3 Statistical analysis
Descriptive statistics were used to analyze the characteristics and distribution of the population. Continuous variables are presented as the means ± standard deviations and were compared with t tests, whereas categorical variables are presented as counts (%s) and were compared with the χ2 test. The serum 25(OH)D concentration was included in the linear and multivariate logistic regression models as a continuous variable (increase in each SD) and as a categorical variable (tertiles, with the first tertile as the reference group). The adjusted variables for multivariate regression analysis were age, city of birth, serum high-density lipoprotein (HDL) level, serum total cholesterol (TC) level, and body mass index (BMI), BMI-SDS (obese: mean BMI SDS >+ 2SD, nonobese: mean BMI<=+ 2SD) and race. The downloaded data were visualized and analyzed using the statistical package R (R.4.3.1), with a two-tailed P<0.05 considered to indicate statistical significance.
3 Results
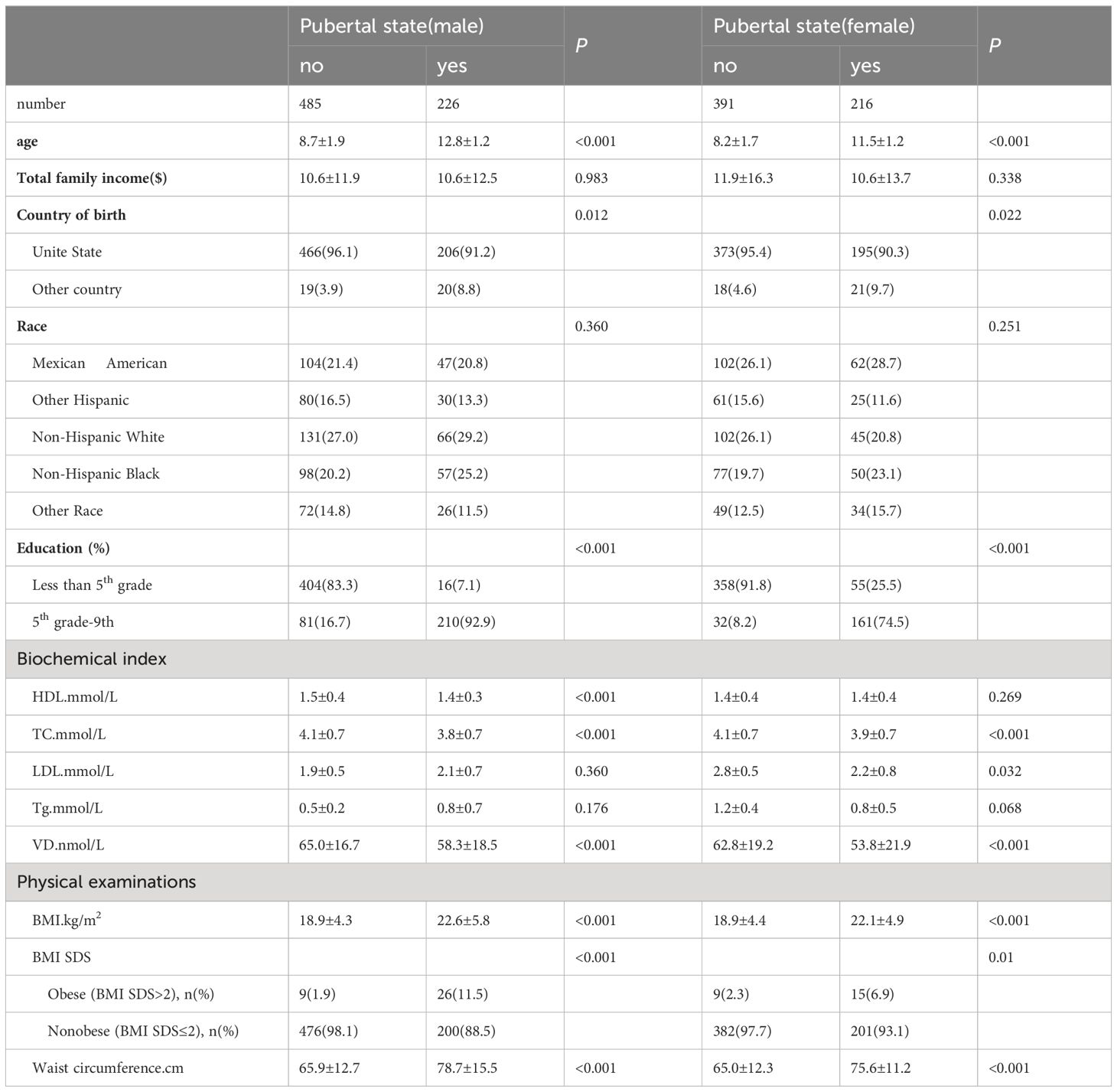
A total of 1318 participants (male: 711, female: 607) were included in this study, for which the male-to-female ratio was 1.17:1. The participant characteristics are shown in Table 1. The median levels of serum vitamin D were 65.0 ± 16.7 nmol/L and 62.8 ± 19.2 nmol/L in pubertal males and females and 58.3 ± 18.5 nmol/L and 53.8 ± 21.9 nmol/L in prepubertal males and females, respectively.
Compared with prepubescent children, male children at puberty were much older; were from other countries; had a higher education level (grade 5 and above); had lower HDL (p<0.001), TC (p<0.001), and serum 25(OH)D levels (p<0.001); and had a greater BMI (p<0.001) and waist circumference (p<0.001). Female children at puberty were older than were those at prepuberty; were from other countries; had higher education levels (grade 5 and above); had lower levels of TC (<0.001), low-density lipoprotein (LDL) (<0.032), and serum 25(OH)D (<0.001); and had greater BMI (<0.001) and waist circumference (<0.001).
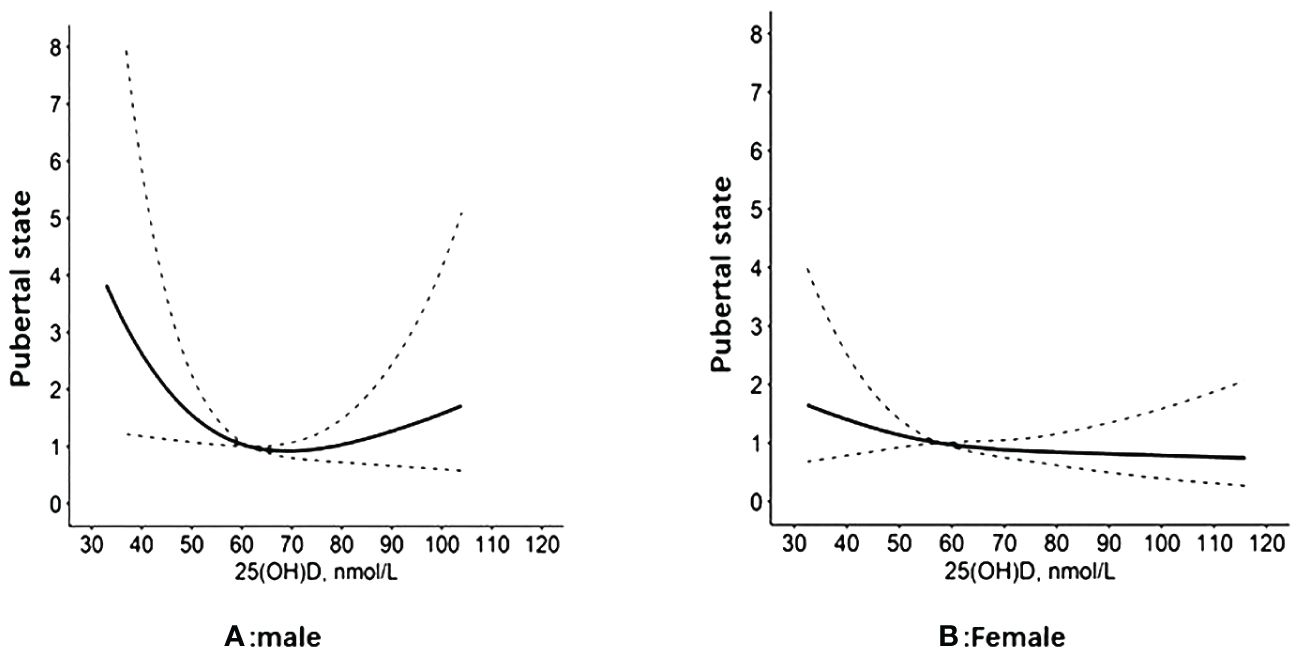
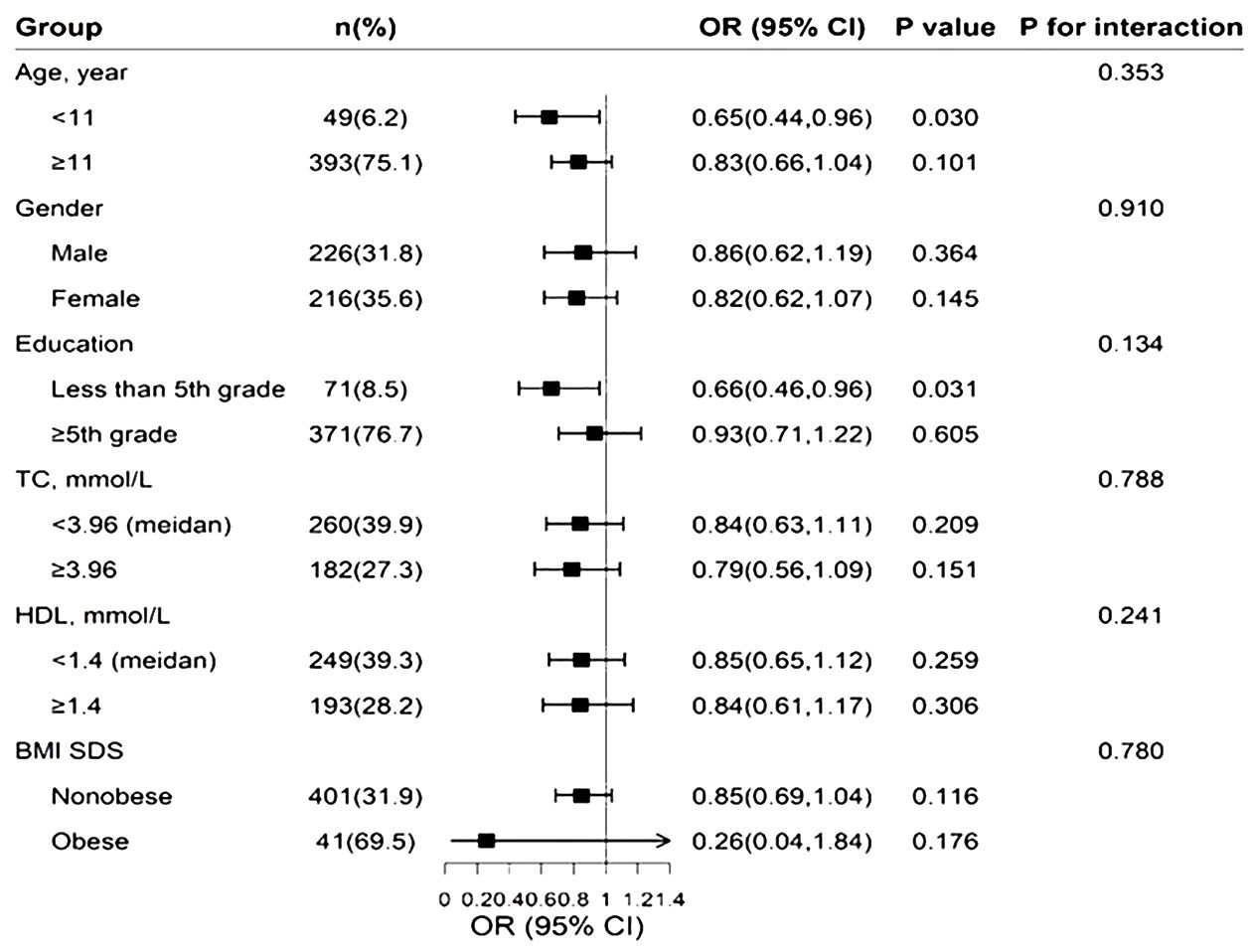
Regression analysis of serum 25(OH)D and pubertal status showed that after adjusting for relevant confounders (age, city of birth, education level, serum HDL levels, serum TC and BMI, race), there was no statistically significant association between one SD and the third quartile of serum 25(OH)D and pubertal status in either the male or female population. Serum 25(OH)D was further grouped according to clinical criteria <50, 50-<75 and ≥75 nmol/L. In the male population, the OR values of serum 25(OH)D and pubertal status between 50 and <75 and ≥75 nmol/L were 0.52 (0.25, 1.08) and 0.64 (0.23, 1.75), respectively, compared with serum 25(OH)D<50 nmol/L. The OR of serum 25(OH)D ≥50 nmol/L compared with <50 nmol/L was 0.54 (0.26, 1.10), and the P value was statistically significant (P=0.048). In the female population, when the serum 25(OH)D concentration was <50 nmol/L, the ORs corresponding to a serum 25(OH)D concentration between 50 and <75 and ≥75 nmol/L were 0.53 (0.29, 0.98) and 0.50 (0.19, 1.30), respectively. The OR of serum 25(OH)D≥50 nmol/L compared with <50 nmol/L was 0.52 (0.19, 0.96), and the P value was statistically significant (P=0.037). The results are shown in Table 2. The RCS revealed an inverse correlation between the serum 25(OH)D concentration and the odds of having reached puberty (Figure 2). Subgroup analysis of 25(OH)D was performed with forest plots of adolescent states, and no significant interaction factors were found (Figure 3).
4 Discussion
In this study, the associations between 25(OH)D and pubertal timing were investigated, and a negative association was found between 25(OH)D and pubertal timing. This association was particularly strong among girls. In girls, 25(OH)D deficiency or insufficiency was associated with earlier puberty, and 25(OH)D deficiency was more likely to be associated with earlier puberty in boys. These findings could lead to clinical and dietary recommendations for individuals with 25(OH) deficiency and insufficiency in the childhood population. To our knowledge, this was the largest sample size in which the link between serum 25(OH)D levels and pubertal timing was examined in both males and females.
Puberty is the physiological process whereby adolescents reach sexual maturity and become capable of reproduction. Puberty onset varies naturally among individuals; in addition to genetic and environmental factors, the importance of nutritional factors, such as iron, zinc, calcium, and vitamin D, increases during puberty (4). 25(OH)D is a key regulator of neuroendocrine and ovarian physiology (5). However, the relationship between 25(OH)D status and pubertal timing is controversial. The findings of several studies are consistent with our results. For example, in one study, the probability of menarche was twice as high in vitamin D-deficient girls than in girls who were vitamin D sufficient. In girls, vitamin D deficiency has been shown to decrease the age of menarche by ∼10 months compared with that in girls with sufficient vitamin D (4, 22). A systematic meta-analysis of six studies showed that vitamin D-deficient individuals were more likely to develop precocious puberty (OR = 2.02 [95% confidence interval 1.65–2.46]) (4, 23). One retrospective study collected data from 221 girls with idiopathic central precocious puberty (ICPP) and 144 girls without ICPP, and the serum 25(OH)D levels in the ICPP group were significantly lower than those in the non-ICPP group (p < 0.001). A low serum 25(OH)D is an independent risk factor for ICPP (4, 24). Another meta-analysis showed that the average serum vitamin D concentration in individuals with precocious puberty was 1.16 ng/mL, which was lower than that in the control group (12).
However, in adult female mice, peripubertal 25(OH)D deficiency was associated with delayed puberty (5). In a study involving 713 (25.0%) Chinese men, participants with hypogonadism had significantly lower 25(OH)D levels and greater BMIs (25). In this study, both adolescent boys and adults were included. In another cross-sectional study, although females who had vitamin D deficiency were more likely to report an early age of menarche (i.e., at or before 9 years of age), this relationship disappeared after controlling for age at screening, race/ethnicity and BMI (4, 26). A retrospective study of 145 girls monitored for ICPP revealed that the mean 25(OH)D concentration was 27.6 ± 17.3 ng/mL, without any correlation with the pubertal characteristics of the subjects (4, 27). Another cross-sectional study showed no significant differences in 25OHD concentrations between ICPP patients and control participants. There were no significant differences in 25(OH)D concentrations between the CPP (25.4 ± 8.6 ng/mL) and control groups (28.2 ± 7.4 ng/mL) (28). However, most studies have described the association between vitamin D and menarche, which occurs in the middle or late stages of puberty. Therefore, one possible explanation for the differences between studies is that our study focused on the association between pubertal status and vitamin D levels in almost healthy participants.
The onset of puberty varies between males and females. Almost all previously published studies included only girls. However, whether vitamin D levels affect the occurrence of puberty and its role in males have been less studied. Our study revealed that the OR of serum 25(OH)D≥50 compared with <50 was 0.54 (0.26, 1.10), and the P value was statistically significant (P=0.048) in males, which was the same trend as that in females. Treatment with active vitamin D3 and VDR showed that VD3/VDR had a positive regulatory effect on Cyp11a1 expression and testosterone secretion. The VDR promotes testosterone synthesis in male mice by upregulating Cyp11a1 expression, which plays an important role in male reproduction (29). Testosterone is produced by Leydig cells and is responsible for male sex characteristics. LH induces steroidogenesis by increasing cyclic AMP production and the intracellular concentration of calcium ions (Ca2+) in Leydig cells, and 1,25-dihydroxyvitamin D3 might influence this calcium-dependent LH response (30, 31).
The prevalence of precocious puberty is sexually dimorphic and greater in girls than in boys (15–20 girls for every boy) (32). Based on our findings, it is possible that both males and females require vitamin D during puberty. Recently, several studies have shown that overweight and obesity are significantly associated with increased odds of ICPP among girls (33, 34). In a cross-sectional study of 220 females and 164 males (aged 7–16 years), vitamin D deficiency was found in 49% of the total patients and was significantly more prevalent in females than in males (33.1% in females; 15.9% in males, P < 0.001). Puberty is an additional risk factor for vitamin D deficiency, especially in girls and obese children (35). Our study showed that even after adjusting for BMI and BMI-SDS, vitamin D still plays an important role in pubertal timing, and that trend occurs in both sexes.
25(OH)D deficiency is prevalent worldwide, but the optimal concentration of serum 25(OH)D has not been determined. There are no data on how much vitamin D is required to prevent vitamin D deficiency in children aged 1–9 years, and no scientific evidence to date has demonstrated an increased requirement for vitamin D for children aged 9–18 years (24). Our study showed that vitamin D supplementation during peripuberty is equally important for males and females. The supplemental doses for males and females may need to be individualized.
There are several limitations to our study. First, since this was an observational study, cause and impact could not be determined. In addition, we did not perform a more detailed, stratified analysis by stage of puberty, and this subgrouping may have had a critical influence on the results.
5 Conclusion
After multivariate adjustment, there was a negative association between 25(OH)D concentrations and pubertal timing. These results highlight the potential advantages of monitoring and evaluating 25(OH)D status during puberty. The systemic effects of 25(OH)D have added another dimension to the endocrinology of puberty.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: The datasets generated and analyzed in the present study are available at the NHANES website (https://www.cdc.gov/nchs/nhanes/index.htm).
Ethics statement
All procedures and study contents were approved by the NCHS Ethics Review Board, and written informed consent was obtained from all participants. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
ZL: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors thank the participants of the NHANES databases.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lerchbaum E, Obermayer-Pietsch B. Vitamin D and fertility: a systematic review. Eur J Endocrinol. (2012) 166:765–78. doi: 10.1530/EJE-11–0984
2. Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. (2005) 29:21–30. doi: 10.1016/j.jchemneu.2004.08.006
3. Lorenzen M, Boisen IM, Mortensen LJ, Lanske B, Juul A, Blomberg Jensen M. Reproductive endocrinology of vitamin D. Mol Cell Endocrinol. (2017) 453:103–12. doi: 10.1016/j.mce.2017.03.023
4. Calcaterra V, Magenes VC, Tagi VM, Grazi R, Bianchi A, Cena H, et al. Association between vitamin D levels, puberty timing, and age at menarche. Children (Basel). (2023) 10:1243. doi: 10.3390/children10071243
5. Dicken CL, Israel DD, Davis JB, Sun Y, Shu J, Hardin J, et al. Peripubertal vitamin D(3) deficiency delays puberty and disrupts the estrous cycle in adult female mice. Biol Reprod. (2012) 87:51. doi: 10.1095/biolreprod.111.096511
6. Pe´rez-Fernandez R, Alonso M, Segura C, Mun˜ oz I, Garcı´aCaballero T, Diguez C. Vitamin D receptor gene expression in human pituitary gland. Life Sci. (1997) 60:35–42. doi: 10.1016/S0024–3205(96)00586–3
7. Agic A, Xu H, Altgassen C, Noack F, Wolfler MM, Diedrich K, et al. Relative expression of 1,25-dihydroxyvitamin D3 receptor, vitamin D 1a-hydroxylase, vitamin D 24-hydroxylase, and vitamin D 25-hydroxylase in endometriosis and gynecologic cancers. Reprod Sci. (2007) 14:486–97. doi: 10.1177/1933719107304565
8. Agic A, Xu H, Altgassen C, Noack F, Wolfler MM, Diedrich K, et al. Relative expression of 1,25-dihydroxyvitamin D3 receptor, vitamin D 1 alpha-hydroxylase, vitamin D 24-hydroxylase, and vitamin D 25-hydroxylase in endometriosis and gynecologic cancers. Reprod Sci. (2007) 14:486–97. doi: 10.1177/1933719107304565
9. Prüfer K, Jirikowski GF. 1.25-Dihydroxyvitamin D3 receptor is partly colocalized with oxytocin immunoreactivity in neurons of the male rat hypothalamus. Cell Mol Biol (Noisy-le-grand). (1997) 43:543–8.
10. Habib FK, Maddy SQ, Gelly KJ. Characterisation of receptors for 1,25-dihydroxyvitamin D3 in the human testis. J Steroid Biochem. (1990) 35:195–9. doi: 10.1016/0022-4731(90)90274-V
11. Corbett ST, Hill O, Nangia AK. Vitamin D receptor found in human sperm. Urology. (2006) 68:1345–9. doi: 10.1016/j.urology.2006.09.011
12. Wu C, Zhang X, Yan F, Cui Y, Song Y, Yan S, et al. Does vitamin D have a potential role in precocious puberty? A meta-analysis. Food Funct. (2023) 14:5301–10. doi: 10.1039/D3FO00665D
13. Cheng H, Chen D, Gao H. An updated meta-analysis of the relationship between vitamin D levels and precocious puberty. Front Endocrinol (Lausanne). (2023) 14:1298374. doi: 10.3389/fendo.2023.1298374
14. Lerchbaum E, Theiler-Schwetz V, Kollmann M, Wölfler M, Pilz S, Obermayer-Pietsch B, et al. Effects of vitamin D supplementation on surrogate markers of fertility in PCOS women: A randomized controlled trial. Nutrients. (2021) 13:547. doi: 10.3390/nu13020547
15. Kalaitzopoulos DR, Samartzis N, Daniilidis A, Leeners B, Makieva S, Nirgianakis K, et al. Effects of vitamin D supplementation in endometriosis: a systematic review. Reprod Biol Endocrinol. (2022) 20:176. doi: 10.1186/s12958–022-01051–9
16. Cheuiche AV, da Silveira LG, de Paula LCP, Lucena IRS, Silveiro SP. Diagnosis and management of precocious sexual maturation: an updated review. Eur J Pediatr. (2021) 180:3073–87. doi: 10.1007/s00431–021-04022–1
17. Wood CL, Lane LC, Cheetham T. Puberty: Normal physiology (brief overview). Best Pract Res Clin Endocrinol Metab. (2019) 33:101265. doi: 10.1016/j.beem.2019.03.001
18. Holick MF. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. (2017) 18:153–65. doi: 10.1007/s11154–017-9424–1
19. National Center for Health Statistics. Centers for Disease Control and Prevention. NCHS research ethics review board (ERB) approval. Available online at: www.cdc.gov/nchs/nhanes/irba98.htm (Accessed 8 January 2022).
20. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. (2011) 96:1911–30. doi: 10.1210/jc.2011–0385
21. Ma Y, Li R, Zhan W, Huang X, Zhou Y, Sun Y, et al. Associations between dietary inflammatory index and sex hormones among 6- to 19-year-old children and adolescents in NHANES 2015–2016. Front Endocrinol (Lausanne). (2022) 10:792114. doi: 10.3389/fendo.2021.792114
22. Villamor E, Marin C, Mora-Plazas M, Baylin A. Vitamin D deficiency and age at menarche: a prospective study. Am J Clin Nutr. (2011) 94:1020–5. doi: 10.3945/ajcn.111.018168
23. Liu S, Zhu X, Wang Y, Yan S, Li D, Cui W. The association between vitamin D levels and precocious puberty: a meta-analysis. J Pediatr Endocrinol Metab. (2020) 33:427–9. doi: 10.1515/jpem-2019–0388
24. Gan DM, Fang J, Zhang PP, Zhao YD, Xu YN. Serum 25-hydroxyvitamin D levels and the risk of idiopathic central precocious puberty in girls. Clinics (Sao Paulo). (2023) 78:100244. doi: 10.1016/j.clinsp.2023.100244
25. Wang N, Han B, Li Q, Chen Y, Chen Y, Xia F, et al. Vitamin D is associated with testosterone and hypogonadism in Chinese men: Results from a cross-sectional SPECT-China study. Reprod Biol Endocrinol. (2015) 13:74. doi: 10.1186/s12958–015-0068–2
26. Hua A. Vitamin D status and age of menarche .Yale University, New Haven, CT, USA (2016). Available at: https://elischolar.library.yale.edu/ysphtdl/1132. Master’s Thesis.
27. Duhil de Bénazé G, Brauner R, Souberbielle JC. There is no association between vitamin D status and characteristics of central precocious puberty in girls. Eur J Pediatr. (2017) 176:1677–80. doi: 10.1007/s00431–017-3022–9
28. Durá-Travé T, Gallinas-Victoriano F. Vitamin D status and parathyroid hormone assessment in girls with central precocious puberty. J Endocrinol Invest. (2022) 45:2069–75. doi: 10.1007/s40618-022-01838-y
29. Hu Y, Wang L, Yang G, Wang S, Guo M, Lu H, et al. VDR promotes testosterone synthesis in mouse Leydig cells via regulation of cholesterol side chain cleavage cytochrome P450 (Cyp11a1) expression. Genes Genomics. (2023) 45:1377–87. doi: 10.1007/s13258-023-01444-z
30. Laaksi A, Laaksi I, Pihlajamäki H, Vaara JP, Luukkaala T, Kyröläinen H. Associations of serum 25(OH)D levels with physical performance and anabolic hormones in young men. Front Physiol. (2023) 14:1049503. doi: 10.3389/fphys.2023.1049503
31. Costa RR, Reis RI, Aguiar JF, Varanda WA. Luteinizing hormone (LH) acts through PKA and PKC to modulate T-type calcium currents and intracellular calcium transients in mice Leydig cells. Cell Calcium. (2011) 49:191–9. doi: 10.1016/j.ceca.2011.02.003
32. Latronico AC, Brito VN, Carel JC. Causes, diagnosis, and treatment of central precocious puberty. Lancet Diabetes Endocrinol. (2016) 4:265–74. doi: 10.1016/S2213–8587(15)00380–0
33. Liu G, Guo J, Zhang X, Lu Y, Miao J, Xue H. Obesity is a risk factor for central precocious puberty: a case-control study. BMC Pediatr. (2021) 21:509. doi: 10.1186/s12887–021-02936–1
34. Song Y, Kong Y, Xie X, Wang Y, Wang N. Association between precocious puberty and obesity risk in children: a systematic review and meta-analysis. Front Pediatr. (2023) 11:1226933. doi: 10.3389/fped.2023.1226933
Keywords: 25(OH)D, pubertal timing, precocious puberty, national health and nutrition examination survey, NHANES
Citation: Liu Z (2024) Association between 25-hydroxyvitamin D concentrations and pubertal timing: 6–14-year-old children and adolescents in the NHANES 2015–2016. Front. Endocrinol. 15:1394347. doi: 10.3389/fendo.2024.1394347
Received: 01 March 2024; Accepted: 10 May 2024;
Published: 22 May 2024.
Edited by:
Artur Mazur, University of Rzeszow, PolandReviewed by:
Lidia Perenc, University of Rzeszow, PolandViktoriya Furdela, Ternopil State Medical University, Ukraine
Otilia Marginean, Victor Babes University of Medicine and Pharmacy, Romania
Copyright © 2024 Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ziqin Liu, MTkzNDg1NTU5N0BxcS5jb20=
 Ziqin Liu
Ziqin Liu