- Assisted Reproduction Center, Northwest Women’s and Children’s Hospital, Xi’an, China
Introduction: In the realm of natural frozen-thawed embryo transfer (FET) cycles, the application of luteal phase support (LPS) is a prevalent practice, primarily due to its beneficial impact on reproductive outcomes. Among the various LPS medications, human chorionic gonadotropin (hCG) is one that exerts its function on both the corpus luteum and the endometrium.
Objective: To evaluate the effect of hCG administration as LPS on reproductive outcomes in natural FET cycles.
Methods: This study was a retrospective cohort analysis conducted at a tertiary care hospital. It included women who underwent natural FET treatment from January 2018 to December 2022. Participants were divided into the hCG LPS group and the non-hCG LPS group on the basis of whether they used hCG as LPS after blastocyst transfer. The primary outcome was the clinical pregnancy and live birth rates. The secondary outcomes included the early miscarriage rate (before 12th gestational week) and total miscarriage rate.
Results: A total of 4762 women were included in the analysis, and 1910 received hCG LPS and 2852 received no hCG LPS (control group). In the general cohort, the clinical pregnancy and live birth rates in the hCG LPS group were significantly lower than those in the control group (63.82% vs 66.41%, aOR 0.872, 95% CI 0.765–0.996, P=0.046; 53.98% vs 57.15%, aOR 0.873, 95% CI 0.766–0.991, P=0.035, respectively). The early miscarriage and total miscarriage rates were similar between the two groups. In a subgroup analysis, in women who received an hCG trigger, there was no significant difference in the clinical pregnancy rate or live birth rate between the two groups. However, in women who ovulated spontaneously, the clinical pregnancy and live birth rates in the hCG LPS group were significantly lower than those in the control group (60.99% vs 67.21%, aOR 0.786, 95% CI 0.652–0.946, P=0.011; 50.56% vs 57.63%, aOR 0.743, 95% CI 0.619–0.878, P=0.001, respectively).
Conclusion: Among women undergoing natural cycle frozen–thawed blastocyst transfer, hCG LPS is associated with lower clinical pregnancy and live birth rates. Additionally, the adverse effect of hCG LPS is more pronounced in women who ovulate spontaneously.
Introduction
In frozen–thawed embryo transfer (FET) treatment, natural cycles refer to the cycles in which follicles develop spontaneously, and subsequently ovulate and shift to the corpus luteum. The corpus luteum produces progesterone and estrogen, which are essential for embryo implantation and subsequent pregnancy. Because of the presence of the corpus luteum, there is a less demand for exogenous administration of luteal support in natural cycles. Although there is little evidence to support the benefit of luteal phase support (LPS) in natural cycles, LPS is commonly used in practice.
Progesterone and human chorionic gonadotropin (hCG) represent the two most frequently utilized luteal phase support (LPS) medications. These can be administered either independently or in conjunction during natural cycles. Notably, hCG serves as one of the earliest signals released by the embryo and detected by the mother, an occurrence documented from the fertilization stage (2PN) within embryonic culture mediums (1, 2). The hCG operates on both the corpus luteum and the endometrium. It effectively regulates multiple metabolic pathways within the decidua, thereby contributing to endometrial receptivity. The hCG has been found to significantly inhibit the intrauterine insulin-like growth factor binding protein-1 (IGF-BP-1) and the macrophage colony-stimulating factor (M-CSF). Conversely, it significantly stimulates the leukemia inhibitory factor (LIF), the vascular endothelial growth factor (VEGF), and the matrix metalloproteinase-9 (MMP-9) (3–5). hCG prompts the corpus luteum to produce progesterone continuously via mimicking LH pulsatility. The mechanism of this process is that hCG binds to the LH receptor of granulosa cells, and subsequently leads to more potent activation of the cyclic AMP protein kinase A pathway, which stimulates progesterone production (6). Studies in vitro have suggested that hCG promotes longevity of the corpus luteum via increasing levels of antiapoptotic BCL-2 and decreasing proapoptotic Bax (7). In addition to the effects of hCG on the corpus luteum, hCG may provide a signal regarding future embryo implantation to the endometrium, foster growth and differentiation of trophoblast cells, and establish placental villous structures (8). The biosynthesis of hCG begins early in embryonic development. Therefore, hCG is detected in relatively high concentrations in the uterine cavity prior to implantation, and exogenous administration of hCG during in vitro fertilization may mimic the local effects of hCG when fertilization occurs naturally (9). Consequently, many researchers originally believed that hCG should be the primary choice for LPS.
However, hCG LPS was reported as non-beneficial in a recently published meta-analysis, which included only two studies examining the use of hCG as the sole regimen for LPS in natural FET cycles (10). These two studies were both conducted by Lee et al. and only cleavage embryos were transferred. Their first study (11) was a retrospective cohort study in which women in the LPS group received two doses of 1500 IU hCG on the day of FET and 6 days later. They found that LPS with hCG did not increase the clinical pregnancy rate. The second study was a placebo-controlled, randomized, controlled trial (RCT), which included 450 women and they used the same regimen for LPS (12). These authors also found a comparable outcome in the LPS and the placebo groups.
In natural FET cycles, the effect of hCG may be related to the dosage and timing of hCG injection. Tmax is 12 h and T1/2 is 23 h for hCG. Therefore, the difference in the dosage and timing of hCG injection may result in different effects on reproductive outcomes. We speculate that the use of continuous hCG injection after embryo transfer may be optimal. Advances in embryo culture media have led to exceeding blastocyst-stage embryo transfer over cleavage-stage embryo transfer. Blastocyst-stage embryo transfer is associated with better pregnancy outcomes than cleavage-stage embryo transfer (13). The effect of hCG LPS on natural cycle FET of blastocyst-stage embryos is unclear.
In this study, we aimed to evaluate the effects of continuous injection of hCG in natural FET. We conducted a retrospective study in women who had blastocysts transferred. We hypothesized that the use of hCG in natural FET as LPS is associated with increased clinical pregnancy and live birth rates.
Materials and methods
Study design and patients
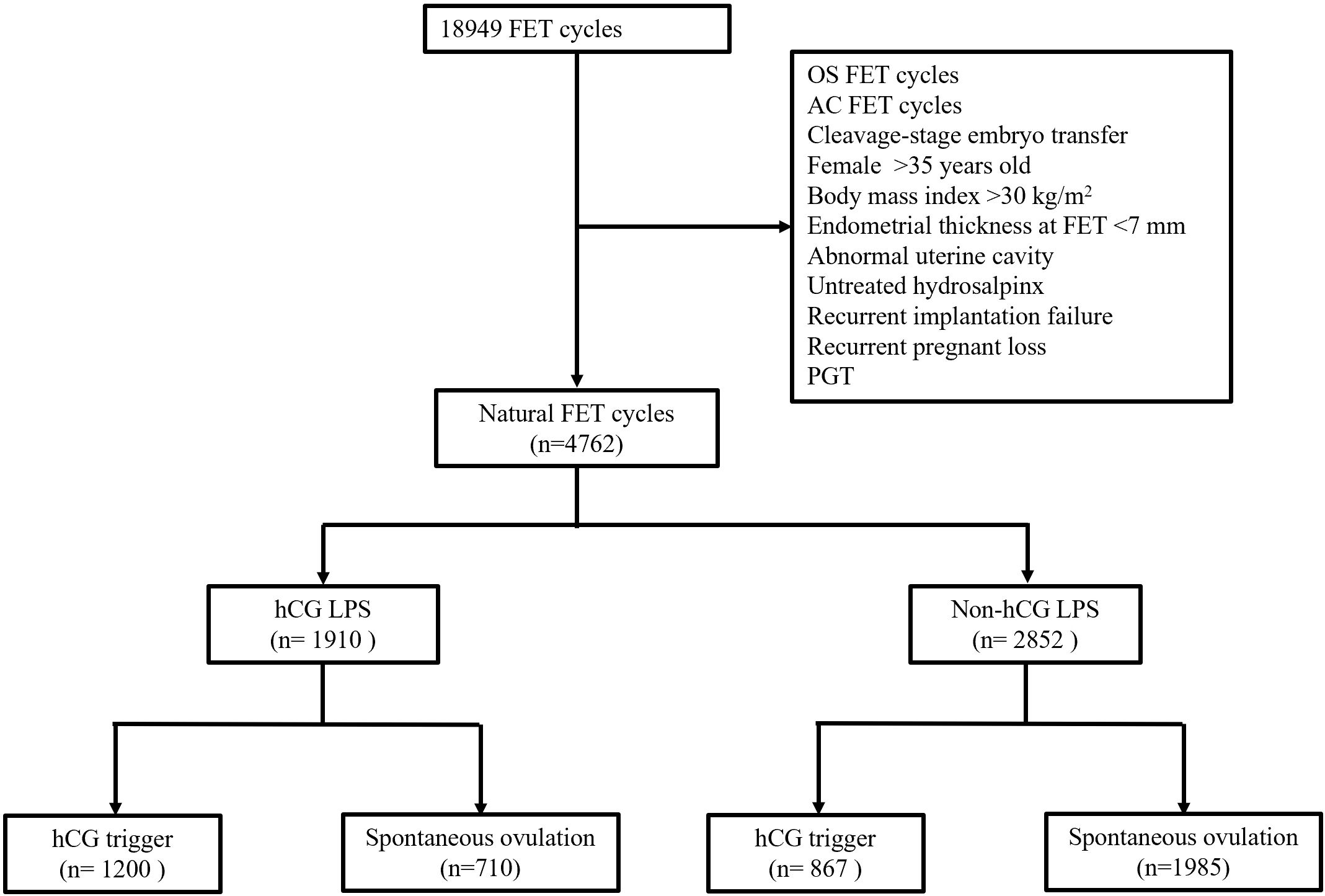
This retrospective cohort study included all patients from a single center who underwent their first natural blastocyst FET cycle from January 2014 to December 2022. The exclusion criteria included: (i) cycles with letrozole or HMG for ovarian stimulation; (ii) female age at FET >35 years old; (iii) body mass index >30 kg/m2; (iv) endometrial thickness at FET <7 mm; (v) with an abnormal uterine cavity or untreated hydrosalpinx; (vi) recurrent implantation failure and recurrent pregnant loss; and (vii) PGT cycles. Data were extracted from electronic medical records. This study was approved by the Ethics Committee of Northwest Women’s and Children’s Hospital (No. 2022007).
Monitoring of ovulation in the natural cycle
Women attended the clinic for ultrasound monitoring of follicular development from the 8th day of menstruation. An hCG trigger (chorionic gonadotropin for injection; Lizhu, China) was provided for ovulation when the leading follicle reached 18 mm for longer than 2 days and transvaginal ultrasound showed anovulation. If the follicle fails to rupture within 2 days following trigger injection, the 36th hour post-injection will be deemed as the day of ovulation. Similarly, in cases where an unruptured follicle is observed post-trigger injection, the 36th hour following injection will also be considered as the day of ovulation. If ovulation was in doubt, further blood serum progesterone concentrations needed to be measured. Serum progesterone concentrations ≥3 ng/ml were considered manifestation of ovulation.
Frozen–thawed blastocyst transfer
Blastocyst-stage embryos were thawed and transferred on the 5th day after ovulation. A maximum of two blastocyst-stage embryos were allowed. Blastocyst evaluation was performed according to Gardner’s grading system.
Luteal support
All women were administered oral progesterone (10 mg tid; Duphaston, Abbot, USA) and vaginal progesterone (90 mg qd, Crinone® 8%; Merck Serono, Switzerland) for luteal support from the day of FET. In the hCG group, women received continuous hCG injection (2000 IU im qd; Lizhu, China) for 5 days from the day of blastocyst transfer. The addition of continuous hCG injection depended on the patients’ and physicians’ preferences. In both groups, oral progesterone and vaginal progesterone were continued until the day of pregnancy testing. If clinical pregnancy was diagnosed by ultrasound at 4 to 5 weeks after the blastocyst transfer, the LPS regimen was continued until 10 weeks of gestation.
Definitions of pregnancy outcomes
Biochemical pregnancy was diagnosed only by the detection of serum beta hCG concentrations >50 mIU/ml at the 13th day after FET. Miscarriage was defined as spontaneous loss of a clinical pregnancy before 22 completed weeks of gestation, and early miscarriage was defined as miscarriage before 12 weeks of gestational age. Preterm birth was defined as birth that occurred after 22 weeks and before 37 completed weeks of gestational age (14).
Statistical analyses
Baseline characteristics are shown as the mean ± standard deviation and count (%), as appropriate. Categorical variables are presented as the proportion and percentage of the total. Comparison of continuous variables between the groups was performed using Student’s t-test or the Mann–Whitney U-test depending on the normality of the distribution. Fisher’s exact test was used to compare categorical variables. A multivariate regression analysis was used to assess the association between hCG LPS and various pregnancy outcomes while adjusting for female age, endometrial thickness, number of blastocysts transferred, hCG trigger administration and good quality blastocyst transfer. A subgroup analysis was performed to assess for potential heterogeneity of the hCG trigger effect on pregnancy outcomes. All data were analyzed using IBM SPSS 22.0 software (IBM Corp., Armonk, NY, USA). The level of significance was set at P<0.05.
Results
Baseline characteristics of the patients
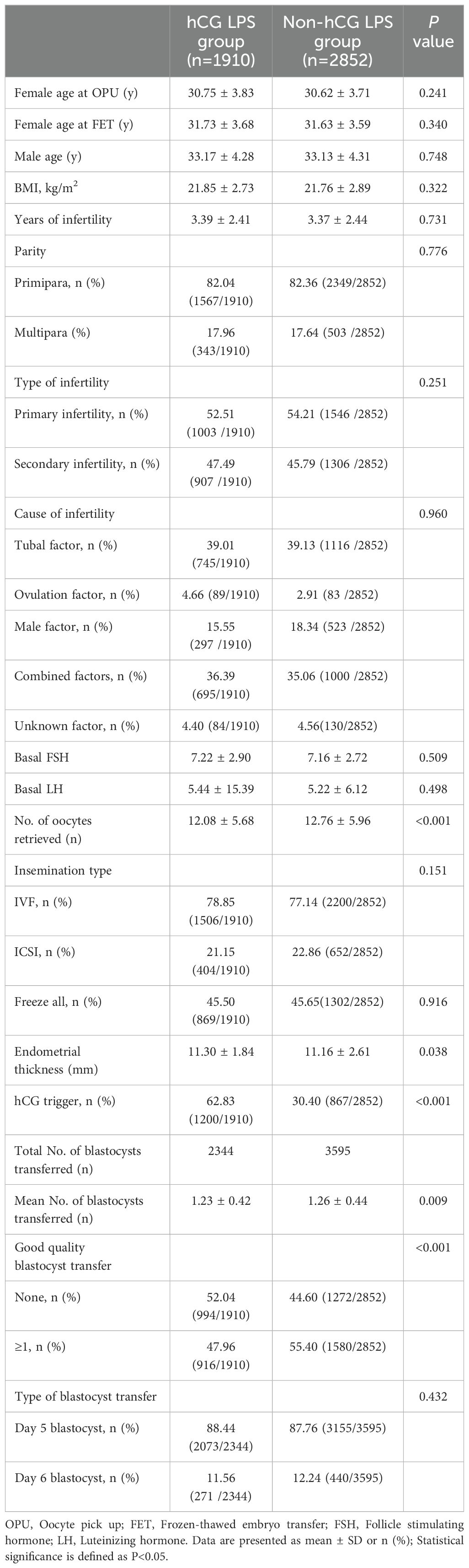
The flowchart of enrolled patients is shown in Figure 1. A total of 4762 women who fulfilled the inclusion and exclusion criteria were included in this study (Table 1). Of these, 1910 and 2852 patients were in the hCG LPS group and the non-hCG LPS group, respectively. Female age, male age, body mass index, infertility duration, parity, cause of infertility, basal FSH concentrations, and basal luteinizing hormone (LH) concentrations were similar in the two groups.
With regard to in vitro fertilization characteristics, the number of oocytes retrieved in the hCG LPS group was significantly greater than that in the non-hCG LPS group (12.08 ± 5.68 vs 12.76 ± 5.96, P<0.001). There was no significant difference in the insemination method or “freeze all” ratio between the groups.
With regard to FET characteristics, the rate of administration of a trigger in the hCG LPS group was 62.3%, which was twice that in the non-hCG LPS group (30.4%, P<0.001). In the hCG LPS group, the average number of blastocysts transferred was significantly less than that in the non-hCG LPS group (1.23 ± 0.42 vs 1.26 ± 0.44, P=0.009). Additionally, the rate of transferring good quality blastocyst transfer was significantly lower than that in the non-hCG LPS group (47.96% vs 55.4%, P<0.001). The type of blastocyst transfer was similar in the two groups.
Pregnancy outcomes
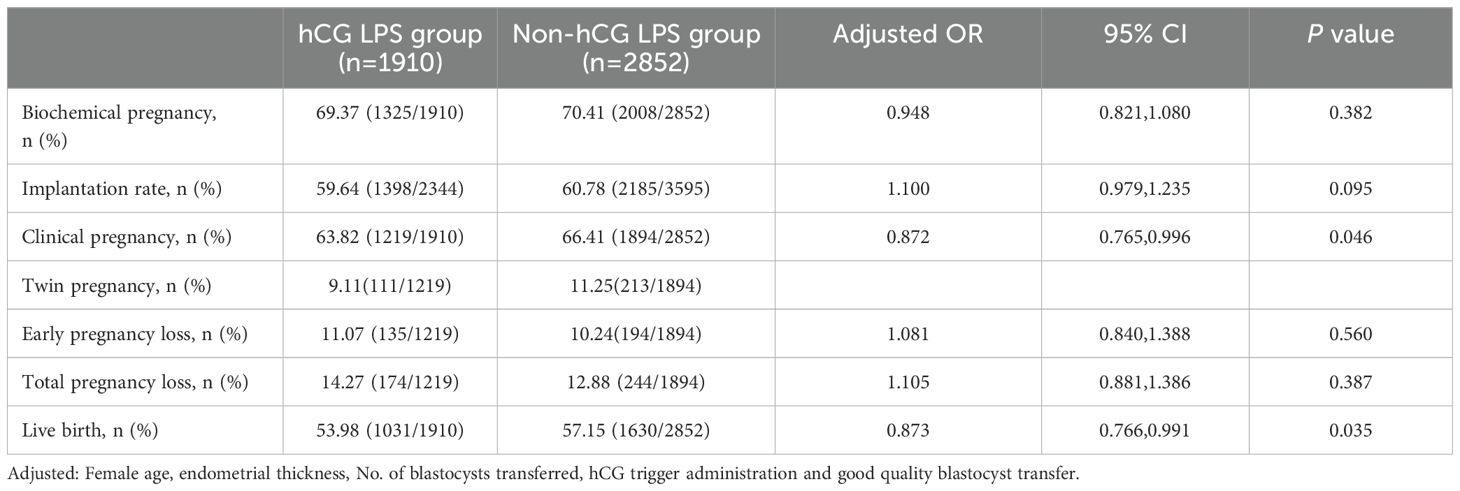
The clinical pregnancy rate in the hCG LPS group was significantly lower than that in the non-hCG LPS group (63.82% vs 66.41%, aOR 0.872, 95% CI 0.765–0.996, P=0.046, Table 2). The live birth rate in the hCG LPS group was significantly lower than that in the non-hCG LPS group (53.98% vs 57.15%, aOR 0.873, 95% CI 0.766–0.991, P=0.035). The rates of biochemical pregnancy, implantation, twin pregnancy early pregnancy loss, and total pregnancy loss were not different between the groups (all P≥0.05).
Subgroup analysis
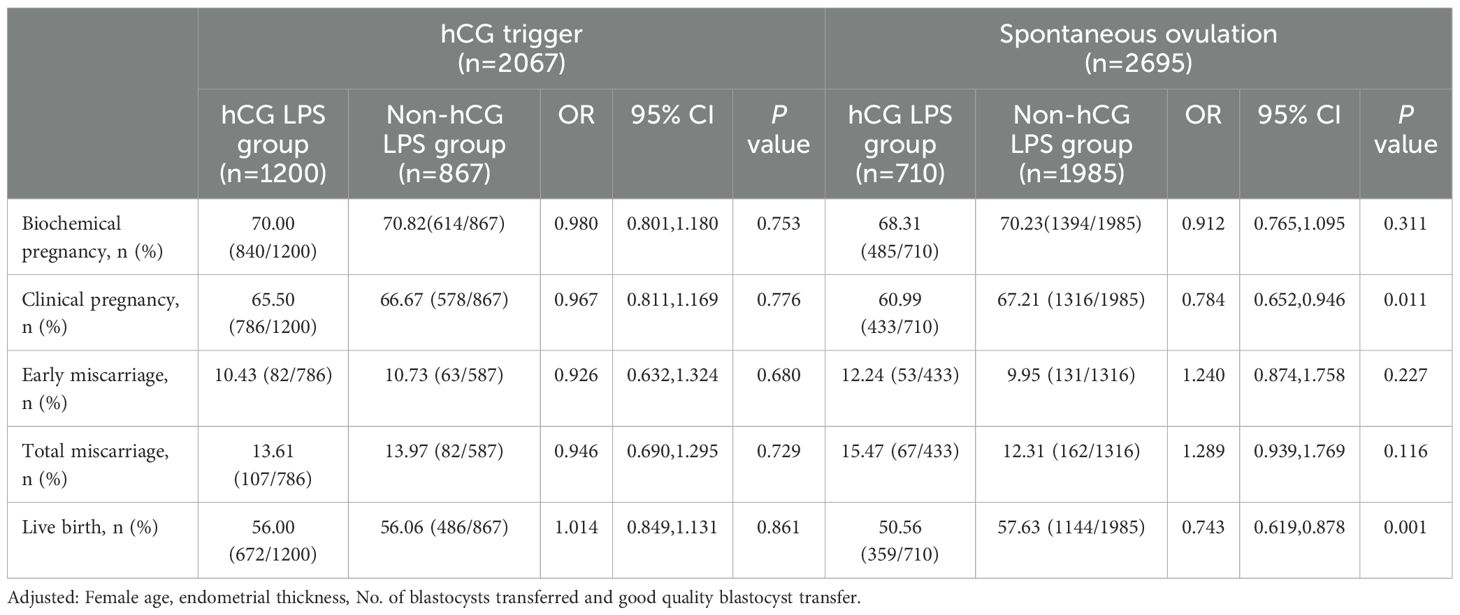
We performed a subgroup analysis by taking into consideration the potential effect of an hCG trigger on reproductive outcomes (Table 3). Among the 2067 women triggered with hCG, 1200 (58.06%) received hCG LPS and 867 (41.94%) did not receive hCG LPS. In women who were triggered with hCG, there was no significant differences in the rate of biochemical pregnancy, clinical pregnancy, early miscarriage, total miscarriage, or live birth between the two subgroups (all P≥0.05).
However, among the 2695 women who ovulated spontaneously, 710 (26.35%) were treated with hCG LPS, and 1985 (73.65%) were not treated with hCG LPS. The rates of clinical pregnancy (60.99% vs 67.21%, aOR 0.786, 95% CI 0.652–0.946, P=0.011) and live birth (50.56% vs 57.63%, aOR 0.743, 95% CI 0.619–0.878, P=0.001) in the hCG LPS group were significantly lower than those in the non-hCG LPS group. The rate of biochemical pregnancy, early miscarriage, or total miscarriage was not different between the two groups (all P≥0.05).
Discussion
The major finding of this study was that the clinical pregnancy and live birth rates in the hCG LPS group were significantly lower than those in the non-hCG LPS group (controls). Continuous hCG supplementation as LPS in natural FET cycles was associated with worse reproductive outcomes. In the subgroup analysis, in women who ovulated spontaneously, the clinical pregnancy and live birth rates in the hCG LPS group were significantly lower than those in the non-hCG LPS group. In women who received an hCG trigger, there was no significant difference in the clinical pregnancy rate or live birth rate between the hCG LPS group and the non-hCG LPS group.
There is some heterogeneity in published studies that examined the use of hCG for LPS. The dose and frequency differed greatly between the different studies, as well as the methods used for detecting ovulation and the use of hCG for triggering ovulation. In 2017, Lee et al. (12) reported that the use of hCG in natural FET cycles did not improve the ongoing pregnancy rate. In their RCT, women in the treatment group received 1500 IU hCG on the day of FET and 6 days after FET. The ongoing pregnancy rate, implantation rate, and miscarriage rate in the treatment group were similar to those in the control group. In 2019, another placebo-controlled RCT reported the same conclusion, although the method of hCG administration was different from previous published RCTs (15). Madani et al. treated women with 10,000 IU urinary hCG to trigger ovulation and 2500 IU hCG every 3 days after embryo transfer. They found that equally effective in terms of implantation, pregnancy, miscarriage, and live birth rates in women with and without hCG LPS. Cleavage-stage embryos were transferred in both studies.
Our study has some differences from previous published studies. First, we recruited women with only blastocyst transfer cycles. The number of blastocyst culture cycles is increasing worldwide owing to the development of embryo culture technology. The rate of implantation and the clinical pregnancy rate of blastocyst-stage embryos are much higher than those of cleavage-stage embryos (16). Therefore, we hypothesize that the effect of hCG LPS on outcomes of blastocyst transfer may be different from those of cleavage-stage embryo transfer. However, our results are in accordance with previous reports, which was unexpected. Second, we treated women in the hCG LPS group with continuous hCG injection after blastocyst-stage embryo transfer (2000 IU daily) for 5 days. Additionally, an hCG trigger was provided for ovulation when transvaginal ultrasound showed anovulation. There is a controversy regarding whether exogenous hCG will result in luteal phase deficiency through a short-loop feedback mechanism (17, 18). Continuous injection of hCG for LPS has been reported to be beneficial (19, 20). However, it is important to note that the hCG used in these studies was administered at a micro dose (100 IU) and the cycles involved fresh embryo transfer. Therefore, it is not certain that the same benefits would be observed in frozen embryo transfer (FET) cycles. In China, 2000 IU and 5000 IU hCG injections are the most common. Taking into consideration that Tmax of an hCG injection is 12 h and T1/2 is 23 h, we continuously treated our patients with 2000 IU hCG injections qd. Intramuscular hCG has a long half-life. Intramuscular hCG achieves circulating serum concentrations for approximately 7 days after administration. Therefore, we added hCG injection for 5 days after blastocyst FET. Third, we monitored ovulation by frequent checking of transvaginal ultrasound and urine LH. Other studies timed ovulation by the serum LH surge. Fourth, we combined hCG with oral and vaginal progesterone for luteal support, while in previous reports (12), only hCG was used as luteal support. The effect of hCG LPS on reproductive outcomes may have been attenuated by other progesterone that we used simultaneously.
Luteal support was beneficial following natural FET cycles, especially progesterone administration. Although hCG could enhance endogenous production of progesterone by the corpus luteum, supraphysiological levels of hCG supplementation did not improve reproductive outcomes.
The effects of an hCG trigger on reproductive outcomes are conflicting (21–23). An hCG trigger can cause an early rise of progesterone and finally lead to advancement of the endometrium and reduced implantation (24). We performed a subgroup analysis to assess the potential heterogeneity of an hCG trigger effect on pregnancy outcomes. In women who received hCG triggering, hCG LPS was not associated with any adverse effect on reproductive outcomes. However, in women who ovulated spontaneously, there was a significant decrease in the clinical pregnancy and live birth rates in women who received hCG LPS. This finding indicates that, in women who can ovulate on their own, extra hCG LPS is detrimental. A possible explanation for the adverse effect of continuous hCG LPS is as follows, in women who can ovulate spontaneously, their pituitary can secrete sufficient LH, and their LH surge can be detected in their serum and urine (17). The administration of continuous external hCG may directly inhibit LH release via negative feedback actions at the hypothalamic–pituitary level. The potential for increasing serum hCG concentrations by augmenting their stimulation with hCG is limited by its capacity. In women in whom an LH surge could not be detected, exogenously added hCG did not cause any harm to their defective pituitary secretion of LH. However, the mechanism of this finding requires further research.
The major strength of our study is that we focused on natural blastocyst FET cycles, eliminating the effects of the developmental stage on reproductive outcomes. There are several limitations to our study. First, this was an observational study, and there was a lack of information regarding the reasons for supplementation of hCG as LPS. Although we used a multivariable logistic regression to control for confounders between the two groups, the findings of our study might have been confounded by unmeasured or unidentified covariates. Second, all data of in vitro fertilization treatment were from a single center. Third, we did not measure serum hormones after blastocyst FET, which meant that we were unable to determine the reason for the decreased clinical pregnancy and live birth rates in the hCG LPS group.
Conclusion
Our study suggests that hCG LPS is non-beneficial in women who receive natural FET cycles with blastocyst transfer. Additionally. the adverse effect of hCG LPS is more pronounced in women who receive no hCG trigger. Further research is required to better understand the mechanisms behind the association.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of Northwest Women’s and Children’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
WW: Investigation, Writing – original draft. NL: Writing – original draft. JS: Conceptualization, Project administration, Writing – review & editing. HZ: Conceptualization, Writing – review & editing. LF: Formal analysis, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by National Center for Women and Children’s Health, China CDC “Maternal and Infants Nutrition and Health Research Programs” (Grant number 2023FYH013) and Shaanxi Provincial Department of Science and Technology (Grant number 2024SF-YBXM-252).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lopata A, Hay DL. The potential of early human embryos to form blastocysts, hatch from their zona and secrete hCG in culture. Hum Reprod. (1989) 4:87–94. doi: 10.1093/humrep/4.suppl_1.87
2. Chen XY, Li J, Jiang D, Li T, Liu XR, Zhuang GL. A highly sensitive electrochemiluminescence immunoassay for detecting human embryonic human chorionic gonadotropin in spent embryo culture media during IVF-ET cycle. J Assist Reprod Genet. (2013) 30:377–82. doi: 10.1007/s10815-012-9923-7
3. Licht P, Losch A, Dittrich R, Neuwinger J, Siebzehnrubl E, Wildt L. Novel insights into human endometrial paracrinology and embryo-maternal communication by intrauterine microdialysis. Hum Reprod Update. (1998) 4:532–8. doi: 10.1093/humupd/4.5.532
4. Fluhr H, Bischof-Islami D, Krenzer S, Licht P, Bischof P, Zygmunt M. Human chorionic gonadotropin stimulates matrix metalloproteinases-2 and -9 in cytotrophoblastic cells and decreases tissue inhibitor of metalloproteinases-1, -2, and -3 in decidualized endometrial stromal cells. Fertil Steril. (2008) 90:1390–5. doi: 10.1016/j.fertnstert.2007.08.023
5. Fluhr H, Carli S, Deperschmidt M, Wallwiener D, Zygmunt M, Licht P. Differential effects of human chorionic gonadotropin and decidualization on insulin-like growth factors-i and -ii in human endometrial stromal cells. Fertil Steril. (2008) 90:1384–9. doi: 10.1016/j.fertnstert.2007.07.1357
6. Casarini L, Lispi M, Longobardi S, Milosa F, La Marca A, Tagliasacchi D, et al. LH and hCG action on the same receptor results in quantitatively and qualitatively different intracellular Signalling. PloS One. (2012) 7:e46682. doi: 10.1371/journal.pone.0046682
7. Sungino N, Suzuki T, Kashida S, Karube A, Takiguchi S, Kato H. Expression of Bcl-2 and Bax in the human corpus luteum during the menstrual cycle and in early pregnancy: regulation by human chorionic gonadotropin. J Clin Endocrinol Metab. (2000) 85:4379–86. doi: 10.1210/jcem.85.11.6944
8. Cole L. Biological functions of hCG and hCG-related molecules. Reprod Biol Endocrinol. (2010) 8:102. doi: 10.1186/1477-7827-8-102
9. Licht P, Fluhr H, Neuwinger J, Wallwiener D, Wildt L. Is human chorionic gonadotropin directly involved in the regulation of human implantation? Mol Cell Endocinol. (2007) 269:85–92. doi: 10.1016/j.mce.2006.09.016
10. Mizrachi Y, Horowitz E, Ganer Herman H, Farhi J, Raziel A, Weissman A. Should women receive luteal support following natural cycle frozen embryo transfer? A systematic review and meta-analysis. Hum Reprod Update. (2021) 27:643–50. doi: 10.1093/humupd/dmab011
11. Lee VCY, Li RHW, Ng EHY, Yeung WSB, Ho PC. Luteal phase support does not improve the clinical pregnancy rate of natural cycle frozen-thawed embryo transfer: a retrospective analysis. Eur J Obstet Gynecol Reprod Biol. (2013) 169:50–3. doi: 10.1016/j.ejogrb.2013.02.005
12. Lee VCY, Li RHW, Yeung WSB, Pak Chung HO, Ng EHY. A randomized double-blinded controlled trial of hCG as luteal phase support in natural cycle frozen embryo transfer. Hum Reprod. (2017) 32:1130–7. doi: 10.1093/humrep/dex049
13. Glujovsky D, Farquhar C, Quinteiro Retamar AM, Alvarez Sedo CR, Blake D. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev. (2016) 2016):CD002118. doi: 10.1002/14651858.CD002118.pub6
14. Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, et al. The international glossary on infertility and fertility care, 2017. Fertil Steril. (2017) 108:393–406. doi: 10.1016/j.fertnstert.2017.06.005
15. Madani T, Ramezanali F, Yahyaei A, Hasani F, Bagheri Lankarani N, Mohammadi Yeganeh L, et al. Live birth rates after different endometrial preparation methods in frozen cleavage−stage embryo transfer cycles: a randomized controlled trial. Arch Gynecol Obstet. (2019) 299:1185–91. doi: 10.1007/s00404-019-05062-7
16. Holden EC, Kashani BN, Morelli SS, Alderson D, Jindal SK, Ohman-Strickland PA, et al. Improved outcomes after blastocyst-stage frozen-thawed embryo transfers compared with cleavage stage: a society for assisted reproductive technologies clinical outcomes reporting system study. Fertil Steril. (2018) 110:89–94.e2. doi: 10.1016/j.fertnstert.2018.03.033
17. Miyake A, Aono T, Kawamura Y, Kurachi K. Suppression of plasma luteinizing hormone-releasing hormone by administration of human chorionic gonadotropin in castrated women. Fertil Steril. (1982) 38:251–2. doi: 10.1016/S0015-0282(16)46468-X
18. Tavaniotou A, Devroey P. Effect of human chorionic gonadotropin on luteal luteinizing hormone concentrations in natural cycles. Fertil Steril. (2003) 80:654–5. doi: 10.1016/S0015-0282(03)00789-1
19. Andersen CY, Fischer R, Giorgione V, Kelsey TW. Micro-dose hCG as luteal phase support without exogenous progesterone administration: mathematical modelling of the hCG concentration in circulation and initial clinical experience. J Assist Reprod Gen. (2016) 33:1311–8. doi: 10.1007/s10815-016-0764-7
20. Carosso AR, Canosa S, Gennarelli G, Sestero M, Evangelisti B, Charrier L, et al. Luteal Support with very low daily dose of human chorionic gonadotropin after fresh embryo transfer as an alternative to cycle segmentation for high responders patients undergoing gonadotropin-releasing hormone agonist-triggered IVF. Pharm (Basel). (2021) 14:228. doi: 10.3390/ph14030228
21. Montagut M, Santos-Ribeiro S, De Vos M, Polyzos NP, Drakopoulos P, Mackens S, et al. Frozen–thawed embryo transfers in natural cycles with spontaneous or induced ovulation: the search for the best protocol continues. Hum Reprod. (2016) 31:2803–10. doi: 10.1093/humrep/dew263
22. Weissman A, Horowitz E, Ravhon A, Steinfeld Z, Mutzafi R, Golan A, et al. Spontaneous ovulation versus HCG triggering for timing natural-cycle frozen-thawed embryo transfer: a randomized study. Reprod BioMed Online. (2011) 23:484–9. doi: 10.1016/j.rbmo.2011.06.004
23. Zhai X, Shu M, Guo Y, Yao S, Wang Y, Han S, et al. Efficacy of low-dose hCG on FET cycle in patients with recurrent implantation failure. Front Endocrinol (Lausanne). (2022) 13:1053592. doi: 10.3389/fendo.2022.1053592
24. Yding Andersen C, Elbaek HO, Alsbjerg B, Laursen RJ, Povlsen BB, Thomsen L, et al. Daily low-dose hCG stimulation during the luteal phase combined with GnRHa triggered IVF cycles without exogenous progesterone: a proof of concept trial. Hum Reprod. (2015) 30(10):2387–95. doi: 10.1093/humrep/dev184
Keywords: luteal phase support, human chorionic gonadotropin, natural cycle, frozen-thawed transfer, live birth rate
Citation: Wen W, Li N, Shi J, Zhou H and Fan L (2024) Use of hCG for luteal support in natural frozen–thawed blastocyst transfer cycles: a cohort study. Front. Endocrinol. 15:1391902. doi: 10.3389/fendo.2024.1391902
Received: 26 February 2024; Accepted: 31 July 2024;
Published: 14 August 2024.
Edited by:
Eytan R. Barnea, BioIncept, LLC, United StatesReviewed by:
Panagiotis Cherouveim, Massachusetts General Hospital and Harvard Medical School, United StatesHongzhan Zhang, Shenzhen Zhongshan Urological Hospital, China
Xiushan Feng, Fujian Medical University, China
Copyright © 2024 Wen, Li, Shi, Zhou and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lijuan Fan, ZHJmYW5saWp1YW5AMTYzLmNvbQ==
 Wen Wen
Wen Wen Na Li
Na Li Juanzi Shi
Juanzi Shi Hanying Zhou
Hanying Zhou Lijuan Fan
Lijuan Fan