- 1Department of Cardiology, First Affiliated Hospital of Gannan Medical University, Key Laboratory of Prevention and Treatment of Cardiovascular and Cerebrovascular Diseases, Ministry of Education, Gannan Medical University, Ganzhou, China
- 2Department of Endocrinology, The Fifth Affiliated Hospital of Guangzhou Medical University, Guangzhou, China
- 3Department of Cardiology, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou, China
- 4Guangdong Provincial Key Laboratory of Coronary Heart Disease Prevention, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China
- 5Department of Biostatistics, School of Public Health, Southern Medical University, Guangzhou, China
- 6Department of Cardiology, Guangdong Provincial People’s Hospital’s Nanhai Hospital, The Second People’s Hospital of Nanhai District, Foshan, China
Purpose: Both glucose and albumin are associated with chronic inflammation, which plays a vital role in post-contrast acute kidney injury (PC-AKI). To explore the relationship between random glucose to albumin ratio (RAR) and the incidence of PC-AKI after percutaneous coronary intervention (PCI) in patients with ST-elevation myocardial infarction (STEMI).
Patients and methods: STEMI patients who underwent PCI were consecutively enrolled from January, 01, 2010 to February, 28, 2020. All patients were categorized into T1, T2, and T3 groups, respectively, based on RAR value (RAR < 3.377; 3.377 ≤ RAR ≤ 4.579; RAR > 4.579). The primary outcome was the incidence of PC-AKI, and the incidence of major adverse clinical events (MACE) was the second endpoint. The association between RAR and PC-AKI was assessed by multivariable logistic regression analysis.
Results: A total of 2,924 patients with STEMI undergoing PCI were finally included. The incidence of PC-AKI increased with the increasing tertile of RAR (3.2% vs 4.8% vs 10.6%, P<0.001). Multivariable regression analysis demonstrated that RAR (as a continuous variable) was associated with the incidence of PC-AKI (adjusted odds ratio (OR) =1.10, 95% confidence interval (CI) =1.04 - 1.16, P<0.001) and in-hospital MACE (OR=1.07, 95% CI=1.02 - 1.14, P=0.012); RAR, as a categorical variable, was significantly associated with PC-AKI (T3 vs. T1, OR=1.70, 95% CI=1.08 - 2.67, P=0.021) and in-hospital MACE (T3 vs. T1, OR=1.63, 95% CI=1.02 - 2.60, P=0.041) in multivariable regression analyses. Receiver operating characteristic curve analysis showed that RAR exhibited a predictive value for PC-AKI (area under the curve (AUC)=0.666, 95% CI=0.625 - 0.708), and in-hospital MACE (AUC= 0.662, 95% CI =0.619 - 0.706).
Conclusions: The high value of RAR was significantly associated with the increasing risk of PC-AKI and in-hospital MACE after PCI in STEMI patients, and RAR offers a good predictive value for those outcomes.
Introduction
Post-contrast acute kidney injury (PC-AKI) is one of the most common comorbidities following percutaneous coronary intervention (PCI), which is significantly higher in patients with ST-segment elevated myocardial infarction (STEMI) than other patients (1, 2). Patients with PC-AKI have higher mortality, and longer hospitalization than patients without PC-AKI (3). However, there is no effective treatment for PC-AKI to date (4), identifying patients at high-risk of PC-AKI and implementing timely preventative measures are critical in avoiding PC-AKI.
In clinical, the constantly updated risk score is used to predict PC-AKI after PCI (5, 6). However, most risk factors for PC-AKI included in the risk score were largely unchangeable and irreversible, making them unsuitable for primary PCI since most of those parameters are not readily available. Investigating some novel and potentially modifiable predictors may help to minimize PC-AKI incidence. Recent studies found non-diabetic patients with elevated pre-procedural random glucose have a higher risk of PC-AKI (7, 8). Both fasting glucose and random glucose can predict in-hospital events in STEMI patients, but random glucose is more convenient in real-time and easier to obtain (9). Low serum albumin has been demonstrated as a potential prognostic marker and predictor of various inflammatory diseases and PC-AKI (10–13). Furthermore, the ratio of fibrinogen to albumin in the blood was successfully used to predict PC-AKI (14). Glucose and albumin have both been adapted as critical parameters for monitoring the dynamic changes in renal function (15, 16). In terms of predicting renal function, a paradox exists between glucose and albumin, with higher glucose and lower albumin indicating worse renal function (15, 16). Therefore, we hypothesize that the random glucose to albumin ratio (RAR) could be a novel predictor for PC-AKI in patients with STEMI which may be helpful for the prevention of PC-AKI. The primary objective of this study was to assess the association between RAR and PC-AKI and other outcomes among patients with STEMI underwent PCI.
Patients and method
Study design and patients
The present study on the predictive value of RAR for PC-AKI was conducted at the Guangdong Provincial People’s Hospital between January 2010 and February 2020. Patients with STEMI undergoing PCI were consecutively enrolled in this observational cohort study. STEMI was diagnosed using the latest criteria from the 2017 ESC Guidelines (1). The following were the exclusion criteria: (1) Patients on renal replacement therapy; (2) Contrast agent allergies; (3) Patients without receiving the percutaneous coronary intervention; (4) A history of severe chronic inflammatory disease or a malignant tumor, and steroidal agents’ treatment recently; (5) Undergoing coronary artery bypass grafting; (6) Random glucose or albumin values were missing. The ethics committee of Guangdong Provincial People’s Hospital approved the study, and all patients signed a written informed consent before the procedure.
Study protocol
The medical information recording systems were used to collect patient demographic and clinical characteristics such as age, sex, smoking status, medical history, laboratory indices, echocardiography, angiographic variables, and medication used during hospitalization. All laboratory examinations were systematically and preoperatively performed in Guangdong Provincial People’s Hospital. RAR was calculated using the random glucose/albumin formula. Random glucose was measured by analyzed biochemically using blood collected before PCI, and the albumin value was measured within 6 hour after PCI. SCr levels were measured before and after PCI for a period of 2–3 days. We evaluated the estimated glomerular filtration rate using the modified Modification of Diet in Renal Disease equation for Chinese patients (17).
PCI was performed using standard guide catheters, guidewires, balloon catheters, and stents via the femoral or radial approach, in accordance with standard clinical practice. All patients received nonionic, low-osmolarity contrast agents. In addition, patients received 0.9% saline (1 ml/kg/h) during the procedure and maintained for 6–12 hours afterward.
Primary and second endpoints
The primary endpoint was PC-AKI development, defined as an increase in serum creatinine (SCr) of more than 44.2 μmol/L (0.5 mg/dL) from baseline in the initial 48 to 72 hours after contrast exposure (4). The second endpoint was the occurrence of major adverse clinical events (MACE), which included all-cause mortality, recurrent myocardial infarction, stroke, or target vessel revascularization during hospitalization. The other definition of PC-AKI, as an increase in SCr of more than 26.4 μmol/L (0.3 mg/dL) from baseline in the initial 48 to 72 hours after contrast exposure, was also reported (4, 18).
Statistical analysis
Baseline characteristics of participants who were divided into three groups according to the tertile of RAR: T1 (n=974, RAR < 3.377), T2 (n=975, 3.377 ≤ RAR ≤ 4.579), and T3 (n=975, RAR > 4.579) were compared. The mean and standard deviation (SD) of normally distributed continuous variables were calculated and analyzed using Student’s t-tests. The Wilcoxon ranksum test was used to analyze nonnormally distributed variables that were expressed as medians or quartile. Categorical variables were represented as percentages and analyzed using the chi-square or Fisher exact test.
For risk factors of PC-AKI and in-hospital MACE, odds ratios (OR) with 95 percent confidence intervals (CI) were calculated using univariable and multivariable logistic regression analyses. Variables that were statistically significant in the univariate analysis and those known to be related to infection (according to previous studies) were adjusted in multivariable logistic regression analyses. Risk factors (age, gender, heart failure, smoke, hypertension, chronic obstructive pulmonary disease, previous myocardial infarction, prior PCI, previous stroke, anemia, estimated glomerular filtration rate) and variables (aspirin, GPIIb/IIIa inhibitor, multi-vessel stenosis, femoral access) which were validated to be meaningful in medical practice, were included in the multiple logistic regression analysis. A cubic spine model (adjusted for age and gender) was also performed to judge the effect of RAR on PC-AKI and MACE. Receiver operating characteristic (ROC) curve analysis was used to evaluate the value of RAR levels for predicting PC-AKI and MACE, and the area under the ROC curve (AUC) was subsequently calculated. The AUC value can be used to evaluate the efficacy of the predictor (AUC <0.6, poor discrimination; 0.6 – 0.75, good discrimination; >0.75, excellent discrimination) (19). The optimal cutoff value was determined by using the Youden index.
SAS version 9.4 (SAS Institute, Cary, NC, USA) was used for all statistical analyses. All probability values were two-tailed, and statistical significance was defined as P value less than 0.05.
Results
Baseline characteristics of all groups
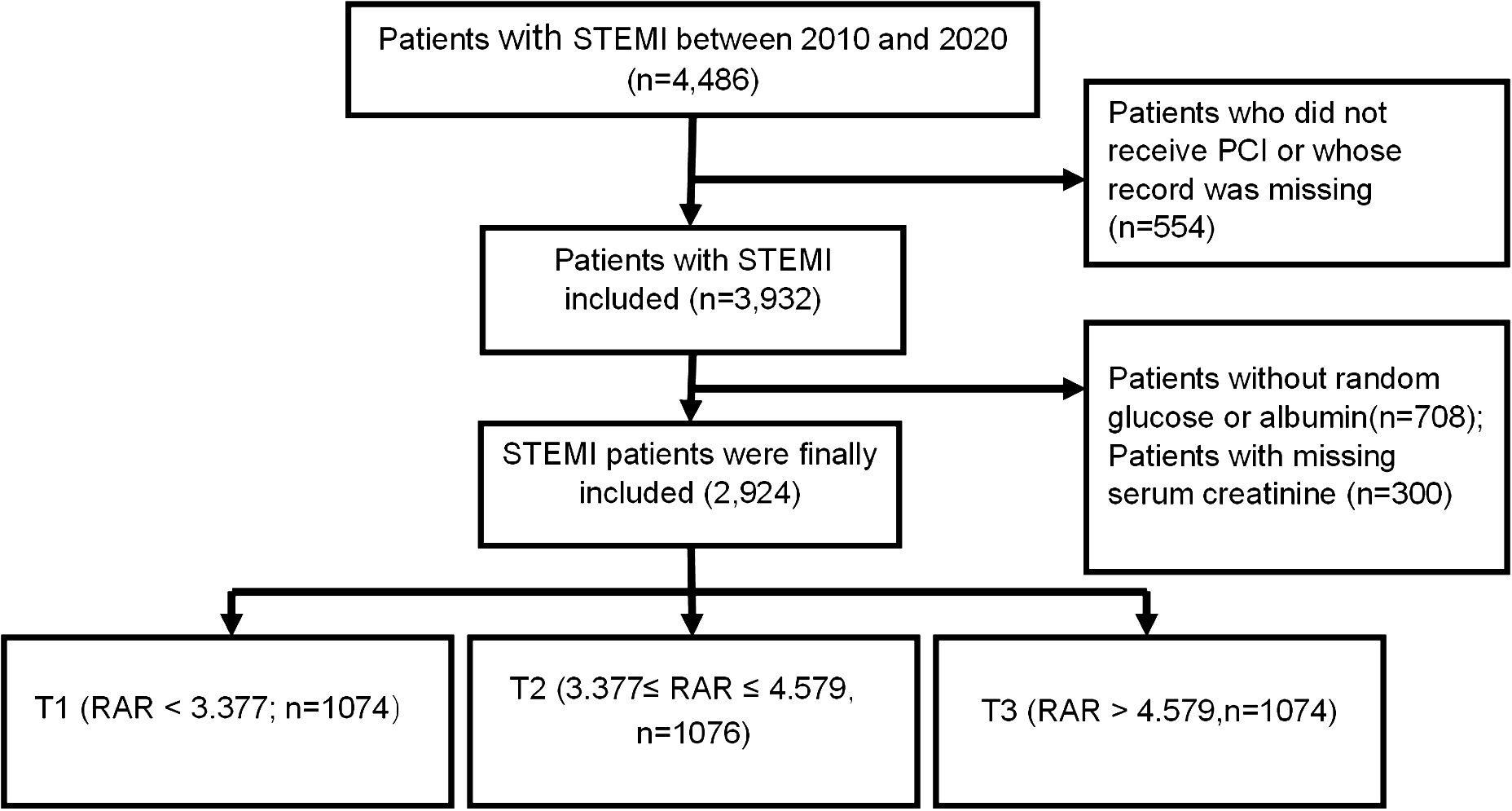
A total of 2,924 STEMI patients undergoing PCI were finally enrolled (Figure 1). The average age was 62.30 ± 12.00 years, 43.39% of the total patients were more than 65 years, and 2,411 (82.46%) were male. Patients were divided into the three groups based on the tertiles of the RAR values: T1(n=974): RAR < 3.377; T2 (n=975): 3.377 ≤ RAR ≤ 4.579; T3 (n=975):RAR > 4.579. Random blood glucose was significantly higher in the T3 group, while, albumin was significantly lower. The percentages of hypertension, heart failure, diabetes, and smoker were higher in the T3 group. Patients with higher RAR received more insulin, clopidogrel, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, aspirin, glycoprotein IIb/IIIa receptor inhibitor, beta-blockers, and calcium channel blocker drugs and more stents; and were more likely to have multi-vessel stenosis than those with lower RAR (Table 1).
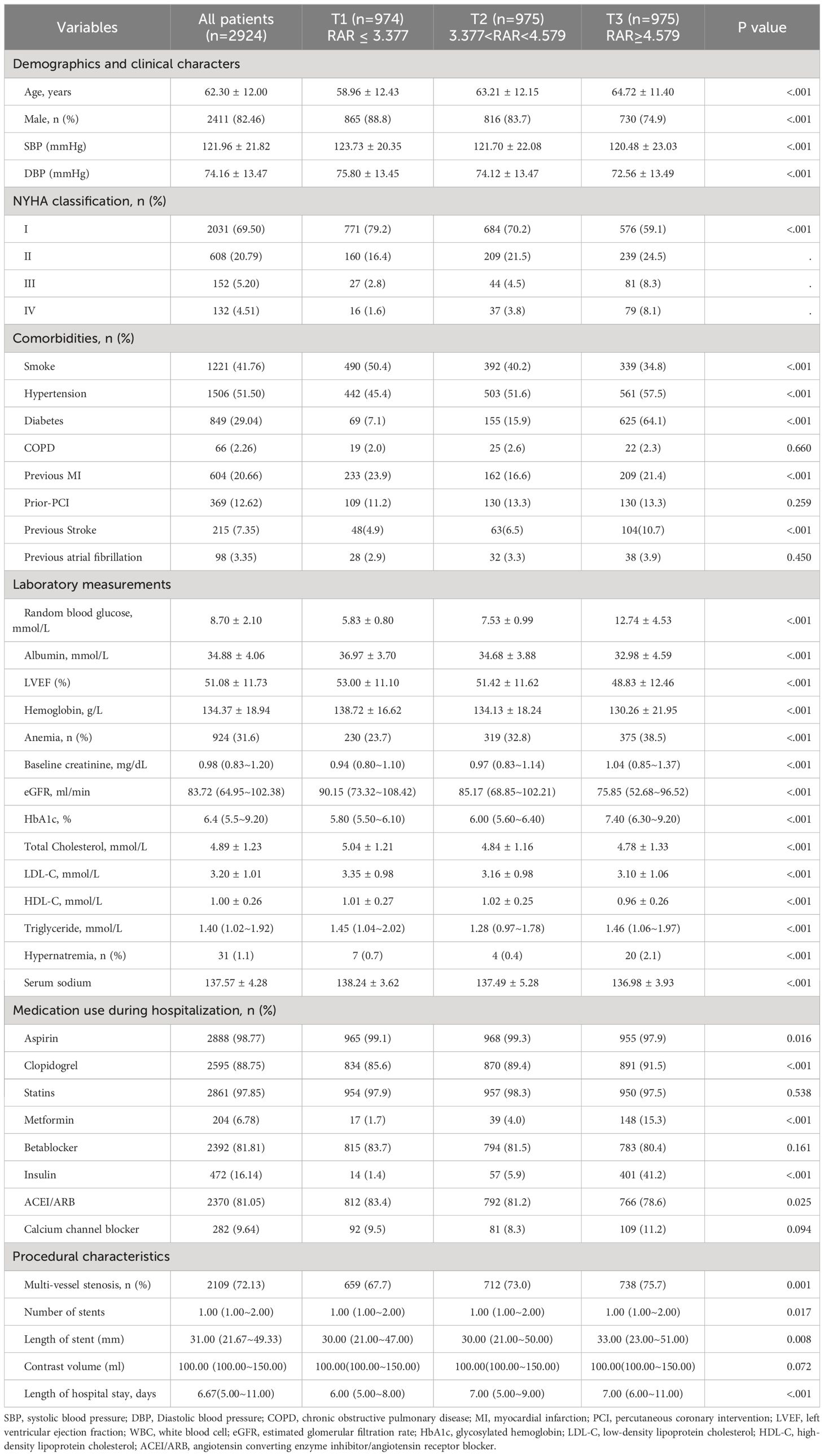
Table 1 Baseline characteristics of patients enrolled in this study were stratified by RAR’s tertile.
RAR correlates with PC-AKI and in-hospital MACE
The incidence of PC-AKI significantly rose with increasing of RAR. Only 3.2% of the patients in T1 developed PC-AKI, while it was as high as 10.6% in the T3 group. Cubic spline models demonstrated no significant non-linear relationship between RAR and MACE (Figures 2A, B). The incidence of each endpoint included in MACE was listed in Supplementary Table 1.
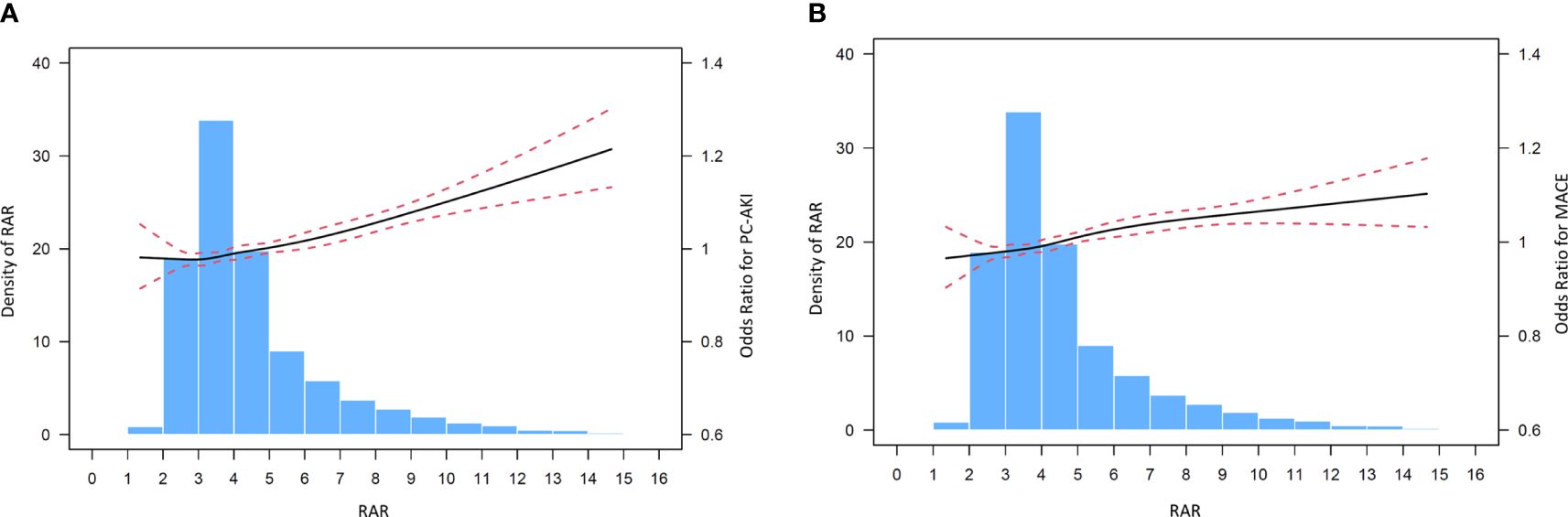
Figure 2 (A) Cubic spine models for the association between RAR and PC-AKI. (B) Cubic spine models for the association between RAR and in hospital MACE.
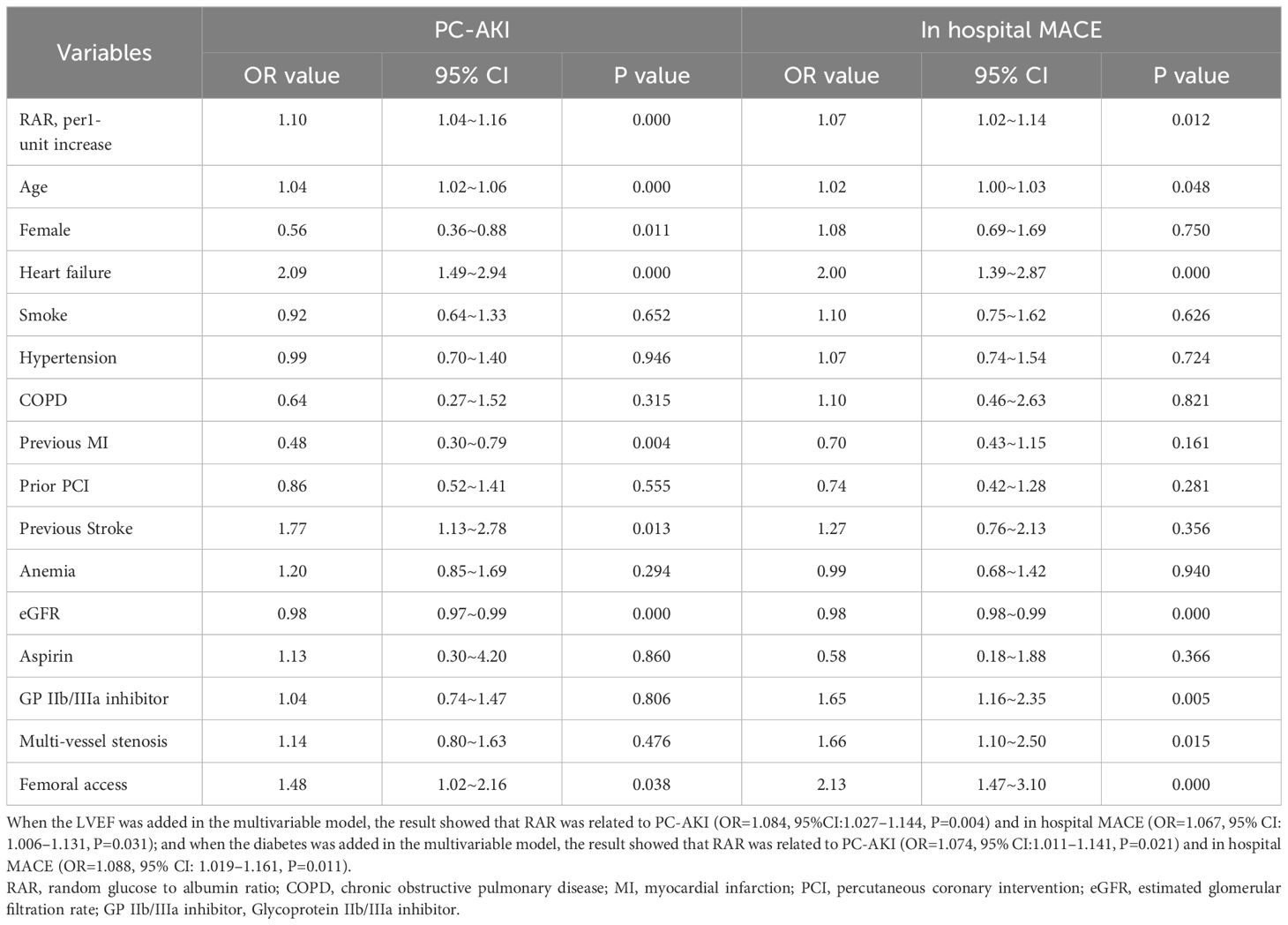
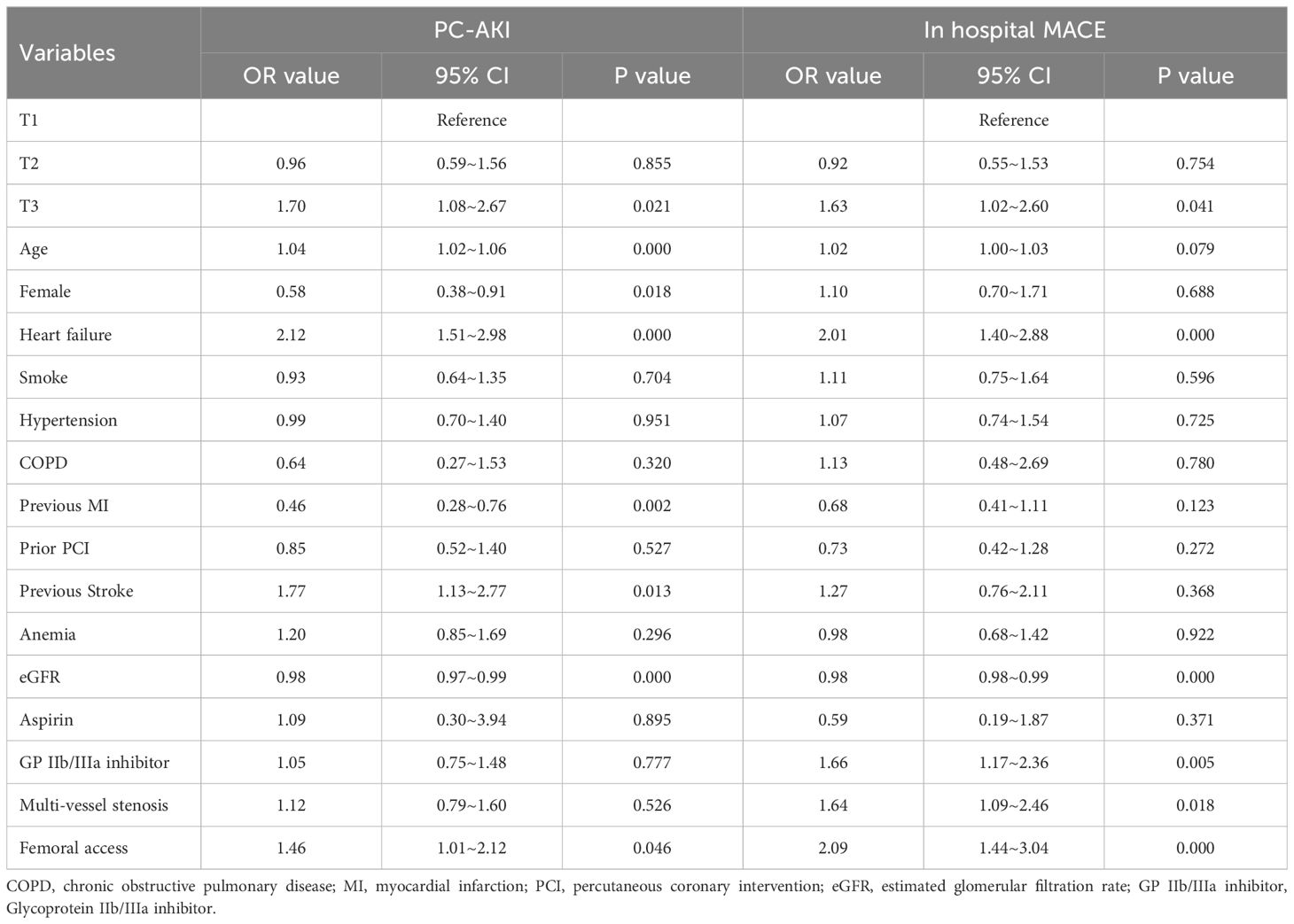
Multivariable regression analyses demonstrated that RAR, as a continuous variable, was associated with the incidence of PC-AKI (OR=1.10, 95% CI=1.04 - 1.16, P<0.001) and in-hospital MACE (OR=1.07, 95% CI=1.02 - 1.14, P=0.012) in patients with STEMI undergoing PCI (Table 2). Furthermore, RAR, as a continuous variable, was also associated with the incidence of other definition of PC-AKI (OR=1.09, 95% CI=1.04 - 1.14, P<0.001) (Supplementary Table 2). Meanwhile, RAR, as a categorical variable, was related to PC-AKI (T3 vs. T1, OR=1.70, 95% CI=1.08 - 2.67, P=0.021), other definition of PC-AKI (T3 vs. T1, OR=1.60, 95% CI=1.14 - 2.26, P=0.007), and in-hospital MACE (T3 vs. T1, OR=1.63, 95% CI=1.02–2.60, P=0.041) in multivariable regression analyses(Table 3 and Supplementary Table 3). Furthermore, the result remained that RAR (as a continuous or categorical variable) was related to PC-AKI and in hospital MACE after adjusting the history of diabetes, and proved that RAR is associated with clinical outcomes independent of history of diabetes (Supplementary Table 4). Another multivariable model also demonstrated similar results (Supplementary Table 5).
Predictive value of RAR for PC-AKI and in-hospital MACE
ROC curve analysis showed that RAR exhibited a good predictive value for PC-AKI (area under the curve (AUC) = 0.666, 95% CI=0.625 - 0.708), and the optimal cutoff point of RAR was 4.351, with a sensitivity of 63.5% and specificity of 63.5% (Figure 3A). Additionally, RAR was demonstrated a similar predictive value for PC-AKI (AUC= 0.633, 95% CI=0.601 - 0.665) based on other definition (Supplementary Figure 1). RAR also revealed a predictive value for in-hospital MACE (AUC= 0.662, 95% CI =0.619 - 0.706) (Figure 3B).
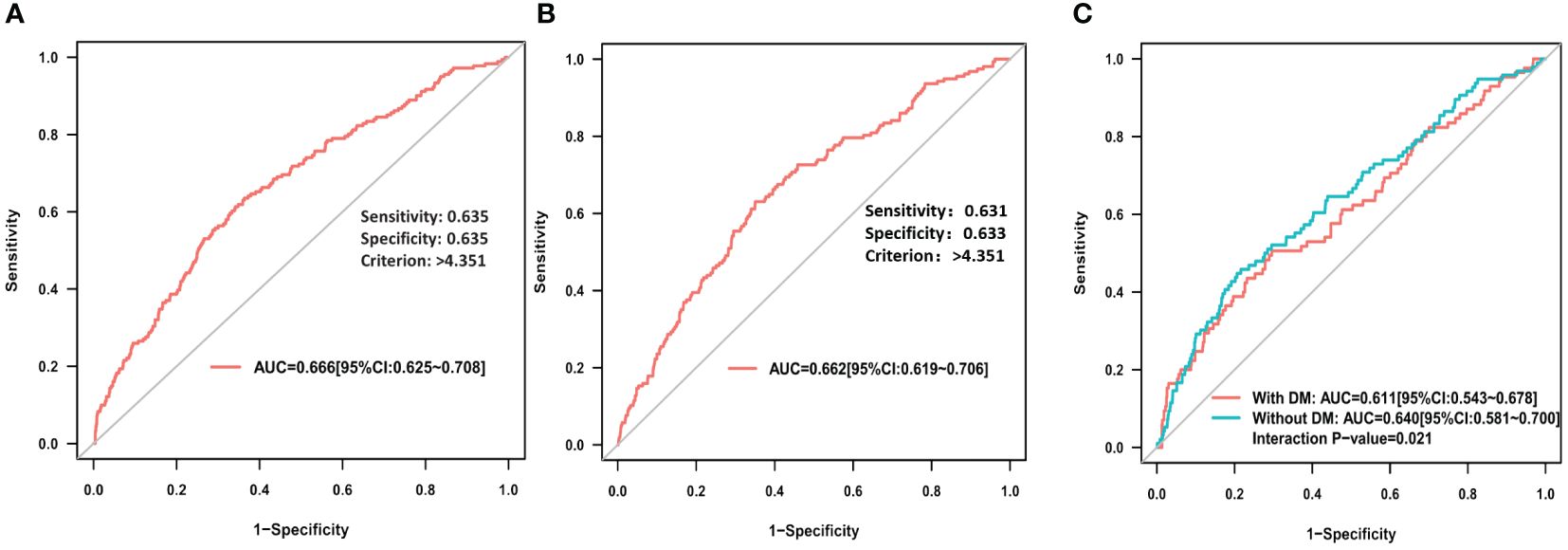
Figure 3 (A) ROC curve analysis of RAR for PC-AKI; (B) ROC curve analysis of RAR for in hospital MACE; (C) ROC curve analysis of RAR for PC-AKI among STEMI patients with or without DM.
Subgroup analysis
The AUC of RAR for predicting PC-AKI in the no diabetes subgroup was significantly higher compared to that in the diabetes subgroup (AUC: 0.640 vs 0.611, P=0.021) (Figure 3C), while, there is not significantly different in subgroup of gender (AUC: 0.659 vs 0.725, P=0.329) (Supplementary Figure 2). However, except for age (p for interaction: <0.001), subgroup analyses of diabetes (p for interaction: 0.554), or gender (p for interaction: 0.533) or hypertension (p for interaction: 0.409) did not identify any significant difference in PC-AKI (Supplementary Table 6). All subgroup analyses did not identify any significant difference in MACE (Supplementary Table 6).
Discussion
To the best of our knowledge, current study was the first to evaluate the relationship between RAR and PC-AKI in patient with STEMI undergoing PCI. Especially, most previous discovered risk factors or predictors are unsuitable for primary PCI since most of those parameters are not readily available (20). Our results found that RAR was an independent predictor for PC-AKI and MACE in patients with STEMI undergoing PCI, and exhibited (more than 60%) good predictive value for PC-AKI in the subgroup of patients without diabetes.
Systemic inflammation is closely related to the development of PC-AKI, and systemic inflammatory indexes like systemic immune-inflammation index (SII) have been used to effectively predict PC-AKI in STEMI patients after PCI (21). However, the SII index lacks specificity in predicting PC-AKI since it was also elevated in various cancer patients (22). Patients with high glucose are prone to chronic inflammation (23). High glucose level causes high expression of pro-inflammatory genes in macrophages in non-diabetes patients (24). Cheuk-Kin Kwan et, al found that high glucose level stimulates inflammation and weakens the pro-resolving response at the cellular level (25). More importantly, study confirmed that acute hyperglycemia could induce renal tubular injury (26).
It is widely acknowledged that diabetes, elevated fasting glucose, and impaired glucose tolerance are vital risk factors for PC-AKI (3), while, glucose tolerance testing and HbA1c are difficult to obtain in patients without diagnosed diabetes or in urgent events like STEMI. Random glucose testing is an optimal option in this situation. In clinical trials, researchers discovered that high random blood glucose significantly increased the risk of PC-AKI after PCI in patients with acute coronary syndrome who did not have diabetes (8). Previous study including 13,3792 non-diabetes patients concluded that an increased random glucose value is a risk factor for diabetes (27) and could predict acceptable overall glycemic control in non-insulin-dependent diabetic patients (28). Qurratul Ain et al. further confirmed that random plasma glucose could effectively reflect glycemic control in adults with type 2 diabetes mellitus (29). A rapid and systemic assessment of glucose metabolism in STEMI patients before coronary radiography and PCI is impossible, while random glucose could turn out to be a reliable option for STEMI patients with normal or unknown abnormal glucose level. Although an increase in random glucose levels was related to PC-AKI, its prognostic value was limited due to its values being easily affected by food intake.
Albumin is an important nutrition subject, and concentration of serum albumin is determined by the absolute rate of albumin synthesis, the fractional catabolic rate, the distribution of albumin between the vascular and extravascular compartments, and exogenous albumin loss (30). The combined effects of inflammation and inadequate protein, and caloric intake induce hypoalbuminemia in patients with chronic diseases such as chronic renal failure (31) and cirrhosis (32). A previous study indicates a negative correlation between albumin and C-response protein levels, as well as between albumin and white blood cell levels (33). Investigators found that inflammation and reduced albumin synthesis are linked to a stable declined of serum albumin in hemodialysis patients (30). Except for low albumin closely associated with inflammation, hypoalbuminemia predicted the risk of AKI in in-hospital patients (34) and non-cardiac surgery (35). Meta-analysis further confirmed that hypoalbuminemia is positively correlated with the risk of AKI (13). However, low protein uptake in renal dysfunction could be the root of low serum albumin.
Ongoing inflammation significantly increased the incidence of PC-AKI in those patients undergoing contrast-enhanced CT (36) and the close relationship between inflammation and PC-AKI has already been widely acknowledged (37–39). As discussed above, random glucose and serum albumin are positively and negatively associated with the pathophysiological process of inflammation, respectively. And a combination of random glucose and serum albumin might be more reliable than random glucose and serum albumin alone in predicting PC-AKI as both random glucose is influenced by daily diet. Notably, the current study provides a reliable foundation for finding potential PC-AKI patients and MACE by RAR, which possesses a higher predictive value in PC-AKI assessments. Considering the analysis conducted, and the metabolic pathways of glucose and albumin are intricately linked to inflammatory processes, which are pivotal in the pathogenesis of PC-AKI. Hence, it is logical to posit that the ratio of glucose to albumin correlates with the incidence of PC-AKI.
Previous researches reported that around 70% of the STEMI patients were male, and 81.3% of them received PCI (40, 41),which is similar to the present study (82.46% male patients receiving PCI). In another more than ten years long-term following up study on STEMI patients, only 17% of them were female (254 in 1498) and there were no differences in the combined patient-oriented endpoint between women and men (42). In addition, although subgroup analyses did not identify any significant difference in PC-AKI after adjustment of other potential factors, the RAR has a little better performance in non-diabetic patients. Several potential reasons should be considered. Firstly, prediabetic patients would be included in the non-diabetic group. Secondly, the random glucose was not affected by anti-diabetic agents in the non-diabetic patients. Thirdly, the operator may pay more attention to those patients with diabetes during the operation since it is well known that diabetes is a main risk factor for PC-AKI. However, the relationship between RAR and PC-AKI in the non-diabetic patients should be evaluated in the future researches with large sample size.
In clinical practice, patients at high risk of PC-AKI based on RAR should be received implementing timely preventative measures avoiding PC-AKI. These patients should be received the standard hydration and monitoring the level of serum creatinine, and avoiding or correcting the low serum albumin. However, further randomized controlled trials with large sample sizes are warranted to validate and optimize the clinical application of RAR on preventing the development of PC-AKI.
Limitation
Firstly, although we have performed the multivariable analysis (including RAR as continuous or categorical variables) and cubic spine model to test the robustness of RAR predictions for PC-AKI, potential bias was inevitable as an observational study, such as metabolic control, the inflammatory state of the population, and nutritional status. Secondly, due to the different diagnostic criteria of PC-AKI (3), the results may not be repeatable when other PC-AKI definition was used. Lastly, only STEMI patients were included in this study, the result may vary for other types of acute coronary syndrome patients.
Conclusion
A high value of RAR was associated with an increased risk of PC-AKI and in-hospital MACE in patients with STEMI undergoing PCI, with RAR showing good predictive value for these outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the ethics committee of Guangdong Provincial People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. The animal study was approved by the ethics committee of Guangdong Provincial People’s Hospital. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
PL: Data curation, Investigation, Writing – original draft, Writing – review & editing. XG: Data curation, Methodology, Writing – original draft. XL: Data curation, Methodology, Writing – original draft. YH: Data curation, Methodology, Writing – original draft. YD: Data curation, Formal analysis, Writing – original draft. CD: Data curation, Investigation, Methodology, Writing – original draft. YL: Investigation, Writing – original draft. WH: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Shuangqing Talent Program Project of Guangdong Provincial people’s Hospital (Grant No. KJ012019095 to YL), and supported by “the Fundamental Research Funds for the Central Universities” (2022ZYGXZR039), and project of Administration of Traditional Chinese Medicine of Guangdong Province (20221007). The National Public Fund for Study Abroad and CSC scholarship (Grant NO.202008360179) (PL).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1390868/full#supplementary-material
Abbreviations
PC-AKI, Post-contrast acute kidney injury; RAR, Random glucose to albumin ratio; SCr, Serum creatinine; MACE, Major adverse clinical events; OR, Odds ratio; CI, Confidence interval; AUC, Area under the curve; STEMI, ST-segment elevated myocardial infarction; PCI, Percutaneous coronary intervention; eGFR, Estimated glomerular filtration rate; GP IIb/IIIa inhibitor, Glycoprotein iib/iiia inhibitor.
References
1. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. (2018) 39:119–77. doi: 10.1093/eurheartj/ehx393
2. Toprak O, Cirit M. Risk factors for contrast-induced nephropathy. Kidney Blood Press Res. (2006) 29:84–93. doi: 10.1159/000093381
3. Wichmann JL, Katzberg RW, Litwin SE, Zwerner PL, De Cecco CN, Vogl TJ, et al. Contrast-induced nephropathy. Circulation. (2015) 132:1931–6. doi: 10.1161/CIRCULATIONAHA.115.014672
4. van der Molen AJ, Reimer P, Dekkers IA, Bongartz G, Bellin MF, Bertolotto M, et al. Post-contrast acute kidney injury - Part 1: Definition, clinical features, incidence, role of contrast medium and risk factors: Recommendations for updated ESUR Contrast Medium Safety Committee guidelines. Eur Radiol. (2018) 28:2845–55. doi: 10.1007/s00330-017-5246-5
5. Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. (2004) 44:1393–9. doi: 10.1016/S0735-1097(04)01445-7
6. Lin KY, Zheng WP, Bei WJ, Chen SQ, Islam SM, Liu Y, et al. A novel risk score model for prediction of contrast-induced nephropathy after emergent percutaneous coronary intervention. Int J Cardiol. (2017) 230:402–12. doi: 10.1016/j.ijcard.2016.12.095
7. Stolker JM, McCullough PA, Rao S, Inzucchi SE, Spertus JA, Maddox TM, et al. Pre-procedural glucose levels and the risk for contrast-induced acute kidney injury in patients undergoing coronary angiography. J Am Coll Cardiol. (2010) 55:1433–40. doi: 10.1016/j.jacc.2009.09.072
8. Kabir MS, Nobi A, Hasem S, Shikder MR, Ali MS, Choudhury AK, et al. Impact of Blood Glucose Levels on Contrast Induced Nephropathy after Percutaneous Coronary Intervention in Patients not known to be Diabetic with Acute Coronary Syndrome. Cardiovasc J. (2013) 6:23–30. doi: 10.3329/cardio.v6i1.16111
9. Qin Y, Yan G, Qiao Y, Wang D, Luo E, Hou J, et al. Predictive value of random blood glucose versus fasting blood glucose on in-hospital adverse events in patients with ST-segment elevation acute myocardial infarction. BMC Cardiovasc Disord. (2020) 20:95. doi: 10.1186/s12872-020-01394-4
10. Hassan K, Fadi H. Is hypoalbuminemia a prognostic risk factor for contrast-induced nephropathy in peritoneal dialysis patients? Ther Clin Risk Manage. (2014) 10:787–95. doi: 10.2147/TCRM
11. Murat SN, Kurtul A, Yarlioglues M. Impact of serum albumin levels on contrast-induced acute kidney injury in patients with acute coronary syndromes treated with percutaneous coronary intervention. Angiology. (2015) 66:732–7. doi: 10.1177/0003319714551979
12. Wang Y, Sun WJ, Ji ZS, Liu CB, Wang R. Serum albumin and the risk of contrast-induced acute kidney injury after percutaneous coronary intervention. Rev Cardiovasc Med. (2020) 21:139–45. doi: 10.31083/j.rcm.2020.01.583
13. Wiedermann CJ, Wiedermann W, Joannidis M. Causal relationship between hypoalbuminemia and acute kidney injury. World J Nephrol. (2017) 6:176–87. doi: 10.5527/wjn.v6.i4.176
14. You Z, Guo T, Lin F, Lin C, Chen J, Li X, et al. Fibrinogen-to-albumin ratio predicts contrast-induced nephropathy in patients after emergency percutaneous coronary intervention. Cardiol Res Pract. (2019) 2019:8260583. doi: 10.1155/2019/8260583
15. MacIsaac RJ, Jerums G, Ekinci EI. Effects of glycaemic management on diabetic kidney disease. World J diabetes. (2017) 8:172–86. doi: 10.4239/wjd.v8.i5.172
16. Haller C. Hypoalbuminemia in renal failure: pathogenesis and therapeutic considerations. Kidney Blood Press Res. (2005) 28:307–10. doi: 10.1159/000090185
17. Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. (2006) 17:2937–44. doi: 10.1681/ASN.2006040368
18. Ad-hoc working group of E, Fliser D, Laville M, Covic A, Fouque D, Vanholder R, et al. A European Renal Best Practice (ERBP) position statement on the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines on acute kidney injury: part 1: definitions, conservative management and contrast-induced nephropathy. Nephrology dialysis transplantation: Off Publ Eur Dialysis Transplant Assoc - Eur Renal Assoc. (2012) 27:4263–72. doi: 10.1093/ndt/gfs375
19. Alba AC, Agoritsas T, Walsh M, Hanna S, Iorio A, Devereaux PJ, et al. Discrimination and calibration of clinical prediction models. JAMA. (2017) 318:1377–84. doi: 10.1001/jama.2017.12126
20. Fiorentino M, Castellano G, Kellum JA. Differences in acute kidney injury ascertainment for clinical. Nephrol Dial Transplant. (2017) 32:1789–805. doi: 10.1093/ndt/gfx002
21. Ozturk R, Inan D, Gungor B. Systemic immune-inflammation index is a predictor of contrast-induced nephropathy in patients with ST-segment elevation myocardial infarction. Angiology. (2022) 73:125–31. doi: 10.1177/00033197211029094
22. Huang Y, Gao Y, Wu Y, Lin H. Prognostic value of systemic immune-inflammation index in patients with urologic cancers: a meta-analysis. Cancer Cell Int. (2020) 20:499. doi: 10.1186/s12935-020-01590-4
23. Tsalamandris S, Antonopoulos AS, Oikonomou E, et al. The role of inflammation in diabetes: current concepts and future perspectives. Eur Cardiol. (2019) 14:50–9. doi: 10.15420/ecr
24. Dissanayake WC, Oh JK, Sorrenson B, Shepherd PR. Glucose regulates expression of pro-inflammatory genes, IL-1beta and IL-12, through a mechanism involving hexosamine biosynthesis pathway-dependent regulation of alpha-E catenin. Biosci Rep. (2021) 41. doi: 10.1042/BSR20211066
25. Kwan CK, Fu SC, Yung PS. A high glucose level stimulate inflammation and weaken pro-resolving response in tendon cells - A possible factor contributing to tendinopathy in diabetic patients. Asia-Pacific J sports medicine arthroscopy Rehabil technology. (2020) 19:1–6. doi: 10.1016/j.asmart.2019.10.002
26. Wang J, Yue X, Meng C, Wang Z, Jin X, Cui X, et al. Acute hyperglycemia may induce renal tubular injury through mitophagy inhibition. Front Endocrinol. (2020) 11:536213. doi: 10.3389/fendo.2020.536213
27. Bowen ME, Xuan L, Lingvay I, Halm EA. Random blood glucose: a robust risk factor for type 2 diabetes. J Clin Endocrinol Metab. (2015) 100:1503–10. doi: 10.1210/jc.2014-4116
28. Gill GV, Hardy KJ, Patrick AW, Masterson A. Random blood glucose estimation in type 2 diabetes: does it reflect overall glycaemic control? Diabetic medicine: J Br Diabetic Assoc. (1994) 11:705–8. doi: 10.1111/j.1464-5491.1994.tb00337.x
29. Ain Q, Latif A, Jaffar SR, Ijaz A. Evaluation of random plasma glucose for assessment of glycaemic control in type 2 diabetes mellitus. J Pak Med Assoc. (2017) 67:1353–6.
30. Kaysen GA, Dubin JA, Muller HG, Rosales L, Levin NW, Mitch WE, et al. Inflammation and reduced albumin synthesis associated with stable decline in serum albumin in hemodialysis patients. Kidney Int. (2004) 65:1408–15. doi: 10.1111/j.1523-1755.2004.00520.x
31. Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin dialysis. (2004) 17:432–7. doi: 10.1111/j.0894-0959.2004.17603.x
32. Arroyo V, Garcia-Martinez R, Salvatella X. Human serum albumin, systemic inflammation, and cirrhosis. J Hepatol. (2014) 61:396–407. doi: 10.1016/j.jhep.2014.04.012
33. Sheinenzon A, Shehadeh M, Michelis R, Shaoul E, Ronen O. Serum albumin levels and inflammation. Int J Biol Macromol. (2021) 184:857–62. doi: 10.1016/j.ijbiomac.2021.06.140
34. Yu MY, Lee SW, Baek SH, Na KY, Chae DW, Chin HJ, et al. Hypoalbuminemia at admission predicts the development of acute kidney injury in hospitalized patients: A retrospective cohort study. PloS One. (2017) 12:e0180750. doi: 10.1371/journal.pone.0180750
35. Li N, Qiao H, Guo JF, Yang HY, Li XY, Li SL, et al. Preoperative hypoalbuminemia was associated with acute kidney injury in high-risk patients following non-cardiac surgery: a retrospective cohort study. BMC Anesthesiol. (2019) 19:171. doi: 10.1186/s12871-019-0842-3
36. Kwasa EA, Vinayak S, Armstrong R. The role of inflammation in contrast-induced nephropathy. Br J Radiol. (2014) 87:20130738. doi: 10.1259/bjr.20130738
37. Lu Z, Cheng D, Yin J, Wu R, Zhang G, Zhao Q, et al. Antithrombin III protects against contrast-induced nephropathy. EBioMedicine. (2017) 17:101–7. doi: 10.1016/j.ebiom.2017.02.009
38. MaChado RA, Constantino Lde S, Tomasi CD, Rojas HA, Vuolo FS, Vitto MF, et al. Sodium butyrate decreases the activation of NF-kappaB reducing inflammation and oxidative damage in the kidney of rats subjected to contrast-induced nephropathy. Nephrology dialysis transplantation: Off Publ Eur Dialysis Transplant Assoc - Eur Renal Assoc. (2012) 27:3136–40. doi: 10.1093/ndt/gfr807
39. Yao Y, Chen S, Cao M, Fan X, Yang T, Huang Y, et al. Antigen-specific CD8(+) T cell feedback activates NLRP3 inflammasome in antigen-presenting cells through perforin. Nat Commun. (2017) 8:15402. doi: 10.1038/ncomms15402
40. Kuehnemund L, Koeppe J, Feld J, Wiederhold A, Illner J, Makowski L, et al. Gender differences in acute myocardial infarction-A nationwide German real-life analysis from 2014 to 2017. Clin Cardiol. (2021) 44:890–8. doi: 10.1002/clc.23662
41. Millett ERC, Peters SAE, Woodward M. Sex differences in risk factors for myocardial infarction: cohort study of UK Biobank participants. BMJ. (2018) 363:k4247. doi: 10.1136/bmj.k4247
Keywords: random glucose, albumin, post-contrast acute kidney injury, ST-segment elevation myocardial infarction, percutaneous coronary intervention
Citation: Lai P, Gu X, Lin X, He Y, Dai Y, Duan C, Liu Y and He W (2024) Association of random glucose to albumin ratio with post-contrast acute kidney injury and clinical outcomes in patients with ST-elevation myocardial infarction. Front. Endocrinol. 15:1390868. doi: 10.3389/fendo.2024.1390868
Received: 24 February 2024; Accepted: 03 June 2024;
Published: 18 June 2024.
Edited by:
Peter Hamar, Semmelweis University, HungaryReviewed by:
Zhenwei Wang, The First Affiliated Hospital of Zhengzhou University, ChinaSonia López-Cisneros, Instituto Nacional de Geriatría, Mexico
Marco Fiorentino, University of Bari Aldo Moro, Italy
Copyright © 2024 Lai, Gu, Lin, He, Dai, Duan, Liu and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenfei He, hwfeidoctor@163.com
†These authors have contributed equally to this work
 Ping Lai
Ping Lai