- Department of Dermatology, Hunan Aerospace Hospital, Changsha, China
Purpose: The aim of this study was to explore the relationship between hemoglobin levels, anemia and diabetic lower extremity ulcers in adult outpatient clinics in the United States.
Methods: A retrospective cross-sectional study was conducted on 1673 participants in the National Health and Nutrition Examination Survey (NHANES) from 1999 to 2004. Three logistic regression models were developed to evaluate the relationship between anemia and diabetic lower extremity ulcers. Model 1 adjusted for demographic and socioeconomic variables (age, sex, race and ethnicity, educational level, family income, and marital status). Model 2 included additional health-related factors (BMI, cardiovascular disease, stroke, family history of diabetes, hyperlipidemia, alcohol and smoking status). Model 3 further included clinical and laboratory variables (HbA1c, CRP, total cholesterol, and serum ferritin levels). Stratified analyses were also conducted based on age, sex, HbA1c level, body mass index (BMI), and serum ferritin level.
Results: The study included 1673 adults aged 40 years and older, with a mean age of 64.7 ± 11.8 years, of whom 52.6% were male. The prevalence of diabetic lower extremity ulcers (DLEU) was 8.0% (136 participants). Anemia was found in 239 participants, accounting for 14% of the study group. Model 1 showed an OR of 2.02 (95% CI=1.28~3.19) for anemia, while Model 2 showed an OR of 1.8 (95% CI=1.13~2.87). In Model 3, the OR for DFU in patients with anemia was 1.79 (95% CI=1.11~2.87). Furthermore, when serum ferritin was converted to a categorical variable, there was evidence of an interaction between DLEU status and serum ferritin in increasing the prevalence of DLEU.
Conclusion: After adjusting for confounding variables, higher levels of anemia were proportionally associated with an increased risk of incident DLEU. These results suggest that monitoring T2DM patients during follow-up to prevent the development of DLEU may be important. However, further prospective studies are needed to provide additional evidence.
Background
The International Diabetes Federation (IDF) has recently published data indicating that there has been a 16% increase (74 million) in the number of adults living with diabetes since 2019. Currently, approximately 537 million adults are affected by this condition. In 2021, T2DM was estimated to cause over 6.7 million deaths in the population aged 20-79 (1). Diabetic foot ulcers are one of the common and serious complications of diabetes mellitus, which can cause severe multi-organ complications leading to high mortality rates and significant health costs (2). Approximately 15% of people with diabetes will eventually develop a diabetic foot ulcers, and 14%-24% of these patients will require amputation due to ulcer-related complications (3).
Previous studies have reported that the prevalence of anemia in patients with DFU is over 50% (4). Common risk factor for foot ulceration include peripheral vascular disease, severity of neuropathy, structural foot deformity, concomitant infection, high plantar pressure, poor glycemic control, duration of diabetes, male gender, and presence of other micro and macrovascular complications. Anemia is also considered a major predictor of the outcome of DFU (5). Research has shown that patients with T2DM are twice as likely to experience anemia compared to those without T2DM (6, 7). The presence of altered microcirculation may exacerbate the negative effects of anemia, hindering ulcer healing and leading to higher rates of amputation and mortality (4, 8–11).
However, there have been no studies conducted on the association between DLEU and anemia in adult outpatients in the United States. The aim of this study was to examine the association between anemia in outpatients with and without DLEU in the NHANES database.
Materials and methods
Study population
The National Health and Nutrition Examination Survey (NHANES) was designed to evaluate the health and nutritional status of non-hospitalized Americans using a stratified, multistage approach. The NHANES received approval from the Ethics Review Committee of the National Center for Health Statistics (NCHS), and all participants provided written informed consent prior to participation. This is a retrospective study based on the NHANES database, which contains data on over 31,126 patients from 1999 to 2004. In the study, 9,970 were adults aged 40 years or older who completed the interview and underwent MEC screening. After excluding 8,297 participants who did not have diabetes (n=8160) and those with missing data on diabetes foot ulcers (n=3) and hemoglobin (n=188), the remaining 1,673 participants were included in the analysis (Figure 1).

Figure 1. Flowchart of the participant selection. NHANES, National Health and Nutrition Examination Survey.
Ascertainment of diabetic lower extremity t ulcers
The primary outcome variable was the status of diabetic lower extremity ulcers (DLEU), defined by the patient’s self-reported answer to the question in Question Data, ‘Have you had an ulcer or sore on your leg or foot that took more than four weeks to heal?’ Type 2 diabetes mellitus (T2DM) was identified based on the American Diabetes Association criteria and a self-report questionnaire. Participants were considered to have T2DM if they met any of the following criteria (12) (1): Glycated hemoglobin (HbA1c) levels of ≥6.5% (2), Fasting plasma glucose (FPG) levels of ≥126 mg/dL (3), 75 g oral glucose tolerance test (OGTT) levels of ≥11.1mmol/L (4), self-reported physician diagnosis of diabetes, or (5) receipt of oral glucose-lowering medicines or insulin.
Ascertainment of hemoglobin level, anemia
The NHANES Laboratory/Medical Technologists Procedures Manual (LPM) provides detailed instructions for sample collection and processing. The study employed the Beckman Coulter method for counting and sizing, combined with an automated diluter and mixer for sample processing and a single-beam photometer for hemoglobinometry to derive complete blood count (CBC) parameters. (https://www.cdc.gov/nchs/nhanes/). Anemia was defined by World Health Organization (WHO) as hemoglobin (Hb) levels <13g/dL for males and <12 g/dL for females (7).
Covariates
Based on the literature, several potential covariates were included in the analysis, such as age, sex, race/ethnicity, education level, marital status, PIR, smoking status, alcohol status, body mass index (BMI), laboratory parameters (total cholesterol and C-reactive protein [CRP], glycosylated hemoglobin [HbA1c], and serum ferritin, and comorbidities (13–16). The comorbidities included family history of diabetes, stroke, coronary heart disease, hyperlipidemia. Marital status was classified as living with a partner, or living alone (15). Family income was divided into three groups according to the poverty income ratio (PIR) as defined by a U.S. government report: low (PIR ≤ 1.3), medium (PIR > 1.3 to 3.5), and high (PIR > 3.5). Alcohol consumption was classified as never (< 12 drinks in lifetime), former (≥12 drinks in 1 year and no drinks in the last year, or no drinks in the previous year but≥12 drinks in lifetime), and current (≥12 drinks and currently drinking). Smoking status was categorized as never (<100 cigarettes in a lifetime), former (≥100 cigarettes but not currently smoking), and current (≥100 cigarettes and currently smoking) (16). Serum ferritin levels were classified as either <100 ng/mL or ≥100 ng/mL, according to previously reported classifications (17). The determination of previous disease (family history of diabetes, stroke, hyperlipidemia, and coronary heart disease) was based on the inquiry in the questionnaire of whether the doctor had been informed of the condition in the past.
Statistical analysis
The statistical analyses were conducted using R Statistical Software (Version 4.2.2, http://www.R-project.org, The R Foundation) and Free Statistics analysis platform (Version 1.9, Beijing, China, http://www.clinicalscientists.cn/freestatistics). The software is intended for reproducible analysis and interactive computing. A two-sided P value < 0.05 was considered statistically significant.
Normally distributed continuous variables were presented as mean ± SD, and skewed continuous variables were presented as median (interquartile range [IQR]). Categorical variables were expressed as frequencies (%). The Student’s t-test or Mann-Whitney U-test was used to compare continuous variables between groups, depending on the normality of the distribution, and categorical data were compared using the chi-squared or Fisher’s exact test, as appropriate.
Crude model was an unadjusted model. Model 1 was adjusted for age, sex, race and ethnicity, educational level, family income and marital status. Model 2 was developed using model 1 and additional factor such as BMI, coronary heart disease, stroke, family history of diabetes, hyperlipidemia, alcohol and smoking status. Model 3 was then developed using model 2 and additional factor such as HbA1c, CRP, total cholesterol, and serum ferritin. Subgroup analysis was conducted to investigate the correlation between anemia and diabetic lower extremity ulcers based on age, sex, BMI, and HbA1C category (<6.5, ≥6.5) as well as serum ferritin category (<100ng/mL, ≥100ng/mL). The percentage of missing values exceeded 20%. To address this issue, missing data for the covariates were imputed using multiple imputation.
Results
Baseline characteristics
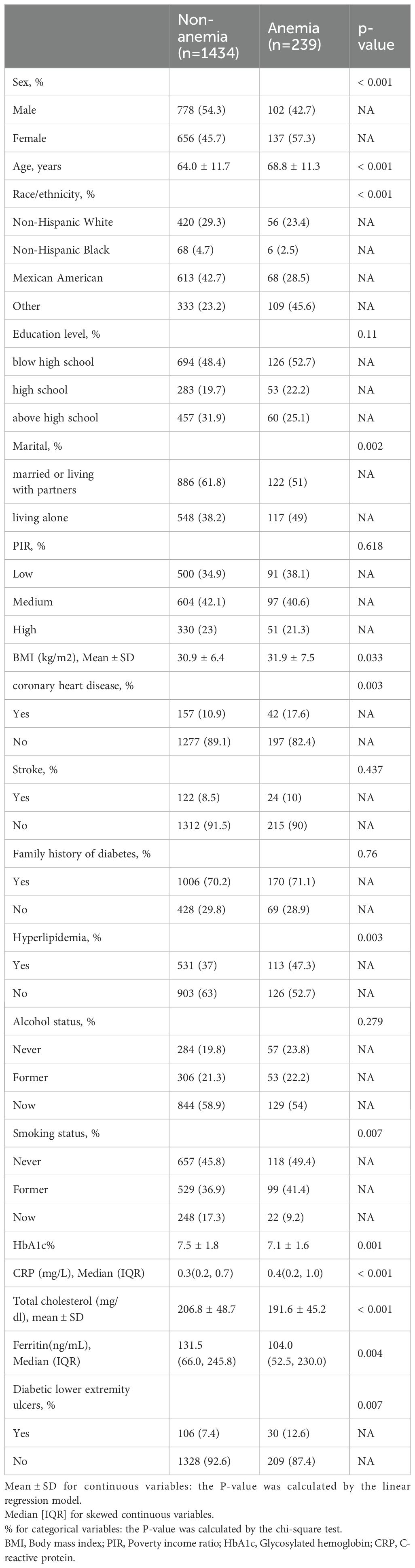
Table 1 displays demographic, socioeconomic, comorbidity, and baseline characteristics by anemia status. The study included 1673 adults aged 40 years and older, with a mean age of 64.7 ± 11.8 years, of whom 52.6% were male. Anemia was found in 239 participants, accounting for 14% of the study group, with a prevalence of 57.3% in women. The prevalence of diabetic lower extremity ulcers was 8.1% (136 participants). The prevalence of diabetic foot ulcers was 12.7% among patients with anemia.
Factor associated with diabetic lower extremity ulcers (DLEU)
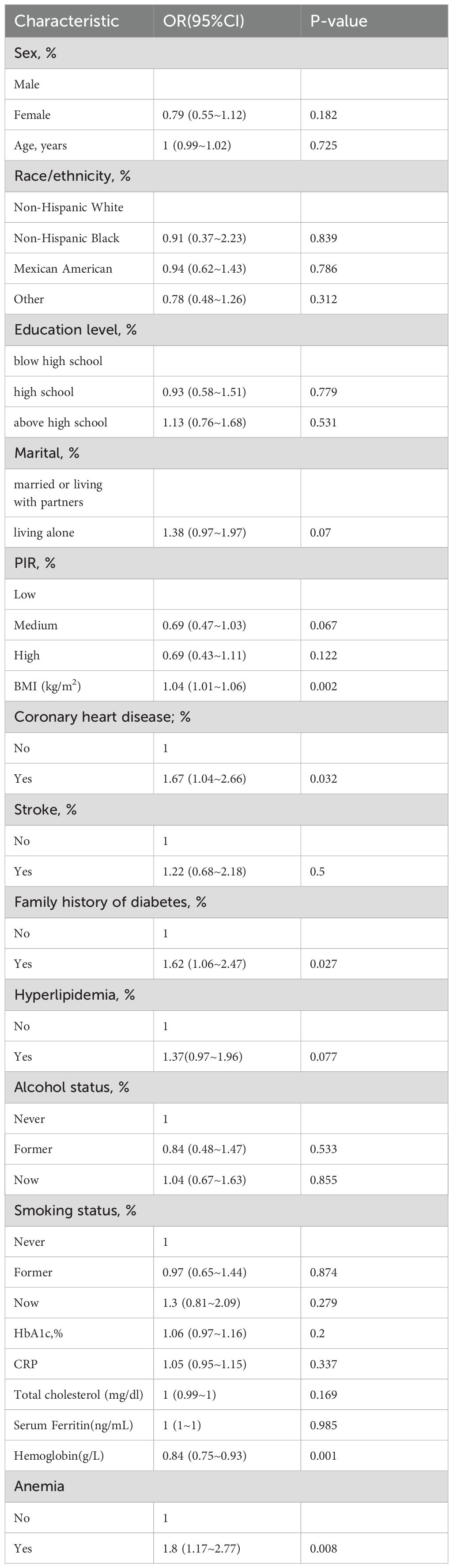
The univariate ordinal regression analysis results indicated that marital status, BMI, coronary heart disease, family history of diabetes, and hyperlipidemia. (P < 0.1; Table 2).
Relationship between hemoglobin levels, anemia status and diabetic lower extremity ulcers
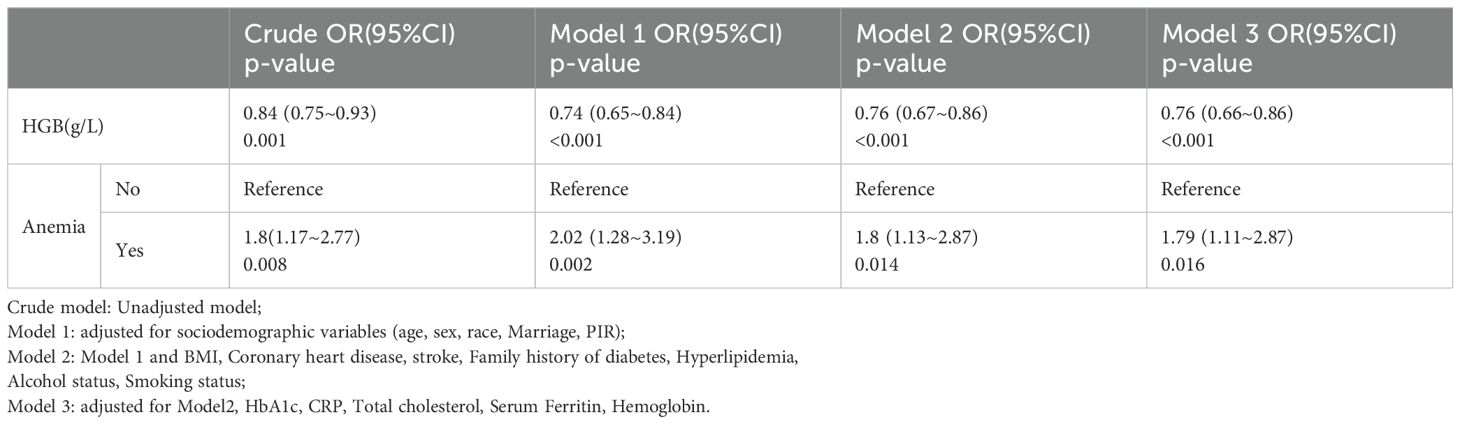
Table 3 presents the odds ratios (OR) and 95% confidence intervals (CI) for the presence of diabetic lower extremity ulcers (DLEU) determined by hemoglobin levels and anemia. When hemoglobin was analyzed as a continuous variable, a significant independent negative association was found between hemoglobin and the risk of DLEU. In the unadjusted model, each 1 unit increase in hemoglobin was associated with a 16% decrease in the presence of DLEU [OR=0.84, 95% CI: (0.75-0.993); p=0.001]. In model 1, 2 and 3, the association between hemoglobin (Hb) and diabetic lower extremity ulcers (DLEU) was marginally significant [OR: 0.74, 95% CI: (0.65-0.84); p<0.001] [OR: 0.76, 95% CI: (0.67-0.86); p<0.001] [OR: 0.76, 95% CI: (0.66-0.86); p<0.001], respectively.
The anemia group had a significantly higher risk of DLEU compared to the non-anemic group [OR: 1.79, 95% CI:(1.11-22.87)]. In Table 3, when hemoglobin levels were categorized as anemic versus non-anemia, anemia was found to be positively associated with the risk of diabetic lower extremity ulcers. The odds ratios (OR) for anemia were calculated for Model 1, Model 2, and Model 3, with the crude model as the reference, using multivariable-adjusted regression and 95% confidence intervals (CIs). The odds ratio (OR) for anemia in Model 1 was [OR=2.02,95% CI:(1.28-185 3.19), P=0.002]. In Model 2, the OR for anemia was [OR=1.8,95% CI:(1.13-2.87), P=0.014] and in Model 3, it was [OR=1.79, 95% CI:(1.11-2.87),p=0.016] (Table 2). Model 3 exhibited the lowest odds ratio (OR) compared to Model 1, which had the highest OR. This suggests a decreasing trend in the risk of diabetic lower extremity ulcers (DLEU). After conducting multivariate logistic regression analysis and smooth curve fitting, it was found that there is a negative association between hemoglobin levels and DLEU incidence when all potential confounders were taken into account (non-linearity: p=0.572).
Subgroup analyses of factor influencing the association between anemia and the presence of diabetic lower extremity ulcers
Stratified analysis was performed in several subgroups to determine the potential effect modifications on the relationship between anemia and DLEU. No significant interactions were found in any subgroup after stratification by sex, age, HbA1c level, and BMI (all P for interaction >0.05). However, results differed between serum ferritin groups for diabetic lower extremity ulcers (P = 0.015 for interaction) (Figure 2).
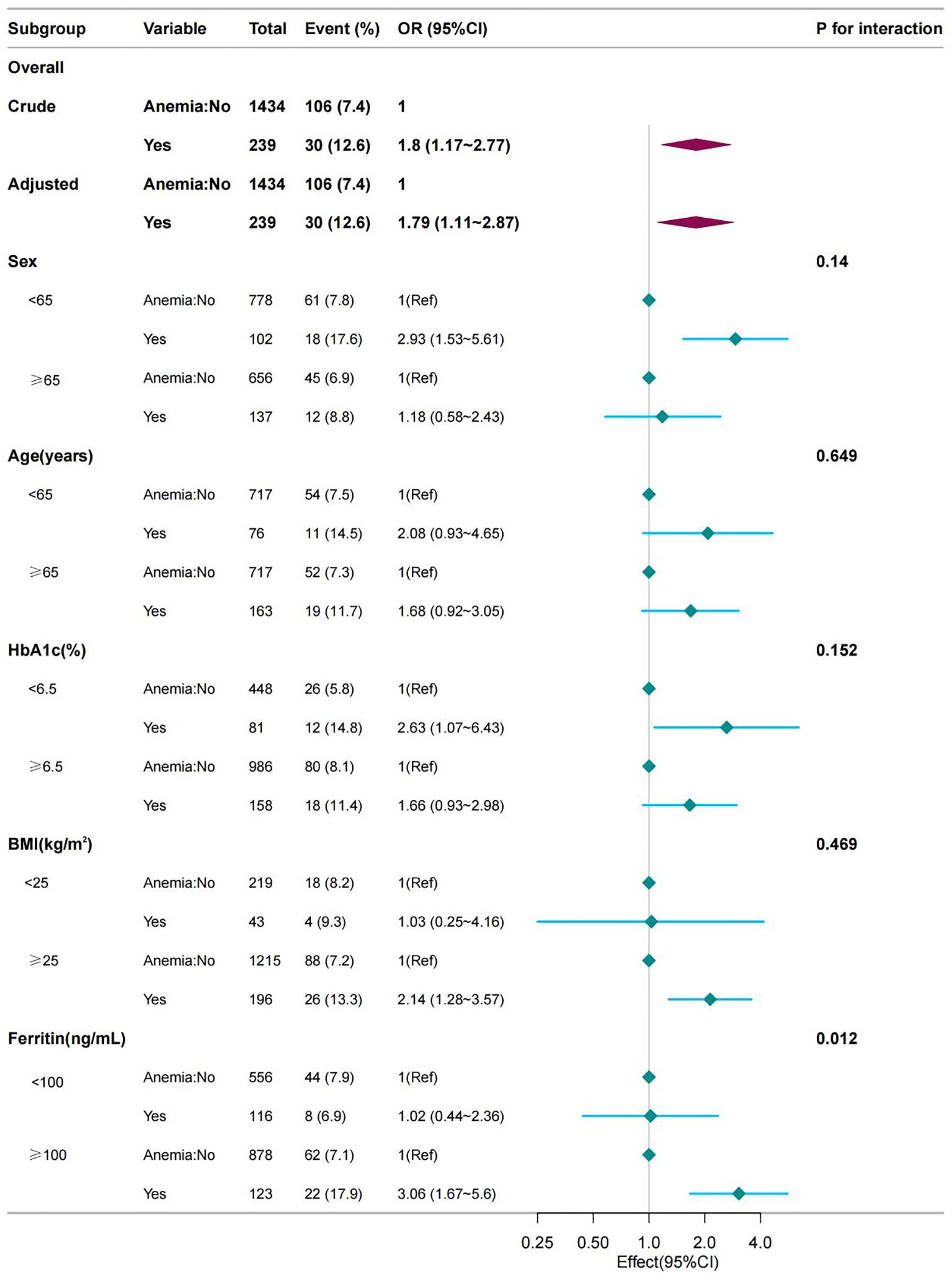
Figure 2. Effect size of anemia on the presence of DLEU in the age, sex, BMI, HbA1c subgroup and serum ferritin level. OR, odds ratio; CI, confidence interval; HGB, hemoglobin.
Discussion
In this cross-sectional study, anemia was found to be positively associated with the incidence of DLEU, and hemoglobin levels were a negative linear association between hemoglobin levels and DLEU Subgroup analysis revealed an interaction between serum ferritin and diabetic lower extremity ulcers, with high serum ferritin identified as a risk factor for diabetic lower extremity ulcers.
In contrast to previous studies that have shown consistency, the incidence of anemia was higher in patients with diabetic foot ulcers than in the non-anemic group (8, 18). Additionally, the prevalence of anemia was higher in women than in men. In this study, the prevalence rate of anemia in the DLEU group was 12.6%, which is higher than the rate in the non-DLEU group (7.4%). DFU can lead to high amputation and mortality rates, particularly in older patients with low hemoglobin levels (10). The more severe the anemia, the greater the impact on ulcer healing, and the higher the amputation rate and mortality (19, 20). Severe anemia can significantly impact ulcer healing and increase the rates of amputation and mortality (8, 21). Anemia is also a predictor of adverse outcomes (21, 22). In our study, the results of the fitted curves suggested a negative linear relationship between hemoglobin levels and the incidence of diabetic foot ulcers.
The results of our subgroup analysis indicate an interaction between serum ferritin and DLEU. It is suggested that high levels of serum ferritin increased the incidence of DLEU risk. Previous studies have shown that ferritin significantly increased with increasing DFU severity (21, 23). Proinflammatory cytokines inhibit the absorption and mobilization of iron from storage into the circulation by down-regulating iron expression in intestinal epithelial cells, macrophages, and hepatocytes. This interference with iron metabolism leads to elevated ferritin expression, which shortens erythrocyte lifespan and impairs EPO production and function, ultimately inhibiting the proliferation and differentiation of normal erythroid progenitor cells (24).
There was significant difference between patients with and without anemia in terms of diabetic microvascular complications (neuropathy, retinopathy, nephropathy) and the related conditions (25–27). However, the mechanism linking anemia and DFU remains unclear. Possible mechanisms include the following: 1) Anemia reduces limb perfusion and exacerbates limb ischemia, which impairs tissue oxygenation and blood flow, ultimately delaying ulcer wound healing (28). 2) Additionally, the presence of anemia induces oxidative stress and hypoxemia with resultant delays in wound healing (29). 3) In DFU patients, the deformability of red blood cells is significantly reduced, and the proportion of non-deformable red blood cells is significantly increased, which can impede capillary flow and lead to thrombosis, which may result in delayed ulcer healing (30). 4) In patients with anemia, blood viscosity decreases, which impairs peripheral circulation, vascular smooth muscle response and EPO levels are destroyed, resulting in damage to the compensatory response of neovascularization and hindering wound healing (31). 5) Pro-inflammatory cytokines released in anemic patients affect iron metabolism, impair the production and function of EPO, and inhibit the proliferation and differentiation of normal red blood cell precursor (24). 6) Reduced tissue oxygenation can lead to increased production of free radicals, endothelial dysfunction and nerve damage (32). 7) Additionally, anemia can accelerate the progression of microvascular and macrovascular complications (28).
This clinical study examines the relationship between anemia and diabetic lower extremity ulcers (DLEU) in adult outpatients in the United States. The study found that Hb levels were a protective factor for DLEU. Anemia is a risk factor for DLEU.
However, the study has several limitations. Firstly, missing data were unavoidable due to the retrospective nature of the study and the data being extracted from the patients’ medical records. Secondly, it does not provide information on the potential causal effect of hemoglobin. Thirdly, larger and prospective studies are needed to overcome this limitation. The study has several limitations. Fourthly, the study was unable to determine other variables such as the severity of DFU and the cause of anemia. Finally, caution should be exercised when extrapolating these findings to other populations as the study focused on a specific population. Interventional studies are necessary to investigate whether clinical correction of anemia reduces the incidence of DLEU and improves its prognosis and prediction.
These findings may have clinical implications, such as better control of hemoglobin concentrations in diabetic patients, especially those diabetic lower extremity ulcers with anemia. It is also important to determine whether correcting anemia reduces the incidence of DLEU and to establish the optimal Hb level required to reduce the risk of diabetic lower extremity ulcers. Well-designed prospective studies are necessary to test the associations and confirm the relationship between anemia and the causation of diabetic lower extremity ulcers.
Conclusion
The study found that hemoglobin level was a protective factor for DLEU, while anemia was an independent risk factor for DLEU in patients with diabetic lower extremity ulcers. Early identification of diabetic lower extremity ulcers risk provides an opportunity to delay or prevent disease onset. Prospective and multicenter studies are needed to explore whether anemia plays a direct role in the development, progression, or adverse outcomes of diabetic lower extremity ulcers.
Therefore, maintaining a higher concentration of hemoglobin is a protective factor that can prevent and ameliorate the development of DLEU.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Centers for Disease Control and Prevention (CDC). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article because NHANES data is publicly available and de-identified to protect the privacy and confidentiality of the participants. As a result, the data is considered to be in the public domain and does not require individual consent for publication.
Author contributions
JC: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JW: Formal analysis, Methodology, Writing – review & editing. SZ: Supervision, Writing – review & editing. GG: Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from Hunan Aerospace Hospital Scientific Research Program (2023YJ16).
Acknowledgments
We gratefully thank Jie Liu of the Department of Vascular and Endovascular Surgery, Chinese PLA General Hospital, Yan Gao of the Department of General Practice, The 960th Hospital of People’s Liberation Army for his contribution to the statistical support, study design consultations, and comments regarding the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1387218/full#supplementary-material
Abbreviations
NHANES, National Health and Nutrition Examination Survey; DLEU, diabetic lower extremity; BMI, Body mass index; PIR, Poverty income ratio; TC, total cholesterol; HbA1c, Glycosylated hemoglobin; HGB, hemoglobin; CRP, C-reactive protein.
References
1. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Available online at: https://pubmed.ncbi.nlm.nih.gov/31518657/ (Accessed January 12, 2024).
2. Everett E, Mathioudakis N. Update on management of diabetic foot ulcers. Ann N Y Acad Sci. (2018) 1411:153–65. doi: 10.1111/nyas.13569
3. Das SK, Roy P, Singh P, Diwakar M, Singh V, Maurya A, et al. Diabetic foot ulcer identification: A review. Diagnostics (Basel). (2023) 13:1998. doi: 10.3390/diagnostics13121998
4. Yammine K, Hayek F, Assi C. Is there an association between anemia and diabetic foot ulcers? A systematic review and meta-analysis. Wound Repair Regeneration. (2021) 29:432–42. doi: 10.1111/wrr.12902
5. Shareef AM, Ahmedani MY, Waris N. Strong association of anemia in people with diabetic foot ulcers (DFUs): Study from a specialist foot care center. Pak J Med Sci. (2019) 35:1216–20. doi: 10.12669/pjms.35.5.1421
6. Anemia complicating type 2 diabetes: Prevalence, risk factors and prognosis. Available online at: https://pubmed.ncbi.nlm.nih.gov/28433448/ (Accessed January 12, 2024).
7. AlDallal SM, Jena N. Prevalence of anemia in type 2 diabetic patients. J Hematol. (2018) 7:57–61. doi: 10.14740/jh411w
8. Li J, Zhang Z, Wei J, Li Y, Cheng C, Ma S, et al. Association between anemia and the risk and outcomes of diabetic foot in patients with type 2 diabetes mellitus. Exp Ther Med. (2023) 26:384. doi: 10.3892/etm.2023.12083
9. Anemia in patients with diabetic foot ulcer and its impact on disease outcome among Nigerians: Results from the MEDFUN study - PMC. Available online at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6917259/ (Accessed January 13, 2024).
10. Costa RHR, Cardoso NA, Procópio RJ, Navarro TP, Dardik A, de Loiola Cisneros L. Diabetic foot ulcer carries high amputation and mortality rates, particularly in the presence of advanced age, peripheral artery disease and anemia. Diabetes Metab Syndr. (2017) 11 Suppl 2:S583–7. doi: 10.1016/j.dsx.2017.04.008
11. Bus SA, Lavery LA, Monteiro-Soares M, Rasmussen A, Raspovic A, Sacco ICN, et al. Guidelines on the prevention of foot ulcers in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res. (2020) 36:e3269. doi: 10.1002/dmrr.3269
12. American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes—2021. Diabetes Care. (2021) 44:S15–33. doi: 10.2337/dc21-S002
13. Wang X, Xu M, Meng L, Song M, Jia Z, Zhao L, et al. The awareness and determinants of diabetic foot ulcer prevention among diabetic patients: insights from NHANES (2011-2018). Prev Med Rep. (2023) 36:102433. doi: 10.1016/j.pmedr.2023.102433
14. Hicks CW, Wang D, Matsushita K, McEvoy JW, Christenson R, Selvin E. Glycated albumin and HbA1c as markers of lower extremity disease in US adults with and without diabetes. Diabetes Res Clin Pract. (2022) 184:109212. doi: 10.1016/j.diabres.2022.109212
15. Liu H, Wang L, Chen C, Dong Z, Yu S. Association between dietary niacin intake and migraine among American adults: National health and nutrition examination survey. Nutrients. (2022) 14:3052. doi: 10.3390/nu14153052
16. Yan S, Luo W, Lei L, Zhang Q, Xiu J. Association between serum klotho concentration and hyperlipidemia in adults: A cross-sectional study from NHANES 2007-2016. Front Endocrinol (Lausanne). (2023) 14:1280873. doi: 10.3389/fendo.2023.1280873
17. Babaei M, Shafiei S, Bijani A, Heidari B, Hosseyni SR, Vakili Sadeghi M. Ability of serum ferritin to diagnose iron deficiency anemia in an elderly cohort. Rev Bras Hematologia e Hemoterapia. (2017) 39:223–8. doi: 10.1016/j.bjhh.2017.02.002
18. Jia W, Weng J, Zhu D, Ji L, Lu J, Zhou Z, et al. Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab Res. (2019) 35:e3158. doi: 10.1002/dmrr.3158
19. Chuan F, Zhang M, Yao Y, Tian W, He X, Zhou B. Anemia in patients with diabetic foot ulcer: prevalence, clinical characteristics, and outcome. Int J Low Extrem Wounds. (2016) 15:220–6. doi: 10.1177/1534734616660224
20. Yammine K, Akiki S, Assi C, Hayek F. Anemia prevalence among patients with diabetic foot ulcers necessitating surgery on admission: a preliminary, retrospective comparative study. Wounds. (2022) 34:216–9. doi: 10.25270/wnds/21073
21. Kumar R, Singh SK, Agrawal NK, Kumar U, Kumar S, Bishnoi A. The prevalence of anemia in hospitalized patients with diabetic foot ulcer (DFU) and the relationship between the severity of anemia and the severity of DFU. Cureus. (2023) 15:e41922. doi: 10.7759/cureus.41922
22. Giangreco F, Iacopi E, Malquori V, Pieruzzi L, Goretti C, Piaggesi A. In blood we trust: anemia as a negative healing prognostic factor in diabetic foot patients. Acta Diabetol. (2023) 61:245–51. doi: 10.1007/s00592-023-02188-8
23. Xu B, Yang CZ, Wu SB, Zhang D, Wang LN, Xiao L, et al. risk factors for lower extremity amputation in patients with diabetic foot. Zhonghua Nei Ke Za Zhi. (2017) 56:24–8. doi: 10.3760/cma.j.issn.0578-1426.2017.01.007
24. Chaparro CM, Suchdev PS. Anemia epidemiology, pathophysiology, and etiology in low- and middle-income countries. Ann N Y Acad Sci. (2019) 1450:15–31. doi: 10.1111/nyas.14092
25. Li X, Chen M. Correlation of hemoglobin levels with diabetic retinopathy in US adults aged ≥40 years: The NHANES 2005–2008. Front Endocrinol (Lausanne). (2023) 14:1195647. doi: 10.3389/fendo.2023.1195647
26. Anemia in diabetic patients reflects severe tubulointerstitial injury and aids in clinically predicting a diagnosis of diabetic nephropathy . Available online at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8170246/ (Accessed February 8, 2024).
27. Tantigegn S, Ewunetie AA, Agazhe M, Aschale A, Gebrie M, Diress G, et al. Time to diabetic neuropathy and its predictors among adult type 2 diabetes mellitus patients in amhara regional state comprehensive specialized hospitals, northwest Ethiopia, 2022: A retrospective follow up study. PloS One. (2023) 18:e0284568. doi: 10.1371/journal.pone.0284568
28. Sahay M, Kalra S, Badani R, Bantwal G, Bhoraskar A, Das AK, et al. Diabetes and Anemia: International Diabetes Federation (IDF) – Southeast Asian Region (SEAR) position statement. Diabetes Metab Syndrome: Clin Res Rev. (2017) 11:S685–95. doi: 10.1016/j.dsx.2017.04.026
29. Grune T, Sommerburg O, Siems WG. Oxidative stress in anemia. Clin Nephrol. (2000) 53:S18–22. doi: 10.1046/j.1464-410x.2000.00494.x
30. Milionis H, Papavasileiou V, Eskandari A, D’Ambrogio-Remillard S, Ntaios G, Michel P. Anemia on admission predicts short- and long-term outcomes in patients with acute ischemic stroke. Int J Stroke: Off J Int Stroke Soc. (2015) 10:224–30. doi: 10.1111/ijs.12397
31. Schmidt-Lucke C, Glattkowski-Schäfer G, Kirchhof C, von Bierbrauer A, Klein HU, Schmidt-Lucke JA. Incidence of cutaneous vasoactivity in patients with anemia and pulmonary hypoxia. VASA Z fur Gefasskrankheiten. (2000) 29:112–5. doi: 10.1024/0301-1526.29.2.112
Keywords: hemoglobin, anemia, diabetic lower extremity ulcers, NHANES, cross-sectional study
Citation: Cao J, Wang J, Zhang S and Gao G (2024) Association between anemia and diabetic lower extremity ulcers among US outpatients in the National Health and Nutrition Examination Survey: a retrospective cross-sectional study. Front. Endocrinol. 15:1387218. doi: 10.3389/fendo.2024.1387218
Received: 17 February 2024; Accepted: 13 August 2024;
Published: 29 August 2024.
Edited by:
Åke Sjöholm, Gävle Hospital, SwedenReviewed by:
Michael Edwin Edmonds, King’s College Hospital NHS Foundation Trust, United KingdomUmesh Kumar, University of Innsbruck, Austria
Copyright © 2024 Cao, Wang, Zhang and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinmin Cao, Y2FvamlubWluX2Nvb2xAMTI2LmNvbQ==
 Jinmin Cao
Jinmin Cao Jingpei Wang
Jingpei Wang